Abstract
Self-reactive T cells have shown to have a potential role as regulators of the immune system preventing or even suppressing autoimmunity. One of the most abundant proteins that can be eluted from human HLA molecules is heat shock protein 70 (HSP70). The aims of the current study are to identify HSP70 epitopes based on published HLA elution studies and to investigate whether T cells from healthy individuals may respond to such self-epitopes. A literature search and subsequent in silico binding prediction based on theoretical MHC binding motifs resulted in the identification of seven HSP70 epitopes. PBMCs of healthy controls proliferated after incubation with two of the seven peptides (H167 and H290). Furthermore H161, H290, and H443 induced CD69 expression or production of cytokines IFNγ or TNFα in healthy controls. The identification of these naturally presented epitopes and the response they elicit in the normal immune system make them potential candidates to study during inflammatory conditions as well as in autoimmune diseases.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-013-0484-1) contains supplementary material, which is available to authorized users.
Keywords: Heat shock protein 70, HSP70, Naturally processed T cell epitopes, Human HSP70 peptides, Autoreactive T cells
Introduction
Autoimmune diseases are characterized by a loss of immune tolerance in one or more organ systems, leading to tissue damage. Autoaggressive T cells can play a role in initiating such a damaging immune response, and thus, for long, it was thought that eliminating self-reactive T cells could benefit patients with autoimmunity (Hasler 2006). In the last decade, however, it has become clear that self-reactive T cells also may play a role as regulators of the immune system preventing or even suppressing autoimmunity (Kronenberg and Rudensky 2005). Irun Cohen proposed already two decades ago that a limited set of dominant antigens forms an “immunological homunculus.” This hypothesis stated that a network of T cells responds to this set of antigens (Cohen 2007) and helps to maintain immune tolerance. Thus self-tolerance is maintained by responding to self and not just by elimination of self-reactive clones.
Which self-antigens are recognized by T cells? To answer this, a number of studies focused on peptides that are presented on HLA class II, the so-called naturally presented epitopes. The peptides eluted from HLA class II were often (85 %) derived from proteins involved in antigen processing, such as HLA, Lamp-2, and heat shock protein 70 (HSP70) (Chicz et al. 1993). Thus, these proteins seem to play a double role here: they are involved in antigen presentation, but at the same time they are being processed themselves, and their peptides are presented. Interestingly, the proteins found to be sources of the peptides identified in these studies show overlap with the proteins that were proposed as part of the immunological homunculus (Cohen 2007). Therefore, it could be hypothesized that self-reactive T cells could respond especially to these abundantly MHC class II presented natural epitopes.
In fact, HSP70 is one of the most frequent HLA II natural ligand sources (Paludan et al. 2005). HSP70 is part of the HSP family, which consists of immunodominant, highly conserved proteins being upregulated during stressful conditions. As such, HSP are indeed proteins that fully fit the immunological homunculus profile. One of the main functions of HSPs is to chaperone other proteins. HSP-chaperoned antigenic peptides can be presented via MHC class I and II molecules inducing enhanced activation of antigen-specific CTL and CD4+ T cells (Haug et al. 2005; Mycko et al. 2004; Singh-Jasuja et al. 2000; Tobian et al. 2004; Udono and Srivastava 1993). Furthermore, HSPs themselves are involved in activating the innate arm of the immune system via different signaling pathways (Asea et al. 2000; Vabulas et al. 2002). In addition, they are capable of inducing antigen-specific T cell responses with immunomodulatory effects (Kamphuis et al. 2005; Pockley 2003; van Eden et al. 2005; Wendling et al. 2000).
From the HSP family, HSP70 is one of the most conserved proteins in evolution (Daugaard et al. 2007). Furthermore, it acts as a chaperone protein for other peptides, such as antigenic peptides from pathogens as well as self-peptides. For instance, E. coli HSP70 molecules were found to bind peptide sequences comprising the shared epitope sequence, which is a conserved sequence found in rheumatoid arthritis susceptible HLA type, DRB1 (Cheetham and Caplan 1998).
The fact that epitopes from HSP70 seem to be presented to T cells so abundantly may suggest that HSP70-reactive T cells have a regulatory role, because otherwise, we continuously would have autoimmune diseases. Therefore, the presence of such T cells could be important for maintenance of self-tolerance. Previously, we and others showed the immunoregulatory capacity of administration of exogenous HSP70 and HSP70-derived peptides in experimental rodent arthritis models (Tanaka et al. 1999; van Herwijnen et al. 2012; Wendling et al. 2000; Wieten et al. 2009). The presence of naturally occurring T cell responses toward HSP70 peptides presented under physiological conditions in humans is, thus far, unknown. In this study, we test the hypothesis that the human healthy immune system contains T cells that are reactive to the peptides of HSP70 that can be eluted from HLA class II.
Materials and methods
Participants
Heparinized blood samples were collected from nine healthy adults. The characteristics of the donors are shown in Table 1.
Table 1.
Age, gender, and HLA-DRB1 type of the donors in Fig. 1
| Donor | Group | Sex | Age | HLA-DRB1 |
|---|---|---|---|---|
| 1 | Healthy | F | 25 | 4/13 |
| 2 | Healthy | F | 27 | 4/16 |
| 3 | Healthy | M | 30 | 7/13 |
| 4 | Healthy | F | 29 | 1/11 |
| 5 | Healthy | F | 25 | 1/4 |
| 6 | Healthy | M | 32 | 4/4 |
| 7 | Healthy | F | 22 | 1/15 |
| 8 | Healthy | F | 25 | 7/11 |
| 9 | Healthy | M | 29 | 1/15 |
Isolation of PBMC
PBMCs were isolated by Ficoll (Pharmacia, Uppsala, Sweden) density gradient. Cells were used fresh.
Peptide selection and synthesis
HSP70 peptide selection is based on literature search on HLA elution studies (Chicz et al. 1993; Dengjel et al. 2005; Friede et al. 1996; Halder et al. 1997; Lippolis et al. 2002; Newcomb and Cresswell 1993; Sanjeevi et al. 2002; Suri et al. 2005; van Herwijnen et al. 2012; Verreck et al. 1996) and secondly by studying their theoretical MHC-binding motifs. For details of the selection procedure, see the “Results” section.
Based on this selection, a total of seven HSP70 peptides were selected. Peptides were prepared by automated simultaneous multiple peptide synthesis (SMPS) as described previously (Zee van der et al. 1995). Peptides were obtained as C-terminal amides after cleavage with 90–95 % TFA/scavenger cocktails. Peptides were analyzed by reversed-phase HPLC and checked via electrospray mass spectrometry on an LCQ ion-trap mass spectrometer (Thermoquest, Breda, the Netherlands). Purity of the peptides ranged between 50 and >95 %. Peptides were dissolved at 2 mg/ml and stored at −20 °C.
Proliferation assays
RPMI 1640 supplemented with 2 mM glutamine, 100 U/ml penicillin and streptomycin (Gibco BRL, Gaithersburg, MD, USA), and 10 % AB-positive human serum (Sanquin Blood Bank, Utrecht, the Netherlands) was used as culture medium. Cells were cultured (2 × 105 cells in 200 μl per well) in triplicate in round-bottom 96-well plates (Nunc, Roskilde, Denmark) for 96 h at 37 °C in 5 % CO2 with 100 % relative humidity, in the absence or presence of 20-μg/ml HSP70 peptides. The mitogen Concanavalin A (Calbiochem, San Diego, CA, USA) and purified Tetanus Toxoid 150Lf/ml (RIVM, Bilthoven, the Netherlands), used in a 1:60 dilution in the well, were used as positive controls. After 96 h, the cells were pulsed overnight with [3H]-thymidine (1 μCi per well; ICN Biomedicals, Amsterdam, the Netherlands), and uptake was measured using a liquid scintillation counter (Betaplate, Wallac, Turku, Finland). The magnitude of the proliferative response is expressed as stimulation index (S.I.), which is calculated as the mean counts per minute of cells cultured with antigen (peptide) divided by the mean counts per minute of cells cultured without antigen.
T cell lines
Similar culture conditions as described above were used for the generation of short-term T cell lines. Cells were cultured (2 × 105 cells in 200 μl per well) in triplicate in round-bottom 96-well plates in the absence or presence of 20 μg/ml HSP70 peptides. At day 4 (96 h), 100 μl of the medium was refreshed with medium containing IL-2 (final concentration IL2 40 IU/ml). At day 8 (192 h), phenotyping of the T cells that respond to the peptides was performed following the procedure described earlier for CMV epitope screening (Bitmansour et al. 2001). In short, antigen presenting cells were derived from freshly thawed PBMC from the same donor. CD3− cells were magnetically isolated from PBMC using CD3 Magnetic Particles (BD Biosciences) and irradiated at 3,500 rad. Cells in culture were spun down, all of the medium was aspirated, and 200 μl new medium (RPMI 10 % AB) was added containing 1.5 × 105 CD3− antigen presenting cells. Then, the same individual peptide as on day 0 and costimulatory molecules anti-CD28 (0.5 μl/ml) and anti-CD49d (0.5 μl/ml) (both from BD Biosciences) were added. Cells were incubated for 6 h at 37 °C in 5 % CO2 with 100 % relative humidity. During the last 4.5 h of culture, GolgiStop (BD Biosciences) was added.
Staining of activation marker CD69 and cytokine analysis by flow cytometry
After this restimulation, the T cell line cells were washed twice in ice-cold PBS, containing 0.1 % natrium-azide and 2 % fetal calf serum, incubated for 5 min in PBS containing 10 % mouse serum, and stained with three combinations of Amcyan, phycoerythrin-cyanin7 (PE-Cy7), or allophycocyanin (APC)-labeled anti-CD3 (BD Biosciences Pharmingen, San Diego, CA, and BioLegend, San Diego, CA) and peridinin-chlorophyll-protein cyanin5.5 (PerCP-Cy5.5) or Pacific Blue (PacB)-labeled anti-CD4 (BioLegend), APC-Cy7-labeled anti-CD8 (BD), fluoroscein isothiocyanate (FITC)-labeled anti-CD25 (BD), Alexa647-APC-labeled CD45RO (BioLegend), phycoerythrin-Cy7 (PE-Cy7)-labeled CD56 (BD), FITC-labeled CD69 (BD), PE-Cy7-labeled Latency Associated Peptide (LAP, BioLegend), or PacB viability stain (eBioscience, San Diego, CA) for 30 min at 4 °C. Subsequently, cells were fixed (Fix/Perm; BD), permeabilized (Permbuffer; BD Biosciences), incubated 15 min in Permbuffer containing 2 % rat serum, and stained with phycoerythrin (PE)-labeled anti-IFNγ (BD), TNFα (eBioscience), or IL-10 (BD) and/or PerCP-Cy5.5-labeled anti-FOXP3 (eBioscience) for 30 min at 4 °C. Cells were fixed in PBS with 1 % paraformaldehyde and kept in PBS with 0.1 % natrium-azide and 2 % fetal calf serum for a maximum of 72 h. Cells were acquired on FACSCalibur or FACSCanto II and analyzed using CellQuest version 3.3 or FACS Diva version 6.1.3 software, respectively (all from BD Biosciences). Data are presented as percentage of CD3 + CD4+ cells positive for the staining, with the background (incubation without peptide) subtracted.
HLA typing
Donors were typed for HLA class II antigen DRB1 by the LABType® SSO DNA typing system according to the manufacturer’s protocol (OneLambda, Los Angeles, CA).
Statistical analysis
For statistical analysis, Prism Software, version 5, was used. For proliferation data (Fig. 1), the one-sample t test was used with “1” (no response) as theoretical mean. For the CD69 and cytokine data group, differences were statistically evaluated using the Friedman test (one-way ANOVA nonparametric repeated measures test) with post hoc comparison to the unstimulated condition using the Dunns test. For differences between DR1/4 positive and negative donors (supplemental Fig. A), the Mann–Whitney test was used (unpaired nonparametric t test).
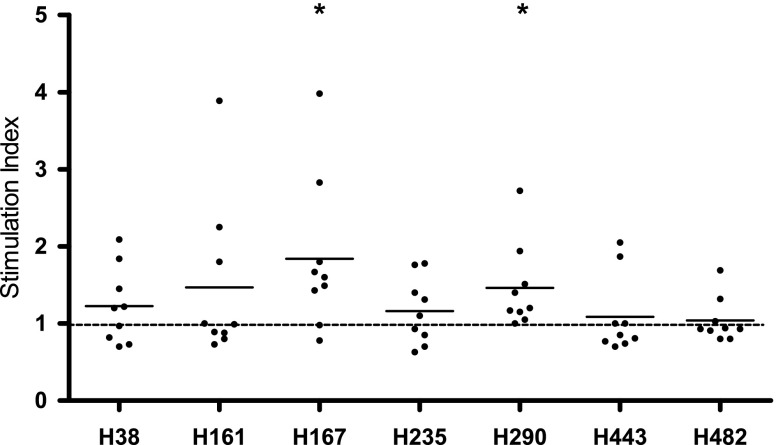
Fig. 1.
Proliferation of donor PBMCs in response to the HSP70 peptides. Each dot represents an individual donor; the mean is shown per column. Proliferation toward H167 and H290 was significant (p < 0.05 as compared to “1” using the one-sample t test)
Results
Peptide selection
Peptide sequence selection was based upon regions of HSP70 described in literature as eluted from HLA (Chicz et al. 1993; Dengjel et al. 2005; Friede et al. 1996; Halder et al. 1997; Lippolis et al. 2002; Newcomb and Cresswell 1993; Sanjeevi et al. 2002; Suri et al. 2005; Verreck et al. 1996). For an overview of the literature, see Table 2. Peptides from these regions were screened for their theoretical MHC-binding motifs with the following three online databases:
Table 2.
HSP70 peptides eluted from human class II MHC molecules (DR and DQ)
| Sequence | Class II type | Species | Source (100 % id) | Entre gene ID | Reference |
|---|---|---|---|---|---|
| QQYLPLPTPKVIGID | HLA-DR10 (DRB1*1001) | Human | HSPA13 (23-37) | 6782 | Alvarez et al. (2008) |
| TPSYVAFTDTERLIG(DA) | HLA-DR7 | Human | HSPA8 (38-52) | 3312 | Chicz et al. (1993) |
| HSPA2 (39-53) | 3306 | ||||
| HSPA1L (40-54) | 3305 | ||||
| HSPA1A (38-52) | 3303 | ||||
| TPSYVAFTDTERLIGD | HLA-DQ2 | Human | As above | As above | Stepniak et al. (2008) |
| DVYVGYESVELADSNPQ | HLA-DQ2 | Human | HSPA13 (77-93) | 6782 | Stepniak et al. (2008) |
| NPTNTVFDAKRLIGRRFD | HLA-DRB1*1104 | Human | HSPA8 (62-79) | 3312 | Verreck et al. (1996) |
| LNVLRIINEPTAAAIAYG | HLA-DRB1*0401 | Human | HSPA8 (167-184) | ||
| HSPA1A (167-184) | |||||
| HSPA1L (169-186) | |||||
| HSPA2 (168-185) | |||||
| NVLRIINEPTAAAIAYG | HLA-DRB1*0401/DRB4*0101 | Human | HSPA8 (168-184) | 3312 | Dengjel et al. (2005) |
| HSPA1A (168-184) | 3303 | ||||
| HSPA1L (170-186) | 3305 | ||||
| HSPA2 (169-185) | 3306 | ||||
| HSPA6 (170-186) | 3310 | ||||
| NVLRIINEPTAAAIA | Multiple HLA mix | Human | HSPA8 (168-184) | 3312 | Halder et al. (1997) |
| HSPA1A (168-184) | 3303 | ||||
| HSPA1L (170-186) | 3305 | ||||
| HSPA2 (169-185) | 3306 | ||||
| HSPA6 (170-186) | 3310 | ||||
| NVMRIINEPTAAAIAYG | Multiple HLA mix | Human | HSPA5 (194-210) | 3309 | Halder et al. (1997) |
| VMRIINEPTAAAIAYG | HLA-DRB1*0401/DRB4*0101 | Human | HSPA5 (195-210) | 3309 | Dengjel et al. (2005) |
| IINEPTAAAIAYGLD | HLA-DQ6 (B*0602) | Human | HSPA8 (172-186) | 3312 | Sanjeevi et al. (2002) |
| HSPA1A (172-186) | 3306 | ||||
| HSPA1L (174-188) | 3305 | ||||
| HSPA2 (173-187) | 3303 | ||||
| HSPA5 (198-212) | 3309 | ||||
| FDVSILTIEDGIFE | HLA-DQ2 | Human | HSPA8 (205-218) | 3312 | Stepniak et al. (2008) |
| VNHFIAEFKRKHKKD | HLA-DR11/w52 | Human | HSPA8 (238-252) | 3312 | Newcomb et al. (1993) |
| XDFYTSITRAXFEE | HLA-DR11/w52 | Human | HSPA8 (291-307) | 3312 | Newcomb et al. (1993) |
| HSPA1A (291-304) | 3303 | ||||
| HSPA1L (293-306) | 3305 | ||||
| HSPA2 (294-307) | 3306 | ||||
| HSPA6 (294-306) | 3310 | ||||
| ADLFRGTLDPVEK | HLA-DQ6 (B*0604) | Human | HSPA8 (307-319) | 3312 | Sanjeevi et al. (2002) |
| KSINPDEAVAYG | HLA-DQ2 | Human | HSPA8 (361-372) | 3312 | Stepniak et al. (2008) |
| HSPA1A (361-372) | 3303 | ||||
| HSPA1L (363-374) | 3305 | ||||
| HSPA2 (364-375) | 3306 | ||||
| HSPA6 (363-374 | 3310 | ||||
| VPTKKSQIFSTASDNQPTVT | HLA-DRB1*0401/DRB4*0101 | Human | HSPA5 (443-462) | 3309 | Muntasell et al. (2004) |
| GERAMTKDNNLLG | HLA-DR4Dw4 | Human | HSPA8 (445-457) | 3312 | Friede et al. (1996) |
| HSPA1A (445-457) | 3303 | ||||
| HSPA1L (447-459) | 3305 | ||||
| HSPA2 (448-460) | 3306 | ||||
| HSPA6 (447-459) | 3310 | ||||
| GERAMTKDNNLLGKFE | HLA-DRB1*0401/DRB4*0101 | Human | HSPA8(445-460) | 3312 | Dengjel et al. (2005) |
| HSPA1A (445-460) | 3303 | ||||
| GERAMTKDNNLLGRFE | HLA-DRB1*0401/DRB4*0101 | Human | HSPA6 (447-462) | 3310 | Dengjel et al. (2005) |
| ANGILNVSAVDKSTGKE | HLA-DRB1*0401 | Human | HSPA8 (482-499) | 3312 | Lippolis et al. (2002) |
| GILNVSAVDKSTGK | HLA-DRB1*0401 | Human | HSPA8 (484-497) | 3312 | Lippolis et al. (2002) |
| GILNVSAVDKSTGKE | HLA-DRB1*0401/DRB4*0101 | Human | HSPA8 (484-498) | 3312 | Dengjel et al. (2005) |
| CNEIINWLDKNQ | HLA-DR4Dw10 | Human | HSPA8 (574-585) | 3312 | Friede et al. (1996) |
| ISWLDKNQTAEKEEFE | HLA-DQ8 (transgenic in NOD) | Human | HSPA8 (578-593) | 15481 | Suri et al. (2005) |
Amino acid sequences of HSP70 peptides eluted from human MHC class II molecules. This literature overview is adapted from van Herwijnen et al. (2012) and the basis for our further search. With the peptides identified, we performed further in silico binding studies, which resulted in the selection of the peptides depicted in Table 3. The HSP70 nomenclature is as proposed by Kampinga et al. (2009)
Syfpeithi (http://www.syfpeithi.de/home), Rankpep (http://bio.dfci.harvard.edu/RANKPEP/), HLA-DR4Pred (http://www.imtech.res.in/raghava/propred/index), and the pan-DR binding computer algorithm kindly provided by Prof A. Sette (Southwood et al. 1998). Good HLA-binding peptides were selected for further study. A total of seven human HSP70-peptides were selected: H38 (38-52), H161 (161-175), H167 (167-181), H 235 (235-249), H 290 (290-304), H 443 (443-457), and H482 (482-496). The peptides and the rationale for their selection are shown in Table 3. Based on literature, three regions (168-186 [H168 best in silico binder], 445-460 [H443], and 482-489 [H482]) were of special interest, because they were identified in more than one study on peptide elution. These three regions were not only found in classical peptide elution studies, but also in a study in which peptide presentation after induction of autophagy was tested (Dengjel et al. 2005). The region around 168-186 is a very conserved area, and in silico binding tests did not only identify peptide H168, but also H161 as “pan-DR binder.” We decided to add H161 to the studies, also because it probably contains the highly conserved binding site for dnaJ (Cheetham and Caplan 1998) Regions 238-252 and 291-304 were only identified in one study (Newcomb and Cresswell 1993), but yielded good in silico binders, especially in DR4 (which is an interesting HLA-type in autoimmunity). Region 38-52 resulted in one DR7 binder [H38]. An extensive overview of all in silico binding scores is depicted in supplementary Table A.
Table 3.
Amino acid sequences of the HSP70 peptides selected for study
| No. | Amino acids (aa) | Sequence | Eluted from | In silico binding | Notes/remarks |
|---|---|---|---|---|---|
| H38 | 38-52 | TPSYVAFTDTERLIG | DR 7 binding epitope (Chicz et al. 1993) | DR 7 binding goodDR1, DR4 binding low | |
| H161 | 161-175 | AGTIAGLNVLRIINE | DR4 (Dengjel et al. 2005; Halder et al. 1997; Sanjeevi et al. 2002) | Pan HLA binders in silico | Possibly include binding site for dnaJ (Cheetham et al. 1998); presented upon autophagy |
| H167 | 167-181 | LNVLRIINEPTAAAI | |||
| H235 | 235-249 | NRMVNHFIAEFKRKH | Frameshift of epitope eluted from DR11 (Newcomb et al. 1993) | Good DR4 binder in silico | |
| H290 | 290-304 | GIDFYTSITRARFEE | DR11 (Newcomb et al. 1993) | Includes also in silico DR1, 4 and 11 binding epitopes | |
| H443 | 443-457 | YEGERAMTKDNNLLG | DR4 and/or DR4/DR53 (Dengjel et al. 2005; Friede et al. 1996) | Mainly DR4 binding epitopes in silico | Presented upon autophagy |
| H482 | 482-496 | ANGILNVSAVDKSTG | Region identified by two groups, from DR4 (Dengjel et al. 2005; Lippolis et al. 2002) | DR4 binding in silico | Presented upon autophagy |
Peptides were named with H (=human) and the position of the first amino acid of the peptide in the Hsc70 protein. Reasons for selecting the peptides are described
T cell proliferation toward H161, H167, and H290
To study whether self-reactive T cells are present in the healthy immune system, with specificity for the peptides that have been eluted from HLA class II, PBMCs from nine healthy donors (Table 1) were isolated and incubated with the different peptides. Proliferation at 96 h was measured using the [3H]-thymidine incorporation assay. Three of the seven tested peptides induced an average T cell response of ≥1.5 relative to the unstimulated condition, respectively mean and standard error of the mean (SEM) of H161 1.5 (0.35), H167 1.8 (0.32), and H290 1.5 (0.18) (Fig. 1). Proliferation towards H167 and H290 was significant (P < 0.05). H167 induced a positive stimulation index above 1.5 in more than 50 % of the donors. The positive control tetanus toxoid full protein induced an average proliferation of 59.2 (35.0) (data not shown).
No correlation of proliferative response and HLA type of individual
Although the peptides were selected on the basis of their pan-DR binding capacity (see Table 3), HLA class of the individual donor may be a predictor of proliferative response (see Table 1 for HLA types of the donors). For the DR1 and DR4 haplotypes, we evaluated if the proliferative response could be predicted by presence or absence of this haplotype. With this low number of donors, we could not show a correlation between the HLA-DR1 and HLA-DR4 compared with the proliferative responses (supplemental Fig. A).
CD69 expression and production of intracellular cytokines IFNγ and TNFα toward H161, H290, and H443 in healthy donors
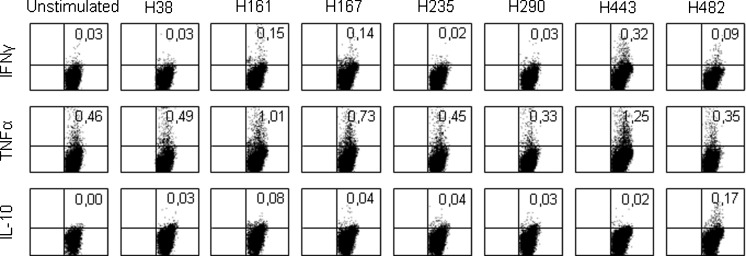
To further characterize peptide-specific T cell responses, CD69 and cytokine expression were analyzed. Restimulation following the protocol of peptide-specific T cell lines resulted in enhanced expression of CD69 and enhanced production of TNFα, IFNγ, and IL-10 by some peptides and in some donors. Figure 2 shows the intracellular cytokine staining in CD3+ CD4+ T cells of one of the donors (donor 5). In this particular donor, peptide H161 induced IFNγ, TNFα, and IL-10; peptides H167 and H443 induced IFNγ and TNFα; and peptide H482 resulted in IL-10 production by CD3+ CD4+ T cells.
Fig. 2.
Intracellular cytokine production of CD4+ T cells from the short-term T cell line of donor 5. Cells were restimulated on day 8 with costimulatory molecules and with or without peptide. In this donor, moderate cytokine production was found in response to H161, H167, H443, and H482. The percentages of CD3+ CD4+ cells positive for the staining are depicted
Pooled data of 13 donors is depicted in Fig. 3. Shown are percentages of CD3+ CD4+ T cells positive for the marker of interest when background (costimulation, but no peptide) is subtracted. CD69 was upregulated in part of the donors after H161 and H290 stimulation, however nonsignificant (Fig. 3, upper left). TNFα production was induced by all peptides from H161 to H443, and it was significantly increased upon stimulation with H161 (P < 0.01), H290 (P < 0.01), and H443 (P < 0.05) (Fig. 3, lower right). IFNγ production was seen after H161 and H235 stimulation (nonsignificant). IL-10 showed small differences compared to the unstimulated condition; nevertheless, in individual donors, populations of cytokine-producing CD3+ CD4+ cells could be identified (Fig. 2). The same was true for TGFβ (as measured by latency-associated peptide [LAP]) and the Treg surface marker combination CD25/FOXP3 (supplemental Fig. B). To test whether the responder cells were naïve or memory type, we stained for CD45RO. We found that T cells that were CD69+ after incubation with the peptides were around 60 % RO+. We found around 60 % CD45RO positivity in the IFNγ-positive T cells after culture and 80 % in the TNFα-positive T cells (supplemental Fig. C). However, when looking at specific samples that showed high IFNγ of TNFα positivity, the percentage of CD45RO was even higher (supplemental Fig. D). At the time of read-out on day 8, we also stained for other cell subsets in the culture. CD4+ T cells were the main population in culture, but also CD8+ T cells and NK cells could be identified (data not shown). Responses to the peptides in CD8+ T cells could not be detected, except for some (nonsignificant) CD69/IFNγ response to H290, lower than in CD4+ (supplemental Fig. E). CD3− CD56+ cells (NK cells) showed (nonsignificant) IFNγ response to peptide H161 (supplemental Fig. F).
Fig. 3.
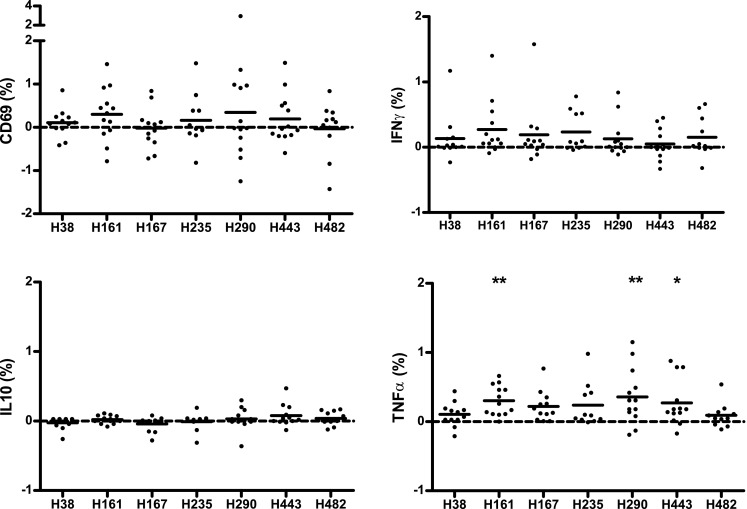
Expression of CD69 and production of IFNγ, IL-10, and TNFα by CD4+ T cells from short-term T cell line restimulated with HSP70 peptides. Data are presented as percentage of CD3+ CD4+ cells positive for the staining, with the background (unstimulated condition) subtracted. Each dot represents an individual donor; the mean is shown per column. TNFα production was significantly increased upon stimulation with H161 (p < 0.01), H290 (p < 0.01), and H443 (p < 0.05) using ANOVA (Friedman test) with post hoc analysis
Discussion
One of the hallmarks of autoimmune diseases is an altered response to self-antigens by T cells. For that reason, many studies have focused on finding the golden bullet: an antigen that triggers autoimmunity. The identification of such antigens, however, has proven to be very difficult in human autoimmune diseases. Possibly, not one antigen, but a set of antigens depending on the genetic background of the individual determines the induction of disease. More importantly, once the disease has progressed, the reactivity of the T cells spreads toward many antigens, a process called “epitope spreading” (Lehmann et al. 1992). It is relevant, therefore, to know which antigens play a role in this stage of perpetuation of the inflammation. In this context, many groups have focused their attention on HSPs for various reasons, among which is their regulatory role in different experimental autoimmune diseases (van Eden et al. 2007).
Of the HSP family, HSP70 is the most conserved protein. HSP70 exists in various highly homologous and conserved structures (Daugaard et al. 2007). It is constitutively expressed in most tissues and is involved in an intracellular process called “chaperone-mediated autophagy.”
While HSP70 autoantibodies have been shown in the human circulation (Abulafia-Lapid et al. 2003; Minota et al. 1988; Salvetti et al. 1996), little is known on potential HSP70 T cell recognition in humans. In this study, we aimed to identify epitopes of HSP70 that are recognized by T cells from healthy individuals.
We chose to focus on epitopes of HSP70 that are earlier found in HLA elution studies. Interestingly, among other proteins, HSP70 epitopes are often eluted from HLA molecules. We questioned whether T cells from healthy individuals recognize these naturally processed HSP70 epitopes. After review of the literature, we further narrowed the selection by running different theoretical binding studies to select epitopes able to bind to more than just one HLA molecule (pan-DR binding epitopes) (Table 3). Indeed, the HSP70 peptides selected were immunogenic, as we found that four peptides, H161, H167, H290, and H443, induced significant proliferation and/or TNFα production. The resulting immune response is not a classical regulator response. The peptide-specific T cells were low proliferative, but did not express the classical regulatory markers. Instead, a large subset of responding T cells was already a memory cell (CD45RO) in the circulation and produced TNFα (and IFNγ). These T cells may be triggered by previous infections or through encounters with self-antigens. Since another significant proportion of responder cells was CD45RO negative, apparently both naïve and “quiescent” memory T cells can respond to the peptide. The T cell responses to the peptides were mainly found in CD4+ T cells. However, H290 also induced some (nonsignificant) response in CD8+ T cells, albeit lower than in CD4+ cells of the same donors. A peptide (TKDNNLLGRFELSG), partly overlapping with H443, has been shown to stimulate human CD94+ NK cells (Multhoff et al. 2001; Gastpar et al. 2004). When we stained for NK cells, we detected IFNγ production toward H161. However, our study and experimental protocol were not designed for detecting NK cell responses, and therefore, it would require further study to test whether the here described peptides can induce NK cell responses.
We found that peptides derived from the most conserved region of HSP70 (both among species and among human HSP70 variants), peptide H161 and H167, showed marked recognition by PBMCs of healthy controls. Interestingly, van Herwijnen et al. recently identified a mycobacterial HSP70 peptide (B29) corresponding with the human H167 region, as one of the immunodominant HSP70 peptides in BALB/c mice (van Herwijnen et al. 2012). Adoptively transferred B29 induced Tregs suppressed ongoing experimentally induced arthritis in mice. In addition, heat shock induced upregulation of the endogenous inducible HSP70 in mouse APC resulting in increased activation of CD4+ hybridoma T cells specific for the identified peptide (Wieten et al. 2010). This study illustrates that stress-induced augmented expression of inducible HSP70 can lead to enhanced presentation of HSP70 peptides on MHC class II. This increased presentation of HSP70 (at the inflammatory site) potentially can be sensed by HSP70-specific T cells, which may subsequently dampen inflammation (van Eden et al. 2005).
The process of autophagy may explain why some peptides from intracellular proteins are found on HLA class II. Chaperone-mediated autophagy may regulate class II presentation of several cytoplasmic antigens. HSP70 as a chaperone molecule (transporting antigens within APCs) plays a central role in chaperone-mediated autophagy. Interestingly, in this process, HSP70 proteins are also being processed, and their peptides are presented themselves. HSP70 is not only functionally, but also genetically, closely linked to the MHC complex (Sargent et al. 1989). Some of the HSP70 epitopes we studied were found on HLA after induction of autophagy in human cells (H161, H167, H443; see Table 3) (Dengjel et al. 2005). It is important to note that these epitopes were also found on HLA in cells that were not starved. Autophagy is also constitutively active in HLA class II-expressing cells (Mizushima et al. 2008). It has been proposed that continuous presentation of self-antigens resulting from autophagy plays a role in maintaining peripheral T cell tolerance (Crotzer and Blum 2009; Levine and Kroemer 2008; Nedjic et al. 2008; Wu et al. 2013). The here presented data, recognition of HSP70 peptides by PBMC of the healthy immune system, may be a first step in unraveling this mechanism. The responder cells in our study did not have the classical phenotype of regulatory T cells, but the fact that the donors did not suffer from autoimmune disease suggests a modulating role in the immune system, rather than a destructive role.
Of interest would be to next look at the role of these HSP70 peptides in activated T cells, e.g., from the dysregulated immune system during active disease and chronic inflammation, for instance in rheumatoid arthritis. T cell reactivity toward HSP70 epitopes is not yet described in human autoimmune diseases. Nevertheless, T cell response toward other self-heat shock protein peptides (DiaPep277, pan-DR binding HSP60, DnaJp1) is present in autoimmune diseases such as in type I diabetes (Huurman et al. 2008; Raz et al. 2001), juvenile idiopathic arthritis, and rheumatoid arthritis (de Jong et al. 2009; Kamphuis et al. 2005; Koffeman et al. 2009; Prakken et al. 2004). Knowledge of T cell responses toward these self-epitopes in chronic inflammation could help find strategies to restore the immune balance in chronic inflammation.
It is conceivable that depending on the immunological context, both pro- and anti-inflammatory HSP-mediated effects can occur (Pockley et al. 2008). On the one hand, HSP may act as danger signals that amplify the inflammatory response (van Wijk and Prakken 2010), while on the other hand, HSP-peptide-specific T cells could assist to control the response (van Eden et al. 2007). Our study shows that healthy donors can show clear immune responses to strongly conserved epitopes of self-HSP70. These responses may be the result of mimicry with microbial antigens and play a role in the defense against pathogens. On the other hand, the fact that these individuals did not suffer from autoimmune diseases underscores that self-reactivity does not equal pathological autoimmunity. Cohen proposed: “We are healthy, not despite autoimmunity, but because of autoimmunity” (Cohen 2007). HSP70 could be an important antigen in balancing the healthy immune system.
Electronic supplementary material
Proliferation of donor PBMCs in response to the HSP70 peptides in relation to the HLA DR1 positivity and to the HLA DR4 positivity. Each dot represents an individual donor; means are shown per column. No differences could be found (Mann-Whitney test, all p values >0,05). (GIF 28 kb)
(EPS 153 kb)
Expression of the combination of CD25 and FOXP3 and expression of LAP (TGF marker) by CD4+ T cells from short term T cells line restimulated with HSP70 peptides. Data are presented as percentage of CD3+ CD4+ cells positive for the staining, with the background (unstimulated condition) subtracted. Each dot represents an individual donor, mean is shown per column. (GIF 18 kb)
(EPS 65 kb)
Percentage of CD45RO positive cells within the population of CD69 or cytokine positive CD4+ T cells. Each dot represents an individual donor, mean is shown per column. (GIF 31 kb)
(EPS 93 kb)
Dot plots of two samples from donors with clear cytokine response by T cells, showing % CD45RO positivity within the population of CD69 or cytokine positive CD4+ T cells. (JPEG 41 kb)
(TIFF 82 kb)
CD4 and CD8 staining of CD3+ cells from short term T cell lines restimulated with HSP70 peptides, showing the expression of CD69 and production of IFN and TNF in the two T cell subsets. Data are presented as percentage of CD3+ CD4+/CD8+ cells positive for the staining, with the background (unstimulated condition) subtracted. Each dot represents an individual donor, mean is shown per column. (GIF 31 kb)
(EPS 147 kb)
Production of IFN by CD3− CD56+ cells from the short term culture restimulated with HSP70 peptides. Data are presented as percentage of CD3− CD56+ cells positive for the staining, with the background (unstimulated condition) subtracted. Each dot represents an individual donor, mean is shown per column. (GIF 5 kb)
(EPS 58 kb)
Expression of CD69 and production of IFN, IL-10 and TNF by CD3+CD4+ T cells from short term T cells line restimulated with HSP70 peptides. Data are presented as Geo Mean of the positive cells. Each dot represents an individual donor, mean is shown per column. (GIF 13 kb)
(EPS 90 kb)
(PDF 353 kb)
Footnotes
Huib de Jong and Eva C. Koffeman contributed equally to this study.
References
- Abulafia-Lapid R, Gillis D, Yosef O, Atlan H, Cohen IR. T cells and autoantibodies to human HSP70 in type 1 diabetes in children. J Autoimmun. 2003;20:313–321. doi: 10.1016/S0896-8411(03)00038-6. [DOI] [PubMed] [Google Scholar]
- Alvarez I, Collado J, Daura X, Colome N, Rodriguez-Garcia M, Gallart T, Canals F, Jaraquemada D. The rheumatoid arthritis-associated allele HLA-DR10 (DRB1*1001) shares part of its repertoire with HLA-DR1 (DRB1*0101) and HLA-DR4 (DRB*0401) Arthritis Rheum. 2008;58:1630–1639. doi: 10.1002/art.23503. [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Bitmansour AD, Waldrop SL, Pitcher CJ, Khatamzas E, Kern F, Maino VC, Picker LJ. Clonotypic structure of the human CD4+ memory T cell response to cytomegalovirus. J Immunol. 2001;167:1151–1163. doi: 10.4049/jimmunol.167.3.1151. [DOI] [PubMed] [Google Scholar]
- Cheetham ME, Caplan AJ. Structure, function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress Chaperones. 1998;3:28–36. doi: 10.1379/1466-1268(1998)003<0028:SFAEOD>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IR. Biomarkers, self-antigens and the immunological homunculus. J Autoimmun. 2007;29:246–249. doi: 10.1016/j.jaut.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Crotzer VL, Blum JS. Autophagy and its role in MHC-mediated antigen presentation. J Immunol. 2009;182:3335–3341. doi: 10.4049/jimmunol.0803458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- de Jong H, Lafeber FF, de Jager W, Haverkamp MH, Kuis W, Bijlsma JW, Prakken BJ, Albani S. PAN-DR-Binding Hsp60 self epitopes induce an interleukin-10-mediated immune response in rheumatoid arthritis. Arthritis Rheum. 2009;60:1966–1976. doi: 10.1002/art.24656. [DOI] [PubMed] [Google Scholar]
- Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Muller M, Kreymborg K, Altenberend F, Brandenburg J, Kalbacher H, Brock R, Driessen C, Rammensee HG, Stevanovic S. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci U S A. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede T, Gnau V, Jung G, Keilholz W, Stevanovic S, Rammensee HG. Natural ligand motifs of closely related HLA-DR4 molecules predict features of rheumatoid arthritis associated peptides. Biochim Biophys Acta. 1996;1316:85–101. doi: 10.1016/0925-4439(96)00010-5. [DOI] [PubMed] [Google Scholar]
- Gastpar R, Gross C, Rossbacher L, Ellwart J, Riegger J, Multhoff G. The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK cells. J Immunol. 2004;172:972–980. doi: 10.4049/jimmunol.172.2.972. [DOI] [PubMed] [Google Scholar]
- Halder T, Pawelec G, Kirkin AF, Zeuthen J, Meyer HE, Kun L, Kalbacher H. Isolation of novel HLA-DR restricted potential tumor-associated antigens from the melanoma cell line FM3. Cancer Res. 1997;57:3238–3244. [PubMed] [Google Scholar]
- Hasler P. Biological therapies directed against cells in autoimmune disease. Springer Semin Immunopathol. 2006;27:443–456. doi: 10.1007/s00281-006-0013-8. [DOI] [PubMed] [Google Scholar]
- Haug M, Dannecker L, Schepp CP, Kwok WW, Wernet D, Buckner JH, Kalbacher H, Dannecker GE, Holzer U. The heat shock protein Hsp70 enhances antigen-specific proliferation of human CD4+ memory T cells. Eur J Immunol. 2005;35:3163–3172. doi: 10.1002/eji.200535050. [DOI] [PubMed] [Google Scholar]
- Huurman VA, van der Meide PE, Duinkerken G, Willemen S, Cohen IR, Elias D, Roep BO. Immunological efficacy of heat shock protein 60 peptide DiaPep277 therapy in clinical type I diabetes. Clin Exp Immunol. 2008;152:488–497. doi: 10.1111/j.1365-2249.2008.03656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis S, Kuis W, De Jager W, Teklenburg G, Massa M, Gordon G, Boerhof M, Rijkers GT, Uiterwaal CS, Otten HG, Sette A, Albani S, Prakken BJ. Tolerogenic immune responses to novel T-cell epitopes from heat-shock protein 60 in juvenile idiopathic arthritis. Lancet. 2005;366:50–56. doi: 10.1016/S0140-6736(05)66827-4. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffeman EC, Genovese M, Amox D, Keogh E, Santana E, Matteson EL, Kavanaugh A, Molitor JA, Schiff MH, Posever JO, Bathon JM, Kivitz AJ, Samodal R, Belardi F, Dennehey C, van den Broek T, van Wijk F, Zhang X, Zieseniss P, Le T, Prakken BA, Cutter GC, Albani S. Epitope-specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis Rheum. 2009;60:3207–3216. doi: 10.1002/art.24916. [DOI] [PubMed] [Google Scholar]
- Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippolis JD, White FM, Marto JA, Luckey CJ, Bullock TN, Shabanowitz J, Hunt DF, Engelhard VH. Analysis of MHC class II antigen processing by quantitation of peptides that constitute nested sets. J Immunol. 2002;169:5089–5097. doi: 10.4049/jimmunol.169.9.5089. [DOI] [PubMed] [Google Scholar]
- Minota S, Cameron B, Welch WJ, Winfield JB. Autoantibodies to the constitutive 73-kD member of the hsp70 family of heat shock proteins in systemic lupus erythematosus. J Exp Med. 1988;168:1475–1480. doi: 10.1084/jem.168.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G, Pfister K, Gehrmann M, Hantschel M, Gross C, Hafner M, Hiddemann W. A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones. 2001;6:337–344. doi: 10.1379/1466-1268(2001)006<0337:AMHPSN>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntasell A, Carrascal M, Alvarez I, Serradell L, van Veelen P, Verreck FA, Koning F, Abian J, Jaraquemada D. Dissection of the HLA-DR4 peptide repertoire in endocrine epithelial cells: strong influence of invariant chain and HLA-DM expression on the nature of ligands. J Immunol. 2004;173:1085–1093. doi: 10.4049/jimmunol.173.2.1085. [DOI] [PubMed] [Google Scholar]
- Mycko MP, Cwiklinska H, Szymanski J, Szymanska B, Kudla G, Kilianek L, Odyniec A, Brosnan CF, Selmaj KW. Inducible heat shock protein 70 promotes myelin autoantigen presentation by the HLA class II. J Immunol. 2004;172:202–213. doi: 10.4049/jimmunol.172.1.202. [DOI] [PubMed] [Google Scholar]
- Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- Newcomb JR, Cresswell P. Characterization of endogenous peptides bound to purified HLA-DR molecules and their absence from invariant chain-associated alpha beta dimers. J Immunol. 1993;150:499–507. [PubMed] [Google Scholar]
- Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Munz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Prakken BJ, Samodal R, Le TD, Giannoni F, Yung GP, Scavulli J, Amox D, Roord S, de Kleer I, Bonnin D, Lanza P, Berry C, Massa M, Billetta R, Albani S. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2004;101:4228–4233. doi: 10.1073/pnas.0400061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- Salvetti M, Ristori G, Buttinelli C, Fiori P, Falcone M, Britton W, Adams E, Paone G, Grasso MG, Pozzilli C. The immune response to mycobacterial 70-kDa heat shock proteins frequently involves autoreactive T cells and is quantitatively disregulated in multiple sclerosis. J Neuroimmunol. 1996;65:143–153. doi: 10.1016/0165-5728(96)00013-6. [DOI] [PubMed] [Google Scholar]
- Sanjeevi CB, Lybrand TP, Stevanovic S, Rammensee HG. Molecular modeling of eluted peptides from DQ6 molecules (DQB1*0602 and DQB1*0604) negatively and positively associated with type 1 diabetes. Ann N Y Acad Sci. 2002;958:317–320. doi: 10.1111/j.1749-6632.2002.tb02995.x. [DOI] [PubMed] [Google Scholar]
- Sargent CA, Dunham I, Trowsdale J, Campbell RD. Human major histocompatibility complex contains genes for the major heat shock protein HSP70. Proc Natl Acad Sci U S A. 1989;86:1968–1972. doi: 10.1073/pnas.86.6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Jasuja H, Toes RE, Spee P, Munz C, Hilf N, Schoenberger SP, Ricciardi-Castagnoli P, Neefjes J, Rammensee HG, Arnold-Schild D, Schild H. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000;191:1965–1974. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, Kubo RT, Chesnut RW, Grey HM, Sette A. Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol. 1998;160:3363–3373. [PubMed] [Google Scholar]
- Stepniak D, Wiesner M, de Ru AH, Moustakas AK, Drijfhout JW, Papadopoulos GK, van Veelen PA, Koning F. Large-scale characterization of natural ligands explains the unique gluten-binding properties of HLA-DQ2. J Immunol. 2008;180:3268–3278. doi: 10.4049/jimmunol.180.5.3268. [DOI] [PubMed] [Google Scholar]
- Suri A, Walters JJ, Gross ML, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity. J Clin Invest. 2005;115:2268–2276. doi: 10.1172/JCI25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Kimura Y, Mitani A, Yamamoto G, Nishimura H, Spallek R, Singh M, Noguchi T, Yoshikai Y. Activation of T cells recognizing an epitope of heat-shock protein 70 can protect against rat adjuvant arthritis. J Immunol. 1999;163:5560–5565. [PubMed] [Google Scholar]
- Tobian AA, Canaday DH, Harding CV. Bacterial heat shock proteins enhance class II MHC antigen processing and presentation of chaperoned peptides to CD4+ T cells. J Immunol. 2004;173:5130–5137. doi: 10.4049/jimmunol.173.8.5130. [DOI] [PubMed] [Google Scholar]
- Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Da CC, Rammensee HG, Wagner H, Schild H. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- van Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann N Y Acad Sci. 2007;1113:217–237. doi: 10.1196/annals.1391.020. [DOI] [PubMed] [Google Scholar]
- van Herwijnen MJ, Wieten L, van der ZR, van Kooten PJ, Wagenaar-Hilbers JP, Hoek A, den Braber I, Anderton SM, Singh M, Meiring HD, van Els CA, Van EW, Broere F (2012) Regulatory T cells that recognize a ubiquitous stress-inducible self-antigen are long-lived suppressors of autoimmune arthritis. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed]
- van Wijk F, Prakken B. Heat shock proteins: Darwinistic immune modulation on dangerous grounds. J Leukoc Biol. 2010;88:431–434. doi: 10.1189/jlb.0410236. [DOI] [PubMed] [Google Scholar]
- Verreck FA, van de Poel A, Drijfhout JW, Amons R, Coligan JE, Konig F. Natural peptides isolated from Gly86/Val86-containing variants of HLA-DR1, -DR11, -DR13, and -DR52. Immunogenetics. 1996;43:392–397. doi: 10.1007/BF02199809. [DOI] [PubMed] [Google Scholar]
- Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–2717. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- Wieten L, Berlo SE, Ten Brink CB, van Kooten PJ, Singh M, van der Zee R, Glant TT, Broere F, van Eden W. IL-10 is critically involved in mycobacterial HSP70 induced suppression of proteoglycan-induced arthritis. PLoS One. 2009;4:e4186. doi: 10.1371/journal.pone.0004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieten L, van der Zee R, Spiering R, Wagenaar-Hilbers J, van Kooten P, Broere F, van Eden W. A novel heat-shock protein coinducer boosts stress protein Hsp70 to activate T cell regulation of inflammation in autoimmune arthritis. Arthritis Rheum. 2010;62:1026–1035. doi: 10.1002/art.27344. [DOI] [PubMed] [Google Scholar]
- Wu C, Aichinger M, Nedjic J, Klein L. Thymic epithelial cells use macroautophagy to turn their inside out for CD4 T cell tolerance. Autophagy. 2013;9:931–932. doi: 10.4161/auto.24374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee van der R, Anderton SM, Buskens C, Alonso de Valasco E, Eden van W (1995) Heat shock protein T cell epitopes as immunogenic carriers in subunit vaccines. H L S Maya (Ed ), Peptides 1994 Proceedings of the Twenty-Third European Peptide Symposium, ESCOM Leiden:841–842.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proliferation of donor PBMCs in response to the HSP70 peptides in relation to the HLA DR1 positivity and to the HLA DR4 positivity. Each dot represents an individual donor; means are shown per column. No differences could be found (Mann-Whitney test, all p values >0,05). (GIF 28 kb)
(EPS 153 kb)
Expression of the combination of CD25 and FOXP3 and expression of LAP (TGF marker) by CD4+ T cells from short term T cells line restimulated with HSP70 peptides. Data are presented as percentage of CD3+ CD4+ cells positive for the staining, with the background (unstimulated condition) subtracted. Each dot represents an individual donor, mean is shown per column. (GIF 18 kb)
(EPS 65 kb)
Percentage of CD45RO positive cells within the population of CD69 or cytokine positive CD4+ T cells. Each dot represents an individual donor, mean is shown per column. (GIF 31 kb)
(EPS 93 kb)
Dot plots of two samples from donors with clear cytokine response by T cells, showing % CD45RO positivity within the population of CD69 or cytokine positive CD4+ T cells. (JPEG 41 kb)
(TIFF 82 kb)
CD4 and CD8 staining of CD3+ cells from short term T cell lines restimulated with HSP70 peptides, showing the expression of CD69 and production of IFN and TNF in the two T cell subsets. Data are presented as percentage of CD3+ CD4+/CD8+ cells positive for the staining, with the background (unstimulated condition) subtracted. Each dot represents an individual donor, mean is shown per column. (GIF 31 kb)
(EPS 147 kb)
Production of IFN by CD3− CD56+ cells from the short term culture restimulated with HSP70 peptides. Data are presented as percentage of CD3− CD56+ cells positive for the staining, with the background (unstimulated condition) subtracted. Each dot represents an individual donor, mean is shown per column. (GIF 5 kb)
(EPS 58 kb)
Expression of CD69 and production of IFN, IL-10 and TNF by CD3+CD4+ T cells from short term T cells line restimulated with HSP70 peptides. Data are presented as Geo Mean of the positive cells. Each dot represents an individual donor, mean is shown per column. (GIF 13 kb)
(EPS 90 kb)
(PDF 353 kb)