Abstract
The pathophysiological effects resulting from many bacterial diseases are caused by exotoxins released by the bacteria. Bacillus anthracis, a spore-forming bacterium, is such a pathogen, causing anthrax through a combination of bacterial infection and toxemia. B. anthracis causes natural infection in humans and animals and has been a top bioterrorism concern since the 2001 anthrax attacks in the USA. The exotoxins secreted by B. anthracis use CMG2 as the major toxin receptor and play essential roles in pathogenesis during the entire course of the disease. This review focuses on the activities of anthrax toxins and their roles in initial and late stages of anthrax infection.
Keywords: Anthrax, Capillary morphogenesis protein 2, edema toxin, lethal toxin, Tumor endothelial marker 8
Bacillus anthracis and anthrax
Bacillus anthracis is a Gram-positive, rod-shaped, spore-forming bacterium, and is the causative agent of anthrax, an acute, rapidly progressing infectious disease that affects both animals and humans. B. anthracis forms spores after the death of infected hosts. The spores can remain dormant for many years in soil and begin to grow again and secrete toxins after gaining entry into susceptible hosts. The B. anthracis spore, the infectious form of the pathogen, has long been considered as a potential warfare agent and has been a top bioterrorism concern since the 2001 anthrax attacks in the USA [1].
In addition to a single chromosome, B. anthracis contains two large extrachromosomal plasmids, pXO1 (182 kb) and pXO2 (96 kb), that are essential for its full virulence [2, 3]. The pXO1 plasmid encodes the three anthrax exotoxin components: protective antigen (PA, 83 kDa), lethal factor (LF, 89 kDa), and edema factor (EF, 90 kDa). Plasmid pXO2 encodes proteins that synthesize the unique poly-D-γ-glutamic acid capsule, which confers resistance to phagocytosis. There are three forms of anthrax disease defined by the route of spore entry into the body: cutaneous, gastrointestinal, and inhalational anthrax. Early studies showed that spores are phagocytosed by resident macrophages and dendritic cells, which may serve as a ‘Trojan horse’ to carry them from peripheral sites to local lymph nodes where they germinate to become toxin-producing vegetative bacteria [4]. Recent studies have shown a rapid localized germination event [5, 6], suggesting the bacteria overcome innate immunity resulting in systemic infection, through what has been termed a ‘jailbreak’ mechanism (for detailed review, see [7]). As major virulence factors of B. anthracis, the anthrax toxins play essential roles during multiple steps of the disease. In this review, we discuss the roles of the anthrax toxins in initiation of anthrax infection as well as their lethal effects at the late stages of the disease.
The anthrax toxin components and receptors
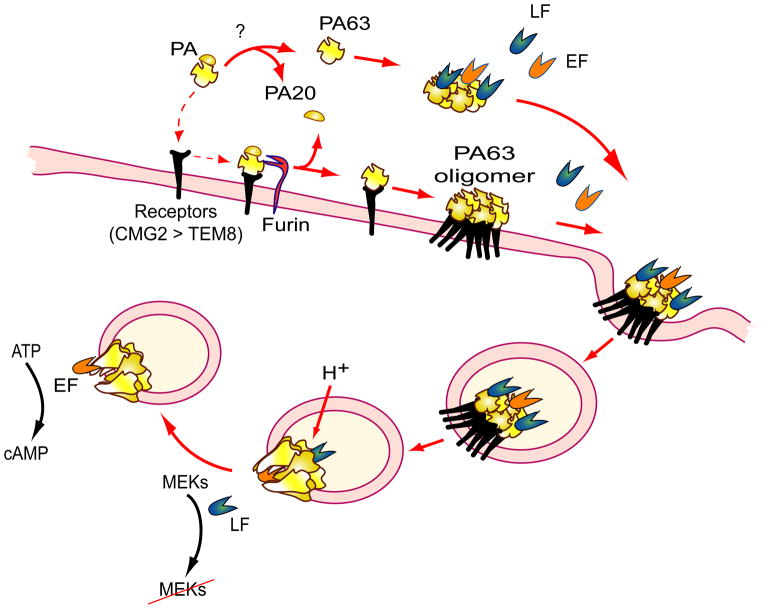
The three components of the anthrax exotoxins, PA, LF, and EF, are individually non-toxic, but they pair to form the two major virulence factors of B. anthracis: lethal toxin (LT, composed of LF + PA) and edema toxin (ET, composed of EF + PA) [8]. PA is the cellular binding moiety, and LF and EF are the catalytic moieties of the toxins. Upon being secreted by B. anthracis during infection, PA binds to its cellular receptors on target host cells and is proteolytically processed by furin or furin-like proteases into the receptor-bound carboxyl-terminal fragment PA63 and the free amino-terminal fragment PA20 (Figure 1). Release of PA20 from PA63 removes steric hindrance and allows PA63 to form a LF/EF-binding competent oligomeric (heptamer or octamer) structure [9] [10]. LF/EF-binding sites are formed by residues located on adjacent PA63 monomers [11]. Each PA63 heptamer and octamer binds 3 or 4 EF and/or LF molecules, respectively, due to steric interference between toxin molecules bound at adjacent sites [11]. The PA63 oligomer/LF and/or EF complex is then internalized through a receptor-mediated endocytic pathway [12]. In endosomes, acidic conditions induce conversion of the PA63 oligomer prepore to a protein-conducting channel through which LF and EF are translocated into the cytosol of the cells to exert their cytotoxic effects (reviewed in detail in [13]). In endosomes, the toxin complex can also be routed into intraluminal vesicles, where LF and EF are sequestered inside the vesicles (for detailed review, see [14]). In this case LF and EF can be released into the cytosol through back fusion of the intraluminal vesicles with endosome membranes. Because PA63 oligomerization triggers receptor-mediated endocytosis, only the EF/LF-binding competent PA63 oligomer, but not cell-surface bound PA monomer is internalized into cells [12, 15].
Figure 1.
Mode of action of anthrax toxins. Following secretion by B. anthracis, the anthrax toxin components distribute quickly into various tissues. Upon binding to cell-surface receptors, PA is cleaved by furin or furin-like proteases, yielding the C-terminal fragment PA63 (dashed arrows), which then spontaneously forms oligomers that gain the ability to bind LF and EF. PA can also be processed in circulation by unidentified proteases, and the resulting PA63 can oligomerize and bind LF and EF prior to binding to the toxin receptors CMG2 and TEM8 to form the PA63 oligomer-receptor complex. CMG2 is the major toxin receptor in vivo, whereas TEM8 plays only a minor role in anthrax toxin pathogenesis. The toxin complex is then internalized through the receptor-mediated endocytosis. Once inside endosomes, the toxin complex encounters an acidic environment, which induces the formation of the PA63 oligomer channel in the membrane, allowing LF and EF to enter the cytosol to exert their cytotoxic effects.
Anthrax toxin receptors
Two cell-surface mammalian proteins were identified as receptors that mediate anthrax toxin entry into host cells: tumor endothelium marker-8 (TEM8), also known as anthrax toxin receptor 1, and capillary morphogenesis protein-2 (CMG2), also named anthrax toxin receptor 2 [16, 17]. TEM8 and CMG2 are homologous cell-surface proteins containing an extracellular von Willebrand factor A (vWA) domain responsible for binding to PA as well as to their natural ligands. CMG2 has been shown to bind collagen IV, laminin, and fibronectin, whereas TEM8 was shown to interact with collagen I, gelatin, and collagen VI [18]. The physiological functions of TEM8 and CMG2 are unknown. TEM8 was first described as a tumor endothelial marker due to its upregulation in human colorectal cancer endothelium, making it a potential target for cancer therapy [19]. CMG2 was identified as one of the genes upregulated in human endothelial cells undergoing tubule formation in a 3D collagen matrix assay, suggesting a role in angiogenesis [20]. Mutations in human CMG2 cause two human autosomal recessive conditions, juvenile hyaline fibromatosis and infantile systemic hyalinosis, now described with the single term hyaline fibromatosis syndrome (HFS) [21]. These conditions are characterized by multiple subcutaneous skin nodules and gingival hypertrophy with excess extracellular protein deposition. Mutations in human TEM8 cause a human autosomal recessive condition called GAPO syndrome [22], characterized by growth retardation, alopecia (hair loss), pseudoanodontia (failure of tooth eruption), and progressive visual impairment. Histology reveals moderate excess of extracellular matrix protein deposition in many tissues from GAPO patients. Because TEM8 and CMG2 mediate receptor-driven toxin internalization, it is tempting to speculate that they may have a parallel function as physiologic receptors for cellular uptake and degradation of certain extracellular proteins, an essential process in maintaining extracellular matrix homeostasis.
CMG2- and TEM8-null mice and double null mice have been generated to characterize the roles of the two receptors in anthrax pathogenesis. Interestingly, these mice recapitulate some of the clinical signs seen in human HFS and GAPO patients. TEM8-null mice display dental dysplasia and moderate excess extracellular matrix deposition [23, 24]. CMG2-null mice have severe collagen deposition in their uteri, disabling their parturition ability [25, 26]. However, both receptor-null mice have less severe phenotypes than human HFS and GAPO patients, perhaps reflecting species differences in extracellular matrix turnover physiology.
Analyzing CMG2- and TEM8-null mice revealed that CMG2 is the physiologically relevant anthrax toxin receptor in vivo [24, 27]. CMG2-null mice are not only resistant to LT and ET challenge but also to B. anthracis infection. In contrast, TEM8-null mice remain susceptible to both the toxins and B. anthracis infection. CMG2 has a 10-fold higher binding affinity for PA than does TEM8, a fact that may in part explain the predominant role of CMG2 in anthrax pathogenesis [24]. Recently, a cell-based analysis was used to implicate another vWA domain-containing protein, integrin β1, as a third anthrax toxin receptor [28]. However, integrin β1 is unlikely to act as an anthrax toxin receptor in vivo, because CMG2 and TEM8 double knockout mice are completely resistant to anthrax toxin challenge and infection [24, 27].
In searching for host proteins required for anthrax toxin action, low density lipoprotein receptor-related protein 6 (LRP6) was reported to be a toxin co-receptor essential for toxin-induced lethality in mammalian cells [29]. However, subsequent genetic studies challenged this conclusion; mouse embryonic fibroblasts from LRP6-null mice and some other cell types with LRP6 knocked down were found to internalize toxin normally and to retain full sensitivity to anthrax toxins [30, 31]. Unfortunately, LRP6 knockout mice are not viable, precluding direct tests of the in vivo role of LRP6 in anthrax pathogenesis [30]. Therefore, the role of LRP6 in anthrax toxin action remains controversial.
Proteolytic activation of PA
The proteolytic processing of PA to PA63 is absolutely required for the action of anthrax toxins. This step was previously thought to occur solely after PA binding to cellular receptors. It is now clear that PA can also be processed by unidentified proteases in the plasma of experimental animals, leading to circulating PA63 oligomers [32]. Thus, it is believed that PA proteolytic activation and cell-surface binding are independent processes, their rates dependent on availabilities of cell-surface receptors and cell-surface and soluble proteases. The soluble PA63 oligomer and the effector proteins LF and EF may assemble a toxin complex before binding to cellular receptors [33]. A cleavage competent mutant PA defective in receptor binding protects mice from LT by forming soluble PA63 oligomers that compete to bind and deplete LF, preventing its binding to functional receptor-bound PA63 oligomers [32]. Within 1 h of PA injection into mice, only PA63 can be detected in plasma. The rapid processing of PA in plasma and tissues was also observed in mice deleted for both the CMG2 and TEM8 receptors, further demonstrating that receptor-independent PA processing occurs in blood. However, it remains uncertain whether the soluble PA63 complexes are subsequently bound and taken up with efficiencies comparable to those of complexes that form on the cell-surface following proteolytic activation of receptor-bound PA.
The biological processes targeted by LT
LF (the catalytic component of LT) is a zinc-dependent metalloproteinase that cleaves and inactivates the mitogen-activated protein kinase kinases (MEKs) 1–4, 6 and 7 [34–37]. The cleavage events result in inactivation of the three key mitogen-activated protein kinase (MAPK) pathways: ERK (through MEK1/2), p38 (through MEK3/6), and Jun N-terminus kinase (JNK) (through MEK4/7). These pathways play crucial roles in responses to a diverse array of stimuli, such as mitogens, proinflammatory cytokines, heat shock and a variety of cellular stresses. Therefore, LT causes a range of effects on cellular functions and is a major virulence factor of B. anthracis [8].
Oncogenic mutations in the RAS–RAF–MEK–ERK pathway are found in 20–25% of all human cancers. In particular, the BRAF Val600 to Glu (BRAFV600E) mutation has been documented in 60% of human melanomas, making the RAS–RAF–MEK–ERK pathway crucial for survival of these tumors [38]. Thus, many small molecule inhibitors of this pathway have been developed for targeted cancer therapy. The BRAFV600E inhibitor Vemurafenib and the MEK inhibitor Trametinib (GSK1120212) have been approved by the Food and Drug Administration (FDA) for the treatment of patients with unresectable or metastatic melanoma with BRAF mutations [39]. Interestingly, human cancer cells with a BRAFV600E mutation are also exceptionally sensitive to LT [40]. This has greatly encouraged the preclinical development of tumor-selective anthrax lethal toxins for cancer therapy [10, 41–44], a subject which is beyond the scope of this review.
Long before identification of MEKs as LF substrates, mouse macrophage cell lines RAW264.7 and J774 were found to be uniquely sensitive to LT. These cells can be killed by LF in as little as 90 min [8]. Thus macrophages were initially thought to be the major target of LT in anthrax infection. Later, macrophages from certain mouse and rat strains (such as BALBc mice and Fischer rats) were found to share the same high sensitivity to LT as RAW264.7 cells, whereas macrophages from other strains (such as C57BL6 mice and Lewis rats) were resistant [2]. The sensitivity of rodent macrophages to LT is not linked to the cleavage of MEKs by LT, but instead is determined by polymorphisms in a single gene, Nlrp1 in rats and Nlrp1b in mice [45, 46]. Nlrp1 encodes the NOD-like receptor (NLR) protein NLRP1, the sensor component of the NLRP1 inflammasome, a multiprotein complex responsible for activation of caspase-1. The rat NLRP1 and mouse NLRP1b proteins from LT-sensitive strains can be directly cleaved by LF at sites near their amino-termini, resulting in activation of the NLRP1 inflammasomes [47–49], eliciting a caspase 1-mediated rapid macrophage cell death, termed pyroptosis. The NLRP1/NLRP1b from LT-resistant strains cannot be cleaved by LT to induce pyroptosis [2].
However, mice are killed by LT over days in a manner independent of their macrophage sensitivity to toxin, suggesting that other cell types play a more important role in LT-induced lethality [50, 51]. In contrast, rats are killed in a manner directly correlated to their NLRP1 sequence and thus, the sensitivity of their macrophages to toxin [45, 46]. Interestingly, because sensitive rats succumb to the toxin in one hour, and not over days, it appears that despite a similar mechanism of inflammasome-mediated macrophage pyroptosis, the two rodent species undergo very different mechanisms leading to lethality. The way in which macrophage sensitivity to LT contributes to the rapid death of rats is not currently understood, while genetic studies described in the next section show that murine macrophage sensitivity does not contribute to LT-induced mouse death.
Paradoxically, a strong inverse correlation between mouse sensitivity to B. anthracis infection and the sensitivity of their macrophages to LT has been observed; mice with LT-sensitive macrophages are more resistant to B. anthracis infection than mice with LT-resistant macrophages [52, 53]. This is likely due to the manner in which LT induces macrophage pyroptosis. Cleavage of Nlrp1b proteins results in activation of the inflammasome and caspase-1 only in mice harboring LT-sensitive macrophages [47–49], and activated caspase-1 releases the inflammatory cytokines, interleukin (IL)-1β and IL-18. These cytokines induce a potent innate immune response against infection which results in a protective effect. Interestingly, human macrophages are resistant to LT-induced pyroptosis, suggesting that humans, like certain mouse and rat strains, may not have the protective pyroptosis-enhanced inflammatory responses against anthrax infection. The striking differences in response to LT among inbred rodent strains, as well as between species, suggests that animals have evolved under strong evolutionary pressures, likely dictated by the inflammatory responses controlled by the toxin.
The biological processes targeted by ET
EF, the catalytic component of ET, is a calmodulin-dependent adenylyl cyclase that elevates intracellular cAMP levels by converting ATP to cAMP, the classical second messenger, thereby causing diverse effects. Whereas subcutaneous injection of ET into mice causes dramatic skin edema, its systemic administration can induce fluid influx into the intestinal lumen, liver edema, extensive tissue damage, and death [54, 55]. For mice, the minimum lethal dose of ET (20~30 μg) is lower than that of LT (40~100 μg), emphasizing the importance of ET as a major virulence factor of B. anthracis. However, the relative importance of ET vs. LT in anthrax infections remains uncertain. LF and EF have been measured in the blood of infected animals. In nearly every successful measurement of toxin levels, LF concentrations exceeded those of EF. A recent analysis of in vivo toxin levels in anthrax-infected rabbits found LF at 10–35 μg/ml by 48 h and EF at about 5-fold lower concentrations [56]. The 5:1 ratio of LF:EF has also been found in other anthrax infection models [57]. Thus, it is possible that lethal doses of ET are not reached in anthrax infection, or only occur at very late stages.
The mechanisms linking cAMP elevation and ET-induced damage are poorly understood. cAMP has at least two distinct intracellular targets: protein kinase A (PKA) and the exchange protein activated by cAMP (Epac), which is a guanine nucleotide exchange factor for the small G-proteins Rap1 and Rap2. The PKA pathway is well studied. PKA exists as an inactive tetramer consisting of two regulatory and two catalytic subunits. Binding of cAMP to the regulatory subunits causes their dissociation from the two catalytic monomers of PKA, which are then activated by ATP binding and are able to phosphorylate serine and threonine residues on many substrate proteins. Cholera toxin, another bacterial toxin that increases cAMP, causes intestinal fluid secretion by activating cystic fibrosis transmembrane conductance regulator (CFTR) [58], suggesting that the ET-induced intestinal fluid influx is likely to follow the same mechanism. CFTR is an ATP-binding cassette transporter-class chloride ion channel found in epithelial cells of many organs, including the lung, liver, digestive tract, and skin. Activated PKA phosphorylates CFTR in intestinal epithelial cells, which leads to ATP-mediated efflux of Cl− ions to the intestinal lumen followed by secretion of Na+ into the intestinal lumen. This increases the total electrolyte concentration in the intestinal lumen, resulting in movement of water out of enterocytes and into intestinal lumen by osmosis [58]. Whether CFTR activation is also the cause of ET-induced liver and skin edema requires further investigation. While all cAMP-mediated effects were originally attributed to PKA activation, it was subsequently recognized that activation of the Epac proteins also plays an important role [59]. It should be noted that PKA and Epac knockout mice have been generated and are viable [60–62]. Analyzing the response of these mice to ET challenges should provide important insights into understanding the mechanisms of ET pathogenesis.
EF is an extremely efficient adenylyl cyclase, able to convert 1000–2000 molecules of ATP to cAMP per second [63]. Therefore, in addition to increasing cellular cAMP, ET may also deplete cellular ATP, thereby affecting a variety of biological processes depending on ATP, such as vesicular trafficking. Through strong expression of either LF or EF in the larval wing of Drosophila melanogaster, a recent study implicated the Rab11/Sec15 exocyst, which acts at the last step of endocytic recycling, as a novel target of both anthrax toxins. EF displayed stronger effects than LF in this model [64]. EF was found to reduce levels of apically localized Rab11 and indirectly block vesicle formation by its binding partner and effector Sec15, whereas LF was found to act more directly to reduce Sec15 vesicles. Although it should be considered that the effects of EF in this model could be partially due to ATP depletion, a recent study also showed similar effects with cholera toxin, with the effects being dependent on direct cAMP effects on downstream G-proteins [65].
In addition to its direct effects on targeted cells, ET can also increase the sensitivity of the host to the anthrax toxins through up-regulation of the toxin receptors [66, 67]. Both TEM8 and CMG2 can be strongly up-regulated by ET through PKA-dependent pathways. ET can also impair general protein clearance in mice through an as-yet-undefined mechanism [67], thus potentially prolonging the plasma half-life of all three anthrax toxin components, PA, LF, and EF. Therefore, ET and LT may coordinately exert their toxic effects in anthrax pathogenesis.
Recently, EF was shown to have broad substrate specificity, cyclizing other nucleotide triphosphates, particularly CTP to cCMP (see review in [68]). However, how these broad activities of EF contribute to pathogenesis remains unknown.
Essential roles of anthrax toxins in anthrax infection
To establish a successful infection, B. anthracis must overcome the powerful scavenger functions of the host innate immune response. Increasing evidence has shown that anthrax LT and ET not only directly cause tissue damage and lethality at late stages of infection but also play essential roles in impairing the host innate immune response at the initial stage of infection to ensure a systemic infection.
The roles of anthrax toxins in the early stage of anthrax infection
Anthrax toxin-based vaccines can efficiently protect experimental animals from B. anthracis infections, indicating that anthrax toxins are major virulence factors at the initial stage of anthrax infection (for review, see [69]). To study the roles of anthrax toxin targeting of phagocytic cells and innate immune first responders early in infection, myeloid-lineage specific CMG2-null mice were generated [70]. Macrophages and neutrophils isolated from these mice are resistant to anthrax toxins, demonstrating that these myeloid-specific CMG2-null mice have an anthrax toxin-resistant innate immune system. Consequently, these myeloid-specific CMG2-null mice are completely resistant to challenge with both B. anthracis Sterne strain spores and vegetative bacteria, whereas wild-type mice are highly susceptible [70] (Figure 2). These myeloid-specific CMG2-null mice retain full sensitivity to both LT and ET [70]. These data reveal that toxin action on myeloid cells is essential for B. anthracis to evade the scavenging functions of the myeloid cells, but is not responsible for anthrax toxin-induced lethality. Among the myeloid cell types, neutrophils appear to be the major player in preventing anthrax infection [52, 70, 71]. To evaluate the relative roles ET and LT play in crippling innate defense, B. anthracis Sterne strains with separate deletions of the PA, LF, and EF genes were tested for mouse infectivity. Deletion of either PA or LF made the B. anthracis Sterne strain completely avirulent, whereas deletion of EF caused only a 10-fold loss of infectivity, indicating that LT plays a more important role in establishing infection [70]. Whether the modest contribution of ET to virulence in this model can be explained by the ET-induced upregulation of toxin receptors and the prolonged plasma half-life of the toxins as discussed above needs to be investigated. A recent study using encapsulated virulent B. anthracis strains suggested a prominent role for ET in B. anthracis dissemination early in infections [72]. The molecular mechanisms underlying the impact of the toxins on the innate immune system remain poorly understood. Both LT and ET have been reported to affect the normal functions of neutrophils, macrophages, dendritic cells, mast cells, and other myeloid cells (please see the recent reviews [73–76]). How these effects, which were mostly described in isolated cell populations, relate to in vivo infections remains to be determined.
Figure 2.
The roles of anthrax toxins in infection. Upon entering a host through various routes, B. anthracis spores may germinate locally or be carried to local lymph nodes and germinate there. The vegetative bacteria then secrete lethal toxin (LT = PA + LF) and edema toxin (ET = PA + EF), which act through binding to CMG2 receptors to incapacitate the scavenger functions of myeloid cells (such as neutrophils and macrophages). This allows the expanding population of vegetative bacteria to evade the powerful anti-bacterial functions of neutrophils and macrophages. At late stages, following dissemination and establishment of a systemic infection, B. anthracis multiplies to high numbers in the blood, producing sufficient amounts of LT and ET to cause host mortality. LT causes host lethality through targeting the cardiovascular system, in particular cardiomyocytes and vascular smooth muscle cells, whereas ET induces lethality mostly by targeting hepatocytes.
How the myeloid-specific CMG2-null mice described above respond to infection with fully virulent encapsulated B. anthracis strains remains to be determined. It should be noted that compared to other experimental animals such as guinea pigs and rabbits, mice are uniquely susceptible to inhalational infection with encapsulated, non-toxinogenic B. anthracis strains, possibly due to the absence in mouse epithelium of the highly bactericidal enzyme sPLA2-IIa (secretory phospholipase A2 group 2a; see review in [77]). Therefore, determining the roles of the toxins in mouse infection models with virulent strains may require careful adjustment of challenge doses administered by various routes.
The roles of anthrax toxins in late stages of infection
Anthrax toxins not only play essential roles in initiation of anthrax infection, but also directly cause host lethality at the terminal stage of infection. Experimental animals challenged with anthrax toxins manifest the symptoms shown in infected animals [8, 50]. Even though B. anthracis bacteria can be successfully eliminated by antibiotic treatment, the toxins released into circulation may still cause host death. LT was shown to induce a hypoxia-mediated toxicity and lethality [50], proposed to be due to its effects on various tissues, including the endothelium, heart, and gastrointestinal tract in various animal models [64, 78–83]. ET can also induce extensive tissue damage and lethality [54, 84]. Because the consequences of targeting specific tissues or cell-types cannot be accurately assessed in systemic challenge models, various cell-type specific CMG2-null mice as well as the corresponding cell-type specific CMG2-expressing mice were generated and used to identify the key tissue targets of LT and ET [55]. Mice lacking CMG2 in cardiomyocytes and vascular smooth muscle cells were found to be highly resistant to LT, and mice with CMG2 expressed only in these cell-types were found to have the same sensitivity to the toxin as wild-type mice. Therefore, the lethality induced by LT occurs primarily through targeting cardiomyocytes and vascular smooth muscle cells rather than other cell-types (Figure 2). Interestingly, hepatocyte-specific CMG2-null mice and endothelial cell-specific CMG2-null mice remain fully sensitive to LT, and the corresponding cell-type specific CMG2-expressing mice are resistant to LT, demonstrating that unlike what had been hypothesized, hepatocytes and endothelial cells are not major targets of LT in vivo.
Studies with cell-type specific receptor-expressing and null mice also identified the liver as a major organ responsible for ET-induced lethality, whereas endothelial cells, again, do not appear to be a major target of this toxin [55]. Interestingly, ET induces significant liver edema by acting directly on hepatocytes rather than on adjacent endothelial and vascular smooth muscle cells [55]. Furthermore, the ET-induced fluid influx into the intestinal lumen could be attributed to targeting intestinal epithelial cells but not endothelial cells and vascular smooth muscle cells. Similarly, ET-induced skin edema is also due to directly targeting cell-types other than endothelial cells and vascular smooth muscle cells. These results suggest that ET-induced tissue edema is not due to direct targeting of endothelial cells, but instead probably results from increased water influx into interstitial compartments through CFTR or other ion channels via an unidentified cAMP-dependent process. Although hepatocyte-specific CMG2-null mice mostly survived an ET dose lethal to wild-type mice, all the challenged mice displayed some temporary symptoms and 20% of the mice eventually succumbed. This indicates that while the liver is a major target, ET also causes significant toxic effects through damaging other unidentified tissues. Although the intestinal epithelial cell-specific CMG2-null mice do not develop intestinal fluid influx, these mice remain susceptible to ET-induced lethality, suggesting that targeting intestines is not absolutely required in ET pathogenesis.
The above data reveal that B. anthracis has evolved to use LT and ET to induce host lethality by coordinately damaging at least two distinct vital systems, cardiovascular system and liver, respectively. However, the mechanisms underlying how LT and ET damage cardiomyocytes and hepatocytes remain to be determined. In addition to the crucial roles of anthrax toxins in anthrax infection as discussed above, the high levels of bacteremia, the structural components of B. anthracis (such as capsule, peptidoglycan, etc.) released into the circulation and the host septic responses at late stages of infection are also believed to contribute to the lethality of anthrax disease (see review in [85]).
Anthrax treatment and prevention
B. anthracis is sensitive to many antibiotics including ciprofloxacin, doxycycline, erythromycin, vancomycin, and penicillin [86]. However, due to the rapid course of the disease, even with intense antibiotic and supportive therapies, mortality rates for inhalational anthrax are still high. This is presumably due to the continuing effects of the anthrax toxins [87]. Therefore, anti-toxin therapies are needed to increase survival. Immune globulin isolated from the plasma of volunteers given BioThrax (anthrax vaccine absorbed, AVA) was shown to improve survival of a patient with naturally-acquired inhalational anthrax [88]. In 2012 the FDA approved Abthrax (Raxibacumab), the first human monoclonal antibody against PA, for treatment of inhalational anthrax [89]. Abthrax binds to domain 4 of PA and blocks toxin binding to its receptors. Several additional human and chimpanzee monoclonal antibodies against PA, LF and EF are in clinical development (for review see [90]). Recently, a fusion of the extracellular domain of human CMG2 and human IgG Fc fragment (CMG2-Fc) was shown to be effective in neutralizing PA and protecting rabbits from B. anthracis infection [91]. One potential advantage of CMG2-Fc is that deliberately engineered B. anthracis strains having variants of PA that evade individual neutralizing anti-PA monoclonal antibodies would remain subject to inhibition by CMG2-Fc. Small molecule inhibitors of LF are also under active development [92]. Adefovir dipivoxyl, an ATP analogue used for hepatitis B treatment in humans, was found to be an effective inhibitor of EF in vitro and in some in vivo models [72, 93, 94]. One obvious advantage of these small molecule inhibitors of LF and EF is that they should be effective against the toxins that have already gained entry into the cytosol of target cells.
Active immunization is pivotal in anthrax pre-exposure prevention. BioThrax is thus far the only FDA-licensed anthrax toxin-based vaccine available for pre-exposure use. BioThrax is also useful for post-exposure prophylaxis. Thus, for treatment of persons with known or suspected exposure to B. anthracis spores, the US Centers for Disease Control recommends a combination of 60-day oral antibiotic treatment with a 3-dose BioThrax vaccination. BioThrax is an incompletely-defined vaccine, produced from culture filtrates of an avirulent, non-encapsulated B. anthracis strain, with PA as the major component. More defined and recombinant PA-based anthrax vaccines are currently under active development [95–98].
Concluding remarks
B. anthracis causes anthrax through a combination of bacterial infection and toxemia. The exotoxins secreted by B. anthracis play essential roles during disease. At the initial stages of infection, LT and ET coordinately impair the host innate immune response, enabling the pathogen to establish a successful infection. At late stages, when high concentrations are reached, LT and ET can directly cause host death through targeting distinct vital systems, in particular the cardiovascular system and the liver. Although the enzymatic activities and the molecular targets of LF and EF have been known for some time, the detailed mechanisms underlying the toxin-induced tissue damage are still poorly understood. Several questions still need to be addressed to fill the gaps in our knowledge (Box 1). Toxin receptor gene-targeted mice have proven very useful in dissecting the roles of the toxins in lethality. Other mouse models having alterations to cytosolic targets and downstream signal transduction pathways can be expected to identify key steps in toxin-induced pathogenesis. In particular, mice having toxin-resistant MEKs or knockouts of PKA or Epac can be used to gain a better understanding of anthrax toxin-mediated pathogenesis.
Box 1. Outstanding questions.
Which specific MEK and MAPK pathways are crucial for LT-induced innate immune suppression?
Which specific MEK and MAPK pathways are crucial for LT-induced lethality?
How does LT affect cardiomyocyte and vascular smooth muscle cell function?
What are the roles of activation of PKA and Epac in ET-induced toxicity?
How does ET affect hepatocyte function?
How does ET induce tissue edema and fluid secretion into intestinal lumen?
Highlights.
CMG2 is the physiologically-relevant receptor for anthrax toxins in vivo.
Targeting of innate immunity by the toxins is essential for initiation of infection
Anthrax toxins directly induce lethality at the terminal stage of infection.
The cardiovascular system and liver are major targets for anthrax toxins.
Acknowledgments
This work was supported by the intramural research programs of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hudson MJ, et al. Bacillus anthracis: balancing innocent research with dual-use potential. Int J Med Microbiol. 2008;298:345–364. doi: 10.1016/j.ijmm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leppla SH. Chapter 281 - Anthrax Lethal Factor. In: Rawlings ND, Salvesen GS, editors. Handbook of Proteolytic Enzymes. 3. Academic Press; 2013. pp. 1257–1261. [Google Scholar]
- 3.Fouet A, Mock M. Regulatory networks for virulence and persistence of Bacillus anthracis. Current opinion in microbiology. 2006;9:160–166. doi: 10.1016/j.mib.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Guidi-Rontani C. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends Microbiol. 2002;10:405. doi: 10.1016/s0966-842x(02)02422-8. [DOI] [PubMed] [Google Scholar]
- 5.Corre JP, et al. In vivo germination of Bacillus anthracis spores during murine cutaneous infection. The Journal of infectious diseases. 2013;207:450–457. doi: 10.1093/infdis/jis686. [DOI] [PubMed] [Google Scholar]
- 6.Okugawa S, et al. Lipoprotein biosynthesis by prolipoprotein diacylglyceryl transferase is required for efficient spore germination and full virulence of Bacillus anthracis. Mol Microbiol. 2012;83:96–109. doi: 10.1111/j.1365-2958.2011.07915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner ZP, Glomski IJ. Updating perspectives on the initiation of Bacillus anthracis growth and dissemination through Its host. Infect Immun. 2012;80:1626–1633. doi: 10.1128/IAI.06061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moayeri M, Leppla SH. Anthrax toxins. In: Bergman NH, editor. Bacillus anthracis and anthrax. 1. John Wiley & Sons, Inc; 2011. pp. 121–156. [Google Scholar]
- 9.Kintzer AF, et al. The protective antigen component of anthrax toxin forms functional octameric complexes. J Mol Biol. 2009;392:614–629. doi: 10.1016/j.jmb.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips DD, et al. Engineering anthrax toxin variants that exclusively form octamers, and their application to targeting tumors. J Biol Chem. 2013;288:9058–9065. doi: 10.1074/jbc.M113.452110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young JA, Collier RJ. Anthrax toxin: receptor-binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 12.Abrami L, et al. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol. 2003;160:321–328. doi: 10.1083/jcb.200211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collier RJ. Membrane translocation by anthrax toxin. Mol Aspects Med. 2009;30:413–422. doi: 10.1016/j.mam.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Der Goot G, Young JA. Receptors of anthrax toxin and cell entry. Mol Aspects Med. 2009;30:406–412. doi: 10.1016/j.mam.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S, Leppla SH. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J Biol Chem. 2003;278:5227–5234. doi: 10.1074/jbc.M210321200. [DOI] [PubMed] [Google Scholar]
- 16.Bradley KA, et al. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 17.Scobie HM, et al. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci USA. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cryan LM, Rogers MS. Targeting the anthrax receptors, TEM-8 and CMG-2, for anti-angiogenic therapy. Front Biosci. 2011;16:1574–1588. doi: 10.2741/3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhary A, et al. TEM8/ANTXR1 blockade inhibits pathological angiogenesis and potentiates tumoricidal responses against multiple cancer types. Cancer Cell. 2012;21:212–226. doi: 10.1016/j.ccr.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell SE, et al. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114:2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- 21.Deuquet J, et al. The dark sides of capillary morphogenesis gene 2. EMBO J. 2012;31:3–13. doi: 10.1038/emboj.2011.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stranecky V, et al. Mutations in ANTXR1 Cause GAPO Syndrome. Am J Hum Genet. 2013;92:792–799. doi: 10.1016/j.ajhg.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullen M, et al. Host-derived tumor endothelial marker 8 promotes the growth of melanoma. Cancer Res. 2009;69:6021–6026. doi: 10.1158/0008-5472.CAN-09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, et al. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci USA. 2009;106:12424–12429. doi: 10.1073/pnas.0905409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reeves CV, et al. Anthrax toxin receptor 2 functions in ECM homeostasis of the murine reproductive tract and promotes MMP activity. PLoS ONE. 2012;7:e34862. doi: 10.1371/journal.pone.0034862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters DE, et al. Capillary morphogenesis protein-2 is required for mouse parturition by maintaining uterine collagen homeostasis. Biochem Biophys Res Commun. 2012;422:393–397. doi: 10.1016/j.bbrc.2012.04.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S, et al. The receptors that mediate the direct lethality of anthrax toxin. Toxins (Basel) 2012;5:1–8. doi: 10.3390/toxins5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martchenko M, et al. Heterodimeric integrin complexes containing {beta}1-integrin promote internalization and lethality of anthrax toxin. Proc Natl Acad Sci USA. 2010;107:15583–15588. doi: 10.1073/pnas.1010145107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei W, et al. The LDL receptor-related protein LRP6 mediates internalization and lethality of anthrax toxin. Cell. 2006;124:1141–1154. doi: 10.1016/j.cell.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 30.Young JJ, et al. LRP5 and LRP6 are not required for protective antigen-mediated internalization or lethality of anthrax lethal toxin. PLoS Pathog. 2007;3:e27. doi: 10.1371/journal.ppat.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan PL, Young JA. Evidence against a human cell-specific role for LRP6 in anthrax toxin entry. PLoS ONE. 2008;3:e1817. doi: 10.1371/journal.pone.0001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moayeri M, et al. Anthrax protective antigen cleavage and clearance from the blood of mice and rats. Infect Immun. 2007;75:5175–5184. doi: 10.1128/IAI.00719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vuyisich M, et al. Binding and cell intoxication studies of anthrax lethal toxin. Mol Biol Rep. 2012;39:5897–5903. doi: 10.1007/s11033-011-1401-2. [DOI] [PubMed] [Google Scholar]
- 34.Duesbery NS, et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 35.Lee CS, et al. MEK2 Is sufficient but not necessary for proliferation and anchorage-independent growth of SK-MEL-28 melanoma cells. PLoS ONE. 2011;6:e17165. doi: 10.1371/journal.pone.0017165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitale G, et al. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- 37.Vitale G, et al. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem J. 2000;352(Pt 3):739–745. [PMC free article] [PubMed] [Google Scholar]
- 38.Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 39.Bollag G, et al. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov. 2012;11:873–886. doi: 10.1038/nrd3847. [DOI] [PubMed] [Google Scholar]
- 40.Abi-Habib RJ, et al. BRAF status and mitogen-activated protein/extracellular signal-regulated kinase kinase 1/2 activity indicate sensitivity of melanoma cells to anthrax lethal toxin. Mol Cancer There. 2005;4:1303–1310. doi: 10.1158/1535-7163.MCT-05-0145. [DOI] [PubMed] [Google Scholar]
- 41.Schafer JM, et al. Efficient targeting of head and neck squamous cell carcinoma by systemic administration of a dual uPA and MMP-activated engineered anthrax toxin. PLoS ONE. 2011;6:e20532. doi: 10.1371/journal.pone.0020532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S, et al. Intermolecular complementation achieves high-specificity tumor targeting by anthrax toxin. Nat Biotechnol. 2005;23:725–730. doi: 10.1038/nbt1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S, et al. Matrix metalloproteinase-activated anthrax lethal toxin demonstrates high potency in targeting tumor vasculature. J Biol Chem. 2008;283:529–540. doi: 10.1074/jbc.M707419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kassab E, et al. Cytotoxicity of anthrax lethal toxin to human acute myeloid leukemia cells is nonapoptotic and dependent on extracellular signal-regulated kinase 1/2 activity. Transl Oncol. 2013;6:25–32. doi: 10.1593/tlo.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newman ZL, et al. Anthrax lethal toxin activates the inflammasome in sensitive rat macrophages. Biochem Biophys Res Commun. 2010;398:785–789. doi: 10.1016/j.bbrc.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newman ZL, et al. Susceptibility to anthrax lethal toxin-induced rat death is controlled by a single chromosome 10 locus that includes rNlrp1. PLoS Pathog. 2010;6:e1000906. doi: 10.1371/journal.ppat.1000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levinsohn JL, et al. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 2012;8:e1002638. doi: 10.1371/journal.ppat.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hellmich KA, et al. Anthrax lethal factor cleaves mouse Nlrp1b in both toxin-sensitive and toxin-resistant macrophages. PLoS ONE. 2012;7:e49741. doi: 10.1371/journal.pone.0049741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chavarria-Smith J, Vance RE. Direct proteolytic cleavage of NLRP1B is necessary and sufficient for inflammasome activation by anthrax lethal factor. PLoS pathogens. 2013;9:e1003452. doi: 10.1371/journal.ppat.1003452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moayeri M, et al. Bacillus anthracis lethal toxin induces TNF-α-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moayeri M, et al. Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect Immun. 2004;72:4439–4447. doi: 10.1128/IAI.72.8.4439-4447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moayeri M, et al. Inflammasome sensor Nlrp1b-dependent resistance to anthrax is mediated by caspase-1, IL-1 signaling and neutrophil recruitment. PLoS Pathog. 2010;6:e1001222. doi: 10.1371/journal.ppat.1001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terra JK, et al. Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol. 2010;184:17–20. doi: 10.4049/jimmunol.0903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Firoved AM, et al. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am J Pathol. 2005;167:1309–1320. doi: 10.1016/S0002-9440(10)61218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S, et al. Key tissue targets responsible for anthrax-toxin-induced lethality. Nature. 2013;501:63–68. doi: 10.1038/nature12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dal Molin F, et al. Ratio of lethal and edema factors in rabbit systemic anthrax. Toxicon. 2008;52:824–828. doi: 10.1016/j.toxicon.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Mabry R, et al. Detection of anthrax toxin in the serum of animals infected with Bacillus anthracis by using engineered immunoassays. Clin Vaccine Immunol. 2006;13:671–677. doi: 10.1128/CVI.00023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muanprasat C, Chatsudthipong V. Cholera: pathophysiology and emerging therapeutic targets. Future medicinal chemistry. 2013;5:781–798. doi: 10.4155/fmc.13.42. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt M, et al. Exchange protein directly activated by cAMP (Epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacological reviews. 2013;65:670–709. doi: 10.1124/pr.110.003707. [DOI] [PubMed] [Google Scholar]
- 60.Kirschner LS, et al. Mouse models of altered protein kinase A signaling. Endocrine-related cancer. 2009;16:773–793. doi: 10.1677/ERC-09-0068. [DOI] [PubMed] [Google Scholar]
- 61.Yan J, et al. Enhanced leptin sensitivity, reduced adiposity, and improved glucose homeostasis in mice lacking exchange protein directly activated by cyclic AMP isoform 1. Molecular and cellular biology. 2013;33:918–926. doi: 10.1128/MCB.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song WJ, et al. Pancreatic beta-cell response to increased metabolic demand and to pharmacologic secretagogues requires EPAC2A. Diabetes. 2013;62:2796–2807. doi: 10.2337/db12-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen Y, et al. Physiological calcium concentrations regulate calmodulin binding and catalysis of adenylyl cyclase exotoxins. EMBO J. 2002;21:6721–6732. doi: 10.1093/emboj/cdf681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guichard A, et al. Anthrax toxins cooperatively inhibit endocytic recycling by the Rab11/Sec15 exocyst. Nature. 2010;467:854–858. doi: 10.1038/nature09446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guichard A, et al. Cholera toxin disrupts barrier function by inhibiting exocyst-mediated trafficking of host proteins to intestinal cell junctions. Cell host & microbe. 2013;14:294–305. doi: 10.1016/j.chom.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maldonado-Arocho FJ, et al. Anthrax oedema toxin induces anthrax toxin receptor expression in monocyte-derived cells. Mol Microbiol. 2006;61:324–337. doi: 10.1111/j.1365-2958.2006.05232.x. [DOI] [PubMed] [Google Scholar]
- 67.Sastalla I, et al. Anthrax edema toxin impairs protein clearance in mice. Infect Immun. 2011;80:529–538. doi: 10.1128/IAI.05947-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gottle M, et al. Bacillus anthracis edema factor substrate specificity: Evidence for new modes of action. Toxins (Basel) 2012;4:505–535. doi: 10.3390/toxins4070505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tournier JN, et al. Anthrax, toxins and vaccines: a 125-year journey targeting Bacillus anthracis. Expert Rev Anti Infect There. 2009;7:219–236. doi: 10.1586/14787210.7.2.219. [DOI] [PubMed] [Google Scholar]
- 70.Liu S, et al. Anthrax toxin targeting of myeloid cells through the CMG2 receptor is essential for establishment of Bacillus anthracis infections in mice. Cell Host Microbe. 2010;8:455–462. doi: 10.1016/j.chom.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weiner ZP, et al. Circulating lethal toxin decreases the ability of neutrophils to respond to Bacillus anthracis. Cellular microbiology. 2013 doi: 10.1111/cmi.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dumetz F, et al. Noninvasive imaging technologies reveal edema toxin as a key virulence factor in anthrax. Am J Pathol. 2011;178:2523–2535. doi: 10.1016/j.ajpath.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tournier JN, et al. Anthrax toxins: a weapon to systematically dismantle the host immune defenses. Mol Aspects Med. 2009;30:456–466. doi: 10.1016/j.mam.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 74.Gnade BT, et al. Emergence of anthrax edema toxin as a master manipulator of macrophage and B cell functions. Toxins (Basel) 2010;2:1881–1897. doi: 10.3390/toxins2071881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turk BE. Manipulation of host signalling pathways by anthrax toxins. Biochem J. 2007;402:405–417. doi: 10.1042/BJ20061891. [DOI] [PubMed] [Google Scholar]
- 76.Baldari CT, et al. Anthrax toxins: a paradigm of bacterial immune suppression. Trends Immunol. 2006;27:434–440. doi: 10.1016/j.it.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 77.Goossens PL. Animal models of human anthrax: the quest for the Holy Grail. Mol Aspects Med. 2009;30:467–480. doi: 10.1016/j.mam.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 78.Ghosh CC, et al. Impaired function of the Tie-2 receptor contributes to vascular leakage and lethality in anthrax. Proc Natl Acad Sci USA. 2012;109:10024–10029. doi: 10.1073/pnas.1120755109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bolcome RE, III, et al. Anthrax lethal toxin induces cell death-independent permeability in zebrafish vasculature. Proc Natl Acad Sci USA. 2008;105:2439–2444. doi: 10.1073/pnas.0712195105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moayeri M, et al. The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS) PLoS Pathog. 2009;4:e1000456. doi: 10.1371/journal.ppat.1000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie T, et al. The effects of anthrax lethal toxin on host barrier function. Toxins (Basel) 2011;3:591–607. doi: 10.3390/toxins3060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fang H, et al. Neutrophil elastase mediates pathogenic effects of anthrax lethal toxin in the murine intestinal tract. J Immunol. 2010;185:5463–5467. doi: 10.4049/jimmunol.1002471. [DOI] [PubMed] [Google Scholar]
- 83.Golden HB, et al. Anthrax toxin: pathologic effects on the cardiovascular system. Front Biosci. 2009;14:2335–2357. doi: 10.2741/3382. [DOI] [PubMed] [Google Scholar]
- 84.Maddugoda MP, et al. cAMP signaling by anthrax edema toxin induces transendothelial cell tunnels, which are resealed by MIM via Arp2/3-driven actin polymerization. Cell Host Microbe. 2011;10:464–474. doi: 10.1016/j.chom.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 85.Coggeshall KM, et al. The sepsis model: an emerging hypothesis for the lethality of inhalation anthrax. Journal of cellular and molecular medicine. 2013;17:914–920. doi: 10.1111/jcmm.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Artenstein AW, Opal SM. Novel approaches to the treatment of systemic anthrax. Clin Infect Dis. 2012;54:1148–1161. doi: 10.1093/cid/cis017. [DOI] [PubMed] [Google Scholar]
- 87.Abrami L, et al. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell reports. 2013;5:986–996. doi: 10.1016/j.celrep.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walsh JJ, et al. A case of naturally acquired inhalation anthrax: clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin Infect Dis. 2007;44:968–971. doi: 10.1086/512372. [DOI] [PubMed] [Google Scholar]
- 89.Mazumdar S. Raxibacumab. mAbs. 2009;1:531–538. doi: 10.4161/mabs.1.6.10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Z, et al. Monoclonal antibody therapies against anthrax. Toxins. 2011;3:1004–1019. doi: 10.3390/toxins3081004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wycoff KL, et al. Recombinant anthrax toxin receptor-Fc fusion proteins produced in plants protect rabbits against inhalational anthrax. Antimicrob Agents Chemother. 2011;55:132–139. doi: 10.1128/AAC.00592-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moayeri M, et al. Small-molecule inhibitors of lethal factor protease activity protect against anthrax infection. Antimicrobial agents and chemotherapy. 2013;57:4139–4145. doi: 10.1128/AAC.00941-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shen Y, et al. Selective inhibition of anthrax edema factor by adefovir, a drug for chronic hepatitis B virus infection. Proc Natl Acad Sci USA. 2004;101:3242–3247. doi: 10.1073/pnas.0306552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, et al. B. anthracis edema toxin increases cAMP levels and inhibits phenylephrine-stimulated contraction in a rat aortic ring model. American journal of physiology. Heart and circulatory physiology. 2013;305:H238–250. doi: 10.1152/ajpheart.00185.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Friedlander AM, Little SF. Advances in the development of next-generation anthrax vaccines. Vaccine. 2009;27(Suppl 4):D28–D32. doi: 10.1016/j.vaccine.2009.08.102. [DOI] [PubMed] [Google Scholar]
- 96.Kaur M, et al. Anthrax vaccines: present status and future prospects. Expert review of vaccines. 2013;12:955–970. doi: 10.1586/14760584.2013.814860. [DOI] [PubMed] [Google Scholar]
- 97.Manish M, et al. A single-dose PLGA encapsulated protective antigen domain 4 nanoformulation protects mice against Bacillus anthracis spore challenge. PloS one. 2013;8:e61885. doi: 10.1371/journal.pone.0061885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yin Y, et al. Deletion modification enhances anthrax specific immunity and protective efficacy of a hepatitis B core particle-based anthrax epitope vaccine. Immunobiology. 2014;219:97–103. doi: 10.1016/j.imbio.2013.08.008. [DOI] [PubMed] [Google Scholar]