Abstract
Co-culture of endothelial cells (EC) and mesenchymal stem cells (MSC) results in robust vascular network formation in constrained 3D collagen/fibrin (COL/FIB) composite hydrogels. However, the ability to form endothelial networks is lost when such gels are allowed to compact via cell-mediated remodeling. In this study, we created co-cultures of human EC and human MSC in both constrained and unconstrained COL/FIB matrices and systematically added nanoparticulate hydroxyapatite (HA, 0–20 mg/mL), a bone-like mineral that has been shown to have pro-vasculogenic effects. Constructs cultured for 7 days were assayed for gel compaction, vascular network formation, and mechanical properties. In vitro, robust endothelial network formation was observed in constrained COL/FIB constructs without HA, but this response was significantly inhibited by addition of 5, 10, or 20 mg/mL HA. In unconstrained matrices, network formation was abolished in pure COL/FIB constructs, but was rescued by 1.25 or 2.5 mg/mL HA, while higher levels again inhibited vasculogenesis. HA inhibited gel compaction in a dose-dependent manner, which was not correlated to endothelial network formation. HA affected initial stiffness of the gels, but gel remodeling abrogated this effect. Subcutaneous implantation of COL/FIB with 0, 2.5, or 20 mg/mL HA in the mouse resulted in increased perfusion at the implant site, with no significant differences between materials. Histology at day 7 showed both host and human CD31-stained vasculature infiltrating the implants. These findings are relevant to the design of materials and scaffolds for orthopaedic tissue engineering, where both vasculogenesis and formation of a mineral phase are required for regeneration.
Keywords: Collagen, Fibrin, Hydroxyapatite, Vasculogenesis, Mesenchymal Stem Cells, Endothelial Cells, In Vivo
1. Introduction
Transplantation and engraftment of engineered tissues requires creation of a vascular supply, either through vasculogenesis, the de novo formation of blood vessels, or through angiogenesis, the creation of new vessels via sprouting from existing vasculature [1]. The diffusive limit for nutrient transport in most tissues has been suggested to be only a few hundred microns, and therefore a new vascular supply to implanted tissue must be created to provide convective transport to the region [2]. A variety of model systems have been created to study the process of vasculogenesis, including 3D systems using extracellular matrix proteins such as collagen, fibrin, and Matrigel® [3–6]. Previous work in our lab has shown composite collagen/fibrin (COL/FIB) matrices to be permissive to endothelial network formation in vitro when human umbilical vein endothelial cells (EC) are co-cultured with bone marrow-derived mesenchymal stem cells (MSC) [7]. The degree of vasculogenesis was shown to be dependent on EC:MSC ratio and the composition of the matrix.
In most studies of vasculogenesis in 3D hydrogels in vitro, volume reduction of the matrix is prevented by constraining the sample at its boundaries. This technique prevents remodeling and compaction of the matrix by the contractile forces exerted by embedded cells. Unconstrained gel compaction leads to increased matrix density and a concomitant increase in matrix stiffness [8, 9]. It is has been suggested that matrix mechanics play an important role in regulating endothelial network formation. Increased stiffness has been shown to promote angiogenesis in some studies [10, 11], while other studies have shown an inverse relationship between increased matrix stiffness and neovessel growth, both in vitro and in vivo [3, 7, 12–14]. In addition, unconstrained gel compaction has been shown to result in the regression of endothelial networks in vitro [16, 17].
Bioceramics have been included in vasculogenesis and angiogenesis models to promote neovessel growth both in vitro and in vivo for bone tissue engineering applications. Bioactive glasses are reactive materials composed of glass-ceramic composites that have been shown to induce mineralization. These materials have also been shown to be proangiogenic at low concentrations, presumably by increasing endothelial cell proliferation via dissolution into ionic components [18–22]. Similarly, hydroxyapatite (HA) is the mineral component of bone, and also has been examined for its ability to promote both vasculogenesis and angiogenesis. Low concentrations of HA have been shown to be compatible with EC, and to maintain the prototypical morphology and biochemical markers associated with normal EC function [23, 24]. HA has also been incorporated into 3D silk scaffolds designed to promote angiogenesis [25], and it has been observed that production of vascular endothelial growth factor (VEGF) from MSC is increased on poly (lactide-co-glycolide)-HA composite scaffolds [26]. In addition to its proangiogenic biochemical effects, it has been suggested that HA can inhibit cell-mediated compaction of protein hydrogels by providing structural integrity to the extracellular matrix [27].
In the current study, we examined the addition of HA to COL/FIB composite hydrogels, as a means to modulate the degree of vasculogenesis by seeded EC and MSC in both constrained and unconstrained model systems. Our motivation was the observation that vasculogenesis is inhibited in unconstrained 3D hydrogels due to matrix compaction, but that HA can have both proangiogenic effects and can reduce gel remodeling. We systematically added HA to 3D composite hydrogels and examined vascular network formation in vitro. We also measured matrix compaction and the mechanical properties of the hydrogels in an effort to understand the relationship between construct morphology and vasculogenic response. Cell-seeded COL/FIB/HA constructs were then implanted subcutaneously into mice to determine whether the effects of HA translated to changes in neovascularization in vivo. These studies demonstrate the use of HA in protein-based composite matrices, and contribute to our understanding of how vasculogenesis can be modulated in bone tissue engineering applications.
2. Materials and Methods
2.1 Cell Culture
Human umbilical vein endothelial cells (EC) were harvested from umbilical cords as previously described [7]. Briefly, umbilical veins were irrigated with sterile phosphate buffered saline (PBS) and then incubated with 0.1% collagenase (Type I, Worthington Biochemical, Lakewood, NJ, USA) at 37°C for 20 min. The digestion product was collected, the vein was washed with PBS, and the resulting suspension was centrifuged. The cell pellet was re-suspended in Endothelial Growth Medium-2 (EGM-2, Lonza Inc., Walkersville, MD, USA) and plated into flasks. After 24 hours, the cells were washed with PBS to remove residual erythrocytes. EC were cultured in EGM-2 and used at passage 4. Human bone marrow-derived mesenchymal stem cells (MSC; Lonza) were cultured in Dulbecco’s modified Eagles medium – low glucose (DMEM; Thermo Scientific; Logan, UT) with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA) and 1% penicillin and streptomyocin (Invitrogen) and used at passage 7. Media was changed every other day for both cells types.
2.2 Formation of Three-Dimensional Collagen/Fibrin/Hydroxyapatite Hydrogels
Collagen/fibrin (COL/FIB) composite hydrogels were created as previously described [7]. Briefly, bovine skin COL Type I (4.0 mg/ml; MP Biomedicals, Solon, OH, USA) was dissolved in 0.02 N acetic acid and bovine fibrinogen (4.0 mg/ml; Sigma Aldrich, St. Louis, MO, USA) was dissolved in EGM-2. Stock solutions of nano-grade hydroxyapatite (HA; Sigma) with a particle size < 200 nm were prepared at concentrations of 0, 12.5, 25, 50, 100, and 200 mg/ml HA in DMEM and sonicated for 1 hour prior to use to facilitate homogenous dispersion in hydrogels [28]. The stock HA solutions were autoclaved at 120°C for 20 min prior to use to maintain sterility. Hydrogels were formed by adding COL and FIB at a mass ratio of 40/60 (total protein concentration 2.5 mg/ml) to a mixture of 10% FBS, 10% 5X-concentrated DMEM, 5% 0.1 N NaOH, 2% thrombin (0.1 UT/ml; Sigma), and 10% HA in DMEM at 4°C. The resulting final concentrations of HA in the hydrogels were 0, 1.25, 2.5, 5, 10, and 20 mg/ml. Matrix mixtures (500 μl) were placed into a 24-well plate and gelled at 37°C for 45 minutes. Both EC and MSC were added directly into the gel mixture to at a ratio of 1:1 EC:MSC and a total cell concentration of (2.4×105 cells/ml). Cells were added prior to gelation to facilitate homogenous distribution of cells throughout the hydrogels. For constrained culture studies, hydrogels were kept in the original 24-well plates and adhered to the walls of the wells. For unconstrained studies, constructs were freed from the well walls immediately after the 45 min incubation period and were transferred to non-tissue culture treated 6-well plates. All hydrogels were cultured in EGM-2 for 7 days at 37°C and 5% CO2.
2.3 Endothelial Network Formation Assay
Vessel-like structure formation was quantified as previously described [7]. Briefly, EC were labeled through a retroviral expression system (Orbigen Inc., San Diego, CA, USA) to enable stable expression of a fluorescent protein (mCherry; Clontech, Mountain View, CA, USA). Cell-seeded hydrogels were imaged at day 7 with a fluorescent microscope (Olympus America Inc., Center Valley, PA, USA). For both constrained and unconstrained hydrogels, five representative images were taken of each gel and analyzed using the Angiogenesis Tube Module in Metamorph Premier Software (Molecular Devices Inc., Sunnyvale, CA, USA). Total network length of vessel-like structures formed in vitro was calculated by setting a minimum width, maximum width, and intensity over background.
2.4 Mechanical Properties Testing
Gel rheology was performed on acellular COL/FIB/HA hydrogels as previously described [7]. Briefly, pre-formed COL/FIB/HA solutions were loaded into a gel rheometer (AR-G2, TA Instruments, New Castle, DE, USA) and a time sweep was conducted for 45 minutes at 37°C. The storage (G′) and loss (G″) moduli were calculated from the final 5 minutes of the time sweep.
Compressive testing was performed by placing hydrogels under uniaxial compression using a 1.5 mm hemispherical indenter mounted on a 50 g load cell in a Test Resources frame (Test Resources Inc., Shakopee, MN, USA), and therefore the system was capable of recording the entire force-displacement curve. Samples were removed from buffer and mounted on a dry rubber block to prevent slipping. Each was compressed at a rate of 0.33 cm/s and force-displacement curves were generated at a sample rate of 200 Hz. Force-displacement curves were truncated to less than 25% compression and the Young’s Modulus (E) was determined from the equation below [29] using a non-linear least squares algorithm implemented in MATLAB.
E is the compressive Young’s modulus
F is the load measured by the load cell
r is the radius of the indenter
ν is the Poisson’s ratio (assumed to be 0.5)
d is the depth of penetration measured by the linear encoder
We assumed a Poisson’s ratio of 0.5 based on the fact that the hydrogels were tested at small strains (<10%), and we consistently analyzed the data within the elastic response of each curve. Under these loads these materials behave as a rubbery elastic solid, due to the highly cross linked nature of the gel and intrinsic elasticity of the fibers.
2.5 Measurement of Gel Compaction and 3-D Ultrasound Imaging
Representative images of unconstrained hydrogels were taken using a standard CCD camera in manual mode with a constant exposure setting at days 0, 1, 3, 5, and 7 to qualitatively demonstrate gel compaction throughout the time course. Ultrasound imaging of the constructs was performed with a Vevo 770 (VisualSonics Inc., Toronto, Canada) using an RMV 707B imaging probe with a nominal 30 MHz center frequency, 15–45 MHz bandwidth (−6 dB), 12.7 mm focal distance, and 2.2 mm depth of focus (−6 dB), as described previously [28]. For each gel, a 3D ultrasound image data containing a series of consecutive B-mode images with a spatial interval of 100 μm was collected. The interval between adjacent A-lines in a B-mode cross-sectional image was 50 μm. Volumes of these gels were measured from the ultrasound images using a semi-automated segmentation procedure, in which an edge detection algorithm identified the contour of the gel in an ultrasound B-mode image. Subsequent contours in adjacent frames or images were drawn semi-automatically until the contours in all frames were drawn, thus providing the volume of the gel within the contoured region.
2.6 Subcutaneous Implants
All animal studies were conducted in accordance with the National Institutes of Health Guidelines following a protocol approved by the University of Michigan Committee on Use and Care of Animals. Seven-week old male C.B.-17/SCID mice (Taconic Labs, Hudson, NY, USA) were administered an anesthetic analgesic drug cocktail containing ketamine (95 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA, USA), xylazine (9.5 mg/kg, Lloyd Laboratories, Shenandoah, IA, USA), and buprenorphine (0.059 mg/kg, Bedford Laboratories, Bedford, OH, USA) via intraperitoneal injection. Each mouse was shaved and sterilized with Betadine® (Thermo Fisher Scientific, Fremont, CA, USA) on its dorsal surface and wiped with alcohol prior to injection. COL/FIB/HA constructs containing 0, 2.5, or 20 mg/ml HA and a total cell concentration of 10.0 × 106 cells/ml were used for the in vivo studies. Acellular constructs served as controls. Two implants per animal were created with gel solutions of 300 μl. After injection, animals were kept stationary for 5 minutes and then placed in fresh cages. A total of five animals were used for each variable investigated.
2.7 Laser Doppler Perfusion Imaging
Mice were anesthetized as previously described and blood flow through the implant was imaged using laser Doppler Perfusion Imaging (LDPI; Perimed AB, Sweden) [30]. Mice were imaged in triplicate and a region of interest (ROI) was overlayed over the implant area to calculate mean perfusion through each implant. Results were calculated as fold change from perfusion values over the baseline (before implantation).
2.8 Histology and Immunohistochemistry
Explants were retrieved at day 7 and then fixed in zinc-buffered formalin (Z-Fix; Anatech Ltd., Battle Creek, MI, USA) overnight. Samples were sent to AML Laboratories (Baltimore, MD, USA) for embedding in paraffin and sectioned into 5 μm sections. Sections were then demineralized in a solution of 10% ethylenediamine tetraacetic acid (EDTA; Sigma) and Z-Fix for 3 hours at 4°C. Tissue sections were rehydrated, steamed in a vegetable steamer for 25 minutes in antigen retrieval solution (Dako, Carpinteria, CA, USA), and then equilibrated in Tris-buffered saline with Tween 20 (TBS-T; Sigma). Sections were then incubated at 4 °C in the primary antibody, either human anti-mouse CD31 (Dako) or human anti-mouse α-SMA (Abcam, Cambridge, MA, USA), and then diluted 1:50 in TBS-T overnight and then treated with horse radish peroxidase-conjugated anti-mouse secondary antibody. Hematoxylin and eosin (H&E) was used as a counterstain.
2.9 Quantification of In Vivo Vessel Formation
Blood vessels found within the implants were quantified manually by three blinded observers. Ten random images per sample at 20X magnification were used to quantify the number of human CD-31 stained vessels (i.e. those that arose from implanted cells) as well as the total number of vessels within the implant region. All vessels were quantified if they displayed a lumen containing erythrocytes and human vessels were identified if they displayed a positive CD31 stain around the lumen.
2.10 Statistical Analysis
All quantitative analyses were performed using a one-way analysis of variance test (ANOVA) with a protected Fisher’s Least Significant Difference post hoc test. Statistical significance was set at p < 0.05. Numerical values and bar charts are presented as mean ± standard error.
3. Results
3.1 Vasculogenesis in Constrained and Unconstrained COL/FIB/HA Hydrogels
Figure 1 shows representative images and quantification of endothelial network formation in constrained COL/FIB/HA materials after 7 days of culture. At low concentrations of HA (1.25 and 2.5 mg/ml), formation of vessel-like structures was similar to control constructs (0 mg/ml HA). However, higher concentrations of HA (5, 10, 20 mg/mL) inhibited vascular network formation. Quantification of total network length in these samples showed no significant differences between the 0, 1.25, and 2.5 mg/ml conditions, but a statistically significant decrease in total network length between the control group and the 5, 10, and 20 mg/ml HA groups.
Figure 1.
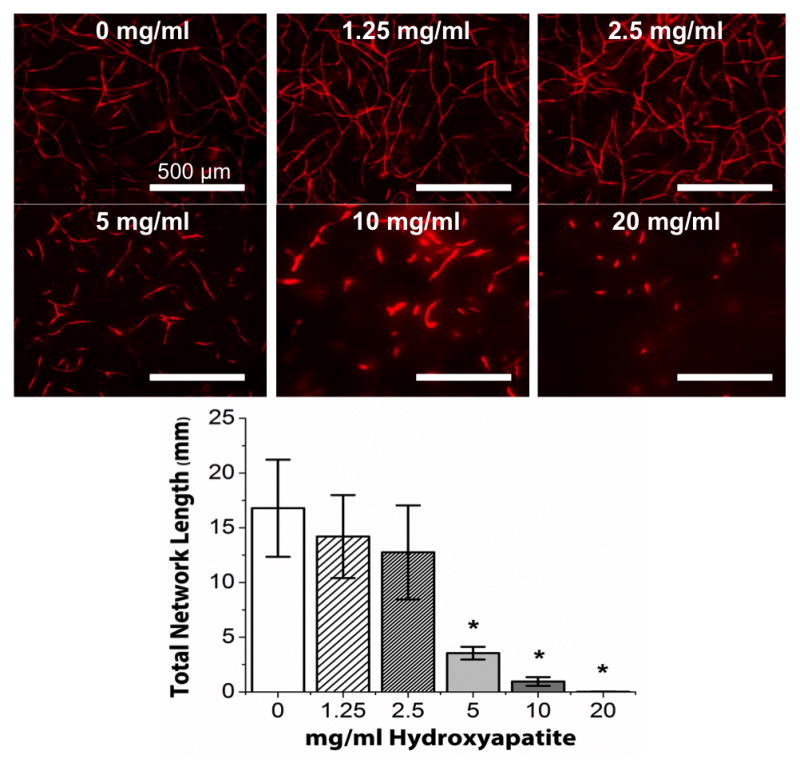
Endothelial network formation in constrained COL/FIB/HA composite hydrogels at day 7. Upper panels show representative images of network structures in COL/FIB matrices at indicated HA concentrations. Graph shows quantification of total network length at each HA concentration. n = 4. *Statistical significance (p<0.05) against 0 mg/ml (pure collagen/fibrin).
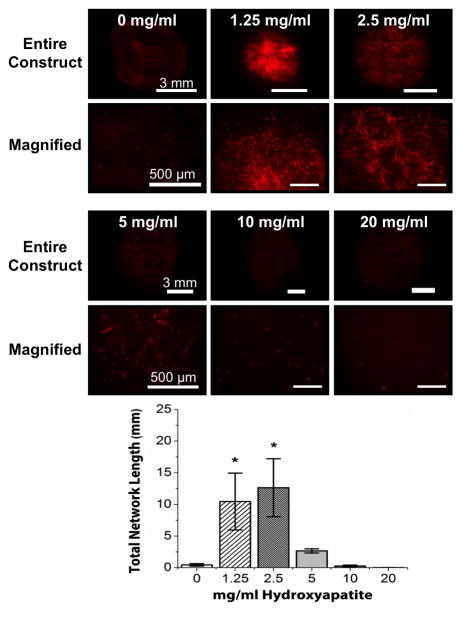
Endothelial network formation in unconstrained COL/FIB/HA hydrogels after 7 days in culture is presented in Figure 2. Images of entire constructs, as well as magnified regions of each construct type are shown in order to clearly show the extent of vasculogenesis in these compacted gels. Endothelial network formation was dramatically reduced in constructs without HA (0 mg/ml), but addition of low concentrations of HA (1.25 and 2.5 mg/ml) lead to a recovery of vascular networks. Similar to the constrained hydrogels, there was a marked decrease in endothelial network formation in the hydrogels containing high concentrations of HA (5, 10 and 20 mg/ml). Quantification of total network length in these samples confirmed that the 1.25, and 2.5 mg/ml conditions were statistically greater than controls, whereas the 5, 10, and 20 mg/ml HA groups were not statistically different from controls. Notably, the degree of vessel formation in the 1.25, and 2.5 mg/ml groups was also statistically the same as in the constrained constructs without HA.
Figure 2.
Endothelial network formation in unconstrained COL/FIB/HA composite hydrogels at day 7. Upper panels show representative images of network structures in COL/FIB matrices at indicated HA concentrations (both entire construct and magnified subsections are presented). Graph shows quantification of total network length at each HA concentration. n = 4. *Statistical significance (p<0.05) against 0 mg/ml (pure collagen/fibrin).
3.2 Hydrogel Compaction
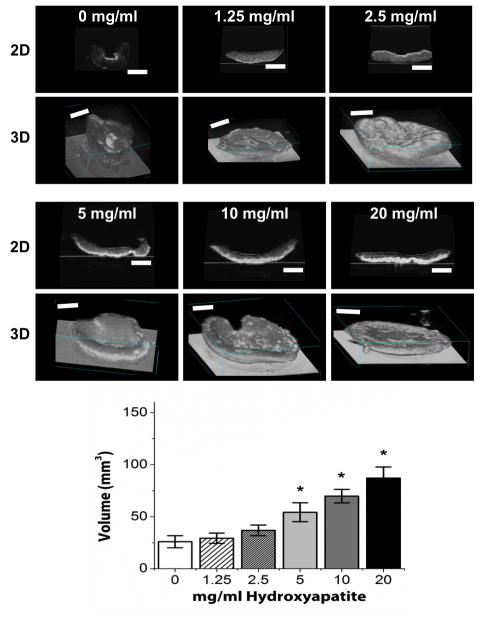
Representative images of COL/FIB/HA hydrogels at day 0 (immediately after gelation) and at day 7 in unconstrained culture are shown in Figure 3. At the initial time point (day 0) all constructs are essentially the same size because cell-mediated compaction has not yet occurred. However, by day 7 the constructs have reduced their volume to varying degrees, depending on the HA content. Resorption of the collagen matrix was not explicitly quantified, but there were no observed matrix degradation over the culture period. Figure 4 shows high-resolution three-dimensional volume renderings and two-dimensional sections of constructs at day 7, obtained through non-invasive ultrasound imaging. Control constructs without HA (0 mg/ml) compacted strongly, and the degree of compaction generally decreased with increasing HA content. Quantification of construct volume from rendered ultrasound images showed that compaction in the 1.25, and 2.5 mg/ml conditions was not statistically different than controls, whereas the 5, 10, and 20 mg/ml HA groups compacted statistically significantly less than controls.
Figure 3.
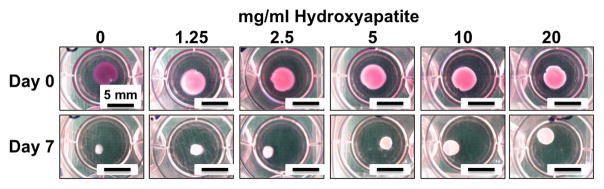
Images of COL/FIB/HA constructs in culture wells immediately after fabrication at day 0 (upper panels) and after 7 days of compaction (lower panels).
Figure 4.
Ultrasound images showing 2D sections (upper panels) and 3D volume renderings (lower panels) of COL/FIB/HA constructs after 7 days of unconstrained culture. Graph shows quantification of construct volume at each HA concentration. n = 4. *Statistical significance (p<0.05) against 0 mg/ml (pure collagen/fibrin). Scale bar = 1 mm.
3.3 Mechanical Properties of COL/FIB/HA Hydrogels
Figure 5 shows mechanical properties data for COL/FIB/HA hydrogels, including the storage moduli at day 0 as determined by gel rheology, and the elastic moduli at day 7 as determined by compressive testing. At the day 0 time point before the gels could compact, there was a statistically significant decrease in the storage modulus of the hydrogels when low concentrations of HA (1.25 and 2.5 mg/ml) were added, compared to the control 0 mg/ml condition. However, at higher HA concentrations (5, 10, 20 mg/ml), the mechanical properties at day 0 were statistically the same as control constructs. After gel compaction had occurred (day 7), compressive testing revealed that hydrogels containing 5 and 10 mg/ml hydroxyapatite were significantly stiffer than the 0 mg/ml condition, though the other conditions were not statistically different from controls without HA.
Figure 5.
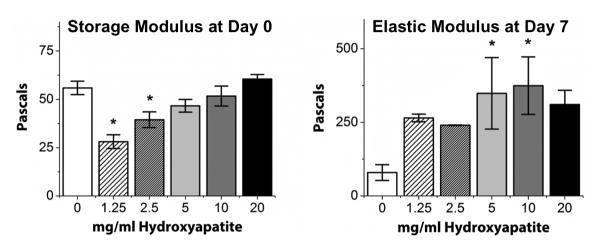
Mechanical property measurements of COL/FIB/HA matrices. Left graph shows average storage (G′) moduli measured at the time of gelation. Right graph shows average elastic moduli of compacted gels measured after 7 days of unconstrained culture. n = 4. *Statistical significance against 0 mg/ml HA (pure collagen/fibrin).
3.4 In Vivo Implantation of COL/FIB/HA Materials
Three HA concentrations, 0, 2.5, and 20 mg/ml, were chosen for implantation experiments to evaluate the effect of HA incorporation on vascularization in vivo. Laser Doppler perfusion imaging (LDPI) allowed for non-invasive quantification of blood perfusion throughout each implant at days 0 (immediately after implantation), 3, and 7, as shown in Figure 6. Perfusion to implants was increased significantly compared to baseline values in all conditions at both days 3 and 7. However, there were no significant differences between the implants containing HA and those without.
Figure 6.
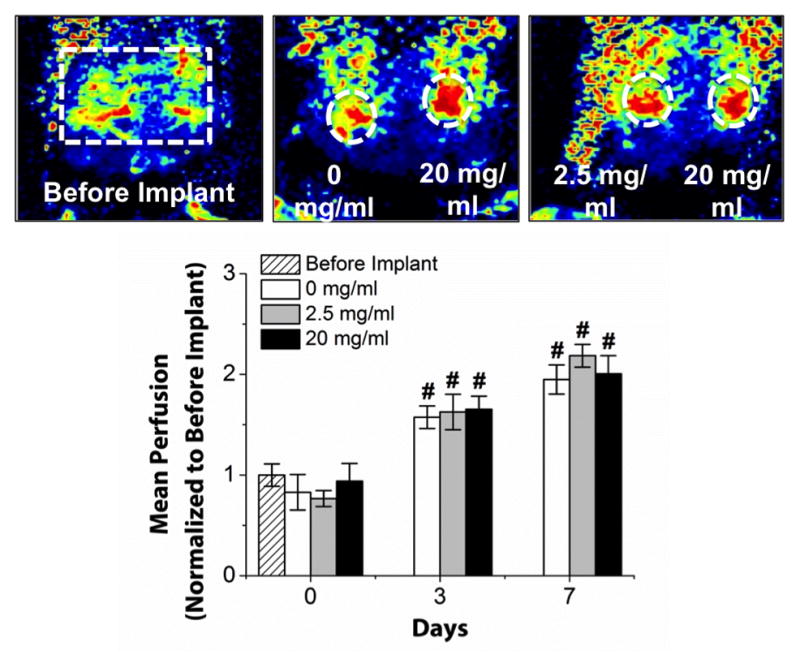
Laser Doppler perfusion imaging (LDPI) of implanted COL/FIB/HA materials. Images show LDPI heat maps indicating degree of perfusion at day 7. Graph shows quantification of mean perfusion at indicated HA concentrations. n = 5. *Statistical significance against 0 mg/ml (pure collagen/fibrin); #Statistical significance against baseline (Before Implantation).
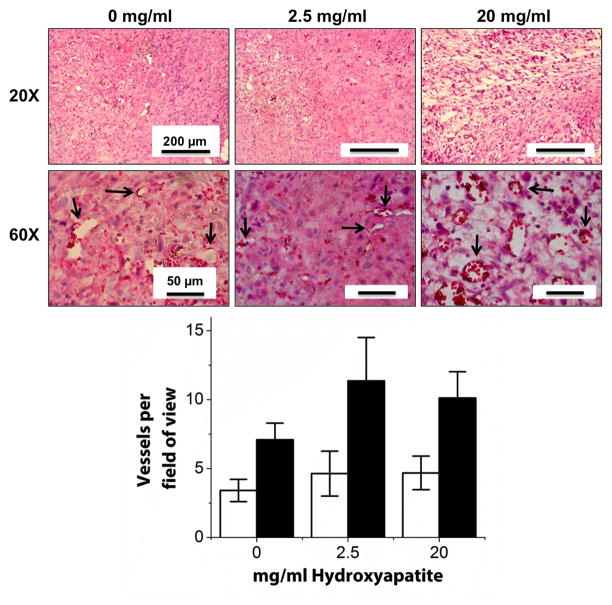
Representative human CD31 and H&E stained sections from cellular implants are shown in Figure 7. In all samples, numerous capillaries containing erythrocytes were evident throughout the implant site. Mononuclear inflammatory cells were present in all three conditions suggesting a potential inflammatory reaction to the implanted materials. Further, free erythrocytes were located throughout the implant region, particularly in the lower HA containing hydrogels. There was also a rich supply of small, positively-stained human capillaries within all of the HA conditions. Pericyte-like cells surrounding the capillaries were also observed, and demonstrated positive human α-SMA staining, confirming that they were of human origin (Figure 8). Quantification of both the number of human vessels and the total number of vessels within the implant area yielded no significant differences between the three implanted conditions (0, 2.5, and 20 mg/ml). In contrast, acellular control constructs with the same amounts of HA demonstrated no capillary formation and therefore vessel number was not quantified (data not shown). Acellular constructs also did not show positive staining for human α-SMA (data not shown).
Figure 7.
Histological analysis of COL/FIB/HA materials after 7 days of subcutaneous implantation in the mouse. Image panels show human CD31 and H&E staining at two magnifications. Arrows point to new vessels. Graph shows quantification of number of human-derived vessels (white bars) and total number of vessels (black bars). n = 5.
Figure 8.
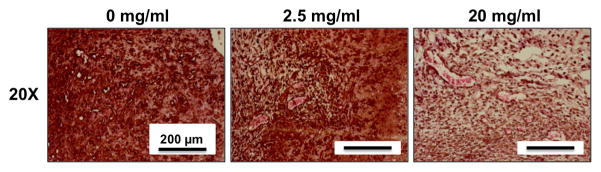
Histological analysis of COL/FIB/HA materials after 7 days of subcutaneous implantation in the mouse. Image panels show human α-SMA staining (brown).
4. Discussion
COL/FIB materials are known to support vasculogenesis, and in this study we examined the effect of incorporating HA into the matrix on endothelial network formation. In vitro, we studied both constrained and unconstrained hydrogels. In constrained constructs, addition of less than 2.5 mg/ml HA resulted in similar levels of vasculogenesis as in COL/FIB hydrogels containing no HA. However, amounts of HA above 5 mg/ml were shown to be detrimental to vasculogenesis in constrained constructs. In unconstrained hydrogels containing no HA, vascular network formation was essentially abolished. Addition of low levels of HA was successful in rescuing the vasculogenic response, even though these hydrogels compacted to a similar degree. However higher HA levels were again detrimental to network formation in unconstrained hydrogels, in spite of diminished gel compaction. This effect may have been due to changes in cell proliferation, or to a decreased paracrine stimulus. The beneficial effect of low levels of HA did not translate to in vivo implants, as all conditions resulted in similar degrees of both xenogeneic and host vasculature within the implant region.
The compaction of protein hydrogels is a well-characterized phenomenon caused by cell contractile forces that result in remodeling of the extracellular matrix. This process has been shown to cause regression of endothelial tube assembly [16] and we observed similar anti-vasculogenic effects in our unconstrained hydrogel studies. The addition of low concentrations of HA to unconstrained COL/FIB hydrogels rescued endothelial network formation to the point where it was statistically indistinguishable from control constrained gels without HA. The mechanism of action of this effect is not clear, but may be related to increased secretion of VEGF from MSC in response to the HA, increased adsorption of proangiogenic growth factors and cytokines to the HA, or modulation of matrix metalloproteinase activity. While it is possible that other calcium phosphate materials would yield a similar response, we have focused on HA because it is a widely used matrix additive in orthopaedic tissue engineering.
The relationship between the degree of vasculogenesis and the bulk mechanical properties of COL/FIB matrices has been previously investigated [7]. In the present study, matrix stiffness at day 0 was slightly reduced by the addition of low levels of HA, possibly through an effect on collagen fiber formation. However, these differences were abolished as the gel compacted. By day 7, the 5 and 10 mg/ml loading levels were stiffer than the control, but the other conditions were statistically equivalent to the control. In addition, quantitative assessment of construct volume using ultrasound imaging showed that low concentrations of HA yielded a similar degree of compaction as hydrogels without HA. Therefore the effect of HA does not seem to be mediated by mechanical properties or by changes in gel compaction, though it may have affected related factors such as matrix architecture, ligand binding density, mass transport, and matrix porosity.
In vivo implantation of COL/FIB/HA composite gels showed no beneficial effect of the HA component on perfusion of tissue in the area of the implant. These experiments used a higher cell concentration to match previous work by our group [30], as required for in vivo experiments. Perfusion values measured through LDPI increased over time in all implants, indicating increased blood flow relative to the pre-implant level. By day 7, human-derived vasculature and pericytes were evident in the implant site in all conditions, as indicated by vessels that stained positive for both human CD31 and human α-SMA. Host vasculature also infiltrated the implants, suggesting possible pro-angiogenic properties of composite COL/FIB/HA matrices. It is interesting to note that while HA was not clearly beneficial to vascular network formation in vivo, neither was it detrimental. Even the relatively high 20 mg/ml HA level, which strongly inhibited vasculogenesis in vitro, showed good perfusion and neovascularization in vivo. The differences between in vitro and in vivo results may be due to the richer and more complex environment in native tissue. Resident and recruited cells such as monocytes, leukocytes, and macrophages may secrete cytokines and other factors that mask or overcome the effects of transplanted HA.
5. Conclusions
HA is used widely as an additive and as a scaffold for promoting bone regeneration. As larger bone defects are targeted, the concomitant creation of a vasculature as the bone heals has become a main goal of orthopaedic tissue engineers. This study has demonstrated that formation of endothelial networks in COL/FIB hydrogels is affected by the culture method, and in particular that unconstrained compaction of these matrices results in a loss of vasculogenesis. This response could be recovered in unconstrained matrices in vitro by the addition of relatively low concentrations of HA. However, the effect of HA did not translate to in vivo subcutaneous implants, which were unaffected by the presence of HA. While the mechanism of HA-enhanced network formation in vitro is not clear, these findings have relevance to the development of biomaterials for bone tissue engineering. Understanding the effect of HA in protein-based and other biomaterial systems will facilitate design of scaffolds that are effective in generating the desired physiological response.
Acknowledgments
This work was supported in part by a National Science Foundation Graduate Research Fellowship (DGE 1256260, to RRR) and by the National Institutes of Health through R01-HL085339 (to AJP), R21-AR062709 (to JPS) and R01-HL118259 (to AJP and JPS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 2.Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63:300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Allen P, Melero-Martin J, Bischoff J. Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks. J Tissue Eng Regen Med. 2011;5:e74–86. doi: 10.1002/term.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietrich F, Lelkes PI. Fine-tuning of a three-dimensional microcarrier-based angiogenesis assay for the analysis of endothelial-mesenchymal cell co-cultures in fibrin and collagen gels. Angiogenesis. 2006;9:111–125. doi: 10.1007/s10456-006-9037-x. [DOI] [PubMed] [Google Scholar]
- 5.Martineau L, Doillon CJ. Angiogenic response of endothelial cells seeded dispersed versus on beads in fibrin gels. Angiogenesis. 2007;10:269–277. doi: 10.1007/s10456-007-9079-8. [DOI] [PubMed] [Google Scholar]
- 6.Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P, Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis. 2012;15:253–264. doi: 10.1007/s10456-012-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez P, Bausch AR. The compaction of gels by cells: a case of collective mechanical activity. Integr Biol (Camb) 2009;1:252–259. doi: 10.1039/b822897c. [DOI] [PubMed] [Google Scholar]
- 9.Lund AW, Bilgin CC, Hasan MA, McKeen LM, Stegemann JP, Yener B, Zaki MJ, Plopper GE. Quantification of spatial parameters in 3D cellular constructs using graph theory. J Biomed Biotechnol. 2009;2009:928286. doi: 10.1155/2009/928286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason BN, Starchenko A, Williams RM, Bonassar LJ, Reinhart-King CA. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 2013;9:4635–46447. doi: 10.1016/j.actbio.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee PF, Bai Y, Smith RL, Bayless KJ, Yeh AT. Angiogenic responses are enhanced in mechanically and microscopically characterized, microbial transglutaminase crosslinked collagen matrices with increased stiffness. Acta Biomater. 2013;9:7178–7190. doi: 10.1016/j.actbio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Critser PJ, Kreger ST, Voytik-Harbin SL, Yoder MC. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res. 2010;80:23–30. doi: 10.1016/j.mvr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghajar CM, Blevins KS, Hughes CC, George SC, Putnam AJ. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12:2875–2888. doi: 10.1089/ten.2006.12.2875. [DOI] [PubMed] [Google Scholar]
- 14.Ghajar CM, Chen X, Harris JW, Suresh V, Hughes CC, Jeon NL, Putnam AJ, George SC. The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys J. 2008;94:1930–1941. doi: 10.1529/biophysj.107.120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kniazeva E, Kachgal S, Putnam AJ. Effects of extracellular matrix density and mesenchymal stem cells on neovascularization in vivo. Tissue Eng Part A. 2011;17:905–914. doi: 10.1089/ten.tea.2010.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis GE, Pintar Allen KA, Salazar R, Maxwell SA. Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci. 2001;114:917–930. doi: 10.1242/jcs.114.5.917. [DOI] [PubMed] [Google Scholar]
- 17.Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118:2325–2340. doi: 10.1242/jcs.02360. [DOI] [PubMed] [Google Scholar]
- 18.Bi L, Jung S, Day D, Neidig K, Dusevich V, Eick D, Bonewald L. Evaluation of bone regeneration, angiogenesis, and hydroxyapatite conversion in critical-sized rat calvarial defects implanted with bioactive glass scaffolds. J Biomed Mater Res A. 2012;100:3267–3275. doi: 10.1002/jbm.a.34272. [DOI] [PubMed] [Google Scholar]
- 19.Gorustovich AA, Roether JA, Boccaccini AR. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences. Tissue Eng Part B Rev. 2010;16:199–207. doi: 10.1089/ten.TEB.2009.0416. [DOI] [PubMed] [Google Scholar]
- 20.Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249–3255. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Leu A, Leach JK. Proangiogenic potential of a collagen/bioactive glass substrate. Pharm Res. 2008;25:1222–1229. doi: 10.1007/s11095-007-9508-9. [DOI] [PubMed] [Google Scholar]
- 22.Leu A, Stieger SM, Dayton P, Ferrara KW, Leach JK. Angiogenic response to bioactive glass promotes bone healing in an irradiated calvarial defect. Tissue Eng Part A. 2009;15:877–885. doi: 10.1089/ten.tea.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pezzatini S, Solito R, Morbidelli L, Lamponi S, Boanini E, Bigi A, Ziche M. The effect of hydroxyapatite nanocrystals on microvascular endothelial cell viability and functions. J Biomed Mater Res A. 2006;76:656–663. doi: 10.1002/jbm.a.30524. [DOI] [PubMed] [Google Scholar]
- 24.Rücker M, Laschke MW, Junker D, Carvalho C, Tavassol F, Mülhaupt R, Gellrich NC, Menger MD. Vascularization and biocompatibility of scaffolds consisting of different calcium phosphate compounds. J Biomed Mater Res A. 2008;86:1002–1111. doi: 10.1002/jbm.a.31722. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Parker ST, Syoji D, Wang X, Lewis JA, Kaplan DL. Direct-write assembly of 3D silk/hydroxyapatite scaffolds for bone co-cultures. Adv Healthc Mater. 2012;1:729–735. doi: 10.1002/adhm.201200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J, Decaris ML, Leach JK. Bioceramic-mediated trophic factor secretion by mesenchymal stem cells enhances in vitro endothelial cell persistence and in vivo angiogenesis. Tissue Eng Part A. 2012;18:1520–1528. doi: 10.1089/ten.tea.2011.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Williams DJ. Incorporation of hydroxyapatite sol into collagen gel to regulate the contraction mediated by human bone marrow-derived stromal cells. IEEE Trans Nanobioscience. 2010;9:1–11. doi: 10.1109/TNB.2009.2034654. [DOI] [PubMed] [Google Scholar]
- 28.Gudur M, Rao RR, Hsiao YS, Peterson AW, Deng CX, Stegemann JP. 2012; Noninvasive, Quantitative, Spatiotemporal Characterization of Mineralization in Three-Dimensional Collagen Hydrogels Using High-Resolution Spectral Ultrasound Imaging. Tissue Eng Part C Methods. 2012;18:935–946. doi: 10.1089/ten.tec.2012.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gent AN. On the relation between indentation hardness and Young’s modulus. IRI Trans. 1958;34:46–57. [Google Scholar]
- 30.Grainger SJ, Carrion B, Ceccarelli J, Putnam AJ. Stromal cell identity influences the in vivo functionality of engineered capillary networks formed by co-delivery of endothelial cells and stromal cells. Tissue Eng Part A. 2013;19:1209–1222. doi: 10.1089/ten.tea.2012.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]