Abstract
Small heat shock proteins constitute the most diverse and least conserved group within the large family of heat shock proteins, which play a crucial role in cell response to environmental insults. Chironomus riparius larvae are widely used in environmental research for testing pollutant toxicity in sediments and freshwater environments. Different genes, such as Hsp70, Hsc70, Hsp90, and Hsp40, have been identified in this species as sensitive biomarkers for xenobiotics, but small Hsps genes remain largely unknown. In this study, the Hsp27 has been characterized in C. riparius and its transcriptional response evaluated under several environmental stimuli. The Hsp27 gene was mapped by FISH on polytene chromosomes at region I-C4 and was found to encode a 195 aa protein, which contains an α-crystallin domain bounded by three conserved regions. This protein shows homology with Drosophila melanogaster HSP27, Ceratitis capitata HSP27, and Sarcophaga crassipalpis HSP25. Real-time reverse transcriptase–polymerase chain reaction analysis showed that heat shock (35 °C) and cadmium dramatically upregulate this gene. Moreover, exposures to triclosan and bisphenol A were able to significantly increase mRNA levels. However, neither nonylphenol nor tributyltin altered Hsp27 gene expression. The transcriptional activity of Hsp27 gene was modulated during cold stress. Interestingly, cold shock (4 °C) significantly reduced Hsp27 transcripts, but this gene was significantly overexpressed during the recovery time at the normal growing temperature. These results show that the Hsp27 gene is sensitive to different environmental stimuli, including endocrine-disrupting pollutants, suggesting its potential as a suitable biomarker for ecotoxicological studies in aquatic systems.
Keywords: Cold heat shock, Cadmium, Bisphenol A (BPA), 4-Nonylphenol (NP), Tributyltin (TBT), Triclosan (TCS)
Introduction
Heat shock proteins (HSPs) are a family of highly conserved proteins from prokaryotes to eukaryotes and ubiquitous in all cell types, two facts that prove their essential role in cells. They have important functions in many physiological processes, and are particularly relevant when cells are under stressful conditions as chaperones for the maintenance of correct protein folding (Nolen and Morimoto 2002; Silver and Noble 2012). HSPs were first discovered under heat shock and were later found to be highly abundant in cells submitted to many other chemical and physical stimuli in a wide range of species. In general, Hsp genes are expressed at low levels under normal growing conditions, but their expression increases considerably in a rapid response to different forms of stressors including heat, hypoxia, chemical exposures, infections, magnetic fields, and tumorigenesis (Parsell and Lindquist 1993; Feder and Hofmann 1999; Bierkens et al. 1998). In arthropods, they are induced by environmental stressors such as heat, desiccation, and heavy metals (Hoffmann and Parsons 1991; Tammariello et al. 1999; Hoffmann et al. 2003). It is known that Hsp genes constitute a subset of a larger group of genes coding for molecular chaperones. Chaperonins play an important role by assisting in the correct folding of nascent peptides, also acting when denatured proteins accumulate in cells, preventing them from irreversible aggregation and misfolding (Hartl and Hayer-Hartl 2002; Sorensen et al. 2003). As HSP induction is critical to the maintenance of cellular homeostasis in response to changes in the environment, these proteins have been proposed as general biomarkers for environmental monitoring (Gupta et al. 2010). Given the large quantity and huge variety of contaminants of anthropogenic origin, the development of alternative methods to animal testing is becoming a real challenge, and the application of toxicogenomic tests is gaining acceptance as a rapid and efficient methodology (Snell et al. 2003). As sensitive stress-sensors, Hsps genes are suitable candidates not only to evaluate the damaging potential of chemicals, but also to analyze the effects of subtle alterations in physical abiotic parameters (i.e., temperature, radiation), which are increasingly acquiring importance as a consequence of the human impact on natural ecosystems. This environmental perspective adds further interest for studying the potential of the Hsp27 gene as a toxicological endpoint.
The larvae of the midge Chironomus riparius (Diptera) have been extensively used as a model for testing pollutant toxicity in sediments and freshwater environments (EPA US 1996; OECD, Organisation for Economic Co-operation and Development 2001). They are abundantly distributed and have strong potency to survive in contaminated environments, even when many other aquatic organisms cannot. Ecotoxicological tests of chemicals have been traditionally performed in laboratory assays using mortality, growth rate, behavior, and reproduction as endpoints (Hatakeyama 1988; Williams et al. 1987). Larval mouthpart deformities are also indicators of anthropogenic stress (Martinez et al. 2003). Moreover, the giant polytene chromosomes from the salivary gland cells are also suitable for analyzing the genotoxic effects of pollutants (Michailova et al. 2006). More recently, chironomids are being used for toxicity testing using molecular endpoints. The utility of gene biomarkers for monitoring both environmental quality and the health of organisms inhabiting polluted ecosystems is gaining increasing attention. In recent years, some genes have been described as biomarkers for toxicant exposures including, among others, those for heat shock proteins (Hsp70, Hsc70, Hsp90, Hsp40), ribosomal proteins, cytochrome P450, and nuclear receptors (Martínez‐Guitarte et al. 2007; Gopalakrishnan et al., 2011; Morales et al. 2011; Planelló et al. 2007; 2008; 2010; 2011; Park and Kwak 2008, 2009, 2010; Martínez-Paz et al. 2012; Ozáez et al. 2013). Hsp70 has been sequenced and evaluated as a biomarker of exposure to metals and insecticides in other species of chironomids, such as Chironomus yoshimatsui and Chironomus dilutus (Yoshimi et al. 2002; Karouna-Renier and Rao 2009). However, there is still scarce DNA sequence information for these aquatic species and, in particular, the family of small Hsps genes has not yet been characterized.
Small heat shock proteins (sHSPs) are stress-inducible molecular chaperones that range in size from 10 to 30 kDa. A low degree of conservation is found among sHSPs, when compared to other proteins of the HSP family, with the exception of an α-crystallin domain of 80–100 amino acids (Denlinger et al. 2001). These proteins are involved in conditions of extreme temperatures, oxidation, UV irradiation, heavy metals, and chemical intoxication (Reineke 2005; Waters et al. 2008). The sHSPs are also involved in some important biological processes such as cell growth, apotosis, differentiation, diapause, lifespan, membrane fluidity, and starvation resistance in insects (Arrigo 1998; Gkouvitsas et al. 2008; Morrow et al. 2004; Tsvetkova et al. 2002; Hao et al. 2007). Yet, in insects, there is only limited information about the expression, regulation and function of sHSPs. Members of the α-crystallin/sHSP superfamily have recently been cloned from a few insect species: Sarcophaga crassipalpis (Rinehart et al. 2007), Venturia canescens (Reineke 2005), Bombyx mori (Sakano et al. 2006), Plutella xylostella (Sonoda et al. 2006), Liriomyza sp. (Huang and Kang 2007), Locusta migratoria (Wang et al. 2007), Ceratitis capitata (Kokolakis et al. 2008), Mamestra brassicae (Sonoda et al. 2007), Sesamia nonagrioides (Gkouvitsas et al. 2008), Liriomyza sativae (Huang et al. 2009), and Macrocentrus cingulum (Xu et al. 2010). In the flesh fly S. crassipalpis, three sHSPs are upregulated during diapause and it has been proposed that they contribute to the survival of this insect at low temperatures (Yocum et al. 1998; Li et al. 2007; Rinehart et al. 2007; Colinet et al. 2010). A slight upregulation of an sHSP has also been observed during diapause of the northern house mosquito, Culex pipiens (Robich et al. 2007). However, this is not the case in all insect species studied to date (Goto and Kimura 2004; Tachibana et al. 2005).
The aim of the present study was to characterize the Hsp27 gene in C. riparius and to analyze the transcriptional regulation of this gene under different temperature shocks and xenobiotic exposures to chemicals frequently found as contaminants in the aquatic environment. Tributyltin (TBT), nonylphenol (NP), triclosan (TCS), bisphenol A (BPA), and cadmium (Cd) were selected. These compounds are all environmental chemicals with estrogenic activity. TBT is a toxic chemical, belonging to the organotin compounds or stannanes, used for disinfection, antifouling and preservation in industrial processes. Due to its widespread use, it is a common contaminant of marine and freshwater environments, and is included on the list of EU priority compounds in water because of its toxic, persistent, bioaccumulative, and endocrine disruptive characteristics (Antizar-Ladislao 2008). Nonylphenol results from the degradation of alkylphenol polyethoxylates used as surfactants in industrial products. It accumulates in environmental compartments with a high organic content, such as sewage sludge and river sediments. NP has been referred to on the list of priority substances in the Water Frame Directive of the EU, and has been classified as an endocrine disrupter (Soares et al. 2008). TCS, a halogenated phenol, is a nonionic broad spectrum antimicrobial widely used as an ingredient in disinfectants, detergent, mouthwash, fabric, deodorant, shampoo, and plastic additives, as well as in innumerable other veterinary, industrial, and household products. The hormonal activity of TCS has been demonstrated by its capability to modulate thyroid hormone-related genes and anuran development (Veldhoen et al. 2006); it also has endocrine disruptive effects in fishes (Raut and Angus 2010). BPA is used in polycarbonate plastics and epoxy resin products, as well as an antioxidant in plasticizers and as an additive in other plastics. The total amount of BPA released into the environment has been estimated, and its predicted concentration in water and sediments has become a primary environmental concern (Cousins et al. 2002). Finally, Cd is a widespread pollutant known to be highly toxic, up to 20 times more toxic than other heavy metals, with numerous detrimental effects on most organisms. It has been ranked sixth on the European Union list of priority hazardous substances (Water Framework Directive 2008/105/EC). Although the effect caused by cadmium at the organism and population levels has been well documented, there are fewer data on sensitive molecular biomarkers for evaluating the impact of this contaminant on aquatic ecosystems. Our study reveals the potential role of the C. riparius Hsp27 gene as a sensitive marker in response to temperature changes and exposure to chemicals in benthic invertebrates.
Material and methods
Animals and treatments
The experimental animals were the aquatic larvae from the midge C. riparius. They were originally collected from natural populations in a non-polluted area of Valencia (Spain), and reared under standard laboratory conditions according to toxicity testing guidelines (EPA US 1996; OECD, Organisation for Economic Co-operation and Development 2001). Larvae were grown from egg masses in aqueous culture medium (0.5 mM CaCl2, 1 mM NaCl, 1 mM MgSO4, 0.1 mM NaHCO3, 0.025 mM KH2PO4, 0.01 mM FeCl3) supplemented with nettle leaves, commercial fish food (TetraMin), and cellulose tissue in polyethylene tanks (500 mL). Cultures were maintained under constant aeration at 20 °C and under standard light–dark periods (16:8). For experimental treatments, the larvae were exposed to the chemicals diluted in culture medium for 24 h with constant aeration at 20 °C. No food or substrate was provided during exposure. Fourth instar larvae were submitted to 0.1, 1, and 10 ng/L tributyltin oxide (TBTO) (Aldrich), 1, 10 and 100 μg/L NP (Fluka), 10, 100, and 1000 μg/L of TCS [5‐chloro‐2‐(2,4‐dichlorophenoxy)phenol] (Sigma), 0.5 and 3 mg/L BPA (Aldrich), and cadmium (Cd) 1 and 10 mM (Fluka). For temperature treatments, larvae were heat shocked at 35 °C for 2 h in preheated and aerated cultured medium, as described previously (Morcillo et al. 1988) and at 4 °C for 2 h in precooled and aerated cultured medium for cold shock. Thereafter, groups of larvae were transferred again to culture medium at 20 °C for 2, 4, and 6 h for recovery experiments after the cold shock. Each treatment consisted of three replicates, and three independent experiments were performed in each analysis using samples from three different control egg masses. The control larvae used in each case were exposed to the same concentration of solvent as the corresponding treatment, and were also measured in triplicate. Larvae were stored at −80 °C until RNA isolation was carried out.
RNA isolation
Total RNA was extracted from control and exposed fourth instar larvae (ten animals for each experiment) using a guanidine isothiocyanate-based method, performed with a commercial kit (Trizol, Invitrogen) according to the manufacturer's protocol. After, RNA was treated with RNase-free DNase (Roche), followed by phenolization. The quality and quantity of total RNA were determined by agarose electrophoresis and absorbance spectrophotometry (Biophotomer Eppendorf). Purified RNA was finally stored at −80 °C.
DNA isolation
Genomic DNA was isolated from 25 control larvae by homogenization in 100 mM NaCl, 25 mM EDTA, 10 mM Tris, pH 8, 0.5 % SDS. The homogenate was then treated with 20 mg/ml of proteinase K (Roche) at 65 °C overnight and 20 mg/ml of Rnase A (Roche) followed by incubation at 37 °C for 1 h. Two phenol/chloroform/isoamylalcohol (25/24/1) extractions were performed followed by ethanol precipitation, and DNA pellets were resuspended in 100 μl of Tris–EDTA (pH 8.0). The quality and quantity of total DNA were determined by agarose electrophoresis and absorbance spectrophotometry (Biophotomer Eppendorf). Purified DNA was finally stored at 4 °C.
Retrotranscription
Reverse transcription was performed with 0.5 μg of the isolated RNA. 0.5 μg oligo dT20 primer (Invitrogen) was used with M-MLV enzyme (Invitrogen), following the procedures described by the manufacturer.
Characterization and isolation of cDNA
Total RNA extracted from C. riparius larvae was used for heat shock protein 27 amplification. The full-length sequence was determined using 5′ and 3′ Rapid Amplification of cDNA Ends (RACE) with commercial kits (Invitrogen), following the manufacturer's instructions. Primer Hsp27 3F was designed based on the conserved sequences of Hsp27 gene from closely related species. The sequences of all gene-specific primers used for Hsp27 amplification are given in Table 1. For 3′ end RACE polymerase chain reaction (PCR), a cDNA template was obtained, as described above, using Adapter Primer (RACE kit, Invitrogen) and PCR was performed with gene-specific primer Hsp27 3F and an adapter primer AUAP (RACE kit, Invitrogen). PCR conditions were one cycle of 94 °C for 5 min, followed by 35 cycles at 94 °C for 30 s, 55 °C for 30 s, 72 °C for 40 s, and finally 72 °C for 10 min. The PCR product was cloned with a TOPO TA Cloning Kit (Invitrogen) and plasmids were purified and sequenced with M13 Forward and M13 Reverse primers. For the 5′ end RACE PCR, new gene-specific primers were designed based on sequence information obtained from the 3′ end fragment. The RNA was transcribed by a 5′ RACE kit (Invitrogen) with gene-specific primer Hsp27 7R, and the cDNA was subsequently amplified with an adapter primer AAP (RACE kit, Invitrogen) and a gene-specific primer Hsp27 6R. The PCR conditions were one cycle of 94 °C for 5 min, followed by 35 cycles at 94 °C for 30 s, 55 °C for 30 s and 72 °C for 40 s; and 72 °C for 10 min. The PCR products were purified and sequenced with hsp27 6R primer.
Table 1.
Primers used for cDNA, genomic DNA sequence and real-time RT–PCR of genes from C. riparius
| PCR objective | Oligo name | Primer DNA sequence | Amplification efficiency (%) |
|---|---|---|---|
| β actin real-time PCR | Actina F | 5′-GATGAAGATCCTCACCGAACG-3′ | 104 |
| Actina 2R | 5′-CGGAAACGTTCATTACCG-3′ | ||
| GAPDH real-time PCR | GAPDH F | 5′-GGTATTTCATTGAATGATCACTTTG-3′ | 96.6 |
| GAPDH R | 5′-TAATCCTTGGATTGCATGTACTTG-3′ | ||
| L13 real-time PCR | L13 F | 5′-AAGCTGCTTTCCCAAGAC-3′ | 107.1 |
| L13 R | 5′-TTGGCATAATTGGTCCAG-3′ | ||
| Hsp27 real-time PCR | Hsp27 rt F | 5′-TCAACACACAGGACCG-3′ | 109.3 |
| Hsp27 rt R | 5′-ATCCTTTATTGGTGATTAATTATG-3′ | ||
| Hsp27 sequence | Hsp27 3 F | 5′-AAGGATGGCTTCCAGGTCTGTATGGA-3′ | |
| Hsp27 6R | 5′-TGTCTTTACAGTAATTTCACTTGG-3′ | ||
| Hsp27 7R | 5′-GTTTTCCTTCAATAACCACC-3′ | ||
| Hsp27 9F | 5′-ATGTCATTAGTTCCTACTTTGTGGA-3′ | ||
| Hsp27 9R | 5′-AAGTCGAGCCGGTCCTGTG-3′ | ||
| Hsp27 10R | 5′-AAGGTCATCCTAAGAACCAACTAATC −3′ |
Hsp27 fragments were sequenced from both strands using ABI Big-Dye 3.1 dye chemistry and ABI 3730XL automated DNA sequencers (PE Biosystems). The complete C. riparius Hsp27 cDNA sequence was deposited in GenBank under accession number # KC495957.
Bioinformatic and phylogenetic analyses
Molecular weight and theoretical isoelectric point were estimated with the Compute pI/Mw tool from ExPASy—Bioinformatics Resource Portal. Multiple sequence alignments and phylogenetic analyses were performed using the Clustal W2 software (http://www.ebi.ac.uk/tools/msa/clustalw2) and compared with C. capitata HSP27, Drosophila melanogaster HSP27 and S. crassipalpis HSP25. The accession numbers for sHsp used in this study are: B. mori Hsp19.9 (AB195970), B. mori Hsp20.4 (AF315318), B. mori Hsp20.1 (AB195971), B. mori Hsp20.8 (AF315317), B. mori Hsp21.4 (AB195972), B. mori Hsp23.7 (AB195973), C. capitata Hsp27 (EU700493.1), D. melanogaster Hsp23 α (AAF50286.1), D. melanogaster Hsp23 β (AFH04365.1), D. melanogaster Hsp26 (AAA28636.1), D. melanogaster Hsp27 (AAA28638), Liriomyza huidobrensis Hsp20 (DQ452370), L. sativae Hsp21.3 (DQ452371), L. migratoria Hsp20.5 (DQ355963), L. migratoria Hsp20.6 (DQ355964), L. migratoria Hsp20.7 (DQ355965), S. crassipalpis Hsp23 (AF156162.1), S. crassipalpis Hsp25 (EF103577), and V. canescens Hsp35 (AY775544).
In situ hybridization and immunodetection
Salivary glands were dissected and fixed in acetic acid/ethanol (3:1), squashed in 50 % acetic acid and the slides dehydrated in absolute ethanol. Subsequently, FISH was carried out essentially as described previously (Martínez‐Guitarte et al. 2007). The squashes were air‐dried and treated with 2× SSC (0.3 M NaCl, 0.03 M sodium citrate) at 65 °C for 45 min, dehydrated in 50–100 % ethanol series for 10 min and treated with 0.07 N sodium hydroxide for 3 min. Finally, the slides were thoroughly washed with 1× PBS before applying the probe. As probe, we used an 874 bp fragment of Hsp27 cDNA from C. riparius (amplified with Hsp27 9F and Hsp27 10R primers), it was labeled by nick translation with Digoxigenin (Roche), denatured by boiling in water for 10 min and rapidly cooled on ice. The hybridization buffer was 50 % deionized formamide and 4× SSC, 0.4 % SDS. Each slide was treated with 95 pmol of probe and incubated overnight at room temperature. After hybridization, the slides were washed twice in PBS, 0.1 % Tween 20 for 10 min. For detection of the probe, slides were incubated for 1 h in anti‐digoxigenin IgG conjugated with fluorescein isothiocyanate (Roche) diluted 1:100 in PBS, 0.1 % Tween 20, and 1 % Blocking reagent (Roche). Following the washes, the slides were stained with 2 μg/ml DAPI (4′,6‐diamino‐2‐phenylindole) for 3 min and mounted in ProLong (Invitrogen) anti‐fading. All slides were examined under a Zeiss Axiohot photomicroscope equipped with an epifluorescence system and a Photometrics Cool Snap CCD camera. Images were processed with Adobe PhotoShop CS5.
Real-Time PCR
Quantitative real-time PCR (qRT–PCR) was used to evaluate the mRNA expression profile of Hsp27 after chemical exposure and cold and heat shock treatments. Actin, GAPDH, and L13 were employed as reference genes. Gene-specific primers used for actin, GAPDH, ribosomal protein L13, and Hsp27 are listed in Table 1. The qRT–PCR was performed using SsoFast EvaGreen Supermix (BioRad) in a CFX96 real-time PCR Detection System (BioRad). Each gene efficiency reaction was carried out with template diluted 1:2 in five steps, and the slope of the regression curves was then calculated (Table 1). The qRT–PCR cycling conditions were: initial denaturation at 95 °C for 3 min, 35 cycles of 95 °C denaturation for 5 s, 58 °C annealing for 15 s and 65 °C elongation for 10 s. To verify the accuracy of each amplicon, a melting curve analysis was performed after amplification. BioRad CFX Manager 3.0 software was used to determine the mRNA levels by normalized gene expression (2−∆∆Cq) against three endogenous reference genes. Each sample was run in duplicate wells and three independent replicates were performed in each experiment.
Amplification of genomic DNA by polymerase chain reaction
Based on the sequences of Hsp27 cDNA, two primers, Hsp27 9F and Hsp27 9R (Table 1), were designed to amplify an Hsp27 genomic DNA fragment from C. riparius. Cycling parameters for PCR amplification were one cycle of 94 °C for 5 min, followed by 35 cycles at 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1.5 min, with a final extension step at 72 °C for 10 min. PCR products were purified and sequenced with the primers Hsp27 9F and Hsp27 9R detailed in Table 1.
Statistical analysis
The levels of Hsp27 gene mRNA in each sample were normalized against the level of actin, GAPDH, and L13 in the same samples based on standard curves. The normalized levels of the specific gene transcripts in treated groups were compared to those of the non‐exposed controls using ANOVA followed by Dunnett's multiple comparison tests using SPSS 19® (IBM). A p < 0.05 was considered to indicate statistical significance.
Results
Characterization and sequence analysis of Hsp27
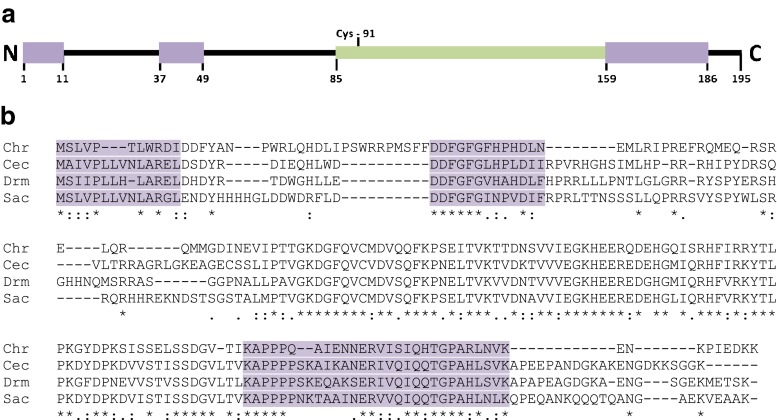
The full length of the C. riparius Hsp27 mRNA was 1,010 bp, containing a 588-bp open-reading frame that encodes a protein of 195 amino acid residues with a calculated molecular weight of 22.8 kDa and an isoelectric point of 6.17. The complete nucleotide and amino acid sequence was deposited in GenBank under accession number # KC495957. The gene included a 5′ untranslated region (UTR) (107 bp) that is rich in adenosine (53.3 %), a characteristic feature of the 5′ UTRs of previously identified HSPs. The 293 bp 3′ UTR (not including the poly A tail) contained three possible polyadenylation signals (AATAAA) and one AU‐rich element (ARE; ATTTA). In addition, there was only one cysteine at position 91 in the entire protein sequence, which is consistent with previous data showing that cysteine residues were rarer in the sequences of molecular chaperones than in other protein families (Fu et al. 2003). Analysis of genomic DNA showed that Hsp27 gene has no introns. A search, via the BLAST software program, showed that the protein belongs to the α-crystallin/sHSP superfamily. Multiple sequence alignment showed that the deduced amino acid sequence of C. riparius shared less similarity with other previously described HSP27s. The C. riparius HSP27 exhibited 47 % identity at the amino acid level with C. capitata, 45 % with D. melanogaster, and 48 % with S. crassipalpis. Sequence comparison revealed three domains of relative high homology between D. melanogaster HSP27, S. crassipalpis HSP25 and C. capitata HSP27 (Fig. 1a). The first domain (aa M1–I11 in ChrHSP27) present in the amino-terminal region is hydrophobic and shows a 29 % amino acid sequence identity in the four proteins (Fig. 1b). The second domain (aa D37–N49 in ChrHSP27) shows 48 % identity among the four HSP27 homologs, and it is specific for heat shock protein 27 (Fig. 1b). The third domain downstream of the α-crystalline domain contains 28 residues (aa K159–K186 in ChrHSP27) and shows 48 % amino acid sequence identity among the four HSP27 homologs (Fig. 1b). This domain is also present in all main Drosophila sHsps (Southgate et al. 1983).
Fig. 1.
a Schematic diagram of the protein domains of C. riparius HSP27 showing a conserved amino-terminal (1–11) and carboxy-terminal (158–186) region, a specific conserved region of HSP27 (37–49), α-crystallin domain (85–158) and the only cysteine (91). b Amino acid sequence comparison of heat shock protein 27 (HSP27) homologs from C. riparius (Chr), C. capitata (Cec), D. melanogaster (Drm) and S. crassipalpis (Sac). Asterisks, double dots, and single dots denote fully conserved, strongly conserved and weakly conserved amino acid residues, respectively. The three conserved regions characteristics of HSP27 are shown in purple
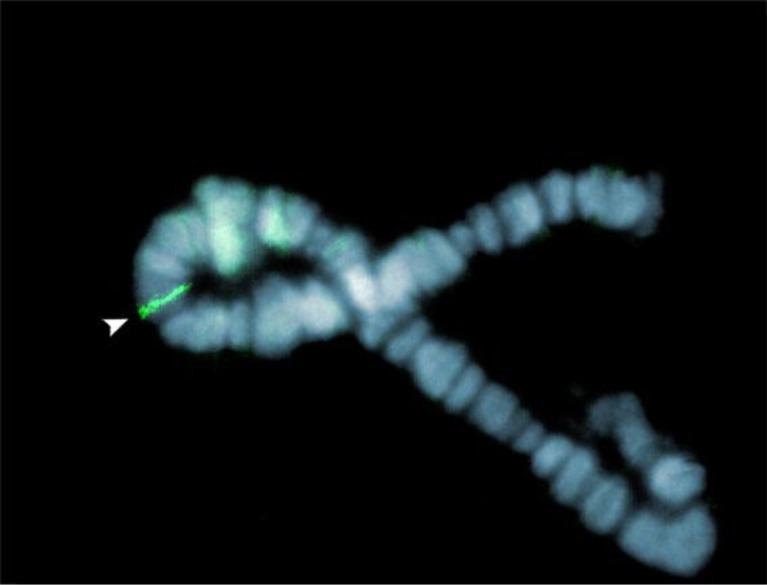
Localization by in situ hybridization of the Hsp27 gene on polytene chromosomes from salivary gland cells showed a single locus located on region I-C4 of the right arm at the polytenic chromosome I (Fig. 2).
Fig. 2.
Localization of the Hsp27 gene in the C. riparius genome. Using digoxigenin-labeled probes, a single signal stained in green with FITC is detected by FISH at polytene location I-C4 on the right arm of the chromosome I counterstained in blue with DAPI. Chromosomes were obtained from salivary gland cells
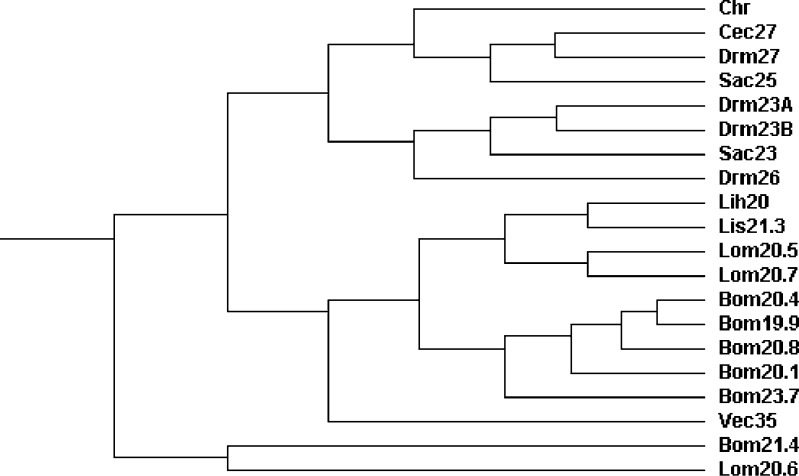
Using ClustalW2, a phylogenetic tree based on the sequence of sHSP from different species was constructed. The relationships displayed in the phylogenetic tree were consistent with the traditional taxonomy of insects (Fig. 3). C. riparius HSP27 was clustered in the same group with D. melanogaster HSP27, C. capitata HSP27 and S. crassipalpis HSP25, and separated from the HSP23 of D. melanogaster and S. crassipalpis. The sHSPs from B. mori and L. migratoria were separated from all other Dipteran sHSPs.
Fig. 3.
Phylogenetic analysis of insect small heat shock protein (sHSP) sequences. The full names of the species are: C. riparius (Chr), C. capitata (Cec), D. melanogaster (Drm), S. crassipalpis (Sac), L. huidobrensis (Lih), L. sativae (Lis), B. mori (Bom), V. canescens (Vec), and L. migratoria (Lom)
Effects of temperature in Hsp27 mRNA levels
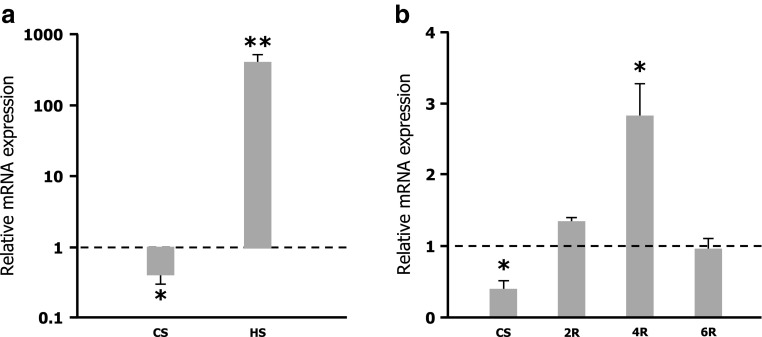
To examine the transcriptional response of Hsp27 gene to cold shock, C. riparius larvae were submitted for 2 h at 4 °C. mRNA levels from control and cold-shocked samples were analyzed by quantitative qRT–PCR, following normalization to avoid random effects on sampling data in relation to actin, GAPDH and rpL13 mRNA levels. A significant reduction in Hsp27 mRNA levels was observed in larvae exposed to 4 °C (Fig. 4a). Nevertheless, when cold-shocked larvae were allowed to recover at control temperature, the expression of Hsp27 gene was reactivated reaching higher mRNA levels than those found in untreated larvae. Within 4 h of recovery, after the shift to 4 °C, the Hsp27 gene appeared to be significantly upregulated. Thereafter, the overexpressed Hsp27 gene returned again to control level within 6 h of recovery at the normal growing temperature (Fig. 4b).
Fig. 4.
a Relative levels of Hsp27 mRNA in larvae exposure at 4 °C (CS) and 35 °C (HS) for 2 h measured by real-time RT–PCR. Note the logarithmic y-axis, where values > 1 indicate upregulation and values < 1 indicate downregulation of the transcript. b Expression of Hsp27 mRNA in larvae exposed at 4 °C (CS) and recovery after exposure for 2 h (2R), 4 h (4R), and 6 h (6R) measured by real-time RT–PCR. The mean ± SEM are shown of measurements taken in three independent biological samples, each with three replicates. The expression level of untreated control larvae was set to 1. Significant differences *p ≤ 0.05, **p ≤ 0.01
The effect of heat shock on the expression profile of the Hsp27 gene was analyzed in C. riparius larvae submitted to 35 °C for 2 h. As shown in Fig. 4a, the Hsp27 gene was clearly upregulated by the temperature shift with a significant increase in mRNA levels up to 400-fold higher than those found in control non-treated larvae.
Effect of different xenobiotics on the expression profile of Hsp27 gene
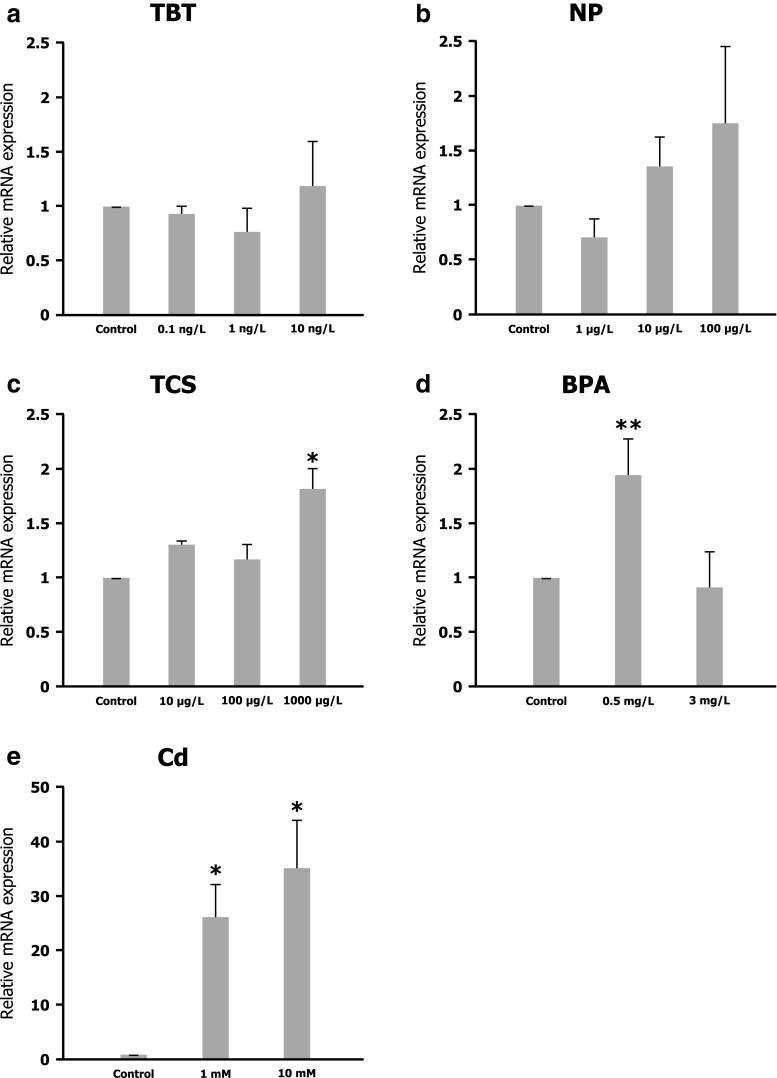
To assess if the Hsp27 gene is a xenobiotic‐responsive gene in C. riparius, the expression profile was analyzed in larvae submitted to different chemicals. TBTO, NP, TCS, BPA, and Cd were assayed for short 24-h exposures at different concentrations. No significant differences in larvae survival were found for the times and concentrations selected for each chemical. The Hsp27 mRNA level was analyzed by real-time RT–PCR, following normalization with reference genes. In each case, gene expression patterns were compared to those obtained from control cultures exposed to the same concentration of solvents. As shown in Fig. 5e, C. riparius larvae exposed to Cd showed a significant increase in the expression of the Hsp27 gene. The mRNA level for 1 and 10 mM Cd-treated larvae were, respectively, 25- and 35-fold higher than that found in untreated control. BPA exposures also resulted in a significant increase in Hsp27 gene expression, doubling that found in control larvae after 24-h 0.5 mg/L BPA treatments (Fig. 5d). When larvae were submitted to TCS, there was also a significant increase in the expression of the Hsp27 gene, after 24 h of exposure at 1,000 μg/L (Fig. 5c). However, neither 4‐NP (Fig. 5b) nor TBT (Fig. 5a) altered Hsp27 gene activity, because there were no significant changes in mRNA levels after any of the assayed treatments.
Fig. 5.
Expression levels of the Hsp27 gene in control larvae and treated larvae with 0.1, 1 and 10 ng/L of tributyltin (a), 1, 10 and 100 μg/L of nonylphenol (b), 10, 100 and 1000 μg/L of triclosan (c), 0.5 and 3 mg/L of bisphenol A (d), and 1 and 10 mM of CdCl2 (e) measured by real-time RT–PCR with primers and reference genes as indicated in “Material and methods”. The expression level of untreated control larvae was set to 1. Significant differences *p ≤ 0.05, **p ≤ 0.01
Discussion
In the present work, the Hsp27 gene from C. riparius has been characterized and its activity evaluated under a variety of experimental treatments, showing for the first time that this gene is differentially regulated by temperature shifts and pollutants in this species. The C. riparius Hsp27 gene is intronless, maps at a single chromosomal locus and the deduced protein contains the typical motifs that have been previously described (Sun and MacRae 2005). Amino acid sequence comparison revealed homology with other dipteran species, finding identities of around 50 % with D. melanogaster HSP27, C. capitata HSP27, and S. crassipalpis HSP 25. This is in contrast with that reported for the C. riparius Hsp70 gene, which appeared highly conserved, at both DNA and protein levels, sharing 80–96 % of overall amino acid identities with homologous sequences from other diptera (Morales et al. 2011). Indeed, members of the sHSPs appear to be the least conserved, with the exception of the α-crystallin domain, when compared with other HSP families (Denlinger et al. 2001). In addition to the α-crystallin domain, three conserved domains were identified in HSP27 homologs of D. melanogaster, S. crassipalpis, C. capitata, and C. riparius (Fig. 2). The first domain, in the amino-terminal region of the protein (aa M1–I11 in ChrHSP27), is very hydrophobic and is the least conserved, showing around 30 % amino acid sequence identity among these four homologs. This hydrophobic domain is also conserved in Drosophila HSP23 and HSP26, but not in Drosophila HSP22 (Southgate et al. 1983), and it has been suggested that it plays a role in oligomerization and chaperoning activity in several sHSPs (Sun and MacRae 2005). The second domain (aa D37–N49 in ChrHSP27) shows around 50 % amino acid sequence identity among the four HSP27 homologs. This domain is very specific as it is only conserved in the HSP27 (Kokolakis et al. 2008), suggesting that it may play a distinct role in this protein. The third domain, located downstream to the α-crystallin domain, contains 28 amino acids (aa K159–K186 in ChrHSP27) and shows around 50 % amino acid sequence identity among the four HSP27 homologs. This domain is present in all the main Drosophila sHSP (Southgate et al. 1983). In addition, C. riparius HSP27 has a conserved cysteine at position 91, which has been reported to have a regulatory role in the apoptotic signaling of HSP27 in human cells (Bruey et al. 2000). Future studies will determine if the conserved cysteine of C. riparius HSP27 functions in a similar manner to that found for mammalian cell culture systems (Fu et al. 2003).
Previous phylogenetic analysis that included vertebrates and plants has already suggested that different sHSPs of the same species are phylogenetically closer than homologous sHSPs between species, pointing to the possibility that sHSPs might have evolved by gene duplication after species divergence (de Jong et al. 1993). However, more recent studies have indicated that several mammalian sHSPs have clearly recognizable orthologs in lower vertebrates (Franck et al. 2004), while the HSP27 homologs from D. melanogaster, C. capitata, and S. crassipalpis have been separated from other dipteran sHSPs (Kokolakis et al. 2008). In agreement with these data, we found that the HSP27 of C. riparius was also separated from other dipteran sHSPs, suggesting that the four proteins are orthologous and may have evolved before divergence of these species.
As C. riparius is one of the invertebrate aquatic species recommended by the OECD for acute toxicity testing, one of the aims of this work was to evaluate the early response of the Hsp27 gene under different environmental stressors, including high and low temperature and short‐term exposures to chemicals. Results are summarized in Table 2 and compared to those previously reported for Hsps70 in this organism. In relation to temperature, the Hsp27 gene appeared to be significantly upregulated by a heat shock at 35 °C, as expected since high temperature is the classic inducer of HSPs. We found levels of mRNA up to 400-fold higher than those from larvae at the normal growing temperature (20 °C). This response of the Hsp27 gene is similar and concomitant with the activation of the Hsp70 gene previously reported during the same temperature shift (Morales et al. 2011). In contrast, it is worth mentioning that the Hsc70 gene maintained a constitutive expression, and it was neither upregulated nor downregulated after 35 °C exposures of C. riparius larvae (Morales et al. 2011). Several studies in D. melanogaster had previously reported that Hsp27 gene and proteins are highly induced over a broad temperature range (30-37 °C) (Lindquist 1980; Vazquez et al. 1993). It has been proposed that overexpression of either Hsp26 or Hsp27 genes extends lifespan in D. melanogaster, and also increases stress resistance in transgenic flies (Wang et al. 2004).
Table 2.
Hsp27 gene expression under different environmental stressors, including high and low temperature and short-term exposures to chemicals
| HS | CS | TBT | NP | TCS | BPA | Cd | |
|---|---|---|---|---|---|---|---|
| Hsp70 | + | nd | = | ++ | nd | + | + |
| Hsc70 | = | nd | = | = | nd | = | = |
| Hsp27 | ++ | − | = | = | + | + | ++ |
HS heat shock; CS cold shock; TBT tributyltin; NP nonylphenol; TCS triclosan; BPA bisphenol A; Cd cadmium
+ upregulated, ++ strongly upregulated, − downregulated; = not altered, nd not determined
A common physiological response to thermal injury is the increased expression of heat shock proteins, but the response to cold stress has been far less studied in insects. In our study, a modulation of C. riparius Hsp27 gene activity was found during cold exposure and also throughout the recovery period. While the Hsp27 gene was significantly downregulated in response to cold exposure at 4 °C, a clear reactivation of the gene took place when larvae recovered at the normal growing temperature. Moreover, a significant overexpression took place 4 h after the cold exposure. It is worth noting that the upregulation of the gene occurred at the normal temperature of 20 °C and, therefore, it could be due to a retarded effect of the cold (4 °C) in activating the Hsp27 gene or, alternatively, due to a thermal shift effect (from 4 °C to 20 °C), even though the final temperature was the normal growing temperature and not strictly a heat shock for these organisms. Thereafter, the overexpressed Hsp27 gene again returned to the control level within 6 h of recovery at the normal temperature. The recovery period is required before the Hsp27 gene is activated, indicating that the gene is not involved in the immediate response to cold shock that leads to rapid cold hardening (Chen et al. 1987; Lee et al. 1987; Clark and Worland 2008). It is interesting to note that cold shock also did not induce the Hsp70 gene in C. riparius (data not shown). These data suggest that some members of the HSP family behave in a different manner during high and low temperature stress. Although there is a consensus regarding the activation of Hsp genes by high temperatures, there is little agreement in their response to cold shocks and previous studies have shown notable differences. A reduction in expression levels of different Hsps genes (Hsp19.7, Hsp20.7, Hsp70, and Hsp90) was found in cultured cells of M. brassicae after 48-h exposures to 0 °C. The expression levels were reactivated during the recovery phases (Sonoda et al. 2007). Sinclair et al. 2007 did not observe any modulation of Hsp23 during exposure to or recovery from a short cold stress in D. melanogaster. In the same species, the transcriptional activity of different sHsps (Hsp22, Hsp23, Hsp26, and Hsp27) was not modulated by cold, but peaks of expression occurred during the recovery phase (Qin et al. 2005; Colinet et al. 2010). In contrast, Yocum et al. 1998 reported that expression of the Hsp23 transcript of the nondiapausing S. crassipalpis was induced in response to cold and low temperature also significantly induced the Hsp27 gene in fishes (Yang et al. 2012).
Regarding the chemical treatments, three out of five compounds tested were able to trigger activation of the Hsp27 gene. All are known for their endocrine activity, and it is worth stressing that previous experiments have shown that the Hsp27 gene is regulated by the hormone 20-hydroxyecdysone in a salivary gland culture of the fruit fly C. capitata (Kokolakis et al. 2008). In C. riparius, the expression of Hsp27 appeared to be differentially regulated by these hormonal disruptors. Tributyltin or nonylphenol did not alter the Hsp27 gene after the exposures assayed in C. riparius. In addition, we have previously found that the Hsp70 gene was not altered by TBT (Morales et al. 2011). Therefore, it seems that exposure to TBT does not elicit a stress response in insects, even though this compound has a high toxicity for the larvae, provokes significant DNA damage and alters the expression pattern of a suite of genes involved in the initial response to steroid hormonal signals (Morales et al. 2013). Yet, nonylphenol was able to induce Hsp70 gene activity in C. riparius (Lee and Choi 2006; Morales et al. 2011). The differential sensitivity of Hsp27 gene to specific chemicals was also reported in Apis cerana; while only some pesticides were able to upregulate this gene others did not alter it or even downregulate transcription (Liu et al. 2012).
In contrast, exposure to triclosan, bisphenol A and, particularly, cadmium significantly increased Hsp27 mRNA levels in C. riparius. This is the first evidence of the ability of TCS, a widely used bactericide and common pollutant of waterways, to alter the activity of a small heat shock protein gene in invertebrates. To date, there are very few studies on the regulation of genes after TCS exposures in aquatic organisms, but it has previously been reported that TCS elicited a cellular stress response, as indicated by the altered CAT and Hsp30 transcript levels in frogs (Hinther et al. 2011). We also found that BPA was able to increase Hsp27 gene expression, with this compound also being an activator of the Hsp70 gene in this organism (Planelló et al. 2008; Morales et al. 2011). Among the toxicants tested in the present study, cadmium was the most potent inducer of the Hsp27 gene, which appeared to be highly overexpressed by up to 35-fold at the higher dose assayed. These data suggest that this gene might be a useful biomarker for assessing cadmium, a widely distributed industrial and environmental toxin, and should be assayed for other heavy metals. In C. riparius, we have previously found that cadmium altered ribosome biogenesis and activated the stress response, as indicated by upregulation of Hsp70 (Martínez‐Guitarte et al. 2007; Morales et al. 2011). The expression of Hsp19.7, Hsp20.7, Hsp70, and Hsp90 genes has been induced by cadmium exposure in M. brassicae (Sonoda et al. 2007). Accumulation of the HSP27 protein was detected in human renal epithelial cells after chronic treatment with low doses of cadmium (Bonham et al. 2003) and, in fishes, the Hsp27 gene also experienced a dramatic upregulation after a combined stress of temperature and cadmium (Yang et al. 2012).
In conclusion, the experimental data from our study indicate that the Hsp27 gene in C. riparius is capable of sensing the cellular stress caused by heat and cold, as well as that caused by various environmental pollutants. The application of HSPs in environmental risk assessment has gained momentum during the last decade and, in particular, the induction of HSP70 has been used as an environmental screening tool (Karouna-Renier and Zehr 2003). Despite increasing evidence to support the role of HSP27 as a biomarker for many diseases including cancer (Wang et al. 2009), more studies are required to address its usefulness in a toxicological context. The induction of the expression of Hsps as a bioindicator for environmental pollution is being advocated by a number of researchers (Gupta et al. 2010). Yet, to date, only a few studies have analyzed small Hsps genes in response to toxicants. Our data suggest that the small Hsp27 gene might play an important role in xenobiotic responses, reflecting the integrated severity of the environmental stress. Its potential use for predicting the toxicity of chemicals merits further research to validate its sensitivity and specificity.
Acknowledgments
The authors wish to thank Dr T. Carretero (University of Zaragoza) and Ted Cater for critical reading of the manuscript. This work was supported by the Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica (Spain), grant CTM2012-37547 from the Ciencias y Tecnologías Medioambientales program. The authors declare that there are no conflicts of interest.
References
- Antizar-Ladislao B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. A review. Environ Int. 2008;34:292–308. doi: 10.1016/j.envint.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- Bierkens J, Maes J, Vander Plaetse F. Dose-dependent induction of heat shock protein 70 synthesis in Raphidocelis subcapitata following exposure to different classes of environmental pollutants. Environ Pollut. 1998;101:91–97. doi: 10.1016/S0269-7491(98)00010-4. [DOI] [PubMed] [Google Scholar]
- Bonham RT, Fine MR, Pollock FM, Shelden EA. Hsp27, Hsp70 and metallothionein in MDCK and LLC-PK1 renal epithelial cells: effects of prolonged exposure to cadmium. Toxicol Appl Pharmacol. 2003;191:63–73. doi: 10.1016/S0041-008X(03)00226-6. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome C. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Chen CP, Denlinger DL, Lee RE. Cold-shock injury and rapid cold hardening in the flesh fly Sarcophaga crassipalpis. Physiol Zool. 1987;60:297–304. [Google Scholar]
- Clark MS, Worland MR. How insects survive the cold: molecular mechanisms. J Comp Physiol B-Biochem Syst Environ Physiol. 2008;178:917–933. doi: 10.1007/s00360-008-0286-4. [DOI] [PubMed] [Google Scholar]
- Colinet H, Lee SF, Hoffmann A. Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult Drosophila melanogaster. FEBS J. 2010;277:174–185. doi: 10.1111/j.1742-4658.2009.07470.x. [DOI] [PubMed] [Google Scholar]
- Cousins IT, Staples CA, Klecka GM, Mackay D. A multimedia assessment of the environmental fate of bisphenol A. Hum Ecol Risk Assess. 2002;8:1107–1135. doi: 10.1080/1080-700291905846. [DOI] [Google Scholar]
- de Jong WW, Leunissen JA, Voorter CE. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993;10:103–126. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]
- Denlinger DL, Rinehart JP, Yocum GD. Stress proteins: a role in insect diapause? In: Denlinger DL, Giebultowicz J, Saunders DS, editors. Insect timing: Circadian rhythmicity to seasonality. Amsterdam: Elsevier; 2001. pp. 155–171. [Google Scholar]
- EPA (US) (1996) Chironomid sediment toxicity test, ecological effects test guidelines. EPA 712-C-96-313; 2nd ed. EPA 600/R-99/064, Washington DC, USA
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Franck E, Madsen O, van Rheede T, Ricard G, Huynen MA, de Jong WW. Evolutionary diversity of vertebrate small heat shock proteins. J Mol Evol. 2004;59:792–805. doi: 10.1007/s00239-004-0013-z. [DOI] [PubMed] [Google Scholar]
- Fu X, Li W, Mao Q, Chang Z. Disulfide bonds convert small heat shock protein Hsp16.3 from a chaperone to a non-chaperone: implications for evolution of cysteine in molecular chaperones. Biochem Biophys Res Commun. 2003;308:627–635. doi: 10.1016/S0006-291X(03)01450-5. [DOI] [PubMed] [Google Scholar]
- Gkouvitsas T, Kontogiannatos D, Kourti A. Differential expression of two small Hsps during diapause in the corn stalk borer Sesamia nonagrioides (Lef.) J Insect Physiol. 2008;54:1503–1510. doi: 10.1016/j.jinsphys.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan Nair PM, Park SY, Lee SW, Choi J (2011) Differential expression of ribosomal protein gene, gonadotrophin relasing hormone gene and Balbiani ring protein gene in silver nanoparticles exposed Chironomus riparius. Aquat Toxicol 101:31–37 [DOI] [PubMed]
- Goto SG, Kimura MT. Heat-shock-responsive genes are not involved in the adult diapause of Drosophila triauraria. Gene. 2004;326:117–122. doi: 10.1016/j.gene.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Shrama A, Mishra M, Mishra RK, Chowdhuri DK. Heat shock proteins in toxicology: how close and how far? Life Sci. 2010;86:377–384. doi: 10.1016/j.lfs.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Hao X, Zhang S, Timakov B, Zhang P. The Hsp27 gene is not required for Drosophila development but its activity is associated with starvation resistance. Cell Stress Chaperones. 2007;12:364–372. doi: 10.1379/CSC-308.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S. Chronic effects of Cu on reproduction of Polypedilum nubifer (Chironomidae) through water and food. Ecotoxicol Environ Saf. 1988;16:1–10. doi: 10.1016/0147-6513(88)90011-5. [DOI] [PubMed] [Google Scholar]
- Hinther A, Bromba CM, Wulff JE, Helbing CC. Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environ Sci Technol. 2011;45:5395–5402. doi: 10.1021/es1041942. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Parsons PA. Evolutionary genetics and environmental stress. New York: Oxford University Press; 1991. [Google Scholar]
- Hoffmann AA, Sørensen JG, Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Therm Biol. 2003;28:175–216. doi: 10.1016/S0306-4565(02)00057-8. [DOI] [Google Scholar]
- Huang LH, Kang L. Cloning and interspecific altered expression of heat shock protein genes in two leafminer species in response to thermal stress. Insect Mol Biol. 2007;16:491–500. doi: 10.1111/j.1365-2583.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- Huang LH, Wang CZ, Kang L. Cloning and expression of five heat shock protein genes in relation to cold hardening and development in the leafminer, Liriomyza sativae. J Insect Physiol. 2009;55:279–285. doi: 10.1016/j.jinsphys.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Karouna-Renier NK, Rao KR. An inducible HSP70 gene from the midge Chironomus dilutus: characterization and transcription profile under environmental stress. Insect Mol Biol. 2009;18:87–96. doi: 10.1111/j.1365-2583.2008.00853.x. [DOI] [PubMed] [Google Scholar]
- Karouna-Renier NK, Zehr JP. Short-term exposures to chronically toxic copper concentrations induce HSP70 proteins in the midge larvae (Chironomus tentans) Sci Total Environ. 2003;312:267–272. doi: 10.1016/S0048-9697(03)00254-7. [DOI] [PubMed] [Google Scholar]
- Kokolakis G, Tatari M, Zacharopoulou A, Mintzas AC. The hsp27 gene of the Mediterranean fruit fly, Ceratitis capitata: structural characterization, regulation and developmental expression. Insect Mol Biol. 2008;17:699–710. doi: 10.1111/j.1365-2583.2008.00840.x. [DOI] [PubMed] [Google Scholar]
- Lee SB, Choi J. Multilevel evaluation of nonylphenol toxicity in fourth-instar larvae of Chironomus riparius (Diptera, Chironomidae) Environ Toxicol Chem. 2006;25:3006–3014. doi: 10.1897/05-601R1.1. [DOI] [PubMed] [Google Scholar]
- Lee RE, Chen CP, Denlinger DL. A rapid cold-hardening process in insects. Science. 1987;238:1415–1417. doi: 10.1126/science.238.4832.1415. [DOI] [PubMed] [Google Scholar]
- Li AQ, Popova-Butler A, Dean DH, Denlinger DL. Proteomics of the flesh fly brain reveals an abundance of upregulated heat shock proteins during pupal diapause. J Insect Physiol. 2007;53:385–391. doi: 10.1016/j.jinsphys.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Varying patterns of protein synthesis in Drosophila during heat shock: implications for regulation. Dev Biol. 1980;77:463–479. doi: 10.1016/0012-1606(80)90488-1. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xi D, Kang M, Guo X, Xu B. Molecular cloning and characterization of Hsp27.6: the first reported small heat shock protein from Apis cerana cerana. Cell Stress Chaperones. 2012;17:539–551. doi: 10.1007/s12192-012-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez EA, Moore BC, Schaumloffel J, Dasgupta N. Morphological abnormalities in Chironomus tentans exposed to cadmium and copper-spiked sediments. Ecotoxicol Environ Saf. 2003;55:204–212. doi: 10.1016/S0147-6513(02)00136-7. [DOI] [PubMed] [Google Scholar]
- Martínez-Guitarte JL, Planelló R, Morcillo G. Characterization and expression during development and under environmental stress of the genes encoding ribosomal proteins L11 and L13 in Chironomus riparius. Comp Biochem Physiol B Biochem Mol Biol. 2007;147:590–596. doi: 10.1016/j.cbpb.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Martínez-Paz P, Morales M, Martínez-Guitarte JL, Morcillo G. Characterization of a cytochrome P450 gene (CYP4G) and modulation under different exposures to xenobiotics (tributyltin, nonylphenol, bisphenol A) in Chironomus riparius aquatic larvae. Comp Biochem Physiol C-Toxicol Pharmacol. 2012;155:333–343. doi: 10.1016/j.cbpc.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Michailova P, Petrova N, Ilkova J, Bovero S, Brunetti S, White K, Sella G. Genotoxic effect of copper on salivary gland polytene chromosomes of Chironomus riparius Meigen 1804 (Diptera, Chironomidae) Environ Pollut. 2006;144:647–654. doi: 10.1016/j.envpol.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Morales M, Planelló R, Martínez-Paz P, Herrero O, Cortés E, Martínez-Guitarte JL, Morcillo G. Characterization of Hsp70 gene in Chironomus riparius: expression in response to endocrine disrupting pollutants as a marker of ecotoxicological stress. Comp Biochem Physiol C-Toxicol Pharmacol. 2011;153:150–158. doi: 10.1016/j.cbpc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Morales M, Martínez-Paz P, Ozáez I, Martínez-Guitarte JL, Morcillo G. DNA damage and transcriptional changes induced by tributyltin (TBT) after short in vivo exposures of Chironomus riparius (Diptera) larvae. Comp Biochem Physiol C-Toxicol Pharmacol. 2013;158:57–63. doi: 10.1016/j.cbpc.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Morcillo G, Barettino D, Carmona MJ, Carretero T, Díez JL. Telomeric-DNA sequences differentially activated by heat-shock in two chironomus subspecies. Chromosoma. 1988;96:139–144. doi: 10.1007/BF00331046. [DOI] [PubMed] [Google Scholar]
- Morrow G, Battistini S, Zhang P, Tanguay RM. Decreased lifespan in the absence of expression of the mitochondrial small heat shock protein Hsp22 in Drosophila. J Biol Chem. 2004;279:43382–43385. doi: 10.1074/jbc.C400357200. [DOI] [PubMed] [Google Scholar]
- Nolen EAA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing heat shock proteins. J Cell Sci. 2002;115:2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- OECD. Organisation for Economic Co-operation and Development (2001) Guideline for testing of chemicals, sediment-water chironomid toxicity test using spiked sediment. 218 pp
- Ozáez I, Martinez-Guitarte JL, Morcillo G. Effects of in vivo exposure to UV filters (4-MBC, OMC, BP-3, 4-HB, OC, OD-PABA) on endocrine signaling genes in the insect Chironomus riparius. Sci Total Environ. 2013;456–457:120–126. doi: 10.1016/j.scitotenv.2013.03.081. [DOI] [PubMed] [Google Scholar]
- Park K, Kwak IS. Characterization of heat shock protein 40 and 90 in Chironomus riparius larvae: effects of di(2-ethylhexyl) phthalate exposure on gene expressions and mouthpart deformities. Chemosphere. 2008;74:89–95. doi: 10.1016/j.chemosphere.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Park K, Kwak IS (2009) Alcohol dehydrogenase gene expression in Chironomus riparius exposed to di(2-ethylhexyl) phthalate. Comp Biochem Comp Biochem Physiol C-Toxicol Pharmaco 150361–367 [DOI] [PubMed]
- Park K, Kwak IS. Molecular effects of endocrine-disrupting chemicals on the Chironomus riparius estrogen-related receptor gene. Chemosphere. 2010;79:934–941. doi: 10.1016/j.chemosphere.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Planelló R, Martínez-Guitarte JL, Morcillo G. Ribosomal genes as early targets of cadmium-induced toxicity in Chironomus riparius larvae. Sci Total Environ. 2007;373:113–121. doi: 10.1016/j.scitotenv.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Planelló R, Martínez-Guitarte JL, Morcillo G. The endocrine disruptor bisphenol A increases the expression of HSP70 and ecdysone-receptor genes in the aquatic larvae of Chironomus riparius. Chemosphere. 2008;71:1870–1876. doi: 10.1016/j.chemosphere.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Planelló R, Martínez-Guitarte JL, Morcillo G. Effect of acute exposure to cadmium on the expression of heat-shock and hormone-nuclear receptor genes in the aquatic midge Chironomus riparius. Sci Total Environ. 2010;408:1598–1603. doi: 10.1016/j.scitotenv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Planelló R, Herrrero O, Martínez-Guitarte JL, Morcillo G. Comparative effects of butyl benzyl phthalate (BBP) and di-2-ethylhexyl phthalate (DEHP) on the aquatic larvae of Chironomus riparius based on gene expression assays related to the endocrine system, the stress response and ribosomes. Aquat Toxicol. 2011;105:62–70. doi: 10.1016/j.aquatox.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Qin W, Neal SJ, Robertson RM, Westwood JT, Walker VK. Cold hardening and transcriptional change in Drosophila melanogaster. Insect Mol Biol. 2005;14:607–613. doi: 10.1111/j.1365-2583.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- Raut SA, Angus RA. Triclosan has endocrine-disrupting effects in male western mosquitofish Gambusia affinis. Environ Toxicol Chem. 2010;29:1287–1291. doi: 10.1002/etc.150. [DOI] [PubMed] [Google Scholar]
- Reineke A. Identification and expression of a small heat shock protein in two lines of the endoparasitic wasp Venturia canescens. Comp Biochem Physiol A-Mol Integr Physiol. 2005;141:60–69. doi: 10.1016/j.cbpb.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Rinehart JP, Li A, Yocum GD, Robich RM, Hayward SA, Denlinger DL. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc Natl Acad Sci U S A. 2007;104:11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robich RM, Rinehart JP, Kitchen LJ, Denlinger DL. Diapause-specific gene expression in the northern house mosquito, Culex pipiens L., identified by suppressive subtractive hybridization. J Insect Physiol. 2007;53:235–245. doi: 10.1016/j.jinsphys.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano D, Li B, Xia Q, Yamamoto K, Banno Y, Fujii H, Aso Y. Genes encoding small heat shock proteins of the silkworm, Bombyx mori. Biosci Biotechnol Biochem. 2006;70:2443–2450. doi: 10.1271/bbb.60176. [DOI] [PubMed] [Google Scholar]
- Silver JT, Noble EG. Regulation of survival gene hsp70. Cell Stress Chaperones. 2012;17:1–9. doi: 10.1007/s12192-011-0290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair BJ, Gibbs AG, Roberts SP. Gene transcription during exposure to, and recovery from, cold and desiccation stress in Drosophila melanogaster. Insect Mol Biol. 2007;16:435–443. doi: 10.1111/j.1365-2583.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- Snell TW, Brogdon SE, Morgan MB. Gene expression profiling in ecotoxicology. Ecotoxicology. 2003;12:475–483. doi: 10.1023/B:ECTX.0000003033.09923.a8. [DOI] [PubMed] [Google Scholar]
- Soares A, Guieysse B, Fefferson B, Cartmell E, Lester JN. Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ Int. 2008;34:1033–1049. doi: 10.1016/j.envint.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Ashfaq M, Tsumuki H. Cloning and nucleotide sequencing of three heat shock protein genes (hsp90, hsc70 and hsp19.5) from the Diamondback moth, Plutella xylostella (L.) and their expression in relation to developmental stage and temperature. Arch Insect Biochem Physiol. 2006;62:80–90. doi: 10.1002/arch.20124. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Ashfaq M, Tsumuki H. A comparison of heat shock protein genes from cultured cells of the Cabbage armyworm, Mamestra brassicae, in response to heavy metals. Arch Insect Biochem Physiol. 2007;65:210–222. doi: 10.1002/arch.20178. [DOI] [PubMed] [Google Scholar]
- Sorensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol Lett. 2003;6:1025–1037. doi: 10.1046/j.1461-0248.2003.00528.x. [DOI] [Google Scholar]
- Southgate R, Ayme A, Voellmy R. Nucleotide sequence analysis of the Drosophila small heat shock gene cluster at locus 67B. J Mol Biol. 1983;165:35–57. doi: 10.1016/S0022-2836(83)80241-1. [DOI] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. Small heat shock proteins: molecular structure and chaperone function. Cell Mol Life Sci. 2005;62:2460–2476. doi: 10.1007/s00018-005-5190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana S, Numata H, Goto SG. Gene expression of heat-shock proteins (Hsp23, Hsp70 and Hsp90) during and after larval diapause in the blow fly Lucilia sericata. J Insect Physiol. 2005;51:641–647. doi: 10.1016/j.jinsphys.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Tammariello SP, Rinehart JP, Denlinger DL. Desiccation elicits heat shock protein transcription in the flesh fly, Sarcophaga crassipalpis, but does not enhance tolerance to high or low temperatures. J Insect Physiol. 1999;45:933–938. doi: 10.1016/S0022-1910(99)00073-6. [DOI] [PubMed] [Google Scholar]
- Tsvetkova NM, Horváth I, Török Z, Wolkers WF, Balogi Z, Shigapova N, Crowe LM, Tablin F, Vierling E, Crowe JH, Vigh L. Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci U S A. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez J, Pauli D, Tissieres A. Transcriptional regulation in Drosophila during heat shock: a nuclear run-on analysis. Chromosoma. 1993;102:233–248. doi: 10.1007/BF00352397. [DOI] [PubMed] [Google Scholar]
- Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, Gunderson MP, Van Aggelen G, Helbing CC. The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat Toxicol. 2006;80:217–227. doi: 10.1016/j.aquatox.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Wang HD, Kazemi-Esfarjani P, Benzer S. Multiple-stress analysis for isolation of Drosophila longevity genes. Proc Natl Acad Sci U S A. 2004;101:12610–12615. doi: 10.1073/pnas.0404648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Wang XH, Zhou CS, Huang LH, Zhang SF, Guo W, Kang L. cDNA cloning of heat shock proteins and their expression in the two phases of the migratory locust. Insect Mol Biol. 2007;16:207–219. doi: 10.1111/j.1365-2583.2006.00715.x. [DOI] [PubMed] [Google Scholar]
- Wang F, Feng M, Xu P, Xiao H, Niu P, Yang X, Bai Y, Peng Y, Yao P, Tan H, Tanguay RM, Wu T. The level of Hsp27 in limphocytes is negatively associated with a higher risk of lung cancer. Cell Stress Chaperones. 2009;14:245–251. doi: 10.1007/s12192-008-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters ER, Aevermann BD, Sanders-Reed Z. Comparative analysis of the small heat shock proteins in three angiosperm genomes. Cell Stress Chaperones. 2008;13:127–142. doi: 10.1007/s12192-008-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Green A, Pascoe DW, Gower DE. Effects of cadmium on oviposition and egg viability in Chironomus riparius (Diptera: Chironomidae) Bull Environ Contam Toxicol. 1987;38:86–90. doi: 10.1007/BF01606563. [DOI] [PubMed] [Google Scholar]
- Xu P, Xiao J, Liu L, Li T, Huang D. Molecular cloning and characterization of four heat shock protein genes from Macrocentrus cingulum (Hymenoptera: Braconidae) Mol Biol Rep. 2010;37:2265–2272. doi: 10.1007/s11033-009-9715-z. [DOI] [PubMed] [Google Scholar]
- Yang QL, Yao CL, Wang ZY. Acute temperature and cadmium stress response characterization of small heat shock protein 27 in large yellow croaker, Larimichthys crocea. Comp Biochem Physiol C-Toxicol Pharmacol. 2012;155:190–197. doi: 10.1016/j.cbpc.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Yocum GD, Joplin KH, Denlinger DL. Upregulation of a 23 kDa small heat shock protein transcript during pupal diapause in the flesh fly, Sarcophaga crassipalpis. Insect Biochem Mol Biol. 1998;28:677–682. doi: 10.1016/S0965-1748(98)00046-0. [DOI] [PubMed] [Google Scholar]
- Yoshimi T, Minowa K, Karouna-Renier NK, Watanabe C, Sugaya Y, Miura T. Activation of a stress-induced gene by insecticides in the midge, Chironomus yoshimatsui. J Biochem Mol Toxicol. 2002;16:10–17. doi: 10.1002/jbt.10018. [DOI] [PubMed] [Google Scholar]