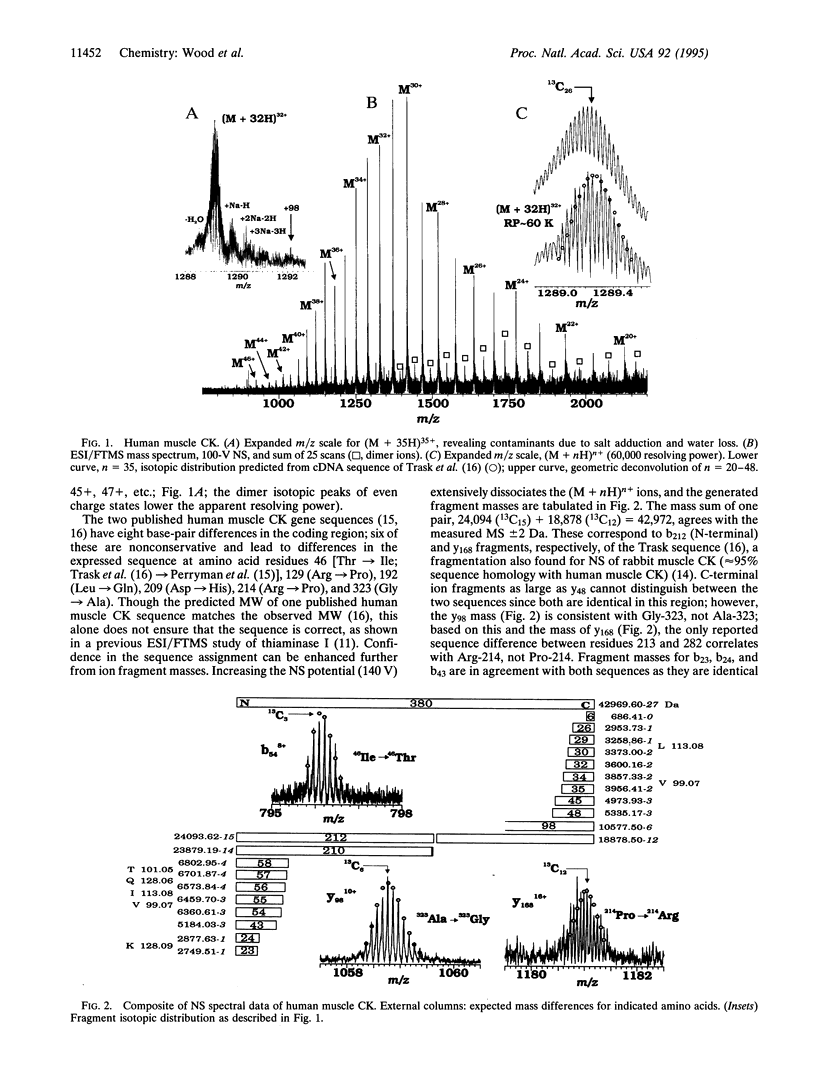
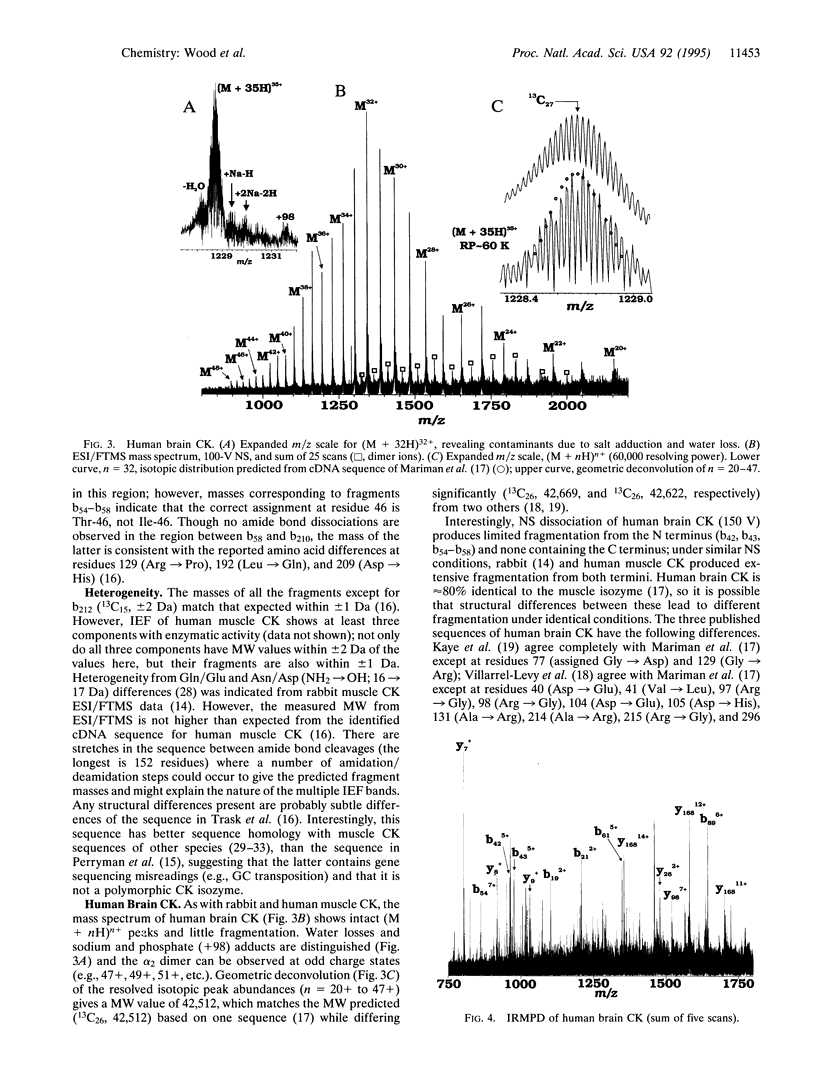
Abstract
Amino acid sequencing by recombinant DNA technology, although dramatically useful, is subject to base reading errors, is indirect, and is insensitive to posttranslational processing. Mass spectrometry techniques can provide molecular weight data from even relatively large proteins for such cDNA sequences and can serve as a check of an enzyme's purity and sequence integrity. Multiply-charged ions from electrospray ionization can be dissociated to yield structural information by tandem mass spectrometry, providing a second method for gaining additional confidence in primary sequence confirmation. Here, accurate (+/- 1 Da) molecular weight and molecular ion dissociation information for human muscle and brain creatine kinases has been obtained by electrospray ionization coupled with Fourier-transform mass spectrometry to help distinguish which of several published amino acid sequences for both enzymes are correct. The results herein are consistent with one published sequence for each isozyme, and the heterogeneity indicated by isoelectric focusing due to 1-Da deamidation changes. This approach appears generally useful for detailed sequence verification of recombinant proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benfield P. A., Henderson L., Pearson M. L. Expression of a rat brain creatine kinase-beta-galactosidase fusion protein in Escherichia coli and derivation of the complete amino acid sequence of rat brain creatine kinase. Gene. 1985;39(2-3):263–267. doi: 10.1016/0378-1119(85)90321-x. [DOI] [PubMed] [Google Scholar]
- Buskin J. N., Jaynes J. B., Chamberlain J. S., Hauschka S. D. The mouse muscle creatine kinase cDNA and deduced amino acid sequences: comparison to evolutionarily related enzymes. J Mol Evol. 1985;22(4):334–341. doi: 10.1007/BF02115689. [DOI] [PubMed] [Google Scholar]
- Chen L. H., Babbitt P. C., Vásquez J. R., West B. L., Kenyon G. L. Cloning and expression of functional rabbit muscle creatine kinase in Escherichia coli. Addressing the problem of microheterogeneity. J Biol Chem. 1991 Jun 25;266(18):12053–12057. [PubMed] [Google Scholar]
- Giraudat J., Devillers-Thiery A., Perriard J. C., Changeux J. P. Complete nucleotide sequence of Torpedo marmorata mRNA coding for the 43,000-dalton nu 2 protein: muscle-specific creatine kinase. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7313–7317. doi: 10.1073/pnas.81.23.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenkamp F., Karas M., Beavis R. C., Chait B. T. Matrix-assisted laser desorption/ionization mass spectrometry of biopolymers. Anal Chem. 1991 Dec 15;63(24):1193A–1203A. doi: 10.1021/ac00024a002. [DOI] [PubMed] [Google Scholar]
- Hossle J. P., Rosenberg U. B., Schäfer B., Eppenberger H. M., Perriard J. C. The primary structure of chicken B-creatine kinase and evidence for heterogeneity of its mRNA. Nucleic Acids Res. 1986 Feb 11;14(3):1449–1463. doi: 10.1093/nar/14.3.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller T., Kaiser R. J., Koop B. F., Hood L. Large-scale and automated DNA sequence determination. Science. 1991 Oct 4;254(5028):59–67. doi: 10.1126/science.1925562. [DOI] [PubMed] [Google Scholar]
- Kaye F. J., McBride O. W., Battey J. F., Gazdar A. F., Sausville E. A. Human creatine kinase-B complementary DNA. Nucleotide sequence, gene expression in lung cancer, and chromosomal assignment to two distinct loci. J Clin Invest. 1987 May;79(5):1412–1420. doi: 10.1172/JCI112969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little D. P., Speir J. P., Senko M. W., O'Connor P. B., McLafferty F. W. Infrared multiphoton dissociation of large multiply charged ions for biomolecule sequencing. Anal Chem. 1994 Sep 15;66(18):2809–2815. doi: 10.1021/ac00090a004. [DOI] [PubMed] [Google Scholar]
- Loo J. A., Edmonds C. G., Smith R. D. Tandem mass spectrometry of very large molecules: serum albumin sequence information from multiply charged ions formed by electrospray ionization. Anal Chem. 1991 Nov 1;63(21):2488–2499. doi: 10.1021/ac00021a018. [DOI] [PubMed] [Google Scholar]
- Loo J. A., Quinn J. P., Ryu S. I., Henry K. D., Senko M. W., McLafferty F. W. High-resolution tandem mass spectrometry of large biomolecules. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):286–289. doi: 10.1073/pnas.89.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariman E. C., Broers C. A., Claesen C. A., Tesser G. I., Wieringa B. Structure and expression of the human creatine kinase B gene. Genomics. 1987 Oct;1(2):126–137. doi: 10.1016/0888-7543(87)90004-8. [DOI] [PubMed] [Google Scholar]
- Ordahl C. P., Evans G. L., Cooper T. A., Kunz G., Perriard J. C. Complete cDNA-derived amino acid sequence of chick muscle creatine kinase. J Biol Chem. 1984 Dec 25;259(24):15224–15227. [PubMed] [Google Scholar]
- Perryman M. B., Kerner S. A., Bohlmeyer T. J., Roberts R. Isolation and sequence analysis of a full-length cDNA for human M creatine kinase. Biochem Biophys Res Commun. 1986 Nov 14;140(3):981–989. doi: 10.1016/0006-291x(86)90732-1. [DOI] [PubMed] [Google Scholar]
- Pickering L., Pang H., Biemann K., Munro H., Schimmel P. Two tissue-specific isozymes of creatine kinase have closely matched amino acid sequences. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2310–2314. doi: 10.1073/pnas.82.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney S., Herlihy W., Royal N., Pang H., Aposhian H. V., Pickering L., Belagaje R., Biemann K., Page D., Kuby S. Rabbit muscle creatine phosphokinase. CDNA cloning, primary structure and detection of human homologues. J Biol Chem. 1984 Dec 10;259(23):14317–14320. [PubMed] [Google Scholar]
- Roman D., Billadello J., Gordon J., Grace A., Sobel B., Strauss A. Complete nucleotide sequence of dog heart creatine kinase mRNA: conservation of amino acid sequence within and among species. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8394–8398. doi: 10.1073/pnas.82.24.8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senko M. W., Beu S. C., McLafferty F. W. High-resolution tandem mass spectrometry of carbonic anhydrase. Anal Chem. 1994 Feb 1;66(3):415–418. doi: 10.1021/ac00075a016. [DOI] [PubMed] [Google Scholar]
- Senko M. W., Speir J. P., McLafferty F. W. Collisional activation of large multiply charged ions using Fourier transform mass spectrometry. Anal Chem. 1994 Sep 15;66(18):2801–2808. doi: 10.1021/ac00090a003. [DOI] [PubMed] [Google Scholar]
- Trask R. V., Strauss A. W., Billadello J. J. Developmental regulation and tissue-specific expression of the human muscle creatine kinase gene. J Biol Chem. 1988 Nov 15;263(32):17142–17149. [PubMed] [Google Scholar]
- Valaskovic G. A., Kelleher N. L., Little D. P., Aaserud D. J., McLafferty F. W. Attomole-sensitivity electrospray source for large-molecule mass spectrometry. Anal Chem. 1995 Oct 15;67(20):3802–3805. doi: 10.1021/ac00116a030. [DOI] [PubMed] [Google Scholar]
- Villarreal-Levy G., Ma T. S., Kerner S. A., Roberts R., Perryman M. B. Human creatine kinase: isolation and sequence analysis of cDNA clones for the B subunit, development of subunit specific probes and determination of gene copy number. Biochem Biophys Res Commun. 1987 May 14;144(3):1116–1127. doi: 10.1016/0006-291x(87)91427-6. [DOI] [PubMed] [Google Scholar]
- Wang R., Chait B. T. High-accuracy mass measurement as a tool for studying proteins. Curr Opin Biotechnol. 1994 Feb;5(1):77–84. doi: 10.1016/s0958-1669(05)80074-6. [DOI] [PubMed] [Google Scholar]



