Abstract
Tendon and ligament (T/L) are dense connective tissues connecting bone to muscle and bone to bone, respectively. Similar to other musculoskeletal tissues, T/L arise from the somitic mesoderm, but they are derived from a recently discovered somitic compartment, the syndetome. The adjacent sclerotome and myotome provide inductive signals to the interposing syndetome, thereby upregulating the expression of the transcription factor Scleraxis, which in turn leads to further tenogenic and ligamentogenic differentiation. These advances in the understanding of T/L development have been sought to provide a knowledge base for improving the healing of T/L injuries, a common clinical challenge due to the intrinsically poor natural healing response. Specifically, the three most common tendon injuries involve tearing of the rotator cuff of the shoulder, the flexor tendon of the hand, and the Achilles tendon. At present, injuries to these tissues are treated by surgical repair and/or conservative approaches, including biophysical modalities such as physical rehabilitation and cryotherapy. Unfortunately, the healing tissue forms fibrovascular scar and possesses inferior mechanical and biochemical properties as compared to native T/L. Therefore, tissue engineers have sought to improve upon the natural healing response by augmenting the injured tissue with cells, scaffolds, bioactive agents, and mechanical stimulation. These strategies show promise, both in vitro and in vivo, for improving T/L healing. However, several challenges remain in restoring full T/L function following injury, including uncertainties over the optimal combination of these biological agents as well how to best deliver tissue engineered elements to the injury site. A greater understanding of the molecular mechanisms involved in T/L development and natural healing, coupled with the capability of producing complex biomaterials to deliver multiple growth factors with high spatiotemporal resolution and specificity, will allow tissue engineers to more closely recapitulate T/L morphogenesis, thereby offering future patients the prospect of T/L regeneration, as opposed to simple tissue repair.
Keywords: tendon development, tendon regeneration, clinical treatment, tendon tissue engineering
INTRODUCTION
Decades of research on tendon and ligament (T/L) injuries have yielded extensive knowledge of the mechanical and biological properties of these dense connective tissues, translating into advances in surgical and conservative therapies that can prevent major disability. However, T/L injuries remain a persistent clinical challenge. In the U.S. alone, tendon, ligament, and joint capsular injuries account for 45% of the 32 million musculoskeletal injuries each year (Butler et al., 2004), with rates rising due to increasing sports participation and an aging population. Unfortunately, current treatment strategies fail to restore the functional, structural, and biochemical properties of repaired T/L to those of native tissue. Consequently, the principal elements of tissue engineering – cells, scaffolds, and bioactive molecules – have been explored in an effort to improve T/L healing. Both in vitro and in vivo studies have expanded the understanding of T/L biology while demonstrating the utility of tissue engineering in enhancing the healing of musculoskeletal tissues. Nevertheless, no tissue engineered construct thus far has achieved complete regeneration of T/L. In response, tissue engineers are looking to the emerging understanding of T/L development in an effort to recapitulate the embryonic events that establish the native structure (Thomopoulos et al., 2010). While researchers are only beginning to explore methods of integrating developmental biology into the design process, such efforts may advance the field of T/L tissue engineering towards its ultimate goal, full restoration of normal mechanical and biological properties (Lenas et al., 2009a). In this review, we will begin with the present understanding of tendon development. Ligament development has not received as much attention as that of tendon, but lessons learned from the latter should be applicable to the former, as tendons and ligaments possess similar ultrastructure and physiology, as well as fulfilling similar functional roles (Tozer and Duprez, 2005). The natural healing cascade of T/L will be summarized, as current therapeutic approaches to the three most common tendon injuries – tears of the rotator cuff, Achilles tendon, and flexor tendon of the hand – will be reviewed. Next, an overview of the current tissue engineering strategies to improve tendon healing, including cells, growth factors, scaffolds, and mechanical stimulation, will be provided. In closing, we will briefly explore the current limitations to tendon and ligament regeneration before offering future directions to address these challenges.
TENDON DEVELOPMENT
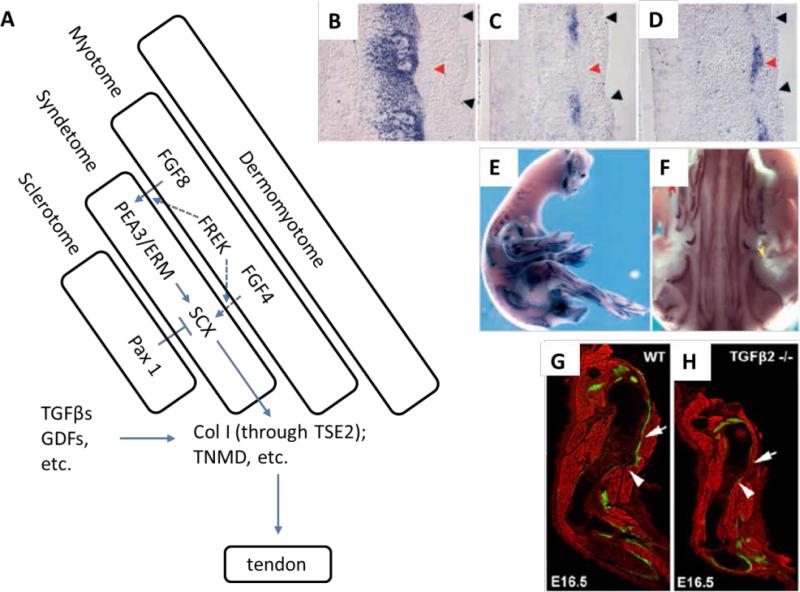
Despite the relative simplicity of tendon structure, understanding of tendon development was limited by the absence of a tendon-specific lineage marker. However, identification of several markers selectively associated with tendon and musculoskeletal tissues has made it possible to trace the formation and maturation of tendons (Figure 1A). Scleraxis (Scx), initially discovered as a sclerotome marker (Cserjesi et al., 1995), and subsequently in all muscle-to-bone attachment sites in chick embryos, is expressed in both tendon progenitor cells and mature tenocytes (Schweitzer et al., 2001). Scx binds a short cis-acting element known as tendon specific element 2 (TSE2) to form the Scx/E47 heterodimer, which in turn activates the collagen type Iα1 (COLI a1) proximal promoter (Carlberg et al., 2000; Lejard et al., 2007). The overexpression of Scx in tendon fibroblasts upregulates the expression of the tenomodulin (Tnmd) gene. This latter gene encodes a transmembrane protein that is expressed selectively in tendons and ligaments, and is considered a late marker of tendon formation (Shukunami et al., 2006). In situ hybridization analysis of the somitic mesoderm that forms along the anterior-posterior axis of the developing embryo in segmented animals provided further insight into the origin of tendon progenitors (Brent et al., 2003; Schweitzer et al., 2001). Scx-expressing progenitor cells first appeared between the myotome and sclerotome (Figure 1B-D). The localization pattern indicated that cells expressing Pax1, an early sclerotome marker, occupy a similar somitic domain as that of Scx-expressing cells. However, Pax1 is expressed more ventromedially while Scx is restricted to those cells nearest the myotome. On the other hand, Scx expression does not overlap with MyoD expression in the myotome. Rather, Scx-expressing progenitor cells are seen immediately adjacent to MyoD-positive cells. They constitute a fourth somitic compartment, syndetome, associated with myotome and sclerotome (Figure 1 A-F).
Figure 1.
An overview of tendon development. (A) Scx-expressing cells of trunk tendons appear between the myotome and sclerotome during early development, constituting a fourth compartment, syndetome, of the somite. Using a chimeric embryo model, syndetome is found emerging from sclerotome. FGF8 and 4 and their receptor, FREK, from myotome are involved in activating Scx-expression, while Pax1 from sclerotome suppresses Scx expression. Scx regulates downstream tendon-related genes including Col I and TNMD. (B-F) Visualized by in situ hybridization, Scx (C, blue) is found expressed in cells between myotomes (red arrowhead) of adjacent somites (black arrowheads), and its expression pattern does not overlap with that of Pax1 (B, blue) or MyoD (D, blue). Scx is expressed in all limb (E) and axial tendons (F) in chick embryos [Reproduced with permission from Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. 2001. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128(19):3855-3866]. A variety of growth factors play critical roles in tendon development and maturation, including members of the TGFβ superfamily. (G, H) White arrows indicate the missing Deltoid tendon (green) in TGFβ2 deficient (TGFβ2 −/−) mouse embryo compared with wild type (WT) [Reproduced with permission from Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R. 2009. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development 136(8):1351-1361].
Further resolution of the origin of Scx-expressing tendon progenitors was found by using a chick-quail chimera model. When quail sclerotomal cells were implanted into chick embryos, quail cells generated Scx-expressing progenitors. Conversely, in embryos transplanted with quail dermamyotomes, quail cells did not contribute to the Scx-expressing progenitors. Although cells of the developing muscle-tendon-bone complex occupy distinct spatial regions, the generation of Scx-expressing progenitors requires signals from both the sclerotome and myotome. Overexpression of Pax1 in sclerotome blocked Scx induction, suggesting that Pax1-positive sclerotome is unable to generate the tendon progenitors. Additionally, no induction of Scx was observed in somites with the dermamyotome removed prior to myotome formation, indicating that the myotome plays a critical role in Scx induction.
Fibroblast growth factor 8 (FGF8), secreted by the myotome, is partly responsible for inducing Scx expression through the Ets transcription factors Pea3 and Erm (Brent and Tabin, 2004). It also functions to downregulate Pax1 in Scx-positive domains in the sclerotome, although such repression alone does not result in Scx expression (Brent et al., 2003). Other FGF family members, such as FGF4, were also found to positively regulate induction of tendon progenitors. FGF4 transcripts are located at the extremities of muscles, close to the attachment sites of tendons in the embryonic chick wing, and the overexpression of FGF4 induces ectopic expression of Scx and tenascin in wing buds (Edom-Vovard et al., 2002).
In addition to FGFs, members of the transforming growth factor-β (TGFβ) superfamily are involved in regulating tendon development, as has been found in various species. For example, TGFβ-2/3 ligand and its receptors were detected throughout the tertiary bundles in the tendon midsubstance and endotenon during the intermediate stages of tendon development in the chick embryo (Kuo et al., 2008). During mouse patellar tendon development, all cells in the tendon were found to respond to TGFβ and BMP signaling at all stages examined, including embryonic and postnatal periods (Liu et al., 2012). In vitro micromass culture of chick mesodermal cells with TGFβ demonstrated significant upregulation of tendon markers Scx and Tnmd, with concurrent reduction in cartilage markers. This trend was lost with the addition of a Smad2/3-specific inhibitor, indicating that the tenogenic effect of TGFβ was mediated by the canonical Smad signaling pathway (Lorda-Diez et al., 2009). Likewise, using a ScxGFP transgenic reporter, disruption of TGFβ signaling in a Tgfb2−/− Tgfb3−/− mouse model was found to result in the loss of most tendons and ligaments (Pryce et al., 2009) (Figure 1 G,H).
Growth and differentiation factors (GDF), members of the bone morphogenetic protein (BMP) family, are additional regulators of tendon development. Subcutaneous implantation of GDF-5, 6, and 7 leads to the formation of neotendon-like connective tissue in rats (Wolfman et al., 1997). Mice deficient in GDF-5 demonstrate inferior matrix composition and mechanical strength in their Achilles tendons (Mikic et al., 2001). Similarly, a null mutation of GDF-6 in mice is associated with substantially lower levels of tail tendon collagen content, which causes impaired mechanical integrity of the tissue (Mikic et al., 2009). However, GDF-7 deficiency does not affect the biochemical composition of mouse tail tendon fascicles, nor does it significantly affect the tensile material properties, suggesting differential effects of GDF isoforms in promoting tendon development (Mikic et al., 2008).
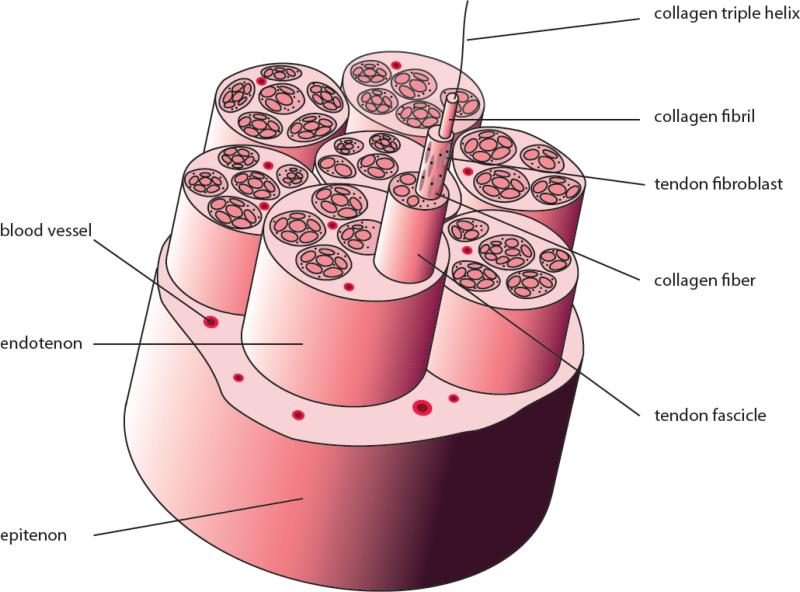
Upon aggregation, tendon progenitors of the developing embryo lay down small diameter collagenous fibrils (Banos et al., 2008; Connizzo et al., 2013), a process which continues after birth, with the fibrils growing in both the linear and lateral directions (Birk et al., 1995; Zhang et al., 2005). Fibrillar collagens, including types I, II, III, V, and XI, constitute the basic structural framework of tendons and ligaments. These collagens share a 300 nm-long triple helical domain comprised of three α chains, each containing about 1,000 amino acid residues. Each chain is rich in glycine and proline. As the smallest amino acid, glycine allows the three helical α chains to pack tightly together, while proline stabilizes the conformation due to its ring structure. Fibrillar collagens are synthesized in a precursor form, procollagen, that possesses a C- and N-terminal propeptide domain. Once secreted into the extracellular space, the propeptide domains are cleaved by specific telopeptidases. Mature fibrillar collagens generated from this modification spontaneously self-assemble into cross-striated fibrils in an entropy-driven manner, yielding a 67 nm repeat that characterizes the axial periodicity of collagen fibrils (Kadler et al., 1996). Collagen fibrils, 100-500 nm in diameter, are bundled into fibers between which tenocytes reside and maintain the extracellular matrix (ECM). The number and diameter of collagen fibers are highly variable among species, lower than 30 μm in rat tail tendon while higher than 300 μm in human tendons (Franchi et al., 2007). The collagen fibers are wrapped by a layer of connective tissue known as endotenon that contains blood vessels, lymphatics, and nerves, to form higher structural units called fascicles, which are surrounded by another connective tissue layer, epitenon, to form the tendon (Amiel et al., 1984; Kastelic et al., 1978) (Figure 2). Many of the tendons are further surrounded by loose areolar connective tissue called paratenon, which functions as an elastic sleeve and permits free movement of the tendon against the surrounding tissues.
Figure 2.
The hierarchical architecture of tendon. Collagen triple-helices self-assemble into fibrils. Bundles of fibrils form fibers, which constitute tendon fascicle. Tendon fibroblasts (tenocytes) reside between collagen fibers. Fascicles are wrapped by endotenon, a layer of connective tissue containing blood vessels, nerves and lymphatics. Multiple fascicles are further surrounded by another connective tissue layer, epitenon, to form the tendon tissue.
Collagen type I is the predominant fibril-forming collagen in tendons and exists as a heterotrimer consisting of two α1 chains and one α2 chain. Collagen type I co-polymerizes with collagen type V, a known regulator of collagen fibrillar structure (Wenstrup et al., 2004). Non-fibrillar collagens, including the fibril-associated collagens with interrupted triple helices (FACIT) such as collagens type XII and XIV, also serve a regulatory role during collagen fibrillogenesis (Ansorge et al., 2009). Collagen type XII has been postulated to integrate adjacent matrix components due to its ability to bind proteoglycans, fibromodulin, and decorin, while interacting with collagen type I fibrils (Zhang et al., 2005). Collagen type XIV integrates fibrils into fibers during development, while collagen type XII assumes the same structural and functional role in mature tendon (Connizzo et al., 2013).
Expression and deposition of small leucine-rich proteoglycans, including decorin, biglycan, fibromodulin, and lumican, also serves to organize fibril assembly and the resulting ultrastructure of the tendon. Decorin-deficient mice developed structurally impaired tendons with abnormal, irregular fibril contours. Moreover, biglycan expression increased dramatically in decorin-deficient mice, suggesting a functional compensation (Zhang et al., 2006). Fibromodulin deficiency alone leads to a significant reduction in tendon stiffness, with further loss in stiffness when combined with lumican deficiency. Fibromodulin might be required to stabilize small-diameter fibrils in early tendon development, with lumican assuming this role later (Chakravarti, 2002). In addition to their regulatory effect on fibrillogenesis, biglycan and fibromodulin were also found to maintain the niche of tendon stem cells. The expression of the tendon marker Scx and of collagen type I was decreased in tendon stem cells from biglycan- and fibromodulin-deficient mice, as compared to cells from wild-type mice (Bi et al., 2007). Glycoproteins are also important constituents of tendon. Tenomodulin (TNMD), the type II transmembrane glycoprotein identified as a marker of mature tenocytes, is positively regulated by Scleraxis. In fact, TNMD is among the most tendon-selective genes in both adult rat and human tendons, compared with other tissues examined (Jelinsky et al., 2010). Loss of Tenomodulin expression in gene-targeted mice abated tenocyte proliferation and led to a reduced tenocyte density. The diameters of collagen fibrils varied significantly and exhibited increased caliber (Docheva et al., 2005). Tenascin C (TNC), member of a family of four ECM glycoproteins in vertebrates, was employed as the primary tendon marker prior to the discovery of Scx (Shukunami et al., 2006). It is an ECM component directly regulated by mechanical stress; induction of its mRNA in stretched fibroblasts is rapid, both in vivo and in vitro (Chiquet et al., 2003). Congruent with these findings of gene expression, the degree of tenascin C deposition is greatest in areas of high mechanical loading (Chiquet-Ehrismann and Tucker, 2004).
As tendons mature, covalent cross-links are formed between collagen fibrils at the overlapping ends of adjacent collagen molecules (Fessel et al., 2012) Marturano et al. (2013) recently showed that collagen cross-links correlate significantly with the increasing elastic modulus of in utero tendon, whereas correlations between mechanical properties and collagen, glycosaminoglycan, and dsDNA content were all weak (Marturano et al., 2013). The formation of collagen type I cross-links in tendons is primarily driven by the enzyme lysyl oxidase, which acts on specific lysine and hydroxylysine residues and results in stable trivalent cross-links that enhance collagen interconnectivity and fibril stability (Bailey, 2001; Eyre et al., 2008). While mechanical properties, collagen cross-links, average fibril diameter, and the distribution of fibril diameter, have all been found to increase with age, the structure-function relationship between these biochemical characteristics and tendon mechanical properties remains inconclusive (Connizzo et al., 2013).
Although perhaps less so than bone and muscle, extensive research on the adaptation of tendons to mechanical stresses has been conducted (Killian et al., 2012b; Wang, 2006). Most investigations have focused on the tendon proper, finding that the nature of loading directs the homeostatic balance between anabolic and catabolic pathways in resident fibroblasts (Killian et al., 2012a). The role of mechanical stimulation in tendon healing is discussed below. In addition, researchers have recently explored the role of mechanical loading on the bone-tendon insertion site (Benjamin et al., 2006). Using a mouse model, Thomopoulos et al. showed that decreased muscle loading delayed maturation of the supraspinatus enthesis during postnatal development. While a fibrocartilage transition was identified in both experimental and control animals at 14 days post-birth, the mice with reduced muscle loading demonstrated less mineral deposition, impaired fibrochondrocyte and matrix organization, and inferior mechanical properties at later time points (Thomopoulos et al., 2010). This study, among others, reinforces the importance of proper mechanical loading in maintaining the health of the mature musculoskeletal system, but also in driving the appropriate maturation of these developing structures early in life. Should tendon injury occur, the implementation of physical rehabilitation protocols is equally important in regaining tendon function, though the innate healing process of tendons is quite poor, as highlighted below.
TENDON INJURY AND NATURAL HEALING
Tendon injuries, broadly categorized as chronic degenerative tendinopathies or acute ruptures, are a common clinical problem. Degenerative tendinopathy often precedes acute ruptures, with the former considered a failed healing response that is characterized by hypervascularity, mucoid degeneration, ectopic bone and cartilage nodules, and disorganized extracellular matrix (Kannus and Jozsa, 1991; Riley, 2008). Given the temporal relationship between chronic and acute tendon injuries, it is arguable that research examining the interaction between both pathologies will provide insights into the common clinical scenario. However, most in vivo studies of the sequelae of acute tendon ruptures are performed in animal models with previously healthy tendons. Therefore the applicability of research findings to the human condition may be questioned. Nevertheless, the current understanding of the innate healing response following acute tendon injury follows.
An estimated 300,000 tendon and ligament repair surgeries are performed annually in the U.S (Pennisi, 2002). In spite of surgical intervention, the natural healing process of tendons is still slow, due to their hypocellular and hypovascular nature (Liu et al., 2011). Even after one year, the structure and function of the resulting tissue remain inferior to uninjured tendons. The healing response is predicable, and is traditionally divided into three overlapping stages – (1) inflammation, (2) proliferation/repair, and (3) remodeling (Hope and Saxby, 2007). In the inflammatory stage, the blood clot that forms immediately following rupture of tendon vessels activates the release of chemoattractants and serves as a preliminary scaffold for invading cells. Inflammatory cells including neutrophils, monocytes, and lymphocytes migrate from surrounding tissues into the wound site, where necrotic debris is digested by phagocytosis (Voleti et al., 2012). Additionally, the recruitment and activation of tenocytes begins, but peaks in the subsequent stage. The second stage, known as the proliferative or reparative phase, begins roughly two days after the injury occurs. Fibroblasts of the paratenon or surrounding synovial sheath are recruited to the wounded area and proliferate, particularly in the epitenon (Garner et al., 1989). Likewise, intrinsic tenocytes located in the endotenon and epitenon migrate to the wound site and begin proliferating. Both sources of tenocytes are important in synthesizing an extracellular matrix and in establishing an internal neovascular network (James et al., 2008). Concurrently, neutrophil levels decline while macrophages continue to release growth factors that direct cell recruitment and activity (Voleti et al., 2012). In this early stage of healing, the matrix synthesized by tenocytes is composed of increased amount of collagen type III (Juneja et al., 2013). Water content and glycosaminoglycan concentrations remain elevated during this stage (Sharma and Maffulli, 2006). Lastly, the remodeling phase commences 1–2 months after injury. Tenocytes and collagen fibers become aligned in the direction of stress. A higher proportion of collagen type I is synthesized (Abrahamsson, 1991), with a corresponding decrease in cellularity and collagen type III and glycosaminoglycan contents (Sharma and Maffulli, 2006). After 10 weeks, fibrous tissue gradually changes to scar-like tendon tissue, a process that continues for years. The repaired tissue never completely regains the biomechanical properties it had prior to injury and the biochemical and ultrastructural characteristics remain abnormal even at 12 months (Miyashita et al., 1997).
Numerous bioactive molecules are involved in orchestrating the cellular response during tendon repair (Andia et al., 2010). A variety of growth factors are markedly upregulated following tendon injury and are active at multiple stages of the healing process, including insulin-like growth factor-I (IGF-I), TGF-β, bFGF, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), BMP, and connective tissue growth factor (CTGF) (Chen et al., 2008; Kobayashi et al., 2006; Molloy et al., 2003; Wurgler-Hauri et al., 2007). IGF-1 is an important mediator during the inflammatory and proliferative phase of tendon healing. IGF-1 mRNA levels were more than 5-fold higher compared with control groups 3 weeks after injury to the rabbit medial collateral ligament (MCL) (Sciore et al., 1998). In a collagenase-induced lesion created in equine flexor tendons, IGF-1 expression and protein levels increased following injury and peaked in weeks 4-8 (Dahlgren et al., 2005). Similarly, TGF-β shows high levels of gene expression and activity throughout the tendon-healing period (Chang et al., 2000; Chen et al., 2008; Natsu-ume et al., 1997) Rabbit flexor tendons subjected to transection and repair exhibited increased signal for TGF-β1 mRNA in both resident tenocytes and infiltrating cells from the tendon sheath (Chang et al., 1997). In a rat tendon injury model, a biphasic pattern was observed in which TGF-β1 expression was highest in the early phases of healing and gradually decreased thereafter, whereas TGF-β3 was upregulated later (Juneja et al., 2013). TGF-β receptors are also upregulated during tendon healing. In a rabbit zone II flexor tendon wound, immunohistochemical staining of transected and repaired tendons demonstrated increased TGF-β receptor type I (TGF-β RI), TGF-β RII, and TGF-β RIII protein levels in the epitenon and along the repair site (Ngo et al., 2001). bFGF, a potent mitogenic and angiogenic growth factor, is also involved in tendon healing. Tendons subjected to transection and repair exhibited an increased signal for bFGF mRNA in both resident tenocytes and in the fibroblasts and inflammatory cells located in the tendon sheath (Chang et al., 1998). Moreover, bFGF significantly accelerated wound closure of a rat patellar tendon defect in vitro (Chan et al., 1997), due in part to its effect on cell proliferation. PDGF was detected in canine digital flexor tendons 3, 10, and 17 days after tendon repairs (Duffy et al., 1995). It is a potent chemoattractant for macrophages and fibroblasts and may stimulate these cells to express other growth factors, including TGF-β, which directly stimulates new collagen synthesis (Pierce et al., 1989). VEGF has been shown to be a potent stimulator of angiogenesis during tendon healing (Petersen et al., 2003) and the importance of angiogenesis in tendon healing is widely recognized (Fenwick et al., 2002). VEGF activity peaks after inflammation, most notably during the proliferative and remodeling phases. Significant expression of VEGF occurred at the canine flexor tendon repair site at 7 days post-operatively, with expression localized to cells within the repair site itself, as opposed to cells of the epitenon (Bidder et al., 2000; Boyer et al., 2001). CTGF showed a high level of gene expression over a 21-day period of early tendon healing in a chicken flexor digitiorum profundus tendon injury (Chen et al., 2008). Likewise, in a rat model of supraspinatus tendon detachment and repair, CTGF was moderately expressed across all time points in both the insertion and midsubstance (Wurgler-Hauri et al., 2007). In the same study, BMP-12, -13, and -14, were dramatically elevated at 1 week and gradually decreased thereafter. These BMPs are established tenogenic factors, capable of driving differentiation of mesenchymal stem cells in vitro (Park et al., 2010) and forming neo-tendon tissue in vivo (Wolfman et al., 1997).
While many studies have investigated the spatial and temporal distribution of growth factors in tendon healing, several other bioactive molecules have been recently recognized as integral mediators of repair. For instance, regulators of extracellular matrix accretion and degradation, including matrix metalloproteinases (MMPs) and their inhibitors (TIMPs), are responsive to tendon injury and repair (Garofalo et al., 2011). In an oft-cited study examining gene expression in a rat flexor tendon laceration model, MMP-9 and MMP-13 expression peaked between days 7 and 14, while MMP-2, MMP-3, and MMP-14 expression remained elevated for 28 days (Oshiro et al., 2003), suggesting that the former pair participates in collagen degradation while the latter three mediate both collagen degradation and remodeling (Sharma and Maffulli, 2006). Markers of reinnervation show an equally complex pattern of expression during tendon healing. In a rat model of acute Achilles tendon rupture, neuronal markers for regenerating (growth associated protein 43, GAP-43) and mature (protein gene product 9.5, PGP9.5) fibers were found in the paratenon of both the ruptured and intact contralateral tendons. Conversely, only the ruptured tendon expressed these markers in the tendon proper. Immunoreactivity was evident as early as 1 week post-injury and peaked at week 6, before declining (Ackermann et al., 2002). As a last example, and in accordance with VEGF expression described above, nitric oxide appears to play a role in tendon healing. Nitric oxide synthase (NOS) levels peaked at 7 days following Achilles tendon tenotomy in a rat model, before returning to baseline at day 14. Furthermore, inhibition of NOS decreased the cross-sectional area and the failure load of healing tendons (Murrell et al., 1997).
A growing understanding of the molecular mediators of tendon healing has led to the supplementation or inhibition of these bioactive molecules as a means of enhancing the quality of the repaired tissue, as discussed below. Others have sought to modulate the inflammatory process itself, thereby affecting the innate healing cascade that produces fibrotic scar in place of regenerated tendon. Unfortunately, the utility of blocking inflammation post-injury remains a topic of debate. For instance, the systemic administration of non-steroidal anti-inflammatory drugs (NSAIDs) for 7 days following Achilles tendon transection in a rat yielded a healing tendon with inferior mechanical properties and a reduced cross-sectional area (Dimmen et al., 2009). Likewise, Virchenko et al. found a similar effect when parecoxib was administered immediately followed surgery (Virchenko et al., 2004). On the other hand, tendon healing was improved when the drug was administered starting 6 days post-surgery. Therefore, the early inflammatory cascade may be needed for the restoration of native tendon characteristics, while late inflammation is detrimental.
An equally debated issue concerns which elements of the adult tissue impair tendon regeneration. Bayer et al. demonstrated embryonic-like fibrillogenesis by adult human tendon fibroblasts when cultured in a fibrin gel contracted around a suture, suggesting that the hormonal/mechanical milieu inhibits the regenerative potential of adult tendons (Bayer et al., 2010). Conversely, Favata et al. transplanted fetal tenocytes contracted around a suture into an adult environment and performed a partial tenotomy on the engineered tendon construct. As compared to adult fibroblasts, the fetal tenocytes filled the defect without scar, suggesting that the adult environment is not an impediment to scarless tendon regeneration, but that such dysfunctional healing may be intrinsic to aged cells (Favata et al., 2006). Likewise, a recent study found that the frequency of tendon-derived stem/progenitor cells (TSPCs) decreases with age. Additionally, the aged TSPCs possess a decreased proliferation rate but an increased propensity for adipogenic differentiation (Zhou et al., 2010). Taken together, it remains unclear why adult tendons fail to regenerate, though such a fact creates a clinical challenge for physicians and patients alike, as outlined below.
CURRENT CLINICAL TREATMENTS FOR COMMON TENDON INJURIES
While tendons anchor every muscle of the body to bone, the most common injuries involve the rotator cuff tendons, Achilles tendon, and flexor tendons of the hand. Furthermore, these three tendons present different challenges to repair, as they possess unique anatomy, function, biomechanical properties, healing capacities, mechanisms of injury, and approaches to rehabilitation (Gott et al., 2011). Current injury rates and clinical treatment strategies for these three tendons are discussed.
Rotator Cuff Tendon
The rotator cuff is a comprised of the interdigitating tendons of four muscles – subscapularis, supraspinatus, infraspinatus, and teres minor – that attach on the lateral aspect of the humeral head, serving important roles in both stabilizing and mobilizing the shoulder. Rotator cuff tears are a common cause of debilitating pain, reduced shoulder function, and weakness, affecting more than 40% of patients older than 60 years of age and resulting in 30,000 to 75,000 repairs performed annually in the United States (Ricchetti et al., 2012). Novel surgical techniques improve repair strength at time 0, but fail to provide superior clinical scores when compared against older approaches (Dines et al., 2010; Lorbach and Tompkins, 2012). Likewise, rehabilitation protocols implementing early mobilization have been developed with the hope of improving the rate of healing while minimizing long-term stiffness. Unfortunately, such approaches show little benefit over more conservative protocols (Kim et al., 2012; Parsons et al., 2010).
Despite advances in surgical techniques and in the understanding of shoulder pathology, chronic tears fail to heal in 20-95% of cases (Derwin et al., 2010; Galatz et al., 2004). In particular, the bone-tendon interface that forms following surgical repair fails to recapitulate the native enthesis, with a fibrovascular scar forming in place of the complex fibrocartilage transition seen between native tendon and bone (Newsham-West et al., 2007). The intra-articular environment may partly explain this poor outcome (Bedi et al., 2009), as the synovial fluid, which contains the anti-adhesive protein lubricin, has been shown to inhibit bone-tendon healing (Funakoshi et al., 2010; Sun et al., 2012).
Tendinopathy and partial tears of the rotator cuff tendons are often treated conservatively with physical therapy and corticosteroid injections. A randomized, controlled study in which patients with full-thickness rotator cuff tears received subacromial injections of triamcinolone reported improved pain relief for at least 3 months, as compared to controls (Gialanella and Prometti, 2011). However, a recent systematic review concluded that there was little reproducible evidence to support the efficacy of subacromial corticosteroid injections in managing rotator cuff disease (Koester et al., 2007). Of further concern, Zhang et al. reported non-tenogenic differentiation (i.e. adipogenic, chondrogenic, osteogenic) of tendon-derived stem cells when treated with dexamethasone (Zhang et al., 2013).
Given the high re-tear rates experienced with current surgical approaches, scaffolds designed to provide mechanical augmentation or enhance biological healing have been investigated in both animal and human studies. Only two prospective randomized, controlled trials have been performed using commercially available devices consisting of decellularized extracellular matrices (Barber et al., 2012; Derwin et al., 2006; Iannotti et al., 2006). While one of these studies found improved subjective clinical scores in patients with scaffold-augmented repairs (Barber et al., 2012), animal models with the same augmented repair did not recapitulate the native bone-tendon interface (Dejardin et al., 2001). Concurrently, many surgeons have augmented rotator cuff repairs with platelet-rich plasma (PRP), an autologous source of concentrated growth factors of known importance in wound repair (Foster et al., 2009). As borne out in multiple prospective clinical studies (Castricini et al., 2011; Ruiz-Moneo et al., 2013), PRP does not have an effect on the overall re-tear rates or shoulder-specific outcome scores after arthroscopic rotator cuff repair (Chahal et al., 2012). As with the anterior cruciate ligament of the knee, PRP may be an insufficient carrier of growth factors as the plasmin found within synovial fluid may quickly degrade the fibrin matrix, thereby allowing diffusion of growth factors away from the repair site (Murray et al., 2009).
Achilles Tendon
The Achilles tendon is the largest and strongest tendon in the body, but one of the most likely to be injured (Calleja and Connell, 2010). Whereas rotator cuff tears are increasingly prevalent with age, Achilles ruptures are most commonly seen in men aged 30-50 (Longo et al., 2009). Achilles tendinopathy is increasingly common due to increasing participation in recreational sports, with Achilles pathology accounting for 30-50% of all sports-related injuries (Sadoghi et al., 2013). Nevertheless, Achilles tendon ruptures are also seen in elite athletes and, despite the research interest this garners, this injury is notorious for its poor quality and slow rate of healing (Maffulli et al., 2011).
Just as full-thickness rotator cuff tears are almost always preceded by partial tears and tendon degeneration (Oh et al., 2011), noninflammatory tendinosis and chronic tendinopathy predispose the Achilles tendon to complete rupture (Hess, 2010). In examining biopsy samples of patients undergoing open repair for torn Achilles tendons, Tallon et al. found that ruptured tendons were significantly more degenerated than tendinopathic tendons (Tallon et al., 2001). Therefore, concurrent research efforts have been made towards promoting healing of both tendinopathic and ruptured tendons.
While a detailed discussion of the etiology and histopathological characteristics of Achilles tendinopathy exceeds the scope of this review, it is worth noting that conservative approaches – including reduced activity, cryotherapy, eccentric loading, deep friction massage, orthotics, and therapeutic ultrasound – produce good to excellent outcomes in up to 75% of cases. In recalcitrant Achilles tendinopathy, surgical excision of adhesion, removal of degenerative nodules, and tenotomies intended to promote angiogenesis, can be performed (Maffulli et al., 2004). Likewise, complete Achilles ruptures can be treated both conservatively and surgically with satisfactory results. However, a recent Cochrane Review found that open surgical repair significantly reduced the re-rupture rate (4.4%) compared with nonoperative treatment (10.6%) (Jones et al., 2012). A percutaneous surgical approach did not reduce the re-rupture rate compared with open repair, but did result in significantly fewer postoperative infections.
As the typical patient suffering from Achilles injuries is often young and active, there is significant research interest in accelerating the healing rate. Recent trends towards earlier weight-bearing and active strengthening have produced comparable or superior results to cast immobilization (Garrick, 2012; Kearney and Costa, 2012). As with rotator cuff tears, surgeons have applied PRP to the surgical site to bolster repair. While Sanchez et al. (Sanchez et al., 2007) reported an accelerated return to sport and a smaller cross-sectional area of PRP-treated surgical repairs in a small cohort of athletes, the only randomized trial to date found no difference in mechanical properties or subjective function scores when comparing PRP-treated to untreated patients (Schepull et al., 2011). Given the long duration for recovery (9-12 months) and the residual impairment following injury and repair, research aimed at improving outcomes is still needed.
Flexor Tendon
Tendons of the flexor pollicis longus, flexor digitorum profundus, and flexor digitorum superficialis muscles attach to the distal and middle phalanges of the hand, allowing flexion at the distal and proximal interphalangeal joints, respectively. Portions of the flexor tendons are enveloped in synovial sheaths, which condense at certain locations to create an arrangement of pulleys that act as fulcrums for the tendon (Griffin et al., 2012). Located immediately beneath the palmar skin and fascia, the flexor tendons are prone to laceration and crush injuries. Rupture of intrasynovial flexor tendons presents unique repair challenges for three reasons: (1) ruptures do not heal without surgical intervention, (2) careful postoperative management of healing tendons is necessary to prevent adhesions and improve gliding function between the tendon and sheath, but mobilization increases the risk of re-rupture, and (3) hypertrophy of healing tendon must be avoided so as to minimize gliding resistance (Griffin et al., 2012).
Since the first report of flexor tendon repair in 1917, suture techniques to optimize surgical results have been extensively explored in animal and cadaveric models (Griffin et al., 2012; Kleinert et al., 1995; Nelson et al., 2012). In light of this body of research, Kim et al. suggested the following surgical techniques to improve upon historical methods: (1) 8-core suture strands with a high-caliber suture material, (2) a purchase length of approximately 1.2 cm, (3) a locking-loop configuration with the knot placed outside the repair site, and (4) a peripheral suture placed deep into the tendon and far away from the cut end (Kim et al., 2010). An equally extensive series of studies examining rehabilitation protocols for flexor tendon repairs also exists (Chesney et al., 2011; Small et al., 1989; Thien et al., 2010). Both early active motion protocols and regimens combining passive flexion with active extension result in low rates of tendon re-rupture and good range of motion following repair. Nevertheless, there exists no universally accepted gold standard for suture material or technique, nor rehabilitation protocol. A recent meta-analysis found rates of re-operation of 6%, re-rupture of 4%, and adhesion formation of 4% (Dy et al., 2012). Consequently, many researchers have explored tissue engineering approaches, as discussed below, aimed at enhancing tendon repair (Chang, 2012; Manning et al., 2013). While several strategies show promise in reducing adhesion formation and increasing tensile strength in animal models of flexor tendon injury, none of these regenerative medicine approaches has been implemented in human clinical trials to date.
TENDON TISSUE ENGINEERING
In cases of severe tendon injury, surgical treatments may be used to repair or replace the damaged tendon with autografts, allografts, xenografts, or prosthetic devices (Lui et al., 2011). However, the clinical outcomes remain unsatisfactory due to limitations including donor site morbidity, high failure rates, risk of injury recurrence and limited long-term function recovery (Klepps et al., 2004; Krueger-Franke et al., 1995; Voleti et al., 2012). These limitations have spurred the development of tissue engineering strategies, which apply a combination of cells, scaffolds, bioactive molecules, and ex vivo mechanical stimulation to create functional replacements or to bolster the innate healing of tendon defects (Kuo et al., 2010). Ultimately, tissue engineering aims at improving the quality of healing in order to promote full restoration of tendon function.
Cells
Cells widely used in tendon tissue engineering include tendon fibroblasts (tenocytes), dermal fibroblasts, and mesenchymal stem cells (MSC). Tenocytes, as reviewed in ‘Tendon Development’ above, synthesize the constituents of extracellular matrix such as collagen, proteoglycans and glycoproteins, and have great influence on collagen fiber formation (Canty and Kadler, 2005; Franchi et al., 2007). Isolated human tenocytes are able to synthesize collagen and upregulate expression of Scx, Tnmd, and Dcn in response to growth factors (Qiu et al., 2013). Tendon defects bridged by an autologous tenocyte-engineered tendon in a hen model demonstrated improved mechanical strength and matrix deposition compared with the cell-free scaffold (Cao et al., 2002). Similarly, decellularized rabbit flexor tendons reseeded with autologous tenocytes had the same elastic modulus as normal tendons, but still possessed a decreased ultimate stress (Chong et al., 2009). Importantly, the seeded tenocytes reduced the degradation of a collagen scaffold incubated in culture medium (Tilley et al., 2012), a mechanism which may partly explain the superior mechanical properties of cell-seeded scaffolds.
Despite the advances in tenoctye-based tendon tissue engineering, harvesting autologous tenocytes may cause secondary tendon defects at the donor site. Therefore, dermal fibroblasts have been considered as an alternative cell source to address this limitation, for they are easily accessible and do not cause major donor site morbidity (Van Eijk et al., 2004). An in vivo study in a porcine model showed that dermal fibroblast- and tenocyte-engineered tendons were similar to each other in their gross morphology, histology, and tensile strength (Liu et al., 2006), indicating the potential of dermal fibroblasts for tendon engineering. Likewise, human tendon-like neotissue was generated by using dermal fibroblasts placed under static strain, producing longitudinally aligned collagen fibers and spindle-shaped cells. Additionally, the average collagen fibril diameter and tensile strength increased with time (Deng et al., 2009). Lastly, an injection of dermal fibroblasts suspended in autologous plasma improved the healing of refractory patellar tendinopathy, as shown in a recent clinical trial (Clarke et al., 2011).
Adult MSCs are another promising cell source for tendon tissue engineering. Due to their self-renewal and multi-lineage differentiation potential, MSCs from a variety of tissues, including bone marrow (Tucker et al., 2010), adipose, and tendon, have been applied to tendon tissue engineering. Bone marrow MSCs (BMSC) seeded in polylactide/glycolide (PLGA) suture material demonstrated higher collagen production and DNA content compared with anterior cruciate ligament fibroblasts (ACLF) and skin fibroblasts (Van Eijk et al., 2004). Similarly, rabbit BMSCs seeded in a silk scaffold showed significantly increased ligament-related ECM expression, including tenascin-C and collagen types I and III, as compared to ACLF-seeded scaffolds (Liu et al., 2008a). In another rabbit model, polyglycolic acid (PGA) sheets seeded with BMSCs had enhanced mechanical strength and expression of collagen type I when compared against the cell-free PGA scaffold (Yokoya et al., 2012).
While the tenogenic potential of BMSCs has been extensively studied, less attention has been paid to adipose-derived mesenchymal stem cells (ASCs). Compared to BMSCs, ASCs are (1) harvested by less invasive procedures, (2) available in greater quantities, and (3) demonstrate similar potential to differentiate along multiple mesenchymal lineages (Gimble et al., 2007). Recently, a report showed that GDF-5-treated rat ASCs expressed tendon-specifics markers Scx and Tnmd (Park et al., 2010). Human ASCs seeded onto a mesh derived from hyaluronan (Hyalonect) and placed under mechanical stress formed a vascularized tendon-like structure (Vindigni et al., 2013). Rabbit Achilles tendon defects repaired by a platelet-rich plasma (PRP) gel mixed with ASCs showed improved tensile strength and collagen type I synthesis, as compared with PRP gels alone (Uysal et al., 2012). In another rabbit tendon defect model, cell proliferation was higher in ASCs than tenocytes, though similar rates of collagen synthesis were found between cell types (Kryger et al., 2007).
Resident, tissue-specific, adult stem cells seem to have a higher regenerative potential for the tissue where they reside (Lui and Chan, 2011). In this regard, tendon-derived stem cells (TDSC) have been identified and applied to tendon tissue engineering. TDSCs are a unique cell population in tendons that have universal stem cell characteristics such as clonogenicity, multipotency and self-renewal capacity (Bi et al., 2007). They also possess a high regenerative potential, which is comparable with that of BMSCs (Randelli et al., 2013). TDSCs in fibrin glue, as compared to a fibrin glue alone, promoted superior tendon repair as measured both histologically and biomechanically (Lui and Chan, 2011). Despite the potential advantages of TDCSs, the isolation of autologous cells would present the same donor site morbidities as tenocytes.
Scaffolds
Scaffolds are another critical factor for tendon tissue engineering. They provide biomechanical support to the healing tissue until endogenous cells have deposited native matrix, thereby preventing re-rupture. In addition, scaffolds with desired functionality can improve tendon healing by facilitating cell proliferation, promoting matrix production, and organizing the matrix into functional tendon tissues. Scaffolds can be further modified to improve tendon healing, including approaches such as cellular hybridization, surface modification, growth factor attachment, and mechanical stimulation-mediated cellular remodeling (Liu et al., 2008b). Three major categories of scaffolds are used: (1) native tendon matrices, (2) synthetic polymers, and (3) derivatives of naturally occurring proteins.
Scaffolds derived from tendon matrices could in theory retain both the normal biomechanical and biochemical properties, thereby serving as the ideal biomaterial to support tendon healing. However, before these tissues can be utilized, the native cells must be removed to prevent disease transmission and an immune response (Badylak et al., 2009; Deeken et al., 2011). Thereafter modified allografts or xenografts could be developed into mechanically functional delivery vehicles for cells, gene therapy vectors, or other biological agents. Optimized processing protocols have allowed acellular tendon scaffolds to maintain similar biomechanical properties compared against native tendon tissues (Pridgen et al., 2011). Moreover, a number of ECM proteins and growth factors are highly preserved in acellular tendon (Ning et al., 2012; Pridgen et al., 2011), suggesting potential bio-compatibility and bio-functionality of these scaffolds. In support, extrinsic fibroblasts were successfully cultured on a tri(n-butyl)phosphate (TnBP)-treated patellar tendon in vitro, creating viable tissue-engineered grafts (Cartmell and Dunn, 2004). Similarly, detergent-treated (sodium dodecyl sulfate) rabbit semitendinosus tendons demonstrated a crimp pattern characteristic of native tendon, yet permitted integration of autologous dermal fibroblasts after cell seeding (Tischer et al., 2007). A great number of biodegradable and biocompatible polymers, in particular the α-hydroxy-polyesters, have been used for tendon tissue engineering, including polyglycolic acid (PGA), poly-L-lactic acid (PLLA) and their copolymer polylactic-co-glycolic acid (PLGA), for their biodegradability and material characteristics. In a rabbit Achilles tendon defect model, knitted PLGA seeded with BMSCs exhibited greater tensile strength and deposition of collagen types I and III, as compared to a cell-free scaffold (Ouyang et al., 2002; Ouyang et al., 2003). As reviewed earlier, PGA was used as scaffold and cell carrier to bridge a tendon defect in a hen model and a porcine model, respectively (Cao et al., 2002; Liu et al., 2006). PGA seeded with mouse skeletal muscle derived cells (MDCs) formed a tendon structure with mature collagen fibrils (Chen et al., 2012a). In a similar study, aligned PLLA scaffolds supported cellular proliferation and tenogenic differentiation (Yin et al., 2010).
Despite the advantages of polyesters, they also suffer from several limitations, including an absence of biochemical motifs for cellular attachment an accompanying inability to fully regulate cell activity (Wan et al., 2003). Scaffolds made from natural proteins and their derivatives may address these issue. Since tendon ECMs are mainly composed of collagen type I, scaffolds based on collagen derivatives are highly biocompatible. Collagen derivatives also exhibit better bio-functionality by supporting cell adhesion and cell proliferation better than polyester materials. Collagen gels seeded with rabbit BMSCs contracted around sutures to improve neotissue formation in a rabbit patellar tendon defect (Awad et al., 2003). Similarly, tissue-engineered constructs created by seeding MSCs in a collagen gel/sponge blend improved the histological and mechanical properties in the same rabbit model (Juncosa-Melvin et al., 2006). Collagen-derived scaffolds are also further modifiable by cross-linking or co-fabricating with other materials to enhance the mechanical strength and water resistance (An et al., 2010; Awad et al., 2003; Fessel et al., 2012; Panzavolta et al., 2011).
Beyond collagen, scaffolds made from silk have been used in tendon tissue engineering. Tenogenesis of MSCs is enhanced when seeded on aligned silk fibroin (SF) electrospun fibers, as evidenced by the upregulation of expression of tendon/ligament-related proteins (Teh et al., 2013). When subjected to mechanical stimulation in vitro, human embryonic stem cell-derived mesenchymal stem cells (hESC-MSCs) within a knitted silk-collagen sponge scaffold exhibited tenocyte-like morphology and positively expressed tendon-related gene markers (Chen et al., 2010). TDSCs within the same scaffold were found to improve rabbit rotator cuff healing, exhibiting increased collagen deposition and better structural and biomechanical properties, as compared to the control group (Shen et al., 2012).
In addition to mechanical properties, the topographical cues provided by scaffolds must be carefully considered when engineering tendon constructs. The micro-/nano-structure of material surfaces has been widely reported to modulate cellular behavior. Since tendon tissue is primarily composed of parallel collagen fibers, alignment is an important topographical characteristic to mimic in tendon tissue engineering. The expression of tenomodulin in tenocytes was rescued after being switched from a smooth to microgrooved silicone membrane (Zhu et al., 2010). Moreover, tendon specific markers such as scleraxis and tenomodulin were significantly increased on electrochemically aligned collagen (ELAC) threads when compared to randomly oriented collagen fibers (Kishore et al., 2012) ELAC scaffold-treated rabbit patellar tendon exhibited improved stiffness and fascicle formation (Kishore et al., 2011). In addition to collagen, aligned polyester materials have also been created for tendon tissue engineering. Cell alignment, distribution, and matrix deposition were found to match the nanofiber organization of a PLGA scaffold designed for rotator cuff repair (Moffat et al., 2009). Likewise, TDSCs seeded on aligned PLLA nanofibrous scaffolds demonstrated increased tenogenesis and collagen deposition, together with suppressed osteogenesis (Yin et al., 2010).
Bioactive molecules
Although numerous growth factors have been shown to be active in both tendon development and healing, the application of growth factors to clinical tendon repair remains challenging. Little is known about the synergistic and antagonistic interactions, nor the optimal spatial and temporal distribution, of growth factors that would produce the best effects. However, some success has been achieved in accelerating the healing process while subsequently enhancing the quality of repaired tissue. While the many bioactive mediators of tendon healing have been reviewed elsewhere (Bedi et al., 2012; Molloy et al., 2003), we highlight here the effects of exogenous application of several growth factor identified to be involved in innate tendon healing (see ‘Tendon Injury and Natural Healing’).
The application of IGF-1 to the site of carrageenan-induced inflammation in a rat Achilles tendon showed that IGF-1 was able to mitigate inflammation-induced functional deficits (Kurtz et al., 1999). Additionally, IGF-1 also stimulates the proliferation and migration of fibroblasts at the site of injury, and subsequently increases the production of collagens and other extracellular matrix structures during the remodeling stages. For example, recombinant human IGF-1 stimulated proteoglycan, collagen, noncollagen protein, and DNA synthesis in a dose-dependent manner in rabbit flexor tendons (Abrahamsson, 1997). In human tendon, the collagen fractional synthesis rate (FSR) and procollagen type I N-terminal propeptide (PINP) content were significantly higher in the IGF-I treated leg compared with the control leg (P < 0.05) (Hansen et al., 2012). Like IGF-1, TGF-β is involved in matrix synthesis in tendon healing. There is a significant increase in collagen types I and III production in rabbit tendon cells with the addition of these TGF-β isoforms (Klein et al., 2002). Additionally, TGF-β signaling is able to drive tenocyte differentiation through the induction of Scx, indicating its potential in stem cell-based tendon tissue engineering. Exposure of equine embryo-derived stem cells (ESCs) to TGF-β in vitro produced an upregulation of Scx at the gene and protein level (Barsby and Guest, 2013). Other TGF-β family members have also been used in tendon tissue engineering with some success. For example, GDF-5 (BMP-14)-treated rat ASCs expressed tendon-specific genes including Scx and Tnmd in both 2D and 3D electrospun matrix systems (James and et al., 2011; Park et al., 2010). Likewise, addition of these growth factors to a transected Achilles tendon improved the mechanical properties (Forslund and Aspenberg, 2001).
Another widely used growth factor in tendon tissue engineering is bFGF. A dose-dependent increase in proliferation and the expression of collagen type III was found 7 days post-injury in a rat patellar tendon treated with bFGF (Chan et al., 2000). Consistent with this finding, addition of bFGF significantly increased the number of equine tendon-derived cells and enhanced collagen type III levels (Durgam et al., 2012). A bFGF-releasing nanofibrous scaffold was developed to facilitate BMSC proliferation and tenogenic differentiation. The loaded bFGF was released over a week and successfully promoted tyrosine phosphorylation of seeded BMSCs, resulting in enhanced expression of tenogenic markers with concurrent increases in the deposition of collagen and tenascin-C on the scaffold (Sahoo et al., 2010). As was seen with IGF-1, PDGF-BB stimulated collagen and noncollagen protein production and DNA synthesis in rabbit tendon. These effects occurred in a dose-dependent manner (Yoshikawa and Abrahamsson, 2001). When delivered by fibrin glue, PDGF-BB improved healing of the rabbit medial collateral ligament (MCL) (Hildebrand et al., 1998).
Mechanical Stimulation
As tendons permit the transmission of force from muscles to bone, their mechanical properties have been extensively studied (Wang, 2006; Woo et al., 2006). In the context of tendon injury and repair, it is recognized that controlled mobilization of healing tendons is needed to improve outcomes, although the optimal timing and magnitude of loading is largely debated (Killian et al., 2012b). In animal models, the complete removal of load in healing tendons results in inferior mechanical properties (Galatz et al., 2009; Murrell et al., 1994), while increased loading in the form of exercise is also detrimental to tendon properties if implemented too quickly (Gimbel et al., 2007; Thomopoulos et al., 2003). Although strong clinical evidence for an optimal rehabilitation protocol for tendon injuries does not exist, it is well accepted that healing tissues should be loaded in a controlled manner to promote favorable remodeling and functional outcomes (Killian et al., 2012b).
More recently, the cellular and molecular basis for loading of healing tendons and ligaments has been explored (Wang et al., 2007). Increased cellular proliferation, collagen production, and tenogenic gene expression is found in both fibroblasts and mesenchymal stem cells exposed to static or cyclic uniaxial tension, with the latter promoting greater effects (Altman et al., 2001; Garvin et al., 2003; Kuo and Tuan, 2008; Yang et al., 2004). Expression of Scx was upregulated in a strain- and cycle-dependent manner in a mesenchymal cell line (C3H10T1/2) seeded in a collagen hydrogel (Scott et al., 2011). Mechanical stretch appears to induce tenogenesis through at least two mechanisms with partially independent downstream targets – integrin-dependent signaling and biochemical (i.e., TGF-β) pathways. Xu et al. showed that RhoA/ROCK and focal adhesion kinase regulate mechanical stretch-induced realignment of MSCs by regulating cytoskeletal organization, thereby driving tenogenesis (Xu et al., 2012). Likewise Maeda et al. found that active TGF-β levels in tendons correlated directly when the extent of physical force and Scx expression.(Maeda et al., 2011). However, the disruption of cytoskeletal organization did not affect TGF-β-mediated Scx expression in tenocytes, suggesting that TGF-β regulates Scx expression independently of cytoskeletal tension. This was supported by Chen et al., who found that force and lentiviral-mediated Scleraxis overexpression synergistically promoted tenogenesis of ESC-MSCs (Chen et al., 2012b). Conversely, tendon overuse injuries are thought to result from upregulated expression of catabolic and inflammatory mediators (Riley, 2008; Wang, 2006). Yang et al. (2005) found 4% uniaxial stretching of fibroblasts decreased COX-2 and MMP-1 expression and PGE2 production, while 8% stretching increased these inflammatory products. Likewise, cyclic stretching at 4% strain promoted tenogenic differentiation of TDSCs, while 8% strain induced greater degrees of adipogenic, chondrogenic, and osteogenic differentiation (Zhang and Wang, 2010). Taken together, these in vitro studies support the clinical strategy of controlled mobilization, where progressively greater frequencies and magnitudes of loads are applied to healing tissues to promote tenogenesis of endogenous progenitor cells, increase extracellular matrix synthesis, and minimize inflammatory and catabolic responses.
Beyond improving the biochemical and material properties of healing tendons, mechanical stimulation can be used to precondition tissue engineered constructs prior to implantation, in turn resulting in improved in vivo outcomes (Thorfinn et al., 2012). Cell-seeded decellularized extracellular matrices demonstrated higher ultimate stresses and elastic moduli when exposed to cyclic stretch in bioreactors (Androjna et al., 2007; Angelidis et al., 2010). The augmented mechanical properties are explained in part by cell-mediated remodeling of the extracellular matrix, with cyclic loading promoting increased deposition of collagen fibrils in the direction of strain (Gilbert et al., 2007; Nguyen et al., 2009). Through a series of studies, Butler et al. (2008) showed that matching the material properties of MSC-seeded collagen sponges with those of native tendon could improve the mechanical and histological characteristics of healed tendon in vivo. Interestingly, MSC-seeded collagen constructs produced better results than tendon autografts or constructs made stiffer through dehydrothermal crosslinking, emphasizing the importance of cellular and topographical composition of constructs in restoring native tendon qualities (Kinneberg et al., 2011; Nirmalanandhan et al., 2009).
As highlighted above, recent research has begun to elucidate the role of mechanical stimulation in improving natural tendon healing and in optimizing the properties of cell-seeded constructs fabricated ex vivo. However, the effects of mechanical loading on the integration and remodeling of tissue engineered constructs implanted in vivo are only beginning to emerge (Ambrosio et al., 2010b). Using a mouse model of muscle injury, Ambrosio et al. showed reduced fibrosis and improved integration of muscle-derived stem cells when mice performed treadmill running for 5 weeks following cell implantation (Ambrosio et al., 2010a). Likewise, the ability of growth factors to improve healing when injected into a tendon lesion is dependent upon the mechanical environment of the tissue following injection (Aspenberg, 2007; Forslund and Aspenberg, 2002; Virchenko and Aspenberg, 2006). Using decellularized porcine small intestinal submucosa for Achilles tendon repair in a rabbit model, Hodde et al. (1997) found that complete immobilization of the ankle joint following surgical repair impaired matrix remodeling. While many questions remain, these studies highlight the role of in vivo mechanical loading in promoting the integration and remodeling of tissue engineered constructs. A thorough understanding will be needed for the promise of regenerative medicine to be realized.
CURRENT CHALLENGES AND FUTURE DIRECTIONS
Past research in tissue engineering for improved tendon healing, as highlighted above, took advantage of concurrent discoveries in molecular biology and material science to explore the benefit of applying cells, growth factors, or scaffolds, independently or in combination. Multiple animal studies confirm the possibility of these strategies. However, advances in biomaterials and nanotechnology now allow bioengineers to more closely replicate the complexities of native tissues, thereby enabling finer control of cellular behavior (Lutolf et al., 2009). Refinement of electrospun nanofibrous scaffolds can support tenogenic differentiation of seeded MSCs (Kishore et al., 2012) while approaching the mechanical properties of native tendon (Moffat et al., 2009), thereby offering the prospect of scaffold-augmented tendon repair in the near future. However, the insertion of tendons into the stiffer bone presents a more complex engineering problem. This problem is being pursued through several approaches. Spalazzi et al. have developed triphasic scaffolds capable of supporting spatially-confined cellular phenotypes germane to tendon-bone insertions – fibroblasts, chondrocytes, and osteoblasts (Spalazzi et al., 2008). Others have adopted dual spinnerette pumps containing different polymer solutions to fabricate a gradual transition along an electrospun scaffold (Ramalingam et al., 2013; Samavedi et al., 2012), while Phillips et al. (Phillips et al., 2008) achieved similar results by controlling the spatial distribution of retrovirus encoding the osteogenic transcription Runx2/Cfba1 on an electrospun scaffold. Conversely, Ma et al. (Ma et al., 2012) have created bone-ligament-bone constructs with a functional enthesis by juxtaposing MSC-derived osteoblasts and fibroblasts that are self-contained in their deposited extracellular matrix. While these advances demonstrate the possibility of fabricating graded interfaces containing different cell phenotypes with surrounding matrices of divergent mechanical and biochemical properties, the integration of these ex vivo constructs with native tissue remains a largely unexplored challenge.
Despite the promise of regenerative medicine that has continued to grow since its inception over two decades ago, translation of basic research into clinical therapies has been frustratingly slow. Treatment of tendon injuries is no exception. Part of the challenge in implementing new regenerative approaches in clinical practice concerns economic and practical matters. In particular, cell-based therapies requiring ex-vivo expansion and manipulation of autologous cells require added expense and multiple procedures for tissue harvesting and re-implantation. Consequently, several researchers have argued that the promise of regenerative medicine will be most quickly and effectively realized by focusing on augmenting the natural healing response. This could be achieved by utilizing off-the-shelf scaffolds or growth factors that could recruit and direct endogenous reparative cells to restore native organ function (Evans et al., 2007; Jakob et al., 2012). Likewise, the concurrent application of biophysical modalities offers a non-invasive means of bolstering healing. For instance, low-intensity pulsed ultrasound (Lovric et al., 2013), pulsed electromagnetic fields (Strauch et al., 2006), and extracorporeal shockwave therapy (Chow et al., 2012), can improve the histological and mechanical properties of healing tendon-bone insertions. While biophysical modalities have long been part of rehabilitation therapy protocols for musculoskeletal injuries, recent research probing the effects of these physical agents on the molecular and cellular functions will allow their optimal use in combination with conventional surgical and tissue engineering approaches (Ambrosio et al., 2010b).
Efforts to facilitate endogenous repair by intraoperative or non-invasive means may overcome some of the hurdles currently impeding the translation from the bench to bedside, but basic research that integrates the growing knowledge of developmental biology into the design process will be needed to fully recapitulate native tissue structure and function (Thomopoulos et al., 2010). In particular, elucidation of the expression patterns of transcription factors involved in tendon development (Liu et al., 2011) would allow tissue engineers to apply growth factors in spatiotemporally defined patterns necessary to restore native structure. To that end, biomaterials capable of delivering multiple growth factors at specified times and locations are needed. While the fabrication of such complex biomaterials is still in its infancy, recent advances in controlled delivery suggest the possibility of implementing these scaffolds in the near future (Richardson et al., 2001; Santo et al., 2013). Furthermore, the construction of mathematical models of biological control circuits, based in part on the burgeoning fields of systems biology and network science, could allow the evaluation of explicit hypotheses that would inform process design (Lenas et al., 2009a; b). This would allow regenerative medicine approaches, for tendons and other tissues, to move beyond a trial-and-error empirical endeavor and towards a technology-based discipline with modifiable design parameters.
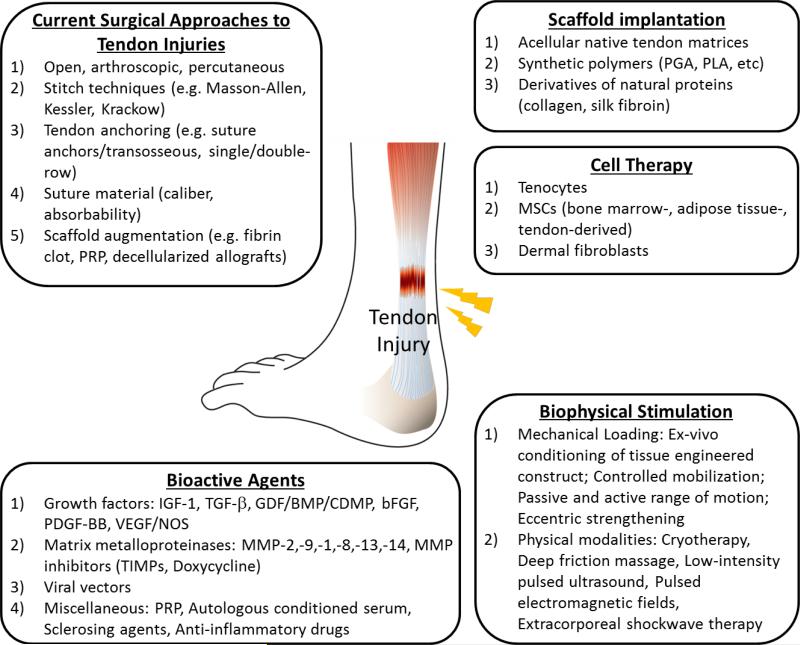
Figure 3.
An overview of approaches for repair of tendon injuries. Briefly, surgical interventions and biophysical stimulation are currently employed in clinical care. Meanwhile, tissue engineering strategies are the cutting-edge of tendon healing and regeneration. Engineered replacement of injured tendon using a combination of cells, bioactive molecules, and scaffolds is under intensive investigation.
ACKNOWLEDGEMENT
This work was supported in part by Commonwealth of Pennsylvania Department of Health (SAP4100050913), NIH (T32-EB001026 and 1R01 AR062947-01A1), and the U.S. Department of Defense (W81XWH-11-2-0143).
REFERENCES
- Abrahamsson SO. Matrix metabolism and healing in the flexor tendon. Experimental studies on rabbit tendon. Scandinavian journal of plastic and reconstructive surgery and hand surgery Supplementum. 1991;23:1–51. [PubMed] [Google Scholar]
- Abrahamsson SO. Similar effects of recombinant human insulin-like growth factor-I and II on cellular activities in flexor tendons of young rabbits: experimental studies in vitro. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1997;15(2):256–262. doi: 10.1002/jor.1100150215. [DOI] [PubMed] [Google Scholar]
- Ackermann PW, Ahmed M, Kreicbergs A. Early nerve regeneration after Achilles tendon rupture - a prerequisite for healing? A study in the rat. Journal of Orthopaedic Research. 2002;20(4):849–856. doi: 10.1016/S0736-0266(01)00159-0. [DOI] [PubMed] [Google Scholar]
- Altman GH, Horan RL, Martin I, Farhadi J, Stark PRH, Volloch V, Richmond JC, Vunjak-Novakovic G, Kaplan DL. Cell differentiation by mechanical stress. The FASEB Journal. 2001 doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- Ambrosio F, Ferrari RJ, Distefano G, Plassmeyer JM, Carvell GE, Deasy BM, Boninger ML, Fitzgerald GK, Huard J. The Synergistic Effect of Treadmill Running on Stem-Cell Transplantation to Heal Injured Skeletal Muscle. Tissue Eng Part A. 2010a;16(3):839–849. doi: 10.1089/ten.tea.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio F, Wolf SL, Delitto A, Fitzgerald GK, Badylak SF, Boninger ML, Russell AJ. The Emerging Relationship Between Regenerative Medicine and Physical Therapeutics. Phys Ther. 2010b;90(12):1807–1814. doi: 10.2522/ptj.20100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel D, Frank C, Harwood F, Fronek J, Akeson W. TENDONS AND LIGAMENTS A MORPHOLOGICAL AND BIOCHEMICAL COMPARISON. Journal of Orthopaedic Research. 1984;1(3):257–265. doi: 10.1002/jor.1100010305. [DOI] [PubMed] [Google Scholar]
- An K, Liu H, Guo S, Kumar DN, Wang Q. Preparation of fish gelatin and fish gelatin/poly(L-lactide) nanofibers by electrospinning. International journal of biological macromolecules. 2010;47(3):380–388. doi: 10.1016/j.ijbiomac.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Andia I, Sanchez M, Maffulli N. Tendon healing and platelet-rich plasma therapies. Expert Opinion on Biological Therapy. 2010;10(10):1415–1426. doi: 10.1517/14712598.2010.514603. [DOI] [PubMed] [Google Scholar]
- Androjna C, Spragg RK, Derwin KA. Mechanical Conditioning of Cell-Seeded Small Intestine Submucosa: A Potential Tissue-Engineering Strategy for Tendon Repair. Tissue Engineering. 2007;13(2):233–243. doi: 10.1089/ten.2006.0050. [DOI] [PubMed] [Google Scholar]
- Angelidis IK, Thorfinn J, Connolly ID, Lindsey D, Pham HM, Chang J. Tissue Engineering of Flexor Tendons: The Effect of a Tissue Bioreactor on Adipoderived Stem Cell-Seeded and Fibroblast-Seeded Tendon Constructs. Journal of Hand Surgery-American. 2010;35A(9):1466–1472. doi: 10.1016/j.jhsa.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Ansorge HL, Meng X, Zhang G, Veit G, Sun M, Klement JF, Beason DP, Soslowsky LJ, Koch M, Birk DE. Type XIV Collagen Regulates Fibrillogenesis PREMATURE COLLAGEN FIBRIL GROWTH AND TISSUE DYSFUNCTION IN NULL MICE. Journal of Biological Chemistry. 2009;284(13):8427–8438. doi: 10.1074/jbc.M805582200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review. International Orthopaedics. 2007;31(6):783–789. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad HA, Boivin GP, Dressler MR, Smith FN, Young RG, Butler DL. Repair of patellar tendon injuries using a cell-collagen composite. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2003;21(3):420–431. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta biomaterialia. 2009;5(1):1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev. 2001;122(7):735–755. doi: 10.1016/s0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Banos CC, Thomas AH, Kuo CK. Collagen fibrillogenesis in tendon development: Current models and regulation of fibril assembly. Birth Defects Research. 2008;84(3):228–244. doi: 10.1002/bdrc.20130. [DOI] [PubMed] [Google Scholar]
- Barber FA, Burns JP, Deutsch A, Labbe MR, Litchfield RB. A Prospective, Randomized Evaluation of Acellular Human Dermal Matrix Augmentation for Arthroscopic Rotator Cuff Repair. Arthroscopy. 2012;28(1):8–15. doi: 10.1016/j.arthro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- Barsby T, Guest D. Transforming Growth Factor Beta3 Promotes Tendon Differentiation of Equine Embryo-derived Stem Cells. Tissue engineering Part A. 2013 doi: 10.1089/ten.TEA.2012.0372. [DOI] [PubMed] [Google Scholar]
- Bayer ML, Yeung CYC, Kadler KE, Qvortrup K, Baar K, Svensson RB, Magnusson SP, Krogsgaard M, Koch M, Kjaer M. The initiation of embryonic-like collagen fibrillogenesis by adult human tendon fibroblasts when cultured under tension. Biomaterials. 2010;31(18):4889–4897. doi: 10.1016/j.biomaterials.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi A, Kawamura S, Ying L, Rodeo SA. Differences in tendon graft healing between the intraarticular and extra-articular ends of a bone tunnel. HSS journal : the musculoskeletal journal of Hospital for Special Surgery. 2009;5(1):51–57. doi: 10.1007/s11420-008-9096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi A, Maak T, Walsh C, Rodeo SA, Grande D, Dines DM, Dines JS. Cytokines in rotator cuff degeneration and repair. J Shoulder Elbow Surg. 2012;21(2):218–227. doi: 10.1016/j.jse.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208(4):471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature medicine. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- Bidder M, Towler DA, Gelberman RH, Boyer MI. Expression of mRNA for vascular endothelial growth factor at the repair site of healing canine flexor tendon. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2000;18(2):247–252. doi: 10.1002/jor.1100180212. [DOI] [PubMed] [Google Scholar]
- Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in-situ - fibril segments undergo postdepositional modifications resulting in linear and lateral growth during matrix development. Developmental Dynamics. 1995;202(3):229–243. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- Boyer MI, Watson JT, Lou J, Manske PR, Gelberman RH, Cai SR. Quantitative variation in vascular endothelial growth factor mRNA expression during early flexor tendon healing: an investigation in a canine model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2001;19(5):869–872. doi: 10.1016/S0736-0266(01)00017-1. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113(2):235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Brent AE, Tabin CJ. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development. 2004;131(16):3885–3896. doi: 10.1242/dev.01275. [DOI] [PubMed] [Google Scholar]
- Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annual review of biomedical engineering. 2004;6:303–329. doi: 10.1146/annurev.bioeng.6.040803.140240. [DOI] [PubMed] [Google Scholar]
- Calleja M, Connell DA. The Achilles Tendon. Seminars in Musculoskeletal Radiology. 2010;14(3):307–322. doi: 10.1055/s-0030-1254520. [DOI] [PubMed] [Google Scholar]
- Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118(Pt 7):1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Cao Y, Liu Y, Liu W, Shan Q, Buonocore SD, Cui L. Bridging tendon defects using autologous tenocyte engineered tendon in a hen model. Plastic and reconstructive surgery. 2002;110(5):1280–1289. doi: 10.1097/01.PRS.0000025290.49889.4D. [DOI] [PubMed] [Google Scholar]
- Carlberg AL, Tuan RS, Hall DJ. Regulation of scleraxis function by interaction with the bHLH protein E47. Molecular Cell Biology Research Communications. 2000;3(2):82–86. doi: 10.1006/mcbr.2000.0195. [DOI] [PubMed] [Google Scholar]
- Cartmell JS, Dunn MG. Development of cell-seeded patellar tendon allografts for anterior cruciate ligament reconstruction. Tissue engineering. 2004;10(7-8):1065–1075. doi: 10.1089/ten.2004.10.1065. [DOI] [PubMed] [Google Scholar]
- Castricini R, Longo UG, De Benedetto M, Panfoli N, Pirani P, Zini R, Maffulli N, Denaro V. Platelet-Rich Plasma Augmentation for Arthroscopic Rotator Cuff Repair A Randomized Controlled Trial. American Journal of Sports Medicine. 2011;39(2):258–265. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- Chahal J, Van Thiel GS, Mall N, Heard W, Bach BR, Cole BJ, Nicholson GP, Verma NN, Whelan DB, Romeo AA. The Role of Platelet-Rich Plasma in Arthroscopic Rotator Cuff Repair: A Systematic Review With Quantitative Synthesis. Arthroscopy. 2012;28(11):1718–1727. doi: 10.1016/j.arthro.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconjugate journal. 2002;19(4-5):287–293. doi: 10.1023/A:1025348417078. [DOI] [PubMed] [Google Scholar]
- Chan BP, Chan KM, Maffulli N, Webb S, Lee KK. Effect of basic fibroblast growth factor. An in vitro study of tendon healing. Clinical orthopaedics and related research. 1997;(342):239–247. [PubMed] [Google Scholar]
- Chan BP, Fu S, Qin L, Lee K, Rolf CG, Chan K. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta orthopaedica Scandinavica. 2000;71(5):513–518. doi: 10.1080/000164700317381234. [DOI] [PubMed] [Google Scholar]
- Chang J. Studies in Flexor Tendon Reconstruction: Biomolecular Modulation of Tendon Repair and Tissue Engineering. J Hand Surg-Am. 2012;37A(3):552–561. doi: 10.1016/j.jhsa.2011.12.028. [DOI] [PubMed] [Google Scholar]
- Chang J, Most D, Stelnicki E, Siebert JW, Longaker MT, Hui K, Lineaweaver WC. Gene expression of transforming growth factor beta-1 in rabbit zone II flexor tendon wound healing: evidence for dual mechanisms of repair. Plastic and reconstructive surgery. 1997;100(4):937–944. doi: 10.1097/00006534-199709001-00016. [DOI] [PubMed] [Google Scholar]
- Chang J, Most D, Thunder R, Mehrara B, Longaker MT, Lineaweaver WC. Molecular studies in flexor tendon wound healing: the role of basic fibroblast growth factor gene expression. The Journal of hand surgery. 1998;23(6):1052–1058. doi: 10.1016/S0363-5023(98)80015-4. [DOI] [PubMed] [Google Scholar]
- Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC. Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg. 2000;105(1):148–155. doi: 10.1097/00006534-200001000-00025. [DOI] [PubMed] [Google Scholar]
- Chen B, Wang B, Zhang WJ, Zhou G, Cao Y, Liu W. In vivo tendon engineering with skeletal muscle derived cells in a mouse model. Biomaterials. 2012a;33(26):6086–6097. doi: 10.1016/j.biomaterials.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Chen CH, Cao Y, Wu YF, Bais AJ, Gao JS, Tang JB. Tendon Healing In Vivo: Gene Expression and Production of Multiple Growth Factors in Early Tendon Healing Period. The Journal of Hand Surgery. 2008;33(10):1834–1842. doi: 10.1016/j.jhsa.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Chen JL, Yin Z, Shen WL, Chen X, Heng BC, Zou XH, Ouyang HW. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials. 2010;31(36):9438–9451. doi: 10.1016/j.biomaterials.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Chen X, Yin Z, Chen JL, Shen WL, Liu HH, Tang QM, Fang Z, Lu LR, Ji JF, Ouyang HW. Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Sci Rep. 2012b;2:9. doi: 10.1038/srep00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney A, Chauhan A, Kattan A, Farrokhyar F, Thoma A. Systematic Review of Flexor Tendon Rehabilitation Protocols in Zone II of the Hand. Plastic and Reconstructive Surgery. 2011;127(4):1583–1592. doi: 10.1097/PRS.0b013e318208d28e. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Renedo AS, Huber F, Fluck M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix biology : journal of the International Society for Matrix Biology. 2003;22(1):73–80. doi: 10.1016/s0945-053x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Tucker RP. Connective tissues: signalling by tenascins. The international journal of biochemistry & cell biology. 2004;36(6):1085–1089. doi: 10.1016/j.biocel.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Chong AK, Riboh J, Smith RL, Lindsey DP, Pham HM, Chang J. Flexor tendon tissue engineering: acellularized and reseeded tendon constructs. Plastic and reconstructive surgery. 2009;123(6):1759–1766. doi: 10.1097/PRS.0b013e3181a65ae7. [DOI] [PubMed] [Google Scholar]
- Chow DHK, Suen PK, Fu LH, Cheung WH, Leung KS, Wong MWN, Qin L. Extracorporeal Shockwave Therapy for Treatment of Delayed Tendon-Bone Insertion Healing in a Rabbit Model A Dose-Response Study. Am J Sports Med. 2012;40(12):2862–2871. doi: 10.1177/0363546512461596. [DOI] [PubMed] [Google Scholar]
- Clarke AW, Alyas F, Morris T, Robertson CJ, Bell J, Connell DA. Skin-derived tenocyte-like cells for the treatment of patellar tendinopathy. The American journal of sports medicine. 2011;39(3):614–623. doi: 10.1177/0363546510387095. [DOI] [PubMed] [Google Scholar]
- Connizzo BK, Yannascoli SM, Soslowsky LJ. Structure-function relationships of postnatal tendon development: A parallel to healing. Matrix biology : journal of the International Society for Matrix Biology. 2013;32(2):106–116. doi: 10.1016/j.matbio.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis - A basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121(4):1099–1110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. Journal of Orthopaedic Research. 2005;23(1):84–92. doi: 10.1016/j.orthres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Deeken CR, White AK, Bachman SL, Ramshaw BJ, Cleveland DS, Loy TS, Grant SA. Method of preparing a decellularized porcine tendon using tributyl phosphate. Journal of biomedical materials research Part B, Applied biomaterials. 2011;96(2):199–206. doi: 10.1002/jbm.b.31753. [DOI] [PubMed] [Google Scholar]
- Dejardin LM, Arnoczky SP, Ewers BJ, Haut RC, Clarke RB. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa - Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29(2):175–184. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- Deng D, Liu W, Xu F, Yang Y, Zhou G, Zhang WJ, Cui L, Cao Y. Engineering human neo-tendon tissue in vitro with human dermal fibroblasts under static mechanical strain. Biomaterials. 2009;30(35):6724–6730. doi: 10.1016/j.biomaterials.2009.08.054. [DOI] [PubMed] [Google Scholar]
- Derwin KA, Baker AR, Iannotti JP, McCarron JA. Preclinical Models for Translating Regenerative Medicine Therapies for Rotator Cuff Repair. Tissue Eng Part B-Rev. 2010;16(1):21–30. doi: 10.1089/ten.teb.2009.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair - Biomechanical, biochemical, and cellular properties. J Bone Joint Surg-Am. 2006;88A(12):2665–2672. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- Dimmen S, Engebretsen L, Nordsletten L, Madsen JE. Negative effects of parecoxib and indomethacin on tendon healing: an experimental study in rats. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):835–839. doi: 10.1007/s00167-009-0763-7. [DOI] [PubMed] [Google Scholar]
- Dines JS, Bedi A, ElAttrache NS, Dines DM. Single-row Versus Double-row Rotator Cuff Repair: Techniques and Outcomes. J Am Acad Orthop Surg. 2010;18(2):83–93. doi: 10.5435/00124635-201002000-00003. [DOI] [PubMed] [Google Scholar]
- Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Molecular and cellular biology. 2005;25(2):699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy FJ, Jr., Seiler JG, Gelberman RH, Hergrueter CA. Growth factors and canine flexor tendon healing: initial studies in uninjured and repair models. The Journal of hand surgery. 1995;20(4):645–649. doi: 10.1016/S0363-5023(05)80284-9. [DOI] [PubMed] [Google Scholar]
- Durgam SS, Stewart AA, Pondenis HC, Yates AC, Evans RB, Stewart MC. Responses of equine tendon- and bone marrow-derived cells to monolayer expansion with fibroblast growth factor-2 and sequential culture with pulverized tendon and insulin-like growth factor-I. American journal of veterinary research. 2012;73(1):162–170. doi: 10.2460/ajvr.73.1.162. [DOI] [PubMed] [Google Scholar]
- Dy CJ, Hernandez-Soria A, Ma Y, Roberts TR, Daluiski A. Complications After Flexor Tendon Repair: A Systematic Review and Meta-Analysis. J Hand Surg-Am. 2012;37A(3):543–551. doi: 10.1016/j.jhsa.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F, Schuler B, Bonnin MA, Teillet MA, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Developmental biology. 2002;247(2):351–366. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- Evans CH, Palmer GD, Pascher A, Porter R, Kwong FN, Gouze E, Gouze JN, Liu F, Steinert A, Betz O, Betz V, Vrahas M, Ghivizzani SC. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Engineering. 2007;13(8):1987–1993. doi: 10.1089/ten.2006.0302. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Weis MA, Wu JJ. Advances in collagen cross-link analysis. Methods. 2008;45(1):65–74. doi: 10.1016/j.ymeth.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata M, Beredjiklian PK, Zgonis MH, Beason DP, Crombleholme TM, Jawad AF, Soslowsky LJ. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. Journal of Orthopaedic Research. 2006;24(11):2124–2132. doi: 10.1002/jor.20271. [DOI] [PubMed] [Google Scholar]
- Fenwick SA, Hazleman BL, Riley GP. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002;4(4):252–260. doi: 10.1186/ar416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessel G, Gerber C, Snedeker JG. Potential of collagen cross-linking therapies to mediate tendon mechanical properties. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons [et al] 2012;21(2):209–217. doi: 10.1016/j.jse.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Forslund C, Aspenberg P. Tendon healing stimulated by injected CDMP-2. Med Sci Sports Exerc. 2001;33(5):685–687. doi: 10.1097/00005768-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Forslund C, Aspenberg P. CDMP-2 induces bone or tendon-like tissue depending on mechanical stimulation. Journal of Orthopaedic Research. 2002;20(6):1170–1174. doi: 10.1016/S0736-0266(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-Rich Plasma From Basic Science to Clinical Applications. American Journal of Sports Medicine. 2009;37(11):2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- Franchi M, Trire A, Quaranta M, Orsini E, Ottani V. Collagen structure of tendon relates to function. TheScientificWorldJOURNAL. 2007;7:404–420. doi: 10.1100/tsw.2007.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi T, Martin SD, Schmid TM, Spector M. Distribution of Lubricin in the Ruptured Human Rotator Cuff and Biceps Tendon: A Pilot Study. Clin Orthop Rel Res. 2010;468(6):1588–1599. doi: 10.1007/s11999-009-1108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg-Am. 2004;86A(2):219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- Galatz LM, Charlton N, Das R, Kim HM, Havlioglu N, Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg. 2009;18(5):669–675. doi: 10.1016/j.jse.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Garner WL, McDonald JA, Koo M, Kuhn C, 3rd, Weeks PM. Identification of the collagen-producing cells in healing flexor tendons. Plastic and reconstructive surgery. 1989;83(5):875–879. doi: 10.1097/00006534-198905000-00018. [DOI] [PubMed] [Google Scholar]
- Garofalo R, Cesari E, Vinci E, Castagna A. Role of Metalloproteinases in Rotator Cuff Tear. Sports Med Arthrosc Rev. 2011;19(3):207–212. doi: 10.1097/JSA.0b013e318227b07b. [DOI] [PubMed] [Google Scholar]
- Garrick JG. Does accelerated functional rehabilitation after surgery improve outcomes in patients with acute achilles tendon ruptures? Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2012;22(4):379–380. doi: 10.1097/JSM.0b013e3182603905. [DOI] [PubMed] [Google Scholar]
- Garvin J, Qi B, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Engineering. 2003;9(5):967–979. doi: 10.1089/107632703322495619. [DOI] [PubMed] [Google Scholar]
- Gialanella B, Prometti P. Effects of Corticosteroids Injection in Rotator Cuff Tears. Pain Med. 2011;12(10):1559–1565. doi: 10.1111/j.1526-4637.2011.01238.x. [DOI] [PubMed] [Google Scholar]
- Gilbert TW, Stewart-Akers AM, Sydeski J, Nguyen TD, Badylak SF, Woo SLY. Gene expression by fibroblasts seeded on small intestinal submucosa and subjected to cyclic stretching. Tissue Engineering. 2007;13(6):1313–1323. doi: 10.1089/ten.2006.0318. [DOI] [PubMed] [Google Scholar]
- Gimbel JA, Van Kleunen JP, Williams GR, Thomopoulos S, Soslowsky LJ. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng-Trans ASME. 2007;129(3):400–404. doi: 10.1115/1.2721075. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circulation research. 2007;100(9):1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gott M, Ast M, Lane LB, Schwartz JA, Catanzano A, Razzano P, Grande DA. Tendon phenotype should dictate tissue engineering modality in tendon repair: a review. Discovery medicine. 2011;12(62):75–84. [PubMed] [Google Scholar]
- Griffin M, Hindocha S, Jordan D, Saleh M, Khan W. An overview of the management of flexor tendon injuries. The Open Orthopaedic Journal. 2012:28–35. doi: 10.2174/1874325001206010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Boesen A, Holm L, Flyvbjerg A, Langberg H, Kjaer M. Local administration of insulin-like growth factor-I (IGF-I) stimulates tendon collagen synthesis in humans. Scandinavian journal of medicine & science in sports. 2012 doi: 10.1111/j.1600-0838.2011.01431.x. [DOI] [PubMed] [Google Scholar]
- Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot & ankle specialist. 2010;3(1):29–32. doi: 10.1177/1938640009355191. [DOI] [PubMed] [Google Scholar]
- Hildebrand KA, Woo SL, Smith DW, Allen CR, Deie M, Taylor BJ, Schmidt CC. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. The American journal of sports medicine. 1998;26(4):549–554. doi: 10.1177/03635465980260041401. [DOI] [PubMed] [Google Scholar]
- Hope M, Saxby TS. Tendon healing. Foot and ankle clinics. 2007;12(4):553–567. v. doi: 10.1016/j.fcl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears - A randomized, controlled trial. J Bone Joint Surg-Am. 2006;88A(6):1238–1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- Jakob F, Ebert R, Rudert M, Noth U, Walles H, Docheva D, Schieker M, Meinel L, Groll J. In situ guided tissue regeneration in musculoskeletal diseases and aging Implementing pathology into tailored tissue engineering strategies. Cell and Tissue Research. 2012;347(3):725–735. doi: 10.1007/s00441-011-1237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R, et al. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomedical Materials. 2011;6(2):025011. doi: 10.1088/1748-6041/6/2/025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R, Kesturu G, Balian G, Chhabra AB. Tendon: Biology, Biomechanics, Repair, Growth Factors, and Evolving Treatment Options. The Journal of Hand Surgery. 2008;33(1):102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28(3):289–297. doi: 10.1002/jor.20999. [DOI] [PubMed] [Google Scholar]
- Jones MP, Khan RJK, Carey Smith RL. Surgical interventions for treating acute achilles tendon rupture: key findings from a recent cochrane review. The Journal of bone and joint surgery American. 2012;94(12):e88–e88. doi: 10.2106/JBJS.J.01829. [DOI] [PubMed] [Google Scholar]
- Juncosa-Melvin N, Boivin GP, Gooch C, Galloway MT, West JR, Dunn MG, Butler DL. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel-collagen sponge constructs used for rabbit patellar tendon repair. Tissue engineering. 2006;12(2):369–379. doi: 10.1089/ten.2006.12.369. [DOI] [PubMed] [Google Scholar]
- Juneja SC, Schwarz EM, O'Keefe RJ, Awad HA. Cellular and molecular factors in flexor tendon repair and adhesions: a histological and gene expression analysis. Connective tissue research. 2013;54(3):218–226. doi: 10.3109/03008207.2013.787418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. The Biochemical journal. 1996;316(Pt 1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon - A controlled-study of 891 patients. J Bone Joint Surg-Am. 1991;73A(10):1507–1525. [PubMed] [Google Scholar]
- Kastelic J, Galeski A, Baer E. MULTICOMPOSITE STRUCTURE OF TENDON. Connect Tissue Res. 1978;6(1):11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Kearney RS, Costa ML. Current concepts in the rehabilitation of an acute rupture of the tendo Achillis. Journal of Bone and Joint Surgery-British. 2012;94B(1):28–31. doi: 10.1302/0301-620X.94B1.28008. [DOI] [PubMed] [Google Scholar]
- Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. Recent advances in shoulder research. Arthritis Res Ther. 2012a;14(3):10. doi: 10.1186/ar3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012b;21(2):228–237. doi: 10.1016/j.jse.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Nelson G, Thomopoulos S, Silva MJ, Das R, Gelberman RH. Technical and Biological Modifications for Enhanced Flexor Tendon Repair. J Hand Surg-Am. 2010;35A(6):1031–1037. doi: 10.1016/j.jhsa.2009.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Chung SW, Kim JY, Ok JH, Park I, Oh JH. Is Early Passive Motion Exercise Necessary After Arthroscopic Rotator Cuff Repair? Am J Sports Med. 2012;40(4):815–821. doi: 10.1177/0363546511434287. [DOI] [PubMed] [Google Scholar]
- Kinneberg KRC, Galloway MT, Butler DL, Shearn JT. Effect of Implanting a Soft Tissue Autograft in a Central-Third Patellar Tendon Defect: Biomechanical and Histological Comparisons. J Biomech Eng-Trans ASME. 2011;133(9):6. doi: 10.1115/1.4004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore V, Bullock W, Sun XH, Van Dyke WS, Akkus O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials. 2012;33(7):2137–2144. doi: 10.1016/j.biomaterials.2011.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore V, Uquillas JA, Dubikovsky A, Alshehabat MA, Snyder PW, Breur GJ, Akkus O. In vivo response to electrochemically aligned collagen bioscaffolds. Journal of biomedical materials research Part B, Applied biomaterials. 2011 doi: 10.1002/jbm.b.31962. [DOI] [PubMed] [Google Scholar]
- Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J. Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. The Journal of hand surgery. 2002;27(4):615–620. doi: 10.1053/jhsu.2002.34004. [DOI] [PubMed] [Google Scholar]
- Kleinert HE, Spokevicius S, Papas NH. History of flexor tendon repair. J Hand Surg-Am. 1995;20 A(3):S46–S52. doi: 10.1016/s0363-5023(95)80169-3. [DOI] [PubMed] [Google Scholar]
- Klepps S, Bishop J, Lin J, Cahlon O, Strauss A, Hayes P, Flatow EL. Prospective evaluation of the effect of rotator cuff integrity on the outcome of open rotator cuff repairs. The American journal of sports medicine. 2004;32(7):1716–1722. doi: 10.1177/0363546504265262. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Itoi E, Minagawa H, Miyakoshi N, Takahashi S, Tuoheti Y, Okada K, Shimada Y. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006;15(3):371–377. doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Koester MC, Dunn WR, Kuhn JE, Spindler KP. The efficacy of subacromial corticosteroid injection in the treatment of rotator cuff disease: A systematic review. J Am Acad Orthop Surg. 2007;15(1):3–11. doi: 10.5435/00124635-200701000-00002. [DOI] [PubMed] [Google Scholar]
- Krueger-Franke M, Siebert CH, Scherzer S. Surgical treatment of ruptures of the Achilles tendon: a review of long-term results. British journal of sports medicine. 1995;29(2):121–125. doi: 10.1136/bjsm.29.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryger GS, Chong AK, Costa M, Pham H, Bates SJ, Chang J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. The Journal of hand surgery. 2007;32(5):597–605. doi: 10.1016/j.jhsa.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Kuo CK, Marturano JE, Tuan RS. Novel strategies in tendon and ligament tissue engineering: Advanced biomaterials and regeneration motifs. Sports medicine, arthroscopy, rehabilitation, therapy & technology : SMARTT. 2010;2:20–20. doi: 10.1186/1758-2555-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CK, Petersen BC, Tuan RS. Spatiotemporal protein distribution of TGF-betas, their receptors, and extracellular matrix molecules during embryonic tendon development. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237(5):1477–1489. doi: 10.1002/dvdy.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CK, Tuan RS. Mechanoactive Tenogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Engineering Part A. 2008;14(10):1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- Kurtz CA, Loebig TG, Anderson DD, DeMeo PJ, Campbell PG. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. The American journal of sports medicine. 1999;27(3):363–369. doi: 10.1177/03635465990270031701. [DOI] [PubMed] [Google Scholar]
- Lejard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MH, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. The Journal of biological chemistry. 2007;282(24):17665–17675. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- Lenas P, Moos M, Jr., Luyten FP. Developmental Engineering: A New Paradigm for the Design and Manufacturing of Cell-Based Products. Part I: From Three-Dimensional Cell Growth to Biomimetics of In Vivo Development. Tissue Eng Part B-Rev. 2009a;15(4):381–394. doi: 10.1089/ten.TEB.2008.0575. [DOI] [PubMed] [Google Scholar]
- Lenas P, Moos M, Jr., Luyten FP. Developmental Engineering: A New Paradigm for the Design and Manufacturing of Cell-Based Products. Part II. From Genes to Networks: Tissue Engineering from the Viewpoint of Systems Biology and Network Science. Tissue Eng Part B-Rev. 2009b;15(4):395–422. doi: 10.1089/ten.TEB.2009.0461. [DOI] [PubMed] [Google Scholar]
- Liu CF, Aschbacher-Smith L, Barthelery NJ, Dyment N, Butler D, Wylie C. What We Should Know Before Using Tissue Engineering Techniques to Repair Injured Tendons: A Developmental Biology Perspective. Tissue Eng Part B-Rev. 2011;17(3):165–176. doi: 10.1089/ten.teb.2010.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CF, Aschbacher-Smith L, Barthelery NJ, Dyment N, Butler D, Wylie C. Spatial and temporal expression of molecular markers and cell signals during normal development of the mouse patellar tendon. Tissue engineering Part A. 2012;18(5-6):598–608. doi: 10.1089/ten.tea.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fan H, Toh SL, Goh JC. A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds. Biomaterials. 2008a;29(10):1443–1453. doi: 10.1016/j.biomaterials.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Liu W, Chen B, Deng D, Xu F, Cui L, Cao Y. Repair of tendon defect with dermal fibroblast engineered tendon in a porcine model. Tissue engineering. 2006;12(4):775–788. doi: 10.1089/ten.2006.12.775. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ramanath HS, Wang DA. Tendon tissue engineering using scaffold enhancing strategies. Trends in biotechnology. 2008b;26(4):201–209. doi: 10.1016/j.tibtech.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Longo UG, Ronga M, Maffulli N. Achilles Tendinopathy. Sports Med Arthrosc Rev. 2009;17(2):112–126. doi: 10.1097/JSA.0b013e3181a3d625. [DOI] [PubMed] [Google Scholar]
- Lorbach O, Tompkins M. Rotator cuff: biology and current arthroscopic techniques. Knee Surgery Sports Traumatology Arthroscopy. 2012;20(6):1003–1011. doi: 10.1007/s00167-012-1901-1. [DOI] [PubMed] [Google Scholar]
- Lorda-Diez CI, Montero JA, Martinez-Cue C, Garcia-Porrero JA, Hurle JM. Transforming growth factors beta coordinate cartilage and tendon differentiation in the developing limb mesenchyme. The Journal of biological chemistry. 2009;284(43):29988–29996. doi: 10.1074/jbc.M109.014811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovric V, Ledger M, Goldberg J, Harper W, Bertollo N, Pelletier MH, Oliver RA, Yu Y, Walsh WR. The effects of Low-intensity Pulsed Ultrasound on tendon-bone healing in a transosseous-equivalent sheep rotator cuff model. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):466–475. doi: 10.1007/s00167-012-1972-z. [DOI] [PubMed] [Google Scholar]
- Lui PP, Chan KM. Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem cell reviews. 2011;7(4):883–897. doi: 10.1007/s12015-011-9276-0. [DOI] [PubMed] [Google Scholar]
- Lui PP, Rui YF, Ni M, Chan KM. Tenogenic differentiation of stem cells for tendon repair-what is the current evidence? Journal of tissue engineering and regenerative medicine. 2011;5(8):e144–163. doi: 10.1002/term.424. [DOI] [PubMed] [Google Scholar]
- Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462(7272):433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JJ, Smietana MJ, Kostrominova TY, Wojtys EM, Larkin LM, Arruda EM. Three-Dimensional Engineered Bone-Ligament-Bone Constructs for Anterior Cruciate Ligament Replacement. Tissue Engineering Part A. 2012;18(1-2):103–116. doi: 10.1089/ten.tea.2011.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, Ilic D, Baskaran H, Sakai T. Conversion of Mechanical Force into TGF-beta-Mediated Biochemical Signals. Current Biology. 2011;21(11):933–941. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffulli N, Longo UG, Maffulli GD, Khanna A, Denaro V. Achilles Tendon Ruptures in Elite Athletes. Foot & Ankle International. 2011;32(1):9–15. doi: 10.3113/FAI.2011.0009. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Sharma P, Luscombe KL. Achilles tendinopathy: aetiology and management. Journal of the Royal Society of Medicine. 2004;97(10):472–476. doi: 10.1258/jrsm.97.10.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning CN, Schwartz AG, Liu W, Xie J, Havlioglu N, Sakiyama-Elbert SE, Silva MJ, Xia Y, Gelberman RH, Thomopoulos S. Controlled delivery of mesenchymal stem cells and growth factors using a nanofiber scaffold for tendon repair. Acta Biomater. 2013;9(6):6905–6914. doi: 10.1016/j.actbio.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc Natl Acad Sci U S A. 2013;110(16):6370–6375. doi: 10.1073/pnas.1300135110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikic B, Entwistle R, Rossmeier K, Bierwert L. Effect of GDF-7 deficiency on tail tendon phenotype in mice. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26(6):834–839. doi: 10.1002/jor.20581. [DOI] [PubMed] [Google Scholar]
- Mikic B, Rossmeier K, Bierwert L. Identification of a tendon phenotype in GDF6 deficient mice. Anat Rec (Hoboken) 2009;292(3):396–400. doi: 10.1002/ar.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikic B, Schalet BJ, Clark RT, Gaschen V, Hunziker EB. GDF-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the Achilles tendon. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2001;19(3):365–371. doi: 10.1016/S0736-0266(00)90018-4. [DOI] [PubMed] [Google Scholar]
- Miyashita H, Ochi M, Ikuta Y. Histological and biomechanical observations of the rabbit patellar tendon after removal of its central one-third. Arch Orthop Trauma Surg. 1997;116(8):454–462. doi: 10.1007/BF00387577. [DOI] [PubMed] [Google Scholar]
- Moffat KL, Kwei AS, Spalazzi JP, Doty SB, Levine WN, Lu HH. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng Part A. 2009;15(1):115–126. doi: 10.1089/ten.tea.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy T, Wang Y, Murrell GAC. The Roles of Growth Factors in Tendon and Ligament Healing. Sports Medicine. 2003;33(5):381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: An in vivo study. Journal of Orthopaedic Research. 2009;27(5):639–645. doi: 10.1002/jor.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell GAC, Lilly EG, Goldner RD, Seaber AV, Best TM. EFFECTS OF IMMOBILIZATION ON ACHILLES-TENDON HEALING IN A RAT MODEL. Journal of Orthopaedic Research. 1994;12(4):582–591. doi: 10.1002/jor.1100120415. [DOI] [PubMed] [Google Scholar]
- Murrell GAC, Szabo C, Hannafin JA, Jang D, Dolan MM, Deng XH, Murrell DF, Warren RF. Modulation of tendon healing by nitric oxide. Inflammation Research. 1997;46(1):19–27. doi: 10.1007/s000110050027. [DOI] [PubMed] [Google Scholar]
- Natsu-ume T, Nakamura N, Shino K, Toritsuka Y, Horibe S, Ochi T. Temporal and spatial expression of transforming growth factor-beta in the healing patellar ligament of the rat. Journal of Orthopaedic Research. 1997;15(6):837–843. doi: 10.1002/jor.1100150608. [DOI] [PubMed] [Google Scholar]
- Nelson GN, Potter R, Ntouvali E, Silva MJ, Boyer MI, Gelberman RH, Thomopoulos S. Intrasynovial flexor tendon repair: A biomechanical study of variations in suture application in human cadavera. Journal of Orthopaedic Research. 2012;30(10):1652–1659. doi: 10.1002/jor.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsham-West R, Nicholson H, Walton M, Milburn P. Long-term morphology of a healing bone-tendon interface: a histological observation in the sheep model. Journal of Anatomy. 2007;210(3):318–327. doi: 10.1111/j.1469-7580.2007.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo M, Pham H, Longaker MT, Chang J. Differential expression of transforming growth factor-beta receptors in a rabbit zone II flexor tendon wound healing model. Plastic and reconstructive surgery. 2001;108(5):1260–1267. doi: 10.1097/00006534-200110000-00025. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Liang R, Woo SLY, Burton SD, Wu CF, Almarza A, Sacks MS, Abramowitch S. Effects of Cell Seeding and Cyclic Stretch on the Fiber Remodeling in an Extracellular Matrix-Derived Bioscaffold. Tissue Eng Part A. 2009;15(4):957–963. doi: 10.1089/ten.tea.2007.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning LJ, Zhang Y, Chen XH, Luo JC, Li XQ, Yang ZM, Qin TW. Preparation and characterization of decellularized tendon slices for tendon tissue engineering. Journal of biomedical materials research Part A. 2012;100(6):1448–1456. doi: 10.1002/jbm.a.34083. [DOI] [PubMed] [Google Scholar]
- Nirmalanandhan VS, Juncosa-Melvin N, Shearn JT, Boivin GP, Galloway MT, Gooch C, Bradica G, Butler DL. Combined Effects of Scaffold Stiffening and Mechanical Preconditioning Cycles on Construct Biomechanics, Gene Expression, and Tendon Repair Biomechanics. Tissue Eng Part A. 2009;15(8):2103–2111. doi: 10.1089/ten.tea.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JH, Jun BJ, McGarry MH, Lee TQ. Does a Critical Rotator Cuff Tear Stage Exist? A Biomechanical Study of Rotator Cuff Tear Progression in Human Cadaver Shoulders. J Bone Joint Surg-Am. 2011;93A(22):2100–2109. doi: 10.2106/JBJS.J.00032. [DOI] [PubMed] [Google Scholar]
- Oshiro W, Lou JR, Xing XY, Tu YZ, Manske PR. Flexor tendon healing in the rat: A histologic and gene expression study. J Hand Surg-Am. 2003;28A(5):814–823. doi: 10.1016/s0363-5023(03)00366-6. [DOI] [PubMed] [Google Scholar]
- Ouyang HW, Goh JC, Mo XM, Teoh SH, Lee EH. The efficacy of bone marrow stromal cell-seeded knitted PLGA fiber scaffold for Achilles tendon repair. Annals of the New York Academy of Sciences. 2002;961:126–129. doi: 10.1111/j.1749-6632.2002.tb03064.x. [DOI] [PubMed] [Google Scholar]
- Ouyang HW, Goh JC, Thambyah A, Teoh SH, Lee EH. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue engineering. 2003;9(3):431–439. doi: 10.1089/107632703322066615. [DOI] [PubMed] [Google Scholar]
- Panzavolta S, Gioffre M, Focarete ML, Gualandi C, Foroni L, Bigi A. Electrospun gelatin nanofibers: optimization of genipin cross-linking to preserve fiber morphology after exposure to water. Acta biomaterialia. 2011;7(4):1702–1709. doi: 10.1016/j.actbio.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Park A, Hogan MV, Kesturu GS, James R, Balian G, Chhabra AB. Adipose-Derived Mesenchymal Stem Cells Treated with Growth Differentiation Factor-5 Express Tendon-Specific Markers. Tissue Eng Part A. 2010;16(9):2941–2951. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons BO, Gruson KI, Chen DD, Harrison AK, Gladstone J, Flatow EL. Does slower rehabilitation after arthroscopic rotator cuff repair lead to long-term stiffness? J Shoulder Elbow Surg. 2010;19(7):1034–1039. doi: 10.1016/j.jse.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Pennisi E. Tending tender tendons. Science. 2002;295(5557):1011. doi: 10.1126/science.295.5557.1011. [DOI] [PubMed] [Google Scholar]
- Petersen W, Unterhauser F, Pufe T, Zantop T, Sudkamp NP, Weiler A. The angiogenic peptide vascular endothelial growth factor (VEGF) is expressed during the remodeling of free tendon grafts in sheep. Archives of orthopaedic and trauma surgery. 2003;123(4):168–174. doi: 10.1007/s00402-002-0462-z. [DOI] [PubMed] [Google Scholar]
- Phillips JE, Burns KL, Le Doux JM, Guldberg RE, Garcia AJ. Engineering graded tissue interfaces. Proc Natl Acad Sci U S A. 2008;105(34):12170–12175. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RM, Deuel TF. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. The Journal of cell biology. 1989;109(1):429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgen BC, Woon CY, Kim M, Thorfinn J, Lindsey D, Pham H, Chang J. Flexor tendon tissue engineering: acellularization of human flexor tendons with preservation of biomechanical properties and biocompatibility. Tissue engineering Part C, Methods. 2011;17(8):819–828. doi: 10.1089/ten.tec.2010.0457. [DOI] [PubMed] [Google Scholar]
- Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136(8):1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Wang X, Zhang Y, Carr AJ, Zhu L, Xia Z, Sabokbar A. Development of a refined tenocyte differentiation culture technique for tendon tissue engineering. Cells, tissues, organs. 2013;197(1):27–36. doi: 10.1159/000341426. [DOI] [PubMed] [Google Scholar]
- Ramalingam M, Young MF, Thomas V, Sun LM, Chow LC, Tison CK, Chatterjee K, Miles WC, Simon CG. Nanofiber scaffold gradients for interfacial tissue engineering. J Biomater Appl. 2013;27(6):695–705. doi: 10.1177/0885328211423783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randelli P, Conforti E, Piccoli M, Ragone V, Creo P, Cirillo F, Masuzzo P, Tringali C, Cabitza P, Tettamanti G, Gagliano N, Anastasia L. Isolation and Characterization of 2 New Human Rotator Cuff and Long Head of Biceps Tendon Cells Possessing Stem Cell-Like Self-Renewal and Multipotential Differentiation Capacity. The American journal of sports medicine. 2013 doi: 10.1177/0363546512473572. [DOI] [PubMed] [Google Scholar]
- Ricchetti ET, Aurora A, Iannotti JP, Derwin KA. Scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2012;21(2):251–265. doi: 10.1016/j.jse.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nature Biotechnology. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- Riley G. Tendinopathy - from basic science to treatment. Nat Clin Pract Rheumatol. 2008;4(2):82–89. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- Ruiz-Moneo P, Molano-Munoz J, Prieto E, Algorta J. Plasma rich in growth factors in arthroscopic rotator cuff repair: a randomized, double-blind, controlled clinical trial. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2013;29(1):2–9. doi: 10.1016/j.arthro.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Sadoghi P, Rosso C, Valderrabano V, Leithner A, Vavken P. The role of platelets in the treatment of Achilles tendon injuries. Journal of Orthopaedic Research. 2013;31(1):111–118. doi: 10.1002/jor.22199. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Ang LT, Cho-Hong Goh J, Toh SL. Bioactive nanofibers for fibroblastic differentiation of mesenchymal precursor cells for ligament/tendon tissue engineering applications. Differentiation; research in biological diversity. 2010;79(2):102–110. doi: 10.1016/j.diff.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Samavedi S, Guelcher SA, Goldstein AS, Whittington AR. Response of bone marrow stromal cells to graded co-electrospun scaffolds and its implications for engineering the ligament-bone interface. Biomaterials. 2012;33(31):7727–7735. doi: 10.1016/j.biomaterials.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Sanchez M, Anitua E, Azofra J, Andia I, Padilla S, Mujika I. Comparison of surgically repaired achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245–251. doi: 10.1177/0363546506294078. [DOI] [PubMed] [Google Scholar]
- Santo VE, Gomes ME, Mano JF, Reis RL. Controlled release strategies for bone, cartilage, and osteochondral engineering-part I: recapitulation of native tissue healing and variables for the design of delivery systems. Tissue engineering Part B, Reviews. 2013;19(4):308–326. doi: 10.1089/ten.teb.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous Platelets Have No Effect on the Healing of Human Achilles Tendon Ruptures A Randomized Single-Blind Study. American Journal of Sports Medicine. 2011;39(1):38–47. doi: 10.1177/0363546510383515. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128(19):3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Sciore P, Boykiw R, Hart DA. Semiquantitative reverse transcription-polymerase chain reaction analysis of mRNA for growth factors and growth factor receptors from normal and healing rabbit medial collateral ligament tissue. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1998;16(4):429–437. doi: 10.1002/jor.1100160406. [DOI] [PubMed] [Google Scholar]
- Scott A, Danielson P, Abraham T, Fong G, Sampaio AV, Underhill TM. Mechanical force modulates scleraxis expression in bioartificial tendons. J Musculoskelet Neuronal Interact. 2011;11(2):124–132. [PubMed] [Google Scholar]
- Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6(2):181–190. [PubMed] [Google Scholar]
- Shen W, Chen J, Yin Z, Chen X, Liu H, Heng BC, Chen W, Ouyang HW. Allogenous tendon stem/progenitor cells in silk scaffold for functional shoulder repair. Cell transplantation. 2012;21(5):943–958. doi: 10.3727/096368911X627453. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Developmental biology. 2006;298(1):234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Small JO, Brennen MD, Colville J. EARLY ACTIVE MOBILIZATION FOLLOWING FLEXOR TENDON REPAIR IN ZONE-2. J Hand Surg-Br Eur. 1989;14B(4):383–391. doi: 10.1016/0266-7681_89_90152-6. [DOI] [PubMed] [Google Scholar]
- Spalazzi JP, Dagher E, Doty SB, Guo XE, Rodeo SA, Lu HH. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. Journal of Biomedical Materials Research Part A. 2008;86A(1):1–12. doi: 10.1002/jbm.a.32073. [DOI] [PubMed] [Google Scholar]
- Strauch B, Patel MK, Rosen DJ, Mahadevia S, Brindzei N, Pilla AA. Pulsed magnetic field therapy increases tensile strength in a rat Achilles’ tendon repair model. J Hand Surg-Am. 2006;31A(7):1131–1135. doi: 10.1016/j.jhsa.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhou XH, Wu B, Tian M. Inhibitory Effect of Synovial Fluid on Tendon-to-Bone Healing: An Experimental Study in Rabbits. Arthroscopy-the Journal of Arthroscopic and Related Surgery. 2012;28(9):1297–1305. doi: 10.1016/j.arthro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Tallon C, Maffulli N, Ewen SWB. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Medicine and Science in Sports and Exercise. 2001;33(12):1983–1990. doi: 10.1097/00005768-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Teh TK, Toh SL, Goh JC. Aligned fibrous scaffolds for enhanced mechanoresponse and tenogenesis of mesenchymal stem cells. Tissue engineering Part A. 2013;19(11-12):1360–1372. doi: 10.1089/ten.TEA.2012.0279. [DOI] [PubMed] [Google Scholar]
- Thien TB, Becker JH, Theis J-C. Rehabilitation after surgery for flexor tendon injuries in the hand. Cochrane Database of Systematic Reviews. 2010;(10) doi: 10.1002/14651858.CD003979.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Genin GM, Galatz LM. The development and morphogenesis of the tendon-to-bone insertion - What development can teach us about healing. J Musculoskelet Neuronal Interact. 2010;10(1):35–45. [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: Differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng-Trans ASME. 2003;125(1):106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- Thorfinn J, Angelidis IK, Gigliello L, Pham HM, Lindsey D, Chang J. Bioreactor optimization of tissue engineered rabbit flexor tendons in vivo. Journal of Hand Surgery-European. 2012;37E(2):109–114. doi: 10.1177/1753193411419439. [DOI] [PubMed] [Google Scholar]
- Tilley JM, Chaudhury S, Hakimi O, Carr AJ, Czernuszka JT. Tenocyte proliferation on collagen scaffolds protects against degradation and improves scaffold properties. Journal of materials science Materials in medicine. 2012;23(3):823–833. doi: 10.1007/s10856-011-4537-7. [DOI] [PubMed] [Google Scholar]
- Tischer T, Vogt S, Aryee S, Steinhauser E, Adamczyk C, Milz S, Martinek V, Imhoff AB. Tissue engineering of the anterior cruciate ligament: a new method using acellularized tendon allografts and autologous fibroblasts. Archives of orthopaedic and trauma surgery. 2007;127(9):735–741. doi: 10.1007/s00402-007-0320-0. [DOI] [PubMed] [Google Scholar]
- Tozer S, Duprez D. Tendon and ligament: Development, repair and disease. Birth Defects Research. 2005;75(3):226–236. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- Tucker BA, Karamsadkar SS, Khan WS, Pastides P. The role of bone marrow derived mesenchymal stem cells in sports injuries. Journal of stem cells. 2010;5(4):155–166. [PubMed] [Google Scholar]
- Uysal CA, Tobita M, Hyakusoku H, Mizuno H. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2012;65(12):1712–1719. doi: 10.1016/j.bjps.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Van Eijk F, Saris DB, Riesle J, Willems WJ, Van Blitterswijk CA, Verbout AJ, Dhert WJ. Tissue engineering of ligaments: a comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblasts as cell source. Tissue engineering. 2004;10(5-6):893–903. doi: 10.1089/1076327041348428. [DOI] [PubMed] [Google Scholar]
- Vindigni V, Tonello C, Lancerotto L, Abatangelo G, Cortivo R, Zavan B, Bassetto F. Preliminary Report of In Vitro Reconstruction of a Vascularized Tendonlike Structure: A Novel Application for Adipose-Derived Stem Cells. Annals of plastic surgery. 2013 doi: 10.1097/SAP.0b013e3182583e99. [DOI] [PubMed] [Google Scholar]
- Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthopaedica. 2006;77(5):806–812. doi: 10.1080/17453670610013033. [DOI] [PubMed] [Google Scholar]
- Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004;32(7):1743–1747. doi: 10.1177/0363546504263403. [DOI] [PubMed] [Google Scholar]
- Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annual review of biomedical engineering. 2012;14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- Wan Y, Chen W, Yang J, Bei J, Wang S. Biodegradable poly(L-lactide)-poly(ethylene glycol) multiblock copolymer: synthesis and evaluation of cell affinity. Biomaterials. 2003;24(13):2195–2203. doi: 10.1016/s0142-9612(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Wang JHC. Mechanobiology of tendon. Journal of Biomechanics. 2006;39(9):1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Wang JHC, Thampatty BP, Lin J-S, Im H-J. Mechanoregulation of gene expression in fibroblasts. Gene. 2007;391(1-2):1–15. doi: 10.1016/j.gene.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. The Journal of biological chemistry. 2004;279(51):53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ, Wozney JM, Rosen V. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. The Journal of Clinical Investigation. 1997;100(2):321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SLY, Abramowitch SD, Kilger R, Liang R. Biomechanics of knee ligaments: injury, healing, and repair. Journal of Biomechanics. 2006;39(1):1–20. doi: 10.1016/j.jbiomech.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Wurgler-Hauri CC, Dourte LM, Baradet TC, Williams GR, Soslowsky LJ. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg. 2007;16(5):198S–203S. doi: 10.1016/j.jse.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu BY, Song GB, Ju Y, Li X, Song YH, Watanabe S. RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. Journal of Cellular Physiology. 2012;227(6):2722–2729. doi: 10.1002/jcp.23016. [DOI] [PubMed] [Google Scholar]
- Yang G, Crawford RC, Wang JHC. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. Journal of Biomechanics. 2004;37(10):1543–1550. doi: 10.1016/j.jbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Yin Z, Chen X, Chen JL, Shen WL, Hieu Nguyen TM, Gao L, Ouyang HW. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31(8):2163–2175. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- Yokoya S, Mochizuki Y, Natsu K, Omae H, Nagata Y, Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. The American journal of sports medicine. 2012;40(6):1259–1268. doi: 10.1177/0363546512442343. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Abrahamsson SO. Dose-related cellular effects of platelet-derived growth factor-BB differ in various types of rabbit tendons in vitro. Acta orthopaedica Scandinavica. 2001;72(3):287–292. doi: 10.1080/00016470152846646. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. Journal of cellular biochemistry. 2006;98(6):1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: Regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005;5(1):5–21. [PubMed] [Google Scholar]
- Zhang J, Wang JHC. Mechanobiological response of tendon stem cells: Implications of tendon homeostasis and pathogenesis of tendinopathy. Journal of Orthopaedic Research. 2010;28(5):639–643. doi: 10.1002/jor.21046. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Keenan C, Wang JHC. The effects of dexamethasone on human patellar tendon stem cells: Implications for dexamethasone treatment of tendon injury. Journal of Orthopaedic Research. 2013;31(1):105–110. doi: 10.1002/jor.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZP, Akinbiyi T, Xu LL, Ramcharan M, Leong DJ, Ros SJ, Colvin AC, Schaffler MB, Majeska RJ, Flatow EL, Sun HB. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010;9(5):911–915. doi: 10.1111/j.1474-9726.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Li J, Wang B, Zhang WJ, Zhou G, Cao Y, Liu W. The regulation of phenotype of cultured tenocytes by microgrooved surface structure. Biomaterials. 2010;31(27):6952–6958. doi: 10.1016/j.biomaterials.2010.05.058. [DOI] [PubMed] [Google Scholar]