Abstract
Given the long-term nature of methadone maintenance treatment, it is important to assess the extent of cognitive side effects. This study investigated cognitive and psychomotor performance in fifty-one methadone maintenance patients (MMP) as a function of time since last methadone dose and maintenance dose level. MMP maintained on doses ranging from 40 to 200 mg (Mean = 97 mg) completed a battery of psychomotor and cognitive measures across two sessions, during peak and trough states, in a double-blind crossover design. Peak sessions were associated with worse performance on measures of sensory processing, psychomotor speed, divided attention, and working memory, compared to trough sessions. The effects of maintenance dose were mixed, with higher dose resulting in worse performance on aspects of attention and working memory, improved performance on executive function, and no effects on several measures. Longer treatment duration was associated with better performance on some measures, but was also associated with increased sensitivity to time since last dose (i.e., worse performance at peak vs. trough) on some measures. The results suggest that cognitive functioning can fluctuate as a function of time since last dose even in MMP who have been maintained on stable doses for an extended time (mean duration in treatment = 4 years), but worsened performance at peak is limited to a subset of functions and may not be clinically significant at these modest levels of behavioral effect. For patients on stable methadone maintenance doses, maintenance at higher doses may not significantly increase the risk of performance impairment.
Keywords: methadone maintenance, substance dependence, opioid, cognition, dose
INTRODUCTION
Methadone maintenance is one of the most widespread treatments for opioid dependence. Daily oral methadone administration suppresses withdrawal symptoms, reduces relapse potential by blocking heroin-induced euphoria, and improves general health outcomes (Tetrault & Fiellin, 2012). Methadone maintenance provides opioid-dependent individuals stability to initiate pro-social lifestyle changes. Due to the long-term nature of methadone treatment, it is important to assess the extent of cognitive side effects that may affect clinical improvement, treatment compliance, and daily functioning.
There is evidence that methadone maintenance patients (MMP) exhibit psychomotor and cognitive impairment compared with matched controls without histories of drug abuse (Mintzer & Stitzer, 2002; Pirastu et al., 2006; Prosser, London, & Galynker, 2009; Specka et al., 2000), or abstinent former opioid abusers (Constantinou et al., 2010; Darke, McDonald, Kaye, & Torok, 2012; Mintzer, Copersino, & Stitzer, 2005; Prosser et al., 2006; Verdejo, Toribio, Orozco, Puente, & Perez-Garcia, 2005). However, conclusions based on observational group comparison studies can be limited due to lack of experimental control, individual differences in patient populations, and selection biases for groups. An experimental design in which cognitive performance is assessed as a function of time relative to methadone dosing would be helpful in determining the effect of methadone itself on cognition.
To date, only three studies have measured cognitive performance in MMP as a function of time relative to methadone dosing (Baewert et al., 2007; Kelley, Welch, & McKnelley, 1978; Lyvers & Yakimoff, 2003). In a within-subject study, Kelley et al. (1978) measured auditory threshold, distance perception, reaction time, time perception, working memory, and attention in MMP during two cognitive testing sessions approximately one week apart. Participants were administered an oral solution of placebo (quinine) or methadone mixed with orange juice in a double-blind fashion one hour before, and immediately after, cognitive testing on each of the two sessions; they were told that each drink was half of their methadone dose. The mean methadone dose was 63 mg, with doses ranging from 20-120 mg. During the peak session (1 hour after methadone dosing), the solution administered before cognitive testing contained methadone and the solution after cognitive testing contained placebo. During the trough session (25 hours after methadone dosing), the pre-testing solution contained placebo and the post-testing solution contained methadone. Performance differences as a function of session (peak vs. trough) were found only on the distance perception measure, and the results were inconsistent, with participants estimating distance less accurately at trough vs. peak for two of the three tested distances, but less accurately at peak vs. trough for the third tested distance.
Baewert et al. (2007) measured roadway traffic-relevant psychomotor and cognitive performance using the Act and React Test System (ART) 2020 in a between-subjects design. MMP who were tested 90 minutes after methadone dosing (estimated time of peak drug plasma levels) performed worse on traffic-specific perception ability compared with MMP who were tested 20 hours after methadone dosing (trough), suggesting an acute effect of methadone on performance. The mean methadone dose was 53 mg, with doses ranging from 21-80 mg. Lyvers and Yakimoff (2003) measured executive function using the Wisconsin Card Sorting Task (WCST) in MMP tested 90 minutes (peak) or 24 hours (trough) after methadone administration (mean dose = 67 mg). The trough group had more perseverative error responses (worse executive function) compared with the peak group, indicating a possible opioid withdrawal effect. Interpretation of the Baewert et al (2007) and Lyvers and Yakimoff (2003) studies is somewhat limited due to the use of between-subjects designs and unblinded dose regimens.
In addition to peak vs. trough differences, methadone maintenance dose level may play a role in the cognitive effects of methadone. Importantly, higher maintenance doses are becoming more prevalent due to the availability of increasingly pure heroin (Tetrault & Fiellin, 2012) and the increasing recognition that higher doses are therapeutically more effective (Faggiano, Vigna-Taglianti, Versino, & Lemma, 2003; Kelly, O'Grady, Mitchell, Brown, & Schwartz, 2011; Strain, Bigelow, Liebson, & Stitzer, 1999). Studies of dose effects on cognition in MMP have produced mixed results. Higher methadone doses were associated with worse cognitive function during peak state on some measures in two studies (Loeber, Kniest, Diehl, Mann, & Croissant, 2008; Rapeli et al., 2007), while other studies have shown improved performance (Bracken et al., 2012) or no association between methadone dose level and cognitive performance (Darke, Sims, McDonald, & Wickes, 2000; Lombardo, Lombardo, & Goldstein, 1976; Soyka et al., 2005). However, to our knowledge no study has evaluated the effects of maintenance dose level as a function of time relative to methadone dosing.
The purpose of the present study was to investigate cognitive performance in MMP as a function of time since last methadone dose (peak vs. trough) and maintenance dose level. MMP maintained on a wide range of doses completed a battery of psychomotor and cognitive measures across two sessions, during peak and trough states, in a double-blind crossover design. Maintenance dose level was included in the analyses. Based on prior studies, we hypothesized that performance would be impacted by time relative to dosing (peak vs. trough), but the direction of the effect (better vs. worse performance at peak or trough) was uncertain given the inconsistencies observed previously (Baewert, et al., 2007; Kelley, et al., 1978; Lyvers & Yakimoff, 2003).
METHODS
Participants
Fifty-one methadone maintenance patients (59% female) in methadone treatment for at least six months were recruited from outpatient methadone maintenance programs in Baltimore, Maryland USA. Daily methadone dose ranged from 40 to 200 mg (M=97.5, SD=34.2). Dose adjusted for weight ranged from 0.6 to 2.3 mg/kg (M=1.2, SD=0.4). Methadone treatment duration ranged from 6 to 274 months (M=48.9, SD=54.7). Potential volunteers were excluded if they had a history of significant medical problems, brain trauma affecting cognitive functioning, or an Axis I psychiatric disorder (except substance abuse or dependence), as assessed by a medical questionnaire, Symptom Checklist (SCL-90; Derogatis, 1977), and the DSM Checklist for Adult Disorders (Hudziak, Copeland, Stanger, & Wadsworth, 2004; Hudziak et al., 1993) (modified for DSM-IV-TR classification) when SLC-90 responses were out of the normal range. Because cognitive impairment induced by acute doses of benzodiazepines and alcohol is well documented (Curran, 1991, 2000; Hammersley, Finnigan, & Millar, 1992), study volunteers with recent benzodiazepine use (based on clinic urinalysis records), a current diagnosis of sedative/hypnotic or alcohol dependence (based on clinic records), or urine screen positive for benzodiazepines or breathalyzer test positive for alcohol at the initial screening interview were also excluded. All participants received detailed information about the study protocol, gave written informed consent, and were paid for their participation. The Institutional Review Board of the Johns Hopkins University School of Medicine approved the study. Demographic variables and drug use characteristics are presented in Table 1.
Table 1.
Demographics and drug use characteristics.
| Demographic variables | Value | Range |
|---|---|---|
| Sample size | 51 | |
| Sex (Male/Female) | 21/30 | |
| Age (years) | 45.80 ± 7.54 | 28 – 55 |
| Education (years) | 11.87 ± 2.01 | 7 – 16 |
| Estimated IQ | 91.94 ± 12.01 | 67 – 111 |
| Methadone dose (mg) | 97.47 ± 34.23 | 40 – 200 |
| Time in treatment (months) | 48.94 ± 54.69 | 6 – 274 |
| Race | ||
| African American | 31 | |
| Caucasian | 18 | |
| Other | 2 |
| Self-reported drug use | ||||
|---|---|---|---|---|
| A. Drug use history. | ||||
| Drug | 30 days | 6 months | 1 year | Lifetime |
| Cocaine | 16 (8) | 24 (12) | 37 (19) | 92 (47) |
| Heroin | 0 | 8 (4) | 33 (17) | 100 (51) |
| Benzodiazepine | 6 (3) | 10 (5) | 20 (10) | 67 (34) |
| Cannabis | 4 (2) | 12 (6) | 20 (10) | 94 (48) |
| Alcohol | 27 (13) | 40 (19) | 49 (25) | 98 (50) |
| Caffeine | 94 (48) | 94 (48) | 94 (48) | 98 (50) |
| Nicotine | 78 (40) | 78 (40) | 80 (40) | 82 (42) |
| Amphetamine | 0 | 0 | 0 | 4 (2) |
| Hallucinogen | 0 | 2 (1) | 2 (1) | 39 (20) |
| B. Drug use severity. | |||
|---|---|---|---|
| Years Used | Cost per day ($) | Cost per week ($) | |
| Cocaine | 16.59 ± 10.59 | 50.31 ± 74.24 | 235.77 ± 465.22 |
| Heroin | 16.21 ± 9.36 | 44.85 ± 45.08 | 306.25 ± 314.96 |
Note. Values for sample size, sex, and race represent n. Values for age, years of education, IQ, methadone dose, and time in treatment represent mean ± 1 standard deviation. Estimated IQ was assessed by the Shipley Institute of Living Scale (Zachary, Paulson, & Gorsuch, 1985).
Note. Values in part A represent % (n); values in part B represent mean ± 1 standard deviation. Data missing for use: benzodiazepine 30 days and 6 months (n = 1), cannabis 30 days and 6 months (n = 1), alcohol 30 days and 6 months (n = 3). Data missing for cost: cocaine (n = 2), heroin (n = 2).
Procedure
Participants completed two outpatient performance-testing sessions approximately one week apart. Prior to each session, the participant’s methadone dose and usual dosing time were confirmed with his/her outpatient clinic. On session days, participants reported to the Johns Hopkins Behavioral Pharmacology Research Unit (instead of their clinic) at their usual daily dosing time. Participants were administered an oral solution in a double-blind fashion 120 min before, and immediately after, the performance testing battery on each of the two sessions. During the peak session, the pre-battery solution contained the participant’s usual methadone dose and the post-battery solution contained placebo. During the trough session, the pre-battery solution contained placebo and the post-battery solution contained methadone. Performance testing began 120 min after the first dosing point so that testing during the peak session would occur during the window of peak methadone effects (Walsh, Preston, Stitzer, Cone, & Bigelow, 1994). The order of the peak and trough sessions was counterbalanced across participants. Methadone doses were mixed with dilute cherry syrup, and placebo doses contained dilute cherry syrup only. The cherry syrup contained 12mcg denatonium benzoate as a bitter-flavored taste mask for blinding purposes. All doses were 40 ml total volume. Participants were instructed to abstain from all drugs (except methadone, and nicotine in smokers) prior to sessions. Participants were tested for drugs 24 hours before each session and on the day of the session with a urine screen (benzodiazepines, opioids, methadone, cocaine) using an EMIT system (Syva Co., Palo Alto, CA) and alcohol breathalyzer. In order to avoid acute heroin or cocaine intoxication effects, participants with current cocaine or heroin use were required to abstain for 24 hours prior to each experimental session. If the drug screen was positive for cocaine or heroin (morphine) 24 hours before the session, quantitative urinalysis procedures were used to compare drug levels on the day of the session to the levels 24 hours earlier; if these procedures indicated ≥ 30% reduction in urine drug levels, the participant was allowed to participate in the session (Preston, Silverman, Schuster, & Cone, 1997). Six participants tested positive for cocaine prior to a session and all showed the specified reduction, indicating absence of recent use. No participants tested positive for heroin (morphine) 24 hours before testing.
Cognitive Battery
Participants completed training on the cognitive battery prior to the first experimental session to reduce potential practice effects. Training included a minimum of 3 practice trials on each task and stable performance (within 20% performance on primary outcome) on 3 consecutive trials. Tasks were administered in the same order for all sessions. During all sessions, a research assistant tested each participant individually and provided standardized instructions prior to each task. Short breaks were given to participants at specified intervals between tasks. Total time to complete the cognitive battery (including breaks) was approximately 90 minutes. All computerized measures were administered on an Apple Macintosh microcomputer (Apple Computer, Cupertino, CA).
Sensory Processing
The Flicker Fusion task assessed sensory acuity and temporal discrimination in the visual system (Simonson & Brozek, 1952). Participants viewed a light stimulus across a range of frequencies and used a toggle switch to indicate, on increasing and decreasing frequency curves, the points at which the stimulus appeared respectively to stop and start flickering. The transition point on the ascending curve is called the threshold of fusion, and the transition point on the decreasing curve is called the threshold of flicker. The average of the flicker and fusion thresholds is the primary outcome variable.
Psychomotor Function
1) A standing balance task measured the ability to stand on one leg with eyes closed; the dependent measure was the number of seconds balanced on each leg (a maximum of 30 sec for each leg). 2) A simple reaction time (RT) task measured the time to respond to a visual stimulus that appeared on the screen at random intervals. The dependent measures were median RT and RT range (absolute difference between maximum and minimum RT) to assess variability). 3 The Circular Lights task involved rapid hand-eye coordinated movements in which the participant pressed a series of 16 buttons (circularly arranged around a 54-cm diameter) as rapidly as possible in response to the randomly sequenced illumination of their associated lights (Griffiths, Bigelow, & Liebson, 1983). The dependent measure was the number of correct button presses during a 60 second trial. 4) In a computerized task analogous to the Trail-Making Test, participants connected numbers in numerical sequence (Trails A) or alternated between numbers and letters (Trails B) (Mintzer, Frey, Yingling, & Griffiths, 1997). Trails A assessed psychomotor speed, and Trails B assessed the ability to shift between sets within working memory and conceptual flexibility (executive function) (Reitan & Wolfson, 1993). Dependent measures were completion time in seconds for Trails A, Trails B, and Trails B minus Trails A (Trails B-A).
Attention
Focused attention was measured using a computerized version of the Digit Symbol Substitution Test (DSST) (McLeod, Bigelow, & Liebson, 1982; Wechsler, 1997). Participants responded to randomly selected digits (1-9) appearing on the computer screen using a numeric keypad to reproduce the corresponding geometric symbols using a digit-symbol code displayed continuously at the top of the screen. The dependent measures were number attempted (speed) and proportion correct (accuracy) during a 90 second trial.
Divided attention was measured using a task that included simultaneous visual tracking, which focused on the center of the screen, and monitoring, which occurred in the corners of the screen (Kleykamp, Griffiths, & Mintzer, 2010). Participants used the mouse to track a diamond stimulus moving horizontally in the center of the computer screen while monitoring a target digit presented at the bottom center of the screen. Participants had to click the mouse each time the digit appearing in one of the four corners of the screen matched the target digit. Dependent measures associated with tracking were tracking moves (number of times the cross hair was moved on the screen), tracking deviation (distance in pixels between the diamond stimulus and cross hair), and tracking overlap (number of times the cross hair and diamond overlapped). Dependent measures associated with monitoring were mean RT (time between digit appearance and participant response), and proportion correct (number of times a mouse press was made when the target digit was presented in the corner of the screen, out of a total possible of 24). Outcomes for this task can be categorized as assessing either speed (tracking moves, mean RT) or accuracy (tracking deviation, tracking overlap, and proportion correct).
Working Memory
Working memory was assessed using: 1) the n-back task (Jonides et al., 1997; Mintzer & Griffiths, 2007) and 2) a modified Sternberg task (Mintzer & Griffiths, 2007; Sternberg, 1969). In the n-back task (0, 1, 2, and 3-back), memory load is manipulated by varying the number of positions ‘n’ back. For each n-back, sixty consonant letters (excluding letters l, w, and y) were presented consecutively on the screen, and participants were instructed to click “yes” whenever the current letter on the screen matched (target) the letter ‘n’ positions back in the sequence, and to click “no” when there was not a match (non-target). The 0-back is a control condition that involves minimal memory and provides a measure of focused attention only; participants were told to click “yes” when the letter on the screen matched a predetermined target letter and click “no” when there was not a match. Dependent measures are the proportion of yes responses made to target letters (hit rate), proportion of yes responses made to non-target letters (false alarm rate), signal detection measures of sensitivity in distinguishing between target and non-target letters (d’) and response bias (C), and median reaction times to correct trials.
In the Sternberg task, a memory set consisting of seven randomly selected and randomly ordered consonant letters (e.g., ZHFKDXW) was presented on the screen followed by a probe consisting of a lower case letter–digit pair (e.g., f–4), and participants were asked to decide whether the probed letter had appeared in the memory set in the ordinal position represented by the digit (e.g., 4 = 4th position in the memory set). Participants completed 24 trials consisting of 12 trials in each of two conditions: non-memory control (i.e., the memory set remained on the screen during probe presentation), and 12-sec delay (between memory set and probe presentation). The accuracy of the response and participants’ RT from the onset of the probe was recorded. Dependent measures were proportion of correct responses and median RT on correct trials.
Episodic Memory and Metamemory
Episodic memory was assessed using word recall and recognition (Mintzer & Stitzer, 2002). Participants studied a list of 70 items, and following a 75-minute delay they completed a free recall test, in which they were given 5 minutes to write down all the words they remembered from the study phase. After free recall, they completed recognition memory testing, which consisted of presentation of the previously studied words and 70 randomly intermixed new words. Participants made confidence judgments about the degree to which they recognized (old) or did not recognize (new) each word from the study phase on a six-point Likert scale (definitely old, probably old, maybe old, maybe new, probably new, definitely new). The outcome measure for free recall performance was the number of correct responses. Dependent measures for recognition memory were proportion of ‘old’ responses made to old words (hit rate), proportion of ‘old’ responses to new words (false alarm rate), signal detection measures of sensitivity in distinguishing between old and new words (d’), and response bias (C; Snodgrass & Corwin, 1988). Metamemory was evaluated by calculating the Goodman-Kruskal gamma correlation between confidence ratings and recognition memory accuracy, collapsed across old and new words for sufficient power (Goodman & Kruskal, 1954).
Data Analysis
A Generalized Estimating Equation (GEE) analysis was conducted for each dependent measure in the cognitive battery. The factors in the model were session time relative to dosing (peak vs. trough session) and weight-adjusted methadone maintenance dose (continuous variable; in mg per kg). Analyses using unadjusted dose (mg) did not differ significantly from those using the weight-adjusted dose. Order (peak session first vs. trough session first) was included as a factor in the model only when it had a significant effect. The covariates included in the initial model were treatment duration (continuous variable; in months), age, sex, and education. However, sex and education were removed from the model due to few effects on performance. On measures where sex was a significant covariate (p<.05), being female was associated with better performance on flicker fusion (threshold frequency), simple RT (RT range), and divided attention (monitoring accuracy: proportion correct), but worse performance on free recall (number correct). Education was not a significant covariate for any measure. In addition to main effects, interactions between the covariate treatment duration and the main factors in the model (session, dose) were also analyzed. The n-back and Sternberg working memory tasks each had an additional factor of condition (memory load and delay, respectively). A priori statistical significance was set at p < 0.05.
RESULTS
GEE analysis results for cognitive measures with significant effects are presented in Table 2. For significant main effects of session, the values presented in the text below for peak and trough sessions represent the fitted means based on the model (not the raw means) and the standard error of the fitted mean, at the mean methadone dose level.
Table 2.
Generalized Estimating Equation (GEE) analysis results for cognitive measures with significant effects.
| Measure | Variable | Factor | B | SE | p |
|---|---|---|---|---|---|
| Flicker Fusion | Threshold frequency | Session | −2.629 | 0.718 | 0.000 |
| Simple RT | Median RT (ms) | Session | 18.255 | 8.671 | 0.035 |
| Median RT (ms) | Session × Duration | 0.459 | 0.143 | 0.001 | |
| RT Range (ms) | Session | 815.687 | 318.525 | 0.010 | |
| Circular Lights | # correct | Session × Duration | −0.027 | 0.013 | 0.037 |
| oTrail Making Test | Trails A time (s) | Session × Order | 11.950 | 5.856 | 0.041 |
| Trails B-A time (s) | Dose | −45.667 | 19.698 | 0.020 | |
| oDSST | # trials attempted | Session | 1.842 | 0.741 | 0.013 |
| # trials attempted | Session × Order | −3.967 | 1.100 | <0.001 | |
| Divided Attention | # tracking moves | Dose | −992.858 | 399.392 | 0.013 |
| Tracking deviation (pixels) | Session | 2.276 | 1.152 | 0.048 | |
| Tracking overlap (accuracy) | Session | −2.433 | 1.112 | 0.029 | |
| RT (monitoring) (ms) | Dose | 0.237 | 0.096 | 0.013 | |
| RT (monitoring) (ms) | Session × Dose | −0.201 | 0.092 | 0.028 | |
| n-Back | Hit rate | Dose | −0.045 | 0.020 | 0.023 |
| C (response bias) | Session × Duration | −0.001 | 0.000 | 0.018 | |
| Median RT (ms) | Session | 37.756 | 16.333 | 0.021 | |
| Sternberg | Proportion correct | Duration | 0.037 | 0.013 | 0.004 |
| oFree Recall | # correct responses | Session | 0.984 | 0.468 | 0.036 |
| # correct responses | Session × Dose | −4.624 | 0.931 | <0.001 | |
| # correct responses | Order × Dose | −5.906 | 2.622 | 0.024 | |
| # correct responses | Session × Order × Dose | 8.631 | 1.969 | <0.001 | |
| # correct responses | Duration | 0.018 | 0.008 | 0.022 | |
| Recognition | Hit rate | Session × Dose | −0.078 | 0.037 | 0.032 |
| Memory | False alarm rate | Duration | −0.001 | 0.000 | 0.023 |
| d′ (sensitivity) | Duration | 0.003 | 0.001 | 0.012 | |
| d′ (sensitivity) | Session × Duration | −0.002 | 0.001 | 0.001 | |
| Metamemory | Gamma | Duration | 0.002 | 0.001 | 0.036 |
Note. RT = Reaction time. Session = peak vs. trough session. Dose = participant's daily methadone maintenance dose (in mg). Duration = months in methadone maintenance treatment, Order = peak session first vs. trough session first. Order was included as a factor in the model only when it had a significant effect, and was only included in the table when there was a significant interaction between Order and another factor.
model included Order as a factor. Positive beta values reflect a higher value on the performance variable for: the peak vs. trough session, higher vs. lower dose, and longer vs. shorter treatment duration.
Sensory Processing
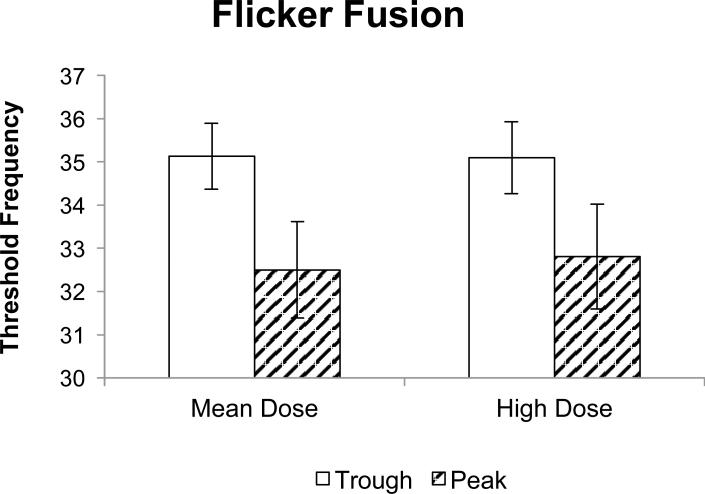
Analysis of the Flicker Fusion Task revealed a significant main effect of session that indicated worse visual acuity (lower threshold) for the peak session (M = 32.50; SE = 0.83) compared to the trough session (M = 35.13; SE = 0.77) (Figure 1).
Figure 1.
Flicker fusion threshold as a function of dose during peak and trough sessions. Data represent fitted (model-based) means ±1 standard error of the mean. High dose is the mean dose +/- 1 standard deviation. GEE analysis showed a significant main effect of session (p < .001).
Psychomotor Function
Assessment of Simple RT showed a significant main effect of session, such that median RT was slower in the peak session (M = 403.52; SE = 10.58) compared to the trough session (M = 385.26; SE = 11.36). There was also a significant session × duration interaction on median RT, such that as treatment duration increased, participants were more sensitive to session, responding more slowly during the peak session and faster during the trough session. A significant main effect of session on RT range revealed greater variability during the peak session (M =1399.55; SE = 279.89) than during the trough session (M = 583.86; SE = 104.92).
Analysis of the Circular Lights task revealed a significant session × duration interaction such that as treatment duration increased, participants were more sensitive to session, with worse performance (lower number correct) during the peak session relative to the trough session.
Analysis of the Trail Making Test revealed a significant session × order interaction on Trails A completion time, indicating that participants who completed the trough session as their first session were faster (lower Trails completion time) during the peak session (their second session) than during the trough session, whereas participants who completed the peak session as their first session were faster during the trough session (their second session) than during the peak session. This pattern seems to reflect a practice effect where participants were faster during their second session. A significant main effect of dose revealed faster Trails B-A completion time as methadone dose increased. No significant effects were found for Trails B completion time or for the Balance task.
Attention
There was a significant main effect of session on DSST number of trials attempted that was qualified by a significant session × order interaction. The interaction revealed that participants who completed the trough session as their first session attempted more trials during the peak session (their second session), whereas participants who completed the peak session as their first session attempted more trials during the trough session (their second session). This pattern seems to reflect a practice effect (similar to that observed for Trails A completion time) where participants attempted more trials during their second session. No significant effects were found for DSST proportion correct.
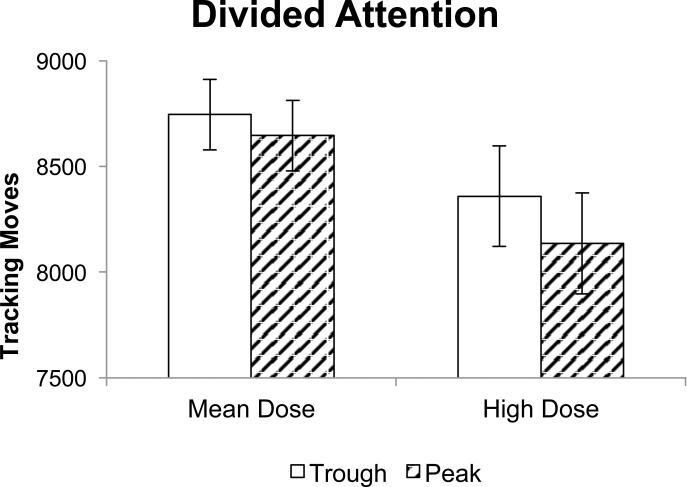
Analysis of Divided Attention Task performance revealed a significant main effect of dose, such that the number of tracking moves decreased (indicating slowing) as dose increased (Figure 2). A significant main effect of session revealed greater tracking deviation and lower tracking overlap (both indicating decreased tracking accuracy) during the peak session [tracking deviation: (M =36.09; SE = 2.32); tracking overlap: (M =55.24; SE = 2.11)] relative to the trough session [tracking deviation: (M = 33.81; SE = 1.99); tracking overlap: (M = 57.68; SE = 2.24)]. A significant main effect of dose on RT (monitoring) indicated that RT increased (indicating slowing) overall as dose increased. A significant session × dose interaction indicated that participants with lower doses were more sensitive to session, with slower RTs in the peak session relative to the trough session. No significant effects were found for proportion correct.
Figure 2.
Divided attention tracking moves as a function of dose during peak and trough sessions. Data represent fitted (model-based) means ±1 standard error of the mean. High dose is the mean dose +/- 1 standard deviation. GEE analysis showed a significant main effect of dose (p < .05).
Working Memory
The n-back test revealed a significant main effect of dose such that hit rate decreased as dose increased. There was a significant session × duration interaction on response bias (C) such that as treatment duration decreased, participants were more sensitive to session, with a higher C (i.e., more conservative response bias; less likely to respond “yes”) during the peak session. A significant main effect of session on median RT revealed that working memory performance was slower (higher median RT) during the peak session (2-back: M =1084.56; SE = 25.62) relative to the trough session (2 back: M = 1052.21; SE = 27.59). Analysis of Sternberg task performance did not show significant effects of session or dose. There was a significant main effect of duration, such that Sternberg proportion correct increased as treatment duration increased. There were no significant interactions between condition (working memory load or delay) and session or dose for either working memory task.
Episodic Memory
For the Free Recall Task, there was a significant main effect of session, and significant interactions of session × dose and order × dose that were qualified by a significant session × order × dose interaction. Such a 3-way interaction that was not predicted is difficult to interpret and needs to be followed up in future studies. Additionally, a main effect of duration revealed that the number of items recalled increased as treatment duration increased.
Analysis of the Recognition Memory Task revealed a significant session × dose interaction such that participants on higher doses had lower hit rates in the peak session than in the trough session, whereas lower dose patients had lower hit rates in the trough session than in the peak session. A significant main effect of duration revealed that the false alarm rate decreased (indicating better performance) as treatment duration increased. Finally, a significant main effect of duration and a significant session × duration interaction revealed that as treatment duration increased, participants were more sensitive to session, with lower d’ (worse performance) during the peak session relative to the trough session. No significant effects were found for proportion correct or C.
Metamemory
Analysis of metamemory revealed a significant main effect of duration, such that gamma scores increased (indicating better metamemory) as treatment duration increased.
DISCUSSION
The present study used a within-subject double-blind design to investigate cognitive and psychomotor performance in methadone maintenance patients as a function of time since the methadone dose (peak vs. trough) and maintenance dose level. Compared to the three previous studies that assessed cognitive performance in methadone patients as a function of time relative to dosing (Baewert et al., 2007; Kelley et al., 1978; Lyvers & Yakomoff, 2003), the present study had a larger sample size and assessed a wider range of psychomotor/cognitive domains. Analysis of the effects of time since dosing showed consistent findings across several measures indicating that performance was often worse during the peak session (approximately 120 minutes after methadone dosing) compared with the trough session (approximately 26 hours after the methadone dose). Specifically, peak sessions were associated with worse performance on measures of sensory processing (Flicker Fusion; Figure 1), psychomotor speed (simple RT), divided attention, and working memory (n-back). The finding of worse performance at peak relative to trough is consistent with the results of Baewert et al. (2007) in which the effects of time relative to dosing were tested in a between-subjects design. In conjunction with previous findings of deficient and slowed performance in MMP relative to matched controls (Mintzer & Stitzer, 2002; Pirastu, et al., 2006; Prosser, et al., 2009; Specka, et al., 2000), these results may have implications for daily performance, particularly for MMP engaged in activities requiring precise timing following dosing. In contrast, performance on episodic memory and metamemory measures did not differ as a function of session, suggesting that long-term memory processes do not fluctuate as a function of time relative to dosing.
Most measures did not show effects of methadone maintenance dose level. However, higher dose was associated with worse performance on two tasks (i.e., slowed divided attention task performance and decreased working memory [n-back] accuracy) and improved performance on a measure of executive function (Trail Making Test B-A time). This pattern is consistent with the literature of mixed effects of methadone dose on performance. Decreased attention and slowed psychomotor speed were observed in MMP with higher doses in one group comparison study (Loeber, et al., 2008), and delayed recall was inversely related to dose in second study (Curran, Kleckham, Bearn, Strang, & Wanigaratne, 2001). However, Bracken et al. (2012) found faster speed on a continuous performance task for higher dose MMP, but no group differences were found on DSST performance. Other studies found no effects of maintenance dose on cognitive performance (Darke, et al., 2000; Lombardo, et al., 1976; Soyka, et al., 2005). To our knowledge, the current study is the first to evaluate the effects of maintenance dose level as a function of time relative to methadone dosing (peak vs. trough session). As with the main effects of dose, most measures did not show significant interactions between dose and session, and the observed interactions (i.e., on divided attention and recognition memory measures) were inconsistent. Taken together, these findings suggest that, for MMP on stable methadone maintenance doses, maintenance at higher doses may not significantly increase the risk of performance impairment.
In addition to the primary variables of interest (time since dosing, maintenance dose), we investigated methadone treatment duration as a covariate of interest. Longer methadone treatment duration was associated with better performance on some measures (Sternberg working memory, free recall, and metamemory), though false alarm rates during recognition memory were increased. These findings are consistent with past correlational and longitudinal studies showing improved cognitive performance with methadone stabilization (Bracken, et al., 2012; Gruber et al., 2006; Soyka et al., 2008; Soyka, Zingg, Koller, & Hennig-Fast, 2010). In a longitudinal study, Gruber et al. (2006) found improved memory and focused attention (DSST), but no change on measures of semantic fluency, psychomotor function (Trail Making Test), or inhibitory control, following two months of treatment compared to the first few weeks of treatment. In a cross-sectional study, Soyka et al. (2010) found improved executive function and visuospatial construction, but no change in attention or memory performance following six months of treatment compared to one month. Whereas previous studies investigated changes in cognitive performance during the initial methadone stabilization period, the present study is the first to extend the assessment of cognitive performance to longer methadone treatment durations (6 months to several years). We also tested the interaction between treatment duration and the main study factors. On a few measures, MMP with longer treatment duration were more sensitive to time since last dose, showing worsened performance (slower Simple RT, less conservative response bias on n-back, and decreased sensitivity on recognition memory) during the peak session relative to the trough session. Although no human studies have investigated this sensitization effect, Allouche et al. (2013) reported the emergence of behavioral sensitization to acute methadone administration after 5 days of methadone administration in mice under certain conditions.
The present findings suggest that cognitive functioning can fluctuate as a function of time relative to daily dosing even in MMP who have been maintained on stable doses for an extended time (mean duration in treatment = 4 years). However, it is important to note that the effects of time since last dose were limited to a subset of functions, primarily sensory processing and speed, and that higher order cognitive functions did not show worse performance during the peak session. Furthermore, the observed worsened performance at peak may not be clinically significant. For example, the 5% increase in reaction time during the Simple RT task at peak relative to trough is low compared to the magnitude of reaction time changes observed in a previous study in our laboratory investigating the acute effects of alcohol in healthy volunteers at doses known to be associated with clinically significant impairment (Kleykamp et al., 2010). In that study, an alcohol dose that resulted in a peak breath alcohol level of approximately .09% was associated with a 213% increase in RT relative to placebo during a Choice Reaction Time task. With respect to maintenance dose level, the present findings suggest that for MMP on stable methadone maintenance doses, maintenance at higher doses may not significantly increase the risk of performance impairment. However, it is important to note that conclusions regarding the effects of dose based on the present study are limited, given that methadone blood levels were not measured; methadone dose and blood levels are not well correlated (Eap et al., 2000). Consistent with previous research, some cognitive functions improved with longer treatment duration, although sensitivity to peak methadone effects increased as a function of treatment duration for some tasks. The few sex differences in this study showed better performance on measures of visual processing, motor speed, and attention by females and better performance on an episodic memory measure (free recall) by males. Given the long-term nature of methadone treatment, additional within-subject studies are needed to provide a more complete assessment of the time course of methadone’s effects on cognitive performance throughout the day, and to better understand the specific effects and interactions of time relative to dosing, maintenance dose level as a function of plasma concentration, and treatment duration on cognitive performance in MMP.
ACKNOWLEDGEMENTS
This work was supported by NIDA grants R01DA17688, R01DA07209, T32DA07209, K24 DA023186, and K23DA027045. We thank Crystal Barnhouser, Julie Garson, Jessica Rhee, and Erica Smearman for protocol management and technical assistance, and John Yingling for computer programming assistance and technical support.
Footnotes
All authors contributed in a significant way to the manuscript and have read and approved the final manuscript. Dr. Mintzer provided overall scientific and administrative leadership to the project, and had supervisory responsibility for the design, conduct, analysis, and publication of the study. Drs. Bigelow, Stitzer, and Strain contributed to the design of the study. Dr. Copersino assisted with writing the protocol. Drs. Vandrey and Kleykamp supervised the conduct of the study including training and supervising the research staff, assisting with patient recruitment, and data management. Dr. Leoutsakos designed the statistical analysis plan, managed the analyses, and assisted with interpretation of the results. Dr. Rass was the primary author, managed the literature searches and summaries of previous related work, and wrote the first draft of the manuscript. All authors contributed to editing the manuscript.
DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- Allouche S, Le Marec T, Noble F, Marie N. Different patterns of administration modulate propensity of methadone and buprenorphine to promote locomotor sensitization in mice. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2013;40:286–291. doi: 10.1016/j.pnpbp.2012.10.013. doi: 10.1016/j.pnpbp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Baewert A, Gombas W, Schindler SD, Peternell-Moelzer A, Eder H, Jagsch R, Fischer G. Influence of peak and trough levels of opioid maintenance therapy on driving aptitude. European Addiction Research. 2007;13(3):127–135. doi: 10.1159/000101548. doi: 10.1159/000101548. [DOI] [PubMed] [Google Scholar]
- Bracken BK, Trksak GH, Penetar DM, Tartarini WL, Maywalt MA, Dorsey CM, Lukas SE. Response inhibition and psychomotor speed during methadone maintenance: Impact of treatment duration, dose, and sleep deprivation. Drug and Alcohol Dependence. 2012;125(1-2):132–139. doi: 10.1016/j.drugalcdep.2012.04.004. doi: 10.1016/j.drugalcdep.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou N, Morgan CJ, Battistella S, O'Ryan D, Davis P, Curran HV. Attentional bias, inhibitory control and acute stress in current and former opiate addicts. Drug and Alcohol Dependence. 2010;109(1-3):220–225. doi: 10.1016/j.drugalcdep.2010.01.012. doi: 10.1016/j.drugalcdep.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Curran HV. Benzodiazepines, memory and mood: A review. Psychopharmacology (Berl) 1991;105(1):1–8. doi: 10.1007/BF02316856. [DOI] [PubMed] [Google Scholar]
- Curran HV. Psychopharmacological approaches to human memory. In: Gazzaniga MS, editor. The Cognitive Neurosciences. 2nd ed. MIT Press; Boston: 2000. pp. 797–804. [Google Scholar]
- Curran HV, Kleckham J, Bearn J, Strang J, Wanigaratne S. Effects of methadone on cognition, mood and craving in detoxifying opiate addicts: A dose-response study. Psychopharmacology (Berl) 2001;154(2):153–160. doi: 10.1007/s002130000628. [DOI] [PubMed] [Google Scholar]
- Darke S, McDonald S, Kaye S, Torok M. Comparative patterns of cognitive performance amongst opioid maintenance patients, abstinent opioid users and non-opioid users. Drug and Alcohol Dependence. 2012;126(3):309–315. doi: 10.1016/j.drugalcdep.2012.05.032. doi: 10.1016/j.drugalcdep.2012.05.032. [DOI] [PubMed] [Google Scholar]
- Darke S, Sims J, McDonald S, Wickes W. Cognitive impairment among methadone maintenance patients. Addiction. 2000;95(5):687–695. doi: 10.1046/j.1360-0443.2000.9556874.x. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. The scl-90 manual i: Scoring, administration and procedures for the scl- 90. Baltimore: Clinical Psychometric Research. 1977 [Google Scholar]
- Eap CB, Bourquin M, Martin J, Spagnoli J, Livoti S, Powell K, Bauman P, Deglon J. Plasma concentrations of the enantiomers of methadone and therapeutic response in methadone maintenance treatment. Drug and Alcohol Dependence. 2000;61(1):47–54. doi: 10.1016/s0376-8716(00)00121-6. [DOI] [PubMed] [Google Scholar]
- Faggiano F, Vigna-Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. The Cochrane Database of Systematic Reviews. 2003;3:CD002208. doi: 10.1002/14651858.CD002208. doi: 10.1002/14651858.CD002208. [DOI] [PubMed] [Google Scholar]
- Goodman LA, Kruskal WH. Measures of association for cross classifications. Journal of the American Statistical Association. 1954;49(268):732–764. doi: Doi 10.2307/2281536. [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson I. Differential effects of diazepam and pentobarbital on mood and behavior. Archives of General Psychiatry. 1983;40(8):865–873. doi: 10.1001/archpsyc.1983.01790070055007. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Tzilos GK, Silveri MM, Pollack M, Renshaw PF, Kaufman MJ, Yurgelun-Todd DA. Methadone maintenance improves cognitive performance after two months of treatment. Experimental and Clinical Psychopharmacology. 2006;14(2):157–164. doi: 10.1037/1064-1297.14.2.157. doi: 10.1037/1064-1297.14.2.157. [DOI] [PubMed] [Google Scholar]
- Hammersley R, Finnigan F, Millar K. Alcohol placebos: You can only fool some of the people all of the time. British Journal of Addiction. 1992;87(10):1477–1480. doi: 10.1111/j.1360-0443.1992.tb01926.x. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Copeland W, Stanger C, Wadsworth M. Screening for DSM-IV externalizing disorders with the child behavior checklist: A receiver-operating characteristic analysis. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2004;45(7):1299–1307. doi: 10.1111/j.1469-7610.2004.00314.x. doi: 10.1111/j.1469-7610.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Helzer JE, Wetzel MW, Kessel KB, McGee B, Janca A, Przybeck T. The use of the DSM-III-R checklist for initial diagnostic assessments. Comparative Psychiatry. 1993;34(6):375–383. doi: 10.1016/0010-440x(93)90061-8. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal working memory load affects regional brain activation as measured by pet. Journal of Cognitive Neuroscience. 1997;9(4):462–475. doi: 10.1162/jocn.1997.9.4.462. doi: Doi 10.1162/Jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Kelley D, Welch R, McKnelley W. Methadone maintenance: An assessment of potential fluctuations in behavior between doses. International Journal of the Addictions. 1978;13(7):1061–1068. doi: 10.3109/10826087809039326. [DOI] [PubMed] [Google Scholar]
- Kelly SM, O'Grady KE, Mitchell SG, Brown BS, Schwartz RP. Predictors of methadone treatment retention from a multi-site study: A survival analysis. Drug and Alcohol Dependence. 2011;117(2-3):170–175. doi: 10.1016/j.drugalcdep.2011.01.008. doi: 10.1016/j.drugalcdep.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleykamp BA, Griffiths RR, Mintzer MZ. Dose effects of triazolam and alcohol on cognitive performance in healthy volunteers. Experimental and Clinical Psychopharmacology. 2010;18(1):1–16. doi: 10.1037/a0018407. doi: 10.1037/a0018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber S, Kniest A, Diehl A, Mann K, Croissant B. Neuropsychological functioning of opiate-dependent patients: A nonrandomized comparison of patients preferring either buprenorphine or methadone maintenance treatment. The American Journal of Drug and Alcohol Abuse. 2008;34(5):584–593. doi: 10.1080/00952990802308239. doi: 10.1080/00952990802308239. [DOI] [PubMed] [Google Scholar]
- Lombardo WK, Lombardo B, Goldstein A. Cognitive functioning under moderate and low dosage methadone maintenance. International Journal of the Addictions. 1976;11(3):389–401. doi: 10.3109/10826087609056158. [DOI] [PubMed] [Google Scholar]
- Lyvers M, Yakimoff M. Neuropsychological correlates of opioid dependence and withdrawal. Addictive Behaviors. 2003;28(3):605–611. doi: 10.1016/s0306-4603(01)00253-2. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Bigelow GE, Liebson IA. Self-regulated opioid detoxification by humans: Effects of methadone pretreatment. NIDA Research Monograph. 1982;41:232–238. [PubMed] [Google Scholar]
- Mintzer MZ, Copersino ML, Stitzer ML. Opioid abuse and cognitive performance. Drug and Alcohol Dependence. 2005;78(2):225–230. doi: 10.1016/j.drugalcdep.2004.10.008. doi: 10.1016/j.drugalcdep.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: A comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behavioural Pharmacology. 1997;8(6-7):561–574. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. A triazolam/amphetamine dose-effect interaction study: Dissociation of effects on memory versus arousal. Psychopharmacology (Berl) 2007;192(3):425–440. doi: 10.1007/s00213-007-0726-y. doi: 10.1007/s00213-007-0726-y. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Stitzer ML. Cognitive impairment in methadone maintenance patients. Drug and Alcohol Dependence. 2002;67(1):41–51. doi: 10.1016/s0376-8716(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Pirastu R, Fais R, Messina M, Bini V, Spiga S, Falconieri D, Diana M. Impaired decision-making in opiate-dependent subjects: Effect of pharmacological therapies. Drug and Alcohol Dependence. 2006;83(2):163–168. doi: 10.1016/j.drugalcdep.2005.11.008. doi: 10.1016/j.drugalcdep.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Schuster CR, Cone EJ. Assessment of cocaine use with quantitative urinalysis and estimation of new uses. Addiction. 1997;92(6):717–727. [PubMed] [Google Scholar]
- Prosser J, Cohen LJ, Steinfeld M, Eisenberg D, London ED, Galynker II. Neuropsychological functioning in opiate-dependent subjects receiving and following methadone maintenance treatment. Drug and Alcohol Dependence. 2006;84(3):240–247. doi: 10.1016/j.drugalcdep.2006.02.006. doi: 10.1016/j.drugalcdep.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser J, London ED, Galynker II. Sustained attention in patients receiving and abstinent following methadone maintenance treatment for opiate dependence: Performance and neuroimaging results. Drug and Alcohol Dependence. 2009;104(3):228–240. doi: 10.1016/j.drugalcdep.2009.04.022. doi: 10.1016/j.drugalcdep.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Rapeli P, Fabritius C, Alho H, Salaspuro M, Wahlbeck K, Kalska H. Methadone vs. Buprenorphine/naloxone during early opioid substitution treatment: A naturalistic comparison of cognitive performance relative to healthy controls. BMC Clinical Pharmacology. 2007;7:5. doi: 10.1186/1472-6904-7-5. doi: 10.1186/1472-6904-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead–Reitan neuropsychological test battery: Theory and clinical interpretation. Neuropsychology Press; Tucson, Az: 1993. [Google Scholar]
- Simonson E, Brozek J. Flicker fusion frequency; background and applications. Physiological Reviews. 1952;32(3):349–378. doi: 10.1152/physrev.1952.32.3.349. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology, General. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Soyka M, Hock B, Kagerer S, Lehnert R, Limmer C, Kuefner H. Less impairment on one portion of a driving-relevant psychomotor battery in buprenorphine- maintained than in methadone-maintained patients: Results of a randomized clinical trial. Journal of Clinical Psychopharmacology. 2005;25(5):490–493. doi: 10.1097/01.jcp.0000178417.60426.60. [DOI] [PubMed] [Google Scholar]
- Soyka M, Lieb M, Kagerer S, Zingg C, Koller G, Lehnert P, Limmer C, Kuefner H, Hennig-Fast K. Cognitive functioning during methadone and buprenorphine treatment: Results of a randomized clinical trial. Journal of Clinical Psychopharmacology. 2008;28(6):699–703. doi: 10.1097/JCP.0b013e31818a6d38. doi: 10.1097/JCP.0b013e31818a6d38. [DOI] [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, Hennig-Fast K. Cognitive function in short- and long-term substitution treatment: Are there differences? The World Journal of Biological Psychiatry. 2010;11(2 Pt 2):400–408. doi: 10.1080/15622970902995604. doi: 10.1080/15622970902995604. [DOI] [PubMed] [Google Scholar]
- Specka M, Finkbeiner T, Lodemann E, Leifert K, Kluwig J, Gastpar M. Cognitive-motor performance of methadone-maintained patients. European Addiction Research. 2000;6(1):8–19. doi: 10.1159/000019004. doi: 19004. [DOI] [PubMed] [Google Scholar]
- Sternberg S. The discovery of processing stages: Extensions of donders' method. ACTA Psychologica. 1969;30:276–315. doi: 10.1016/0001-6918(69)90055-9. [Google Scholar]
- Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate- vs high-dose methadone in the treatment of opioid dependence: A randomized trial. JAMA. 1999;281(11):1000–1005. doi: 10.1001/jama.281.11.1000. [DOI] [PubMed] [Google Scholar]
- Tetrault JM, Fiellin DA. Current and potential pharmacological treatment options for maintenance therapy in opioid-dependent individuals. Drugs. 2012;72(2):217–228. doi: 10.2165/11597520-000000000-00000. doi: 10.2165/11597520-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo A, Toribio I, Orozco C, Puente KL, Perez-Garcia M. Neuropsychological functioning in methadone maintenance patients versus abstinent heroin abusers. Drug and Alcohol Dependence. 2005;78(3):283–288. doi: 10.1016/j.drugalcdep.2004.11.006. doi: 10.1016/j.drugalcdep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Journal of Clinical Pharmacology and Therapeutics. 1994;55(5):569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Administration and scoring manual: Wechsler adult intelligence scale. 3rd ed. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Zachary RA, Paulson MJ, Gorsuch RL. Estimating wais iq from the shipley institute of living scale using continuously adjusted age norms. Journal of Clinical Psychology. 1985;41(6):820–831. doi: 10.1002/1097-4679(198511)41:6<820::aid-jclp2270410616>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]