Abstract
Cessation of drug use often coincides with increased food consumption and weight gain in recovering addicts. However, it is not known whether this phenomenon (particularly the weight gain) is uniquely human, or whether it represents a consequence of drug cessation common across species. To address this issue, rats (n = 10/group) were given systemic injections of D-amphetamine (3 mg/kg) or an equal volume of saline vehicle for nine consecutive days. Beginning two days after the final injection, rats were given free access to a highly palatable food mixture (consisting of sugar and butter) along with their standard chow diet, and food consumption and body weight were measured every 48 hours for 30 days. Consistent with clinical observations, amphetamine-treated rats showed a greater increase in body weight over the course of the 30 days relative to vehicle-treated rats. Surprisingly, there was no difference in highly palatable food consumption between amphetamine- and vehicle-treated groups, but the amphetamine-treated group consumed significantly more standard chow than the control group. The finding that a history of chronic amphetamine exposure increases food consumption is consistent with previous work in humans showing that withdrawal from drugs of abuse is associated with overeating and weight gain. The current findings may reflect amphetamine-induced sensitization of mechanisms involved in reward motivation, suggesting that weight gain following drug cessation in humans could be due to similar mechanisms.
Keywords: Amphetamine, sensitization, eating, addiction
1. Introduction
Cessation of drug abuse following a period of chronic intake is associated with hyperphagia and weight gain [1], which can cause adverse health outcomes beyond those directly associated with drug consumption. It is not known, however, whether this problem is unique to humans (e.g., due to the social contexts or constraints of drug abstinence) or whether it reflects a more fundamental neurobiological consequence of drug cessation. With respect to the latter notion, chronic exposure to drugs of abuse in animal models causes long-lasting neuroadaptations which can persist well beyond the period of drug exposure [2, 3]. For example, psychostimulant administration can lead to enhanced locomotor responses to these drugs (locomotor sensitization), as well as increased self-administration of other drugs of abuse [4–8]. Furthermore, chronic drug exposure can cause persistent increases in behavior directed toward non-drug rewards, including palatable foods (so-called “incentive sensitization”) [9–14]. For instance, chronic amphetamine administration can cause an increase in sugar intake during subsequent abstinence [4] as well as an increase in food consumption stimulated by food-predictive cues [10].
Drugs of abuse act upon the same neural circuitry involved in the motivation to procure and consume food [15]. Acute ingestion of highly palatable food (HPF) as well as various drugs, such as amphetamine, alcohol, and cocaine, increase dopamine neurotransmission in the nucleus accumbens [1, 15–20]. Thus, it is no surprise that drug-induced neural changes may result in alterations in appetitive and consummatory behavior, which could result in hyperphagia and ultimately obesity. Notably, however, despite previous demonstrations in animal models that chronic drug (particularly amphetamine) administration can increase appetitive and consummatory behavior during acute test sessions [4, 9, 10], it is unclear whether this is reflected in sustained (i.e., home cage) increases in food consumption and, ultimately, in increases in body weight. Given the behavioral and neurobiological overlap between drug use and food consumption, elucidating their interactions may have considerable implications for understanding drug abuse and co-morbid hyperphagia and weight gain during withdrawal. To this end, we investigated the effects of a regimen of chronic amphetamine administration on both consummatory behavior and weight gain for a 30 day period following amphetamine cessation.
2. Materials and methods
2.1. Animals and equipment
Subjects were 20 adult male Long-Evans rats (P70; 320–350g) obtained from a commercial supplier (Charles River Laboratories, Raleigh, NC, USA). Rats were individually housed and maintained on a 12 h light/dark cycle (lights on from 0800-2000) with free access to laboratory chow (on the wire cage lid) and water (via an automatic watering system) throughout all points of the experiment. Locomotor activity was measured in standard operant behavioral test chambers equipped with overhead activity monitors (Coulbourn Instruments, Whitehall, PA, USA). These monitors consisted of an array of infrared detectors focused over the entire test chamber. Movement in the test chamber (in x, y, or z planes) was defined as a relative change in the infrared energy falling on the different detectors. All behavioral procedures were carried out in accordance with the University of Florida Institutional Animal Care and Use Committee and NIH guidelines.
2.2. Amphetamine administration
Rats were randomly divided into two groups (n=10) that received intraperitoneal injections of either D-amphetamine (AMPH; 3 mg/kg body weight; provided by the Drug Supply Program at the National Institute on Drug Abuse) or 0.9% saline vehicle (SAL) once per day for nine consecutive days during the rats’ light cycle (1600–1800 h). Injections were administered at a volume of 1 ml/kg. This amphetamine regimen was based on data showing that milder regimens are effective in producing both locomotor and incentive sensitization [9] and in enhancing food consumption during acute test sessions [10]. On days 1 and 9, rats were placed in the operant test chambers 10 min after injections and locomotor activity was monitored for 1 h. Rats were returned to their home cages immediately following injections on days 2–8. One rat in the saline group was excluded from subsequent analyses due to the fact that his baseline body weight (prior to any injections) was greater than 2 SD from the mean of the saline group.
2.3. Food consumption
To avoid assessing potential acute withdrawal effects and/or drug-induced anorexia, rats were left undisturbed for 48 h after the last injection. After that time had elapsed, rats were weighed, and in their home cages, given access to a highly palatable food mixture (HPF; 50 g), which consisted of 12 cups of confectioner’s sugar per 1 lb butter (35.7% fat and 64.3% sugar, by kcals; 4.48 kcal/g; [21]). In addition to the HPF, rats still had concurrent access to their standard laboratory chow (100g; Harlan Irradiated mouse/rat diet; protein 19.1%, fat 5.8%, fiber 4.6%, 3.1 kcal/g). The laboratory chow was placed on the wire cage lid, whereas HPF was placed in a glass jar hung inside the cage. Water was freely available at all times. Every two days, both the rats and the remaining HPF and chow were weighed. Fresh chow and HPF were given to the rats at this time. This regimen was maintained across a 30 day period, yielding fifteen body weight measurements and fourteen food consumption measurements across the 30 days.
2.4. Data analysis
Data analyses were conducted in SPSS 21.0. Locomotor activity was analyzed with a repeated measures ANOVA (treatment day X drug condition). Weight gain and food consumption (both chow and HPF) were analyzed with a repeated measures ANOVA (day X drug condition).
3. Results
3.1. Chronic amphetamine administration causes weight gain and increased chow consumption
There were no differences in body weight between SAL and AMPH rats prior to injections [SAL: 333.2 g, AMPH: 333.1 g; t (1, 17) = 0.02, p > 0.05] or 48 h after the last injection [SAL: 377.8 g, AMPH: 372.9 g; t (1, 17) = 0.60, p > 0.05]. However, AMPH rats gained more weight than SAL rats over the 30 days following drug administration. Figure 1 shows body weight data for SAL and AMPH rats across the 30 days (fifteen timepoints). A two-factor repeated measures ANOVA revealed a significant interaction between drug condition and day [F (14, 238) = 3.80, p < 0.01] such that AMPH rats gained significantly more weight across the 30 days of free access to chow and HPF compared to SAL control rats. The same analysis also revealed a significant main effect of day [F (14, 238) = 1110.21, p < 0.01) indicating that all rats gained weight over the course of the 30 days, but no main effect of drug condition [F (1, 17) = 0.54, n.s.].
Figure 1.
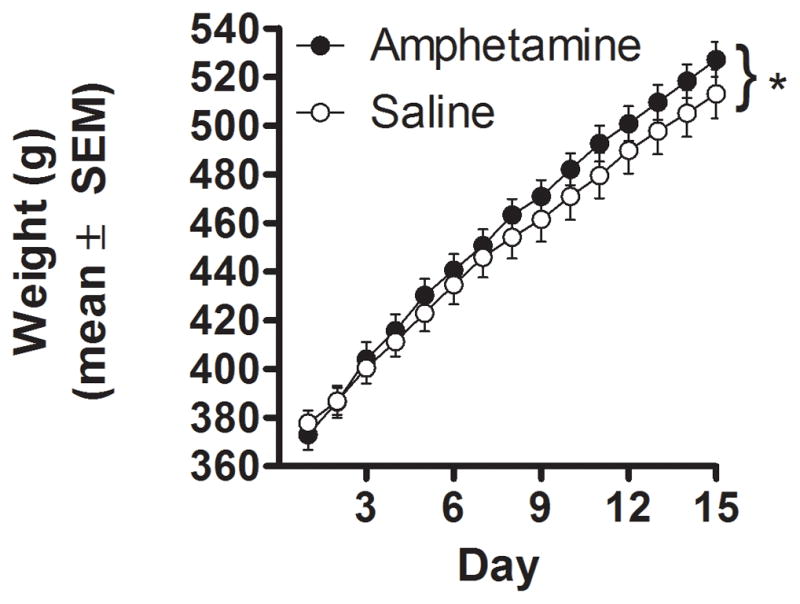
Body weight measurements for AMPH and SAL rats. Measurements were taken over a thirty day period that followed 9 days of AMPH or SAL administration. Each measurement was taken every 48 hours, resulting in 15 data points. Data are represented as means ± SEM. * = significant interaction between day and drug condition.
Despite the greater increase in body weight in the AMPH compared to the SAL rats, chronic amphetamine did not affect HPF consumption (Figure 2A). A two-factor repeated measures ANOVA conducted on HPF consumption data revealed neither a main effect of drug condition [F (1, 17) = 1.04, n.s.] nor an interaction between drug condition and day [F (13, 221) = 0.48, n.s.], although there was a significant main effect of day [F (13, 221) = 7.40, p < 0.01]. In contrast, chronic amphetamine significantly increased chow consumption (Figure 2B). A two-factor repeated measures ANOVA conducted on chow consumption data revealed a main effect of drug condition [F (1, 17) = 5.26, p < 0.05], as well as a main effect of day [F (13, 221) = 4.10, p < 0.01], but no interaction between the two variables [F (13, 221) = 1.24, n.s.].
Figure 2.
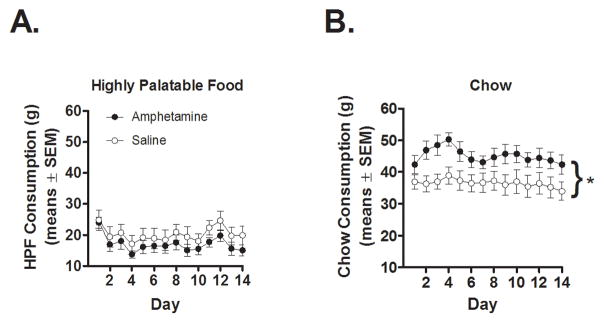
Consumption of (A) highly palatable food (HPF) and (B) laboratory chow in AMPH and SAL rats. Measurements were taken over a thirty day period that followed chronic AMPH or SAL exposure. Each measurement was taken every 48 hours; note that the first measurement was taken on the third day (after 48 h of HPF availability), resulting in 14 data points. Data are represented as means ± SEM. * = significant main effect of drug condition.
3.2. Effects of amphetamine administration on locomotor activity
Locomotor activity on days 1 and 9 of drug administration was assessed by summing activity in the test chamber across the 60 min session (Figure 3). As expected, AMPH rats exhibited significantly greater activity than SAL controls [F (1, 17) = 31.17, p < 0.01], indicating the efficacy of the injections. However, the interaction between drug and day (which would indicate the presence of locomotor sensitization) was not quite significant [F (1, 17) = 4.19, p = 0.06]. Together, these data show that amphetamine administration caused an overall elevation in locomotor activity, but the measures employed did not provide strong evidence for locomotor sensitization under these conditions.
Figure 3.
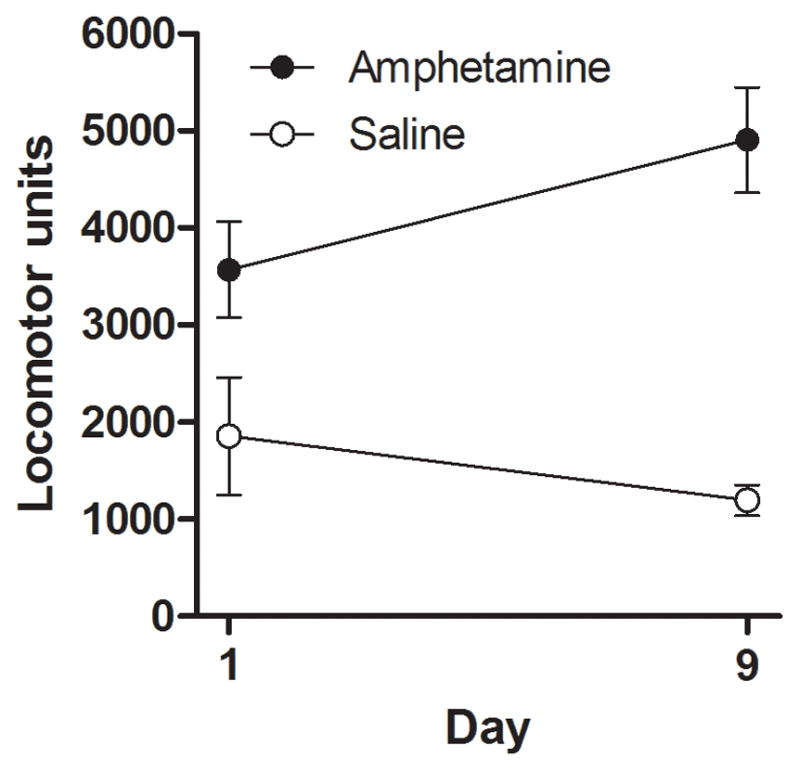
Locomotor activity counts (in arbitrary units) in AMPH and SAL rats on the first (day 1) and last (day 9) day of drug administration. Data are represented as means ± SEM.
4. Discussion
The data presented here indicate that chronic amphetamine exposure causes persistent changes in eating behavior following drug cessation. Across a 30 day period following a 9 day amphetamine regimen, AMPH rats gained significantly more weight than SAL controls. Additionally, AMPH rats ate significantly more laboratory chow than their SAL counterparts. These findings are consistent with human empirical data in which withdrawal from amphetamine use results in rebound hyperphagia and weight gain [1], and suggest that such effects in humans may be due in part to neuropharmacological consequences of drug withdrawal that are common across species (rather than being unique to the conditions of human drug cessation).
In humans, increased food consumption may reflect the use of food as a substitute for drug use to reduce cravings during withdrawal [22, 23]. Indeed, individuals in early recovery from drug abuse report gaining excessive weight and using food to replace their former drug use and regulate their moods [22]. Neither drug withdrawal nor craving was assessed in the present experiment. However, chronic stimulant administration is reported to cause long-lasting changes in mesolimbic dopamine (DA) neurotransmission [24] such that dopamine D1 receptor binding is increased, basal DA transmission within the nucleus accumbens is decreased, and subsequent stimulant administration leads to enhanced DA release compared to non-drug-exposed controls (i.e., DA release is “sensitized”; [18, 25, 26]). Given that food consumption itself causes release of DA within the mesolimbic system [16, 27–30], it is possible that the increase in body weight after amphetamine exposure is due to the effects of this DA release on a drug-sensitized brain. In a similar vein, withdrawal from amphetamine is reported to result in a “reward deficit” [31–33] mediated by changes in the mesolimbic DA system [34–36], which might be alleviated by food consumption. Notably, some investigators have reported that stimulant withdrawal can be associated with a decrease rather than an increase in reward consumption [31, 33]. However, these effects occur soon after drug cessation and appear to be transient (resolving within a week or so). In contrast, sensitization-induced changes in dopamine signaling and behavior tend to emerge several weeks after drug cessation [25], a phenomenon that parallels the observed weight gain in the AMPH rats (although not the effects on chow consumption, which were evident on the first measurement day). It will be of considerable interest in future studies to attempt to dissociate the contributions of sensitization vs. withdrawal on food consumption (e.g., via comparisons of different timepoints following drug cessation).
A surprising outcome of this study was that the increase in food consumption in AMPH rats was specific to laboratory chow rather than HPF. Previous work showed that chronic amphetamine exposure can cause an increase in sucrose consumption during acute test sessions, although this occurred in normal weight animals [4]. Moreover, given the choice between a sucrose solution or laboratory chow, normal rats will binge on sucrose rather than chow [37]. Although these findings were unexpected, there are several possible explanations. First, it is not unusual for rats to eat less of a food with a higher caloric density compared to one with a lower caloric density [21, 38]. As such, in the current study, rats may have consumed less of the HPF relative to the chow because the HPF had a higher caloric density (4.48 kcal/g) than chow (3.1 kcal/g). Second, not only is AMPH withdrawal accompanied by hyperphagia, but it is also associated with anhedonia and depression-like behaviors [1, 39]. Indeed, AMPH cessation has been shown to cause a significant increase in immobility in the forced swim test, a common measure of depression in rodents [31]. Thus, it is possible that a prolonged anhedonic state after AMPH cessation contributed to the choice of chow over HPF, a substance that drug-naïve animals would typically find more desirable [37, 40]. Nevertheless, we do not believe that this explanation is likely, as AMPH-induced anhedonia has primarily been observed shortly after drug cessation [31–33].
Third, though ingestion of HPF and sugar has been shown to activate the mesolimbic DA system, this activation is driven most strongly by novelty [23]; once the food has lost its novelty, DA release decreases. Since AMPH-treated rats were given free access to the HPF, it is possible that the novelty of this food rapidly became attenuated, resulting in a decrease in DA release and a subsequent decrease in the motivational properties that HPF consumption may have first elicited. Along these same lines, restricted rather than free access to HPF has been shown to induce behaviors that are commonly associated with drug self-administration, such as hyperactivity and increased breakpoints in responding to obtain food [23]. Thus, the schedule of availability of the HPF may be a critical factor in determining whether stimulant cessation results in increased consumption. Notably, the availability of chow was constant throughout amphetamine administration and the 30 days after drug cessation and thus seems in contrast to the notion of novelty-induced activation of the DA system. Given that standard laboratory chow can also cause mesolimbic DA release [23, 41], it may be that the amount of DA release in response to novelty varies with different foods. Further investigation is warranted to explore this possibility.
It is also possible that rats negatively associated the cessation of amphetamine administration with the introduction of the HPF, and that this aversive association may have driven the rats to bias their food choice away from the HPF and toward the familiar laboratory chow. This possibility seems unlikely, however, as the timing of drug cessation and HPF availability (48 hour interval) was not conducive to such associative learning. Furthermore, this type of food aversion typically results from administration of stimulants (such as amphetamine or cocaine) rather than their cessation [42–45]. Lastly, several studies have shown that stimulant cessation can produce a persistent increase in palatable food consumption during acute testing [4, 9]. Thus, it seems unlikely that a negative association between amphetamine cessation and HPF availability mediated the lack of an increase in HPF consumption.
Finally, it is possible that fat-rich foods simply do not elicit sensitized consumption following amphetamine administration, as has been shown previously for sugar solutions [4]. Indeed, we recently showed that unlike access to sugar solutions alone, prolonged access to sweet-fat foods (including the HPF mixture used in the current study) does not result in evidence of withdrawal symptoms, suggesting that the potential for cross-sensitization and “addiction-like” behavior is greater for sweet rather than fat-rich foods [4, 21, 37, 41, 46]. It will be important in future work to directly compare the effects of drug cessation on consumption of a variety of palatable foods in order to identify the nutrients and access conditions which are most affected by prior drug exposure.
An alternate interpretation of the effects of amphetamine on body weight and food consumption is that they are due to amphetamine-induced changes in metabolic rate rather than the sensitization of the motivational salience of food. Amphetamine use causes weight loss, a decrease in energy consumption [47] and a decrease in metabolic rate [48], consistent with the anorexic properties of the drug [1, 49]. As such, it is possible that after amphetamine cessation, there is a rebound increase in metabolic rate congruent with the reported rebound hyperphagia. Given that we did not collect measures of metabolic rate, we are unable to evaluate this hypothesis, although this could be explored in future experiments. Regardless of mechanism, however, the current findings demonstrate that chronic stimulant use leads to weight gain and an increase in food consumption, which have important implications for the treatment of addiction.
An additional possible mechanism that might account for the increased chow consumption arises from the fact that rats were returned to their home cages following the majority of the amphetamine injections (days 2–8). Such pairings of amphetamine with the contextual cues of the home cage could have led to the home cage context acquiring heightened incentive salience [50]. It is well-established that incentive cues can potentiate food consumption [51], but this account of the effects of amphetamine on chow consumption seems unlikely for several reasons. First, the laboratory chow was present in the home cage environment for several weeks prior to amphetamine, rendering it part of the environment itself and less likely to be modulated by environmental cues after amphetamine exposure. Second, and more importantly, it is not clear why the effects of amphetamine-enhanced contextual incentive cues would be specific to chow, and not HPF consumption. Nevertheless, to address this possible mechanism, future studies should compare changes in consummatory behavior between groups given amphetamine injections in different contexts.
The amphetamine administration regimen used in the present study (3 mg/kg/day for 9 consecutive days) was chosen on the basis of data showing that even milder regimens (lower doses or shorter duration) can induce locomotor sensitization as well as enhanced food consumption during acute test sessions [4, 9, 10]. As expected, amphetamine administration caused acute increases in activity compared to saline on days 1 and 9, indicating the efficacy of the injections; however, the interaction between day and drug condition, which would indicate the presence of locomotor sensitization, did not quite reach statistical significance (p=0.06). There are a number of possible reasons for this, including the high dose of amphetamine (which produces a high baseline level of activity against which it can be challenging to observe further increases) and the likely development of motor stereotypy (which tends to register as less activity using automated measures indeed, the two rats with the greatest response to amphetamine on day 1 exhibited a decrease in activity on day 9 [19, 25, 26]). Importantly, however, the absence of significant locomotor sensitization to amphetamine does not directly bear on the effects of amphetamine on weight gain and food consumption, which were the measures of interest in this experiment. It will be interesting in future work to evaluate relationships between the locomotor and appetitive/consummatory consequences of stimulant administration.
In conclusion, the data presented here indicate that chronic exposure to a stimulant drug of abuse can lead to an increase in food consumption and associated weight gain as long as a month after drug cessation. This finding has considerable public health implications as obesity alone is thought to confer a larger morbidity risk than some drugs of abuse [52]. Ultimately, understanding both the behavioral and neural ramifications of addiction will result in the development of better treatment methods and a healthier life for recovering addicts.
Highlights.
Overeating and weight gain during recovery from addiction is a clinical concern
It is unclear whether this is unique to humans, or if it can be modeled in animals
Rats received amphetamine or saline for 9 days, followed by abstinence
Drug-treated rats gained more weight and consumed more chow than saline rats.
Drug-induced increases in weight and eating may reflect sensitization processes.
Acknowledgments
We thank the Drug Supply Program at NIDA for kindly providing D-amphetamine, and Ms. Bonnie McLaurin for technical assistance. Supported by DA03123 (NMA), DA024671 (BS), and the University of Florida Foundation and University of Florida Alumni Association (MSG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edge PJ, Gold MS. Drug withdrawal and hyperphagia: lessons from tobacco and other drugs. Curr Pharm Des. 2011;17(12):1173–9. doi: 10.2174/138161211795656738. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, et al. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78(3):610–24. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND, et al. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9(6):557–69. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 4.Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacol Biochem Behav. 2003;74(3):635–9. doi: 10.1016/s0091-3057(02)01050-x. [DOI] [PubMed] [Google Scholar]
- 5.Liguori A, et al. Subjective effects of oral caffeine in formerly cocaine-dependent humans. Drug Alcohol Depend. 1997;49(1):17–24. doi: 10.1016/s0376-8716(97)00133-6. [DOI] [PubMed] [Google Scholar]
- 6.Hubbell CL, Mankes RF, Reid LD. A small dose of morphine leads rats to drink more alcohol and achieve higher blood alcohol concentrations. Alcohol Clin Exp Res. 1993;17 (5):1040–3. doi: 10.1111/j.1530-0277.1993.tb05661.x. [DOI] [PubMed] [Google Scholar]
- 7.Nichols ML, et al. Morphine increases intake of beer among rats. Alcohol. 1991;8(4):237–40. doi: 10.1016/0741-8329(91)90273-y. [DOI] [PubMed] [Google Scholar]
- 8.Volpicelli JR, Ulm RR, Hopson N. Alcohol drinking in rats during and following morphine injections. Alcohol. 1991;8(4):289–92. doi: 10.1016/0741-8329(91)90401-h. [DOI] [PubMed] [Google Scholar]
- 9.Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci. 2001;21(19):7831–40. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendez IA, et al. Long-lasting sensitization of reward-directed behavior by amphetamine. Behav Brain Res. 2009;201(1):74–9. doi: 10.1016/j.bbr.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roesch MR, et al. Ventral striatal neurons encode the value of the chosen action in rats deciding between differently delayed or sized rewards. J Neurosci. 2009;29(42):13365–76. doi: 10.1523/JNEUROSCI.2572-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roesch MR, et al. Neural correlates of variations in event processing during learning in basolateral amygdala. J Neurosci. 2010;30(7):2464–71. doi: 10.1523/JNEUROSCI.5781-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gisabella B, et al. Amygdala-dependent regulation of electrical properties of hippocampal interneurons in a model of schizophrenia. Biol Psychiatry. 2009;65(6):464–72. doi: 10.1016/j.biopsych.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of pavlovian approach behavior in rats: Differential effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine (“ecstasy”) Biological Psychiatry. 2001;50(2):137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- 15.Volkow ND, et al. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 16.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 17.Blumenthal DM, Gold MS. Neurobiology of food addiction. Curr Opin Clin Nutr Metab Care. 2010;13(4):359–65. doi: 10.1097/MCO.0b013e32833ad4d4. [DOI] [PubMed] [Google Scholar]
- 18.Robinson TE, et al. Persistent Sensitization of Dopamine Neurotransmission in Ventral Striatum (Nucleus Accumbens) Produced by Prior Experience with (+)-Amphetamine - a Microdialysis Study in Freely Moving Rats. Brain Research. 1988;462(2):211–222. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- 19.Camp DM, Browman KE, Robinson TE. The Effects of Methamphetamine and Cocaine on Motor Behavior and Extracellular Dopamine in the Ventral Striatum of Lewis Versus Fischer-344 Rats. Brain Research. 1994;668(1–2):180–193. doi: 10.1016/0006-8993(94)90523-1. [DOI] [PubMed] [Google Scholar]
- 20.Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999;11(12):4389–97. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- 21.Bocarsly ME, et al. Rats that binge eat fat-rich food do not show somatic signs or anxiety associated with opiate-like withdrawal: implications for nutrient-specific food addiction behaviors. Physiol Behav. 2011;104(5):865–72. doi: 10.1016/j.physbeh.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowan J, Devine C. Food, eating, and weight concerns of men in recovery from substance addiction. Appetite. 2008;50(1):33–42. doi: 10.1016/j.appet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Avena NM, et al. Overlaps in the nosology of substance abuse and overeating: the translational implications of “food addiction”. Curr Drug Abuse Rev. 2011;4(3):133–9. doi: 10.2174/1874473711104030133. [DOI] [PubMed] [Google Scholar]
- 24.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151(2–3):99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 25.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396(2):157–98. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 26.Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol Biochem Behav. 1987;26(4):821–7. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- 27.Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35(1–2):227–63. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- 28.Bassareo V, Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurosci. 1997;17(2):851–61. doi: 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanda GL, Di Chiara G. A dopamine mu(1) opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. European Journal of Neuroscience. 1998;10(3):1179–1187. doi: 10.1046/j.1460-9568.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- 30.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286(1):R31–7. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 31.Der-Avakian A, Markou A. Withdrawal from chronic exposure to amphetamine, but not nicotine, leads to an immediate and enduring deficit in motivated behavior without affecting social interaction in rats. Behav Pharmacol. 2010;21(4):359–68. doi: 10.1097/FBP.0b013e32833c7cc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parkinson JA, et al. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19(6):2401–11. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;141(1):99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhri N, et al. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35(3):783–91. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blaiss CA, Janak PH. The nucleus accumbens core and shell are critical for the expression, but not the consolidation, of Pavlovian conditioned approach. Behav Brain Res. 2009;200(1):22–32. doi: 10.1016/j.bbr.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambroggi F, et al. Roles of nucleus accumbens core and shell in incentive-cue responding and behavioral inhibition. J Neurosci. 2011;31(18):6820–30. doi: 10.1523/JNEUROSCI.6491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berner LA, Avena NM, Hoebel BG. Bingeing, self-restriction, and increased body weight in rats with limited access to a sweet-fat diet. Obesity (Silver Spring) 2008;16(9):1998–2002. doi: 10.1038/oby.2008.328. [DOI] [PubMed] [Google Scholar]
- 39.Gold MS. From bedside to bench and back again: a 30-year saga. Physiol Behav. 2011;104(1):157–61. doi: 10.1016/j.physbeh.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 40.Levine AS, Kotz CM, Gosnell BA. Sugars and fats: The neurobiology of preference. Journal of Nutrition. 2003;133(3):831s–834s. doi: 10.1093/jn/133.3.831S. [DOI] [PubMed] [Google Scholar]
- 41.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737–44. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 42.Lin JY, et al. Reduced palatability in drug-induced taste aversion: I. Variations in the initial value of the conditioned stimulus. Behav Neurosci. 2012;126(3):423–32. doi: 10.1037/a0027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang AC, Hsiao S. Re-examination of amphetamine-induced conditioned suppression of tastant intake in rats: the task-dependent drug effects hypothesis. Behav Neurosci. 2008;122(6):1207–16. doi: 10.1037/a0013511. [DOI] [PubMed] [Google Scholar]
- 44.Gomez F. Induction of conditioned taste aversion with a self-administered substance in rats. Brain Res Brain Res Protoc. 2001;8(2):137–42. doi: 10.1016/s1385-299x(01)00097-6. [DOI] [PubMed] [Google Scholar]
- 45.Goudie AJ, Dickins DW, Thornton EW. Cocaine-induced conditioned taste aversions in rats. Pharmacol Biochem Behav. 1978;8(6):757–61. doi: 10.1016/0091-3057(78)90279-4. [DOI] [PubMed] [Google Scholar]
- 46.Avena NM, Long KA, Hoebel BG. Sugar-dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav. 2005;84(3):359–62. doi: 10.1016/j.physbeh.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Jones JR, Caul WF, Hill JO. The effects of amphetamine on body weight and energy expenditure. Physiol Behav. 1992;51(3):607–11. doi: 10.1016/0031-9384(92)90187-7. [DOI] [PubMed] [Google Scholar]
- 48.Bushnell PJ. Differential effects of amphetamine and related compounds on locomotor activity and metabolic rate in mice. Pharmacol Biochem Behav. 1986;25(1):161–70. doi: 10.1016/0091-3057(86)90248-0. [DOI] [PubMed] [Google Scholar]
- 49.Cole SO. Brain mechanisms of amphetamine-indcued anorexia, locomotion, and stereotypy: A review. Neurosci Biobehav Rev. 1978;2:89–100. [Google Scholar]
- 50.Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20(21):8122–30. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrovich GD. Forebrain networks and the control of feeding by environmental learned cues. Physiol Behav. 2013;121:10–8. doi: 10.1016/j.physbeh.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mokdad AH, et al. Actual causes of death in the United States, 2000. Jama-Journal of the American Medical Association. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]


