Abstract
The NLRP3 inflammasome is a cytosolic complex that activates Caspase-1, leading to maturation of IL-1β and IL-18 and induction of a pro-inflammatory cell death in sentinel cells of the innate immune system. Diverse stimuli have been shown to activate the NLRP3 inflammasome during infection and metabolic diseases, thus implicating the pathway in triggering both adaptive and maladaptive inflammation in a variety of clinically important settings. Here I discuss the emerging model that signals associated with mitochondrial destabilization may critically activate the NLRP3 inflammasome. Together with studies indicating an important role for Ca2+ signaling, these findings suggest that many stimuli engage Ca2+ signaling as an intermediate step to trigger mitochondrial destabilization, thus generating the mitochondria-associated ligands that activate the NLRP3 inflammasome.
Keywords: Mitochondrial destabilization, mitochondrial damage, calcium signaling, NLRP3 inflammasome
Activating the NLRP3 inflammasome
The NLRP3 inflammasome was first identified about a decade ago[1]. Informed by recent characterization of other inflammasome complexes (including NLRP1), NLRP3 was shown to assemble a cytoplasmic complex in which interaction with the adaptor protein Asc enables recruitment and activation of Caspase-1. Soon afterwards, the generation of NLRP3−/− mice provided genetic confirmation for a role of this complex in Caspase-1 activation and consequent IL-1β and IL-18 maturation. These studies also uncovered several stimuli that can trigger inducible activation of this complex, including extracellular ATP, nigericin, RNA, and uric acid crystals[2–4]. Since then, many additional stimuli have been shown to activate the NLRP3 inflammasome, thus implicating the pathway in a variety of clinically important settings, including host defense where its activity is beneficial as well as metabolic diseases where it may play a pathophysiological role[5–9](Table 1). In addition, mutations in NLRP3 lead to aberrantly increased NLRP3 inflammasome activity and inflammation-mediated impairment of multiple tissues in Cryopyrin-Associated Periodic Syndrome (CAPS), a group of rare, inherited autoinflammatory diseases[10].
Table 1.
Diverse stimuli activate the NLRP3 inflammasome.
| Activating stimulus | Distal/upstream steps in NLRP3 inflammasome activation | Mechanisms of Ca2+ mobilization | Mitochondrial process engaged | Ref |
|---|---|---|---|---|
| Extracellular ATP | P2X7R | P2X7R forms Ca2+-permeant pore, PLC activation, ER Ca2+ release | mROS production, Ca2+ uptake, mtDNA release, cardiolipin externalization | 16, 17, 18, 23, 42, 44, 47 |
| Nigericin | K+ ionophore (K+/H+ exchange) | ER Ca2+ release | Mitochondrial hyperpolarization followed by depolarization; mROS production; mtDNA release | 16, 41, 47 |
| Crystals (e.g. uric acid crystals, silica, cholesterol crystals, alum) | Phagocytosis followed by phagolysosomal destabilization | TRPM2 opening and perhaps Ca2+ release via lysosomal damage, ER Ca2+ release | mROS production and cardiolipin externalization | 16, 18, 44 |
| Lysosomotropic peptides | Osmotic swelling and rupture of lysosomal compartment | ER Ca2+ release implicated, perhaps via TRPM2 opening and/or Ca2+ release via lysosomal damage | Mitochondrial membrane permeabilization as a result of increased lysosomal permeability? | 16 |
| High extracellular Ca2+ levels | CaSR, GPRC6A | GPCR-mediated activation of PLC and Ca2+ signaling, ER Ca2+ release | ? | 17, 22 |
| RNA | DHX33-mediated RNA recognition | ? | ? | 53 |
| EMCV | EMCV viroporin 2B activity | EMCV viroporin 2B-mediated Ca2+ flux | Mitofusin-2 has been implicated (intact membrane potential?) | 21 |
| Sendai virus | RIG-I? | ? | Mitochondrial- resident protein MAVS has been implicated | 52 |
| Cell swelling | Ionic fluxes | TRPM7 and TRV2 opening | ? | 24 |
| Complement membrane attack complex | Plasma membrane pore formation | Ca2+ influx across damaged membranes, ER Ca2+ release | Mitochondrial Ca2+ uptake via MCU, loss of membrane potential | 19 |
| Cyclic dinucleotides | ? | Not known, but inflammasome activation sensitive to Ca2+ signaling inhibitors | ? | 20 |
| Pore forming toxins (e.g. pneumolysin) | Pore and/or membrane damage | Ca2+ influx across pore and/or damaged membranes | ? | |
| Fatty acid | AMPK and autophagy inactivation | ? | mROS production | 51 |
Intriguingly, many NLRP3 inflammasome activators are chemically and structurally distinct from each other and/or known to engage the cell in disparate ways (Table 1). For example, extracellular ATP signals through the cell surface receptor P2X7R; nigericin is a bacterial toxin with potassium ionophore activity; and crystal formation is necessary for several stimuli to activate the NLRP3 inflammasome (e.g. uric acid, silica). Therefore, it is generally believed that these stimuli engage the NLRP3 inflammasome indirectly, by triggering some signal(s) associated with cellular stress that can be recognized by the complex (see below)[6, 7, 11]. Activation of the NLRP3 inflammasome also requires a priming step, typically provided by LPS pretreatment, which is thought to be needed for transcriptional upregulation of the NLRP3 subunit. Common assays for inflammasome activation include analysis of Caspase-1 processing, which indicates Caspase-1 activation in most settings; Caspase-1 activity as reflected in processing and secretion of IL-1β and IL-18; and complex formation (including formation of puncta that are visible by immunofluorescence)[6, 7, 11].
Understanding how the NLRP3 inflammasome pathway is activated by diverse stimuli has been an outstanding question in the field and the focus of considerable attention in the last several years. At least a few models have been advanced[6, 7, 11]. One model proposes a key role for ROS production, but later studies suggest that this process is likely important for the priming step rather than regulation of complex assembly. Other models implicate phagolysosomal damage (in the case of crystals) and K+ efflux. However, how these processes lead to cytosolic assembly of the NLRP3 inflammasome has remained unclear[6, 7, 11]. Here I discuss recent studies that implicate mitochondria-associated signal(s), generated by mitochondrial destabilization or dysfunction, in many settings of NLRP3 inflammasome activation. In particular, there is compelling evidence to indicate that mtDNA released into the cytosol and externalized cardiolipin can interact with and activate the NLRP3 inflammasome, potentially serving as ligands of the complex. I also discuss a potential role for Ca2+ signaling in triggering such mitochondrial destabilization, in response to diverse upstream stimuli.
Ca2+ signaling and NLRP3 inflammasome activation
Mobilizing Ca2+
Early studies linked Ca2+ mobilization to NLRP3 inflammasome activation largely based on the ability of BAPTA-AM to inhibit IL-1β secretion[12–14]. These studies did not address the critical Ca2+-regulated step, and Ca2+ signaling was speculated to control IL-1β secretion but not NLRP3 inflammasome activation[12]. Ca2+ mobilization was first implicated in the upstream steps of this pathway in the context of Mycobacterium abscessus infection[15]. Subsequently, two studies revealed a general requirement for Ca2+ signaling in the proximal steps upstream of NLRP3 inflammasome activation[16, 17]. Multiple NLRP3 inflammasome activators mobilize Ca2+, including ATP, nigericin, and high levels of extracellular Ca2+. This is also true of crystals and lysosomotropic peptides, indicating that lysosomal destabilization or rupture is sufficient to trigger Ca2+ mobilization. Importantly, disrupting Ca2+ signaling inhibits complex assembly, Caspase-1 processing, and IL-1β processing during NLRP3 inflammasome activation, but does not have similar effects on the AIM2 inflammasome pathway[16, 17]. Several follow-up studies identified additional stimuli that activate the NLRP3 inflammasome in a manner dependent on Ca2+ signaling [18–22].
These studies raised the question of how Ca2+ is mobilized during NLRP3 inflammasome activation. As in many other settings of Ca2+ signaling, Ca2+ appears to be mobilized from the extracellular space as well as intracellular stores. At least for some stimuli, blocking either attenuates inflammasome activation, suggesting contributions of both processes[16, 18]. The ER is a major reservoir of intracellular Ca2+ and indeed ER Ca2+ release is critical, since pharmacological inhibition or shRNA knockdown of IP3R, a ER-resident Ca2+ release channel, blocks NLRP3 inflammasome activation by many stimuli, including extracellular ATP, high extracellular Ca2+, crystals, lysosomotropic peptides, and nigericin[16, 17]. The IP3R is gated by IP3, a product of PLC-mediated PIP2 cleavage, and consistently pharmacological inhibition of PLC proteins also reduces inflammasome activation[16, 17]. Other Ca2+ release channels, such as ryanodine receptors (RyRs), may also liberate ER Ca2+ during inflammasome activation[19]. Therefore, many NLRP3 inflammasome activators may critically promote ER Ca2+ release through IP3R and other Ca2+ channels.
The mechanisms of Ca2+ mobilization upstream of ER Ca2+ release are less clear. Lee et al proposed a critical role for the Calcium Sensing Receptor (CaSR), a G-protein coupled receptor (GPCR) that is known to be activated by high levels of extracellular Ca2+. In support, CaSR knockdown reduces Ca2+ mobilization as well as inflammasome activation by many stimuli, including extracellular ATP, nigericin, crystals, and high extracellular Ca2+ levels[17]. However, a subsequent study implicated CaSR and the related family member GPRC6A in extracellular Ca2+- but not ATP-mediated inflammasome activation[22], in line with a dominant role for the P2X7R in extracellular Ca2+ influx during ATP stimulation[18, 23]. In general, how the CaSR may be engaged by stimuli such as ATP, crystals, and nigericin would be an important issue to resolve.
In other studies, the Ca2+-permeant plasma membrane channel TRPM2 was important for Ca2+ mobilization and NLRP3 inflammasome activation by crystals and charged liposomes[18], while additional members of the same family (TRPM7 and TRPV2) were linked to inflammasome activation during cell swelling[24]. Finally, store-operated Ca2+ entry (SOCE), which is often coupled to ER Ca2+ release, was also implicated with pharmacological inhibitors[16], warranting the use of genetic models to confirm a role for this process in NLRP3 inflammasome activation. Thus it appears likely that Ca2+ is mobilized by multiple pathways distal to NLRP3 inflammasome activation (Table 1).
Taken together, these findings support a model whereby many stimuli may engage Ca2+ signaling as a common denominator in activation of the NLRP3 inflammasome pathway (Fig. 1 and Table 1). Distal to the NLRP3 inflammasome complex, the mechanisms of Ca2+ mobilization are likely to be varied, reflecting the diversity of activating stimuli; examples include Ca2+-permeant channels (e.g. P2X7R and TRPM2 in the case of ATP and crystals respectively), G-protein coupled receptors (e.g. CaSR and GPRC6A in the case of extracellular Ca2+) and membrane and/or lysosomal damage leading to increased Ca2+ permeability (e.g. crystals). In contrast, Ca2+ liberation from the ER may be common to NLRP3 inflammasome activation in many settings, given the ability of IP3R and/or RyR inhibitors to inhibit inflammasome activation by most stimuli. How might NLRP3 inflammasome activators converge on ER Ca2+ release? Upstream of IP3R, PLC can be activated by GPCRs or Ca2+ influx across the plasma membrane, and Ca2+ itself can regulate opening of the RyR [25, 26].
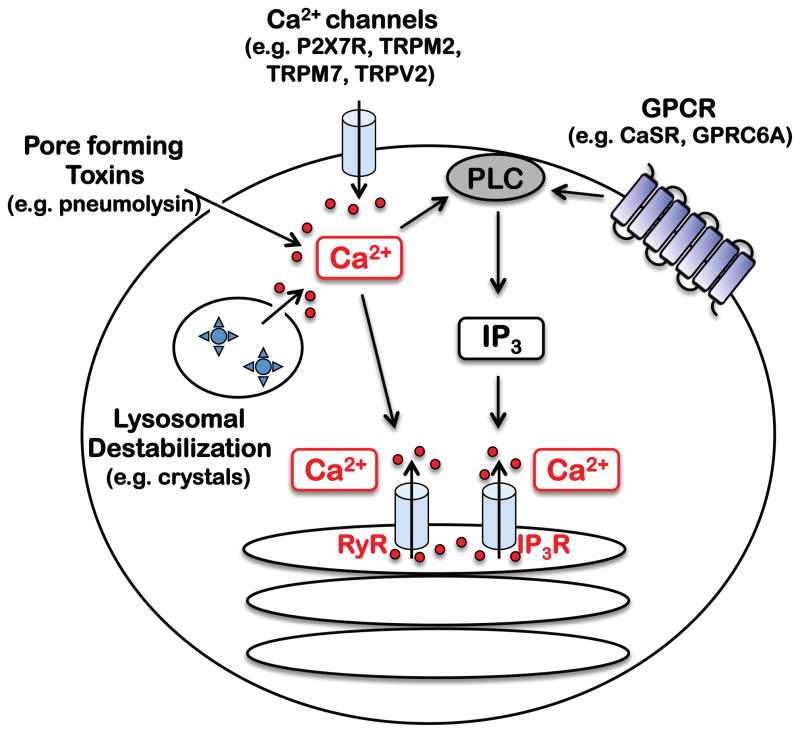
Fig 1. Mechanisms of Ca2+ mobilization during NLRP3 inflammasome activation.
NLRP3 inflammasome activators initiate Ca2+ signaling in a context dependent manner. GPCRs such as CaSR trigger Ca2+ signaling via PLC activation, IP3 generation, and opening of the IP3R. Alternatively, Ca2+ can enter via Ca2+ channels and pores (e.g. P2X7R, TRPM2, and pore forming toxins) or damaged membranes. Such Ca2+ influx can activate PLC to lead to IP3R opening, and/or act directly on RyR, another ER resident Ca2+ channel. Therefore, many stimuli may converge on ER Ca2+ release proximal to inflammasome activation, allowing for amplification of Ca2+ signaling and coordination of mitochondrial Ca2+ uptake, dynamics, and destabilization.
It is worth noting here that many studies have relied on pharmacological inhibitors to link Ca2+ signaling to NLRP3 inflammasome activation. This is acceptable given the redundancy in the mechanisms of Ca2+ mobilization (e.g. multiple PLC proteins) and the lethality of mice lacking critical regulators (e.g. IP3R1), but such findings are greatly strengthened if Ca2+ signaling inhibitors can be shown to block Ca2+ mobilization in the given experimental system.
The role of Ca2+ signaling
Although Ca2+ mobilization is necessary to activate the NLRP3 inflammasome, it can not be sufficient given that Ca2+ signaling is triggered in many settings without coincident inflammasome activation. Different models have been proposed to explain such a role for Ca2+ signaling. Lee et al suggested that Ca2+ binds to the NLRP3 inflammasome complex to regulate assembly, since addition of Ca2+ to lysates of LPS-stimulated macrophages induced co-immunoprecipitation of Asc and NLRP3. This study also described an inhibitory role for cAMP, levels of which fell during NLRP3 inflammasome activation. Increasing cAMP production inhibited activation of the NLRP3 inflammasome, likely via direct regulation of complex assembly and/or activity since cAMP was shown to interact with NLRP3. Many biological processes are controlled by coordinate activation of Ca2+ and cAMP signaling[27], and Lee et al proposed that a balance between the levels of Ca2+ and cAMP, and their respective positive and negative effects, determine the outcome of NLRP3 inflammasome activation[17] (Fig. 2). Additional studies investigating the effects of Ca2+ and cAMP binding on conformational changes and/or complex assembly would further support this model.
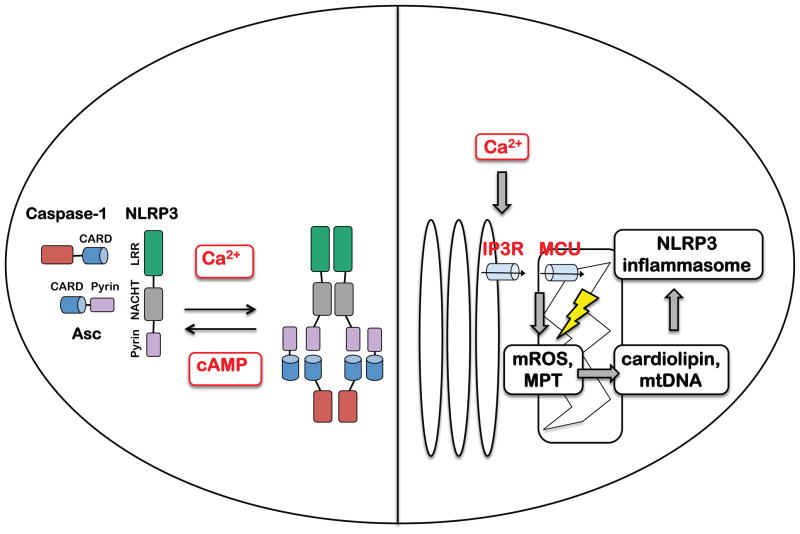
Fig 2. Role of Ca2+ signaling during NLRP3 inflammasome activation.
Ca2+ and cAMP may bind directly to the NLRP3 inflammasome complex to positively and negatively regulate assembly and/or activation respectively, such that the balance of Ca2+ and cAMP signaling determines the outcome of inflammasome activation (left). Alternatively, mitochondrial Ca2+ uptake (via ER Ca2+ release channels and mitochondrial MCU) can lead to Ca2+ overload and associated mitochondrial destabilization, including mROS production and induction of MPT, thus facilitating cardiolipin externalization and mtDNA release (right).
An alternative model is that Ca2+ mobilization induces mitochondrial damage as an intermediate step in activation of the NLRP3 inflammasome[16] (Fig. 2). At sites of ER-mitochondria contact, ER Ca2+ release is closely coordinated with mitochondrial Ca2+ uptake to regulate multiple aspects of mitochondria biology and function[28]. While physiological Ca2+ uptake increases mitochondrial metabolism, excessive and/or sustained Ca2+ influx can trigger mitochondrial Ca2+ overload, a pathophysiological condition characterized by multiple parameters of mitochondrial damage such as high levels of mROS production and induction of MPT[29–31] (see Glossary Box). Therefore, Ca2+ signaling per se is insufficient and only Ca2+ mobilization that leads to Ca2+ overload and/or mitochondrial damage can trigger NLRP3 inflammasome activation. Supporting this model, ER-mitochondrial juxtaposition, which is critical for Ca2+ transfer from the ER to the mitochondria, is necessary for NLRP3 inflammasome activation[32]. Mitochondrial dysfunction is observed in many settings of NLRP3 inflammasome activation (see below), and inhibiting Ca2+ mobilization reduces mROS production and depolarization in addition to inflammasome activation[16]. Finally, mitochondria take up Ca2+ during NLRP3 inflammasome activation by the membrane attack complex, and shRNA knockdown of the mitochondrial calcium uniporter (MCU)[33, 34], which reduces mitochondrial Ca2+ uptake, diminished IL-1β production[19]. This supports an intra-mitochondrial rather than a cytoplasmic role for Ca2+ in inflammasome activation, because defects in mitochondrial Ca2+ buffering (i.e. uptake of cytoplasmic Ca2+) should increase cytoplasmic Ca2+. The hypothesis that some stimuli may engage Ca2+-dependent mitochondrial destabilization and/or damage to activate the inflammasome could be tested further by examining mitochondrial Ca2+ uptake during various settings of NLRP3 inflammasome activation, and determining consequences of perturbing this process for mitochondrial destabilization and generation of the putative NLRP3 inflammasome ligands, cytosolic mtDNA and externalized cardiolipin (see below).
Finally, because Ca2+ controls many aspects of mitochondrial biology, Ca2+ mobilization may modulate inflammasome activation by impacting other aspects of mitochondrial function. For example, Ca2+ regulates mitochondrial motility[32, 35], which is of interest given that microtubule-driven apposition of mitochondria to the ER is necessary for assembly of the NLRP3 inflammasome complex[32]. Ca2+ is also known to control a related process of mitochondrial fusion and fission[35]. Although fusion-fission dynamics has not been directly linked to NLRP3 inflammasome activation, Mitofusin 2, a critical regulator of mitochondrial fusion, has been implicated[36]. Therefore, in addition to stimulus-dependent mitochondrial destabilization, it is plausible that Ca2+ signaling regulates basal mitochondrial activities such as motility and fusion and fission to govern NLRP3 inflammasome activation.
Cryopyrin Associated Periodic syndrome (CAPS)
Interestingly, Ca2+ signaling contributes to dysregulated NLRP3 inflammasome activation in CAPS, a spectrum of autoinflammatory diseases caused by mutations in NLRP3[10]. In monocytes from CAPS patients, the NLRP3 inflammasome is aberrantly active such that LPS stimulation or cold exposure is sufficient to trigger Caspase-1 activation and IL-1β maturation, presumably because of a reduced threshold for activation. Importantly, Lee et al showed that Ca2+ signaling inhibitors, including an inhibitor of the IP3R, blocks LPS-mediated NLRP3 inflammasome activation in CAPS PBMCs, and that CAPS mutations decrease cAMP binding[17]. It would also be informative to determine if CAPS mutations reduce the threshold for Ca2+ binding and/or recognition of the putative NLRP3 inflammasome ligands, cytosolic mtDNA and externalized cardiolipin.
Ca2+ mobilization and K+ efflux
It is worth discussing the role of Ca2+ mobilization vis-à-vis K+ efflux, since the latter has been linked to NLRP3 inflammasome activation[37, 38]. Many stimuli, including ATP, nigericin and crystals, can trigger K+ efflux. Buffers containing high, supraphysiological K+ levels block NLRP3 inflammasome activation by most/all stimuli, while lowering cytoplasmic K+ levels is sufficient for activation[38]. Such decreases in cytoplasmic K+ may allow complex assembly since physiological levels of K+ inhibit complex formation in vitro[37]. Alternatively, since manipulating K+ levels will perturb Ca2+ mobilization, it is possible that K+ fluxes impinge on Ca2+ signaling to control inflammasome activation, at least in some settings; plerhaps consistently, Ca2+ signaling inhibitors[17] but not high K+ buffers[38] attenuate activation of NLRP3 inflammasomes harboring CAPS mutations. Finally, the mitochondrial potassium cycle and various mitochondrial K+ channels regulate multiple aspects of mitochondrial physiology, including mitochondrial volume, Ca2+ uptake, and mROS generation[39, 40]. Thus it may be useful to examine the effects of experimentally manipulating extracellular K+ levels on Ca2+ signaling and generation of putative NLRP3 inflammasome ligands such as cardiolipin (see below).
The role of mitochondria in NLRP3 inflammasome activation
An increasing number of studies implicate mitochondrial damage, including mROS production and MPT induction, in activation of the NLRP3 inflammasome in a variety of settings. Because the precise mitochondrial processes involved remain unclear and may vary depending on the context (see below), I use the terms destabilization, damage, and dysfunction interchangeably in this review to refer to some perturbation of normal mitochondrial function.
Mitochondrial destabilization or dysfunction
Zhou et al were the first to link mitochondrial damage to NLRP3 inflammasome activation. Blocking mitophagy, which leads to the accumulation of damaged mitochondria, increased activation of the NLRP3 inflammasome. Elevated mitochondrial ROS (mROS) production was suggested to be the relevant signal of mitochondrial damage, since disruption of the electron transport chain was sufficient to activate the NLRP3 inflammasome[41]. Moreover, stimulation with nigericin, uric acid crystals, and ATP increased mROS levels, and mROS scavenging by treatment with MitoTempo attenuated inflammasome activation[41–43]. Although the precise role of mROS is not clear, these findings are provocative because increased mROS production is a feature of mitochondrial dysfunction in many settings[29].
Other studies indicated a role for mitochondrial permeability transition (MPT), another common feature of damaged mitochondria, in activating the NLRP3 inflammasome[42, 44]. MPT is the increase in mitochondrial permeability caused by opening of the permeability transition pore (PTP), and can be triggered by high/sustained Ca2+ influx and/or mROS production[30, 45]. MPT was implicated based on the ability of Cyclosporin A to block inflammasome activation by several stimuli. Given that Cyclosporin A has other cellular targets in addition to the PTP and that induction of MPT was not demonstrated in these studies, the role of MPT could be examined further. These experiments could employ the use of macrophages deficient in Cyclophilin D, a subunit of the PTP, with the caveat that MPT is triggered independently of Cyclophilin D in some settings[46].
Mitochondria-derived ligands
If the NLRP3 inflammasome is activated by mitochondrial destabilization and damage, what signals associated with this process are being recognized? Recent studies have revealed two putative ligands, mtDNA and cardiolipin. mtDNA is released into the cytosol during NLRP3 inflammasome activation by ATP, while depleting mitochondria of mtDNA (by ethidium bromide treatment) impairs inflammasome activation. Furthermore, NLRP3 can co-immunoprecipitate mtDNA that is either exogenously introduced (via transfection) or released from the mitochondria during ATP stimulation[42, 47]. Interestingly, two models have been proposed with respect to the role of mtDNA. Shimada et al place mtDNA release upstream of NLRP3 inflammasome activation and argue that it initiates activation of this pathway[47]. In contrast, Nakahira et al show that ATP-mediated mtDNA release is dependent on the NLRP3 inflammasome, thus mtDNA amplifies inflammasome activation downstream of the initial trigger (proposed to be mROS production). How might mtDNA be released from the mitochondria to enable NLRP3 inflammasome activation? The study suggests that mtDNA release is mediated by MPT based on sensitivity to Cyclosporin A[42], which is plausible if MPT is followed by osmotic swelling and mitochondrial rupture (since the PTP allows permeability only to low molecular weight solutes).
Additionally, a recent study suggests that cardiolipin[48, 49], a phospholipid abundant in the mitochondria, may be a ligand for the NLRP3 inflammasome[44]. In vitro studies indicate direct interactions between cardiolipin and recombinant NLRP3, mediated by the latter’s leucine rich repeat (LRR) domain. In a broken cell system, addition of cardiolipin containing liposomes is sufficient for NLRP3 inflammasome activation. Moreover, inhibiting cardiolipin synthesis, either through palmitate treatment or knockdown of cardiolipin synthase, reduces NLRP3 inflammasome activation by ATP and silica. Although not addressed in this study, cardiolipin externalization is presumably prerequisite for inflammasome activation since the phospholipid is normally sequestered in the inner membrane of the mitochondria. Future studies to demonstrate such externalization and to understand how it occurs during inflammasome activation are warranted. Interestingly, a recent study showed that mitochondrial damage induced by rotenone and CCCP treatment (which induce mROS production and membrane depolarization respectively) are sufficient to induce cardiolipin externalization[50]. Another regulated aspect of cardiolipin biology that may be worth exploring in the context of NLRP3 inflammasome activation is peroxidation, since the peroxidized forms of the phospholipid are thought to be important in triggering mitochondrial damage and cell death in other settings. Although not examined by Iyer et al[44], the high unsaturated content of cardiolipin renders it very susceptible to oxidation by mROS, which is elevated in many settings of NLRP3 inflammasome activation. Perhaps not surprisingly, mitochondrial Ca2+ influx has been linked to cardiolipin peroxidation[48, 49].
Therefore, mitochondrial dysfunction may be critical for NLRP3 inflammasome activation in some contexts[16, 19, 41–44, 47, 51], and two putative ligands associated with this process have been identified[42, 44, 47]. In contrast, other studies indicate no role for mROS production and/or MPT induction in NLRP3 inflammasome activation. In response to some stimuli, the NLRP3 inflammasome can be activated independent of detectable increases in mROS generation, and mROS or total ROS scavengers are not able to block NLRP3 inflammasome activation in some settings[36, 38, 44]. Induction of MPT is also unlikely to be a universal requirement for activating the NLRP3 inflammasome, given that dissipating the membrane potential can inhibit inflammasome activation, at least in some settings[36]. These differences could be reconciled if the proximal signals for NLRP3 inflammasome activation are triggered by multiple mechanisms, including but likely not limited to mROS production and MPT induction. Such mechanisms could be differentially engaged depending on the context, and/or act redundantly to regulate the availability of the NLRP3 inflammasome ligand(s), consistent with the intimate connection between multiple parameters of mitochondrial damage. Altogether, it is likely that in many settings of NLRP3 inflammasome activation, some perturbation of normal mitochondrial function may be needed to generate the proximal signals that activate this pathway (Fig. 2). This model can be readily tested with the recent identification of cytosolic mtDNA and externalized cardiolipin, by examining their role in NLRP3 inflammasome activation by various stimuli, as well as their generation by Ca2+ signaling and mitochondrial destabilization.
Alternative models?
An alternative (but not mutually exclusive) role for mitochondria in NLRP3 inflammasome activation may be to provide a platform for complex assembly. Recently, the mitochondrial-resident protein MAVS was shown to facilitate NLRP3 oligomerization and inflammasome activation during infection by Sendai virus[52]. Regulation of inflammasome activation by mitofusin-2[36] could also be related to such a scaffolding function for the mitochondria, given the changes in mitochondrial morphology and dynamics when fusion is impaired[35]. Finally, a recent study showed that DHX33, a RNA helicase of the DExD/H-box family, bridges RNA ligands with NLRP3 to enable inflammasome activation[53], reminiscent of NAIP proteins which confer specificity to NLRC4 inflammasome activation by bacterial products[54, 55]. Therefore, engagement of accessory proteins may increase the repertoire of NLRP3 inflammasome activators, perhaps to include those that do not trigger mitochondrial destabilization or otherwise engage the mitochondria.
Mitochondrial surveillance by the inflammasome and apoptosome
Interestingly, the emerging view that some mitochondria associated signal (e.g. mtDNA or externalized cardiolipin) is necessary for NLRP3 inflammasome activation bears striking parallels to activation of the apoptosome during apoptosis (Fig 3). Activation of Caspase-9 in this complex is mediated by binding of cytochrome c to the regulatory subunit Apaf, and thus is dependent on cytochrome c release from the mitochondrial intermembrane space. Such cyt c release is a consequence of mitochondrial outer membrane permeabilization (MOMP), which is regulated by the Bcl-2 family of proteins[56]. Indeed, modulation of NLRP3 inflammasome activation by Bcl-2 proteins[41, 47] represents another parallel with the apoptosome and presumably reflects a related role of these proteins in mitochondrial stabilization.
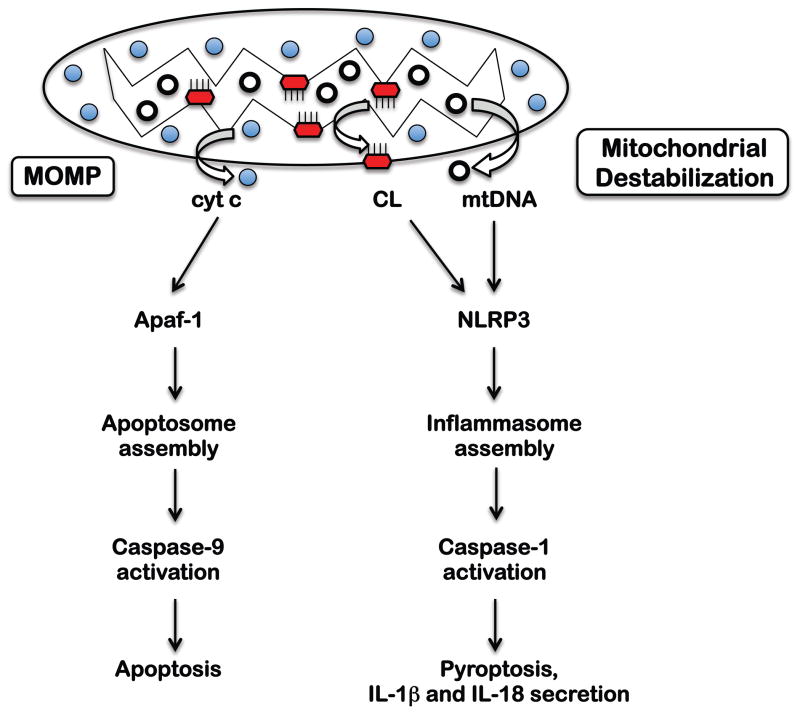
Fig 3. Activation of the NLRP3 inflammasome and the apoptosome.
Following MOMP, release of cyt c from the mitochondrial intermembrane space leads to activation of the apoptosome and consequent Caspase-9-mediated apoptosis. In contrast, the NLRP3 inflammasome is activated by mitochondrial destabilization-associated cardiolipin externalization and mtDNA release, followed by Caspase-1-mediated pro-inflammatory cell death (called pyroptosis).
Thus, both the apoptosome and the NLRP3 inflammasome can be considered sensors of mitochondrial integrity. However, the two complexes are activated by distinct signals (cytochrome c versus cardiolipin and mtDNA) that are likely associated with different modes of mitochondrial destabilization. Although cardiolipin has been linked to activation of the apoptosome in addition to the inflammasome, presumably its differential modification (e.g. peroxidation) or other context dependent signals would enable specific activation of one or the other pathway. Additionally, the different modes of mitochondrial dysfunction may have consequences for the inflammatory nature of the ensuing cell death, which is anti-inflammatory in the case of apoptosome-mediated apoptosis and pro-inflammatory in the case of inflammasome-mediated pyroptosis. Indeed the mitochondria is a source of Danger Associated Molecular Patterns (DAMPs) that can be externalized and/or released extracellularly, including not only mtDNA but also N-formylated peptides, cardiolipin, and mitochondrial transcription factor A[57]. Thus the apoptosome and the NLRP3 inflammasome may survey mitochondrial integrity in a context dependent manner to regulate anti-inflammatory versus pro-inflammatory cell death.
Concluding Remarks
In conclusion, mitochondrial destabilization or dysfunction is likely critical for activation of the NLRP3 inflammasome by many stimuli. Diverse forms of cellular stress, including plasma membrane damage, lysosomal damage and ionic imbalance, are likely to impinge on the mitochondria to activate the NLRP3 inflammasome (Table 1). Consistently, the NLRP3 inflammasome is localized to the mitochondria, facilitating its function as a mitochondrial surveillance system analogous to the apoptosome. In some settings, mROS production and MPT induction play key roles in inflammasome activation, likely by generating proximal signals of inflammasome activation (e.g. mtDNA and cardiolipin). Increased mROS and MPT induction are not observed and/or dispensable in other conditions, perhaps because of redundancy in the mechanisms for triggering mitochondrial destabilization, and suggesting that additional mechanisms exist. Recent studies implicate Ca2+ signaling in activation of the NLRP3 inflammasome, perhaps by facilitating mitochondrial destabilization. While diverse stimuli may mobilize Ca2+ through distinct mechanisms distal to inflammasome activation, Ca2+ transfer at ER-mitochondria contact sites is likely to be of particular significance. This process is closely associated with induction of mitochondrial damage, including mROS production and MPT induction. Additional support for such a role for Ca2+ signaling could come from more careful analysis of cells deficient in mitochondrial Ca2+ uptake. Moreover, it would be informative to revisit the role of Ca2+ vis-à-vis mtDNA release and cardiolipin externalization, and conversely, determine if introducing the ligands can bypass the requirement for Ca2+ mobilization. Ca2+ signaling could also regulate other aspects of mitochondrial biology (e.g. ER-mitochondrial apposition, fusion-fission, motility) or complex assembly to control NLRP3 inflammasome activation. There is compelling evidence to indicate that mtDNA and cardiolipin can serve as proximal signals for NLRP3 inflammasome activation, downstream of mitochondrial destabilization (although as noted above mtDNA may not be the initiating trigger in some contexts). In addition to functional data, biochemical studies indicate direct interactions with the inflammasome complex, consistent with their role as bona fide ligands. Such identification of the proximal signals warrants an examination of their role in various settings of NLRP3 inflammasome activation, and additionally will enable clarification of other related questions, including the role played by Ca2+ signaling and mitochondrial destabilization. These recent findings also raise important new questions. For example, of the two putative ligands that have been identified, which one(s) is relevant in various settings of NLRP3 inflammasome activation? Are there other ligands yet to be uncovered? Are there additional accessory proteins that can interact with NLRP3 to diversify the repertoire of ligands? Second, can mitochondrial Ca2+ uptake, mitochondrial destabilization, and cardiolipin externalization and mtDNA release be manipulated therapeutically in CAPS and other diseases associated with aberrant activation of the NLRP3 inflammasome? Could these processes be targeted by pathogens to evade NLRP3 inflammasome recognition? Finally, one consequence of apoptosome activation by MOMP is feedback amplification of mitochondrial damage. Is this also true of NLRP3 inflammasome activation, and if so, what are the implications for cell death and the inflammatory nature of such cell death? Can the NLRP3 inflammasome and the apoptosome be activated coordinately, and if so, what determines if the ensuing cell death is inflammatory or not?
Highlights.
The NLRP3 inflammasome is activated by mitochondrial (mt) destabilization and dysfunction
Recognition of mtDNA and externalized cardiolipin by NLRP3 inflammasome may be direct
Ca2+ is mobilized in many settings of NLRP3 inflammasome activation
Intercepting Ca2+ mobilization inhibits inflammasome activation
Ca2+ may trigger mt destabilization leading to NLRP3 inflammasome activation
Acknowledgments
The author is supported by NIH grant R01AI102964.
Glossay Box
- Mitochondrial Ca2+ uptake and overload
In addition to shaping spatiotemporal patterns of Ca2+ in the cytosol, mitochondrial uptake and release of Ca2+ during Ca2+ signaling has important consequences for mitochondrial function. Mitochondrial Ca2+ uptake boosts oxidative phosphorylation and mROS production, but excessive and/or sustained Ca2+ influx, especially along with increased mROS production, leads to mitochondrial Ca2+ overload, a dysfunctional state that can promote further increases in mROS and MPT induction[30, 31]. The ER is also a key player in these processes, since it is an important source of Ca2+ release and is a feedback target of mROS in modulating such Ca2+ release[28, 29]
- Mitochondrial permeability transition (MPT)
MPT is defined as an increase in permeability of the mitochondria to small solutes and ions (<1.5kD), and is mediated by opening of the permeability transition pore (PTP) in the mitochondrial inner membrane. The best-characterized triggers of MPT are mitochondrial Ca2+ uptake and mROS generation, which are thought to act synergistically[30, 45]. Although the molecular basis of the PTP remains poorly defined, one important subunit is cyclophilin D, which is critical for pore opening in response to mitochondrial Ca2+ accumulation and oxidative stress and is a target of Cyclosporin A[46]. Irreversible MPT dissipates the membrane potential; disrupts oxidative phosphorylation; promotes mROS production; and may lead to osmotic swelling and mitochondrial rupture
- Mitochondrial ROS (mROS)
Various processes can increase mROS production, and likely to be most relevant for rapid, mROS-mediated NLRP3 inflammasome activation by stimuli such as ATP are mitochondrial Ca2+ uptake and MPT. Mitochondrial Ca2+ influx stimulates Ca2+ sensitive matrix dehydrogenases thus increasing oxidative phosphorylation and mROS production, while MPT promotes mROS production by disrupting electron transport. Conversely, elevated mROS generation can modify redox-sensitive sites in key regulatory proteins to increase mitochondrial Ca2+ uptake and trigger MPT. Thus mROS, Ca2+ and MPT form integral components of an amplification loop that triggers irreversible mitochondrial damage[29, 31]
- Mitochondrial Outer Membrane Permeabilization (MOMP)
Induction of MOMP commits a cell to die in the extrinsic pathway of apoptosis. It is regulated by the antagonistic activities of the pro- and anti- apoptotic members of the Bcl-2 family of proteins, and triggers some features of mitochondrial stress similar to MPT (e.g. depolarization)[56]. However, limited mitochondrial destabilization during MOMP permits the release of proteins in the intermembrane space (e.g. cyt c), in contrast to MPT which can result in mitochondrial rupture and liberation of matrix components (e.g. Ca2+, mtDNA)
- Mitophagy
Mitophagy is the process by which mitochondria are targeted to and degraded by the autophagy pathway. In many settings, the critical targeting step is mediated by PINK1 and Parkin proteins, which couple recognition of damaged mitochondria, and particularly those with loss of membrane potential, to the autophagy machinery[58]
- Pyroptosis
Pyroptosis is the term used to describe Caspase-1 mediated cell death. Since it is associated with loss of plasma membrane integrity and release of DAMPs such as HMGB1, pyroptosis is a pro-inflammatory form of cell death. How Caspase-1 regulates pyroptosis is poorly understood[59]
- Mitochondrial fusion/fission
Mitochondria do not exist as individual organelles but as part of a dynamic network maintained by constant fusion and fission. These antagonistic activities promote a healthy pool of mitochondria (by allowing mixing of mitochondrial contents) and also enables sequestration of damaged mitochondria (which are unable to fuse). Thus mitochondrial fusion and fission impact mitophagy, stress responses, and apoptosis[60]
- Cardiolipin
Cardiolipin is a phospholipid abundant in the mitochondria. It normally resides in in the inner membrane, where it interacts with a number of proteins such as components of the electron transport chain to regulate key mitochondrial processes. During apoptosis, cardiolipin oxidation and redistribution to the outer mitochondrial membrane not only disrupts mitochondrial function, but appears to actively trigger cell death through multiple mechanisms, including regulation of MOMP induction, cytochrome c release, Caspase-8 activation, and as discussed here, NLRP3 inflammasome activation[48, 49]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agostini L, et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20(3):319–25. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 2.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 3.Martinon F, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 4.Kanneganti TD, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440(7081):233–6. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 5.Franchi L, et al. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature immunology. 2009;10(3):241–7. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamkanfi M, V, Dixit M. Inflammasomes and their roles in health and disease. Annual review of cell and developmental biology. 2012;28:137–61. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 7.Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39(3):432–41. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strowig T, et al. Inflammasomes in health and disease. Nature. 2012;481(7381):278–86. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 9.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nature immunology. 2012;13(4):333–2. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman HM, Brydges SD. Genetic and molecular basis of inflammasome-mediated disease. The Journal of biological chemistry. 2011;286(13):10889–96. doi: 10.1074/jbc.R110.135491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin C, Flavell RA. Molecular mechanism of NLRP3 inflammasome activation. J Clin Immunol. 2010;30(5):628–31. doi: 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- 12.Brough D, et al. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol. 2003;170(6):3029–36. doi: 10.4049/jimmunol.170.6.3029. [DOI] [PubMed] [Google Scholar]
- 13.Feldmeyer L, et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17(13):1140–5. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 14.Chu J, et al. Cholesterol-dependent cytolysins induce rapid release of mature IL-1beta from murine macrophages in a NLRP3 inflammasome and cathepsin B-dependent manner. J Leukoc Biol. 2009;86(5):1227–38. doi: 10.1189/jlb.0309164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HM, et al. Mycobacterium abscessus activates the NLRP3 inflammasome via Dectin-1-Syk and p62/SQSTM1. Immunology and cell biology. 2012;90(6):601–10. doi: 10.1038/icb.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami T, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):11282–7. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee GS, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492(7427):123–7. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Z, et al. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nature communications. 2013;4:1611. doi: 10.1038/ncomms2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Triantafilou K, et al. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. Journal of cell science. 2013;126(Pt 13):2903–13. doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Sater AA, et al. Cyclic-di-GMP and cyclic-di-AMP activate the NLRP3 inflammasome. EMBO reports. 2013;14(10):900–6. doi: 10.1038/embor.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito M, Yanagi Y, Ichinohe T. Encephalomyocarditis virus viroporin 2B activates NLRP3 inflammasome. PLoS pathogens. 2012;8(8):e1002857. doi: 10.1371/journal.ppat.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossol M, et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nature communications. 2012;3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Castejon G, et al. P2X(7) receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J Immunol. 2010;185(4):2611–9. doi: 10.4049/jimmunol.1000436. [DOI] [PubMed] [Google Scholar]
- 24.Compan V, et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37(3):487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Lau BW, et al. Deoxycholic acid activates protein kinase C and phospholipase C via increased Ca2+ entry at plasma membrane. Gastroenterology. 2005;128(3):695–707. doi: 10.1053/j.gastro.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 26.Witting A, et al. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc Natl Acad Sci U S A. 2004;101(9):3214–9. doi: 10.1073/pnas.0306707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borodinsky LN, Spitzer NC. Second messenger pas de deux: the coordinated dance between calcium and cAMP. Science’s STKE: signal transduction knowledge environment. 2006;2006(336):pe22. doi: 10.1126/stke.3362006pe22. [DOI] [PubMed] [Google Scholar]
- 28.Patergnani S, et al. Calcium signaling around Mitochondria Associated Membranes (MAMs) Cell Commun Signal. 2011;9:19. doi: 10.1186/1478-811X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Csordas G, Hajnoczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta. 2009;1787(11):1352–62. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemasters JJ, et al. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787(11):1395–401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camello-Almaraz C, et al. Mitochondrial reactive oxygen species and Ca2+ signaling. American journal of physiology Cell physiology. 2006;291(5):C1082–8. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 32.Misawa T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nature immunology. 2013;14(5):454–60. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 33.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476(7360):341–5. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Stefani D, et al. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476(7360):336–40. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Hajnoczky G. Ca2+-dependent regulation of mitochondrial dynamics by the Miro-Milton complex. The international journal of biochemistry & cell biology. 2009;41(10):1972–6. doi: 10.1016/j.biocel.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichinohe T, et al. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(44):17963–8. doi: 10.1073/pnas.1312571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrilli V, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14(9):1583–9. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 38.Munoz-Planillo R, et al. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–53. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szabo I, et al. Physiology of potassium channels in the inner membrane of mitochondria. Pflugers Archiv: European journal of physiology. 2012;463(2):231–46. doi: 10.1007/s00424-011-1058-7. [DOI] [PubMed] [Google Scholar]
- 40.Nowikovsky K, Schweyen RJ, Bernardi P. Pathophysiology of mitochondrial volume homeostasis: potassium transport and permeability transition. Biochimica et biophysica acta. 2009;1787(5):345–50. doi: 10.1016/j.bbabio.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Zhou R, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 42.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–30. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lupfer C, et al. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nature immunology. 2013;14(5):480–8. doi: 10.1038/ni.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iyer SS, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39(2):311–23. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernardi P, et al. The mitochondrial permeability transition. Biofactors. 1998;8(3–4):273–81. doi: 10.1002/biof.5520080315. [DOI] [PubMed] [Google Scholar]
- 46.Elrod JW, Molkentin JD. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circulation journal: official journal of the Japanese Circulation Society. 2013;77(5):1111–22. doi: 10.1253/circj.cj-13-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimada K, et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity. 2012 doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paradies G, et al. Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium. 2009;45(6):643–50. doi: 10.1016/j.ceca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. American journal of physiology Cell physiology. 2007;292(1):C33–44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 50.Chu CT, et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nature cell biology. 2013;15(10):1197–205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature immunology. 2011;12(5):408–15. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park S, et al. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. Journal of immunology. 2013;191(8):4358–66. doi: 10.4049/jimmunol.1301170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitoma H, et al. The DHX33 RNA helicase senses cytosolic RNA and activates the NLRP3 inflammasome. Immunity. 2013;39(1):123–35. doi: 10.1016/j.immuni.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477(7366):596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 55.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477(7366):592–5. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305(5684):626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 57.Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nature reviews Molecular cell biology. 2012;13(12):780–8. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- 58.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nature reviews Molecular cell biology. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamkanfi M, V, Dixit M. Manipulation of host cell death pathways during microbial infections. Cell host & microbe. 2010;8(1):44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 60.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annual review of genetics. 2012;46:265–87. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]