Summary
Non-treponemal tests such as the rapid plasma reagin (RPR) assay are mainstays of syphilis diagnosis, but false-positive tests are common. We identified false-positive RPR titers in 8.2% of patients with malaria due to Plasmodium vivax in northern Peru. Similar rates were not detected in patients with other acute febrile illnesses.
Keywords: Syphilis, Vivax malaria, Diagnostic test
Introduction
The prevalence of syphilis has increased greatly over the past decade, with an estimated worldwide prevalence of 12 million cases, of which 90% are believed to occur in developing countries [1]. The diagnosis of syphilis is complicated by the inability to culture its causative agent, Treponema pallidum pallidum, and by the protean nature of its symptoms. The sequelae of untreated syphilis make early treatment paramount. T. pallidum has defied vaccination and eradication efforts, despite the effectiveness and availability of benzathine penicillin as first-line treatment. In Peru, 0.4–0.5% of young Peruvian adults have serologic evidence of syphilis infection [2]. The lesions of symptomatic early syphilis are associated with increased transmission of human immunodeficiency virus (HIV) and other sexually transmitted infections, partially due to erosion of mucosal genital surfaces [3].
The detection of syphilis is complicated by frequent false positives on screening tests in patients with inflammatory disorders. The rapid plasma reagin (RPR) is the most commonly used screening test for blood, while the Venereal Disease Research Laboratory (VDRL) is used to screen both blood and cerebrospinal fluid (CSF) specimens. Both assays detect nonspecific antibodies to host cardiolipin antigens, and as such are referred to as non-treponemal assays. Positive RPR and VDRL results are confirmed with a more specific treponemal assay, such as the T. pallidum hemagglutination (TPHA) or fluorescent treponemal antibody-absorption (FTA-ABS) tests, which measure specific antibodies to treponemal antigens and differentiate true from false-positive RPRs or VDRLs. Recently, the syphilis diagnostic algorithm has come under reconsideration, with some organizations considering the use of treponemal tests as an initial screening tool, to be followed by RPR or VDRL to estimate disease activity and severity [4]. The main motivation for this is cost and automation, as the RPR and VDRL assays are manual tests whereas the newer treponemal enzyme immunoassays (EIAs) can be run on automated instruments.
The RPR was used as a screening tool in a recent collaboration between the Naval Medical Research Center (NMRC, Silver Spring, Maryland) and Naval Medical Research Unit No. 6 (NAMRU-6, Lima, Peru) as part of ongoing studies of acute febrile illness and Plasmodium vivax infection in northern coastal Peru.
After providing informed consent, blood from patients with acute vivax malaria was offered to female anopheline mosquitos through an in vitro feeding apparatus; the mosquitoes were shipped to NMRC for analysis and use in human P. vivax challenge model development. Infected donors in Peru were screened for bloodborne infections as part of their enrollment, including testing for HIV, hepatitis B and C, and syphilis. In the course of this study, patients with active vivax malaria were observed to have a disproportionate frequency of positive RPRs on screening serologies. Confirmatory testing with TPHA demonstrated these positive RPRs to be false positives. Similar false positives were not demonstrated in the control population, who were Peruvian adults with non-malarious febrile illnesses. Based on this observation, a case–control study of RPR reactivity was conducted to quantify this phenomenon in acutely febrile patients with and without vivax malaria.
Methods
These studies were conducted following ethical review and approval by the Peruvian Ministry of Health and by the Institutional Review Boards of NMRC and NAMRU-6, in accordance with United States Federal and Peruvian regulations for the protection of human subjects (protocols NMRCD.2008.0004, NMRCD.2000.0006, and PJT.NMRCD.068). Patients were offered enrollment into an ongoing febrile surveillance project in the cities of Tumbes and Sullana, in northern coastal Peru, upon presentation to an affiliated health center with an undifferentiated fever of ≥38.0 °C for ≤7 days.
Upon obtaining informed consent, patients were initially evaluated for malaria by microscopy and then later confirmed by PCR [5]. Parasite density was calculated by counting the number of asexual parasites per 200 white blood cells in the thick smear, assuming a mean white blood cell count of 6000 per µL. Seventy-three patients with malaria, all with P. vivax infection, were identified; no cases of falciparum malaria were diagnosed in this sample. In patients without malaria, serum specimens were tested by viral culture and PCR for arboviral pathogens as well as by paired acute and convalescent IgM ELISA for viral antibodies [6]. A sequential sample of 76 such patients was selected from the same time period and geographic region as the patients with malaria to serve as controls. Testing with RPR (RPRnosticon II kit, bioMérieux, Marcy l’Etoile, France) and TPHA (TPHA 100, bioMérieux) was then performed on all samples.
A confirmed case of syphilis was defined as an RPR titer ≥1:1 with a positive TPHA result. All positive results, including syphilis diagnoses, were communicated with patients and attending clinicians in order to provide appropriate therapy. Groups were compared for significance by two-tailed Fisher’s exact test or t-test, as appropriate. Significance was defined as a p-value of ≤0.05.
Results
Demographics and test results for patients with malaria and for febrile controls without malaria are presented in Table 1. Those patients with malaria were more likely to be male and were slightly older than malaria-uninfected controls, but these differences did not achieve statistical significance. Positive RPR titers were detected in 8/73 (11.0%) patients with malaria. Of these, 2/73 patients (2.7%) with malaria had a positive TPHA consistent with syphilis, while 6/73 (8.2%) patients had false-positive RPR titers. All six of these patients were men; no false-positives were detected in women in the sample. False-positive RPR titers ranged from 1:1 to 1:16 (Table 2). A positive RPR titer was detected in 1/76 (1.3%) of patients without malaria; this single patient had a positive TPHA confirming syphilis. No false-positive RPRs were detected among the control group. No blood type differences were observed between groups. No significant differences in degree of parasitemia were noted in malaria-infected participants with and without false-positive RPRs.
Table 1.
Patient demographics and test results.
| Patients with malaria (n = 73) |
Controls (n = 76) | p-Value | |
|---|---|---|---|
| Mean age (years) | 31.5 (±12.6) | 28.9 (±14.2) | 0.24 |
| Sex | 55% male | 41% male | 0.10 |
| RPR+/TPHA+ (true positives) | 2/73 (2.7%) | 1/76 (1.3%) | 0.61 |
| RPR+/TPHA− (false-positives) | 6/73 (8.2%) | 0/76 (0%) | 0.01 |
| Mean parasitemia (par/µl) – false-positive RPR | 3572 (95% CI 1527–5616) | n/a | |
| Mean parasitemia (par/µl) – other patients with malaria | 5396 (95% CI 3363–7128) | n/a |
RPR = rapid plasma reagin; TP-HA = Treponema pallidum hemagglutination test; par/µl = parasites per microliter of whole blood. : “Other patients with malaria” refers to patients with malaria who lack false-positive RPR tests and includes both patients with confirmed syphilis (RPR+/TPHA+) and those without syphilis (RPR−).
Table 2.
Demographics and test results of patients with reactive rapid plasma reagin assays.
| Number | Age | Sex | RPR titer | TP-HA | Parasitemia (par/µl) |
Comments |
|---|---|---|---|---|---|---|
| 1 | 20 | Male | 1:16 | Negative | 2900 | False-positive RPR |
| 2 | 23 | Male | 1:1 | Negative | 2000 | False-positive RPR |
| 3 | 20 | Male | 1:16 | Negative | 3369 | False-positive RPR |
| 4 | 21 | Male | 1:4 | Negative | 5567 | False-positive RPR |
| 5 | 62 | Male | 1:8 | Negative | 1353 | False-positive RPR |
| 6 | 21 | Male | 1:1 | Negative | 6240 | False-positive RPR |
| 7 | 45 | Male | 1:2 | Positive | 1253 | True case of syphilis; referred for care |
| 8 | 37 | Female | 1:4 | Positive | 7100 | True case of syphilis; referred for care |
| 9 | 60 | Female | 1:2 | Positive | 0 | True case of syphilis detected in control patient without evidence of malaria; referred for care |
RPR = rapid plasma reagin; TP-HA = Treponema pallidum hemagglutination test; par/µl = parasites per microliter of whole blood.
Discussion
Prior to advent of penicillin, therapeutic infection of patients suffering from neurosyphilis with Plasmodium was conducted to induce fever with the intention of denaturing spirochetal proteins [7]. Since the introduction of penicillin, the hazardous work and uncertain results of malariotherapy were rapidly supplanted by more effective antimicrobial therapy, but observations of the serologic effect of malaria on syphilis diagnostics date from this period. The phenomenon of false-positive RPRs in patients with malaria was described in the 1930s and 1940s in both natural and experimental infections. Between 2 and 4% of European patients in Africa with treated, naturally- acquired Plasmodium falciparum malaria demonstrated reactive non-treponemal tests [8]. In patients without syphilis, 5–23% developed abnormal Wasserman tests (a non-treponemal flocculation assay, similar in principle to the RPR) following experimental infection with Plasmodium malariae, with abnormal Wasserman reactions becoming more common with more prolonged periods of malarious fever [9].
More recently, Ghosh et al. described false-positive RPR results in 6.6–9% of patients with both falciparum and vivax malaria, in addition to other false-positive serologic results to include rheumatoid factor, Widal, and Coombs tests. Titer results and confirmatory treponemal testing were not reported, but their overall false-positive results are similar to those described here [10].
Vivax malaria-induced false-positive RPRs in our series often presented with titers of 1:8 or greater (Table 2), unlike many other causes of biologic false-positive syphilis tests that often present with lower titers [11]. An RPR titer in excess of 1:16 may be concerning for the presence of neurosyphilis and is an indication for lumbar puncture (LP), cerebrospinal fluid analysis, and potentially more-aggressive treatment [12]. Furthermore, patients with malaria frequently have nonspecific symptoms that may increase the clinical suspicion for neurosyphilis in this setting [13], potentially resulting in unnecessary LPs and the risk of procedural complications.
While there is little clinical similarity between syphilis and vivax malaria, syphilis is endemic worldwide, including in malarious regions. Sexually transmitted diseases are often prevalent in developing countries, and the medical facilities available to low income peoples may treat presumptively if an RPR is positive due to a perceived high pretest probability. Furthermore, some populations require a significant amount of follow-up, such as patients thought to have neurologic disease, HIV-infected individuals, or pregnant women [1].
Maternal and congenital syphilis are significant public health concerns in developing and industrialized settings alike, and pregnancy represents an opportunity for effective screening. Pregnant women in P. vivax-endemic areas are of particular concern, given the potentially devastating effects of both congenital syphilis and malaria and the impact of ongoing efforts to identify and treat peripartum syphilis. Congenital infection develops in approximately 15% of children born to mothers with untreated syphilis, with a 21% increased absolute risk for fetal loss and 9.3% increased absolute risk for neonatal deaths per a recent meta-analysis [14]. RPR- and VDRL-based screening is effective, although the provision of therapy may be hampered by inadequate follow-up and delays in confirmatory testing [15].
Asymptomatic malaria was noted in a significant percentage of pregnant women in a malaria-hypoendemic region of Peru [16], which could confuse diagnostic efforts for both diseases. Specifically, asymptomatic malaria could cause a false-positive RPR during a routine syphilis screen during pregnancy leading to unnecessary treatment for presumed syphilis and a missed opportunity to treat the real infection with its potential for negative outcomes on the fetus or neonate [17].
As in all patients, confirmatory treponemal testing should be performed in pregnant women prior to initiation of treatment for syphilis, due to the risk of false-positive RPR tests from malaria and other causes. In malarious areas, a blood smear should be considered, even on asymptomatic patients, to determine one potential cause of the false-positive RPR and provide an opportunity to prevent the potential poor outcomes of malaria during pregnancy.
Non-treponemal tests for syphilis such as the RPR detect nonspecific antibodies to cardiolipin. These antibodies are thought to be produced in response to various lipoidal antigens released from damaged host cells but also present in T. pallidum. These antigens are not unique to syphilis, however, and positive RPR assays may occur in autoimmune disorders, certain viral infections, following immunizations, or in pregnancy [18]. B-cell activation and hyper-gammaglobulinemia are common findings in malaria. In P. falciparum, this process is mediated in part by interaction of the erythrocyte membrane protein 1 (PfEMP1) with host B-cells, leading to polyclonal B-cell expansion [19]. Plasmodium infections are also associated with a loss of T-cell control and subsequent B-cell disinhibition [20]. A similar polyclonal expansion is seen in P. vivax infection [21], possibly via similar mechanisms. Elevated levels of anti-cardiolipin antibodies are reported in both vivax and falciparum malaria; the metabolism of host phospholipids by Plasmodium with the subsequent exposure of altered lipid antigen on the host erythrocyte surface may serve as a trigger for antibody production [22].
All of the false-positive RPR assays detected in our study occurred in men. The reason for this is not clear, although men outnumbered women in the malaria arm of the study. It is interesting to note that the earlier studies of treponemal tests in patients undergoing malariotherapy during the 1940s [9,23] were conducted in state hospitals and prisons where male patients may have predominated. False-positive non-treponemal tests are more common in women than in men [18,24], however, and our finding here may be coincidental.
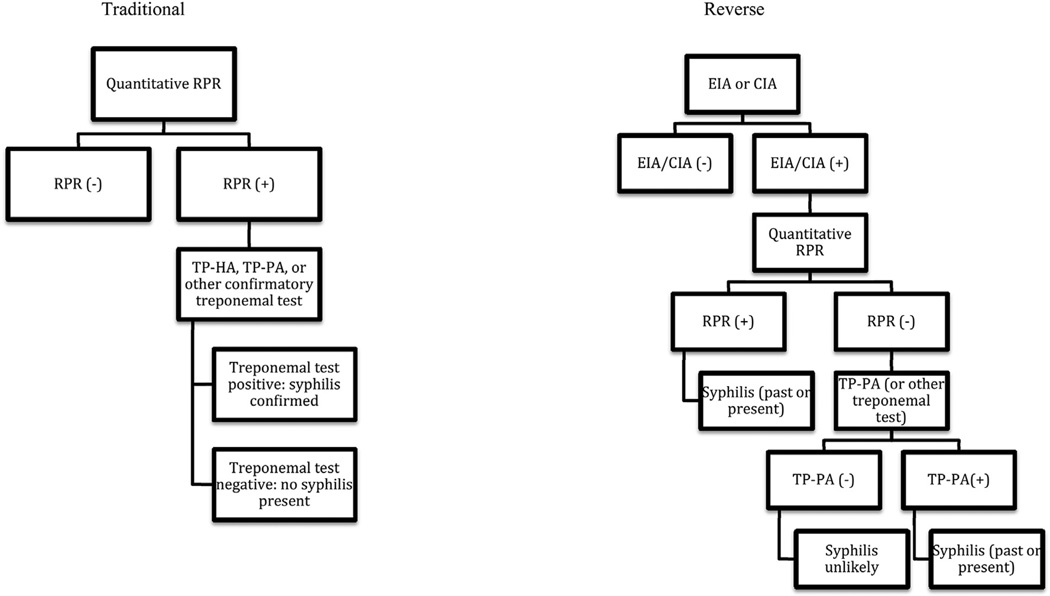
Given the limitations of RPR-based syphilis diagnostics, many clinical laboratories are shifting from the use of nontreponemal tests for syphilis screening to the use of treponemal tests in a “reverse algorithm”. In this method, patient specimens are tested initially using a specific serologic assay, such as enzyme immunoassays (EIA) or chemiluminescence immunoassays (CIA) for the detection of anti-T. pallidum IgG. Positive treponemal tests are then followed by RPR testing with titer to assess the extent of infection, risk for neurosyphilis, and response to therapy (if applicable) (Fig. 1). This methodology avoids biological false-positive syphilis test results as well as false-negative results due to the prozone phenomenon, as well as permitting for rapid automated testing of screening samples [25].
Figure 1.
Centers for Disease Control and Prevention diagnostic algorithms for syphilis. In the traditional algorithm, a screening quantitative RPR is followed by a confirmatory treponemal assay. In the reverse algorithm, an EIA or CIA specific for Treponema pallidum replaces the RPR as the screening test; a positive EIA or CIA is followed by a confirmatory RPR. If that RPR is negative, a second treponemal test is performed to verify the results. RPR = rapid plasma reagin; TP-HA = T. pallidum hemoagglutination test; TP-PA = T. pallidum particle agglutination test; EIA = enzyme immunoassay; CIA = chemiluminescence immunoassay.
The high sensitivity and increased specificity of the reverse algorithm, however, may be offset in part by increased initial equipment and reagent costs. Additionally, test specificity for the reverse algorithm may be decreased in low-prevalence populations [26]. While RPR and VDRL remain the standard for syphilis screening in most developing settings, novel point of care tests may permit rapid and specific diagnosis with prompt therapy during a single prenatal visit [27].
Malaria incidence remains high, with 258 million suspected cases worldwide in 2011 and 219 million estimated cases in 2010 [28]. Although P. falciparum is responsible for the majority of these cases, P. vivax is increasingly recognized as a neglected public health problem, with 40% of the world’s population at risk. Deaths due to vivax malaria are comparatively less frequent than those due to falciparum malaria, but the capacity of P. vivax for chronic infection and relapses in the absence of radical cure gives it a disproportionate impact. Individuals in endemic areas may suffer 10–30 attacks over their lifetimes, and global costs due to both direct medical expenses and lost productivity from vivax malaria may reach US$4 billion per year [29].
In conclusion, vivax malaria was associated with false-positive RPR test results in this sample of febrile patients in northern coastal Peru, often with higher titers than are traditionally associated with false positivity. As the syphilis epidemic continues, the association between malaria and the potential for false-positive RPRs may confound the diagnosis of both disorders. These false positives, if not recognized as such, may result in the potential overtreatment of syphilis and under-treatment of malaria. Medical professionals and epidemiologists may consider using treponemal, rather than non-treponemal, tests as initial screening in malarious regions [11,30]. Positive nontreponemal test results should be interpreted with caution in malaria-endemic settings.
Acknowledgments
We thank the members of the Parasitology, Virology, and Bacteriology Laboratories at NAMRU-6 for their excellent technical assistance. We thank Edith Guerrero and colleagues in Sullana and Tumbes for the collection of specimens. This work was supported by the Military Infectious Disease Research Program and by the Global Emerging Infections Surveillance and Response System, a division of the Armed Forces Health Surveillance Center.
Footnotes
This data was previously presented at the 59th annual meeting of the American Society of Tropical Medicine and Hygiene, November 6, 2010, Atlanta, Georgia, United States (abstract #903).
The authors are employees of the United States Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Departments of the Navy, Army, or Air Force, the Department of Defense, nor the U.S. Government.
Conflict of interest
None.
Disclosure
None of the authors has a financial or personal conflict of interest related to this study. The corresponding author had full access to all data in the study and final responsibility for the decision to submit this scientific work.
References
- 1.Tucker JD, Bu J, Brown LB, Yin YP, Chen XS, Cohen MS. Accelerating worldwide syphilis screening through rapid testing: a systematic review. Lancet Infect Dis. 2010;10:381–386. doi: 10.1016/S1473-3099(10)70092-X. [DOI] [PubMed] [Google Scholar]
- 2.Carcamo CP, Campos PE, Garcia PJ, Hughes JP, Garnett GP, Holmes KK, et al. Prevalences of sexually transmitted infections in young adults and female sex workers in Peru: a national population-based survey. Lancet Infect Dis. 2012;12:765–773. doi: 10.1016/S1473-3099(12)70144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis. 2007;44:1222–1228. doi: 10.1086/513427. [DOI] [PubMed] [Google Scholar]
- 4.Owusu-Edusei K, Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1–7. doi: 10.1097/OLQ.0b013e3181ec51f1. [DOI] [PubMed] [Google Scholar]
- 5.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–292. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- 6.Forshey BM, Guevara C, Laguna-Torres VA, Cespedes M, Vargas J, Gianella A, et al. Arboviral etiologies of acute febrile illnesses in Western South America, 2000–2007. PLoS Negl Trop Dis. 2010;4:e787. doi: 10.1371/journal.pntd.0000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin SC, Stolley PD, Lasky T. The history of malariotherapy for neurosyphilis. Modern parallels. J Am Med Assoc. 1992;268:516–519. [PubMed] [Google Scholar]
- 8.Nelson MG. Serological tests for syphilis in treated Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1947;41:127–132. doi: 10.1016/s0035-9203(47)90207-1. [DOI] [PubMed] [Google Scholar]
- 9.Burney LE, Mays JR, Iskrant AP. Results of serologic tests for syphilis in non-syphilitic persons inoculated with malaria. Am J Public Health Nations Health. 1942;32:39–47. doi: 10.2105/ajph.32.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh K, Javeri KN, Mohanty D, Parmar BD, Surati RR, Joshi SH. False-positive serological tests in acute malaria. Br J Biomed Sci. 2001;58:20–23. [PubMed] [Google Scholar]
- 11.Nandwani R, Evans DT. Are you sure it’s syphilis? A review of false positive serology. Int J STD AIDS. 1995;6:241–248. doi: 10.1177/095646249500600404. [DOI] [PubMed] [Google Scholar]
- 12.Workowski KA, Berman S Centers for Disease C, Prevention. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110. [PubMed] [Google Scholar]
- 13.Rogerson SJ, Carter R. Severe vivax malaria: newly recognised or rediscovered. PLoS Med. 2008;5:e136. doi: 10.1371/journal.pmed.0050136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez GB, Kamb ML, Newman LM, Mark J, Broutet N, Hawkes SJ. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217–226. doi: 10.2471/BLT.12.107623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Doare K, Gannon H, Handforth J. Missed opportunities to treat: syphilis in pregnancy. Sex Transm Infect. 2012;88:594. doi: 10.1136/sextrans-2012-050684. [DOI] [PubMed] [Google Scholar]
- 16.Parekh FK, Hernandez JN, Krogstad DJ, Casapia WM, Branch OH. Prevalence and risk of Plasmodium falciparum and P vivax malaria among pregnant women living in the hypoendemic communities of the Peruvian Amazon. Am J Trop Med Hyg. 2007;77:451–457. [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkes S, Matin N, Broutet N, Low N. Effectiveness of interventions to improve screening for syphilis in pregnancy: a systematic review and meta-analysis. Lancet Infect Dis. 2011;11:684–691. doi: 10.1016/S1473-3099(11)70104-9. [DOI] [PubMed] [Google Scholar]
- 18.Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1–21. doi: 10.1128/cmr.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donati D, Zhang LP, Chene A, Chen Q, Flick K, Nystrom M, et al. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect Immun. 2004;72:5412–5418. doi: 10.1128/IAI.72.9.5412-5418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troye-Blomberg M. Human T-cell responses to blood stage antigens in Plasmodium falciparum malaria. Immunol Lett. 1994;41:103–107. doi: 10.1016/0165-2478(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 21.Fesel C, Goulart LF, Silva Neto A, Coelho A, Fontes CJ, Braga EM, et al. Increased polyclonal immunoglobulin reactivity toward human and bacterial proteins is associated with clinical protection in human Plasmodium infection. Malar J. 2005;4:5. doi: 10.1186/1475-2875-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Consigny PH, Cauquelin B, Agnamey P, Comby E, Brasseur P, Ballet JJ, et al. High prevalence of co-factor independent anticardiolipin antibodies in malaria exposed individuals. Clin Exp Immunol. 2002;127:158–164. doi: 10.1046/j.1365-2249.2002.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan BI, Brightman IJ. The course of the serologic tests during therapeutic malaria in patients with syphilis. Am J Public Health Nations Health. 1943;33:1073–1082. doi: 10.2105/ajph.33.9.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geusau A, Kittler H, Hein U, Dangl-Erlach E, Stingl G, Tschachler E. Biological false-positive tests comprise a high proportion of Venereal Disease Research Laboratory reactions in an analysis of 300,000 sera. Int J STD AIDS. 2005;16:722–726. doi: 10.1258/095646205774763207. [DOI] [PubMed] [Google Scholar]
- 25.Binnicker MJ. Which algorithm should be used to screen for syphilis? Curr Opin Infect Dis. 2012;25:79–85. doi: 10.1097/QCO.0b013e32834e9a3c. [DOI] [PubMed] [Google Scholar]
- 26.Loeffelholz MJ, Binnicker MJ. It is time to use treponema-specific antibody screening tests for diagnosis of syphilis. J Clin Microbiol. 2012;50(1):2–6. doi: 10.1128/JCM.06347-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mabey DC, Sollis KA, Kelly HA, Benzaken AS, Bitarakwate E, Changalucha J, et al. Point-of-care tests to strengthen health systems and save newborn lives: the case of syphilis. PLoS Med. 2012;9:e1001233. doi: 10.1371/journal.pmed.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. World malaria report 2012. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 29.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 30.Owusu-Edusei K, Jr, Koski KA, Ballard RC. The tale of two serologic tests to screen for syphilis–treponemal and nontreponemal: does the order matter? Sex Transm Dis. 2011;38:448–456. doi: 10.1097/OLQ.0b013e3182036a0f. [DOI] [PubMed] [Google Scholar]