Abstract
Heat-shock protein 70 (HSP70) is one of the most important heat-shock proteins that helps organisms to modulate stress response via over-expression. The HSP70 gene from Pomacea canaliculata was cloned using the RACE approach; the gene is 2,767 bp in length and contains an open reading frame of 1,932 bp, which is encoded by a polypeptide of 643 amino acids. BLAST analysis showed that the predicted amino acid sequence of the P. canaliculata HSP70 gene shared a relatively high similarity with that of other known eukaryotic species that display conserved HSP characteristics. The phylogeny demonstrated a separate clustering of the apple snail HSP70 with other constitutive members from other mollusk species. Quantitative real-time RT-PCR was used to detect the differential expression of HSP70 in both sexes of P. canaliculata at different temperature conditions. These results showed that HSP70 transcript levels decreased slightly under cold shock and increased significantly under heat-shock conditions in both sexes compared to normal temperatures (26 °C). Under cold-shock treatment, the sex effect was not significant. With heat treatment, HSP70 expression could be induced at 36 °C in both females and males, and it peaked at 42 and 39 °C in females and males, respectively. In addition, a clear time-dependent HSP70 expression pattern of the apple snail exposed to the same high temperature (36 °C) was observed at different time points. The maximal induction of HSP70 expression appeared at 12 and 48 h in males and females after heat shock, respectively. The maximal induction in females was significantly higher compared to males under heat stimulus. Taken together, these results strongly suggested that males were more susceptible to heat than females and provided useful molecular information for the ecological adaptability of P. canaliculata against extreme environmental stress.
Keywords: Pomacea canaliculata, HSP70, Gene expression, Temperature stress
Introduction
Golden apple snails (Pomacea canaliculata) are native to the freshwater wetlands in South America and belong to the Ampullariidae family. In the late 1970s, they were introduced from Argentina to Taiwan as human food or aquarium pets (Hayes et al. 2008; Mochida 1991) and were then subsequently introduced to numerous countries throughout southern and eastern Asia. However, as apple snails gradually lost their commercial value, they were discarded or escaped. Because of their omnivorous behavior, strong environmental adaptability, high reproductive capacity, and lack of natural enemies, Pomacea canaliculata rapidly spread through natural waterways and irrigation canals and unexpectedly developed into a vicious pest that has posed massive agricultural economic losses and serious ecological threats for more than 30 years in Asia (Joshi 2007; Cowrie 2002). Pomacea canaliculata is widely distributed in southern China, including more than 11 provinces, and may have expanded northward due to environmental adaptation and climate change, which caused serious damage for humans and the environment (Li et al. 2009; Song et al. 2010). In addition, Pomacea canaliculata have been identified as a major intermediate host of Angiostrongylus cantonensis, which infects humans in China (Lv et al. 2011). Many studies investigating the effect of temperature on Pomacea canaliculata have been performed (Ito 2002; Matsukura et al. 2009; Syobu et al. 2001; Teo 2004; Wada and Matsukura 2007; Yoshida et al. 2009). The activity of the apple snail increased with rising water temperature, and their crawling velocity positively correlated with temperature. Higher temperatures shortened the developmental time and improved the growth rate in apple snails. Water temperature also exhibits an important effect on other aspects of the biology of snails, including aerial respiration and survival, among other factors. Thus, temperature plays a cardinal role on the geographic distribution and spread of these snails. In addition, Pomacea canaliculata can resist pH 4.5–9.4 acid-based erosion and various types of chemical, bacterial, and parasitic threats. The ongoing expansion of its potential habitat may also increase the effect of Pomacea canaliculata (Lv et al. 2006), thus making it crucial to increase our understanding of the molecular mechanisms behind their invasion phenomenon.
Heat-shock proteins (HSPs) are a family of proteins expressed in response to a wide range of biotic and abiotic stressors and is thus also referred to as stress proteins (Srivastava 2002) that can help organisms to modulate the stress response and protect organisms from environmentally induced cellular damage. HSPs have been classified into several families according to their molecular mass, including HSP90 (85–90 kDa), HSP70 (68–73 kDa), HSP60, HSP47, and low molecular mass HSPs (16–24 kDa). HSP70 is one of the most conserved HSPs and is regulated at the tissue-, time- and dose-dependent expression level via various chemical and biological stressors in all organisms ranging from bacteria to plants to mammals (Ahrens and Issels 2002; Beere and Green 2001; Sørensen et al. 2003; Srivastava 2002). In the gastropod mollusk, the HSP70 gene has been cloned from several species, such as Haliotis tuberculata (Farcy et al. 2007), Haliotis discus hannai (Cheng et al. 2007), Biomphalaria glabrata (Laursen et al. 1997), and Cellana toreuma (Han et al. 2013), which have all demonstrated the synthesis and amplified expression of HSP70 under environmental stress responses or temperature shock. Understanding the response of Pomacea canaliculata to environmental challenges can provide us with further information of the anti-stress mechanism used by Pomacea canaliculata and can help to develop strategies and approaches to control the expansion of their invasion.
Pomacea canaliculata occurs naturally in Argentina in regions of temperate climate and extends into the tropical and subtropical eastern Asia and the Pacific, which possess unique adaptations to maximize fitness. The successful invasion of Pomacea canaliculata may be due to the thermal resistance ability that is one of the potential mechanisms underlying the expansion of apple snails (Pan et al. 2008). In a survey of rice field invaded by Pomacea canaliculata in Malaysia, Teo (2004) found the male to female ratio was 1:5 (Teo 2004). The females had a higher survival rate than males at high temperatures and different populations of apple snails have different resistances in response to heat (Banpavichit et al. 1994; Dong et al. 2010). The differences between males and females are not restricted to the morphology of sexual structures involved in mating or fertilization. The female Pomacea canaliculata with high fecundity actively select habitats that offer optimal conditions for growth and production of offspring and need to avoid all kinds of different environmental risks. Unlike females, males do not need to attain a large size or gather as much energy for reproduction, and encounter less environmental stress than females. These indicated that there were likely to be sex-related differences in the thermal resistance in Pomacea canaliculata and in the molecular response of HSPs to changes in temperature experienced by this species. To our knowledge, there is little information on the thermal resistance of different sexes in apple snail. In this study, the full sequence of HSP70 complementary deoxyribonucleic acid (cDNA) was cloned from P. canaliculata, and the differential expression of the HSP70 gene of two sex groups under thermo and cold challenges was analyzed using quantitative real-time polymerase chain reaction (RT-PCR). The results of this study indicate that the different thermal resistance of the two sexes in apple snail result in different patterns of HSP70 production, leading us to hypothesize that the enhanced ability of the female to adapt to high-temperature conditions may play an important role in its distribution and expansion.
Materials and methods
Animal and temperature stimulus
Golden apple snails (body weight = 25–30 g) were collected from the wetlands in the Aquatic Invasive Risk Assessment Center of Pearl River Fisheries Research Institute in Guangzhou City. Prior to the experiments, all apple snails were placed in 45 × 27 × 30-cm tanks containing 12 L of freshwater in a chamber and were allowed to acclimatize for at least 3 days prior to any experiments. For the temperature gradient treatment, 10 snails (female/male ratio of 1:1) were randomly selected and exposed to each temperature (4, 7, 10, 16, 26, 33, 36, 39, 42, and 45 °C) for 1 h. Next, 8 individuals (female/male ratio of 1:1) were sampled and decapitated in each group. Seventy snails were used for the temporal expression experiment, four male and four female snails were sampled and decapitated at 2, 6, 12, 24, 48, 72, and 96 h, after the same temperature challenge (36 °C). The foot muscle from each dissected individual was sampled for RNA extraction.
RNA extraction and cDNA synthesis
Total RNA was immediately extracted from 30-mg foot muscle of each sample using TRIzol reagent (Takara, Dalian, China) according to the manufacturer’s instructions. The integrity of the RNA was verified by electrophoresis on 1.2 % agarose gels. The quantity and purity of total RNA were measured by BioPhotometer (Eppendorf, Hamburg, Germany). Each RNA sample was treated with DNase I (Promega, Madison, WI) to remove any genomic DNA contamination prior to reverse transcription. Single-stranded cDNA was synthesized from 1 μg of total RNA of each sample using the PrimeScript® 1st Strand cDNA Synthesis Kit (Takara, Japan) according to the manufacturer’s instructions. The reaction system contained 2-μL MgCl2, 1-μL 10× RT buffer, 1-μL dNTP, 0.5-μL Oligo dT-Adaptor primer, 0.25-μL RNase Inhibitor, 0.5-μL AMV reverse transcriptase, and 1-μg total RNA in a final volume of 10 μL with supplement of H2O. The reaction conditions were 42 °C for 30 min, 99 °C for 5 min, and 4 °C for 5 min.
Full-length HSP70 cDNA cloning
To clone the core region of HSP70, one primer pair of HSP70-c-sf and HSP70-c-sr (Table 1) was designed based on the conserved nucleotide sequence of the known HSP70s of the other mollusk species. Reactions were performed in a 50-μL volume containing 5 μL of cDNA template from one sample, 5-μl 10× buffer (with Mg2+), 2 μM each primer, 0.25 mM each dNTP, and 0.5 U of Taq DNA polymerase (Takara, Dalian, China). PCR amplification was performed with an initial denaturation at 95 °C for 4 min, followed by 30 cycles of denaturation for 30 s at 95 °C, annealing for 30 s at 55 °C, extension for 1 min at 72 °C, and a final extension for 7 min at 72 °C.
Table 1.
Name, sequence, and technique for polymerase chain reaction (PCR) primers used in the cloning and expression of the HSP70 gene from Pomacea canaliculata
| Primer | Sequence (5′–3′) | Technique |
|---|---|---|
| HSP70-3-1 | TGCCGACCTGTTCCGTAAGACCCTG | HSP70 3′-RACE |
| HSP70-3-2 | ACGACAGATCCACGAAGTGGTGCTG | HSP70 3′-RACE |
| M13 | GTTTTCCCAGTCACGAC | HSP70 3′-RACE |
| HSP70-5-1 | CGTCTTGGTCTCGCCCTTGTATTGC | HSP70 5′-RACE |
| HSP70-5-2 | TCGTCAAAACGCCTGCCGATCAGTC | HSP70 5′-RACE |
| HSP70-c-sf1 | GTSGAAATCATCGCSAACGACCARGG | Amplification of HSP70 core region |
| HSP70-c-sf1 | GCDGCACCATARGCWACAGCYTC | Amplification of HSP70 core region |
| Rt-hsp70-sf | GTGTGCCGACCTGTTCCGTAA | Real-time PCR |
| Rt-hsp70-sr | TGGATTTGTTCAGCTCTTTGC | Real-time PCR |
| 18S-sf | CCGTCCCTTTTGGTGACTCTG | Real-time PCR |
| 18S-sr | GGATGTGGTAGCCGTTTCTC | Real-time PCR |
To obtain 3′-RACE, two forward primers, HSP70-3-1 and HSP70-3-2, were designed according to the known partial cDNA sequence of HSP70 (Table 1). The 3′ region of HSP70 was amplified with the HSP70-3-1 and M13 reverse primer provided in the RNA PCR Kit (AMV) Ver.3.0 (Takara, Dalian, China). The reaction conditions were performed according to the manufacturer’s instructions. Amplification was performed with an initial denaturation at 94 °C for 3 min, followed by 32 cycles of denaturation for 30 s at 94 °C, annealing for 30 s at 55 °C, extension for 30 s at 72 °C and a final extension for 5 min at 72 °C. Nested PCR were performed the HSP70-3-2 and M13 reverse primers.
For 5′-RACE, two reverse primers, HSP70-5-1 and HSP70-5-2 (Table 1), were designed according to the known partial cDNA sequence of HSP70. The 5′-RACE was performed using the SmartTM RACE cDNA Synthesis Kit (BD, Biosciences Clontech). Briefly, the first-strand cDNA was synthesized from foot muscle total RNA and used as a template according to the manufacturer’s recommendations. Two rounds of PCR amplifications were performed with the specific primers and two adapter primers provided in the kit. All the PCR products were separated using electrophoresis, and the desired DNA band was excised and purified using a gel extraction kit (Omega, Norcross, GA). Then, the DNA fragment was subcloned into the pMD18-T vector (Takara, Dalian, China). Following transfection into Escherichia coli DH5a-competent cells, recombinants were identified by blue and white spot selection. The positive clones were sequenced by Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, China).
Sequence comparison and phylogenetic analysis
Searches for nucleotide and amino acid sequence similarities were performed using BLAST programs on the NCBI website (http://www.ncbi.nlm.gov/BLAST/). The open reading frame (ORF) was identified using the ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf html), and the motif sequences were searched using the InterPro software (Hunter et al. 2009). The molecular weight was calculated using the Swiss-Prot (ExPASy server) program (Kiefer et al. 2009). The amino acid sequences used in the phylogenetic study included HSP70 sequences of Biomphalaria glabrata (AAB99911.1), Crassostrea virginica (AAB34577.1), Cristaria plicata (ADM64336.1), Crassostrea hongkongensis (ACH95805.1), Crassostrea virginica (CAB89802.1), Haliotis diversicolor (ACH95805.1), Cristaria plicata (ADM64336.1), Brissopsis lyrifera (CBJ55211.1), Daphnia magna (ACB11340.1), Harpegnathos saltator (EFN75098.1), Macrocentrus cingulum (ACD84945.1), Paralvinella grasslei (ABU63808.1), Aedes aegypti (ACJ64194.1), Anatolica polita borealis (ABQ39970.1), Bemisia tabaci (ACH85197.1), Plutella xylostella (BAF95560.1), Danaus plexippus (EHJ73891.1), Bombyx mori (NP_001037396.1), and Spodoptera litura (ADV03160.1). The phylogenetic tree was constructed using the neighbor-joining method with software MEGA 5.0 (Tamura et al. 2011).
Quantitative real-time PCR analysis of HSP70 mRNA expression
Expression of the HSP70 transcript in foot muscle of each sampled individual was measured using fluorescent quantitative RT-PCR. A pair of HSP70 gene-specific primers (rt-hsp70-sf and rt-hsp70-sr; Table 1) was designed to amplify a product of 173 bp. The apple snail 18S RNA gene was used as a reference to normalize the HSP70 gene, and the primer sequences (18 s-sf and 18 s-sr; Table 1) were designed according the reported sequence (EU520448.1). The PCR products were excised, purified, and subcloned into the pMD19-T vector for sequencing to confirm whether it was target gene. The purified plasmids were quantified using a Biophotometer, and standard samples were constructed with a 10-fold (10−1 pmol/L–10−8 pmol/L) serial dilution. All standard curves exhibited a correlation coefficient higher than 0.99. Real-time PCR was performed on an ABI PRISM 7300 instrument (Applied Biosystems, Foster City, CA) using the Power SYBR Green Master Mix (Applied Biosystems). Reactions were performed in a 20-μL volume containing 1-μL cDNA template, 10-μL Power SYBR Green Master Mix, 0.5-μL forward and reverse primers (10 pmol/L), and 8-μL ddH2O. The PCR was performed as follows: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. A denaturing step of 15 s at 95 °C was added after the amplification, and melting curve analysis was performed over a range of 60–95 °C to confirm that a single PCR product was generated at the end of the assay. Each cDNA sample was performed in triplicate biological replicates. The concentration of the target gene was determined based on the threshold cycle number (CT). The cDNA concentration in each sample was determined according to the gene-specific standard curve. The relative gene expression of HSP70 was calculated using the following formula: F = a/b (where a represents the concentration of the target gene and b represents the concentration of the reference gene). The data were expressed as the mean ± SD (n = 4). All statistical analyses were performed using SPSS package (version16). Significant difference between the two sexes at the same temperature or at the same time point was identified by an independent-sample t test. The statistical variation at the different temperatures or at the different time points for the same sex were determined using one-way ANOVA followed by the LSD multiple range test. P < 0.05 (*) was considered significant and P < 0.01 (**) was considered highly significant.
Results
Molecular characteristics of the HSP70 gene
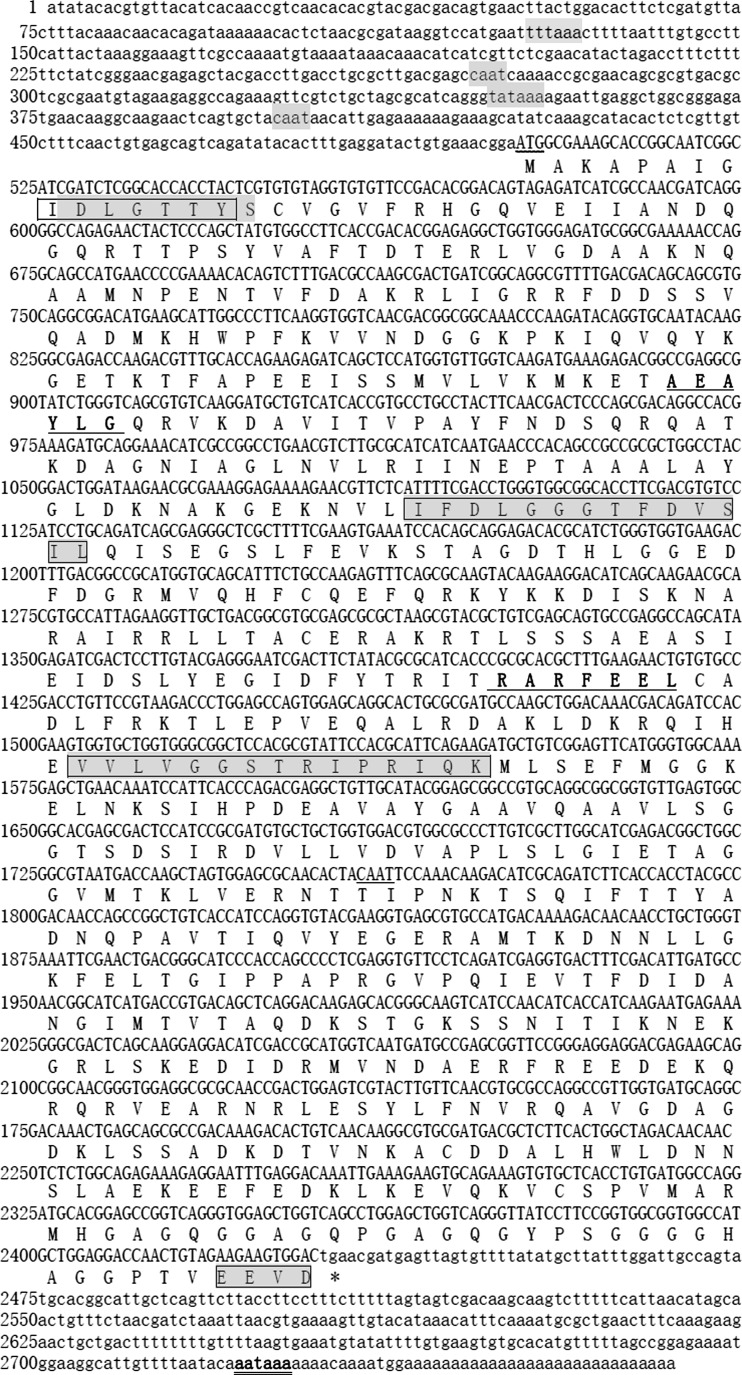
The cDNA sequence of the apple snail HSP70 gene was deposited in GenBank under the accession number KF356182. The full-length HSP70 gene was 2,767 bp in length, including a 5′ terminal untranslated region (UTR) of 524 bp with a putative TATA box (TATTAAA) and CAAT box, a 3′ terminal UTR of 338 bp with an atypical polyadenylation signal sequence AATAAA and poly (A), and an ORF of 1,932 bp, encoding a polypeptide of 643 amino acids with a predicted molecular mass of 70.23 kDa and theoretical isoelectric point of 5.65.
Using PROSITE of InterPro searches, three signature motifs were identified in the HSP70 amino acid sequences (aa 9-16 IDLGTTYS, aa 197-210IFDLGGGTFDVSIL, and aa 335-349 VVLVGGSTRIPRIQK) (Fig. 1), which were defined as highly conserved regions present in all HSP70 family members. The cytoplasmic Hsp70 C-terminal region of EEVD was found at position 640-643 (Fig. 1). Two additional specific motifs, the putative adenosine triphosphate (ATP)-guanosine triphosphate (GTP) binding domain (AEAYLG) and the potential non-organelle eukaryotic consensus motif (RARFEEL) were found.
Fig. 1.
Nucleotide and deduced amino acid sequences of HSP70 in Pomacea canaliculata. Lowercase represents 5′ and 3′ untranslation region. Uppercase represents translation region, above: nucleotide sequence, below: amino acid sequence. The start codon is shown with the curved underlining, and the stop codon is indicated with an asterisk. The characteristic HSP70 family signatures and the cytoplasmic C-terminal region (EEVD) are shown in gray boxes. The putative ATP-GTP binding domain (AEAYLG) and non-organelle eukaryotic consensus motif (RARFEEL) are underlined. The TATA boxes (TTTAAA and TATAAA elements) and two CAAT box are shown in gray. The consensus polyA signal AATAAA is double underlined
Sequence comparison and phylogenetic analysis
The deduced amino acid sequence of the apple snail HSP70 shared a close homology to a relative high similarity with other organisms, including the HSP70s of Biomphalaria glabrata (80 %), Crassostrea virginica (79 %), Bombyx mori (79 %), Plutella xylostella (78 %), Spodoptera litura (77 %), Bemisia tabaci (77 %), Anatolica polita borealis (77 %), Danaus plexippus (76 %), Aedes aegypti (76 %), Crassostrea hongkongensis (76 %), Canis lupus familiaris (74 %), Paralvinella grasslei (73 %), Mytilus galloprovincialis (73 %), Gallus gallus (73 %), Capra hircus (73 %), and Dicentrarchus labrax (72 %), among others (Table 2). The multiple sequence alignment revealed that they were highly conserved, particularly in the regions of the HSP70 family signatures and ATP-GTP binding domain.
Table 2.
Putative HSP70 amino acid homologies between Pomacea canaliculata and other eukaryotic species determined using Clustal W
| Species | Family | Phylum | GenBank no. | Identity (%) |
|---|---|---|---|---|
| Bombyx mori | Bombycidae | Arthropoda | NP_001037396.1 | 79 |
| Spodoptera litura | Noctuidae | Arthropoda | ADV03160.1 | 77 |
| Danaus plexippus | Nymphalidae | Arthropoda | EHJ73891.1 | 76 |
| Plutella xylostella | Plutellidae | Arthropoda | BAF95560.1 | 78 |
| Bemisia tabaci | Aleyrodidae | Arthropoda | ACH85197.1 | 77 |
| Anatolica polita borealis | Tenebrionidae | Arthropoda | ABQ39970.1 | 77 |
| Aedes aegypti | Culicidae | Arthropoda | ACJ64194.1 | 76 |
| Paralvinella grasslei | Scolecida | Annelida | ABU63809.1 | 73 |
| Biomphalaria glabrata | Planorbidae | Mollusca | AAB99911.1 | 80 |
| Mytilus galloprovincialis | Mytilidae | Mollusca | AAW52766.1 | 73 |
| Crassostrea hongkongensis | Ostreidae | Mollusca | ACH95805.1 | 76 |
| Crassostrea virginica | Ostreidae | Mollusca | AAB34577.1 | 79 |
| Gallus gallus | Phasianidae | Vertebrata | AAN18282.1 | 73 |
| Dicentrarchus labrax | Moronidae | Vertebrata | AAR01102.2 | 72 |
| Capra hircus | Bovidae | Vertebrata | AEX55800.1 | 73 |
| Canislupus familiaris | Canidae | Vertebrata | BAB78505.1 | 74 |
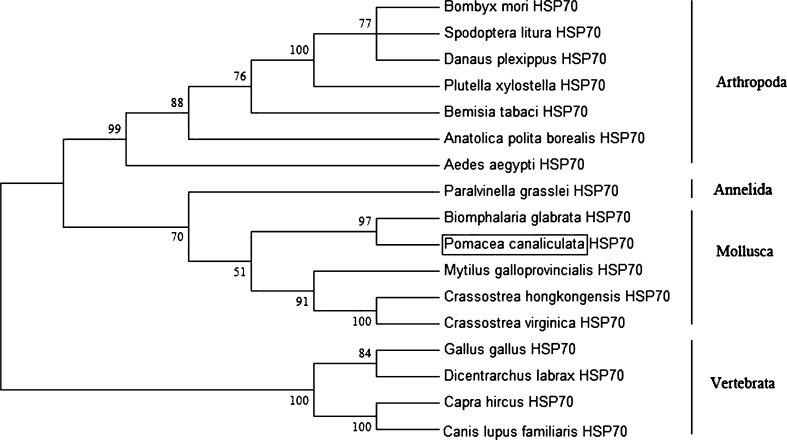
Based on the amino acid sequences of HSP70, a phylogenetic tree was constructed using MEGA 5.0 with the neighbor-joining method (Fig. 2). Sixteen HSP70 family members were selected for the analysis. Mollusca, including Pomacea canaliculata, Biomphalaria glabrata, Mytilus galloprovincialis, and two oysters were clustered together and formed a sister group with annelids, and then clustered to arthropods, and finally formed a group independent from vertebrates. Vertebrate and invertebrate HSP70s were separated and formed two distinct branches in the tree. The phylogenetic relationships displayed in the phylogenic tree were consistent with the traditional classification.
Fig. 2.
Phylogenetic relationships between the amino acid sequences of HSP70 from Pomacea canaliculata and those from 16 other species. The tree was constructed using MEGA 5.1 with the neighbor-joining method, and bootstrap confidence values (percentage of 500 replicates) are shown at each branch point. The P. canaliculata HSP70 sequence identified in this study is labeled with a box
Expression of HSP70 mRNA in response to temperature gradient-shocked stress
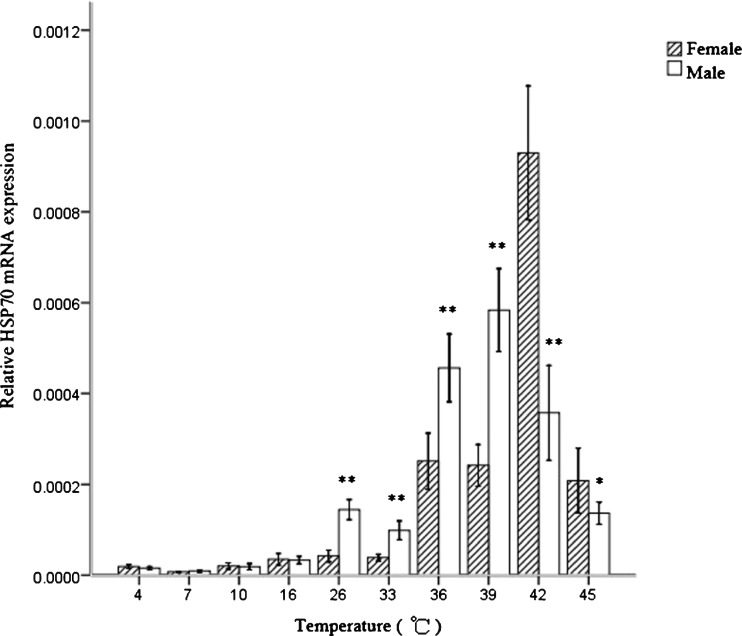
Quantitative RT-PCR was used to examine the expression pattern of HSP70 in the foot muscle of Pomacea canaliculata under normal temperature (26 °C), high temperature (30, 33, 36, 39, 42, and 45 °C) and low temperature (4, 7, 10, and 16 °C) treatment (Fig. 3). Under cold stress conditions, the HSP70 relative expression level was slightly decreased in the foot muscle compared to normal temperature, and the sex effect was not significant. Under heat stress conditions, The HSP70 mRNA level could not be induced by 2-h shock at 33 °C compared to the normal temperature in the two sexes, but it was significantly up-regulated when temperature was at 36 °C to 45 °C. The increase in HSP70 expression with temperature for males was less steep compared to females. The highest expression level that occurred at 39 °C was 4-fold of that at 26 °C in males, and at 42 °C, it was 22-fold of that at 26 °C in females. The maximal induction in females was significantly higher compared to males. After the maximal induction, the expression level was observed to decrease significantly in both sexes. The HSP70 expression was higher in males compared to females at 26, 33, 36, and 39 °C (P < 0.01); however, it was lower in males compared to females at 42 °C (P < 0.01) and 45 °C (P < 0.05).
Fig. 3.
The mRNA expression analysis of HSP70 in Pomacea canaliculata males and females under the different temperature stimuli by quantitative real-time PCR with SYBR Green. The values are expressed as the means ± SD (n = 4). The asterisks above the bars within each temperature treatment indicate significant differences (*P < 0.05; **P < 0.01) between males and females in an independent-sample t test
Temporal expression profile of apple snail HSP70 transcripts after a thermal challenge
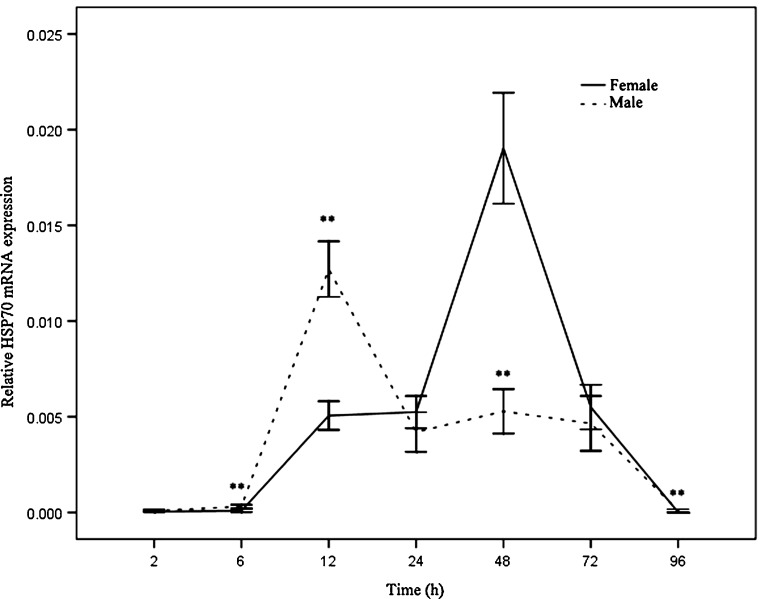
RT-PCR was used to examine the time-dependent expression pattern of the HSP70 gene in the foot muscle of Pomacea canaliculata at 2, 6, 12, 24, 48, 72, and 96 h after thermal stress treatment (36 °C). A clear time-dependent expression pattern of HSP70 was observed in both male and female Pomacea canaliculata (Fig. 4). After heat shock, the expression of HSP70 gene was up-regulated and reached the highest level at 48 h in females. However, after 72 h, it decreased gradually and returned to its baseline value after 96 h. However, the expression of HSP70 began to be induced after 6 h in males and peaked at 12 h. It decreased significantly after 24 h and returned to its baseline level after 96 h. The highest expression level was markedly higher in females compared to males (Fig. 4). The HSP70 expression levels at 6, 12, 48, and 96 h were significantly different between the sexes.
Fig. 4.
The mRNA expression analysis of HSP70 in Pomacea canaliculata males and females at different time points after thermal challenge (36 °C). Transcript levels for all samples were assessed by Quantitative real-time PCR with SYBR Green. The values are expressed as the means ± SD (n = 4). The asterisks above the lines indicate significant differences (*P < 0.05; **P < 0.01) between males and females at the same time point in an independent-sample t test
Discussion
The gold apple snail Pomacea canaliculata is one of the most important agricultural pests in Asia and has been ranked in the top 100 of the worst invasive species in the world (Lowe et al. 2000). In the invasion process, enormous populations have developed an ecologically adaptive mechanism in response to all types of stresses, and this species’ temperature adaptability is thought to be one of the most important attributes (Yusa et al. 2006). HSP70 is one of the most conserved HSP family members and is found in all organisms from bacteria to plants to mammals. Increasing evidence has demonstrated the importance of HSPs as molecular chaperones involved in the resistance to heat, cold, and a range of other biotic and abiotic stressors. HSP70’s conservation and universality demonstrate its evolutionary importance and protective effect on cells (Hunt and Morimoto 1985; Mayer and Bukau 2005). Currently, only several HSP70 genes of the Gastropoda species have been reported, including Haliotis tuberculata (Farcy et al. 2007), Haliotis discus hannai (Cheng et al. 2007), Haliotis diversicolor (GenBank: FJ812177.1), Biomphalaria glabrata (Laursen et al. 1997) and Cellana toreuma (Han et al. 2013).
In this study, we determined the full-length cDNA sequence of the HSP70 gene from the gold apple snail Pomacea canaliculata. The sequence alignment and structural features suggested that the gene sequence obtained encoded HSP70. The identification of a notable structural characteristic of non-organellar consensus motif (RARFEEL), as well as the extreme C-terminal domain EEVD, which enables HSP70 to bind other co-chaperones, strongly indicated that HSP70 was a cytosolic 70-kDa HSP. The GGMP repeats were considered to function in forming a structural entity together with the helical sub-domain and the EEVD motif to mediate chaperone cofactor binding (Demand et al. 1998). Some of these repetitive peptides have been shown to be antigenic (Engman et al. 1990). In the present study, the tetrapeptide motif GGMP was absent in the apple snail HSP70. Similar results for the lack of the GGMP motif were obtained in the HSP70 gene from Littorina keenae, Mytilus galloprovincialis, and Pinctada fucata (Kourtidis et al. 2006; Kourtidis and Scouras 2005; Lee and Boulding 2010; Wang et al. 2009). In the Pacific oyster Crassostrea gigas and European flat oyster Ostrea edulis, both HSP70 and HSC70 contained two repeats of the GGMP tetrapeptide (Boutet et al. 2003; Boutet et al. 2003). However, the tetrapeptide was present in heat-shock cognate 70 (HSC70) but not HSP70 in Pomacea canaliculata (Zheng et al. 2012). In addition, changes in the GGMP motif between inducible HSP70 and the constitutive HSC70 sequence may be partially responsible for the functional differences in response to stress as hypothesized in vertebrates (Fuertes et al. 2004), and thus, further studies are needed to further confirm whether such structural variations affect the expression patterns of HSP70 and HSC70.
The expression of HSP70 mRNA decreased slightly under cold conditions but increased significantly under heat-shock conditions in the apple snail. This finding suggested that a specific amount of HSP70 mRNA was present in foot muscle tissues both under stressed and non-stressed conditions, which might play an important role in physiological processes and the prevention of cellular damage under temperature stressors. However, the expression level of the HSP70 gene under cold stress was not changed significantly compared to normal temperatures. The same expression pattern has been demonstrated in Drosophila melanogaster (Sejerkilde et al. 2003), Danio rerio (Airaksinen et al. 2003), and Oncorhynchus mykiss (Currie et al. 2000). It seems to that HSP70 play less of a role in cold resistance and knockdown heat resistance (Hoffmann et al. 2003). However, the chronic acclimation of sea bream to cold temperature resulted in an up-regulation of HSP70 in comparison to levels in sea bream kept at a warmer temperature (Deane and Woo 2005). The up-regulation under long-term cold stress might be triggered by the accumulation of chilling injuries to some extent (Fujikake et al. 2005). Treatment at temperatures of 26 to 33 °C could not induce HSP70 in either females or males. Moreover, heat treatments performed at 36 °C induced a three- to five-fold increase in the expression levels of HSP70 in both sexes, indicating that 36 °C was closer to the threshold temperature for the induction of HSP70. This up-regulated expression pattern of HSP70 genes was found in some mollusk species, such as Haliotis tuberculata (Farcy et al. 2007), Mytilus galloprovincialis (Cellura et al. 2006), Ostrea edulis (Piano et al. 2002), and Haliotis discus hannai (Cheng et al. 2007) after high-temperature stress. The expression of HSP70 in Crassostrea gigas, Pomacea canaliculata and Ostrea edulis was induced at 38, 36, and 32 °C after heat shock, respectively (Clegg et al. 1998; Piano et al. 2002). The lethal temperature of Crassostrea gigas, Pomacea canaliculata and Ostrea edulis was 44, 40, and <38 °C, respectively (Piano et al. 2002; Zhou et al. 2003). It was speculated that the higher lethal temperature might be in agreement with the higher induction temperature of HSP70 expression in some mollusk species. After exposure to a 30 °C heat shock, the induced thermotolerance of Pomacea canaliculata remained for 4 days. However, the high level of HSP70 in Argopecten irradians, Crassostrea gigas, Haliotis discus hannai, and Apostichopus japonicas after heat shock could maintain for 28, 14, 4, and approximately 3 days, with or without recovery at normal temperature, respectively (Cheng et al. 2007; Clegg et al. 1998; Dong and Dong 2008; Song et al. 2006). The difference in the temporal patterns of HSP70 in different species might be attributed to the duration and severity of thermal stress and to the stability of HSP70 mRNA, which may vary as a function of temperature (Helmuth and Hofmann 2001). Based on the function of the HSP70 gene, this transcriptional up-regulation related to stresses strongly suggested that HSP70 was an important factor in cell protection and survival in Pomacea canaliculata.
The temperatures for the onset or maximal induction of HSP70 expression were used to interpret the heat tolerance (Huang and Kang 2007; Piano et al. 2002; Yu et al. 2012). Using heat-shock treatments, HSP70 could not be induced either in females or males at 26–33 °C. At higher temperatures, the expression level of HSP70 gene transcripts increased significantly in both sexes and peaked at 42 °C in females and 39 °C in males, thus suggesting that males were more susceptible to heat than females. Previous studies have also revealed that there was a positive relationship between HSP expression levels and heat tolerance in organisms (Cui et al. 2008; Downs et al. 1998; Fangue et al. 2006; Sørensen et al. 2001). High expression levels of the HSP70 gene enabled organisms to survive during thermal stress by repairing the denatured proteins, and organisms could adapt to a wide range of temperature changes and could resist thermal stress. The maximal induction of HSP70 expression was 1.5 and 1.6 times higher in females compared to males under heat shock of different temperature gradients and long-term heat shock, respectively. This finding indicated that the female might be more resistant to heat than the male, which was consistent with the observations that females had a higher survival rate than males at high temperatures (Banpavichit et al. 1994). Furthermore, heat tolerance is a crucial factor in the adaptation and expansion of the apple snail in the process of invasion in Asia (Zheng et al. 2012). The enhanced ability of the female to adapt to high-temperature conditions may play an important role in its distribution and expansion (Lü and Wan 2008). Apple snail populations may have suffered short-term extreme high temperatures in the summer, which resulted in a higher number of females surviving compared to males. The increased number of offspring produced by females also increased the population size after high-temperature periods (Banpavichit et al. 1994). Thus, it was assumed that apple snail females with higher heat resistance are partially able to adjust to the dynamics of the sex ratio to increase the population. Our identification of the HSP70 gene under heat-shock conditions in both sexes might be used to better understand the invasion mechanism.
The HSP70 gene in the apple snail exhibited a clear time-dependent expression pattern under thermal stress. This result was consistent with the expression pattern of HSP70 in the Pacific abalone, where the HSP70 mRNA levels were up-regulated under heat stimulus. However, we found that the pattern of HSP70 expression was not identical for the sexes. The maximal induction of HSP70 expression was 48 h in females and 12 h in males after heat shock (Fig. 4). In addition it was 1.6 times higher in females compared to males. This finding also indicated that the males were more susceptible to heat than females. After long-term heat treatment, the induced expression of HSP70 is reduced. This reduction or down-regulation of the induced expression level might enable the organism to avoid the harmful effects that have been found to be associated with HSP70 expression, e.g., reduced cell growth rates (Feder et al. 1992) reduction in productivity (Krebs and Loeschcke 1994). Previous studies have shown that different sexes and populations of apple snails have different resistances in response to heat (Banpavichit et al. 1994; Dong et al. 2010)Thus, heat stress resistance is a complex trait. It is assumed that many differentially expressed genes might be involved in the difference in heat resistance in apple snail females and males. Thus, it was necessary to identify the differentially expressed genes under heat-shock conditions to further understand the molecular mechanism underlying the difference in heat resistance in Pomacea canaliculata of the two sexes.
Taken together, the present study provides additional insights for future molecular studies on the resistance mechanism and ecological adaptability of invasive Pomacea canaliculata. The differential expression patterns and potential roles in sustaining the heat-resistance response of the HSP70 gene detected in male and female snails needs to be further confirmed using additional techniques, such as RNA interference, combined with further studies investigating the sex ratio.
Acknowledgments
This work was supported by funds obtained from the Natural Science Foundation of China (31300468), Agricultural Biological Resources Protection and Utilization Project (2130108), and the Natural Science Foundation of China-Guangdong Joint Funds (u1131006).
References
- Ahrens B, Issels RD. Heat shock protein 70: role in antigen presentation and immune stimulation. Int J Hyperther. 2002;18:563–575. doi: 10.1080/02656730210166140. [DOI] [PubMed] [Google Scholar]
- Airaksinen S, Jokilehto T, Råbergh CM, Nikinmaa M. Heat-and cold-inducible regulation of HSP70 expression in zebrafish ZF4 cells. Comp Biochem Phys B. 2003;136:275–282. doi: 10.1016/S1096-4959(03)00205-7. [DOI] [PubMed] [Google Scholar]
- Banpavichit S, Keawjam RS, Upatham ES. Sex ratio and susceptibility of the golden apple snail, Pomacea canaliculata. Southeast Asian J Trop Med Public Health. 1994;25:387–391. [PubMed] [Google Scholar]
- Beere HM, Green DR. Stress management-heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001;11:6–10. doi: 10.1016/S0962-8924(00)01874-2. [DOI] [PubMed] [Google Scholar]
- Boutet I, Tanguy A, Moraga D. Organization and nucleotide sequence of the European flat oyster Ostrea edulis heat shock cognate 70 (hsc70) and heat shock protein 70 (hsp70) genes. Aquat Toxicol. 2003;65:221–225. doi: 10.1016/S0166-445X(03)00137-1. [DOI] [PubMed] [Google Scholar]
- Boutet I, Tanguy A, Rousseau S, Auffret M, Moraga D. Molecular identification and expression of heat shock cognate 70 (hsc70) and heat shock protein 70 (hsp70) genes in the Pacific oyster Crassostrea gigas. Cell Stress Chaperon. 2003;8:76. doi: 10.1379/1466-1268(2003)8<76:MIAEOH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellura C, Toubiana M, Parrinello N, Roch P. HSP70 gene expression in Mytilus galloprovincialis hemocytes is triggered by moderate heat shock and Vibrio anguillarum, but not by V. splendidus or Micrococcus lysodeikticus. Dev Comp Immunol. 2006;30:984–997. doi: 10.1016/j.dci.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Cheng P, Liu X, Zhang G, He J. Cloning and expression analysis of a HSP70 gene from Pacific abalone (Haliotis discus hannai) Fish Shellfish Immun. 2007;22:77–87. doi: 10.1016/j.fsi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Clegg JS, Uhlinger KR, Jackson SA, Cherr GN, Rifkin E, Friedman CS. Induced thermotolerance and the heat shock protein-70 family in the Pacific oyster Crassostrea gigas. Mol Mar Biol Biotechnol. 1998;7:21–30. [Google Scholar]
- Cowrie RH. Apple snails (Ampullariidae) as agricultural pests: their biology, impacts and management. In: Cowrie RH, editor. Molluscs as crop pests. Wallingford: CABI; 2002. [Google Scholar]
- Cui X, Wan F, Xie M, Liu T. Effects of heat shock on survival and reproduction of two whitefly species, Trialeurodes vaporariorum and Bemisia tabaci biotype B. J Insect Sci. 2008;8:24. doi: 10.1673/031.008.2401. [DOI] [Google Scholar]
- Currie S, Moyes CD, Tufts BL. The effects of heat shock and acclimation temperature on hsp70 and hsp30 mRNA expression in rainbow trout: in vivo and in vitro comparisons. J Fish Biol. 2000;56:398–408. doi: 10.1111/j.1095-8649.2000.tb02114.x. [DOI] [Google Scholar]
- Deane EE, Woo N. Cloning and characterization of the hsp70 multigene family from silver sea bream: modulated gene expression between warm and cold temperature acclimation. Biochem Bioph Res Co. 2005;330:776–783. doi: 10.1016/j.bbrc.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Demand J, Lüders J, Höhfeld J. The carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of chaperone cofactors. Mol Cell Biol. 1998;18:2023–2028. doi: 10.1128/mcb.18.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Dong S. Induced thermotolerance and expression of heat shock protein 70 in sea cucumber Apostichopus japonicus. Fisheries Sci. 2008;74:573–578. doi: 10.1111/j.1444-2906.2008.01560.x. [DOI] [Google Scholar]
- Dong S, Bai X, Pan Y, Yu X. Effects of temperature stress on development and survival rate of the apple snail, Pomacea canaliculata (Lamarck) from different geographical populations in China. Hubei Agric Sci. 2010;11:93. [Google Scholar]
- Downs C, Heckathorn S, Bryan J, Coleman J. The methionine-rich low-molecular-weight chloroplast heat-shock protein: evolutionary conservation and accumulation in relation to thermotolerance. Am J Bot. 1998;85:175–183. doi: 10.2307/2446306. [DOI] [PubMed] [Google Scholar]
- Engman DM, Dragon EA, Donelson JE. Human humoral immunity to hsp70 during Trypanosoma cruzi infection. J Immunol. 1990;144:3987–3991. [PubMed] [Google Scholar]
- Fangue NA, Hofmeister M, Schulte PM. Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J Exp Biol. 2006;209:2859–2872. doi: 10.1242/jeb.02260. [DOI] [PubMed] [Google Scholar]
- Farcy E, Serpentini A, Fiévet B, Lebel J. Identification of cDNAs encoding HSP70 and HSP90 in the abalone Haliotis tuberculata: transcriptional induction in response to thermal stress in hemocyte primary culture. Comp Biochem Phys B. 2007;146:540–550. doi: 10.1016/j.cbpb.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Feder JH, Rossi JM, Solomon J, Solomon N, Lindquist S. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Gene Dev. 1992;6:1402–1413. doi: 10.1101/gad.6.8.1402. [DOI] [PubMed] [Google Scholar]
- Fuertes MA, Pérez JM, Soto M, Menéndez M, Alonso C. Thermodynamic stability of the C-terminal domain of the human inducible heat shock protein 70. BBA-Proteins Proteom. 2004;1699:45–56. doi: 10.1016/j.bbapap.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Fujikake N, Nagai Y, Popiel HA, Kano H, Yamaguchi M, Toda T. Alternative splicing regulates the transcriptional activity of Drosophila heat shock transcription factor in response to heat/cold stress. Febs Lett. 2005;579:3842–3848. doi: 10.1016/j.febslet.2005.05.074. [DOI] [PubMed] [Google Scholar]
- Han G, Zhang S, Marshall DJ, Ke C, Dong Y. Metabolic energy sensors (AMPK and SIRT1), protein carbonylation, and cardiac failure as biomarkers of thermal stress in an intertidal limpet: linking energetic allocation with environmental temperature during aerial emersion. J Exp Biol. 2013 doi: 10.1242/jeb.084269. [DOI] [PubMed] [Google Scholar]
- Hayes KA, Joshi RC, Thiengo SC, Cowie RH. Out of South America: multiple origins of non-native apple snails in Asia. Divers Distrib. 2008;14:701–712. doi: 10.1111/j.1472-4642.2008.00483.x. [DOI] [Google Scholar]
- Helmuth BS, Hofmann GE. Microhabitats, thermal heterogeneity, and patterns of physiological stress in the rocky intertidal zone. Biol Bull. 2001;201:374–384. doi: 10.2307/1543615. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Sørensen JG, Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Therm Biol. 2003;28:175–216. doi: 10.1016/S0306-4565(02)00057-8. [DOI] [Google Scholar]
- Huang LH, Kang L. Cloning and interspecific altered expression of heat shock protein genes in two leafminer species in response to thermal stress. Insect Mol Biol. 2007;16:491–500. doi: 10.1111/j.1365-2583.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- Hunt C, Morimoto RI. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985;82:6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S, Apweiler R, Attwood TK, Bairoch A, Bateman A, Binns D, Bork P, Das U, Daugherty L, Duquenne L. InterPro: the integrative protein signature database. Nucleic Acids Res. 2009;37:D211–D215. doi: 10.1093/nar/gkn785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K. Environmental factors influencing overwintering success of the golden apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae), in the northernmost population of Japan. Appl Entomol Zool. 2002;37:655–661. doi: 10.1303/aez.2002.655. [DOI] [Google Scholar]
- Joshi RC (2007) Problems with the management of the golden apple snail Pomacea canaliculata: an important exotic pest of rice in Asia., Area-Wide Control of Insect Pests. Springer, pp. 257-264.
- Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtidis A, Scouras ZG. Analysis and characterization of the transcriptional unit of a new Mytilus galloprovincialis (Mollusca: Bivalvia) hsp70 gene. Mitochondrial DNA. 2005;16:36–43. doi: 10.1080/10425170400024391. [DOI] [PubMed] [Google Scholar]
- Kourtidis A, Drosopoulou E, Nikolaidis N, Hatzi VI, Chintiroglou CC, Scouras ZG. Identification of several cytoplasmic Hsp70 genes from the Mediterranean mussel (Mytilus galloprovincialis) and their long-term evolution in Mollusca and Metazoa. J Mol Evol. 2006;62:446–459. doi: 10.1007/s00239-005-0121-4. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Loeschcke V. Effects of exposure to short-term heat stress on fitness components in Drosophila melanogaster. J Evolution Biol. 1994;7:39–49. doi: 10.1046/j.1420-9101.1994.7010039.x. [DOI] [Google Scholar]
- Laursen JR, di Liu H, Wu X, Yoshino TP. Heat-shock response in a molluscan cell line: characterization of the response and cloning of an inducible HSP70 cDNA. J Invertebr Pathol. 1997;70:226–233. doi: 10.1006/jipa.1997.4686. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Boulding EG. Latitudinal clines in body size, but not in thermal tolerance or heat-shock cognate 70 (HSC70), in the highly-dispersing intertidal gastropod Littorina keenae (Gastropoda: Littorinidae) Biol J Linn Soc. 2010;100:494–505. doi: 10.1111/j.1095-8312.2010.01450.x. [DOI] [Google Scholar]
- Li X, Hu Y, Song H, Wang P, Wang X, Mu X, Liu C, Luo J. Invasion and monitoring methods of Pomacea canaliculata in China. Chin Agric Sci Bull. 2009;25:229–232. [Google Scholar]
- Lowe S, Browne M, Boudjelas S, De Poorter M. 100 of the world’s worst invasive alien species: a selection from the global invasive species database. New Zealand: Invasive Species Specialist Group Auckland; 2000. [Google Scholar]
- Lü Z, Wan F. Differential gene expression in whitefly (Bemisia tabaci) B-biotype females and males under heat-shock condition. Comp Biochem Phys D. 2008;3:257–262. doi: 10.1016/j.cbd.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Lv S, Zhou X, Zhang Y, Liu H, Zhu D, Yin W, Steinmann P, Wang X, Jia T. The effect of temperature on the development of Angiostrongylus cantonensis (Chen 1935) in Pomacea canaliculata (Lamarck 1822) Parasitol Res. 2006;99:583–587. doi: 10.1007/s00436-006-0198-8. [DOI] [PubMed] [Google Scholar]
- Lv S, Zhang Y, Steinmann P, Yang GJ, Yang K, Zhou XN, Utzinger J. The emergence of angiostrongyliasis in the People’s Republic of China: the interplay between invasive snails, climate change and transmission dynamics. Freshwater Biol. 2011;56:717–734. doi: 10.1111/j.1365-2427.2011.02579.x. [DOI] [Google Scholar]
- Matsukura K, Tsumuki H, Izumi Y, Wada T. Physiological response to low temperature in the freshwater apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae) J Exp Biol. 2009;212:2558–2563. doi: 10.1242/jeb.031500. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida O. Spread of freshwater Pomacea snails (Pilidae, Mollusca) from Argentina to Asia. Micronesica. 1991;3:51–62. [Google Scholar]
- Pan Y, Dong S, Yu X. Effects of temperature stress on development, feeding and survival of the apple snail, Pomacea canaliculata (Lamarck) Acta Phytophylacica Sin. 2008;35:224–239. [Google Scholar]
- Piano A, Asirelli C, Caselli F, Fabbri E. Hsp70 expression in thermally stressed Ostrea edulis, a commercially important oyster in Europe. Cell Stress Chaperon. 2002;7:250. doi: 10.1379/1466-1268(2002)007<0250:HEITSO>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejerkilde M, Sørensen JG, Loeschcke V. Effects of cold-and heat hardening on thermal resistance in Drosophila melanogaster. J Insect Physiol. 2003;49:719–726. doi: 10.1016/S0022-1910(03)00095-7. [DOI] [PubMed] [Google Scholar]
- Song L, Wu L, Ni D, Chang Y, Xu W, Xing K. The cDNA cloning and mRNA expression of heat shock protein 70 gene in the haemocytes of bay scallop (Argopecten irradians, Lamarck 1819) responding to bacteria challenge and naphthalin stress. Fish Shellfish Immun. 2006;21:335–345. doi: 10.1016/j.fsi.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Song H, Hu Y, Wang P, Mou X, Li X, Wang X, Luo J. Sequencing cytochrome oxidase subunit I of mitochondrial DNA and the taxonomic status of apple snails. Chin J Zool. 2010;45:1–7. [Google Scholar]
- Sørensen JG, Dahlgaard J, Loeschcke V. Genetic variation in thermal tolerance among natural populations of Drosophila buzzatii: down regulation of Hsp70 expression and variation in heat stress resistance traits. Funct Ecol. 2001;15:289–296. doi: 10.1046/j.1365-2435.2001.00525.x. [DOI] [Google Scholar]
- Sørensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol Lett. 2003;6:1025–1037. doi: 10.1046/j.1461-0248.2003.00528.x. [DOI] [Google Scholar]
- Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- Syobu S, Mikuriya H, Yamaguchi J, Matsuzaki M, Zen S, Wada T. Estimating the overwintering mortality of the apple snail, Pomacea canaliculata (Lamarck) (Gastropoda: Ampullariidae) in a paddy field of Southern Japan using temperature data. Jpn J Appl Entomol Z. 2001;45:203–207. doi: 10.1303/jjaez.2001.203. [DOI] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo SS. Biology of the golden apple snail, Pomacea canaliculata (Lamarck, 1822), with emphasis on responses to certain environmental conditions in Sabah, Malaysia. Molluscan Research. 2004;24:139–148. doi: 10.1071/MR04009. [DOI] [Google Scholar]
- Wada T, Matsukura K. Seasonal changes in cold hardiness of the invasive freshwater apple snail, Pomacea canaliculata (Lamarck)(Gastropoda: Ampullariidae) Malacologia. 2007;49:383–392. doi: 10.4002/0076-2997-49.2.383. [DOI] [Google Scholar]
- Wang Z, Wu Z, Jian J, Lu Y. Cloning and expression of heat shock protein 70 gene in the haemocytes of pearl oyster (Pinctada fucata, Gould 1850) responding to bacterial challenge. Fish Shellfish Immun. 2009;26:639. doi: 10.1016/j.fsi.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Hoshikawa K, Wada T, Yusa Y. Life cycle of the apple snail Pomacea canaliculata (Caenogastropoda: Ampullariidae) inhabiting Japanese paddy fields. Appl Entomol Zool. 2009;44:465–474. doi: 10.1303/aez.2009.465. [DOI] [Google Scholar]
- Yu H, Wan F, Guo J. cDNA cloning of heat shock protein genes and their expression in an indigenous cryptic species of the whitefly Bemisia tabaci complex from China. J Integr Agric. 2012;11:293–302. doi: 10.1016/S2095-3119(12)60013-6. [DOI] [Google Scholar]
- Yusa Y, Sugiura N, Wada T. Predatory potential of freshwater animals on an invasive agricultural pest, the apple snail Pomacea canaliculata (Gastropoda: Ampullariidae), in southern Japan. Biol Invasions. 2006;8:137–147. doi: 10.1007/s10530-004-1790-4. [DOI] [Google Scholar]
- Zheng G, Dong S, Hou Y, Yang K, Yu X. Molecular characteristics of HSC70 gene and its expression in the golden apple snails, Pomacea canaliculata (Mollusca: Gastropoda) Aquaculture. 2012;15:41–49. doi: 10.1016/j.aquaculture.2012.06.002. [DOI] [Google Scholar]
- Zhou W, Wu Y, Yang J. Viability of the ampullaria snail in China. Fujian J Agric Sci. 2003;18:25–28. [Google Scholar]