Abstract
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that controls cell proliferation, growth, survival, metabolism, and migration by activating the PI3K (phosphoinositide 3-kinase)-AKT and ERK (extracellular signal regulated kinase)-RSK (ribosomal S6 kinase) pathways. EGFR signaling to these pathways is temporally and spatially regulated. Endocytic trafficking controls the access of EGFR to these downstream effectors and also its degradation, which terminates EGFR signaling. Here, we showed that AKT facilitated the endocytic trafficking of EGFR to promote its degradation. Interfering with AKT signaling reduced both EGFR recycling and the rate of EGFR degradation. In AKT-impaired cells EGFRs were unable to reach the cell surface or the lysosomal compartment and accumulated in the early endosomes, resulting in prolonged signaling and increased activation of ERK and RSK. Upon EGF stimulation, AKT phosphorylated and activated the kinase PIKfyve, which promotes vesicle trafficking to lysosomes. PIKfyve activation promoted EGFR degradation. Similar regulation occurred with platelet-derived growth factor receptor (PDGFR), suggesting that AKT phosphorylation and activation of PIKfyve is likely to be a common feedback mechanism for terminating receptor tyrosine kinase signaling and reducing receptor abundance.
INTRODUCTION
EGFR is a major regulator of cell proliferation, growth, survival, metabolism and motility and is over-expressed or inappropriately activated in many cancers (1, 2). EGFR carries out these functions by activating multiple signaling cascades, including the phosphatidylinositol-3 kinase (PI3K)-AKT, mammalian target of rapamycin complex 1 – p70 ribosomal S6 kinase (mTORC1-S6K), and extracellular signal regulated kinase - p90 ribosomal S6 Kinase (ERK-RSK) pathways. PI3K, mTORC1 and ERK variably induce distinct cellular functions depending the cell type, cell-cycle time, and magnitutde and duration of pathway activation (3). Upon ligand binding, EGFR molecules trans-phosphorylate each other on multiple tyrosine residues to create docking sites for the Grb2 and GAB1 adapter proteins (4). GAB1 recruitment results in EGFR activation of type 1 PI3Ks, which generate phosphatidylinositol -3,4,5- trisphosphate (PI(3,4,5)P3). Pleckstrin homology (PH) domains in AKT, PDK1 and other molecules recognize PI(3,4,5)P3 and the interaction brings AKT and PDK1 to the plasma membrane. PDK1 and mammalian target of rapamycin complex 2 (mTORC2) then activate AKT by phosphorylating AKT at Thr308 and Ser473, respectively. Grb2 recruitment to EGFR results in activation of the RAS-RAF-MEK-ERK-RSK signaling cascade. In response to EGF stimulation, AKT, ERK and RSK all contribute to the activation of the mTORC1 – S6K pathway. EGFR and these downstream signaling pathways are regulated through a network of feedback and cross-talk mechanisms (3).
Receptor endocytosis is a regulatory mechanism that promotes sustained and spatially-regulated signaling by localizing receptors to signaling endosomes and by promoting receptor recycling to the cell surface (5–7). Alternatively, endocytosis can lead to signal attenuation by culminating in receptor degradation. EGFR endocytosis is initiated by EGF binding to EGFR dimers at the plasma membrane (8). Stabilization of EGFR dimers promotes EGFR activation and trans-phosphorylation. Active EGFR is ubiquitinated by the E3 ligase Cbl, a posttranslational modification that recruits the endocytic machinery. Both clathrin-dependent (9) and clathrin-independent (10, 11) pathways contribute to EGFR endocytosis. Receptor internalization is followed by localization to early endosome antigen 1 (EEA.1)-positive endosomes, where cargo destined for recycling or degradation are separated (12, 13). EGFR molecules are recycled back to the plasma membrane from the early endosomes and the limiting membrane of multivesicular bodies (MVBs) in a Rab4- and Rab11-dependent manner. Recycled EGFRs engage in additional rounds of endocytosis and signaling (14). Alternatively, protein tyrosine phosphatase 1B (PTP1B) can dephosphorylate EGFR at the limiting membrane of MVBs (15, 16). De-phosphorylated EGFRs enter the MVBs lumen through the endosomal sorting complex for transport (ESCRT) complexes (10, 17). These EGFRs are dissociated from signal-transducing molecules and signaling is terminated. These EGFRs are destined for degradation in the lysosomes.
Proteins involved in EGFR sorting and degradation such as EEA.1 and ESCRT proteins are recruited to the endocytic vesicles through their interaction with phosphoinositides. For example, endomembranes contain phosphatidyl-inositol-3-phosphate (PI3P), which is recognized by FYVE (Fab1, YOTB, Vac1, EEA.1) domains found in these respective proteins. Fab1 is a phosphoinositide kinase that phosphorylates PI3P to generate PI(3,5)P2. The phosphoinositide phosphatase SAC3 dephosphorylates PI(3,5)P2 at the 5 position to generate PI3P (18). In yeast, deletion of Fab1 disrupts cargo sorting to the yeast vacuoles (19). The human homolog of Fab1 is called PIKfyve [FYVE-containing phosphoinositide 3-phosphate (PI3P) 5 kinase], and forms a complex with ArPIKfyve (associated regulator of PIKfyve) and SAC3 at the endomembranes. PIKfyve, facilitates and SAC3 inhibits the progression of early endosomes towards MVBs, suggesting that PI(3,5)P2 promotes and PI3P inhibits vesicle progression (20). However, the regulation of the PIKfyve-ArPIKfyve-SAC3 complex in mammalian cells is not well understood.
Previous work has implicated type I PI3Ks, which are activated by Insulin and EGF, in the modulation of vesicular trafficking. Mutagenesis of the binding site of platelet derived growth factor receptor (PDGFR) for the type I PI3K regulatory subunit p85 blocks degradation of PDGFR (21). The recruitment of the PI3K catalytic subunit p110 to the plasma membrane accelerates clathrin coat dynamics (22). Injection of p110α-blocking antibodies causes transferrin to accumulate within the cells, suggesting the antibodies inhibit transferrin recycling (23). The PI3K inhibitor wortmannin also suppresses transferrin recycling in cells and reduces the rate of endosomal sorting in cell free systems (24, 25). Two developments prompted us to investigate whether PI3K signals through AKT to modulate trafficking: AKT inhibitors increase the protein abundance of several RTKs and AKT knockdown reduces transferrin and EGF uptake (26, 27).
We investigated the role of AKT in EGFR trafficking and discovered a negative feedback loop in which EGF-mediated activation of AKT promotes EGFR progression through the early endosomes and EGFR degradation by activating PIKfyve. Multiple AKT inhibitors and AKT knockdown reduce EGFR progression through the early endosomes, the rate of EGFR degradation and PIKfyve activity in vitro and in cells. Similarly, knocking down PIKfyve or its activator ArPIKfyve or treatment with an inhibitor of PIKfyve reduce the rate of EGFR degradation. The reduced rate of EGFR degradation produced by AKT inhibition is rescued by knockdown of the phosphatase SAC3. Further, expressing a PIKfyve mutant that cannot be phosphorylated by AKT reduces EGFR degradation. We propose a model in which AKT phosphorylates and activates PIKfyve to facilitate EGFR endosomal progression, thereby increasing EGFR degradation and dampening EGFR signaling.
RESULTS
AKT promotes EGFR degradation
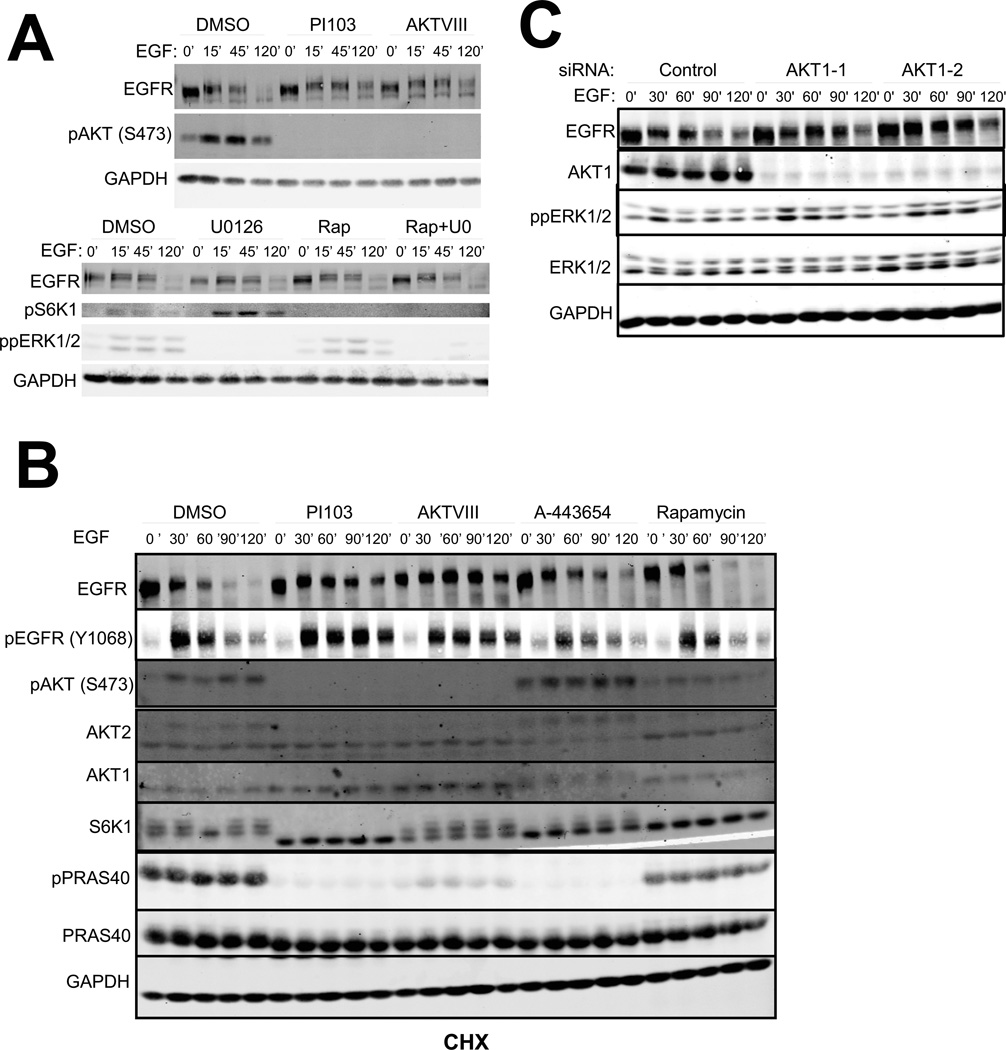
EGF stimulation causes EGFR degradation by delivery to the lysosomes (14). To address the role of AKT in EGFR degradation, we assessed EGFR degradation in human mammary epithelial cells (HMECs), which have 4.5×105 EGFR molecules per cell (28). This concentration of EGFRs is within the range of other cell lines commonly used in endocytosis studies. HeLa cells have 1.7×105 EGFRs per cell (28). MDA-MB-468 and A431 cells, which harbor amplified EGFR, express three and six-fold more EGFR than HMECs, respectively (29–31). Stable over-expression of EGFR in HMECs led to a 25–50% increase in the total EGFR abundance (fig. S1A), so the approximate number of EGFR molecules is 6×105. We used the latter cells for our studies as they were more tractable to interrogation and exhibited an intermediate EGFR abundance compared to the above mentioned commonly-used cell lines. Stimulation of these HMECs with a high concentration of EGF (100 ng/mL) induced a decrease in the abundance of EGFR in total cell lysate, which was abrogated by pre-treatment with the lysosomal inhibitor chloroquine (fig. S1B and C). Similar to HeLa cells, a low dose of EGF (1 ng/mL) did not cause EGFR degradation (fig. S1B), suggesting a specific degree of EGFR activation is needed to either induce degradation or detect the degradation. We hypothesized that perturbations in EGFR trafficking to the lysosomes would alter its degradation rate. We treated cells with vehicle (DMSO), the dual PI3K-mTOR inhibitor PI103 (32) or the highly specific allosteric AKT inhibitor AKTVIII (33). PI103 interferes with PI3K activity and thus reduces PI(3,4,5)P3 concentrations and phosphorylation of AKT at Ser473 and Thr308, leading to AKT inactivation. AKTVIII stabilizes an AKT conformation in which the PH and kinase domains are locked together (34), which is proposed to interfere with AKT membrane recruitment and the access of PDK1 and mTORC2 to AKT. The inhibitor therefore reduces the phosphorylation of AKT at Ser473 and Thr308 and AKT activation. Membrane recruitment of the PH domain containing protein GRP1 is unaffected by AKTVIII suggesting the drug specifically interferes with membrane recruitment of AKT (35).
In HMECs pretreated with DMSO, EGF stimulation caused more than 80% of EGFR to be degraded within 120 min (Fig. 1A). Pre-treatment with AKTVIII and PI103 blocked phosphorylation of AKT at Ser473 as expected. Furthermore, pretreatment of cells with these inhibitors reduced the rate of EGF-induced EGFR degradation and a substantial amount of EGFR was detected even after 120 min of EGF stimulation. This phenotype was specific to AKT inhibition, because blocking other signaling pathways downstream of EGFR, such as the mTORC1-S6K pathway with rapamycin and the ERK-RSK pathway with U0126, did not affect EGFR degradation (Fig. 1A).
Figure 1. AKT facilitates EGFR degradation.
A. Starved HMECs were stimulated with EGF for the indicated times in the presence or absence of indicated inhibitors. Western blots are representative of at least 3 independent experiments. B. Cells were stimulated as above in the presence of CHX. Western blots are representative of at least 2 independent experiments. EGFR degradation curves are in fig. S1E. C. Cells transfected with siRNAs targeting AKT1 or with Non-targeting Allstars control siRNA were deprived of growth factors for 24 hours, treated with CHX for 30 min and stimulated with EGF. Western blots are representative of at least 3 independent experiments. EGFR degradation curves are in fig. S1F.
Our observation that both PI103 and AKTVIII stabilized EGFR suggests PI3K acted through AKT to promote EGFR degradation by modulating the trafficking of EGFR-containing vesicles or by modulating EGFR protein synthesis. To test this, we repeated the EGFR degradation assays in the presence of protein synthesis inhibitor cycloheximide (CHX). Co-treatment of cells with CHX and PI103, AKTVIII, or a structurally distinct ATP-competitive AKT catalytic inhibitor A-443654 reduced EGFR degradation rates (Fig. 1B and fig S1E). The effect of A-443654 on EGFR degradation was not as potent as AKTVIII, potentially due to its decreased specificity (36). Consistent with our previous experiments, rapamycin did not alter EGFR degradation. These data indicate AKT promotes EGFR degradation in a protein synthesis-independent manner. AKTVIII reduced EGFR degradation in T47D breast cancer cells, which has low EGFR abundance (37) suggesting AKT stimulates the degradation of EGFR independent of its abundance (fig. S1D). We used siRNA knockdown of AKT1 to further validate that AKT controls EGFR degradation and found that two different AKT1 siRNAs significantly increased the percent of EGFR left undegraded after 120 min (Fig. 1C and Fig S1F).
AKT inhibition prolongs EGF-EGFR occupancy in the early endosomes
Stimulation of cells with PKC or G-protein coupled receptor agonists sequesters EGFR in a perinuclear region (38), which blocks the access of EGF to EGFR and inhibits ligand-induced degradation. To determine if AKT controls EGFR degradation by facilitating EGF access to EGFR and the resulting EGFR internalization, we quantified the colocalization of EEA.1 with Alexa-488-labeled EGF. AKTVIII treatment did not significantly alter EEA.1 co-localization with EGF (fig. S2A) or with EGFR after EGF stimulation and did not cause EGFR accumulation in the perinuclear region (fig. S2B). Together with the observation that AKT inhibition does not prevent EGFR phosphorylation (Fig. 1B), these data suggest AKT does not promote EGFR degradation by controlling EGF-EGFR binding or their subsequent internalization.
To determine if AKT promotes EGFR degradation by controlling vesicle trafficking, we sought to identify the subcellular location of the EGFR that accumulates upon AKT inhibition. In the presence of AKTVIII, EGFR molecules that were not degraded were still phosphorylated (Fig. 1B). Because PTP1B dephosphorylates EGFR before EGFR is sorted into the lumen of the MVBs (15), we hypothesized the un-degraded EGFR accumulates at the early endosomes or the limiting membrane of MVBs. Indeed, AKTVIII treatment increased EEA.1 co-localization with EGF and EGFR 60 min after EGF stimulation (Fig. 2A and B). In cells in which internalization was synchronized. AKTVIII treatment increased EEA.1 co-localization with EGF and EGFR as well (fig. S2C). To verify that the increased EEA.1-EGF and EEA.1-EGFR co-localization indicated increased EGF and EGFR in EEA.1 positive endosomes, we quantified the integrated intensities of the EGF and EGFR signal in each endosome throughout confocal Z-stacks of DMSO and AKTVIII treated cells. We plotted the cumulative probability distribution of endosomes against integrated EGF/EGFR intensity values. In AKTVIII cells, endosomal EGF and EGFR intensities were significantly higher for the same probability value, suggesting AKTVIII treatment increased the amount of EGF and EGFR in each endosome (Fig. 2C and D). Thus we conclude that upon AKT inhibition, undegraded EGF and EGFR molecules accumulate in EEA.1-positive early endosomes.
Figure 2. AKT regulates the endocytic progression of EGF/EGFR.
A. Cells plated on coverslips were deprived of growth factors, stimulated with Alexa-488 labeled EGF (green) for 60 min and labeled with EEA.1 (red) antibody (n>10 fields of cells; P<0.005). B. Cells were stimulated with unlabeled EGF for 60 min and labeled with EEA.1 (red) and EGFR (green) (n>15 fields of cells; P<0.01). EGF/EGFR co-localization with EEA.1 is depicted in yellow and indicated by arrows. Scale bar, 35 μm. Values are averages of percent co-localization ± standard deviation (SD). P<0.005. Panels to the right are boxplots. The '+' signs indicate outliers and whiskers indicate maximum and minumum values. Lower end of the boxes, the middle line and the upper edge of the boxes are 25th, 50th and 75th quantiles, respectively. The median values indicated by the red lines are statistically significantly different at 95% confidence level. C. Cells were treated as in (A) and (B). Images were acquired throughout the Z-plane. Endosomes were created from EEA.1 pixels in the 568nm channel above a local background with a continuous shape. Endosomes were transferred onto the Alexa-488 EGF image. Integrated intensity of the Alexa-488 EGF signal in each endosome was calculated for every other Z-plane in the Z-stack. Graph represents the cumulative distribution function plotted against integrated EGF intensities. X-axis gives the probability of a randomly selected endosome to have an EGF intensity value less than or equal to the EGF intensity value on the Y-axis. AKTVIII treatment decreases this probability because it creates endosomes with significantly higher EGF intensity values in endosomes (n>2300 endosomes; P<10−7) D. Analysis in (C) repeated for EGFR intensity in endosomes. AKTVIII treatment increases the EGFR intensity for any given probabilty (n>900 endosomes; P<10−9).
AKT promotes EGFR recycling
Disruption of receptor recycling with a dominant-negative Rab4 decreases EGF degradation (39). This is thought to be due to the inhibition of continuous rounds of receptor endocytosis in which a subset of the EGFRs would have been degraded with each cycle. We found that Rab11 knockdown reduced EGFR degradation (fig. S3). This suggests that interfering with vesicle recycling induces intracellular retention of vesicles, which prohibits additional rounds of EGF binding to EGFR and therefore additional rounds of the recycling or degradation decision. We hypothesized AKT facilitates EGFR degradation by promoting receptor recycling and ensuring continuity of the internalization and degradation cycles driven by the EGF in the media. We used an established method to assay receptor recycling in which we measured median cell surface EGFR staining using flow cytometry at different time points: before EGF stimulation (Total), after 15 min EGF stimulation (Pulse) and after washing the EGF-pulsed cells with acid to remove surface EGF and transferring the cells back to 37 °C for 10–20 min to allow EGFR recycling back to the plasma membrane (Chase) (40) (fig. S4). We found that AKT inhibition reduced the rate of EGF-induced EGFR recycling (Fig. 3A, 3B). Because EGF induces both degradation and the recycling of EGFR, we also assayed EGFR recycling in response to TGFα, an EGFR ligand that promotes EGFR recycling without substantial degradation (41). TGFα induced more robust EGFR recycling than EGF and TGFα-induced EGFR recycling was also reduced by AKTVIII treatment (Fig. 3A, 3C).
Figure 3. AKT regulates EGFR recycling.
Percent recycling was calculated in cells that were pre-treated with AKTVIII for 20 minutes and stimulated with EGF or TGFα for 15 minutes. (A) Percent recycling values are averages of three independent experiments with standard error of means. * P =0.05. (B) and (C) Flow-cytometry histograms are representative of three independent experiments. Solid purple histograms and T represent total EGFR surface labeling prior to any growth factor stimulation. Green histograms with 0' represent surface EGFR labeling after 15 min of EGF stimulation. Pink (10') and Blue (20') histograms represent surface labeling of EGFR after allowing cells to recycle EGFR at 37°C after 0, 10 and 20 minutes of chase time following a pulse of 15 minutes with growth factors.
AKT promotes the lysosomal progression of EGFR
AKT1 promotes the localization of CD89 targeted antigen to the lysosome associated membrane protein 1 (LAMP1)-containing vesicles (42, 43). This suggests that AKT is also involved in lysosomal sorting. To determine if the AKTVIII-induced reduction in EGFR degradation could be due to reduced lysosomal sorting, we quantified the co-localization of LAMP2 with Alexa-488 EGF. EGF stimulation for 30 min caused a small amount of LAMP2 to co-localize with Alexa-488 EGF. However, AKT inhibition did not significantly change the colocalization of LAMP2 with EGF (fig. S5A).
The small extent of LAMP2 and EGF co-localization suggested the possibility of EGF degradation in the lysosomal compartment. To reduce the loss of EGF signal, we used chloroquine to inhibit lysosomal degradation (fig. S1C). In the presence of chloroquine, AKTVIII significantly decreased the co-localization of LAMP2 with EGF, suggesting that AKT promotes the sorting of EGF into lysosomes (Fig. 4A and 4B). Synchronization of EGF binding to receptors by stimulating the cells at 4°C before internalization also resulted in similar amounts of LAMP2 co-localization with EGF, which was also reduced by AKTVIII pretreatment (fig. S5B). Because EGF causes both EGFR recycling and lysosomal sorting, we also repeated the degradation assay with betacellulin, an EGFR ligand that induces lysosomal sorting and degradation of EGFR without detectable recycling (41). Consistent with a role for AKT in EGFR lysosomal trafficking, AKT inhibition reduced the rate of betacellulin-induced EGFR degradation (Fig. 4C).
Figure 4. AKT regulates lysosomal progression of EGFR.
A. Cells were pre-treated with chloroquine and stimulated with Alexa-488 EGF for 30 min. LAMP2 is pseudo-colored in red and EGF in green, and yellow represents co-localization. Average percent co-localization values and standard deviations are given (n>25 fields of cells; P<0.005). Scale bar, 35 μm. B. Boxplot of LAMP2 co-localization with EGF as in Fig. 2. C. EGFR degradation was calculated in cells that were starved overnight, pre-treated with DMSO or AKTVIII for 45 min and CHX for the last 30 min of inhibitor treatment and stimulated with betacellulin for the indicated time points. N=2 independent experiments.
AKT promotes EGFR degradation by phosphorylating and activating PIKfyve
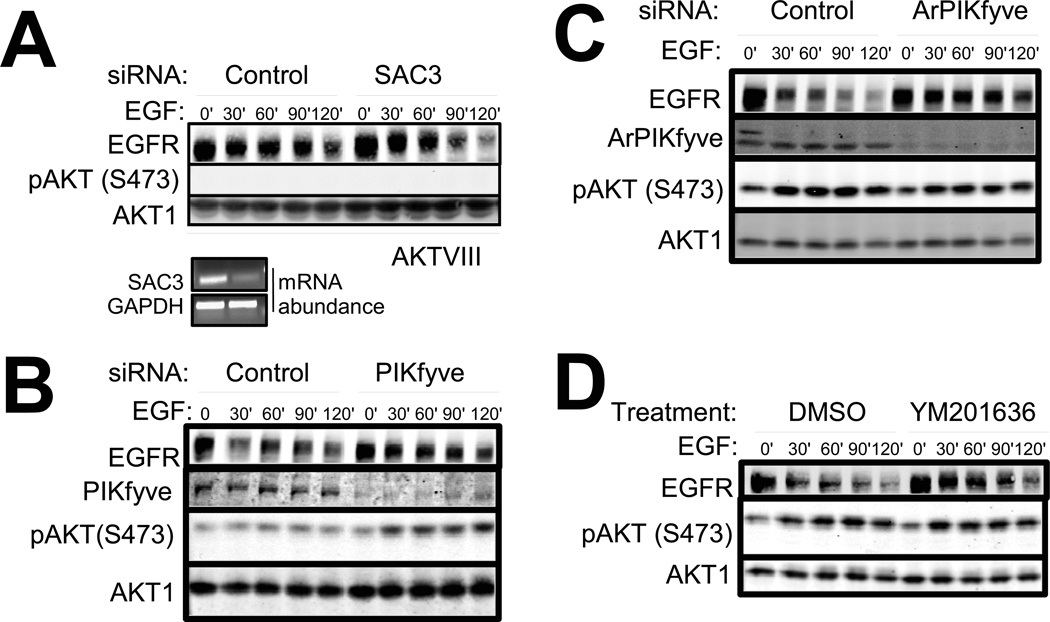
We next investigated the mechanism by which AKT controls endocytic vesicle progression. Endosomal identity and ability to progress within the vesicular trafficking system is dictated by the phosphoinositide composition of vesicles (20, 44). For example, increases in the concentration of the phosphoinositide PI(3,5)P2 promote the progression of early endosomes into MVBs. Insulin stimulation, which activates AKT, increases the concentration of PI(3,5)P2 by inhibiting the phosphatase activity of SAC3 (45). Thus, we tested whether AKT functioned upstream of or in a pathway parallel with SAC3 to facilitate early endosome progression and EGFR degradation. Whereas SAC3 knockdown did not affect the EGFR degradation rate in untreated HMECs (fig. S6A), it rescued the reduction in EGFR degradation induced by AKT inhibition (Fig. 5A and fig.S7A). When de-convolved, 3 out of 4 siRNAs in the SAC3 siRNA pool reproduced this phenotype, confirming acceleration of EGFR degradation was a specific effect of the SAC3 siRNAs (fig. S6B). These data indicate SAC3 functions downstream of or in a pathway parallel with AKT to regulate EGFR degradation.
Figure 5. AKT phosphorylates PIKfyve to facilitate EGFR degradation.
A. Cells were transfected with non-targeting Allstars control siRNA or siRNA pool targeting SAC3. Cells were pre-treated with AKTVIII for 45 min and CHX with 30 min and stimulated with EGF for the indicated time points. Lower panel shows efficient knockdown of SAC3 as assessed by SAC3 mRNA abundance. N=2 independent experiments. (B and C) PIKfyve (B) or its activator ArPIKfyve (C) was knocked down with siRNA and cells were stimulated with EGF as in (A). D . Cells were pretreated with DMSO or YM201636 for 45 min and with CHX for the last 30 min and stimulated with EGF. Representative blots from 4 or more independent experiments. See fig. S7 for the EGFR degradation decay graphs.
Because insulin stimulation inhibits SAC3, we tested if AKT directly regulated the phosphatase activity of SAC3 (45). We immuno-precipitated the SAC3-ArPIKfyve-PIKfyve complex from human embryonic kidney 293T (HEK 293T) cells pretreated with or without AKT inhibitor. Pretreatment of cells with AKT inhibitor did not change the phosphatase activity of SAC3 (fig. S6C). Scansite and Phosphosite programs do not indicate a consensus AKT phosphorylation motif in SAC3, collectively suggesting that AKT does not directly regulate SAC3 (46, 47). However, the SAC3 binding partner PIKfyve is phosphorylated by AKT in vitro and in cells (48, 49). We perturbed PIKfyve function to test if PIKfyve regulates EGFR degradation in a similar manner to AKT. Knockdown of PIKfyve or its activator ArPIKfyve or pharmacological suppression of PIKfyve activity with the inhibitor YM201636 (50) reduced the rate of EGFR degradation (Fig. 5B - D and fig. S7B - D). Together these results suggest that similar to AKT activity, PIKfyve activity also facilitates EGFR degradation.
Incubation of AKT with PIKfyve in vitro increases PIKfyve activity towards PI3P to generate PI(3,5)P2, suggesting AKT activates PIKfyve (48). To confirm that AKT regulates PIKfyve phosphorylation, we immunoblotted FLAG-PIKfyve immunoprecipitates with phospho-AKT substrate (pAS) antibody, which recognizes the consensus AKT phosphorylation motifs (RXRXXS/T) only when the Ser or Thr residues are phosphorylated, and detected a band co-migrating with the FLAG PIKfyve protein (Fig. 6A). AKTVIII treatment or mutation of the AKT consensus phosphorylation site Ser318 to an alanine substantially reduced the pAS signal (Fig. 6A), confirming AKT that phosphorylates Ser318 in PIKfyve.
Figure 6. AKT phosphorylates and activates PIKfyve.
A. 293T cells that were transfected with FLAG-PIKfyve WT or S318A mutant PIKfyve were untreated or pretreated with AKTVIII before lysis. FLAG immunoprecipitates containing PIKfyve were immunoblotted with anti pAS antibody to determine the phosphorylation status of PIKfyve and anti-FLAG antibody to determine the total amount of over-expressed PIKfyve. Results of 2 independent experiments are shown. B. HMECs were metabolically labeled with 3H-myo-inositol, deprived of growth factors, and treated with indicated inhibitors for the last 30 min of the growth factor starvation. Cells were then stimulated with EGF for 30 min in the continued presence of inhibitors. Radioactively labeled phosphoinositides were extracted and quantified. Results of 2 independent experiments are shown. C. HEK293T cells were transfected as in (A) and the WT PIKfyve complex was immunoprecipitated from cells treated with or without AKTVIII and stimulated with or without EGF (10 ng/mL). 1/11th of the immunoprecipitate was immunoblotted to ensure equal isolation of the PIKfyve complex. The rest was used in a PIKfyve kinase activity assay using PI3P as a substrate. PI(3,5)P2 was extracted and quantifications of PI(3,5)P2 with Quantity One software are presented in the graph. Error bars represent standard error of mean from three independent experiments. D. HEK293T cells were transfected with indicated PIKfyve complexes and stimulated with EGF (10ng/mL). EGFR degradation was quantified and is presented in fig. S7E. Results are representative of 8 independent experiments are shown.
When activated, PIKfyve phosphorylates PI(3)P to generate PI(3,5)P2, which is then dephosphorylated by the myotubularin family of phosphatases to generate PI(5)P (51, 52). PIKfyve depletion in fibroblasts or treatment with PIKfyve inhibitor results in approximately 85% decrease in the concentration of PI(5)P, suggesting that most cellular PI(5)P is generated by the sequential action of PIKfyve and myotubularins (52). To test whether AKT inhibition and reduced PIKfyve phosphorylation correlated with PIKfyve activity in cells, we measured 3H-labeled phosphoinositide species isolated from HMECs (53). Due to its low abundance in cells we could not detect PI(3,5)P2 (53). However, the concentration of PI(5)P was reduced upon PIKfyve inhibitor treatment, confirming that it reflects PIKfyve activity (Fig. 6B). AKT inhibitor treatment also reduced the concentration of PI(5)P to varying extents, suggesting that PIKfyve activity is regulated by AKT in cells.
To determine if AKT directly regulates PIKfyve, we tested the effect of AKT inhibition or mutation of the AKT phosphorylation sites on the in vitro kinase activity of PIKfyve by immuno-precipitating the PIKfyve-ArPIKfyve-SAC3 complex from cells stimulated with or without EGF and pre-treated with DMSO or AKTVIII. EGF stimulation increased PIKfyve kinase activity which was reduced by pre-treatment of cells with AKT inhibitor, suggesting AKT is required for PIKfyve activation upon EGF stimulation (Fig. 6C). Taken together with the observations that AKT regulates PIKfyve phosphorylation and PIKfyve activity in cells these data suggest AKT directly activates the phosphoinositide kinase activity of PIKfyve.
To determine the role of AKT-mediated phosphorylation of PIKfyve in EGFR degradation, we transiently-expressed wild-type PIKfyve or the non-phosphorylatable S318A mutant PIKfyve together with SAC3 and ArPIKfyve, in HEK 293T cells. Expression of the S318A mutant caused a small but statistically significant reduction in EGFR degradation and increase in the phosphorylation of EGFR compared to wild-type PIKfyve (Fig. 6D and S7D). In addition to EGFR degradation, PDGFRβ degradation was also reduced by PIKfyve and AKT inhibitors suggesting that this pathway is likely to be functional in degradation of other RTKs (fig. S8).
AKT Reduces ERK Signaling by Facilitating EGFR Degradation
Because receptor endocytosis can regulate receptor signaling to downstream pathways, we hypothesized AKT-regulated degradation of EGFR constitutes a negative feedback loop that reduces EGFR signaling. In this model, EGF stimulation activates EGFR, which activates AKT. AKT directly phosphorylates and activates PIKfyve, which then promotes progression of early endosomes containing EGFR into the degradation path. This activated endocytic trafficking promotes EGFR degradation and would therefore result in reduced EGFR signaling to the RAS-RAF-MEK-ERK-RSK pathway. We tested the functionality of this negative feedback loop in the MCF10A normal breast epithelial cell line, which is a well-established system to study the cross-talk between AKT and ERK pathways (54). In these cells, AKT inhibition reduced degradation of EGFR and led to more sustained ERK signaling as judged by EGFR abundance, phosphorylation of ERK and phosphorylation of RSK following EGF stimulation (Fig. 7A). To confirm that AKT feeds back to EGFR rather than a parallel pathway that regulates ERK signaling, we tested whether increased ERK activity by AKT inhibition could be suppressed by EGFR inhibition. We treated MCF10A cells with or without AKTVIII in combination with an EGFR inhibitor. Consistent with the hypothesis that AKT regulates EGFR signaling to modulate ERK activity, EGFR inhibition reduced phosphorylation of ERK even in the presence of AKTVIII (Fig. 7B).
Figure 7. AKT inhibits EGFR and ERK signaling by promoting EGFR degradation.
A. MCF10As cells were deprived of EGF and serum overnight and stimulated with EGF in the presence of CHX for the indicated times. Results are representative of 3 independent experiments. EGFR degradation curves are in fig. S8A. B. MCF10 cells were deprived of EGF and serum overnight, pretreated with AKTVIII, gefitinib or the combination and stimulated with EGF. Results are representative of 3 independent experiments. Quantification is in fig. S8B. C. Model for how AKT promotes EGFR degradation. AKT phosphorylates and activates PIKfyve for proper EGFR progression through the early endosomes towards the lysosomes. When AKT is inhibited PIKfyve is less efficient and EGFR progression from the early endosomes is reduced, which leads to reduced rates of EGFR degradation. Reduced EGFR degradation also correlates with increased phosphorylation of EGFR and ERK.
DISCUSSION
A Role for AKT in EGFR Trafficking and Turnover
Although PI3Ks and their lipid products have been implicated in endocytic trafficking and trafficking of EGFR, the PI(3,4,5)P3–binding proteins that mediate these effects have not been identified and characterized. Here we showed that AKT regulates EGFR trafficking and determined the molecular mechanism behind this regulation. Our results uncover a feedback loop by which AKT promotes EGFR degradation. Inhibition of AKT reduces recycling and lysosomal sorting of EGFR, which correlates with an increase in the localization of EGFR in early endosomes and decreased PIKfyve activity in vitro and in cells. Reduced AKT activity enhances ERK and RSK activation in an EGFR dependent manner. Thus, we propose a model (Fig. 7C) in which AKT negatively feeds back to EGFR to inhibit EGFR signaling to its downstream pathways: (i) AKT phosphorylates and activates PIKfyve which generates PI(3,5)P2, (ii) PI(3,5)P2 facilitates the progression of EGFR-containing early endosomes to MVBs and late endosomes, and (iii) EGFR degradation increases and EGFR signaling to ERK decreases. Because AKT is upstream of the general endocytic regulator PIKfyve, this model suggests AKT may regulate the degradation of other RTKs as well. Supporting this hypothesis we found AKT and PIKfyve also regulate PDGFRβ degradation.
Role for the PIKfyve-ArPIKfyve-SAC3 complex in Receptor Tyrosine Kinase Degradation
Previous studies on the role of PIKfyve in RTK degradation have been inconclusive. PIKfyve and its product PI(3,5)P2 regulate retro-grade trafficking and vesicle budding and degradation of Notch and the voltage gated calcium channel Cav1.2 (18). Yet, although two structurally-similar PIKfyve inhibitors reduce EGFR degradation and sorting to the lysosomes (50, 55), PIKfyve knockdown does not interfere with EGFR degradation in HeLa cells (56). The different results obtained by different groups could be due to cell type specificity, non-specific effects of the inhibitors or inefficient RNAi knockdown. Alternatively, manipulating the abundance of one component of the PIKfyve-SAC3-ArPIKfyve complex can change the stoichiometry of proteins within this complex and therefore could produce a phenotype different than the one observed by directly inhibiting the kinase activity of an intact complex. For example, in contrast to the aberrant vacuolation observed after PIKfyve inhibition (50, 55), expressing a PIKfyve mutant (K2000E) with lowered phosphoinositide kinase activity does not cause a morphological defect unless it is expressed together with SAC3 and ArPIKfyve (57). Similarly, knockdown of SAC3 increases PI(3,5)P2 (20, 45); however SAC3 knockout decreases the concentration of PI(3,5)P2 possibly due to disruption of the complex integrity or function (52).
Given that relative abundances of these proteins in cells determine the phenotypes observed, we utilized knockdown and exogenous expression together with pharmacological inhibition to determine if the PIKfyve-ArPIKfyve-SAC3 complex regulates EGFR degradation. We showed SAC3 knockdown, which increases the concentration of PI(3,5)P2, rescued the reduction in EGFR degradation produced by AKT inhibitor treatment. Conversely, knockdown of ArPIKfyve or PIKfyve, both of which are required for PI(3,5)P2 generation, reduced EGFR degradation. Inhibiting PIKfyve kinase activity and expressing the AKT phosphosite PIKfyve mutant reduced EGFR degradation. Thus, we conclude PIKfyve regulates EGFR degradation by generating PI(3,5)P2. The observations that PIKfyve inhibitors reduce c-MET (55) and PDGFRβ degradation suggest that PI(3,5)P2 and enzymes involved in its metabolism are general regulators of RTK degradation. It is likely that PI(3,5)P2 controls RTK degradation either by recruitment of effector proteins that are involved in endosomal sorting or by directly activating mucolipin 1 and two-pore channel proteins TCP1 and TCP2 ion channels thereby increasing endosome and lysosome fusion events (58, 59).
Receptor Endocytosis and Signaling
EGFR signaling to the PI3K-AKT and ERK-RSK pathways is regulated by endocytosis at multiple levels. Blocking EGFR internalization by expressing EGFR mutants or dominant-negative dynamin reduces ERK and AKT activation (9, 60). In addition, the route of EGFR internalization determines signal duration. Clathrin-dependent EGFR endocytosis promotes receptor recycling and sustained AKT activation, whereas non-clathrin dependent EGFR endocytosis promotes transient AKT activation and EGFR degradation (40). Once internalized, EGFR remains associated with the signaling adapter Grb2 in the early endosomes (61). EGFR signaling from the endosomes is sufficient to induce DNA synthesis and activates ERK and AKT in HeLa cells and regulates apoptosis during zebrafish development. (6, 7, 62). EGFR signaling to the ERK pathway is also regulated at the late endosomes by the p14-MEK1-MP1 complex (63, 64).
Our finding that AKT regulates EGFR trafficking and degradation suggests AKT can regulate EGFR signaling by regulating EGFR trafficking. AKT inhibition increases phosphorylation of EGFR at Tyr1068 and phosphorylation of ERK in MCF10A cells. The AKT1 isoform inhibits ERK signaling in these cells through an unknown mechanism (54). We show that the AKT inhibitor-induced increase in ERK activation requires active EGFR, consistent with AKT facilitating EGFR degradation as a mechanism to reduce ERK signaling. AKT also inhibits ERK by phosphorylating and inhibiting B-Raf (65, 66) and our data add to this mechanism of pathway cross-inhibition.
Trafficking and degradation are important components of oncogenic RTK signaling. Mutant EGFRs that drive lung cancer proliferation degrade at a slower rate than wild-type EGFRs, associate to a reduced extent with the ubiquitination machinery, and co-localize to a greater extent with markers of the recycling endosomes (67, 68). Constitutively active c-Met mutants require receptor endocytosis to promote migration and in vivo transformation (69). Our data suggest that therapeutic targeting of AKT in settings where RTKs are hyperactive should be performed with caution, due to the ability of AKT to facilitate EGFR trafficking and degradation and inhibit ERK signaling. Thus, inhibiting AKT signaling in these cells may actually promote tumorigenesis by reducing the ability of cells to clear surface receptors and inducing continued RTK signaling from the endosomes. Determining the contribution of the AKT-mediated feedback to multiple RTKs in the context of various cancer cell types will enhance our understanding of RTK biology and the intricate signaling networks that govern cellular response to the extracellular environment.
MATERIALS AND METHODS
Cell culture and transfections
Human mammary epithelial cells were immortalized, maintained and infected with pBabeNeo EGFR retrovirus as previously described (70). For knockdown experiments cells were plated at a density of 200–250 thousand cells per 6 cm dishes 24 hours before transfection with Allstars non-targeting control siRNA (Qiagen) or siRNAs targeting AKT1, RAB11a, and ArPIKFyve, PIKfyve (Qiagen) or SAC3 (Dharmacon) using Lipofectamine RNAimax reagent (Invitrogen). MCF10A cells were cultured as previously described (54). HEK 293T cells were cultured in DMEM containing 10% FBS. Calcium phosphate transfections in HEK293T cells were done as previously described (70).
Antibodies and reagents
EGFR, pEGFR (Tyr1068), pAKT (Ser473), pS6K (Thr389), pPRAS40, PRAS40, ubiquitin antibodies were from Cell Signaling Technologies. Antibodies were from the following companies GAPDH (Ambion),FLAG tag, ppERK1/2 (Sigma), S-tag (Novagen), EEA-1 (Santa Cruz Biotechnologies). Rab11 (BD biosciences). S6K1 and ERK antibodies were made in house. EGFR antibody used for immuno-precipitation, immune-fluorescence and flow cytometry (Calbiochem), ArPIKfyve and PIKfyve (Abcam). Secondary Alexa-Fluor 488, 674 and 568 antibodies and Alexa-488 labeled EGF were purchased from Molecular Probes, Invitrogen. Secondary antibodies for Western blotting was from Li-COR. Gefitinib (2µM final), PI103 (5µM final) and U0126 (20 µM Final) was from Selleck Biochemical. AKTVIII (10µM final) and YM201636 (200nM final) were from Calbiochem. DMSO, N.E.M (N-ethylamimide), Cyclohexmide and Chloroquine was from Sigma-Aldrich. EGF (100ng/mL for stimulation experiments unless otherwise noted), Insulin was from Pepro-Tech. AKT catalytic inhibitor A-443656 was a kind gift from Dr. Nathaniel Gray.
Cell Lysis, immuno-precipitation and western-blotting
Cell lysis was performed as previously described in the presence of protease and phosphatase inhibitors (70). For ubiquination experiments 10µM n-ethylmaleimide was added to the lysis buffer. EGFR was immuno-precipitated with EGFR antibody or mouse IgG and with a 1:1 mixture of Protein A (Sigma-Aldrich) and Protein G Sephrose Beads (GE Health Care) at 4 °C for 90 minutes. FLAG PIKfyve was immune-precipitated using agarose conjugated FLAG antibodies (Sigma, Aldrich). Beads were spun at 5000rpm for 1 minute and the flow-through was discarded. Beads were washed three times with lysis buffer and boiled with SDS loading buffer at 100 °C for 6 minutes. Western blotting and quantification using the Odyssey Li-COR imaging system was carried out as previously described (71)
Immuno fluorescence and Image Analysis
Cells were plated on HCl treated coverslips as previously described (70) at a density of 50,000 cells per well in 6-well plate, 48 hours prior to assay. Cells were deprived of growth factors 24 hours prior to staining and treated with inhibitors as indicated. Where indicated, EGF entry into the cells was synchronized by stimulating cells with EGF at 4 °C for 60 min on ice prior to moving them to 37 °C. All other EGF stimulation experiments were carried out at 37 °C. Cells were fixed with 3.7% Formaldehyde and stained overnight with primary antibody for EEA.1 (1:100) and EGFR (1:100). Images were taken using Nikon Ti inverted microscope with Yokagawa CSU-10 spinning disk confocal microscope at 60X magnification. Images were taken from the focal plane of the nucleus or as Z-stacks at a 0.3µm step size. Percent co-localization values were calculated using the measure co-localization function of the MetaMorph software. Pixels with intensity values above a fixed threshold from each channel were used for calculations. Statistical analysis of the percent co-localization values was carried out using Bootstrap permutation test (72). Box plots were generated using MATLAB ® (R2011b, Natick, MA). For measurement of integrated intensities of EGF and EGFR in EEA.1 positive early endosomes, we created early endosomes from EEA.1 pixels that are above the local background and form a continuous shape with a fixed minimum and maximum size. These endosomes were transferred over to the images that contain the EGF/EGFR signal and only the EGF/EGFR signal within these regions were calculated as integrated intensities after a constant background subtraction. Integrated intensities from every other Z-plane were pooled for an entire field. Integrated EGF-EGFR intensities in DMSO and AKTVIII treated cells were compared using Kolmogrov-Smirnov analysis for non-normal distributions. Cumulative distribution function plots were generated using MATLAB ® .
EGFR Recycling assay
EGFR recycling was assessed as previously described in (40) with the following changes. 3 plates of cells were pulsed with 100ng/mL EGF or TGFα for 15 minutes at 37 °C to allow for internalization and 1 plate was left un-stimulated (to measure total surface EGFR). Internalization was stopped by moving cells to 4 °C. Excess EGF on the cell surface was washed away with an acid treatment (150mM NaCl, 50mM Glycine at pH 3.0) for 3 minutes at 4 °C and washed with PBS three times. One plate was left at 4 °C (Pulse). Two plates were moved back to 37 °C to allow for recycling for 10 or 20 minutes (Chase). Cells were moved back to 4 °C, fixed with 1% Formaldehyde in PBS, washed once with PBS and twice with STE (10mM Tris-Cl, pH7.5, 10mM NaCl, 1mM EDTA) and collected. Cell suspension was blocked with 3% BSA in PBS at 4 °C for 30 minutes and labeled with EGFR antibody (1:100) at 4 °C for one hour in 3% BSA and with Alexa-488 donkey anti mouse antibody for 30 minutes at 4 °C. Using flow-cytometry 10,000 cells were evaluated for surface EGFR staining. Percent recycling at either 10 or 20 minute time points was calculated using the formula ((Chase – Pulse) /(Total Surface EGFR – Pulse)) * 100 from median values of each treatment group.
Phosphoinositide Phosphatase Assays
FLAG-PIKfyve, S-SAC3 and HA-ArPIKfyve complex were expressed in HEK293T cells. The complex was immunoprecipitated with anti-HA antibodies (30 min at 4 degrees) and Protein A beads (1 h at 4 degrees). Immunoprecipitates were washed twice with lysis buffer and twice with phosphatase buffer (50mM Tris-Cl pH7.4, 1mM MgCl2 , 1mM Dithiothreitol). Beads were then resuspended with 35uL of reaction mixture (31uL of phosphatase buffer and 4uL of di-C8 PI(3,5)P2 (Echelon-inc Biosciences). Reactions were carried out at 37 C degrees for 15 min for WT SAC3 and for 60 min for D488A mutant. 25uL of the reaction was mixed with malachite green assay buffer (Echelon-inc Biosciences). Amount of free phosphate liberated from PI(3,5)P2 via SAC3 activity in each reaction was read at 660nm along with free phosphate standards and calculated as instructed by the manufacturer.
Phosphoinositide Kinase Assay
PI3P (Avanti Polar Lipids) vesicles was generated by sonication for 10 min in HEPES/EGTA buffer. FLAG-PIKfyve, S-SAC3 and HA-ArPIKfyve complex expressed in 293T cells was immunoprecipitated as above, washed twice with lysis buffer and twice with TNE buffer (). After the first wash 1/11th of the imunnoprecipated beads were used for western-blotting to ensure equal loading, and the rest was used in the phosphoinositide kinase assay. After the second wash the beads were resuspended in TNE buffer, incubated with PI3P vesicles for 5 min on ice and incubated with the ATP reaction mix (HEPES, ATP, MnCl2, MgCl2, β-glycerophosphate) and 32P-ATP (Perking Elmer) for 15 min at room temperature. The reaction was stopped by adding 4N HCl and phosphoinositides were extracted using 1:1 Methanol:Chloroform. Phosphoinositides were spotted on Silica thin layer chromatography (TLC) (Milipore) and separated using 65:35 2M Acetic acid:n-proponal. Membrane was dried and exposed to Phospho-imager screen (Bio-Rad) and the volumes of radioactively labeled PI(3,5)P2 spots were quantified using Quantity One® software (Bio-Rad).
Measurement of Phosphoinositides in Cells
HMECs are plated at 1 million cells per 10 cm plate and phosphoinositides were radioactively labeled by growing cells with inositol free DMEM/F-12 media (U.S. Biologicals) supplemented 10µCi/mL 3H-myo-inositol (American Radiolabeled Chemicals) for 48 hours with. Cells were then starved for growth factors by incubation in inositol free DMEM/F12 media without EGF and Insulin for 1 hour. Inhibitors were added at the last 30 min of starvation. Cells were then stimulated with EGF (100ng/mL) for 30 min. Phosphoinositides were then extracted, deactylated and separated by high performance liquid chromatography as described previously(53).
Supplementary Material
Supplemental Figure 1. Stimulation with high EGF concentrations induces lysosomal degradation of EGFR independently of EGFR abundance.
Supplemental Figure 2. Internalization of EGF/EGFR does not require AKT activity.
Supplemental Figure 3. RAB11a knockdown reduces the rate of EGFR degradation.
Supplemental Figure 4. The EGFR recycling assay.
Supplemental Figure 5. Effects of AKTVIII on the colocalization of EGF with LAMP2.
Supplemental Figure 6. SAC3 promotes EGFR degradation but is not regulated by AKT.
Supplemental Figure 7. The PIKfyve-ArPIKfyve-SAC3 complex promotes EGFR degradation.
Supplemental Figure 8. AKT reduces EGFR signaling to ERK.
Supplemental Figure 9. PIKfyve and AKT promote PDGFRβ degradation in HMECs.
ACKNOWLEDGEMENTS
We would like to thank Dr. Nathaniel Gray for the AKT catalytic inhibitor, A-443656, Dr. Alex Toker, Dr. Lewis Cantley, Dr. Jean Zhao and Dr. Steve Gygi for critical discussion, Dr. Pier Paolo Di Fiore and Dr. Sara Sigismund for providing the detailed protocol for the EGFR recycling assay, Dr Atsuo Sasaki for his advice and providing a detailed protocol for the in vitro PI3K assay, and Dr. Marco Vilela for his help with statistical analysis. We thank the Nikon Imaging Facility at Harvard Medical School for their help with light microscopy and the members of the Blenis Lab for critical discussion.
FUNDING: This work was funded by Susan G. Komen for the Cure (to M.C.M.) and the National Cancer Institute (NCI) grant R37CA46595 (to J.B.). J.B. is an Established Investigator of the LAM Foundation.
Footnotes
AUTHOR CONTRIBUTIONS: E.E.E, M.C.M and J.B designed the experiments, analyzed the data, and wrote the manuscript. E.E.E. performed the experiments. A.M.M and L.E.R designed and performed the HPLC experiments and analyzed the data. L.E.R made corrections to the manuscript.
COMPETING INTERESTS: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. published online EpubFeb ( [DOI] [PubMed] [Google Scholar]
- 2.Riese DJ, 2nd, Gallo RM, Settleman J. Mutational activation of ErbB family receptor tyrosine kinases: insights into mechanisms of signal transduction and tumorigenesis. Bioessays. 2007;29:558–565. doi: 10.1002/bies.20582. published online EpubJun ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. published online EpubJun ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaeper U, Vogel R, Chmielowiec J, Huelsken J, Rosario M, Birchmeier W. Distinct requirements for Gab1 in Met and EGF receptor signaling in vivo. Proc Natl Acad Sci U S A. 2007;104:15376–15381. doi: 10.1073/pnas.0702555104. published online EpubSep 25 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. published online EpubAug ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, Brand M, Zerial M. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. published online EpubMay 2 ( [DOI] [PubMed] [Google Scholar]
- 7.Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De Camilli P. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. published online EpubMar 20 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung I, Akita R, Vandlen R, Toomre D, Schlessinger J, Mellman I. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature. 2010;464:783–787. doi: 10.1038/nature08827. published online EpubApr 1 ( [DOI] [PubMed] [Google Scholar]
- 9.Goh LK, Huang F, Kim W, Gygi S, Sorkin A. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J Cell Biol. 2010;189:871–883. doi: 10.1083/jcb.201001008. published online EpubMay 31 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. published online EpubFeb 22 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orth JD, McNiven MA. Get off my back! Rapid receptor internalization through circular dorsal ruffles. Cancer Res. 2006;66:11094–11096. doi: 10.1158/0008-5472.CAN-06-3397. published online EpubDec 1 ( [DOI] [PubMed] [Google Scholar]
- 12.Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. published online EpubMar 10 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leonard D, Hayakawa A, Lawe D, Lambright D, Bellve KD, Standley C, Lifshitz LM, Fogarty KE, Corvera S. Sorting of EGF and transferrin at the plasma membrane and by cargo-specific signaling to EEA1-enriched endosomes. J Cell Sci. 2008;121:3445–3458. doi: 10.1242/jcs.031484. published online EpubOct 15 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scita G, Di Fiore PP. The endocytic matrix. Nature. 2010;463:464–473. doi: 10.1038/nature08910. published online EpubJan 28 ( [DOI] [PubMed] [Google Scholar]
- 15.Eden ER, White IJ, Tsapara A, Futter CE. Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat Cell Biol. 2010;12:267–272. doi: 10.1038/ncb2026. published online EpubMar ( [DOI] [PubMed] [Google Scholar]
- 16.Haj FG, Verveer PJ, Squire A, Neel BG, Bastiaens PI. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science. 2002;295:1708–1711. doi: 10.1126/science.1067566. published online EpubMar 1 ( [DOI] [PubMed] [Google Scholar]
- 17.Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. published online EpubMay ( [DOI] [PubMed] [Google Scholar]
- 18.Ho CY, Alghamdi TA, Botelho RJ. Phosphatidylinositol-3,5-Bisphosphate: No Longer the Poor PIP(2) Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01246.x. published online EpubJul 7 (10.1111/j.1600-0854.2011.01246.x) [DOI] [PubMed] [Google Scholar]
- 19.Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. published online EpubDec 11 ( [DOI] [PubMed] [Google Scholar]
- 20.Sbrissa D, Ikonomov OC, Fu Z, Ijuin T, Gruenberg J, Takenawa T, Shisheva A. Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex. J Biol Chem. 2007;282:23878–23891. doi: 10.1074/jbc.M611678200. published online EpubAug 17 ( [DOI] [PubMed] [Google Scholar]
- 21.Joly M, Kazlauskas A, Fay FS, Corvera S. Disruption of PDGF receptor trafficking by mutation of its PI-3 kinase binding sites. Science. 1994;263:684–687. doi: 10.1126/science.8303278. published online EpubFeb 4 ( [DOI] [PubMed] [Google Scholar]
- 22.Nakatsu F, Perera RM, Lucast L, Zoncu R, Domin J, Gertler FB, Toomre D, De Camilli P. The inositol 5-phosphatase SHIP2 regulates endocytic clathrin-coated pit dynamics. J Cell Biol. 2010;190:307–315. doi: 10.1083/jcb.201005018. published online EpubAug 9 (jcb.201005018 [pii] 10.1083/jcb.201005018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddhanta U, McIlroy J, Shah A, Zhang Y, Backer JM. Distinct roles for the p110alpha and hVPS34 phosphatidylinositol 3'-kinases in vesicular trafficking, regulation of the actin cytoskeleton, and mitogenesis. J Cell Biol. 1998;143:1647–1659. doi: 10.1083/jcb.143.6.1647. published online EpubDec 14 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiro DJ, Boll W, Kirchhausen T, Wessling-Resnick M. Wortmannin alters the transferrin receptor endocytic pathway in vivo and in vitro. Mol Biol Cell. 1996;7:355–367. doi: 10.1091/mbc.7.3.355. published online EpubMar ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barysch SV, Aggarwal S, Jahn R, Rizzoli SO. Sorting in early endosomes reveals connections to docking- and fusion-associated factors. Proc Natl Acad Sci U S A. 2009;106:9697–9702. doi: 10.1073/pnas.0901444106. published online EpubJun 16 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. published online EpubJan 18 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collinet C, Stoter M, Bradshaw CR, Samusik N, Rink JC, Kenski D, Habermann B, Buchholz F, Henschel R, Mueller MS, Nagel WE, Fava E, Kalaidzidis Y, Zerial M. Systems survey of endocytosis by multiparametric image analysis. Nature. 2010;464:243–249. doi: 10.1038/nature08779. published online EpubMar 11 ( [DOI] [PubMed] [Google Scholar]
- 28.Spangler JB, Neil JR, Abramovitch S, Yarden Y, White FM, Lauffenburger DA, Wittrup KD. Combination antibody treatment down-regulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc Natl Acad Sci U S A. 2010;107:13252–13257. doi: 10.1073/pnas.0913476107. published online EpubJul 27 (10.1073/pnas.0913476107 0913476107 [pii]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merlino GT, Xu YH, Ishii S, Clark AJ, Semba K, Toyoshima K, Yamamoto T, Pastan I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984;224:417–419. doi: 10.1126/science.6200934. published online EpubApr 27 ( [DOI] [PubMed] [Google Scholar]
- 30.Filmus J, Pollak MN, Cailleau R, Buick RN. MDA-468, a human breast cancer cell line with a high number of epidermal growth factor (EGF) receptors, has an amplified EGF receptor gene and is growth inhibited by EGF. Biochem Biophys Res Commun. 1985;128:898–905. doi: 10.1016/0006-291x(85)90131-7. published online EpubApr 30 (0006-291X(85)90131-7 [pii]) [DOI] [PubMed] [Google Scholar]
- 31.Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, Lee J, Yarden Y, Libermann TA, Schlessinger J, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. published online EpubMay 31-Jun 6 ( [DOI] [PubMed] [Google Scholar]
- 32.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. published online EpubMay 19 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, Duggan ME. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15:761–764. doi: 10.1016/j.bmcl.2004.11.011. published online EpubFeb 1 ( [DOI] [PubMed] [Google Scholar]
- 34.Calleja V, Laguerre M, Parker PJ, Larijani B. Role of a novel PH-kinase domain interface in PKB/Akt regulation: structural mechanism for allosteric inhibition. PLoS Biol. 2009;7:e17. doi: 10.1371/journal.pbio.1000017. published online EpubJan 20 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green CJ, Goransson O, Kular GS, Leslie NR, Gray A, Alessi DR, Sakamoto K, Hundal HS. Use of Akt inhibitor and a drug-resistant mutant validates a critical role for protein kinase B/Akt in the insulin-dependent regulation of glucose and system A amino acid uptake. J Biol Chem. 2008;283:27653–27667. doi: 10.1074/jbc.M802623200. published online EpubOct 10 ( [DOI] [PubMed] [Google Scholar]
- 36.Okuzumi T, Fiedler D, Zhang C, Gray DC, Aizenstein B, Hoffman R, Shokat KM. Inhibitor hijacking of Akt activation. Nat Chem Biol. 2009;5:484–493. doi: 10.1038/nchembio.183. published online EpubJul ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charafe-Jauffret E, Ginestier C, Monville F, Finetti P, Adelaide J, Cervera N, Fekairi S, Xerri L, Jacquemier J, Birnbaum D, Bertucci F. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene. 2006;25:2273–2284. doi: 10.1038/sj.onc.1209254. published online EpubApr 6 (1209254 [pii] 10.1038/sj.onc.1209254) [DOI] [PubMed] [Google Scholar]
- 38.Idkowiak-Baldys J, Baldys A, Raymond JR, Hannun YA. Sustained receptor stimulation leads to sequestration of recycling endosomes in a classical protein kinase C- and phospholipase D-dependent manner. J Biol Chem. 2009;284:22322–22331. doi: 10.1074/jbc.M109.026765. published online EpubAug 14 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCaffrey MW, Bielli A, Cantalupo G, Mora S, Roberti V, Santillo M, Drummond F, Bucci C. Rab4 affects both recycling and degradative endosomal trafficking. FEBS Lett. 2001;495:21–30. doi: 10.1016/s0014-5793(01)02359-6. published online EpubApr 20 ( [DOI] [PubMed] [Google Scholar]
- 40.Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. published online EpubAug ( [DOI] [PubMed] [Google Scholar]
- 41.Roepstorff K, Grandal MV, Henriksen L, Knudsen SL, Lerdrup M, Grovdal L, Willumsen BM, van Deurs B. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic. 2009;10:1115–1127. doi: 10.1111/j.1600-0854.2009.00943.x. published online EpubAug ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang GA, Lang ML. Protein kinase Balpha is required for vesicle trafficking and class II presentation of IgA Fc receptor (CD89)-targeted antigen. J Immunol. 2006;176:3987–3994. doi: 10.4049/jimmunol.176.7.3987. published online EpubApr 1 ( [DOI] [PubMed] [Google Scholar]
- 43.Lang ML, Shen L, Gao H, Cusack WF, Lang GA, Wade WF. Fc alpha receptor cross-linking causes translocation of phosphatidylinositol-dependent protein kinase 1 and protein kinase B alpha to MHC class II peptide-loading-like compartments. J Immunol. 2001;166:5585–5593. doi: 10.4049/jimmunol.166.9.5585. published online EpubMay 1 ( [DOI] [PubMed] [Google Scholar]
- 44.Poccia D, Larijani B. Phosphatidylinositol metabolism and membrane fusion. Biochem J. 2009;418:233–246. doi: 10.1042/BJ20082105. published online EpubMar 1 (BJ20082105 [pii] 10.1042/BJ20082105) [DOI] [PubMed] [Google Scholar]
- 45.Ikonomov OC, Sbrissa D, Ijuin T, Takenawa T, Shisheva A. Sac3 is an insulin-regulated phosphatidylinositol 3,5-bisphosphate phosphatase: gain in insulin responsiveness through Sac3 down-regulation in adipocytes. J Biol Chem. 2009;284:23961–23971. doi: 10.1074/jbc.M109.025361. published online EpubSep 4 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. published online EpubJul 1 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, Sullivan M. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr1122. published online EpubDec 1 (gkr1122 [pii] 10.1093/nar/gkr1122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berwick DC, Dell GC, Welsh GI, Heesom KJ, Hers I, Fletcher LM, Cooke FT, Tavare JM. Protein kinase B phosphorylation of PIKfyve regulates the trafficking of GLUT4 vesicles. J Cell Sci. 2004;117:5985–5993. doi: 10.1242/jcs.01517. published online EpubDec 1 ( [DOI] [PubMed] [Google Scholar]
- 49.Hill EV, Hudson CA, Vertommen D, Rider MH, Tavare JM. Regulation of PIKfyve phosphorylation by insulin and osmotic stress. Biochem Biophys Res Commun. 2010;397:650–655. doi: 10.1016/j.bbrc.2010.05.134. published online EpubJul 9 ( [DOI] [PubMed] [Google Scholar]
- 50.Jefferies HB, Cooke FT, Jat P, Boucheron C, Koizumi T, Hayakawa M, Kaizawa H, Ohishi T, Workman P, Waterfield MD, Parker PJ. A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 2008;9:164–170. doi: 10.1038/sj.embor.7401155. published online EpubFeb ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oppelt A, Lobert VH, Haglund K, Mackey AM, Rameh LE, Liestol K, Oliver Schink K, Marie Pedersen N, Wenzel EM, Haugsten EM, Brech A, Erik Rusten T, Stenmark H, Wesche J. Production of phosphatidylinositol 5-phosphate via PIKfyve and MTMR3 regulates cell migration. EMBO Rep. 2013;14:57–64. doi: 10.1038/embor.2012.183. published online EpubJan 3 (10.1038/embor.2012.183 embor2012183 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zolov SN, Bridges D, Zhang Y, Lee WW, Riehle E, Verma R, Lenk GM, Converso-Baran K, Weide T, Albin RL, Saltiel AR, Meisler MH, Russell MW, Weisman LS. In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc Natl Acad Sci U S A. 2012;109:17472–17477. doi: 10.1073/pnas.1203106109. published online EpubOct 23 (10.1073/pnas.1203106109 1203106109 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarkes D, Rameh LE. A novel HPLC-based approach makes possible the spatial characterization of cellular PtdIns5P and other phosphoinositides. Biochem J. 2010;428:375–384. doi: 10.1042/BJ20100129. published online EpubJun 15 (10.1042/BJ20100129 BJ20100129 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. published online EpubDec 19 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Lartigue J, Polson H, Feldman M, Shokat K, Tooze SA, Urbe S, Clague MJ. PIKfyve regulation of endosome-linked pathways. Traffic. 2009;10:883–893. doi: 10.1111/j.1600-0854.2009.00915.x. published online EpubJul ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rutherford AC, Traer C, Wassmer T, Pattni K, Bujny MV, Carlton JG, Stenmark H, Cullen PJ. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J Cell Sci. 2006;119:3944–3957. doi: 10.1242/jcs.03153. published online EpubOct 1 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikonomov OC, Sbrissa D, Fenner H, Shisheva A. PIKfyve-ArPIKfyve-Sac3 core complex: contact sites and their consequence for Sac3 phosphatase activity and endocytic membrane homeostasis. J Biol Chem. 2009;284:35794–35806. doi: 10.1074/jbc.M109.037515. published online EpubDec 18 (10.1074/jbc.M109.037515 M109.037515 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu MX, Clapham DE, Ren D, Xu H. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. published online EpubOct 12 (10.1016/j.cell.2012.08.036 S0092-8674(12)01105-1 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun. 2010;1:38. doi: 10.1038/ncomms1037. 10.1038/ncomms1037 ncomms1037 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. published online EpubDec 20 ( [DOI] [PubMed] [Google Scholar]
- 61.Di Guglielmo GM, Baass PC, Ou WJ, Posner BI, Bergeron JJ. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. Embo J. 1994;13:4269–4277. doi: 10.1002/j.1460-2075.1994.tb06747.x. published online EpubSep 15 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pennock S, Wang Z. Stimulation of cell proliferation by endosomal epidermal growth factor receptor as revealed through two distinct phases of signaling. Mol Cell Biol. 2003;23:5803–5815. doi: 10.1128/MCB.23.16.5803-5815.2003. published online EpubAug ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teis D, Taub N, Kurzbauer R, Hilber D, de Araujo ME, Erlacher M, Offterdinger M, Villunger A, Geley S, Bohn G, Klein C, Hess MW, Huber LA. p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis. J Cell Biol. 2006;175:861–868. doi: 10.1083/jcb.200607025. published online EpubDec 18 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teis D, Wunderlich W, Huber LA. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev Cell. 2002;3:803–814. doi: 10.1016/s1534-5807(02)00364-7. published online EpubDec ( [DOI] [PubMed] [Google Scholar]
- 65.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. published online EpubNov 26 (8027 [pii]) [DOI] [PubMed] [Google Scholar]
- 66.Guan KL, Figueroa C, Brtva TR, Zhu T, Taylor J, Barber TD, Vojtek AB. Negative regulation of the serine/threonine kinase B-Raf by Akt. J Biol Chem. 2000;275:27354–27359. doi: 10.1074/jbc.M004371200. published online EpubSep 1 (10.1074/jbc.M004371200 M004371200 [pii]) [DOI] [PubMed] [Google Scholar]
- 67.Shtiegman K, Kochupurakkal BS, Zwang Y, Pines G, Starr A, Vexler A, Citri A, Katz M, Lavi S, Ben-Basat Y, Benjamin S, Corso S, Gan J, Yosef RB, Giordano S, Yarden Y. Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene. 2007;26:6968–6978. doi: 10.1038/sj.onc.1210503. published online EpubOct 25 ( [DOI] [PubMed] [Google Scholar]
- 68.Chung BM, Raja SM, Clubb RJ, Tu C, George M, Band V, Band H. Aberrant trafficking of NSCLC-associated EGFR mutants through the endocytic recycling pathway promotes interaction with Src. BMC Cell Biol. 2009;10:84. doi: 10.1186/1471-2121-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joffre C, Barrow R, Menard L, Calleja V, Hart IR, Kermorgant S. A direct role for Met endocytosis in tumorigenesis. Nat Cell Biol. 2011;13:827–837. doi: 10.1038/ncb2257. published online EpubJul ( [DOI] [PubMed] [Google Scholar]
- 70.Mendoza MC, Er EE, Zhang W, Ballif BA, Elliott HL, Danuser G, Blenis J. ERK-MAPK drives lamellipodia protrusion by activating the WAVE2 regulatory complex. Mol Cell. 2011;41:661–671. doi: 10.1016/j.molcel.2011.02.031. published online EpubMar 18 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mendoza MC, Er EE, Blenis J. ERK-MAP Kinase signaling in the cytoplasm. Methods Mol Biol. 2010;661:185–203. doi: 10.1007/978-1-60761-795-2_11. 10.1007/978-1-60761-795-2_11). [DOI] [PubMed] [Google Scholar]
- 72.Efron B, Tibshirani R. An introduction to the bootstrap. Monographs on statistics and applied probability. Chapman & Hall; New York: 1993. p. xvi.p. 436. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Stimulation with high EGF concentrations induces lysosomal degradation of EGFR independently of EGFR abundance.
Supplemental Figure 2. Internalization of EGF/EGFR does not require AKT activity.
Supplemental Figure 3. RAB11a knockdown reduces the rate of EGFR degradation.
Supplemental Figure 4. The EGFR recycling assay.
Supplemental Figure 5. Effects of AKTVIII on the colocalization of EGF with LAMP2.
Supplemental Figure 6. SAC3 promotes EGFR degradation but is not regulated by AKT.
Supplemental Figure 7. The PIKfyve-ArPIKfyve-SAC3 complex promotes EGFR degradation.
Supplemental Figure 8. AKT reduces EGFR signaling to ERK.
Supplemental Figure 9. PIKfyve and AKT promote PDGFRβ degradation in HMECs.