Abstract
Circadian rhythms are prominent in many physiological and behavioral functions. Circadian disruptions either by environmental or molecular perturbation can have profound health consequences, including the development and progression of addiction. Both animal and humans studies indicate extensive bidirectional relationships between the circadian system and drugs of abuse. Addicted individuals display disrupted rhythms, and chronic disruption or particular chronotypes, may increase the risk for substance abuse and relapse. Moreover, polymorphisms in circadian genes and an evening chronotype have been linked to mood and addiction disorders, and recent efforts suggest an association with the function of reward neurocircuitry. Animal studies are beginning to determine how altered circadian gene function results in drug induced neuroplasticity and behaviors. Many studies suggest a critical role for circadian rhythms in reward-related pathways in the brain and indicate that drugs of abuse directly affect the central circadian pacemaker. In this review, we highlight key findings demonstrating the importance of circadian rhythms in addiction, and how future studies will reveal important mechanistic insights into the involvement of circadian rhythms in drug addiction.
Keywords: Circadian rhythms, addiction, reward, dopamine, alcohol
Introduction
A growing body of literature connects perturbations in circadian rhythms and the genes that control the molecular clock to the development and progression of addictive disorders. Clinical studies have found that individuals with addictive disorders have highly disrupted rhythms and it is likely that genetic and/or environmental disruptions to the normal sleep/wake cycle increase the vulnerability for addiction (Bolelli et al., 1979; Conroy et al., 2012; Fakier & Wild, 2011; Kovanen et al., 2010; Sjoholm et al., 2010; Vescovi, Coiro, Volpi, & Passeri, 1992). Conversely, self-medication through drug or alcohol abuse is considered to be an attempt to ameliorate sleep and mood-related problems found with general circadian rhythm disruption (Staddon, 2013). Several studies have also found that an evening chronotype (i.e. “night owls”) is associated with higher rates of depression, sleep problems, and substance abuse (Adan, 1994; Broms et al., 2011; Fisk & Montgomery, 2009; Levandovski et al., 2011). During adolescence, there is a natural shift towards an evening preference, which coincides with the developmental window in which individuals are most vulnerable to mood and addiction disorders (Hasler & Clark, 2013). One concept put forth to help understand these phenotypes is the “social jet lag hypothesis”. Social jet lag occurs when an individual is up early for work or school during the week and then sleeps late on the weekends, creating a situation that is similar to flying overseas and back each week (Hasler & Clark, 2013; Touitou, 2013; Wittmann, Dinich, Merrow, & Roenneberg, 2006). Studies generally find correlations between the degree of social jet lag, depression, and drug and alcohol use (Foster et al., 2013; Hasler & Clark, 2013; Wittmann et al., 2006). More work is necessary to determine the importance of circadian misalignment to the vulnerability for addiction. The increased impulsivity of night owls and adolescents also often puts them into situations in which drug and alcohol use is prevalent and may predispose them to substance abuse (Selvi et al., 2011; Stautz & Cooper, 2013). Additionally, while environmentally-derived circadian disturbances (i.e. shift work) have been associated with heightened risk for substance abuse (Bildt & Michelsen, 2002); it is difficult to determine if these effects can be attributed to circadian misalignment per se or the effects of stress or other predisposing factors. Furthermore, we now know that circadian genes are directly involved in the regulation of dopaminergic reward circuitry (Akhisaroglu, Kurtuncu, Manev, & Uz, 2005; Schade et al., 1995; Shieh, Chu, & Pan, 1997; Sleipness, Sorg, & Jansen, 2007b; Weber, Lauterburg, Tobler, & Burgunder, 2004), thus disruptions to the circadian system change the reward value and motivation for addictive substances through direct effects on reward circuits (Abarca, Albrecht, & Spanagel, 2002; Andretic, Chaney, & Hirsh, 1999; Liu et al., 2005; McClung et al., 2005; Roybal et al., 2007; Spanagel et al., 2005a; Zghoul et al., 2007). This regulation by the circadian system is through both indirect projections from the master pacemaker of the suprachiasmatic nucleus (SCN) to the ventral tegmental area (VTA), and through local circadian gene expression within dopaminergic neurons (Luo & Aston-Jones, 2009; McClung, 2007a; Sleipness et al., 2007b). Thus it appears that vulnerability to addiction is dependent on the circadian system in multiple ways.
Once an individual starts abusing drugs or alcohol, this exposure produces both acute and lasting changes to circadian rhythms and sleep, creating a vicious cycle for someone who already started with a circadian rhythm abnormality (Irwin, Valladares, Motivala, Thayer, & Ehlers, 2006; Jones, Knutson, & Haines, 2003; Morgan et al., 2006; Shibley, Malcolm, & Veatch, 2008; Wasielewski & Holloway, 2001). These changes to rhythms and sleep persist even after administration of the abused substance has stopped, and this very often contributes to relapse. Indeed, insomnia is the most common complaint from alcoholics after they stop drinking (Spanagel, Rosenwasser, Schumann, & Sarkar, 2005b; Zhabenko, Wojnar, & Brower, 2012). It is possible that circadian rhythm and sleep stabilization would help decrease addiction vulnerability and/or reduce the risk for relapse in those with addictive disorders (Arnedt, Conroy, & Brower, 2007; Brower et al., 2011). Thus, it is important to understand how circadian rhythm disruptions lead to increased vulnerability for addiction, and what changes occur to the molecular clock following chronic drug use. This review will focus on studies aimed at understanding the influence of specific circadian genes, as well as rhythm disruptions as a whole, on addiction-related behavior. We will also discuss some of the mechanisms by which circadian genes regulate reward-related pathways in the brain, altering the response to drugs and alcohol. Finally, we will highlight some of the changes that occur in circadian gene expression in response to drugs and alcohol, and what studies are needed moving forward to advance our understanding of the connection between the circadian system and reward.
The molecular clock
At the cellular level, circadian rhythms are generated by 24 hour autoregulatory transcriptional/translational feedback loops consisting of ‘circadian’ genes and their protein products (Bae et al., 2001; Jin et al., 1999; Shearman, Zylka, Reppert, & Weaver, 1999). In mammals, the feedback loop begins in the cell nucleus where Circadian Locomotor Output Cycles Kaput (CLOCK), or Neuronal PAS Domain Protein 2 (NPAS2), and Brain and Muscle ARNT like Protein 1 (BMAL1) proteins heterodimerize and drive the transcription of the Period (Per1, Per2 and Per3) and Cryptochrome (Cry1 and Cry2) genes by binding to the E-box (CACGTG) domain on their gene promoters. When CLOCK expression is low in the SCN, NPAS2 compensates to regulate normal circadian transcription (Debruyne et al., 2006). Under normal conditions, NPAS2 is largely expressed in the forebrain and liver, with very little expression in the SCN (Bertolucci et al., 2008; DeBruyne, Weaver, & Reppert, 2007; O’Neil et al., 2013; Reick, Garcia, Dudley, & McKnight, 2001). Once translated, PER and CRY proteins build in the cytoplasm of the cell over the course of the day, and eventually form hetero- and homodimers that feed back to the cell nucleus to inhibit CLOCK(NPAS2):BMAL1-mediated transcription. The timing of nuclear entry is balanced by regulatory kinases that phosphorylate the PER and CRY proteins, leading to their degradation (Lowrey & Takahashi, 2000; Mohawk & Takahashi, 2011; Wang, Ko, Koletar, Ralph, & Yeomans, 2007). CRY1 and CRY2 degradation is also regulated by FBXL3, which regulates the period and robustness of the clock(Busino et al., 2007; Godinho et al., 2007; Shi et al., 2013). The molecular clock regulates two other promoter elements, DBP/E4BP4 binding elements (D boxes) and REV-ERBα/ROR binding elements (RREs)(Ueda et al., 2005). REV-ERBα, an orphan nuclear receptor, negatively regulates the activity of the CLOCK:BMAL1. The same mechanism controlling Per and Cry gene transcription also controls transcription of REV-ERBα. Similarly, the transcription factor DPB is positively regulated by the CLOCK:BMAL1 complex (Ripperger & Schibler, 2006) and acts as an important output mechanism, driving rhythmic transcription of other output genes via a PAR basic leucine zipper (PAR bZIP) (Lavery et al., 1999). In addition to these cyclical feedback loops, clock controlled genes (CCG’s) present in the CNS and periphery, are regulated in a similar fashion, via E-Box mediated transcription. In this manner, the clock gene machinery regulates the rhythmic control of countless genes in the brain and periphery. Indeed, between 10–15% of all transcripts in various tissues exhibit circadian rhythms and are believed to be directly controlled by the molecular clock (Duffield et al., 2002; Panda, Hogenesch, & Kay, 2002; Storch et al., 2002).
Circadian gene mutations, cocaine seeking and reward
In clinical studies, patients with substance abuse disorders exhibit disturbances in sleep patterns and circadian rhythms, indicating that drug use may perturb the circadian system and/or disturbances in the circadian system may contribute to the development of drug dependence. Association studies in clinical populations between clock gene polymorphisms and substance abuse disorders have been useful in determining if circadian disruptions are risk factors for addiction susceptibility. Malison and colleagues initially determined that there was no association between CLOCK, PER1 or PER2 single-nucleotide polymorphisms (SNPs) and susceptibility to cocaine addiction (Malison, Kranzler, Yang, & Gelernter, 2006). A caveat of this study, however, is that all patients comorbid for other psychiatric diseases, including bipolar disorder and depression, were excluded from the addiction cohort. The extensive associations between clock gene polymorphisms and mood disorders likely excluded many patients that would yield significant differences in clock gene SNPs compared to healthy controls. A subsequent study of polymorphic repeat variation of the PER2 gene using variable number tandem repeat (VNTR) analysis determined that variation in repeat allele frequencies in intron 3 of the PER2 gene was significantly associated with vulnerability to cocaine addiction (Shumay et al., 2012a). Interestingly, the shorter allele, which was characteristic of the cocaine abusers, was also associated with lower D2R availability (Shumay et al., 2012a), which is consistently associated with an increased risk for drug addiction (Volkow, Wang, Fowler, Tomasi, & Telang, 2011). This raises the intriguing possibility that a Per2 mutation may in turn lead to the disruption of D2R, although such associative studies are limited in interpreting the directionality of these effects. Preclinical studies that can utilize molecular techniques to systematically disrupt clock gene function have provided insight into the potential causal connection between circadian disturbances and limbic function.
The molecular components of the circadian clock described above are widely expressed throughout the brain, including the mesocorticolimbic system central to reward processing and susceptibility to drugs of abuse (Reppert & Weaver, 2002; Rosenwasser, 2010). Consequently, these nuclei exhibit circadian patterns of activity in neuronal firing and neurochemical transcription and release in mice and rats (McClung, 2007b), suggesting functional regulation of the circadian system over these processes. Additionally, many behaviors associated with the limbic system, including drug-seeking behavior and locomotor sensitization, exhibit rhythmic patterns despite no change in the metabolic rates of the drugs themselves (Akhisaroglu et al., 2005; Baird & Gauvin, 2000). Collectively, these phenomena point to a direct connection between circadian gene expression and reward, leading to the possibility that disruptions in the circadian system may directly impact risk for drug dependence and addiction.
Clock
The circadian gene, Clock, is expressed in the ventral tegmental area (VTA) and nucleus accumbens (NAc) (McClung et al., 2005; Uz, Akhisaroglu, Ahmed, & Manev, 2003) and has been implicated in modulating reward processing, providing a candidate target for ameliorating critical risk factors in models of addiction and bipolar disorder. Polymorphisms in the Clock gene are associated with aberrant behavioral outcomes in mice and humans, including manic phenotypes and profound increases in drug seeking behavior (Benedetti et al., 2003; Roybal et al., 2007). Specifically, mice harboring a mutation in the Clock gene that results in a 51 amino acid deletion of exon 19 (ClockΔ19), eliminating the transactivational domain sequence (King et al., 1997), exhibit a number of behavioral responses to cocaine that are associated with a hyperhedonic phenotype. The characterization of mesolimbic alterations associated with this mutation lends insight into the mechanisms by which the Clock gene normally regulates reward circuitry, and can uncover candidate targets connecting mutations in circadian genes to aberrant reward processing. ClockΔ19 mice exhibit a conditioned place preference for cocaine (CPP) at a dose that is lower than that which is sufficient to generate a CPP response in WT littermates (McClung et al., 2005). Additionally, ClockΔ19 mice exhibit decreased acquisition latency and increased intake of cocaine under an FR1 self-administration paradigm compared to WT mice (Ozburn, Larson, Self, & McClung, 2012). ClockΔ19 mice also exhibit significantly higher cocaine breakpoints under a progressive ratio schedule, collectively indicating that this mutation increases the motivational and reinforcing properties of cocaine (Ozburn et al., 2012). These results are striking given the typically low drug self-administration behavior in BALB/c mice, the background on which these mice are bred. The consistent behavioral profile in Clock mutant mice across paradigms in the context underscores the critical role of the Clock gene in modulating cocaine reward in WT animals.
Period genes
Disruptions in the Period (Per) genes yield different behavioral outcomes in measures of cocaine reward and sensitization. While the psychomotor responses to acute cocaine injections in Per1 or Per2 mutant mice do not differ from WT littermates, chronic cocaine administration results in different levels of behavioral sensitization (Abarca et al., 2002). Per1 mutant mice do not show locomotor sensitization to repeated cocaine injections compared to WT controls, which is comparable to the response of Drosophila with a mutation in the Period genes (Andretic et al., 1999), suggesting a conserved influence of Per1 on behavioral sensitization. Conversely, Per2 mutant mice exhibit enhanced locomotor sensitization to cocaine compared to WT and Per1 mice (Abarca et al., 2002). In WT mice, striatal PER1 rhythms are associated with changes in cocaine sensitization, where high Per1 expression is associated with high cocaine sensitization (Uz et al., 2003). Moreover, cocaine sensitization diminishes during the night when striatal Per1 expression is at its nadir (Uz et al., 2003). If Per1 rhythms in melatonin-proficient mice are abolished through pinealectomy, this results in a loss of diurnal rhythms in sensitization. These results, along with the presence of melatonin receptors in the striatum, indicate that pineal hormones directly influence striatal Per1 rhythms and cocaine sensitization and that the rhythm in Per1 expression is critical for diurnal differences in behavioral sensitization (Uz et al., 2003). Given that behavioral sensitization is thought to be a critical component of drug craving that leads to dependence (Robinson & Berridge, 1993), understanding the precise connection between the circadian system and drug sensitization will lend insight into the influence of circadian disturbances as a risk factor for drug taking and addiction.
In addition to the differential effects of Per1 and Per2 on cocaine sensitization, Per1 and Per2 mutant mice exhibit opposing conditioned place preference (CPP) responses to cocaine, where Per1 mice do not show cocaine CPP compared to WT or Per2 mice (Abarca et al., 2002). In contrast, Per2 mice exhibit a significant place preference to cocaine, although this does not differ from WT mice, suggesting the role of Per2 in regulating locomotor responses to cocaine may differ from its role in processing its hedonic value (Abarca et al., 2002). Interestingly, further examination of drug-seeking behavior in Per1 mutant mice found that this mutation does not abolish motivation for cocaine self-administration or cocaine reinstatement following extinction (Halbout, Perreau-Lenz, Dixon, Stephens, & Spanagel, 2011). These differences suggest that each circadian gene mutation may differentially modulate cocaine reward depending on the paradigm used. This discrepancy adds to the voluminous body of literature indicating distinct neural substrates may underlie various components of drug reward. Unlike ClockΔ19 mice, which exhibit alterations in both cocaine CPP and self-administration (McClung et al., 2005; Ozburn et al., 2012), the role of Per1 in regulating cocaine reward clearly depends on the particular behavioral paradigm measured; for example, whether the drug administration is passive or active. This also suggests that Per1 may regulate sub-components of the neurobiological underpinnings of reward. Indeed, the potential mechanisms by which circadian genes regulate mesolimbic circuitry are numerous, and therefore, global circadian gene mutations likely involve pleiotropic effects on reward-related processing.
Circadian gene mutations and alcohol seeking and reward
Human clinical studies suggest bidirectional relationships between alcoholism and circadian rhythms. Excessive alcohol drinking may disrupt circadian rhythms, while circadian disruption or desynchrony may promote and maintain excessive alcohol drinking (Spanagel et al., 2005b). In chronic alcoholics, circadian disruption is known to persist during extended periods of abstinence (Drummond, Gillin, Smith, & DeModena, 1998; Landolt & Gillin, 2001), which may precipitate relapse to drinking (Brower, 2003; Brower, Aldrich, Robinson, Zucker, & Greden, 2001). On the other hand, alterations to sleep-wake cycles, among other rhythms, may predict initial susceptibility to alcohol abuse and alcoholism (Crum, Ford, Storr, & Chan, 2004; Crum, Storr, Chan, & Ford, 2004) Circadian desynchrony itself may be a risk factor for alcoholism (Harma, Tenkanen, Sjoblom, Alikoski, & Heinsalmi, 1998; Trinkoff & Storr, 1998). Human genetic studies have found mutations in a few of the circadian genes that are associated with alcohol addiction (Comasco et al., 2010; Dong et al., 2011; Spanagel et al., 2005a). Evidence from circadian mutant mice have begun to uncover the role of these genes in alcohol related behaviors.
Period genes
Several studies have suggested the role of Per2 in alcohol drinking behavior in humans and animal models. Schumann and colleagues were the first to demonstrate that a SNP in Per2 (rs56013859) was associated with high levels of alcohol drinking in alcohol dependent patients (Spanagel et al., 2005a). Interestingly, this SNP is not associated with the amount of alcohol consumed, or measures of alcohol misuse, among social drinkers (Kovanen et al., 2010), suggesting Per2 has a specific role in alcohol dependence. Although direct biological significance of this SNP is unknown, it is hypothesized to reduce Per2 gene expression by impairing transcription factor binding (Partonen et al., 2007; Spanagel et al., 2005a). Evidence from Per2 mutant mice (Per2−/−) further supports Per2 as a candidate gene for elevated alcohol intake. Per2−/− mice consume high levels of alcohol, display increased preference for alcohol at higher concentrations and alcohol-seeking behaviors, and elevated behavioral sensitivity to acute alcohol administration (Brager, Prosser, & Glass, 2011a; Perreau-Lenz, Zghoul, de Fonseca, Spanagel, & Bilbao, 2009; Spanagel et al., 2005a). These behavioral phenotypes seem to persist regardless of genetic background, indicating the Per2 mutation has high penetrance (Gamsby et al., 2013).
Per2−/− mice also show brain hyperexcitability caused by decreased efficiency to clear synaptic glutamate and elevated dopamine levels in the striatum (Hampp et al., 2008), which may contribute to their elevated alcohol intake (Spanagel et al., 2005a). Acamprosate (Campral), a pharmacological treatment for relapse in recovering alcoholics, is thought to exert its therapeutic effects by attenuating hyperglutamatergic signaling induced by repeated binge and withdrawal episodes (Spanagel & Kiefer, 2008; Spanagel et al., 2005b). Acamprosate reduces extracellular glutamate levels in the brain and reduces alcohol drinking during free-choice (Brager, Prosser, & Glass, 2011c; Spanagel et al., 2005b), and relapse paradigms in Per2−/− mice (Brager et al., 2011a). These effects appear to be independent of alterations to locomotor and drinking rhythms (Brager et al., 2011a; Gamsby et al., 2013), suggesting acamprosate’s ability to reduce alcohol drinking may not involve direct modulation of the circadian pacemaker. However, infusion of acamprosate directly into the SCN in Per2−/− mice reduced alcohol intake during a relapse paradigm that was similar to the reductions observed after infusion into the VTA, NAc, or peduculopontine tegmentum (PPT) (Brager et al., 2011c). It will be important to determine whether acamprosate reduces alcohol drinking by altering or modulating synaptic communication between circadian and reward related neurocircuitry.
The majority of human studies reporting associations between circadian gene polymorphisms and alcohol drinking have revealed novel interactions between genes and environment, or other psychiatric disorders. Several recent genetic studies from the Mannheim Study of Children at Risk, a large ongoing epidemiological cohort study of the outcome of early risk factors from infancy to adulthood (Dong et al., 2011), link polymorphisms in circadian genes with alcohol use and stress. The roles of Per1 and Per2 in stress-related alcohol drinking may involve both the development of compulsive alcohol drinking in adolescents and the maintenance of heavy alcohol drinking in adults. A SNP in the Per1 promoter (rs3027172) is associated with heavy drinking among adolescents and alcohol dependent adults (Dong et al., 2011). Among heavy drinking adolescents exposed to high psychosocial stress, the rs2037172 genotype (minor C allele) was significantly associated with both frequency of drinking and the amount of alcohol consumed (Dong et al., 2011). Similarly, adolescents homozygous for the major A allele of the Per2 rs56013859 genotype were more likely to report harmful and risky alcohol drinking patterns if they were exposed to high stress in both “experienced” and “inexperienced” alcohol users (Blomeyer et al., 2013), and were more likely to report disrupted sleep (Comasco et al., 2010). Stress-related psychiatric disorders are also quite high among alcoholics, and comorbid anxiety and depression disorders often lead to relapse drinking (De Witte, Pinto, Ansseau, & Verbanck, 2003; Driessen et al., 2001). As circadian gene mutations are also associated with mood disorders (Kennaway, 2010), these could be an underlying contributor to heightened stress response, alcohol drinking, and anxiety and depression.
Animal studies suggest that lowered Per gene function is associated with elevated alcohol drinking, increased anxiety-like behaviors, and altered stress response. However, the effect of either the Per1 or Per2 mutation on alcohol and anxiety-like behaviors requires more systematic study because the penetrance of the mutation seems to depend on genetic background of the mouse. When the Per1 mutation is backcrossed to a high alcohol preferring C57BL/6J background, these mice display elevated levels of alcohol intake and reward similar to the Per2 mutation on either a mixed (129SvEv x C57BL/6) or pure (C57BL/6) background (Gamsby et al., 2013). In contrast, the Per1 mutation on a mixed background fail to display increased alcohol drinking or lever pressing, elevated drinking following deprivation, or enhanced sensitivity to alcohol (Perreau-Lenz et al., 2009; Zghoul et al., 2007). However, Per1−/− mice on a mixed background consume more alcohol than wild-type animals following social defeat stress (Dong et al., 2011). Preliminary data suggests Per2−/− mice do not show an increase in alcohol drinking following repeated restraint stress (Sarkar, 2012). Additive effects of Per1 and Per2 knockdown on anxiety-like behaviors have been observed (Spencer et al., 2012a), but not on alcohol drinking or reward (Gamsby et al., 2013), although genetic background may contribute to these discrepancies.
Systematic characterization of the Per mutant mice across multiple behavioral domains in combination with targeted brain region gene knockdown and overexpression approaches are necessary to better understand the role of Per genes in alcohol, anxiety, and depression phenotypes. There is very little known regarding the molecular mechanisms in the brain affected by changes in Per expression that give rise to changes in behavior. Per genes seem to be protective against elevated alcohol drinking under baseline and stress conditions. Glucocorticoid release following stress directly regulates the transcription of Per1 and Per2 by transcription factor binding to glucocorticoid receptor response elements and other binding motifs (Cheon, Park, Cho, & Kim, 2013; Pezuk, Mohawk, Wang, & Menaker, 2012; Reddy, Gertz, Crawford, Garabedian, & Myers, 2012). The CC genotype identified in high drinking chronically stressed adolescents significantly reduces glucocorticoid induced transcriptional activation of Per1 in human B-lymphoblastoid cells (Dong et al., 2011). Impaired Per gene upregulation in response to stress may impact downstream mechanisms that alter neuronal function and responses contributing to elevated alcohol drinking and dependence (Agapito, Mian, Boyadjieva, & Sarkar, 2010; Dong et al., 2011; Sarkar, 2012).
Clock
The Clock gene has also been identified as a potential candidate for alcohol addiction and comorbid disorders. Several polymorphisms in Clock are linked to alcohol dependence and depression, among other psychiatric disorders. However, studies have failed to find associations between any Clock polymorphism and alcohol dependence independent of other psychiatric disorders (Kovanen et al., 2010; Sjoholm et al., 2010). A haplotype formed by Clock SNPs rs3805151, rs2412648, rs2412464, and rs11240 was associated with an increased risk for comorbid depression and alcohol use disorders (Sjoholm et al., 2010). Among the SNPs within the haplotype, the most suggestive of the polymorphisms is rs11240, which is in linkage disequilibrium with rs1801260, a well-studied Clock SNP (3111T/C). The C allele is associated with bipolar disorder, schizophrenia, sleep disorders, evening chronotypes, and obesity (Barclay et al., 2011; Benedetti et al., 2007; Benedetti et al., 2003; McCarthy, Nievergelt, Kelsoe, & Welsh, 2012; Soria et al., 2010; Tsuchimine, Yasui-Furukori, Kaneda, & Kaneko, 2013; Zhang et al., 2011). This polymorphism is located within the 3′ untranslated region of the Clock gene, where it is likely to affect RNA stability or protein translation.
As mentioned previously, the ClockΔ19 mutant mice have an increase in dopaminergic activity in the VTA, and exhibit increased cocaine preference, reward, and seeking behaviors (Coque et al., 2011; Ozburn et al., 2012; Roybal et al., 2007). They also exhibit reduced anxiety- and depression-like behavior, hyperhedonia, hyperactivity, circadian rhythm disruptions, and decreased sleep, which is similar to a “manic-like” behavioral state (Arey & McClung, 2012; Roybal et al., 2007). Indeed, substance use disorders, including alcohol, are often comorbid with bipolar disorder (Fossey et al., 2006; Regier et al., 1990). Many of the anxiety- and depression-like behaviors are rescued by restoring CLOCK expression specifically in the VTA, or can be recapitulated by reducing CLOCK expression in the VTA (Mukherjee et al., 2010; Roybal et al., 2007). Recently, we demonstrated Clock is also important for modulating alcohol intake and preference (Ozburn et al., 2013). ClockΔ19 mutant mice consume more alcohol and prefer alcohol at higher concentrations during two-bottle free choice conditions than their wild-type littermates. Similar to other behaviors, Clock knockdown in the VTA of wild-type mice recapitulates the alcohol preferring phenotype of ClockΔ19 mutant mice. ClockΔ19 mutant mice are also hypersensitive to the sedative effects of alcohol as well as ketamine, an NMDA antagonist, but not pentobarbital, during both inactive and active phases (Ozburn et al., 2013). Unlike Per2−/− mice, acamprosate fails to reduce alcohol intake and preference in ClockΔ19 mutant mice (Ozburn et al., 2013; Spanagel et al., 2005a). Thus, glutamatergic transmission is involved in the sedative and hypnotic effects of alcohol, whereas altered dopaminergic transmission likely contributes to the elevated alcohol drinking in ClockΔ19 mice (McClung, 2007b; Ozburn et al., 2012; Spencer et al., 2012b). A hyperdopaminergic state may also underlie the mood- and reward-related behaviors in these mice (Russo & Nestler, 2013).
Other circadian genes
Other circadian genes are implicated in human alcohol abuse and alcoholism, and comorbid disorders. For example, SNPs in Per3 are associated with sleep disruption, bipolar disorder, novelty seeking, and alcohol behaviors (Archer et al., 2003; Artioli et al., 2007; Benedetti et al., 2008; Kripke, Nievergelt, Joo, Shekhtman, & Kelsoe, 2009; Viola et al., 2007; Wang et al., 2012). Using BXD recombinant inbred mouse strains, a cis-acting expression QTL was mapped to Per3 that is linked to stress, anxiety, and alcohol traits (Wang et al., 2012). Per3 may have tissue specific function in circadian rhythmicity (Pendergast, Niswender, & Yamazaki, 2012). In the mouse hippocampus, Per3 regulates multiple downstream genes associated with circadian rhythms, stress response, synaptic functions, and alcoholism (Wang et al., 2012). Notably, one of those genes, calpastatin (Cast), was identified as a susceptibility gene for human alcohol dependence (Treutlein et al., 2009). A study from a population-based sample of controls and individuals with alcohol use disorder analyzing multiple SNPs failed to identify any significant associations between circadian genes and alcohol dependence (Kovanen et al., 2010). However, a SNP in Arntl2 (Bmal2, rs4964057) was nominally associated with alcohol abuse, and Arntl (Bmal1, rs6486120) was nominally associated with alcohol abuse among social drinkers (Kovanen et al., 2010). Both Bmal1 and Bmal2 are involved in normal circadian rhythms (Shi et al., 2010). Polymorphisms in Bmal1 have also been associated with bipolar and depressive disorders (Mansour et al., 2006; Utge et al., 2010); however, there have been few studies that have examined the role of Bmal1 in drug-seeking behavior. Both In peripheral blood and mononuclear cells collected from alcohol dependent and socially drinking individuals, Bmal1 expression was significantly reduced (Ando et al., 2010; Huang et al., 2010). In chronically drinking animals, Per expression rhythms are altered in the hypothalamus (Chen, Kuhn, Advis, & Sarkar, 2004), but the effects of alcohol on Bmal1 or Bmal2 expression in mood and reward related brain regions are unknown. Disrupted or reduced expression of Bmal1 or Bmal2 could alter the transcriptional mechanisms driven by CLOCK and BMAL complexes that impact alcohol drinking or withdrawal behaviors. Alcohol related phenotypes have not been explored in BMAL1-deficient mice.
Both casein-kinase 1 episolon and delta (CK1ε and CK1δ, respectively) are additional circadian genes tied to alcoholism and alcohol relapse (Al-Housseini et al., 2008; Perreau-Lenz et al., 2012). We will refer to both isoforms as CK1ε/δ because most studies do not distinguish between the two. Both isoforms belong to the superfamily of serine/threonine-specific kinases that play a major role in regulating and sustaining the pace of the molecular clock by phosphorylating circadian proteins, BMAL1, CRY, PER1 and PER2 (Eide, Kang, Crapo, Gallego, & Virshup, 2005; Meng et al., 2008). PER2 is rhythmically destabilized by CK1ε/δ phosphorylation (Eide et al., 2005; Etchegaray et al., 2009; Meng et al., 2008), and pharmacological inhibition of CK1ε/δ induces phase delays of circadian locomotor activity, enhances PER2 protein levels, and inhibits nuclear translocation of PER3 (Badura et al., 2007; Sprouse et al., 2010). CK1ε/δ inhibition also inhibits PER3 nuclear translocation (Sprouse et al., 2010). CK1ε/δ is associated with behavioral responses to other drugs of abuse (Bryant et al., 2009; Bryant et al., 2012). In chronic alcoholics, CK1ε/δ protein levels are moderately elevated in the superior frontal cortex (Al-Housseini et al., 2008).
Pharmacological inhibition of CK1ε/δ in rats undergoing a relapse-like alcohol drinking paradigm, during which chronic alcohol drinking is interrupted with repeated deprivation phases (Vengeliene, Bachteler, Danysz, & Spanagel, 2005), prevented the alcohol deprivation effect at higher doses with some non-specific effects on water and saccharin consumption (Perreau-Lenz et al., 2012). CK1ε/δ inhibition attenuated the elevated alcohol intake during the day that is often observed during the reintroduction of alcohol following a period of deprivation, and induced an almost 12 h phase shift in locomotor activity (Perreau-Lenz et al., 2012). CK1ε/δ inhibition also normalizes anxiety-like behaviors and has partial effects on depression-like behaviors, but not hyperactivity, in ClockΔ19 mutant mice (Arey & McClung, 2012). Future studies will determine if changes or stabilization of circadian rhythms are necessary for the therapeutic effects of CK1ε/δ inhibition on mood and alcohol related behaviors in ClockΔ19 mutant mice. More studies are needed to systematically probe alcohol related phenotypes in mice with mutations in circadian genes.
Circadian gene mutations and other drugs of abuse
Besides cocaine and alcohol, there are few studies specifically testing the role of circadian genes in the behavioral responses to other types of drugs. Opiate dependent patients often report sleep-wake disturbances (Oyefeso, Sedgwick, & Ghodse, 1997), and morphine withdrawal in rodents disrupts circadian behavioral rhythms (Caille, Espejo, Koob, & Stinus, 2002; Stinus, Robert, Karasinski, & Limoge, 1998). Chronic opiate withdrawal enhances Per2 gene and protein expression in the limbic forebrain and frontal cortex (Ammon, Mayer, Riechert, Tischmeyer, & Hollt, 2003; Ammon-Treiber & Hollt, 2005; Hood, Cassidy, Mathewson, Stewart, & Amir, 2011). Per2Brdm1 mutant and wild-type mice responded similarly to acute morphine, but the mutant mice exhibited reduced tolerance to the analgesic and withdrawal effects (Perreau-Lenz, Sanchis-Segura, Leonardi-Essmann, Schneider, & Spanagel, 2010), suggesting Per2 is involved in the sensitivity to the behavioral effects of morphine.
Chronic methamphetamine treatment enhanced the amplitude of locomotor rhythms and desynchronized the activity rhythm from the LD cycle, which was correlated with desynchronized expression of circadian genes between the striatum and SCN (Honma & Honma, 2009). In arrhythmic animals, regardless of the perturbation (i.e., SCN lesion, LL, or mutant and knockout), methamphetamine restores locomotor rhythmicity (Honma, Honma, & Hiroshige, 1986), suggesting the mechanisms that underlie methamphetamine-restored rhythmicity are substantially different from that of the molecular clock in other brain regions or tissues, and completely independent from the SCN (Mohawk, Baer, & Menaker, 2009). It is unknown whether circadian gene mutations have any effects on methamphetamine-induced locomotor sensitization, reward, or withdrawal, but studies using other psychostimulants, such as cocaine, would suggest so.
Human chronotypes, circadian genes, and reward neurocircuitry
In humans, defining the role of the circadian system in addiction is complicated, and reliably measuring robust markers of circadian phase and chronotype is particularly critical. Objective measures of circadian phase or entrainment typically rely on self-report, rather than objective biological measures. Sleep-wake preferences are often assessed by questionnaires that use subjective ratings of hypothetical situations, comparisons to the habits of others, and feelings of “best rhythm” (Roenneberg, Wirz-Justice, & Merrow, 2003). Participants are typically dichotomously categorized into either “morningness” (larks), or “eveningness” (owls) preferring (Horne & Ostberg, 1976). Morning chronotypes prefer daytime activity, with peak performance and alertness associated with morning hours, whereas evening chronotypes prefer the opposite. These approaches are evolving in order to become closer to the underlying biology (Roenneberg et al., 2003). There are a few studies showing a genetic basis for human chronotypes. For example, polymorphisms in Clock (3111T/C) are associated with eveningness (Katzenberg et al., 1998), and polymorphisms in Per1 (2424T/C), Per2 (111C/G), and Per3 (647G) are associated with morningness (Carpen, Archer, Skene, Smits, & von Schantz, 2005; Carpen, von Schantz, Smits, Skene, & Archer, 2006; Johansson et al., 2003), among cohorts from different ethnic backgrounds. Several of these polymorphisms are also related to major depression, bipolar disorder, and addiction disorders (Kennaway, 2010; McClung, 2007c; Soria et al., 2010; Wang et al., 2012). The interpretation of these genetic findings remains difficult because chronotype is a complex polygenic trait (Duffy, Dijk, Hall, & Czeisler, 1999; Vink, Groot, Kerkhof, & Boomsma, 2001), and more advanced genetics analyses have the potential to cope with these issues and even reveal novel genes underlying these interactions (McCarthy et al., 2012).
Emerging evidence indicates there are shared genetic and biological factors between chronotype, mood disorders, and addiction. Chronotype is linked to affective disturbances (Hasler, Allen, Sbarra, Bootzin, & Bernert, 2010; Mansour, Monk, & Nimgaonkar, 2005), and reward circuitry is critical to the pathophysiology of mood disorders (Forbes et al., 2009; Nestler & Carlezon, 2006). In bipolar patients, evening preference is more prevalent, especially in those who experience rapid mood swings (Ahn et al., 2008; Hatonen, Forsblom, Kieseppa, Lonnqvist, & Partonen, 2008). Clinically depressed individuals who are also evening chronotypes display delayed and blunted positive affect rhythms (Boivin et al., 1997; Murray et al., 2009), and report poorer emotional adjustment and elevated rates of substance abuse (Giannotti, Cortesi, Sebastiani, & Ottaviano, 2002). Chronotype, circadian gene polymorphisms, and brain reward circuitry appear to be related. Using positron emission tomography, Hasler and colleagues measured diurnal variations of the rate of glucose uptake within brain regions associated with positive affect and reward in patients with insomnia who are either morning or evening types (Hasler et al., 2012a). When compared to morning types, evening chronotypes exhibited a delayed and reduced diurnal peak of positive affect that correlated with a dampened morning to evening variation of glucose uptake in both the medial prefrontal cortex (mPFC) and the striatum (Hasler et al., 2012a), supporting the notion that altered diurnal variation of mood and reward related brain circuitry function underlies the differences in positive affect between chronotypes. Circadian gene polymorphisms also mediate the correlation between decreased mPFC activity and reward. Healthy adolescents carrying a specific G allele (Per2 SNP rs2304672) were more likely to display reduced activity in the mPFC during a reward task (Forbes et al., 2012). In response to reward outcome, mPFC activation was strongly negatively correlated with ventral striatal response in carriers of the G allele (Forbes et al., 2012). Moreover, later sleep midpoints, was associated with reduced mPFC activity, but not Per2 polymorphisms (Forbes et al., 2012). Thus, chronotype and circadian genes may mediate cortico-striatal activation following a rewarding stimulus (Haber & Knutson, 2010; Hasler & Clark, 2013). Future studies will require systematic and objective measurement of circadian phase, sleep-wake cycles, and environmental factors, to determine the extent to which circadian and/or sleep homeostatic processes impact reward circuitry activation. It will also be important to identify robust biological markers of circadian phase, which could be used to more appropriately characterize diurnal variations in reward circuitry activation and mood.
Circadian control of brain reward mechanisms
The molecular components of the circadian clock are expressed in the mesocorticolimbic system central to reward processing and susceptibility to drugs of abuse (Panda et al., 2002; Rosenwasser, 2010). Consequently, these nuclei exhibit circadian patterns of activity in neuronal firing and neurochemical transcription and release in mice and rats (McClung, 2007b), suggesting functional regulation of the circadian system over these processes. Additionally, many behaviors regulated by the limbic system, including drug-seeking behavior and locomotor sensitization, exhibit rhythmic patterns despite no change in the metabolic rates of the drugs themselves. Collectively, these phenomena point to a direct connection between circadian gene expression and reward, leading to the possibility that disruptions in the circadian system may directly impact risk for drug dependence and addiction.
The VTA
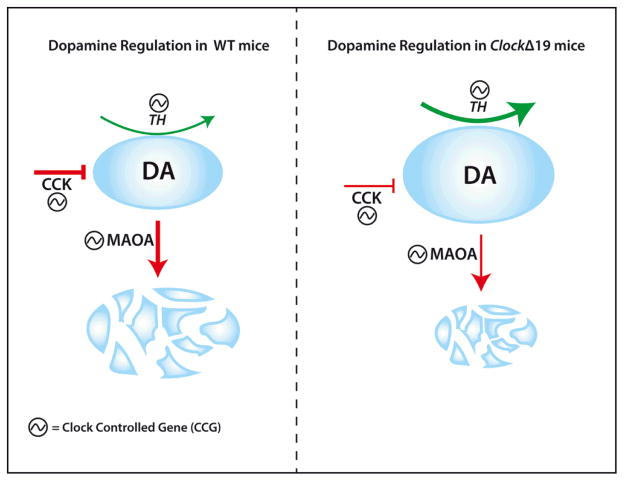
In the VTA, a number of processes associated with dopamine transmission are controlled by circadian genes and express diurnal rhythms. The expression of tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis, exhibits diurnal rhythms in both mice and rats (McClung et al., 2005; Webb, Baltazar, Lehman, & Coolen, 2009). Additionally, the expression of dopamine receptors and monoamine oxidase (MAOA) the primary enzyme responsible for dopamine degradation express diurnal rhythms (Hampp et al., 2008; McClung et al., 2005; Wirz-Justice, 1987). The canonical E-Box sites (CACGTG) present in Maoa, Drd1/Drd2 and TH genes suggest these are direct clock-controlled genes (CCGs) allowing for transcriptional control of these genes by core circadian proteins. Indeed, chromatin immunoprecipitation (ChIP) studies reveal that CLOCK and BMAL bind the E-box promoters of TH and Maoa and regulate their transcription (Hampp et al., 2008; Sleipness et al., 2007b). Despite their heterogeneous mechanisms of action, all drugs of abuse impinge upon the brain’s reward circuitry, the majority of which increase DA concentrations in VTA projection sites, as well as within the VTA itself (Robison & Nestler, 2011). Disruptions in any of the myriad regulatory mechanisms that regulate baseline DA in the VTA increase the vulnerability to the rewarding properties of drugs. Therefore, it is not surprising that the behavioral aberrations associated with clock gene mutations in the context of reward are also associated with disruptions in dopamine transmission within the VTA or its primary projection sites. ClockΔ19 mice exhibit increased TH mRNA expression, higher dopamine firing and increased bursting activity in the VTA (Coque et al., 2011; McClung et al., 2005). CLOCK-specific shRNA delivered locally into the VTA recapitulates these findings (Mukherjee et al., 2010), suggesting that CLOCK is potentially exerting its effects on dopamine transmission locally. The increase in TH, in ClockΔ19 mice suggests that the WT CLOCK protein acts as a modulator of dopamine synthesis, providing a potential mechanistic insight into the circadian control of reward. The role of CLOCK as an inhibitor of TH transcription is compelling, given the predominantly activational role of CLOCK in the CNS and periphery. The proximity of the E-box in the TH promoter to adjacent CRE sites may underlie the interference of the CLOCK protein for other transactivational targets (Manev & Uz, 2006). In addition to TH, other circadian-controlled transcriptional targets within the VTA may regulate DA output. Cholecystokinin (CCK), a neuropeptide co-localized with NAc-projecting DA neurons in the VTA (Hokfelt et al., 1980; Lanca, De Cabo, Arifuzzaman, & Vaccarino, 1998), negatively modulates dopaminergic transmission in vivo (Hokfelt et al., 1980; Schade et al., 1995). CCK expresses an E-box in the promoter and is positively regulated by the CLOCK protein (Hansen, 2001)
Additionally, Cck mRNA is significantly downregulated in ClockΔ19 mice, and the behavioral phenotype of these mice can be recapitulated by VTA-specific knockdown of Cck (Arey et al., 2013). These results implicate Cck as a critical CCG that regulates DA output and contributes to reward-related behavioral outcomes. Most neurons in the VTA are dopaminergic or GABAergic interneurons that exert local inhibitory control over DA output signals. In ClockΔ19 mice, or following CLOCK RNAi in the VTA, a number of GABAergic associated genes are downregulated (Arey et al., 2013; McClung et al., 2005; Mukherjee et al., 2010), suggesting the potential loss of inhibitory control contributes to the increase in DA and associated hyperhedonia in these mice. Future studies examining the role of circadian genes on regulating GABAergic transmission in the VTA are necessary to determine if the loss of GABAergic control contributes to the molecular and behavioral phenotype of Clock mutants.
The NAc
The mechanisms by which drugs of abuse impinge upon the NAc and regulate reward and drug dependence are complicated by the cellular and molecular heterogeneity of this nucleus. Glutamatergic input from multiple regions, including the PFC and amygdala, and dopaminergic input from the VTA converge on sub-sets of neurons within the accumbens and potentially regulate different components of reward and motivation (Robison & Nestler, 2011). While 95% of NAc neurons are medium spiny neurons, they differ in the expression profiles of dopamine D1 or D2 type receptors, and the net effect of drugs of abuse in this nucleus greatly depends on the specific receptor expression profiles and afferent networks involved. Many of these critical components are additionally regulated by circadian transcriptional mechanisms that modulate the response to drugs of abuse. Daily rhythms in DA release in the NAc, as measured by microdialysis (Schade et al., 1995), reveal circadian influence, although these rhythms may be the result of signaling from afferent networks. For example, Clock RNAi delivered into the VTA results in a net increase of DA release in the accumbens (Mukherjee et al., 2010), and TH and DAT rhythms in the NAc are abolished following SCN lesions (Sleipness et al., 2007b). However, in addition to afferent regulation, circadian genes within the accumbens likely regulate local dopaminergic tone. In Per2 mutant mice, Maoa expression is lower in the accumbens, and dopamine levels are elevated in these mice compared to wild-type littermates (Hampp & Albrecht, 2008). Given that Maoa is a CCG, the elevation in DA directly connects a circadian mutation to disrupted DA metabolism, which likely contributes to the aberrant behavioral mutant phenotype (see above). The ratio of D1:D2 in Per2 mice is significantly decreased, which may be the result of compensatory processes to the elevated DA tone in the NAc (Hampp & Albrecht, 2008). This effect suggests that disruptions in the circadian system will impact multiple downstream processes in addition to the direct impact on CCGs. In ClockΔ19 mice, total dopamine levels in NAc are elevated, although this may be the result of increased firing and bursting of VTA-originating dopamine neurons that terminate in the NAc (Coque et al., 2011). Potentially as a result of the increase in DA tone in the NAc of these mice, there is a dramatic decrease in the D1/D2 ratio, specifically due to a robust upregulation in D2 receptor expression (Spencer et al., 2012b). Consequently, the locomotor response to a D1 agonist is blunted in ClockΔ19 mice, despite their normal locomotor response to cocaine. Conversely, the response to a D2 agonist is increased in these mice, which reflects the D1/D2 shift and a potential compensatory shift in DRD2 expression as a result of the inability of the mutant CLOCK protein to modulate DA transcription and release. As with Per2 mice, further studies will be necessary to determine the relative impact of local clock gene regulation versus afferent signaling in contributing to the hyperhedonic phenotype in ClockΔ19 mice.
The SCN
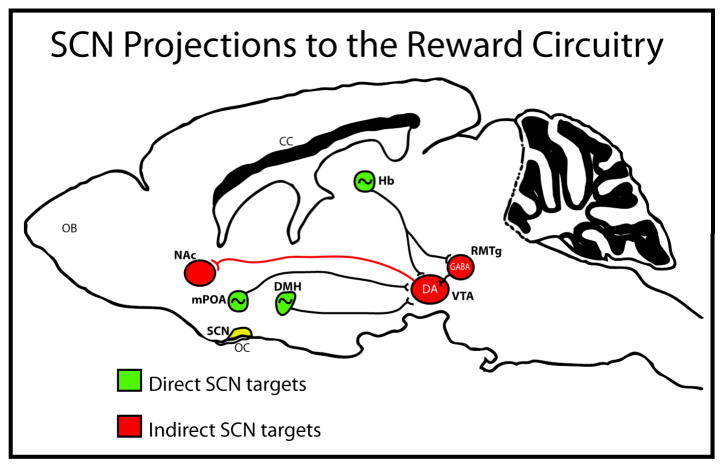
In addition to the local regulation of mesolimbic circuitry by circadian genes, the rhythmic expression of dopamine and glutamate in these regions may be controlled at the circuit-level through indirect connections originating in the SCN. Indeed, the SCN sends multisynaptic projections to the VTA through the mPOA (Cheng, Leslie, & Zhou, 2006; Jhou, Fields, Baxter, Saper, & Holland, 2009; Luo & Aston-Jones, 2009) providing an anatomical connection between the central pacemaker and rhythmic neurobiological processes in the VTA (see below). Lesions to the SCN in rats have an antidepressant effect, suggesting the master clock may exert control over mood and affect through connections to limbic circuits (Tataroglu, Aksoy, Yilmaz, & Canbeyli, 2004). Additionally, the efficacy of the “antidepressant” drug, agomelatine, a melatonin receptor agonist and a serotonin (5-HT2C) antagonist, on social defeat-induced anxiety requires an intact SCN in rats (Tuma, Strubbe, Mocaer, & Koolhaas, 2005), pointing to a direct role of this nucleus in mediating the response to stress and anxiety.
More studies are needed to determine if SCN lesions impact reward and drug taking, although the indirect projections to limbic circuitry reveals functional control of the master clock over nuclei associated with reward and motivation. For example, the discovery of novel, ‘wide –spike’ VTA neurons that exhibit strong circadian rhythms in firing activity, with selective spiking during the active phase of the diurnal cycle, suggest entrainment by the master clock (Luo & Aston-Jones, 2009; Luo, Georges, & Aston-Jones, 2008). Given that neither Per nor Clock mRNA expression is rhythmic in in vitro preparations of VTA tissue (Abe et al., 2002) the regulation of circadian processes within the VTA in vivo may involve multisynaptic inputs from the SCN. The rhythms of both DAT and TH are attenuated in the NAc following SCN lesions in rats, leading to constitutively higher levels of both DAT and TH across the diurnal cycle (Sleipness et al., 2007b). This suggests that the SCN is exerting inhibitory control over dopamine rhythms in the NAc. However, it is possible that an extra-SCN oscillator is responsible for regulating circadian variation in specific drug-related behaviors (Sleipness, Sorg, & Jansen, 2007a).
In addition to neuronal control, the SCN may communicate with reward circuitry through humoral factors that are believed to contribute to motivation and affect. Prokineticin 2 (PK2) is a signaling molecule expressed in the SCN that is considered to be critical for the generation of circadian locomotor rhythms (Cheng et al., 2002). Intriguingly, all primary SCN targets express the PK2 receptor, including regions that project directly to the VTA, such as the lateral habenula and mPOA (Masumoto et al., 2006). PK2 knockout mice exhibit decreased anxiety and depression like behaviors, which are rescued by ICV infusions of PK2 (Li, Hu, & Zhou, 2009). These results provide further evidence of the influence of SCN signaling over mood and affect. While the contribution of PK2 to reward and drug seeking requires further testing, these mice raise the intriguing possibility that SCN-derived neurochemicals can regulate limbic structures and associated behaviors.
The effects of drugs of abuse on circadian rhythms and circadian genes in humans and animals
Chronic drug administration in humans
Human drug addiction is linked to altered circadian rhythms, while circadian disruption, either by genetic mutation or environmental desynchrony, may be a risk factor for addiction, relapse, and withdrawal severity. Acute and chronic drug exposure alters molecular, physiological and behavioral rhythms, which may involve SCN-dependent or SCN-independent (i.e., extra-SCN oscillators). Few human studies have examined the effects of drugs of abuse on molecular and physiological rhythms in tissues collected from addicted individuals. Peripheral blood mononuclear cells (PBMCs) collected from human alcoholics immediately prior to hospital admission and one week into withdrawal showed a marked reduction in circadian gene expression compared to healthy controls (Huang et al., 2010). During the course of the withdrawal week, circadian gene expression tended to increase, however, these levels failed to approach that of controls and were not correlated with withdrawal severity (Huang et al., 2010). Skin fibroblasts collected from alcohol dependent patients that were transfected with a Per2:luc construct to monitor real-time bioluminescence rhythms, displayed similar rhythm amplitude and period as controls (McCarthy, Fernandes, Kranzler, Covault, & Welsh, 2013). However, within the alcohol dependent cohort, period was inversely correlated with illness severity, such that patients with a shorter period may be more severely alcohol dependent than those with longer periods (McCarthy et al., 2013). More extensive studies are necessary to determine whether these changes in molecular rhythms are robust across alcohol dependent cohorts, and/or if they vary depending on cell type, physiological state, and psychiatric subtype.
In chronically alcohol drinking mice and rats, changes in circadian gene expression depend on alcohol paradigm, length and dose of alcohol exposure, and brain region or peripheral tissue. In rats, chronic ethanol liquid diet shifted the gene expression rhythms of Per2 and Per3 in the SCN and Per1 and Per2 in the arcuate nucleus (Chen et al., 2004), but minimally affected circadian gene expression in the SCN (Filiano et al., 2013). However, chronic alcohol drinking for extended periods (e.g., 6 to 12 months) reduced gene expression of arginine vasopressin (AVP), vasoactive-intestinal polypeptide (VIP), and somatostatin (SST) in the SCN and the number of SCN neurons expressing these neuropeptides, which persisted during withdrawal from alcohol (Madeira et al., 1997; Madeira & Paula-Barbosa, 1999). Both AVP and VIP are differentially distributed across the ventral (core) and dorsal (shell) SCN, respectively (Antle & Silver, 2005), which contributes to their distinct functional roles in the SCN (Aton, Colwell, Harmar, Waschek, & Herzog, 2005; Yan et al., 2007). Reductions of AVP and VIP signaling in the SCN provide a plausible mechanism by which chronic alcohol drinking or during alcohol withdrawal alters circadian responses to photic stimuli and circadian period (Logan, McCulley, Seggio, & Rosenwasser, 2012; Rosenwasser, Logan, & Fecteau, 2005b; Seggio, Fixaris, Reed, Logan, & Rosenwasser, 2009; Seggio, Logan, & Rosenwasser, 2007).
Acute and chronic treatment of rodents with psychostimulants, opiates, and other drugs of abuse alter expression of circadian genes in the SCN, and reward and stress related brain regions. Acute and repeated methamphetamine injections in mice increases Per1 and Per2 expression in the SCN, and alters the expression rhythms of Per1, Per2, Bmal1, and Rev-erbα in the striatum, which are both likely mediated by dopaminergic and glutamatergic signaling (Iijima, Nikaido, Akiyama, Moriya, & Shibata, 2002; Li et al., 2008; Nikaido, Akiyama, Moriya, & Shibata, 2001; Sanchis-Segura, Jancic, Jimenez-Minchan, & Barco, 2009; Webb et al., 2009; Wongchitrat, Mukda, Phansuwan-Pujito, & Govitrapong, 2013). Altered expression of circadian genes in the dorsal striatum and the NAc persist during acute and protracted withdrawal from methamphetamine and heroin. Repeated morphine administration stably enhances Per1 and Per2 expression in the frontal cortex and striatum (Ammon-Treiber & Hollt, 2005; Li et al., 2008). Inhibiting Per1 activation in the frontal cortex, hippocampus, and striatum, prevents morphine CPP and activation of ERK and CREB signaling pathways (Li et al., 2008), indicating this mechanism is important for morphine reward.
Chronic cocaine administration differentially affects circadian gene expression across many mood- and reward-related brain regions. In the hippocampus, chronic cocaine increased the expression of Per1 and Per2, and decreased the expression of Bmal1 and Cry1, whereas in the caudate putamen, only Per1 and Clock were increased (Uz et al., 2005). Also, Per2, Cry1, Bmal1, and Clock were increased in the dorsal striatum following chronic cocaine (Lynch, Girgenti, Breslin, Newton, & Taylor, 2008). We recently demonstrated that chronic, but not acute cocaine administration, increased the expression and altered the diurnal variation of the circadian transcription factor, Npas2, along with Per1 and Per3 expression in the NAc (Falcon, Ozburn, Mukherjee, Roybal, & McClung, 2013). Chronic cocaine also enhanced the binding of NPAS2 to both the Per1 and Per3 promoters in the NAc and dorsal striatum, but had no effect on CLOCK binding, or the expression of Clock and Bmal1 (Falcon et al., 2013). Overall these results suggest that in response to chronic cocaine, CLOCK and NPAS2, may be differentially regulated depending on the brain region and signaling mechanism.
There is extensive crosstalk between the molecular clock and dopaminergic pathways. For example, the circadian variation of the locomotor response to a D2/D3 receptor agonist correlates with the peak (day) and troth (night) of D2:D3 receptor ratios in the striatum (Akhisaroglu et al., 2005). Furthermore, activation of D2/D3 receptors in the mouse striatum inhibits Clock and Per1 expression, whereas D1 receptor activation stimulates Npas2, Clock, Bmal1, and Per1 expression (Imbesi et al., 2009). However, there is very little known about the downstream molecular mechanisms that are affected by changes in circadian gene expression following chronic drug administration, and whether these changes lead to brain region specific alterations or plasticity that plays a role in the addiction process.
The master circadian pacemaker of the SCN is also vulnerable to drugs of abuse. Drugs of abuse directly affect neuronal firing within the SCN and modulate afferent and efferent pathways for photic and nonphotic entrainment. Ethanol treatment blocks glutamate induced phase delays of SCN firing, while co-treatment with ethanol and 8-OH-DPAT (5HT1AR agonist) enhances phase advances of SCN firing (McElroy, Zakaria, Glass, & Prosser, 2009; Prosser & Glass, 2009). Ethanol also disrupts circadian phase resetting and alters the period (Brager, Ruby, Prosser, & Glass, 2010, 2011b; Seggio et al., 2007). Other drugs of abuse, including methamphetamine, cannabinoids, nicotine, and cocaine, alter the pattern and rate of SCN firing (Acuna-Goycolea, Obrietan, & van den Pol, 2010; Biello & Dafters, 2001; Yang, Wang, Cheng, Kuo, & Huang, 2010). Nicotine phase advances SCN firing rhythms during the subjective night, presumably through activation of M1 and M4 acetylcholine receptors (Liu & Gillette, 1996; Yang et al., 2010). Circadian variation of behavioral sensitivity to nicotine may involve SCN dependent (e.g., melatonin) or independent (e.g., mesolimbic dopamine) mechanisms (Ferguson, Kennaway, & Moyer, 1999; Pietila, Laakso, & Ahtee, 1995). Cocaine also impacts normal SCN functioning. An acute cocaine injection directly into the SCN induces large phase advances during the mid-subjective day, which can be prevented using a serotonin receptor antagonist, and attenuates photic phase delays during the early subjective night (Brager et al., 2011b).
Most of the aforementioned studies address the acute effects of drug administration on SCN functioning. There is very little known about the effects of repeated administration or withdrawal from drugs of abuse on SCN functioning. A few recent studies suggest that the SCN develops rapid tolerance to acute drug effects that potentially underlies the consequences of chronic drug use on circadian rhythms. Indeed, pretreating the SCN with either ethanol or methamphetamine prevents glutamate and serotonin induced phase shifts in neuronal firing (Biello & Dafters, 2001; Ruby, Prosser, DePaul, Roberts, & Glass, 2009), indicating the SCN may develop rapid tolerance to acute drug exposure. Although, withdrawal from chronic drug administration produces long-lasting changes to SCN firing, suggesting chronic drug-induced plasticity in the SCN (Cutler, Mundey, & Mason, 1999). Following chronic intermittent ethanol vapor exposure, C3H/HeJ, but not C57BL/6J, mice display a significantly shortened period of running wheel activity rhythms, a behavioral output of SCN-driven rhythms (Logan et al., 2012). Thus, there are complex genetic factors underlying the acute and chronic effects of drugs of abuse on SCN functioning and overt behavioral rhythms. Future studies will need to expand on previous work in order to identify the mechanisms underlying rapid drug tolerance in the SCN, the circadian effects of acute and chronic drug exposure, and whether these changes directly or indirectly affect SCN outputs.
Developmental exposure to drugs of abuse
During the prenatal period, exposure to drugs of abuse has a deleterious impact on fetal development that consequently enhances the risk for the emergence of diseases later in life, including psychiatric diseases, such as depression and anxiety (Behnke, Smith, Committee on Substance, Committee on, & Newborn, 2013; Molteno, Jacobson, Carter, Dodge, & Jacobson, 2014; O’Connor & Kasari, 2000; Pei, Denys, Hughes, & Rasmussen, 2011; Streissguth et al., 2004; Treit et al., 2013). A clinical diagnosis of depression or anxiety is often preceded by motor, cognitive, sleep and behavioral problems in early childhood and adolescence (Kelly, Day, & Streissguth, 2000; Logan, Brown, & Hayes, 2013a; Nanson & Hiscock, 1990; Streissguth et al., 2004). These behavioral issues, including attention and social deficits, impulsivity, hyperactivity, and anxiety, may be associated with stress hyper-responsiveness and circadian disruptions (Gruber, Sadeh, & Raviv, 2000; McClung, 2013; Sher, 2003; Verma, Hellemans, Choi, Yu, & Weinberg, 2010). A few lines of evidence support a link between prenatal drug exposure and an increased risk for substance abuse later in life (Alati et al., 2006; Baer, Barr, Bookstein, Sampson, & Streissguth, 1998). For example, disturbances in sleep-wake patterns and stress responsiveness are often observed in children with Fetal Alcohol Spectrum Disorder (FASD), who are at an increased risk for alcohol abuse during adulthood (Alati et al., 2006; Chen, Olson, Picciano, Starr, & Owens, 2012; Troese et al., 2008; Verma et al., 2010). In humans, there is very little known about the structural and functional effects of prenatal drug exposure on circadian physiology and behaviors and their possible contribution to the risk of developing certain diseases. A majority of the evidence showing the circadian system is particularly malleable when exposed to drugs of abuse during early development stems from animal studies.
Animal models of developmental alcohol exposure indicate that the circadian system is particularly vulnerable to alcohol. In rats, developmental alcohol exposure during the human “equivalent” of the last trimester (i.e., postnatal days 4–9), alters circadian responses to light-induced phase shifts, entrainment, and re-entrainment, and shortens the free-running period during constant conditions (Allen et al., 2005a; Allen, West, Chen, & Earnest, 2005b; Farnell, West, Chen, Allen, & Earnest, 2004; Sakata-Haga et al., 2006). Similarly, adult flies reared on ethanol containing solutions during the third larval instar stage display significantly shortened free-running periods (Seggio, Possidente, & Ahmad, 2012). The degree of period shortening depends on the dose of ethanol, such that the highest dose (20%) results in the shortest period (Seggio et al., 2012). The shortened free-running period in adult rats and flies exposed to alcohol during development suggests there are similar molecular mechanisms underlying these seemingly permanent alterations to the circadian system.
Prenatal alcohol exposure also impacts the development of functional components of the circadian system, such as the retina (Chmielewski et al., 1997; Tenkova, Young, Dikranian, Labruyere, & Olney, 2003), optic tracts (Parson & Sojitra, 1995) and dorsal and medial raphe nuclei (Sari & Zhou, 2004; Sliwowska, Song, Bodnar, & Weinberg, 2013). In the SCN, the structural effects of alcohol exposure during development appear to be minimal. Alcohol exposure during postnatal days 4–9 in rats reduces SCN neuronal density, without affecting cell number or volume (Farnell et al., 2004). Loss of myelinated optic axons or raphe neurons, and reduced neuronal density in the SCN may alter circadian entrainment under entrained conditions and re-entrainment following a light pulse.
In addition to structural changes, there is growing evidence that developmental alcohol exposure targets components of the molecular clock, and other signaling neuropeptides involved in circadian functions. In the SCN of adult rats exposed to alcohol during postnatal days 4–9, the expression and rhythm amplitude of the neurotrophin, brain-derived neurotrophic factor (BDNF), was significantly reduced. BDNF modulates the activation of NMDA glutamate receptors located in the SCN and on terminals from the retinohypothalamic tract, which mediates the circadian response to photic phase resetting and photic phase shifting (Kim, Choi, & Colwell, 2006; Liang, Allen, & Earnest, 2000). It is likely that BDNF, in part, contributes to the altered phase resetting of animals exposed to alcohol during development.
Developmental alcohol exposure impacts the expression of circadian genes in the hypothalamus and other brain regions. Recent evidence suggests a primary role of the Per genes in circadian and stress phenotypes associated with developmental alcohol exposure. In the SCN, developmental alcohol exposure reduced the amplitude of the Per2 and Cry1 expression rhythms and enhanced the amplitude of Per1 and Bmal1 (Farnell et al., 2008). Less dramatic changes in expression rhythms were found in the cerebellum (Farnell et al., 2008). A recent study by Seggio and colleagues revealed that period effects of developmental alcohol exposure depends on the inherent circadian period and correlates with changes in Per2 expression (Ahmad, Steinmetz, Bussey, Possidente, & Seggio, 2013). In another study, mutant flies that displayed either a shortened (Per2S) or a lengthened (Per2L) activity period were reared in an ethanol-containing solution. Developmental alcohol exposure shortened and lengthened the periods of Per2L and Per2S mutant flies, respectively, while decreasing Per2 in Per2L flies and increasing Per2 in Per2S flies (Ahmad et al., 2013), suggesting Per2, in particular, may be a primary target for alcohol to permanently impact the circadian system. In the mediobasal hypothalamus, the overall expression levels and rhythm amplitudes of Per1 and Per2 were significantly reduced in animals exposed to alcohol during early development (Agapito, Barreira, Logan, & Sarkar, 2012; Chen, Kuhn, Advis, & Sarkar, 2006). The lack of Per2 reduced the stimulatory response of ethanol on hypothalamic genes and neuropeptides involved in metabolism and stress regulation (Agapito et al., 2010). Developmental alcohol exposure may alter Per2 expression and subsequent downstream pathways by a number of molecular mechanisms, such as accelerating degradation by CK1ε and epigenetic modifications.
Most likely, other drugs of abuse impact the development of the circadian system. Cocaine exposure during development reduces the response to a light pulse during the early subjective night (Strother, Vorhees, & Lehman, 1998), and alters the expression of circadian genes Per3 and Bmal1 (Shang & Zhdanova, 2007). The effects of cocaine on the development of the circadian system likely involve Drd1 receptors. Prenatal administration of Drd1 agonists, including cocaine, activate c-fos expression in the fetal SCN (Ferguson, Rowe, Krupa, & Kennaway, 2000; Weaver, Rivkees, & Reppert, 1992) and alter the response to light closely following birth and in adulthood (Ferguson & Kennaway, 2000; Ferguson et al., 2000; Strother et al., 1998). Other stimulants, such as caffeine and nicotine, induce c-Fos in the fetal SCN, although distinct neurotransmitter mechanisms are involved in entraining the fetal SCN (Shearman & Weaver, 2001). The impact of drugs of abuse on the developmental trajectory of the fetal circadian system may have profound health consequences (Brooks & Canal, 2013). Future studies will need to determine the extent to which the development of the circadian system is involved in the emergence, progression, and comorbidity of psychiatric diseases, such as anxiety, depression, and addiction, across the lifespan.
Genetic linkages between circadian and alcohol phenotypes
Shared genetic linkages between the circadian clock and alcohol-related behaviors are suggested by studies showing that rats and mice selectively bred for alcohol preference, withdrawal, and behavioral responses to acute alcohol, exhibit different circadian phenotypes. In high and low alcohol-preferring mice (HAP-1 and LAP-1, respectively), HAP-1 mice have shorter circadian periods in constant darkness (DD) than LAP-1 mice (Hofstetter, Grahame, & Mayeda, 2003). Other high drinking mouse lines, such as the High Drinking in the Dark (HDID-1 and 2), (Crabbe et al., 2009; Crabbe, Spence, Brown, & Metten, 2011; Rhodes, Best, Belknap, Finn, & Crabbe, 2005), display shorter free-running periods and less coherent activity rhythms in constant light (LL) relative to the genetically heterogeneous progenitor line (Hs/Npt) controls (McCulley, Ascheid, Crabbe, & Rosenwasser, 2013). In selectively bred rat lines for high and low alcohol drinking, high preferring (P) and high alcohol drinking (HAD-2) rats have shorter circadian periods in LL and DD, respectively, than their low drinking counterparts (NP and LAD-2) (Rosenwasser et al., 2005a). Moreover, under gradually shortening light-dark (LD) cycles, NP rats are more readily able to entrain to changing LD cycles compared to P rats (Rosenwasser et al., 2005a).
Selective breeding for alcohol withdrawal severity also influences circadian phenotype. The Withdrawal Seizure Prone (WSP-1 and 2) and Withdrawal Seizure-Resistant (WSR-1 and 2) mice were selected for high and low handling induced convulsions during alcohol vapor withdrawal (Crabbe & Phillips, 1993; Kosobud & Crabbe, 1986). In DD, WSP-2 mice have longer circadian periods than the WSR-2 mice (McCulley et al., 2013). Overall it appears that selective breeding for alcohol drinking or other alcohol traits seems to alter responsiveness to light, such as in the case of the P rats, or disrupt the innate circadian period, as in the case of the HAP-1 mice and the HAD-2 rats. Similar mechanisms could be involved in the withdrawal resistant and prone mice. The high drinking P and HAD-2 rats are derived from distinct progenitor stocks and genetic backgrounds, and exhibit different circadian phenotypes in DD and LL, yet identifying the genetic loci underlying these phenotypes is difficult because of the inherent polygenic complexity of both circadian and alcohol phenotypes (Crabbe, Phillips, Buck, Cunningham, & Belknap, 1999; McCulley et al., 2013; Shimomura et al., 2001).
A few studies have pointed towards GABAergic neurotransmission as a potential common mechanism underlying circadian and alcohol-related phenotypes. In the SCN, the molecular clock regulates GABAergic neurotransmission, which is important for coordinated neuronal firing (Belenky, Yarom, & Pickard, 2008; Freeman, Krock, Aton, Thaben, & Herzog, 2013; Moore & Speh, 1993). Both GABA-mediated neurotransmission and GABA-A receptors are also important for many alcohol-related behaviors (Buck & Finn, 2001; Lovinger, 2008; Sprow & Thiele, 2012). For example, QTL mapping studies in WSP and WSR mice and recent gene co-expression analyses in High Drinking in the Dark (HDID) mice, strongly suggest the role of GABA-A receptors in their selected phenotypes (Bergeson et al., 2003; Buck & Finn, 2001; Iancu et al., 2013). As addressed above, most of the selectively bred mouse lines for alcohol-related phenotypes display divergent circadian behavioral phenotypes. New genetic tools and analyses will help identify interactions between the GABAergic system and the molecular clock. Using high-throughput genome sequencing, or other array based approaches, in selectively bred lines, researchers can now construct gene networks that could help to identify the mechanisms at the intersection of circadian and alcohol phenotypes.
Circadian photoperiod, environmental desynchrony, and drug-related behavior
Circadian photoperiod and desynchrony impacts drug administration and responses. Experimental manipulation of the day length and chronic shifting of the LD cycle (shift-lag) can increase or decrease drug related behaviors depending on the paradigm, species, and behavior. In HAD-1 rats, repeated 6 hour LD phase advances at 3 to 4 week intervals decreases alcohol drinking in males and increases drinking in females (Clark, Fixaris, Belanger, & Rosenwasser, 2007). A similar paradigm reduces alcohol drinking in male and female Fischer and Lewis rats (Rosenwasser, Clark, Fixaris, Belanger, & Foster, 2010), but increases voluntary consumption in Spraque Dawley rats (Gauvin et al., 1997). Photoperiod changes (i.e., day length) and chronic shifting paradigms have little effect on alcohol intake in mice, although both DD and LL reduce voluntary consumption in mice and hamsters (Rosenwasser & Fixaris, 2013; Trujillo, Do, Grahame, Roberts, & Gorman, 2011). Cocaine reinstatement is also unresponsive to long days in rats; however, shorter photoperiods profoundly suppress cocaine reinstatement, and this persists even after the animals are transferred back to a standard 12:12 LD cycle (Sorg, Stark, Sergeeva, & Jansen, 2011). It will be important to elucidate how changes in photoperiod alter reward circuit function and drug-induced plasticity and clarify whether these effects are SCN dependent.
Changes in day length (e.g., short vs. long days) and phase of light onset (i.e., shifting paradigms) activates glucocorticoid release and receptor expression in the brain and peripheral tissues, which is thought to aid in the resetting and adjustment of the circadian system to the environment (Gibson, Wang, Tjho, Khattar, & Kriegsfeld, 2010; Kiessling, Eichele, & Oster, 2010; Pezuk et al., 2012). Circadian glucocorticoid oscillations in the brain have a role in neuroplasticity (Liston et al., 2013) and modulate the functional output of regions downstream from the SCN (Segall & Amir, 2010). Indeed, TH and DAT in the mPFC and dorsal striatum respond to changes in photoperiod, but not in the NAc or the VTA (Sorg et al., 2011). A recent study demonstrated that photoperiod mediates anxiety- and depression-like behavior by directly mediating dopamine signaling in the hypothalamus. Interestingly, a short day photoperiod (which is less stressful for a nocturnal mouse) increased the number of dopaminergic neurons in the periventricular (PeVN) and paraventricular (PVN) hypothalamic nuclei (Dulcis, Jamshidi, Leutgeb, & Spitzer, 2013). In response to long day photoperiods (a more stressful situation), a subset of hypothalamic neurons actually “switched” their neurotransmitter production from primarily dopamine to somatostatin (SST) (Dulcis et al., 2013). Both dopaminergic and SST interneurons synapse onto PeVN corticotropin-releasing factor (CRF) neurons (Kumar, 2007), which may regulate stress-induced psychiatric diseases. The decrease in the number of dopaminergic neurons following a long day photoperiod reduced the density of D2 receptors on CRF containing terminals (Dulcis et al., 2013). Reduced postsynaptic density of D2 receptors on CRF neurons dramatically elevated CRF levels in the CSF following a long day photoperiod (Dulcis et al., 2013). The opposite was found following a short day photoperiod, indicating a bidirectional photoperiodic modulation of neurotransmitter switching in the hypothalamus that regulates CRF response and behavior. Indeed, a short day photoperiod decreased anxiety- and depression-related behaviors, whereas a long day photoperiod had the opposite behavioral effect (Dulcis et al., 2013). The behavioral effects of a long day photoperiod seemed to be mediated directly by dopaminergic signaling, as dopaminergic lesions in the hypothalamus similarly increased anxiety- and depression-like behavior (Dulcis et al., 2013), and, quite strikingly, the behavioral effects of dopaminergic lesions were partly reversed by the reemergence of dopaminergic neurons following a short day photoperiod (Dulcis et al., 2013). The ability of a short day photoperiod to reverse the behavioral effect of dopaminergic lesions was blocked by the administration of D1 and D2 antagonists (Dulcis et al., 2013). For the first time, these results provide a mechanism by which photoperiod modulates behavior that may contribute to mood and addiction disorders (LeGates et al., 2012). These studies provide novel insights into how changes in day length or environmental desynchrony potentially modulate drug taking and response.
Circadian rhythms of drug self-administration: animal models of “loss of control”
Drug self-administration follows a robust diurnal pattern in humans and animals. Voluntary alcohol consumption and cocaine self-administration in nocturnal rodents is primarily consolidated into the dark phase (active), with peaks typically occuring during the early night (Aalto & Kiianmaa, 1987; Agabio et al., 1996; Boyle, Smith, & Amit, 1997; Dole, Ho, & Gentry, 1985; Lynch & Taylor, 2004; Pasley, Powell, & Halberg, 1987; Roberts, Brebner, Vincler, & Lynch, 2002; Trujillo, Roberts, & Gorman, 2009). Cocaine self-administration also free-runs under constant conditions, indicating an endogenous oscillatory regulator of drug intake (Bass, Jansen, & Roberts, 2010). The circadian pattern of drug self-administration persists under constant conditions (Bass et al., 2010). In the general human population, alcohol consumption peaks during the early evening, whereas alcohol-dependent individuals report craving for their first drink peaks during the early morning (Danel, Jeanson, & Touitou, 2003), suggesting a temporal “shift” or loss of “normal” diurnal alcohol drinking. Thus, loss of normal circadian rhythm of drug-self-administration may be an informative marker for the transition from use to abuse to addiction.
The progression towards addiction is characterized by the transition from sporadic drug use to consistent drug taking, intense binging, and persistent drug seeking. A “loss of control” is the hallmark of an addicted state. In animals, experimental paradigms where access to a drug is presented intermittently or continuously but separated by periods of deprivation over long periods of time (i.e., alcohol deprivation effect, ADE) escalates drug seeking and drug taking (Holter & Spanagel, 1999; Perreau-Lenz et al., 2012; Roberts et al., 2002; Sinclair & Senter, 1968; Tornatzky & Miczek, 2000). The few studies that have investigated the diurnal patterns of drug intake using these methods indicate alterations in the circadian pattern of drug self-administration. Extended continuous access to nicotine reduces the peak of self-administration during the night (active phase) and increases intake during the day (inactive phase) (O’Dell et al., 2007). Daily cocaine intake is also increased in rats allowed to self-administer during controlled intermittent access trials (Lynch & Taylor, 2004). The circadian pattern of cocaine self-administration seems to depend on the dose and availability of the drug (Roberts et al., 2002). Increasing the allowable dose of cocaine during daily intermittent access eventually leads to a loss of the daily rhythmicity of self-administration (Roberts et al., 2002). In addition, ADE results in the complete loss of normal circadian rhythmicity of alcohol self-administration (Perreau-Lenz et al., 2012; Vengeliene, Noori, & Spanagel, 2013). These are the most impressive pieces of evidence suggesting a loss of rhythmicity of drug self-administration may represent a “loss of control”. It will be important to determine whether chronotherapeutics and compounds that are known to target certain components of the molecular clock reduce drug self-administration by altering the circadian pattern of drug intake (Perreau-Lenz et al., 2012; Vengeliene et al., 2013).
Future Directions
The challenge of future research is to further tease apart the mechanisms by which the circadian system modulates affective and addictive phenotypes. For example, circadian genes may modulate behavior by classic circadian (i.e. SCN driven) or novel mechanisms. Certain behavioral phenotypes in circadian mutant mice may be “secondary” to alterations in circadian rhythms at the cellular or systems level, rather than the action of the circadian gene on an entity that is “independent” of the molecular clock (Rosenwasser, 2010). Studies using mice with global circadian gene mutations are somewhat incapable of reliably distinguishing between these two possible roles. However, it is important to consider that not all of the circadian mutations produce the same behavioral phenotypes, even if the mutation is expected to have similar roles in the molecular clock.
The use of site-specific disruption of circadian genes through RNAi or clock gene/protein rescue experiments in transgenic mice, are useful methodologies in determining the local contribution that these genes play in regulating reward and susceptibility to drug administration. Additionally, studies investigating other clock genes or clock-controlled targets will be useful to determine the regulatory mechanisms by which individual circadian genes or circadian rhythmicity as a whole differentially regulate drug-related phenotypes. For example, Clock knockdown in the VTA of wild-type mice recapitulates both the mood- and drug-related behavior, but not the circadian phenotypes, of ClockΔ19 mice (Mukherjee et al., 2010; Ozburn et al., 2013; Ozburn et al., 2012). Similarly Per1 and Per2 knockdown in the NAc recapitulates the anxiety-like behaviors of Per1−/−/Per2−/− mice (Spencer et al., 2013). Although these studies provide regional specificity for the action of a particular circadian gene, the question remains if these gene manipulations alter cellular rhythms that give rise to the behavioral phenotypes. Current studies are determining whether the behavioral phenotypes following circadian gene knockdown are due to loss of molecular clock function in the mesolimbic circuitry, or whether these directly or indirectly regulate pathways that influence the central clock in the SCN.
Developing tools and techniques that allow for the spatial and temporal control of neural circuits will be critical for determining the site-specific influence of the circadian system over reward and drug seeking behaviors. Specifically, isolating the effects of circuit-level vs. intra-nucleus disruptions of circadian rhythms and their impact on behavior will be greatly aided by the implementation of cutting-edge tools that are already contributing to our knowledge of reward and addiction. Our understanding of the mechanisms that control the intercellular communication within the SCN, which is critical for generating rhythmic output, has been greatly enhanced by the use of Designer Receptors Exclusively Activated by Designer Drugs (DREADDs). Brancaccio and colleagues utilized this technology as a mechanism to activate G-coupled pathways in the SCN, and discovered that Gq-Ca+ signaling is required for the control of cellular rhythm coherence in the SCN, and is dependent upon VIP signaling (Brancaccio, Maywood, Chesham, Loudon, & Hastings, 2013). The augmentation of clock gene signals through these peptidergic cues reveals extra-circadian control over overt rhythms, which adds an additional layer of complexity connecting the network-related properties to circadian rhythmicity. Utilizing DREADDs to augment intercellular rhythms in distinct nuclei will provide novel insight into the mechanisms connecting cellular rhythms to network function in the context of reward. For example, desynchronized phase coupling in ClockΔ19 mice may be rescued through DREADD technology in order to determine the relative contribution in phase uncoupling to the manic behaviors in these mice. In addition to DREADD’s, optogenetic technology may be utilized in the future to determine the contribution of SCN efferents to limbic structures in the regulation of reward circuitry. This technology has already uncovered many circuit level mechanisms that contribute to drug reward, motivation and aversion (Stamatakis & Stuber, 2012).
The long-term adaptations to limbic circuits following acute exposure to drugs of abuse implicate transcriptional modifications as a mechanism of drug dependence. It has become clear that in addition to classic transcriptional factors, epigenetic modifications to DNA are critical regulatory processes that result in myriad behavioral adaptations to drug exposure (Renthal & Nestler, 2008). The ability of circadian genes to modify chromatin structure provides a compelling bridge between circadian genes and the underpinnings of motivation and reward. The discovery that the CLOCK protein exhibits histone acetyl transferase (HAT) activity (Doi, Hirayama, & Sassone-Corsi, 2006) has greatly enhanced our understanding of the mechanisms by which circadian genes can regulate gene transcription. Given the potent ability of epigenetic modifiers to pharmacologically alter reward and drug seeking behaviors, the connection between circadian genes and chromatin state may reveal numerous therapeutic targets for treating drug dependence. For example, the HDAC Sirtuin1 (SIRT1) is expressed in the NAc, where it significantly enhances the behavioral effects of cocaine in mice (Renthal et al., 2009). The enzymatic activity of SIRT1 requires the cofactor NAD+, which is connected to the circadian machinery by the NAD rate-limiting enzyme NAMPT (Nakahata, Sahar, Astarita, Kaluzova, & Sassone-Corsi, 2009), a CCG. In turn, SIRT1 can directly affect clock gene expression, as BMAL1 and PER2 are themselves SIRT1 targets (Asher et al., 2008; Nakahata et al., 2008). The connection between the circadian system and epigenetic modifiers highlights the importance of examining mechanisms beyond classical E-Box control of transcription. Here, the direct regulation of an HDAC by the circadian system can itself impact the influence of drugs of abuse in the NAc, and alter the adaptive response to drugs through non-canonical clock targets, in addition to the direct effects of circadian genes on CCGs. The circadian regulation of the epigenome yields highly dynamic changes in transcriptional access to DNA (Bellet, Orozco-Solis, Sahar, Eckel-Mahan, & Sassone-Corsi, 2011), which likely includes genes associated with reward and motivation. Modifications to this circadian epigenome, therefore, will likely yield differential responses to drugs of abuse, depending on permissiveness of the chromatin-DNA complex. Future studies exploring the relationship between circadian genes and the epigenome in limbic structures will undoubtedly advance our understanding of the connection between the circadian system and reward.
For over a decade, large-scale gene expression studies have identified circadian regulated genes using microarrays in brain and peripheral tissues. More recently, newer high-throughput sequencing approaches have begun to construct more comprehensive perspectives on circadian regulation of tissue specific signaling pathways (Eckel-Mahan et al., 2012; Hughes, Grant, Paquin, Qian, & Nitabach, 2012; Koike et al., 2012; Rey et al., 2011). These tools are poised to reveal novel connections between gene regulation and addiction (Hitzemann et al., 2013; Renthal & Nestler, 2008), and have already been used to study genome-wide transcriptional changes associated with chronic cocaine (Maze et al., 2011) and morphine treatments (Sun et al., 2012). Such a comprehensive and integrative approach has yet to be employed in the mammalian brain within the context of circadian rhythms. For example, ChIP-seq will help identify targets of circadian transcription factors in reward related brain areas following acute and chronic drug treatments. There is very little known about how changes in circadian gene or protein expression alter downstream outputs that are involved in drug induced behaviors and neuroplasticity. Integrative approaches using transcriptome and proteome data collected from specific cell types, or brain regions across circadian time or in connection with circadian proteins following acute and chronic drug exposure will surely determine the extent to which localized clocks are involved in drug related neurocircuitry. To uncover the differential role of circadian genes in drug related behaviors, these approaches can be used with circadian mutant mice or cell and region specific viral-mediated gene knockdown or overexpression. Systematic behavioral phenotyping in response to drugs of abuse of other circadian mutants besides the Per and Clock mutants, such as Cry, Rev-erbα, Bmal1, and Fbxl3, will also aid in this endeavor. Indeed, it was recently reported that Afterhours mice carrying a mutation in the circadian gene Fbxl3 display a long circadian period (~27 h) and reduced anxiety- and depression-like behavior (Keers et al., 2012). Genome wide analyses revealed an associated between a variant in Fbxl3 and bipolar disorder (Keers et al., 2012).
Whole genome approaches such as deep-sequencing and proteomics are useful tools for identifying novel gene and protein regulatory networks that could be either modifiers of the molecular clock, or outputs of the clock. Newly available genetically and phenotypically heterogeneous mouse populations, such as the Collaborative Cross (Threadgill & Churchill, 2012) and Diversity Outbred (Svenson et al., 2012), provide a valuable resource for mapping novel genomic loci to behaviors related to mood and addiction (Logan et al., 2013b). High-density haplotype mapping and high-throughput genotyping are readily accessible tools for studying the genetics underlying inherent circadian and addiction phenotypes. Recently, in silico locus mapping, gene expression, and behavioral phenotype identified specific Per3 variants as a candidate gene for circadian, stress, and addiction (Wang et al., 2012). Furthermore, eQTL mapping, where gene expression levels are mapped across the genome similar to a behavioral phenotype, constructed a hierarchical gene network that is downstream of Per3 (Wang et al., 2012). Future studies using computational and biological strategies have the potential to build models for system level interactions that may be involved in circadian disruption and drug taking.
While it is often daunting to consider the numerous “ways” by which circadian rhythms regulate mood and reward neurocircuitry (Benedetti & Terman, 2013; McClung, 2013), the advent of new technologies accompanied by novel analytical approaches will help overcome past shortcomings. As technology continues to improve and becomes more accessible, researchers are more likely to adopt a systems approach that combines multiple technologies across species, such as neuroimaging and optogenetics, genetics, epigenetics, human post-mortem, self-report, and sleep and activity monitoring (Edgar & McClung, 2013; Forbes & Dahl, 2012; Hasler & Clark, 2013; Hasler et al., 2012b; Li et al., 2013; Shumay, Logan, Volkow, & Fowler, 2012b). Integrative translational systems approaches will be necessary to understand the role of the circadian system in substance use and abuse disorders and will be absolutely critical for determining whether treatments that target the circadian system are effective for treating addiction.
Figure 1. Proposed regulatory mechanisms of dopamine by circadian genes that contribute to the hyper-dopaminergic phenotype of clock mutant mice.
(Left) The synthesis of dopamine is controlled by the rate-limiting enzyme, tyrosine hydroxylase (TH), inhibited through the regulatory peptide, cholecystokinin (CCK), and degraded by the enzyme monoamine oxidase (MAOA). Collectively, these regulatory processes, which are all controlled by circadian clock genes, augment baseline dopamine signaling in VTA cell bodies and terminals in the NAc. (Right) In ClockΔ19 mice, DA synthesis is heightened through an increase in TH mRNA, and disinhibited by a decrease in cck mRNA. Additionally, DA degradation is attenuated in NAc synapses via the downregulation of mao-a transcription. Collectively, these disruptions contribute to the increased reward seeking behaviors in these mice.
Figure 2. Indirect connections between the Master Clock and Reward Circuitry.
The circadian control of reward and motivation may involve connections between the SCN reward circuitry. The SCN projects to three hypothalamic/epithalamic regions that directly project to the VTA, which in turn sends dopaminergic efferents to the NAc (red). These targets play significant roles in modulating reward and drug seeking behavior. The direct targets include the medial preoptic area (mPOA), dorsomedial hypothalamus (DMH) and lateral habenula (LHb), all of which express clock genes that may exert local circadian control of these processes. Direct SCN targets receive signals through monosynaptic afferents and/or diffusible signals (see text).
Table 1.
Phenotypes circadian gene mutant mice
| Genotype | Background | Circadian Phenotype (DD) | Drug Behaviors | References |
|---|---|---|---|---|
| ClockΔ19 | BALB/c | Longer period; arrhythmic |
Cocaine: hypersensitization, increased CPP, reduced latency to acquire self-administration, increased seeking and motivation Alcohol: elevated two-bottle choice drinking and preference, hypersensitive to sedative effects (LORR) |
McClung et al., 2005; Ozburn et al., 2012; Vitaterna et al., 1994; Lowrey and Takahashi, 2004 |
| Per1Brdm1 | 129SvEvBrd/C57BL/6-Tyrc-Brd | Shorter period; rhythmic |
Cocaine: normal sensitization and self-administration and reinstatement, abolished CPP Alcohol: normal sensitivity to sedative effects (LORR), normal seeking, reinstatement, and alcohol deprivation effect, elevated two-bottle choice drinking following social defeat stress |
Abarca et al., 2002; Dong et al., 2011; Halbout et al., 2011; Zghoul et al., 2007; Zheng et al., 1999, 2001 |
| Per2Brdm1 | 129SvEvBrd/C57BL/6-Tyrc-Brd | Shorter period; becomes arrhythmic |
Cocaine: hypersensitization, normal CPP, Alcohol: elevated two-bottle choice drinking and preference, increased seeking and motivation, hypersensitive to sedative effects (LORR) Morphine: reduced tolerance and withdrawal |
Perreau-Lenz et al., 2009, 2010; Spanagel et al., 2005b |
| Per1Brdm1 | C57BL/6J | Shorter period; rhythmic | Alcohol: elevated two-bottle choice drinking and preference, increased CPP | Gamsby et al., 2013 |
| Per2Brdm1 | C57BL/6J or C57BL/6-Tyrc-Brd | Shorter period; becomes arrhythmic | Alcohol: elevated two-bottle choice drinking and preference that is not increased by chronic restraint stress, increased CPP | Brager et al., 2011; Logan et al., 2012; Gamsby et al., 2013 |
Acknowledgments
The authors would like to acknowledge, NIDA, NIMH, NINDS, the McKnight Foundation, NARSAD and IMHRO for the funding of studies from our lab discussed in this review.
Footnotes
The authors declare no conflicts of interest.
References
- Aalto J, Kiianmaa K. Role of brain monoaminergic systems in the increased ethanol drinking caused by REM-sleep deprivation. Alcohol and Alcoholism Supplement. 1987;1:313–317. [PubMed] [Google Scholar]
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):9026–9030. doi: 10.1073/pnas.14203909999/13/9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Block GD. Circadian rhythms in isolated brain regions. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(1):350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuna-Goycolea C, Obrietan K, van den Pol AN. Cannabinoids excite circadian clock neurons. Journal of Neuroscience. 2010;30(30):10061–10066. doi: 10.1523/JNEUROSCI.5838-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adan A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction. 1994;89(4):455–462. doi: 10.1111/j.1360-0443.1994.tb00926.x. [DOI] [PubMed] [Google Scholar]
- Agabio R, Cortis G, Fadda F, Gessa GL, Lobina C, Reali R, Colombo G. Circadian drinking pattern of Sardinian alcohol-preferring rats. Alcohol and Alcoholism. 1996;31(4):385–388. doi: 10.1093/oxfordjournals.alcalc.a008166. [DOI] [PubMed] [Google Scholar]
- Agapito MA, Barreira JC, Logan RW, Sarkar DK. Evidence for Possible Period 2 Gene Mediation of the Effects of Alcohol Exposure During the Postnatal Period on Genes Associated with Maintaining Metabolic Signaling in the Mouse Hypothalamus. Alcoholism, Clinical and Experimental Research. 2012 doi: 10.1111/j.1530-0277.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agapito M, Mian N, Boyadjieva NI, Sarkar DK. Period 2 gene deletion abolishes beta-endorphin neuronal response to ethanol. Alcoholism, Clinical and Experimental Research. 2010;34(9):1613–1618. doi: 10.1111/j.1530-0277.2010.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad ST, Steinmetz SB, Bussey HM, Possidente B, Seggio JA. Larval ethanol exposure alters free-running circadian rhythm and per Locus transcription in adult D. melanogaster period mutants. Behavioural Brain Research. 2013;241:50–55. doi: 10.1016/j.bbr.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn YM, Chang J, Joo YH, Kim SC, Lee KY, Kim YS. Chronotype distribution in bipolar I disorder and schizophrenia in a Korean sample. Bipolar disorders. 2008;10(2):271–275. doi: 10.1111/j.1399-5618.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- Akhisaroglu M, Kurtuncu M, Manev H, Uz T. Diurnal rhythms in quinpirole-induced locomotor behaviors and striatal D2/D3 receptor levels in mice. Pharmacology, biochemistry, and behavior. 2005;80(3):371–377. doi: 10.1016/j.pbb.2004.11.016. doi: S0091-3057(04)00401-010.1016/j.pbb.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Al-Housseini AM, Sivanandam TM, Bradbury EL, Tannenberg RK, Dodd PR, Gu Q. Upregulation of beta-catenin levels in superior frontal cortex of chronic alcoholics. Alcoholism, Clinical and Experimental Research. 2008;32(6):1080–1090. doi: 10.1111/j.1530-0277.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- Alati R, Al Mamun A, Williams GM, O’Callaghan M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Archives of General Psychiatry. 2006;63(9):1009–1016. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- Allen GC, Farnell YZ, Maeng JU, West JR, Chen WJ, Earnest DJ. Long-term effects of neonatal alcohol exposure on photic reentrainment and phase-shifting responses of the activity rhythm in adult rats. Alcohol. 2005a;37(2):79–88. doi: 10.1016/j.alcohol.2005.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GC, West JR, Chen WJ, Earnest DJ. Neonatal alcohol exposure permanently disrupts the circadian properties and photic entrainment of the activity rhythm in adult rats. Alcoholism, Clinical and Experimental Research. 2005b;29(10):1845–1852. doi: 10.1097/01.alc.0000183014.12359.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammon S, Mayer P, Riechert U, Tischmeyer H, Hollt V. Microarray analysis of genes expressed in the frontal cortex of rats chronically treated with morphine and after naloxone precipitated withdrawal. Brain Research: Molecular Brain Research. 2003;112(1–2):113–125. doi: 10.1016/s0169-328x(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Ammon-Treiber S, Hollt V. Morphine-induced changes of gene expression in the brain. Addiction Biology. 2005;10(1):81–89. doi: 10.1080/13556210412331308994. [DOI] [PubMed] [Google Scholar]
- Ando H, Ushijima K, Kumazaki M, Eto T, Takamura T, Irie S, Fujimura A. Associations of metabolic parameters and ethanol consumption with messenger RNA expression of clock genes in healthy men. Chronobiology International. 2010;27(1):194–203. doi: 10.3109/07420520903398617. [DOI] [PubMed] [Google Scholar]
- Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285(5430):1066–1068. doi: 10.1126/science.285.5430.1066. 7747. [DOI] [PubMed] [Google Scholar]
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends in Neurosciences. 2005;28(3):145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, von Schantz M. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26(4):413–415. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- Arey R, McClung CA. An inhibitor of casein kinase 1 epsilon/delta partially normalizes the manic-like behaviors of the ClockDelta19 mouse. Behavioural Pharmacology. 2012;23(4):392–396. doi: 10.1097/FBP.0b013e32835651fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arey RN, Enwright JF, 3rd, Spencer SM, Falcon E, Ozburn AR, Ghose S, McClung CA. An important role for Cholecystokinin, a CLOCK target gene, in the development and treatment of manic-like behaviors. Molecular Psychiatry. 2013 doi: 10.1038/mp.2013.47mp201347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnedt JT, Conroy DA, Brower KJ. Treatment options for sleep disturbances during alcohol recovery. Journal of Addictive Diseases. 2007;26(4):41–54. doi: 10.1300/J069v26n04_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artioli P, Lorenzi C, Pirovano A, Serretti A, Benedetti F, Catalano M, Smeraldi E. How do genes exert their role? Period 3 gene variants and possible influences on mood disorder phenotypes. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2007;17(9):587–594. doi: 10.1016/j.euroneuro.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134(2):317–328. doi: 10.1016/j.cell.2008.06.050S0092-8674(08)00837-4. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nature Neuroscience. 2005;8(4):476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura L, Swanson T, Adamowicz W, Adams J, Cianfrogna J, Fisher K, Sprouse J. An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. Journal of Pharmacology and Experimental Therapeutics. 2007;322(2):730–738. doi: 10.1124/jpet.107.122846. jpet.107.12284610.1124/jpet.107.122846. [DOI] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30(2):525–536. doi: 10.1016/s0896-6273(01)00302-6. S0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. Journal of Studies on Alcohol. 1998;59(5):533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- Baird TJ, Gauvin D. Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacology, Biochemistry and Behavior. 2000;65(2):289–299. doi: 10.1016/s0091-3057(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Barclay NL, Eley TC, Mill J, Wong CC, Zavos HM, Archer SN, Gregory AM. Sleep quality and diurnal preference in a sample of young adults: associations with 5HTTLPR, PER3, and CLOCK 3111. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2011;156B(6):681–690. doi: 10.1002/ajmg.b.31210. [DOI] [PubMed] [Google Scholar]
- Bass CE, Jansen HT, Roberts DC. Free-running rhythms of cocaine self-administration in rats held under constant lighting conditions. Chronobiology International. 2010;27(3):535–548. doi: 10.3109/07420521003664221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke M, Smith VC Committee on Substance, Abuse, Committee on, Fetus, & Newborn. Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics. 2013;131(3):e1009–1024. doi: 10.1542/peds.2012-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky MA, Yarom Y, Pickard GE. Heterogeneous expression of gamma-aminobutyric acid and gamma-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. Journal of Comparative Neurology. 2008;506(4):708–732. doi: 10.1002/cne.21553. [DOI] [PubMed] [Google Scholar]
- Bellet MM, Orozco-Solis R, Sahar S, Eckel-Mahan K, Sassone-Corsi P. The time of metabolism: NAD+, SIRT1, and the circadian clock. Cold Spring Harbor Symposia on Quantitative Biology. 2011;76:31–38. doi: 10.1101/sqb.2011.76.010520. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Colombo C, Pirovano A, Marino E, Smeraldi E. A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neuroscience Letters. 2008;445(2):184–187. doi: 10.1016/j.neulet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Fulgosi MC, Lorenzi C, Serretti A, Barbini B, Smeraldi E. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144B(5):631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E, Smeraldi E. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2003;123B(1):23–26. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Terman M. Much ado about...a moody clock. Biological Psychiatry. 2013;74(4):236–237. doi: 10.1016/j.biopsych.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Bergeson SE, Kyle Warren R, Crabbe JC, Metten P, Gene Erwin V, Belknap JK. Chromosomal loci influencing chronic alcohol withdrawal severity. Mammalian Genome. 2003;14(7):454–463. doi: 10.1007/s00335-002-2254-4. [DOI] [PubMed] [Google Scholar]
- Bertolucci C, Cavallari N, Colognesi I, Aguzzi J, Chen Z, Caruso P, Pinotti M. Evidence for an overlapping role of CLOCK and NPAS2 transcription factors in liver circadian oscillators. Molecular and Cellular Biology. 2008;28(9):3070–3075. doi: 10.1128/MCB.01931-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biello SM, Dafters RI. MDMA and fenfluramine alter the response of the circadian clock to a serotonin agonist in vitro. Brain Research. 2001;920(1–2):202–209. doi: 10.1016/s0006-8993(01)03070-0. [DOI] [PubMed] [Google Scholar]
- Bildt C, Michelsen H. Gender differences in the effects from working conditions on mental health: a 4-year follow-up. International Archives of Occupational and Environmental Health. 2002;75(4):252–258. doi: 10.1007/s00420-001-0299-8. [DOI] [PubMed] [Google Scholar]
- Blomeyer D, Buchmann AF, Lascorz J, Zimmermann US, Esser G, Desrivieres S, Laucht M. Association of PER2 genotype and stressful life events with alcohol drinking in young adults. PloS One. 2013;8(3):e59136. doi: 10.1371/journal.pone.0059136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, Waterhouse JM. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Archives of General Psychiatry. 1997;54(2):145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- Bolelli G, Lafisca S, Flamigni C, Lodi S, Franceschetti F, Filicori M, Mosca R. Heroin addiction: relationship between the plasma levels of testosterone, dihydrotestosterone, androstenedione, LH, FSH, and the plasma concentration of heroin. Toxicology. 1979;15(1):19–29. doi: 10.1016/0300-483x(79)90016-7. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Smith BR, Amit Z. A descriptive analysis of the structure and temporal pattern of voluntary ethanol intake within an acquisition paradigm. Journal of Studies on Alcohol. 1997;58(4):382–391. doi: 10.15288/jsa.1997.58.382. [DOI] [PubMed] [Google Scholar]
- Brager AJ, Prosser RA, Glass JD. Circadian and acamprosate modulation of elevated ethanol drinking in mPer2 clock gene mutant mice. Chronobiology International. 2011a;28(8):664–672. doi: 10.3109/07420528.2011.601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcoholism, Clinical and Experimental Research. 2010;34(7):1266–1273. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Acute ethanol disrupts photic and serotonergic circadian clock phase-resetting in the mouse. Alcoholism, Clinical and Experimental Research. 2011b;35(8):1467–1474. doi: 10.1111/j.1530-0277.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager A, Prosser RA, Glass JD. Acamprosate-responsive brain sites for suppression of ethanol intake and preference. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2011c;301(4):R1032–1043. doi: 10.1152/ajpregu.00179.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaccio M, Maywood ES, Chesham JE, Loudon AS, Hastings MH. A Gq-Ca2+ axis controls circuit-level encoding of circadian time in the suprachiasmatic nucleus. Neuron. 2013;78(4):714–728. doi: 10.1016/j.neuron.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broms U, Kaprio J, Hublin C, Partinen M, Madden PA, Koskenvuo M. Evening types are more often current smokers and nicotine-dependent-a study of Finnish adult twins. Addiction. 2011;106(1):170–177. doi: 10.1111/j.1360-0443.2010.03112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks E, Canal MM. Development of circadian rhythms: role of postnatal light environment. Neuroscience and Biobehavioral Reviews. 2013;37(4):551–560. doi: 10.1016/j.neubiorev.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Medicine Reviews. 2003;7(6):523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. American Journal of Psychiatry. 2001;158(3):399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, McCammon RJ, Wojnar M, Ilgen MA, Wojnar J, Valenstein M. Prescription sleeping pills, insomnia, and suicidality in the National Comorbidity Survey Replication. The Journal of clinical psychiatry. 2011;72(4):515–521. doi: 10.4088/JCP.09m05484gry. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Graham ME, Distler MG, Munoz MB, Li D, Vezina P, Palmer AA. A role for casein kinase 1 epsilon in the locomotor stimulant response to methamphetamine. Psychopharmacology. 2009;203(4):703–711. doi: 10.1007/s00213-008-1417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Parker CC, Zhou L, Olker C, Chandrasekaran RY, Wager TT, Palmer AA. Csnk1e is a genetic regulator of sensitivity to psychostimulants and opioids. Neuropsychopharmacology. 2012;37(4):1026–1035. doi: 10.1038/npp.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck KJ, Finn DA. Genetic factors in addiction: QTL mapping and candidate gene studies implicate GABAergic genes in alcohol and barbiturate withdrawal in mice. Addiction. 2001;96(1):139–149. doi: 10.1080/09652140020017012. [DOI] [PubMed] [Google Scholar]
- Busino L, Bassermann F, Maiolica A, Lee C, Nolan PM, Godinho SI, Pagano M. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316(5826):900–904. doi: 10.1126/science.1141194. [DOI] [PubMed] [Google Scholar]
- Caille S, Espejo EF, Koob GF, Stinus L. Dorsal and median raphe serotonergic system lesion does not alter the opiate withdrawal syndrome. Pharmacology, Biochemistry and Behavior. 2002;72(4):979–986. doi: 10.1016/s0091-3057(02)00810-9. [DOI] [PubMed] [Google Scholar]
- Carpen JD, Archer SN, Skene DJ, Smits M, von Schantz M. A single-nucleotide polymorphism in the 5′-untranslated region of the hPER2 gene is associated with diurnal preference. Journal of Sleep Research. 2005;14(3):293–297. doi: 10.1111/j.1365-2869.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- Carpen JD, von Schantz M, Smits M, Skene DJ, Archer SN. A silent polymorphism in the PER1 gene associates with extreme diurnal preference in humans. Journal of Human Genetics. 2006;51(12):1122–1125. doi: 10.1007/s10038-006-0060-y. [DOI] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. Journal of Neurochemistry. 2004;88(6):1547–1554. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Prenatal ethanol exposure alters the expression of period genes governing the circadian function of beta-endorphin neurons in the hypothalamus. Journal of Neurochemistry. 2006;97(4):1026–1033. doi: 10.1111/j.1471-4159.2006.03839.x. [DOI] [PubMed] [Google Scholar]
- Chen ML, Olson HC, Picciano JF, Starr JR, Owens J. Sleep problems in children with fetal alcohol spectrum disorders. Journal of Clinical Sleep Medicine. 2012;8(4):421–429. doi: 10.5664/jcsm.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417(6887):405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Leslie FM, Zhou QY. Expression of prokineticins and their receptors in the adult mouse brain. The Journal of comparative neurology. 2006;498(6):796–809. doi: 10.1002/cne.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon S, Park N, Cho S, Kim K. Glucocorticoid-mediated Period2 induction delays the phase of circadian rhythm. Nucleic Acids Research. 2013;41(12):6161–6174. doi: 10.1093/nar/gkt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski CE, Hernandez LM, Quesada A, Pozas JA, Picabea L, Prada FA. Effects of ethanol on the inner layers of chick retina during development. Alcohol. 1997;14(4):313–317. doi: 10.1016/s0741-8329(97)87948-7. [DOI] [PubMed] [Google Scholar]
- Clark JW, Fixaris MC, Belanger GV, Rosenwasser AM. Repeated light-dark phase shifts modulate voluntary ethanol intake in male and female high alcohol-drinking (HAD1) rats. Alcoholism, Clinical and Experimental Research. 2007;31(10):1699–1706. doi: 10.1111/j.1530-0277.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- Comasco E, Nordquist N, Gokturk C, Aslund C, Hallman J, Oreland L, Nilsson KW. The clock gene PER2 and sleep problems: association with alcohol consumption among Swedish adolescents. Upsala Journal of Medical Sciences. 2010;115(1):41–48. doi: 10.3109/03009731003597127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy DA, Hairston IS, Arnedt JT, Hoffmann RF, Armitage R, Brower KJ. Dim light melatonin onset in alcohol-dependent men and women compared with healthy controls. Chronobiology International. 2012;29(1):35–42. doi: 10.3109/07420528.2011.636852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, McClung CA. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockDelta19 mouse model of mania. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36(7):1478–1488. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu CH, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biological Psychiatry. 2009;65(8):662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ. Selective breeding for alcohol withdrawal severity. Behavior Genetics. 1993;23(2):171–177. doi: 10.1007/BF01067422. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK. Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends in Neurosciences. 1999;22(4):173–179. doi: 10.1016/s0166-2236(99)01393-4. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Spence SE, Brown LL, Metten P. Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol. 2011;45(5):427–440. doi: 10.1016/j.alcohol.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum RM, Ford DE, Storr CL, Chan YF. Association of sleep disturbance with chronicity and remission of alcohol dependence: data from a population-based prospective study. Alcoholism, Clinical and Experimental Research. 2004;28(10):1533–1540. doi: 10.1097/01.alc.0000141915.56236.40. [DOI] [PubMed] [Google Scholar]
- Crum RM, Storr CL, Chan YF, Ford DE. Sleep disturbance and risk for alcohol-related problems. American Journal of Psychiatry. 2004;161(7):1197–1203. doi: 10.1176/appi.ajp.161.7.1197. [DOI] [PubMed] [Google Scholar]
- Cutler DJ, Mundey MK, Mason R. Electrophysiological effects of opioid receptor activation on Syrian hamster suprachiasmatic nucleus neurones in vitro. Brain Research Bulletin. 1999;50(2):119–125. doi: 10.1016/s0361-9230(99)00069-6. [DOI] [PubMed] [Google Scholar]
- Danel T, Jeanson R, Touitou Y. Temporal pattern in consumption of the first drink of the day in alcohol-dependent persons. Chronobiology International. 2003;20(6):1093–1102. doi: 10.1081/cbi-120025533. [DOI] [PubMed] [Google Scholar]
- De Witte P, Pinto E, Ansseau M, Verbanck P. Alcohol and withdrawal: from animal research to clinical issues. Neuroscience and Biobehavioral Reviews. 2003;27(3):189–197. doi: 10.1016/s0149-7634(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50(3):465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nature Neuroscience. 2007;10(5):543–545. doi: 10.1038/nn1884. nn1884 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125(3):497–508. doi: 10.1016/j.cell.2006.03.033. S0092-8674(06)00444-210.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Dole VP, Ho A, Gentry RT. Toward an analogue of alcoholism in mice: criteria for recognition of pharmacologically motivated drinking. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(10):3469–3471. doi: 10.1073/pnas.82.10.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Bilbao A, Laucht M, Henriksson R, Yakovleva T, Ridinger M, Schumann G. Effects of the circadian rhythm gene period 1 (per1) on psychosocial stress-induced alcohol drinking. The American journal of psychiatry. 2011;168(10):1090–1098. doi: 10.1176/appi.ajp.2011.10111579. [DOI] [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol and Alcoholism. 2001;36(3):249–255. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcoholism, Clinical and Experimental Research. 1998;22(8):1796–1802. [PubMed] [Google Scholar]
- Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Current biology: CB. 2002;12(7):551–557. doi: 10.1016/s0960-9822(02)00765-0. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. Journal of Investigative Medicine. 1999;47(3):141–150. [PMC free article] [PubMed] [Google Scholar]
- Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340(6131):449–453. doi: 10.1126/science.1234152. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-Corsi P. Coordination of the transcriptome and metabolome by the circadian clock. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(14):5541–5546. doi: 10.1073/pnas.1118726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar N, McClung CA. Major depressive disorder: a loss of circadian synchrony? Bioessays. 2013;35(11):940–944. doi: 10.1002/bies.201300086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide EJ, Kang H, Crapo S, Gallego M, Virshup DM. Casein kinase I in the mammalian circadian clock. Methods in Enzymology. 2005;393:408–418. doi: 10.1016/S0076-6879(05)93019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray JP, Machida KK, Noton E, Constance CM, Dallmann R, Di Napoli MN, Weaver DR. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Molecular and Cellular Biology. 2009;29(14):3853–3866. doi: 10.1128/MCB.00338-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakier N, Wild LG. Associations among sleep problems, learning difficulties and substance use in adolescence. Journal of Adolescence. 2011;34(4):717–726. doi: 10.1016/j.adolescence.2010.09.010S0140-1971(10)00148-X. [DOI] [PubMed] [Google Scholar]
- Falcon E, Ozburn A, Mukherjee S, Roybal K, McClung CA. Differential Regulation of the Period Genes in Striatal Regions following Cocaine Exposure. PloS One. 2013;8(6):e66438. doi: 10.1371/journal.pone.0066438. PONE-D-13-07654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnell YZ, Allen GC, Nahm SS, Neuendorff N, West JR, Chen WJ, Earnest DJ. Neonatal alcohol exposure differentially alters clock gene oscillations within the suprachiasmatic nucleus, cerebellum, and liver of adult rats. Alcoholism, Clinical and Experimental Research. 2008;32(3):544–552. doi: 10.1111/j.1530-0277.2007.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnell YZ, West JR, Chen WJ, Allen GC, Earnest DJ. Developmental alcohol exposure alters light-induced phase shifts of the circadian activity rhythm in rats. Alcoholism, Clinical and Experimental Research. 2004;28(7):1020–1027. doi: 10.1097/01.alc.0000130807.21020.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SA, Kennaway DJ. Prenatal exposure to SKF-38393 alters the response to light of adult rats. Neuroreport. 2000;11(7):1539–1541. [PubMed] [Google Scholar]
- Ferguson SA, Kennaway DJ, Moyer RW. Nicotine phase shifts the 6-sulphatoxymelatonin rhythm and induces c-Fos in the SCN of rats. Brain Research Bulletin. 1999;48(5):527–538. doi: 10.1016/s0361-9230(99)00033-7. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Rowe SA, Krupa M, Kennaway DJ. Prenatal exposure to the dopamine agonist SKF-38393 disrupts the timing of the initial response of the suprachiasmatic nucleus to light. Brain Research. 2000;858(2):284–289. doi: 10.1016/s0006-8993(99)02392-6. [DOI] [PubMed] [Google Scholar]
- Filiano AN, Millender-Swain T, Johnson R, Jr, Young ME, Gamble KL, Bailey SM. Chronic Ethanol Consumption Disrupts the Core Molecular Clock and Diurnal Rhythms of Metabolic Genes in the Liver without Affecting the Suprachiasmatic Nucleus. PloS One. 2013;8(8):e71684. doi: 10.1371/journal.pone.0071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk JE, Montgomery C. Sleep impairment in ecstasy/polydrug and cannabis-only users. American Journal on Addictions. 2009;18(5):430–437. doi: 10.3109/10550490903077762. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Research Review: altered reward function in adolescent depression: what, when and how? Journal of Child Psychology and Psychiatry and Allied Disciplines. 2012;53(1):3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE, Almeida JR, Ferrell RE, Nimgaonkar VL, Mansour H, Phillips ML. PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biological Psychiatry. 2012;71(5):451–457. doi: 10.1016/j.biopsych.2011.10.012. S0006-3223(11)00970-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossey MD, Otto MW, Yates WR, Wisniewski SR, Gyulai L, Allen MH, Step BD Investigators. Validity of the distinction between primary and secondary substance use disorder in patients with bipolar disorder: data from the first 1000 STEP-BD participants. American Journal on Addictions. 2006;15(2):138–143. doi: 10.1080/10550490500528423. [DOI] [PubMed] [Google Scholar]
- Foster RG, Peirson SN, Wulff K, Winnebeck E, Vetter C, Roenneberg T. Sleep and circadian rhythm disruption in social jetlag and mental illness. Progress in Molecular Biology and Translational Science. 2013;119:325–346. doi: 10.1016/B978-0-12-396971-2.00011-7B978-0-12-396971-2.00011-7. [DOI] [PubMed] [Google Scholar]
- Freeman GM, Jr, Krock RM, Aton SJ, Thaben P, Herzog ED. GABA networks destabilize genetic oscillations in the circadian pacemaker. Neuron. 2013;78(5):799–806. doi: 10.1016/j.neuron.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamsby JJ, Templeton EL, Bonvini LA, Wang W, Loros JJ, Dunlap JC, Gulick D. The circadian Per1 and Per2 genes influence alcohol intake, reinforcement, and blood alcohol levels. Behavioural Brain Research. 2013;249:15–21. doi: 10.1016/j.bbr.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV, Baird TJ, Vanecek SA, Briscoe RJ, Vallett M, Holloway FA. Effects of time-of-day and photoperiod phase shifts on voluntary ethanol consumption in rats. Alcoholism, Clinical and Experimental Research. 1997;21(5):817–825. [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. Journal of Sleep Research. 2002;11(3):191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. Experimental ‘jet lag’ inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PloS One. 2010;5(12):e15267. doi: 10.1371/journal.pone.0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho SI, Maywood ES, Shaw L, Tucci V, Barnard AR, Busino L, Nolan PM. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316(5826):897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- Gruber R, Sadeh A, Raviv A. Instability of sleep patterns in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(4):495–501. doi: 10.1097/00004583-200004000-00019. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbout B, Perreau-Lenz S, Dixon CI, Stephens DN, Spanagel R. Per1Brdm1 mice self-administer cocaine and reinstate cocaine-seeking behaviour following extinction. Behavioural Pharmacology. 2011;22(1):76–80. doi: 10.1097/FBP.0b013e328341e9ca. [DOI] [PubMed] [Google Scholar]
- Hampp G, Albrecht U. The circadian clock and mood-related behavior. Communicative & Integrative Biology. 2008;1(1):1–3. doi: 10.4161/cib.1.1.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Albrecht U. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Current biology: CB. 2008;18(9):678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Hansen TV. Cholecystokinin gene transcription: promoter elements, transcription factors and signaling pathways. Peptides. 2001;22(8):1201–1211. doi: 10.1016/s0196-9781(01)00443-0. [DOI] [PubMed] [Google Scholar]
- Harma M, Tenkanen L, Sjoblom T, Alikoski T, Heinsalmi P. Combined effects of shift work and life-style on the prevalence of insomnia, sleep deprivation and daytime sleepiness. Scandinavian Journal of Work, Environment and Health. 1998;24(4):300–307. doi: 10.5271/sjweh.324. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Allen JJ, Sbarra DA, Bootzin RR, Bernert RA. Morningness-eveningness and depression: preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatry Research. 2010;176(2–3):166–173. doi: 10.1016/j.psychres.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Clark DB. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcoholism, Clinical and Experimental Research. 2013;37(4):558–565. doi: 10.1111/acer.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Dahl RE, Holm SM, Jakubcak JL, Ryan ND, Silk JS, Forbes EE. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biological Psychology. 2012a;91(3):334–341. doi: 10.1016/j.biopsycho.2012.08.008. S0301-0511(12)00173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Germain A, Nofzinger EA, Kupfer DJ, Krafty RT, Rothenberger SD, Buysse DJ. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. Journal of Sleep Research. 2012b;21(5):515–526. doi: 10.1111/j.1365-2869.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatonen T, Forsblom S, Kieseppa T, Lonnqvist J, Partonen T. Circadian phenotype in patients with the co-morbid alcohol use and bipolar disorders. Alcohol and Alcoholism. 2008;43(5):564–568. doi: 10.1093/alcalc/agn057. [DOI] [PubMed] [Google Scholar]
- Hitzemann R, Bottomly D, Darakjian P, Walter N, Iancu O, Searles R, McWeeney S. Genes, behavior and next-generation RNA sequencing. Genes Brain Behav. 2013;12(1):1–12. doi: 10.1111/gbb.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter JR, Grahame NJ, Mayeda AR. Circadian activity rhythms in high-alcohol-preferring and low-alcohol-preferring mice. Alcohol. 2003;30(1):81–85. doi: 10.1016/s0741-8329(03)00095-8. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K. Evidence for coexistence of dopamine and CCK in meso-limbic neurones. Nature. 1980;285(5765):476–478. doi: 10.1038/285476a0. [DOI] [PubMed] [Google Scholar]
- Holter SM, Spanagel R. Effects of opiate antagonist treatment on the alcohol deprivation effect in long-term ethanol-experienced rats. Psychopharmacology. 1999;145(4):360–369. doi: 10.1007/s002130051069. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S. The SCN-independent clocks, methamphetamine and food restriction. European Journal of Neuroscience. 2009;30(9):1707–1717. doi: 10.1111/j.1460-9568.2009.06976.x. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S, Hiroshige T. Disorganization of the rat activity rhythm by chronic treatment with methamphetamine. Physiology and Behavior. 1986;38(5):687–695. doi: 10.1016/0031-9384(86)90265-9. [DOI] [PubMed] [Google Scholar]
- Hood S, Cassidy P, Mathewson S, Stewart J, Amir S. Daily morphine injection and withdrawal disrupt 24-h wheel running and PERIOD2 expression patterns in the rat limbic forebrain. Neuroscience. 2011;186:65–75. doi: 10.1016/j.neuroscience.2011.04.045. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4(2):97–110. [PubMed] [Google Scholar]
- Huang MC, Ho CW, Chen CH, Liu SC, Chen CC, Leu SJ. Reduced expression of circadian clock genes in male alcoholic patients. Alcoholism, Clinical and Experimental Research. 2010;34(11):1899–1904. doi: 10.1111/j.1530-0277.2010.01278.x. ACER1278. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Grant GR, Paquin C, Qian J, Nitabach MN. Deep sequencing the circadian and diurnal transcriptome of Drosophila brain. Genome Research. 2012;22(7):1266–1281. doi: 10.1101/gr.128876.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iancu OD, Oberbeck D, Darakjian P, Metten P, McWeeney S, Crabbe JC, Hitzemann R. Selection for drinking in the dark alters brain gene coexpression networks. Alcoholism, Clinical and Experimental Research. 2013;37(8):1295–1303. doi: 10.1111/acer.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Nikaido T, Akiyama M, Moriya T, Shibata S. Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. European Journal of Neuroscience. 2002;16(5):921, 929. doi: 10.1046/j.1460-9568.2002.02140.x. 2140. [DOI] [PubMed] [Google Scholar]
- Imbesi M, Yildiz S, Dirim Arslan A, Sharma R, Manev H, Uz T. Dopamine receptor-mediated regulation of neuronal “clock” gene expression. Neuroscience. 2009;158(2):537–544. doi: 10.1016/j.neuroscience.2008.10.044. S0306-4522(08)01586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Valladares EM, Motivala S, Thayer JF, Ehlers CL. Association between nocturnal vagal tone and sleep depth, sleep quality, and fatigue in alcohol dependence. Psychosomatic Medicine. 2006;68(1):159–166. doi: 10.1097/01.psy.0000195743.60952.00. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61(5):786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96(1):57–68. doi: 10.1016/s0092-8674(00)80959-9. S0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- Johansson C, Willeit M, Smedh C, Ekholm J, Paunio T, Kieseppa T, Partonen T. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28(4):734–739. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- Jones EM, Knutson D, Haines D. Common problems in patients recovering from chemical dependency. American Family Physician. 2003;68(10):1971–1978. [PubMed] [Google Scholar]
- Katzenberg D, Young T, Finn L, Lin L, King DP, Takahashi JS, Mignot E. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21(6):569–576. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- Keers R, Pedroso I, Breen G, Aitchison KJ, Nolan PM, Cichon S, Fernandes C. Reduced anxiety and depression-like behaviours in the circadian period mutant mouse afterhours. PloS One. 2012;7(6):e38263. doi: 10.1371/journal.pone.0038263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicology and Teratology. 2000;22(2):143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway DJ. Clock genes at the heart of depression. J Psychopharmacol. 2010;24(2 Suppl):5–14. doi: 10.1177/1359786810372980. [DOI] [PubMed] [Google Scholar]
- Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. Journal of Clinical Investigation. 2010;120(7):2600–2609. doi: 10.1172/JCI41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Choi HJ, Colwell CS. Brain-derived neurotrophic factor regulation of N-methyl-D-aspartate receptor-mediated synaptic currents in suprachiasmatic nucleus neurons. Journal of Neuroscience Research. 2006;84(7):1512–1520. doi: 10.1002/jnr.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell. 1997;89(4):641–653. doi: 10.1016/s0092-8674(00)80245-7. S0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosobud A, Crabbe JC. Ethanol withdrawal in mice bred to be genetically prone or resistant to ethanol withdrawal seizures. Journal of Pharmacology and Experimental Therapeutics. 1986;238(1):170–177. [PubMed] [Google Scholar]
- Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lonnqvist J, Partonen T. Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol and Alcoholism. 2010;45(4):303–311. doi: 10.1093/alcalc/agq035. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. 1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar U. Colocalization of somatostatin receptor subtypes (SSTR1-5) with somatostatin, NADPH-diaphorase (NADPH-d), and tyrosine hydroxylase in the rat hypothalamus. Journal of Comparative Neurology. 2007;504(2):185–205. doi: 10.1002/cne.21444. [DOI] [PubMed] [Google Scholar]
- Lanca AJ, De Cabo C, Arifuzzaman AI, Vaccarino FJ. Cholecystokinergic innervation of nucleus accumbens subregions. Peptides. 1998;19(5):859–868. doi: 10.1016/s0196-9781(98)00032-1. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs. 2001;15(5):413–425. doi: 10.2165/00023210-200115050-00006. [DOI] [PubMed] [Google Scholar]
- Lavery DJ, Lopez-Molina L, Margueron R, Fleury-Olela F, Conquet F, Schibler U, Bonfils C. Circadian expression of the steroid 15 alpha-hydroxylase (Cyp2a4) and coumarin 7-hydroxylase (Cyp2a5) genes in mouse liver is regulated by the PAR leucine zipper transcription factor DBP. Molecular and Cellular Biology. 1999;19(10):6488–6499. doi: 10.1128/mcb.19.10.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandovski R, Dantas G, Fernandes LC, Caumo W, Torres I, Roenneberg T, Allebrandt KV. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiology International. 2011;28(9):771–778. doi: 10.3109/07420528.2011.602445. [DOI] [PubMed] [Google Scholar]
- Li JD, Hu WP, Zhou QY. Disruption of the circadian output molecule prokineticin 2 results in anxiolytic and antidepressant-like effects in mice. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34(2):367–373. doi: 10.1038/npp.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, Bunney WE. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Wang ZR, Li J, Peng ZG, Zhou W, Zhou M, Lu L. Inhibition of Period1 gene attenuates the morphine-induced ERK-CREB activation in frontal cortex, hippocampus, and striatum in mice. American Journal of Drug and Alcohol Abuse. 2008;34(6):673–682. doi: 10.1080/00952990802308197. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Allen G, Earnest D. Role of brain-derived neurotrophic factor in the circadian regulation of the suprachiasmatic pacemaker by light. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20(8):2978–2987. doi: 10.1523/JNEUROSCI.20-08-02978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nature Neuroscience. 2013;16(6):698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Gillette MU. Cholinergic regulation of the suprachiasmatic nucleus circadian rhythm via a muscarinic mechanism at night. Journal of Neuroscience. 1996;16(2):744–751. doi: 10.1523/JNEUROSCI.16-02-00744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Wan C, Zhou W, Peng T, Wang Z, Halberg F. The role of mPer1 in morphine dependence in mice. Neuroscience. 2005;130(2):383–388. doi: 10.1016/j.neuroscience.2004.09.012. S0306-4522(04)00837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan BA, Brown MS, Hayes MJ. Neonatal abstinence syndrome: treatment and pediatric outcomes. Clinical Obstetrics and Gynecology. 2013a;56(1):186–192. doi: 10.1097/GRF.0b013e31827feea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, McCulley WD, 3rd, Seggio JA, Rosenwasser AM. Effects of withdrawal from chronic intermittent ethanol vapor on the level and circadian periodicity of running-wheel activity in C57BL/6J and C3H/HeJ mice. Alcoholism, Clinical and Experimental Research. 2012;36(3):467–476. doi: 10.1111/j.1530-0277.2011.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Robledo RF, Recla JM, Philip VM, Bubier JA, Jay JJ, Chesler EJ. High-precision genetic mapping of behavioral traits in the diversity outbred mouse population. Genes Brain Behav. 2013b;12(4):424–437. doi: 10.1111/gbb.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Communication networks in the brain: neurons, receptors, neurotransmitters, and alcohol. Alcohol Res Health. 2008;31(3):196–214. [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Genetics of the mammalian circadian system: Photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annual Review of Genetics. 2000;34:533–562. doi: 10.1146/annurev.genet.34.1.533. [DOI] [PubMed] [Google Scholar]
- Luo AH, Aston-Jones G. Circuit projection from suprachiasmatic nucleus to ventral tegmental area: a novel circadian output pathway. The European journal of neuroscience. 2009;29(4):748–760. doi: 10.1111/j.1460-9568.2008.06606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Georges FE, Aston-Jones GS. Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. The European journal of neuroscience. 2008;27(2):408–422. doi: 10.1111/j.1460-9568.2007.05985.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Girgenti MJ, Breslin FJ, Newton SS, Taylor JR. Gene profiling the response to repeated cocaine self-administration in dorsal striatum: a focus on circadian genes. Brain Research. 2008;1213:166–177. doi: 10.1016/j.brainres.2008.02.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2004;29(5):943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Andrade JP, Lieberman AR, Sousa N, Almeida OF, Paula-Barbosa MM. Chronic alcohol consumption and withdrawal do not induce cell death in the suprachiasmatic nucleus, but lead to irreversible depression of peptide immunoreactivity and mRNA levels. Journal of Neuroscience. 1997;17(4):1302–1319. doi: 10.1523/JNEUROSCI.17-04-01302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira MD, Paula-Barbosa MM. Effects of alcohol on the synthesis and expression of hypothalamic peptides. Brain Research Bulletin. 1999;48(1):3–22. doi: 10.1016/s0361-9230(98)00131-2. [DOI] [PubMed] [Google Scholar]
- Malison RT, Kranzler HR, Yang BZ, Gelernter J. Human clock, PER1 and PER2 polymorphisms: lack of association with cocaine dependence susceptibility and cocaine-induced paranoia. Psychiatric Genetics. 2006;16(6):245–249. doi: 10.1097/01.ypg.0000242198.59020.ca. [DOI] [PubMed] [Google Scholar]
- Manev H, Uz T. Clock genes as a link between addiction and obesity. European Journal of Human Genetics. 2006;14(1):5. doi: 10.1038/sj.ejhg.5201524. 5201524. [DOI] [PubMed] [Google Scholar]
- Mansour HA, Monk TH, Nimgaonkar VL. Circadian genes and bipolar disorder. Annals of Medicine. 2005;37(3):196–205. doi: 10.1080/07853890510007377. [DOI] [PubMed] [Google Scholar]
- Mansour HA, Wood J, Logue T, Chowdari KV, Dayal M, Kupfer DJ, Nimgaonkar VL. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 2006;5(2):150–157. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- Masumoto KH, Nagano M, Takashima N, Hayasaka N, Hiyama H, Matsumoto S, Shigeyoshi Y. Distinct localization of prokineticin 2 and prokineticin receptor 2 mRNAs in the rat suprachiasmatic nucleus. The European journal of neuroscience. 2006;23(11):2959–2970. doi: 10.1111/j.1460-9568.2006.04834.x. [DOI] [PubMed] [Google Scholar]
- Maze I, Feng J, Wilkinson MB, Sun H, Shen L, Nestler EJ. Cocaine dynamically regulates heterochromatin and repetitive element unsilencing in nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3035–3040. doi: 10.1073/pnas.1015483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ, Fernandes M, Kranzler HR, Covault JM, Welsh DK. Circadian Clock Period Inversely Correlates with Illness Severity in Cells from Patients with Alcohol Use Disorders. Alcoholism, Clinical and Experimental Research. 2013 doi: 10.1111/acer.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ, Nievergelt CM, Kelsoe JR, Welsh DK. A survey of genomic studies supports association of circadian clock genes with bipolar disorder spectrum illnesses and lithium response. PloS One. 2012;7(2):e32091. doi: 10.1371/journal.pone.0032091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacology and Therapeutics. 2007a;114(2):222–232. doi: 10.1016/j.pharmthera.2007.02.003. S0163-7258(07)00036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Circadian rhythms, the mesolimbic dopaminergic circuit, and drug addiction. ScientificWorldJournal. 2007b;7:194–202. doi: 10.1100/tsw.2007.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Clock genes and bipolar disorder: implications for therapy. Pharmacogenomics. 2007c;8(9):1097–1100. doi: 10.2217/14622416.8.9.1097. [DOI] [PubMed] [Google Scholar]
- McClung CA. How Might Circadian Rhythms Control Mood? Let Me Count the Ways. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.02.019. S0006-3223(13)00187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9377–9381. doi: 10.1073/pnas.0503584102. 0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulley WD, 3rd, Ascheid S, Crabbe JC, Rosenwasser AM. Selective breeding for ethanol-related traits alters circadian phenotype. Alcohol. 2013;47(3):187–194. doi: 10.1016/j.alcohol.2013.01.001. S0741-8329(13)00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy B, Zakaria A, Glass JD, Prosser RA. Ethanol modulates mammalian circadian clock phase resetting through extrasynaptic GABA receptor activation. Neuroscience. 2009;164(2):842–848. doi: 10.1016/j.neuroscience.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, Brown TM, Loudon AS. Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58(1):78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Baer ML, Menaker M. The methamphetamine-sensitive circadian oscillator does not employ canonical clock genes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3519–3524. doi: 10.1073/pnas.0813366106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Takahashi JS. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends in Neurosciences. 2011 doi: 10.1016/j.tins.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteno CD, Jacobson JL, Carter RC, Dodge NC, Jacobson SW. Infant emotional withdrawal: a precursor of affective and cognitive disturbance in fetal alcohol spectrum disorders. Alcoholism, Clinical and Experimental Research. 2014;38(2):479–488. doi: 10.1111/acer.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neuroscience Letters. 1993;150(1):112–116. doi: 10.1016/0304-3940(93)90120-a. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug and Alcohol Dependence. 2006;82(3):238–249. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, McClung CA. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biological Psychiatry. 2010;68(6):503–511. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, Trinder J. Nature’s clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9(5):705–716. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324(5927):654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanson JL, Hiscock M. Attention deficits in children exposed to alcohol prenatally. Alcoholism, Clinical and Experimental Research. 1990;14(5):656–661. doi: 10.1111/j.1530-0277.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. S0006-3223(05)01243-6. [DOI] [PubMed] [Google Scholar]
- Nikaido T, Akiyama M, Moriya T, Shibata S. Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Molecular Pharmacology. 2001;59(4):894–900. doi: 10.1124/mol.59.4.894. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Kasari C. Prenatal alcohol exposure and depressive features in children. Alcoholism, Clinical and Experimental Research. 2000;24(7):1084–1092. [PubMed] [Google Scholar]
- O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Koob GF. Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. Journal of Pharmacology and Experimental Therapeutics. 2007;320(1):180–193. doi: 10.1124/jpet.106.105270. jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- O’Neil D, Mendez-Figueroa H, Mistretta TA, Su C, Lane RH, Aagaard KM. Dysregulation of Npas2 leads to altered metabolic pathways in a murine knockout model. Molecular Genetics and Metabolism. 2013;110(3):378–387. doi: 10.1016/j.ymgme.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyefeso A, Sedgwick P, Ghodse H. Subjective sleep-wake parameters in treatment-seeking opiate addicts. Drug and Alcohol Dependence. 1997;48(1):9–16. doi: 10.1016/s0376-8716(97)00097-5. [DOI] [PubMed] [Google Scholar]
- Ozburn AR, Falcon E, Mukherjee S, Gillman A, Arey R, Spencer S, McClung CA. The Role of Clock in Ethanol-Related Behaviors. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Larson EB, Self DW, McClung CA. Cocaine self-administration behaviors in ClockDelta19 mice. Psychopharmacology. 2012;223(2):169–177. doi: 10.1007/s00213-012-2704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417(6886):329–335. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- Parson SH, Sojitra NM. Loss of myelinated axons is specific to the central nervous system in a mouse model of the fetal alcohol syndrome. Journal of Anatomy. 1995;187(Pt 3):739–748. [PMC free article] [PubMed] [Google Scholar]
- Partonen T, Treutlein J, Alpman A, Frank J, Johansson C, Depner M, Schumann G. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Annals of Medicine. 2007;39(3):229–238. doi: 10.1080/07853890701278795. [DOI] [PubMed] [Google Scholar]
- Pasley JN, Powell EW, Halberg F. Strain differences in circadian drinking behaviors of ethanol and water in rats. Progress in Clinical and Biological Research. 1987;227B:467–471. [PubMed] [Google Scholar]
- Pei J, Denys K, Hughes J, Rasmussen C. Mental health issues in fetal alcohol spectrum disorder. J Ment Health. 2011;20(5):438–448. doi: 10.3109/09638237.2011.577113. [DOI] [PubMed] [Google Scholar]
- Pendergast JS, Niswender KD, Yamazaki S. Tissue-specific function of period3 in circadian rhythmicity. PloS One. 2012;7(1):e30254. doi: 10.1371/journal.pone.0030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau-Lenz S, Sanchis-Segura C, Leonardi-Essmann F, Schneider M, Spanagel R. Development of morphine-induced tolerance and withdrawal: involvement of the clock gene mPer2. European Neuropsychopharmacology. 2010;20(7):509–517. doi: 10.1016/j.euroneuro.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Perreau-Lenz S, Vengeliene V, Noori HR, Merlo-Pich EV, Corsi MA, Corti C, Spanagel R. Inhibition of the casein-kinase-1-epsilon/delta prevents relapse-like alcohol drinking. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37(9):2121–2131. doi: 10.1038/npp.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau-Lenz S, Zghoul T, de Fonseca FR, Spanagel R, Bilbao A. Circadian regulation of central ethanol sensitivity by the mPer2 gene. Addiction Biology. 2009;14(3):253–259. doi: 10.1111/j.1369-1600.2009.00165.x. [DOI] [PubMed] [Google Scholar]
- Pezuk P, Mohawk JA, Wang LA, Menaker M. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology. 2012;153(10):4775–4783. doi: 10.1210/en.2012-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietila K, Laakso I, Ahtee L. Chronic oral nicotine administration affects the circadian rhythm of dopamine and 5-hydroxytryptamine metabolism in the striata of mice. Naunyn-Schmiedebergs Archives of Pharmacology. 1995;353(1):110–115. doi: 10.1007/BF00168923. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Glass JD. The mammalian circadian clock exhibits acute tolerance to ethanol. Alcoholism, Clinical and Experimental Research. 2009;33(12):2088–2093. doi: 10.1111/j.1530-0277.2009.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy TE, Gertz J, Crawford GE, Garabedian MJ, Myers RM. The hypersensitive glucocorticoid response specifically regulates period 1 and expression of circadian genes. Molecular and Cellular Biology. 2012;32(18):3756–3767. doi: 10.1128/MCB.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293(5529):506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, Nestler EJ. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62(3):335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends in Molecular Medicine. 2008;14(8):341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biology. 2011;9(2):e1000595. doi: 10.1371/journal.pbio.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology and Behavior. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nature Genetics. 2006;38(3):369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug and Alcohol Dependence. 2002;67(3):291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain research Brain research reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nature reviews Neuroscience. 2011;12(11):623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. Journal of Biological Rhythms. 2003;18(1):80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM. Circadian clock genes: non-circadian roles in sleep, addiction, and psychiatric disorders? Neuroscience and Biobehavioral Reviews. 2010;34(8):1249–1255. doi: 10.1016/j.neubiorev.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Clark JW, Fixaris MC, Belanger GV, Foster JA. Effects of repeated light-dark phase shifts on voluntary ethanol and water intake in male and female Fischer and Lewis rats. Alcohol. 2010;44(3):229–237. doi: 10.1016/j.alcohol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW, Reed JD, Cotter SJ, Seggio JA. Circadian activity rhythms in selectively bred ethanol-preferring and nonpreferring rats. Alcohol. 2005a;36(2):69–81. doi: 10.1016/j.alcohol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC. Chronobiology of alcohol: studies in C57BL/6J and DBA/2J inbred mice. Physiology and Behavior. 2013;110–111:140–147. doi: 10.1016/j.physbeh.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Logan RW, Fecteau ME. Chronic ethanol intake alters circadian period-responses to brief light pulses in rats. Chronobiology International. 2005b;22(2):227–236. doi: 10.1081/cbi-200053496. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, McClung CA. Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(15):6406–6411. doi: 10.1073/pnas.0609625104. 0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. American journal of physiology Regulatory, integrative and comparative physiology. 2009;296(2):R411–418. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature Reviews: Neuroscience. 2013;14(9):609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata-Haga H, Dominguez HD, Sei H, Fukui Y, Riley EP, Thomas JD. Alterations in circadian rhythm phase shifting ability in rats following ethanol exposure during the third trimester brain growth spurt. Alcoholism, Clinical and Experimental Research. 2006;30(5):899–907. doi: 10.1111/j.1530-0277.2006.00105.x. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Jancic D, Jimenez-Minchan M, Barco A. Inhibition of cAMP responsive element binding protein in striatal neurons enhances approach and avoidance responses toward morphine--and morphine withdrawal-related cues. Frontiers in Behavioral Neuroscience. 2009;3:30. doi: 10.3389/neuro.08.030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Zhou FC. Prenatal alcohol exposure causes long-term serotonin neuron deficit in mice. Alcoholism, Clinical and Experimental Research. 2004;28(6):941–948. doi: 10.1097/01.alc.0000128228.08472.39. [DOI] [PubMed] [Google Scholar]
- Sarkar DK. Circadian genes, the stress axis, and alcoholism. Alcohol Res. 2012;34(3):362–366. doi: 10.35946/arcr.v34.3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade R, Vick K, Ott T, Sohr R, Pfister C, Bellach J, Lemmer B. Circadian rhythms of dopamine and cholecystokinin in nucleus accumbens and striatum of rats--influence on dopaminergic stimulation. Chronobiology International. 1995;12(2):87–99. doi: 10.3109/07420529509064504. [DOI] [PubMed] [Google Scholar]
- Segall LA, Amir S. Glucocorticoid regulation of clock gene expression in the mammalian limbic forebrain. Journal of molecular neuroscience: MN. 2010;42(2):168–175. doi: 10.1007/s12031-010-9341-1. [DOI] [PubMed] [Google Scholar]
- Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. Journal of Biological Rhythms. 2009;24(4):304–312. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacology, Biochemistry and Behavior. 2007;87(3):297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Possidente B, Ahmad ST. Larval ethanol exposure alters adult circadian free-running locomotor activity rhythm in Drosophila melanogaster. Chronobiology International. 2012;29(1):75–81. doi: 10.3109/07420528.2011.635236. [DOI] [PubMed] [Google Scholar]
- Selvi Y, Aydin A, Atli A, Boysan M, Selvi F, Besiroglu L. Chronotype differences in suicidal behavior and impulsivity among suicide attempters. Chronobiology International. 2011;28(2):170–175. doi: 10.3109/07420528.2010.535938. [DOI] [PubMed] [Google Scholar]
- Shang EH, Zhdanova IV. The circadian system is a target and modulator of prenatal cocaine effects. PloS One. 2007;2(7):e587. doi: 10.1371/journal.pone.0000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman LP, Weaver DR. Distinct pharmacological mechanisms leading to c-fos gene expression in the fetal suprachiasmatic nucleus. Journal of Biological Rhythms. 2001;16(6):531–540. doi: 10.1177/074873001129002222. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Zylka MJ, Reppert SM, Weaver DR. Expression of basic helix-loop-helix/PAS genes in the mouse suprachiasmatic nucleus. Neuroscience. 1999;89(2):387–397. doi: 10.1016/s0306-4522(98)00325-x. S0306-4522(98)00325-X. [DOI] [PubMed] [Google Scholar]
- Sher L. Developmental alcohol exposure, circadian rhythms, and mood disorders. Canadian Journal of Psychiatry Revue Canadienne de Psychiatrie. 2003;48(6):428. doi: 10.1177/070674370304800619. [DOI] [PubMed] [Google Scholar]
- Shi G, Xing L, Liu Z, Qu Z, Wu X, Dong Z, Xu Y. Dual roles of FBXL3 in the mammalian circadian feedback loops are important for period determination and robustness of the clock. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(12):4750–4755. doi: 10.1073/pnas.1302560110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hida A, McGuinness OP, Wasserman DH, Yamazaki S, Johnson CH. Circadian clock gene Bmal1 is not essential; functional replacement with its paralog, Bmal2. Current Biology. 2010;20(4):316–321. doi: 10.1016/j.cub.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibley HL, Malcolm RJ, Veatch LM. Adolescents with insomnia and substance abuse: consequences and comorbidities. Journal of Psychiatric Practice. 2008;14(3):146–153. doi: 10.1097/01.pra.0000320113.30811.46. [DOI] [PubMed] [Google Scholar]
- Shieh KR, Chu YS, Pan JT. Circadian change of dopaminergic neuron activity: effects of constant light and melatonin. Neuroreport. 1997;8(9–10):2283–2287. doi: 10.1097/00001756-199707070-00037. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Low-Zeddies SS, King DP, Steeves TD, Whiteley A, Kushla J, Takahashi JS. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Research. 2001;11(6):959–980. doi: 10.1101/gr.171601. [DOI] [PubMed] [Google Scholar]
- Shumay E, Fowler JS, Wang GJ, Logan J, Alia-Klein N, Goldstein RZ, Volkow ND. Repeat variation in the human PER2 gene as a new genetic marker associated with cocaine addiction and brain dopamine D2 receptor availability. Translational psychiatry. 2012a;2:e86. doi: 10.1038/tp.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumay E, Logan J, Volkow ND, Fowler JS. Evidence that the methylation state of the monoamine oxidase A (MAOA) gene predicts brain activity of MAO A enzyme in healthy men. Epigenetics: official journal of the DNA Methylation Society. 2012b;7(10):1151–1160. doi: 10.4161/epi.21976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Quarterly Journal of Studies on Alcohol. 1968;29(4):863–867. [PubMed] [Google Scholar]
- Sjoholm LK, Kovanen L, Saarikoski ST, Schalling M, Lavebratt C, Partonen T. CLOCK is suggested to associate with comorbid alcohol use and depressive disorders. Journal of circadian rhythms. 2010;8:1. doi: 10.1186/1740-3391-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Contribution of the suprachiasmatic nucleus to day:night variation in cocaine-seeking behavior. Physiology and Behavior. 2007a;91(5):523–530. doi: 10.1016/j.physbeh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: dependence on the suprachiasmatic nucleus. Brain Research. 2007b;1129(1):34–42. doi: 10.1016/j.brainres.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Sliwowska JH, Song HJ, Bodnar T, Weinberg J. Prenatal Alcohol Exposure Results in Long-Term Serotonin Neuron Deficits in Female Rats: Modulatory Role of Ovarian Steroids. Alcoholism, Clinical and Experimental Research. 2013 doi: 10.1111/acer.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Stark G, Sergeeva A, Jansen HT. Photoperiodic suppression of drug reinstatement. Neuroscience. 2011;176:284–295. doi: 10.1016/j.neuroscience.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria V, Martinez-Amoros E, Escaramis G, Valero J, Perez-Egea R, Garcia C, Urretavizcaya M. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35(6):1279–1289. doi: 10.1038/npp.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Kiefer F. Drugs for relapse prevention of alcoholism: ten years of progress. Trends in Pharmacological Sciences. 2008;29(3):109–115. doi: 10.1016/j.tips.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nature Medicine. 2005a;11(1):35–42. doi: 10.1038/nm1163. nm1163. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body’s biological clock. Alcoholism, Clinical and Experimental Research. 2005b;29(8):1550–1557. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- Spencer S, Falcon E, Kumar J, Krishnan V, Mukherjee S, Birnbaum SG, McClung CA. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. The European journal of neuroscience. 2012a doi: 10.1111/ejn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Falcon E, Kumar J, Krishnan V, Mukherjee S, Birnbaum SG, McClung CA. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. The European journal of neuroscience. 2013;37(2):242–250. doi: 10.1111/ejn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Torres-Altoro MI, Falcon E, Arey R, Marvin M, Goldberg M, McClung CA. A mutation in CLOCK leads to altered dopamine receptor function. Journal of Neurochemistry. 2012b;123(1):124–134. doi: 10.1111/j.1471-4159.2012.07857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Kleiman R, Tate B, Swanson TA, Pickard GE. Chronic treatment with a selective inhibitor of casein kinase I delta/epsilon yields cumulative phase delays in circadian rhythms. Psychopharmacology. 2010;210(4):569–576. doi: 10.1007/s00213-010-1860-5. [DOI] [PubMed] [Google Scholar]
- Sprow GM, Thiele TE. The neurobiology of binge-like ethanol drinking: evidence from rodent models. Physiology and Behavior. 2012;106(3):325–331. doi: 10.1016/j.physbeh.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon P. Mental health problems who use alcohol to self-medicate. Ment Health Today. 2013:16. [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Optogenetic strategies to dissect the neural circuits that underlie reward and addiction. Cold Spring Harbor Perspectives in Medicine. 2012;2(11) doi: 10.1101/cshperspect.a011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stautz K, Cooper A. Impulsivity-related personality traits and adolescent alcohol use: a meta-analytic review. Clinical Psychology Review. 2013;33(4):574–592. doi: 10.1016/j.cpr.2013.03.003. S0272-7358(13)00043-3. [DOI] [PubMed] [Google Scholar]
- Stinus L, Robert C, Karasinski P, Limoge A. Continuous quantitative monitoring of spontaneous opiate withdrawal: locomotor activity and sleep disorders. Pharmacology, Biochemistry and Behavior. 1998;59(1):83–89. doi: 10.1016/s0091-3057(97)00319-5. [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417(6884):78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental and Behavioral Pediatrics. 2004;25(4):228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Strother WN, Vorhees CV, Lehman MN. Long-term effects of early cocaine exposure on the light responsiveness of the adult circadian timing system. Neurotoxicology and Teratology. 1998;20(5):555–564. doi: 10.1016/s0892-0362(98)00014-2. [DOI] [PubMed] [Google Scholar]
- Sun H, Maze I, Dietz DM, Scobie KN, Kennedy PJ, Damez-Werno D, Nestler EJ. Morphine epigenomically regulates behavior through alterations in histone H3 lysine 9 dimethylation in the nucleus accumbens. Journal of Neuroscience. 2012;32(48):17454–17464. doi: 10.1523/JNEUROSCI.1357-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson KL, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ, Churchill GA. High-resolution genetic mapping using the Mouse Diversity outbred population. Genetics. 2012;190(2):437–447. doi: 10.1534/genetics.111.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataroglu O, Aksoy A, Yilmaz A, Canbeyli R. Effect of lesioning the suprachiasmatic nuclei on behavioral despair in rats. Brain Research. 2004;1001(1–2):118–124. doi: 10.1016/j.brainres.2003.11.063. S0006899303041398. [DOI] [PubMed] [Google Scholar]
- Tenkova T, Young C, Dikranian K, Labruyere J, Olney JW. Ethanol-induced apoptosis in the developing visual system during synaptogenesis. Investigative Ophthalmology and Visual Science. 2003;44(7):2809–2817. doi: 10.1167/iovs.02-0982. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Churchill GA. Ten years of the Collaborative Cross. Genetics. 2012;190(2):291–294. doi: 10.1534/genetics.111.138032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Cocaine self-administration “binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology. 2000;148(3):289–298. doi: 10.1007/s002130050053. [DOI] [PubMed] [Google Scholar]
- Touitou Y. Adolescent sleep misalignment: a chronic jet lag and a matter of public health. Journal of Physiology, Paris. 2013 doi: 10.1016/j.jphysparis.2013.03.008. S0928-4257(13)00010-7. [DOI] [PubMed] [Google Scholar]
- Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, Beaulieu C. Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. Journal of Neuroscience. 2013;33(24):10098–10109. doi: 10.1523/JNEUROSCI.5004-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Cichon S, Ridinger M, Wodarz N, Soyka M, Zill P, Rietschel M. Genome-wide association study of alcohol dependence. Archives of General Psychiatry. 2009;66(7):773–784. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkoff AM, Storr CL. Work schedule characteristics and substance use in nurses. American Journal of Industrial Medicine. 1998;34(3):266–271. doi: 10.1002/(sici)1097-0274(199809)34:3<266::aid-ajim9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Troese M, Fukumizu M, Sallinen BJ, Gilles AA, Wellman JD, Paul JA, Hayes MJ. Sleep fragmentation and evidence for sleep debt in alcohol-exposed infants. Early Human Development. 2008;84(9):577–585. doi: 10.1016/j.earlhumdev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Trujillo JL, Do DT, Grahame NJ, Roberts AJ, Gorman MR. Ethanol consumption in mice: relationships with circadian period and entrainment. Alcohol. 2011;45(2):147–159. doi: 10.1016/j.alcohol.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo JL, Roberts AJ, Gorman MR. Circadian timing of ethanol exposure exerts enduring effects on subsequent ad libitum consumption in C57 mice. Alcoholism, Clinical and Experimental Research. 2009;33(7):1286–1293. doi: 10.1111/j.1530-0277.2009.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimine S, Yasui-Furukori N, Kaneda A, Kaneko S. The CLOCK C3111T polymorphism is associated with reward dependence in healthy Japanese subjects. Neuropsychobiology. 2013;67(1):1–5. doi: 10.1159/000342383. [DOI] [PubMed] [Google Scholar]
- Tuma J, Strubbe JH, Mocaer E, Koolhaas JM. Anxiolytic-like action of the antidepressant agomelatine (S 20098) after a social defeat requires the integrity of the SCN. European Neuropsychopharmacology. 2005;15(5):545–555. doi: 10.1016/j.euroneuro.2005.02.004. S0924-977X(05)00040-410.1016/j.euroneuro.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nature Genetics. 2005;37(2):187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- Utge SJ, Soronen P, Loukola A, Kronholm E, Ollila HM, Pirkola S, Paunio T. Systematic analysis of circadian genes in a population-based sample reveals association of TIMELESS with depression and sleep disturbance. PloS One. 2010;5(2):e9259. doi: 10.1371/journal.pone.0009259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2003;28(12):2117–2123. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- Uz T, Arslan AD, Kurtuncu M, Imbesi M, Akhisaroglu M, Dwivedi Y, Manev H. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Research: Molecular Brain Research. 2005;136(1–2):45–53. doi: 10.1016/j.molbrainres.2005.01.002. S0169-328X(05)00011-2. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48(6):822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Noori HR, Spanagel R. The use of a novel drinkometer system for assessing pharmacological treatment effects on ethanol consumption in rats. Alcoholism, Clinical and Experimental Research. 2013;37(Suppl 1):E322–328. doi: 10.1111/j.1530-0277.2012.01874.x. [DOI] [PubMed] [Google Scholar]
- Verma P, Hellemans KG, Choi FY, Yu W, Weinberg J. Circadian phase and sex effects on depressive/anxiety-like behaviors and HPA axis responses to acute stress. Physiology and Behavior. 2010;99(3):276–285. doi: 10.1016/j.physbeh.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi PP, Coiro V, Volpi R, Passeri M. Diurnal variations in plasma ACTH, cortisol and beta-endorphin levels in cocaine addicts. Hormone Research. 1992;37(6):221–224. doi: 10.1159/000182316. [DOI] [PubMed] [Google Scholar]
- Vink JM, Groot AS, Kerkhof GA, Boomsma DI. Genetic analysis of morningness and eveningness. Chronobiology International. 2001;18(5):809–822. doi: 10.1081/cbi-100107516. [DOI] [PubMed] [Google Scholar]
- Viola AU, Archer SN, James LM, Groeger JA, Lo JC, Skene DJ, Dijk DJ. PER3 polymorphism predicts sleep structure and waking performance. Current Biology. 2007;17(7):613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ko CH, Koletar MM, Ralph MR, Yeomans J. Casein kinase I epsilon gene transfer into the suprachiasmatic nucleus via electroporation lengthens circadian periods of tau mutant hamsters. The European journal of neuroscience. 2007;25(11):3359–3366. doi: 10.1111/j.1460-9568.2007.05545.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Mozhui K, Li Z, Mulligan MK, Ingels JF, Zhou X, Lu L. A promoter polymorphism in the Per3 gene is associated with alcohol and stress response. Translational psychiatry. 2012;2:e73. doi: 10.1038/tp.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasielewski JA, Holloway FA. Alcohol’s interactions with circadian rhythms. A focus on body temperature. Alcohol Res Health. 2001;25(2):94–100. [PMC free article] [PubMed] [Google Scholar]
- Weaver DR, Rivkees SA, Reppert SM. D1-dopamine receptors activate c-fos expression in the fetal suprachiasmatic nuclei. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(19):9201–9204. doi: 10.1073/pnas.89.19.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb IC, Baltazar RM, Lehman MN, Coolen LM. Bidirectional interactions between the circadian and reward systems: is restricted food access a unique zeitgeber? European Journal of Neuroscience. 2009;30(9):1739–1748. doi: 10.1111/j.1460-9568.2009.06966.x. [DOI] [PubMed] [Google Scholar]
- Weber M, Lauterburg T, Tobler I, Burgunder JM. Circadian patterns of neurotransmitter related gene expression in motor regions of the rat brain. Neuroscience Letters. 2004;358(1):17–20. doi: 10.1016/j.neulet.2003.12.053. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A. Circadian rhythms in mammalian neurotransmitter receptors. Progress in Neurobiology. 1987;29(3):219–259. doi: 10.1016/0301-0082(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiology International. 2006;23(1–2):497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Wongchitrat P, Mukda S, Phansuwan-Pujito P, Govitrapong P. Effect of amphetamine on the clock gene expression in rat striatum. Neuroscience Letters. 2013;542:126–130. doi: 10.1016/j.neulet.2013.03.009. S0304-3940(13)00214-0. [DOI] [PubMed] [Google Scholar]
- Yan L, Karatsoreos I, Lesauter J, Welsh DK, Kay S, Foley D, Silver R. Exploring spatiotemporal organization of SCN circuits. Cold Spring Harbor Symposia on Quantitative Biology. 2007;72:527–541. doi: 10.1101/sqb.2007.72.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JJ, Wang YT, Cheng PC, Kuo YJ, Huang RC. Cholinergic modulation of neuronal excitability in the rat suprachiasmatic nucleus. Journal of Neurophysiology. 2010;103(3):1397–1409. doi: 10.1152/jn.00877.2009. [DOI] [PubMed] [Google Scholar]
- Zghoul T, Abarca C, Sanchis-Segura C, Albrecht U, Schumann G, Spanagel R. Ethanol self-administration and reinstatement of ethanol-seeking behavior in Per1(Brdm1) mutant mice. Psychopharmacology. 2007;190(1):13–19. doi: 10.1007/s00213-006-0592-z. [DOI] [PubMed] [Google Scholar]
- Zhabenko N, Wojnar M, Brower KJ. Prevalence and correlates of insomnia in a polish sample of alcohol-dependent patients. Alcoholism, Clinical and Experimental Research. 2012;36(9):1600–1607. doi: 10.1111/j.1530-0277.2012.01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liao G, Liu C, Sun L, Liu Y, Wang Y, Wang Z. The association of CLOCK gene T3111C polymorphism and hPER3 gene 54-nucleotide repeat polymorphism with Chinese Han people schizophrenics. Molecular Biology Reports. 2011;38(1):349–354. doi: 10.1007/s11033-010-0114-2. [DOI] [PubMed] [Google Scholar]