Abstract
Organisms have evolved to survive rigorous environments and are not prepared to thrive in a world of caloric excess and sedentary behavior. A realization that physical exercise (or lack of it) plays a pivotal role in both the pathogenesis and therapy of type 2 diabetes mellitus (t2DM) has led to the provocative concept of therapeutic exercise mimetics. A decade ago, we attempted to simulate the beneficial effects of exercise by treating t2DM patients with 3 weeks of daily hyperthermia, induced by hot tub immersion. The short-term intervention had remarkable success, with a 1 % drop in HbA1, a trend toward weight loss, and improvement in diabetic neuropathic symptoms. An explanation for the beneficial effects of exercise and hyperthermia centers upon their ability to induce the cellular stress response (the heat shock response) and restore cellular homeostasis. Impaired stress response precedes major metabolic defects associated with t2DM and may be a near seminal event in the pathogenesis of the disease, tipping the balance from health into disease. Heat shock protein inducers share metabolic pathways associated with exercise with activation of AMPK, PGC1-a, and sirtuins. Diabetic therapies that induce the stress response, whether via heat, bioactive compounds, or genetic manipulation, improve or prevent all of the morbidities and comorbidities associated with the disease. The agents reduce insulin resistance, inflammatory cytokines, visceral adiposity, and body weight while increasing mitochondrial activity, normalizing membrane structure and lipid composition, and preserving organ function. Therapies restoring the stress response can re-tip the balance from disease into health and address the multifaceted defects associated with the disease.
What if we had a new paradigm to explain the metabolic syndrome and type 2 diabetes? What if our focus on the importance of glucotoxicity, lipotoxicity, and inflammation could be addressed in a new perspective of a disease that so threatens global health? We propose that loss of cellular stress response in insulin responsive tissues is the near seminal event that disrupts metabolic homeostasis, leading to a cascade of pathological outcomes. As a valid paradigm, it necessarily would occur very early in the disease process and be a fundamental factor in the pathological features of the disease—namely, obesity, inflammation, beta-cell malfunction, insulin resistance, dyslipidemia, mitochondrial dysfunction, and organ vulnerability. Importantly, correcting the defect through a variety of means would restore metabolic homeo-dynamics and improve functioning of diverse organ systems adversely affected by type 2 diabetes mellitus (liver, muscle, kidney, heart, brain, and beta-cell). Conversely, inducing the defect would induce the disease. Indeed, defects in the stress response occur prior to the development of glucose intolerance, and restoration of the stress response aids in the resolution of all of the abnormalities associated with the metabolic syndrome and t2DM—yielding more robust organelles, organs, and, ultimately, organisms. Herein, we propose that impaired Hsp activity is a near seminal event in the pathogenesis of t2DM—tipping the balance from health into disease.
Background: exercise, hyperthermia, and diabetes
Lifestyle modification is a primary intervention improving all of the major features of the disease: glycemia, dyslipidemia, obesity, and hypertension. Indeed, the Diabetes Prevention Program found that lifestyle modification to be more effective than drug therapy (Diabetes Prevention Program Research Group et al. 2009). We hypothesized that attempting to mimic the physiological effects of exercise by warming the body might duplicate the beneficial effects of exercise on glycemic control in t2DM. We reasoned that skeletal muscle is the major organ that consumes glucose in response to insulin and predicted that simply warming muscle would improve glucose indices. Indeed, when we treated traditionally managed t2DM with partial submersion in a hot tub for 30 min, 6 out of 7 days/week for 3 weeks. The results exceeded our expectations with improvements in fasting glucose, a 1 % drop in HbA1, a trend toward weight loss, and relief of neuropathic symptoms (Hooper 1999). The neuropathic improvement suggested that heat did more than just improve blood flow to muscles; therefore, we explored cellular mechanisms that surround the heat response and found a vast literature outside of clinical medicine concerning heat shock proteins (HSPs). A Hungarian colleague, Kurucz, reflected on the hot tub study and then examined messenger RNA (mRNA) of heat shock protein70 (Hsp 70) in skeletal muscle of patients with t2DM, subjects with glucose intolerance, and euglycemic identical twins of subjects with glucose intolerance. All subjects, including the euglycemic twins, had lower muscle mHsp70 than control subjects and the levels positively correlated with insulin sensitivity (Kurucz et al. 2002). Subsequently, as we will discuss below, nearly all of the pathological features of t2DM and its complications can be treated by induction of HSPs—ranging from whole body hyperthermia, to genetic modification, to pharmacological agents.
What are Hsps?
Hsps are almost as old as life itself, 2.6 billion years old, and remarkably evolutionarily conserved. Even plant Hsp70 remains 70 % homologous with human Hsp70(Hooper et al. 2010). The heat shock protein molecular chaperones protect cells and their organelles from succumbing to stressful insults, whether from heat, cold, oxidation, free radicals, toxins, or hypoxia. They guard protein integrity by aiding in protein folding, preventing aggregation, or degrading nonfunctional proteins from the cytoplasm. They are often anti-inflammatory inside the cell but can be pro-inflammatory outside the cell, acting as a danger signal that alerts the body to foreign threats. They preserve surface membranes, mitochondria, endoplasmic reticulum, and nuclear fidelity, and participate in intracellular transport. Hsps aid in wound healing, ischemia–reperfusion injury, and sepsis survival (Chen et al. 2007).
Hsp levels in diabetes
In both types of diabetes, iHSP levels [(iHsp70 (iHsp72, HSPA1A), iHsp27, and hemeoxygenase (iHsp 32)] and their response to stress are low in tissues that are insulin sensitive, particularly skeletal muscle, the heart, liver, and monocytes (Kurucz et al. 2002; Atalay et al. 2004a; Bruce et al. 2003; Nakhjavani et al. 2012; Rodrigues-Krause et al. 2012; McClung et al. 2008; Kavanagh et al. 2009; Figueredo et al. 1996). The fall in iHSPs is likely a consequence of the deactivation of the major regulator of iHSPs, HSF1 (Kavanagh et al. 2009, 2011; Atalay et al. 2004b). Within skeletal muscle types, HSP expression is associated with oxidative capacity. Slow twitch fibers (highly oxidative) have higher HSP expression levels, lower activation of inflammatory cytokines, and better insulin signaling compared to fast twitch fibers (Gupte et al. 2008). Moreover, diabetics have a decreased amount of slow twitch fibers and correlated to the severity of insulin resistance (Stuart et al. 2013). Further debilitating the function of Hsp70 is its glycation, which blocks its protein refolding ability (Bathaie et al. 2010). Loss of insulin signaling itself promotes deactivation of HSF1 via an inhibitory phosphorylation of HSF1 by glycogen synthase kinase 3-β (GSK-3β). A low Hsp state then promotes increased activation of inflammatory cytokines, c-Jun N-terminal kinase (JNK) and IkappaB kinase (pIKK-β), which phosphorylate serine 307 of IRS1 and further interfere with insulin signaling. Thus, a vicious cycle is created in which inflammation-induced insulin resistance leads to lower Hsps and further inflammation (Hooper and Hooper 2009). iHSP levels are inversely correlated with glucose disposal rate, insulin resistance, inflammatory cytokines, GLUT4 levels, and mitochondrial function (Bruce et al. 2003). The HSP response is delayed and diminished in diabetic wound healing (McMurtry et al. 1999). Finally, not all studies have found low iHsps in diabetes (Ugurlucan et al. 2010). Not infrequently, these studies were in drug-induced, streptozotocin, diabetes, and the acute stress of the sudden diabetic state could raise the levels initially and with time fall to lower levels (Bathaie et al. 2010).
Low levels of iHsps contribute to an impaired stress response in a disease disrupted by protein glycation and oxidation, free radical formation, protein aggregation, and inflammation, and may be a clue to the etiology of the disease itself for it sets up for disruption of homeostasis and induction of pathology. On the other hand, appropriately serum extracellular HSPs are higher than normal in t2DM and rise further with longer duration of t2DM, higher glycemia, and inflammation (Nakhjavani et al. 2010, 2012).
Obesity and t2DM are associated with ER stress. Hsp72 directly binds to IRE1α and enhances IRE1α-XBP1 signaling at the ER and thus improves adaptation to ER stress and cell survival (Gupta et al. 2010). Furthermore, reduction of ER stress with agents that augment ER chaperones is associated with amelioration of obesity and diabetes (Lee et al. 2003, 2011).
HSF1 directly stimulates expression of the tight junction protein, occludin (Dokladny et al. 2008). Diabetes induces defects in intestinal tight junctions that lead to a chronic endotoxemia and exacerbate systemic inflammation (Geurts et al. 2013). Impaired HSF1 activity in t2DM may contribute to the gut endotoxemia. Tight junction defects also contribute to renal and retinal pathology in diabetes (Hara et al. 2009; Silva et al. 2013), again perhaps exacerbated by a low HSF1 state.
Aging, diabetes, and Hsps
t2DM is an age-related disease that reduces longevity and in many ways accelerates many of the features associated with aging. HSPs are thought to play a fundamental role in longevity and aging (Murshid et al. 2013). Cytoplasmic Hsp70 levels have been examined across many different species, and higher levels confer longer maximum life spans (Rincon et al. 2005; Salway et al. 2011). For example, in invertebrates, overexpression of HSP70 confers a more than 40 % extension in lifespan (Yokoyama et al. 2002). In Caenorhabditis elegans, the transcription factor that regulates Hsps, HSF, is required for daf-2 mutants to express their longevity phenotype. These mutants have reduced insulin-like growth factor receptor function and double the expected lifespan (Hsu et al. 2003). Aging is generally associated with lower iHSPs; however, in our long-term studies of non-human primates, we found that development of insulin resistance via a high fat diet conferred lower iHsps, not age itself (Kavanagh et al. 2007, 2012).
Interestingly, neurodegenerative diseases like Parkinson’s and Alzheimer’s diseases have a higher prevalence in patients with t2DM (Garcia-Lara et al. 2010; Hu et al. 2007). These diseases share a common loss of insulin signaling in brain and in the t2DM pancreatic beta cell with amyloid precursor accumulation and aggregation (Hooper and Hooper 2005; Frame and Zheleva 2006). Insulin sensitivity as a central aging mechanism is supported by the longest lived mouse models, which have high insulin sensitivity through genetic modification of growth hormone biology (Brown-Borg and Bartke 2012). While iHSPs have not been measured in neurons or beta cells in vivo, loss of insulin signaling likely reduces iHSPs in these tissues, resulting in abnormal protein accumulation and function. Administering insulin and Hsp70 can reduce amyloid accumulation in the brain (Tang et al. 2013; Huang et al. 2014; Bobkova et al. 2013).
Like aging, t2DM accelerates the loss of genome-protecting telomeres (Garagnani et al. 2013; Balasubramanyam et al. 2007) because preservation of telomeres is dependent on a functioning cell stress response (Strub et al. 2008). Disruption of Hsps by t2DM could accelerate aging (Pandita et al. 2004; Tzanetakou et al. 2012). Not surprisingly, telomere shortness and t2DM are both tied to malignant transformation (Ornish et al. 2013). Finally, aging and diabetes are both associated with fluidity reduction and micro-domain remodeling (Adak et al. 2008; Vigh et al. 2007a). These changes in membrane organization can nullify healthy membrane-perturbing signaling and attenuating the heat shock response leading to a vicious cycle whereby aging reduces Hsp induction, which promotes aging through reduced cell survival and accumulation of oxidized proteins (Horvath and Vigh 2010; Vigh et al. 2007b; Török et al. 2013).
Hsp induction—importance of the membrane
We were intrigued how membrane composition could modulate the stress response and act as a temperature and/or stress sensor to activate the cellular stress response. We found evidence for a direct correlation between membrane fluidization and the Hsp response in mammalian cells. The thermal shift of membrane fluidity induced by heat was duplicated by membrane fluidizers (like benzyl alcohol and heptanol). The formation of isofluid membrane states in response to the chemical agents increased the expression of Hsp70 at physiological temperatures. Importantly, we demonstrated that the activation of Hsp expression by membrane fluidizers was not induced by a protein-unfolding signal (Balogh et al. 2013).
Saturated fats from animals are solid, while most unsaturated plant or marine origin fats are liquid at room temperature. Intuitively, it is not surprising that there is an association between type of dietary fats consumed, membrane fatty acid composition, and the development of diabetes (Weijers 2012). Less obvious is the notion that endurance exercise alone can change the membrane fatty acid content with a reduction of saturated fat and increased membrane fluidity (Marini et al. 2011). Conversely, patients with t2DM have higher saturated/cis-unsaturated fatty acid ratio in their membranes (resulting in lower membrane fluidity)(Weijers 2012). A high oleic acid intake normalizes the saturated/unsaturated fatty acid ratio, resets the proper membrane fluidity, and improves glycemia (Perona et al. 2007). Consumption of trans unsaturated fats, whose structure and effects on the membrane structure are closer to those of saturated fats, is also associated with diabetes and other health problems (Kavanagh et al. 2007; Bhardwaj et al. 2011). On the basis of the membrane sensor hypothesis, we speculate that a diet rich in cis-unsaturated fatty acids can be useful in the treatment of diabetes by remodeling membranes and thus upregulating Hsp70 (Vigh et al. 2007b; Balogh et al. 2013; Török et al. 2013).
Our present interest focuses on better defining a heat receptor and understanding the acute changes in membrane fluidity induced by its activation. Evidence is now gathering that a membrane calcium channel transient receptor potential vanilloid (TRPV), can react to heat and the herb capsaicin and produce a calcium influx into the cell resulting in activation of HSF-1 and thus the cellular stress response (Bromberg et al. 2013). Interestingly, capsaicin ingestion can improve mitochondrial biogenesis, improve exercise performance, block fat-induced insulin resistance, and protect against ischemic events (Luo et al. 2012; Xu et al. 2011). Perhaps, it is no surprise that knock out of TRP channels leads to a t2DM phenotype (Zhu et al. 2011).
Finally, in addition to the general lipid dietary approach, single membrane lipid or lipid molecular species can also be important. The ganglioside GM3 blocks insulin signaling, causing a dissociation of the insulin receptor and caveolin-1 complex in the surface membrane microdomains (rafts) (Kabayama et al. 2007). Thus, a novel therapeutic intervention aimed at normalizing the elevated level of GM3 through inhibiting GM3 synthase could prove beneficial for the treatment of t2DM.
Exercise, diabetes, and Hsps
Exercise offers great potential for improving the complications associated with obesity and diabetes. Exercise can maintain optimal blood glucose, lipid, and blood pressure profiles, which prevent or delay chronic complication of diabetes (American Diabetes Association 2010; Eriksson 1999; Zanuso et al. 2010; Ostergard et al. 2006). Acute and chronic exercise induces mechanical and cellular changes that affect metabolism and organ structure. Acute bouts, depending on intensity of exercise, can result in structural damage to tissues that lead to an adaptive response of tissue repair. Repeated acute bouts lead to enhanced cardiovascular and skeletal muscle functioning (Harber et al. 2012; Gollnick et al. 1972; Hamilton and Booth 2000). Moreover, endurance exercise training increases skeletal muscle mitochondrial enzyme activity (Holloway et al. 2006; Dudley et al. 1982; Gollnick et al. 1973; Holloszy 1975) and respiratory control via oxidative phosphorylation (Holloszy 1967), which improves fatigue resistance (Conlee and Fisher 1979) by modifying fiber type characteristics (Gollnick et al. 1973). Exercise induces metabolic adaptations include increased insulin sensitivity and muscle glycogen content (Manabe et al. 2013) and improved fatty acid oxidation and synthesis of acid cycle enzymes (Harber et al. 2012). Repeated exercise bouts will enhance cardiovascular function such as increasing the absolute and relative left ventricular mass (Longhurst et al. 1981; Wernstedt et al. 2002) as well as vascular density in skeletal muscles (Lash and Bohlen 1992), while physical inactivity deconditions the skeletal muscle and cardiovascular system.
While the etiology of t2DM is not well understood, evidence suggests that progressive insulin resistance is associated with damaged pancreatic β-cell function (Alonso-Magdalena et al. 2011; Sheng and Yang 2008). These impairments may be the cause of physical inactivity and increase calorie intake due to lipotoxicity and excess fatty acids accumulation, and resulting in a chronic pro-inflammatory state (Eckel et al. 2005; Sheng and Yang 2008; Furuhashi et al. 2011). This has been supported in obese type 2 diabetics and the descendants of patients with t2DM having a defective mitochondrial oxidative phosphorylation capacity and increased triglycerides and lipids in skeletal muscle (Eckel et al. 2005). Elevated lipid metabolites (ceremide and diacyglycerol) can directly activate inflammatory pathways (i.e., JNK, nuclear factor-κB, and IKK) (Copps and White 2012; Tanti et al. 2012). Moreover, an increase in inflammatory proteins impairs the insulin receptor substrate in t2DM and disrupts the downstream signal for the translocation of GLUT4 protein from the vesicles to the cell membrane, thus impairing glucose transport (Eckel et al. 2005; Hotamisligil 2005; Ozcan et al. 2004).
An acute bout of exercise improves whole-body insulin sensitivity and glucose tolerance (Wojtaszewski et al. 2002; Sakamoto and Goodyear 2002) 24–48 h after the bout (Hawley and Lessard 2008; Zierath 2002; Schneider et al. 1984). The precise mechanisms are not well understood; however, muscle contraction leads to an insulin independent effect via activation of 5′ adenosine monophosphate-activated protein kinase (AMPK) that likewise cause the translocation of GLUT4 to the cell membrane as well as increases GLUT4 gene expression (Daugaard and Richter 2001; Zisman et al. 2000; Hussey et al. 2012; Lehnen et al. 2011; Holloszy 2008; O’Gorman et al. 2006; Kraniou et al. 2006; Holmes and Dohm 2004; Daugaard et al. 2000), thereby improving glucose tolerance and insulin sensitivity (Chen et al. 2003; Richter et al. 2004; Frosig et al. 2004). AMPK is a key regulator of skeletal muscle metabolism and gene expression and is believed to be an important signaling molecule for adaptations caused by exercise training (Russell et al. 2014; Richter and Hargreaves 2013). Furthermore, exercise is known to have an anti-inflammatory effect with reduce pro-inflammatory cytokines in obese and diabetic humans (Belotto et al. 2010; TeixeiradeLemos et al. 2009; Petersen and Pedersen 2006; Gielen et al. 2003). Moreover, acute exercise reduces JNK activity and restores insulin sensitivity by modulating IRS (pSER) in humans (Pauli et al. 2010; Kiraly et al. 2010; Teixeira-Lemos et al. 2011) rat models (Kiraly et al. 2010; Ropelle et al. 2006; Berdichevsky et al. 2010), and cell cultures (Berdichevsky et al. 2010). Hsp72 functions as a natural inhibitory protein of JNK (Park et al. 2001; Volloch et al. 2000) and improvements attribute to limiting inflammatory kinase disruption of insulin signaling (Gabai et al. 1997).
Muscle mitochondrial function in diabetes mellitus is impaired with fiber-type-specific defects in insulin signal transduction for glucose transport (Song et al. 1999). Specifically, GLUT4 is reduced in type I muscle fibers of type 2 diabetic patients (Gaster et al. 2001; Tanner et al. 2002). These impairments are associated with a low aerobic exercise capacity in obese type 2 diabetics (Kadoglou et al. 2009; Leite et al. 2009). Physical inactivity causes a deconditioning effect and a diminished capacity of skeletal muscle and the cardiovascular system, as observed in bed rest studies (Ringholm et al. 2011; Adami et al. 2013; Brocca et al. 2012). Moreover, in healthy populations, 2 weeks of inactivity can likewise impair peripheral insulin sensitivity and cardiovascular fitness (Olsen et al. 2008). This deconditioned effect may be associated with a decreased HSPs expression and HSF1 gene, which is observed in obese type 2 diabetic humans (Rodrigues-Krause et al. 2012).
Exercise will increase iHsps in response to a varied stress response such as muscle contraction (Liu and Steinacker 2001), ischemia (Bushell et al. 2002; Lepore et al. 2000; Liu et al. 2002), metabolism (Ndisang 2014), oxidative stress (Fittipaldi et al. 2014), and glycogen depletion (Febbraio and Koukoulas 2000; Khassaf et al. 2001). Moreover, the extent of such changes is dependent on training status, intensity, duration, mode, damaging/nondamaging, and fiber recruitment (see reviews: Liu and Steinacker 2001; Morton et al. 2009). Animal studies have shown that acute exercise increases iHsp70 in tissues such as skeletal muscle, lymphocytes, spleen, heart, brain, and liver (Lollo et al. 2013; Touchberry et al. 2012; Salo et al. 1991; Pahlavani et al. 1995; Mikami et al. 2004; Campisi et al. 2003). Interestingly, high-intensity exercise of short duration raises iHsps as effectively as longer duration exercise and produces similar positive metabolic effects on skeletal muscle (Bartlett et al. 2012). Moreover, resistance exercise, which can cause significant muscle damage, has demonstrated that mammalian target of rapamycin (mTOR) signaling is important for inducing hypertrophy (Farnfield et al. 2012; Apro et al. 2013). Recently, mTOR has been implicated as a key protein for the activation of HSF1 in cell cultures (Chou et al. 2012). Exercise induced heat shock proteins have been extensively studied in cardiac tissues and are thought to serve as a cardio protective role for ischemia–reperfusion injury. In fact, a single exercise bout will increase iHsp70 in large and small vessels (Milne et al. 2012) and myocytes and improved ischemia recovery and reduce infarct size (Dillmann and Mestril 1995; Mestril et al. 1994a, b; Nishizawa et al. 1996). Similarly, a cross-tolerance response (Whitley et al. 1999), such that an exposure to one stress (exercise) can protect against other stresses (i.e., hypoxia and or ischemia) or cross-talk (Vigh et al. 2007b), may also occur in skeletal muscle.
In conclusion, we believe that exercise can play a major role in enhancing the endogenous defense system against mechanical and metabolic muscle damage, which has the potential for cross-talk mechanisms for improving insulin signaling and reducing inflammatory induced insulin resistance. Ultimately, exercise can provide a model for developing new therapeutic options to overcome or limit the metabolic impairments of t2DM.
Hyperthermia, diabetes, and Hsps
While a tradition of treating diabetes with healing hot waters has thrived for centuries, particularly in Eurasia, only in the past decade have we invested scientific attention to understand the therapeutic effects of hyperthermia in treating diabetes. Table 1 highlights the results of studies examining hyperthermia and/or other nonpharmaceutical HSP induction methods in animal models of diabetes. One striking observation is how a brief heat shock—as short as 15 min and as infrequent as once a week—results in remarkable improvements in the metabolic state. A variety of techniques have been used to induce hyperthermia—hot water immersion, warm electric blanket, sauna, and infrared box. Mild, direct electrical current stimulation has also been used to augment heat-induced rises in HSPs. Relevantly, whole-body hyperthermia raises baseline iHSPs (Shinohara et al. 2006; Singleton and Wischmeyer 2006). Provocatively (but not recommended by us), it has been observed that low-dose gamma radiation, known to raise HSPs (Seo et al. 2006), applied to diabetic genotype animals over a lifetime is associated with longer lifespan and less renal disease than nonirradiated animals(Nomura et al. 2011).
Table 1.
Hyperthermia and/or other nonpharmaceutical HSP induction methods and therapies for diabetes/metabolic syndrome
| Species and metabolic state or model | Heat or stress applied, intensity, duration frequency | Therapeutic result |
|---|---|---|
| Type 2 diabetes patients (Hooper 1999) | Hot tub: oral temperature rose 0.8 °C, 30 min, for 3 weeks, 6 out of 7 days/week | 1 % fall in HbA1 1.3 mmol/l, weight loss trend, symptoms of neuropathy improved |
| Obese subjects (Biro et al. 2003) | Sauna: rectal temperature rose 1.0 °C, 15 min at 60 °C, daily, 2 weeks | Fasting blood sugar fell 0.3 mmol/l, weight loss 0.3 kg, BP fell 4 mmHg systolic and 5 mmHg diastolic |
| Obese subjects’ ex vivo monocytes (Simar et al. 2004) | Cells incubated for 2 h at 42 °C | Decreases in pJNK, pIKK-β, and inhibitory serine IRS-1 phosphorylation |
| Type 2 diabetes patients (Beever 2010) | Sauna: far-infrared, 20 min, 3 times/week for 3 months | Increased quality of life: reduced stress, fatigue, increased health perception, and social functioning |
| Fat fed mice—model of t2DM (Chung et al. 2008) | Warming blanket: rectal temperature 41.5 °C for 15 min, weekly, 16 weeks | Prevented fat induction of fasting glucose, glucose intolerance, hyperinsulinemia, insulin resistance, and pJNK |
| Fat fed rats (Gupte et al. 2009a) | Hot water immersion: rectal temperature 41.0 °C for 20 min, weekly, 3 months | Improved glucose tolerance, insulin-stimulated glucose uptake, increased insulin signaling in slow twitch skeletal muscle, decreased pJNK, pIKK-β, increased mitochondrial enzyme levels |
| Aged insulin resistant rats 24 months old (Gupte et al. 2011) | Warming blanket: rectal temperature of 41–41.5 °C for 20 min, tested 24 h later | Increase in insulin-stimulated glucose uptake in slow twitch skeletal muscle |
| Aged insulin resistant rats 24 months old, in vitro soleus muscle (Gupte et al. 2011) | Incubated: 30 min at 42 °C | Inhibited anisomycin-induced activation of JNK: effect blocked by specific Hsp72 inhibitor |
| L-6 rat skeletal muscle cell line treated with tumor necrosis factor alpha to induce insulin resistance (Gupte et al. 2011) | Incubated: 43 °C for 20 min, tested 24 h later | Preserved ATP-coupled oxygen consumption, and fatty acid oxidation, i.e., enhanced mitochondrial function |
| db/db mice (Kokura et al. 2007) | Far infrared light: rectal temperature of 38 °C for 30 min, 3 times/week, 3 months | Improved glycemia, triglycerides, free fatty acid levels, urinary protein excretion, histological kidney damage, GLUT4 expression |
| db/db mice and fat mice (Morino et al. 2008) | Heat and mild electric stimulation: 42 °C electrodes and 12 V direct current (55 pps of 0.1 ms duration), 2 times/week for 12–15 weeks | Improved glycemic, reduced insulin levels, reduced liver and body fat, decreased size of adipocytes |
| HepG2 cells in high glucose medium (Morino-Koga et al. 2013) | Incubation at 42 °C and MES for 10 min | Increased activating phosphorylation IRS and Akt, increased accumulation of insulin on lipid rafts |
| db/db mice (Kondo et al. 2012) | Heat and mild electric stimulation: 42 °C electrodes and 12 V direct current (55 pps of 0.1 ms duration), 2 times/week for 12–15 weeks | Reduced beta cell apoptosis and ER stress, increased insulin response to glucose challenge, reduced cytokine activation |
| Low-dose streptozotocin rat(Bathaie et al. 2010) | Hot water immersion: rectal temperature 41.0 °C for 20 min, 3 times/week, 5 months | Lowered fasting glucose, Hb A1c AGE, triglycerides, low-density lipoprotein cholesterol, increased high-density lipoprotein cholesterol, and insulin secretion |
As noted in Table 1, hyperthermic and nonpharmaceutical Hsp induction studies demonstrate multiple physiological improvements: notably increased GLUT4 transport and AMPK activation, improved adipokine profile, and reduced C-reactive protein, triglycerides, low-density lipoprotein (LDL), advanced glycosylation end product (AGE) formation, body weight, abdominal fat, liver fat, and blood pressure. Lastly, heat shock has been shown to protect from loss of organ function (liver, kidney, pancreatic beta cell, and peripheral nerves). The uniform amelioration of so many of the pathological features associated with t2DM with Hsp induction through nonpharmacologic methods reflects the fundamental role in the initiation and progression of the disease.
Herein, we will review, beyond our initial hot tub therapy study (Hooper 1999), the hyperthermia-metabolic syndrome studies as they differ considerably from the animal model studied, the tissues examined, and the method of Hsp induction. In collaboration with Febbraio and colleagues, we studied Hsp inducers in a fat diet mice model of t2DM. Fifteen minutes of weekly increasing body temperature to 41.5 °C via a warm blanket resulted in a transient rise in Hsp72 in skeletal muscle, liver, and adipose tissue over a 24-h period. After 16 weeks, heat therapy prevented fat induction of fasting glucose, glucose intolerance, hyperinsulinemia, insulin resistance, and phosphorylation of JNK (Chung et al. 2008). Gupte, Geiger, and coworkers studied fat fed male Wister rats by weekly immersion for 20 min in hot water to raise rectal temperature in a range of 41–41.5 °C for 12 weeks. The heat treatment did not alter body weight, but it did reduce epididymal fat accumulation compared to control fat fed animals. While fasting glucose levels were not altered by the diet, heat treatment, compared to fat fed control animals, reduced insulin levels, improved glucose clearance, improved mitochondrial function, increased insulin-stimulated glucose in both fast and slow twitch muscles, increased insulin signaling with activating phosphorylation of IRS and Akt, augmented GLUT4 translocation, reduced JNK activation (which was blocked by Hsp72 inhibitor, KNK437), and reduced activation of pIKK-β in fast twitch EDL muscle but not in slow twitch soleus muscle. Heat treatment phosphorylated Hsp25 in EDL muscle and restored low Hsp60 in the mitochondria. A single bout of heat treatment in a nonfat fed rat, 41 °C for 20 min, resulted in a rise in insulin stimulated glucose uptake 24 h after the heat shock. Finally, the authors studied the effect of heat (43 °C for 20 min) in vitro L6 muscles and found at 24 h improved oxygen consumption and fatty acid oxidation compared to sham-treated muscle (Gupte et al. 2009a). In a separate study by the same authors, a single bout of heat, 41 °C for 20 min, improved insulin-stimulated glucose uptake in aging Fisher 344 rats in slow twitch soleus muscle 24 h after the heat shock. In vitro heat treatment, 42 °C for 30 min, of soleus muscle applied to 3-month and older 24 month soleus muscles increased expression of Hsp72 and inhibited anisomycin-induced activation of JNK. Inhibition of Hsp72 transcription with KNK437 blocked the ability of heat treatment to reduce JNK activation, which suggests that heat treatment’s ability to inhibit JNK activation in skeletal muscle is dependent on increased Hsp72 expression (Gupte et al. 2011).
Kokura and coworkers studied the rodent model of t2DM, db/db mice, treated three times per week for 12 weeks with rectal temperature of 38 °C for 30 min using far infrared light therapy. Heat treatment decreased fasting blood glucose, insulin, and triglycerides levels, and improved glucose tolerance and GLUT4 mRNA in muscle as compared with untreated db/db mice. The rise in urinary albumin and histological kidney damage observed in the db/db mice was inhibited by heat therapy (Kokura et al. 2007).
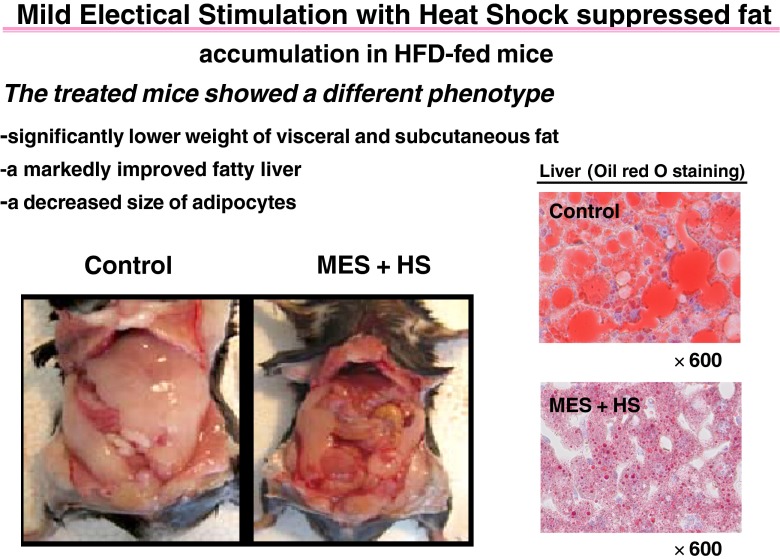
Kai, Kondo, and colleagues have developed a method that combines heat and mild electrical stimulation that maximizes Hsp72 expression in tissues. In their study, they heated fat fed mice to 42 °C in a warming box and applied electrodes with a 12-V direct current (55 pps of 0.1 ms duration), two times/week for 12–15 weeks. They observed lower fasting glucose, insulin and tumor necrosis factor alpha levels, but raised adiponectin levels, and improved glucose tolerance. Despite the same level of activity and calorie consumption, the treated animals had less liver and body fat, and weighed less with a change in the diet induced t2DM phenotype (see Fig. 1). At 15 weeks, fat cell size decreased and brown fat increased, as did uncoupling protein. The treated animals demonstrated improved insulin signaling in the liver (IRS phosphorylation, Akt activation), lower JNK activation, and higher Hsp72 expression. When Hsp 72 was knocked down with small interfering RNA, insulin signaling, and reduction in JNK activation by the treatment were blocked (Morino et al. 2008). When the same researchers used heat and electrical stimulation applied to db/db mice for 12 weeks, compared to sham treatment, insulin secretion was improved in response to a glucose challenge. Levels of HSP72, insulin, pancreatic duodenal homeobox-1, glucose transporter type 2, and insulin receptor substrate-2 were up regulated in the pancreatic islets of treated mice. On the other hand, JNK phosphorylation, nuclear translocation of forkhead box class O-1, and nuclear factor-κB p65 were reduced. Apoptotic signals, ER stress, and oxidative stress markers were attenuated. Thus, the therapy preserved beta cell function in addition to improving insulin signaling and body composition (Kondo et al. 2012). Finally, the same research group directed their attention to insulin-resistant liver cells, HepG2 cells in high glucose medium, by applying heat at 42 °C and MES for 10 min. The treatment activated the insulin receptor and improved insulin signaling in the absence of insulin by accumulating insulin receptors within lipid rafts (Morino-Koga et al. 2013).
Fig. 1.
Ventral aspect of high fat-fed mice sham-treated (control) or treated with heat shock and mild electrical stimulation [42 °C electrodes and 12 V direct current (55 pps of 0.1 ms duration) 10 min, two times per week] after 15 weeks of treatment with exposed peritoneal cavity, showing decrease in visible adipose tissues in treated mice. The diet induced t2DM phenotype is normalized by therapy [from open access journal, Plos1 (Morino et al. 2008)]. Similarly, but not shown here, heme oxygenase stimulation with cobalt protoporphyrin in the Zucker fat rat alters the t2DM phenotype to a thin, smaller rat (Nicolai et al. 2009)
Bathaie and colleagues studied streptozotocin-induced diabetic rats, which are the not ideal animal model to study t2DM, but can add insight into impact of hypoinsulinemia and hyperglycemia and long-term pathological impact. Rats were immersed in a circulating water bath (42 °C for 30 min) to obtain a core body temperature of 41 °C and repeated three times a week for 5 months. The treated diabetic rats compared to untreated animals had improvement in lipid profile [lower cholesterol, triglycerides, and higher density lipoprotein (HDL) cholesterol), better antioxidant capacity, insulin secretion, normalization of serum Hsp70 level and a decrease in AGE formation. The effect on fasting glucose was minimal. Glycated Hsp70 lost its chaperoning ability to reactivate the denatured luciferase. While fewer rats died in the intervention group, the reduction was not statistically significant (Bathaie et al. 2010).
Regarding the limited human studies, Biro and coworkers studied subjects with lifestyle-related diseases (12 % diabetes, 32 % hypertension, 60 % smoking, and 36 % obesity), which might be considered subjects with metabolic syndrome; however, the authors did not use that term. Patients were seated in a far-infrared sauna for 15 min, followed by a warm blanket for 30 min. The treatment raised the body temperature by 1.0 °C. Two weeks of therapy significantly reduced fasting glucose (99 to 94 mg/dl), body weight, and systolic and diastolic blood pressure, while serum lipids remained unchanged (Biro et al. 2003). In a study of insulin-resistant obese subjects with body mass index (BMI) > 30 kg/m2, compared to thinner, insulin-sensitive subjects with average BMIs of 21 kg/m2 fasting blood was collected and incubated for 2 h ex vivo at 42 °C. Monocytes were separated and tested for insulin signaling and inflammatory markers. At baseline, obese subjects had increased phosphorylation and thus activation of JNK and pIKK-β with increased inhibitory serine phosphorylation of IRS-1 and reduced GLUT4 response to insulin. In response to the hyperthermia, monocyte iHsp25 and iHsp27 rose less in the obese group than in the thin cohort. The heat lowered phosphorylation of JNK and pIKK-β and reduced serine IRS-1 in the obese group. GLUT4 response to insulin after the heat challenge was not retested (Simar et al. 2012).
A study of t2DM patients who were treated with far infrared therapy three times a week for 3 months examined the psychosocial impact of the intervention. Heat therapy was associated with increased quality of life: reduced stress, fatigue, increased health perception, and social functioning (Beever 2010). Clearly, larger, clinical interventional hyperthermia studies are needed in humans.
Induction of Hsps with bioactive compounds and diabetes therapy
Background
In 1997, we showed that the cyto-protective properties of an Hsp inducer (bimoclomol) improved diabetic wound healing and cardiac ischemia (Vígh et al. 1997). Soon afterward, bimoclomol demonstrated amelioration of diabetic neuropathy, retinopathy, and nephropathy. Subsequently, we found that bimoclomol and other hydroximic acids modified membrane lipid domains where thermally or chemically induced perturbation of lipid phase is sensed and transduced into a cellular signal, leading to enhanced activation of heat shock genes (Török et al. 2003). Later, we were able to replicate the therapeutic effects of hyperthermia on t2DM with an Hsp coinducer (BGP 15) by blocking fat fed induction of t2DM in rodents—improving insulin signaling, lowering fasting glucose levels, and reducing cytokine levels. Overexpressing the Hsp72 gene prevented fat feeding-induced impairments in insulin signaling via reduced cytokine release (Chung et al. 2008).
Overview of bioactive inducers
The diverse compounds that raise Hsps and improve many of the pathologies associated with t2DM. These compounds vary widely in their structure and mechanism of Hsp induction and, like hyperthermia, their effects are diverse and consistently metabolically restorative. Table 2 summarizes the agents and their biological effects in t2DM subjects and t2DM animal models. While some of the agents are impractical for therapeutic use in t2DM, many are nontoxic and effective when administered orally. Relevantly, some of these compounds also enhance exercise endurance and, in some cases, longevity.
Table 2.
HSPs inducers: therapeutic effect on diabetes/metabolic syndrome
| HSP inducer | Therapeutic result |
|---|---|
| Hydroximic acids (Crul et al. 2013) | Improves glycemia, insulin signaling, anti-inflammatory, mitochondria generation renal, and nerve protection; blocks weight gain to antipsychotic medications; ischemia protection; improves diabetic wound healing; reduces liver fat; improves dyslipidemia |
| Geranylgeranylacetone (Kavanagh et al. 2011) | Improves glycemia, insulin signaling, anti-inflammatory |
| Alpha-lipoic acid (Gupte et al. 2009b) | Improves glycemia, insulin signaling, anti-inflammatory |
| Xenohormetic plant compounds (Hooper et al. 2010)-curcumin (Sahin et al. 2012; Maradana et al. 2013), carvacrol (Cho et al. 2012; Wieten et al. 2010), resveratrol (Han et al. 2012; Ito-Nagahata et al. 2013), metformin (Tsuei and Martinus 2012), astaxanthine (Lee et al. 2010; Yuan et al. 2011), naringin (Sharma et al. 2011), rhodiola (Wang et al. 2012; Panossian et al. 2009), capsaicin (Luo et al. 2012; Joo et al. 2010) | Improves glycemia, insulin signaling, and anti-inflammatory; reduce fatty liver; improves exercise performance; reduces fatigue; preserves kidney function; reduces diabetes-related cancer risk; improves endothelial function; extends life span of nematode |
| Hemeoxygenase inducers (Li et al. 2008) | Improves glycemia, insulin signaling, anti-inflammatory |
| Hsp90 inhibitors (Lee et al. 2013; Farmer et al. 2012) | Improves glycemia, insulin signaling, anti-inflammatory |
| Chemical chaperones (Kars et al. 2010; Raciti et al. 2010) | Improves glycemia, insulin signaling, anti-inflammatory |
| GLP agonist (Cunha et al. 2009) | Protects islets cells |
| Hsp27 (Dai et al. 2009) and Hsp72 (Chung et al. 2008) gene overexpression | Improves glycemia, improves insulin signaling, reduces inflammatory cytokines, reduces body fat, preserves beta cells |
Hydroximic acids
This group of compounds, and BGP15 in particular, has received the most research as potentially therapeutic HSP inducers in diabetes. As coinducers of Hsps, they augment HSP induction through enhancing membrane fluidization, acting as raft stabilizers (Gombos et al. 2011) and thereby activating specific “heat sensors” in the membranes (Brameshuber et al. 2010; Török et al. 2013), which initiate a cascade of events resulting in HSF1 activation, IRS laden lipid raft formation, GLUT4 translocation, Akt phosphorylation, mTOR activation, glucose uptake, AMPK activation, SIRT1-like deacetylation, mitochondrial preservation, and reduced JNK activation (see Fig. 2). Relevantly, Rac1 inhibitors almost completely block the hsp-coinducer effect of BGP-15. Beyond improvements in metabolic homeostasis, this group of drugs demonstrate potential efficacy in treating complications of diabetes and providing renal, eye, kidney, nerve, endothelial function, and heart protection. Finally, animal disease models of previously untreatable diseases like muscular dystrophy and ALS respond to this class of drugs. Problems with drug tolerability and/or toxicity have not been identified in either animal or human trials (Crul et al. 2013).
Fig. 2.
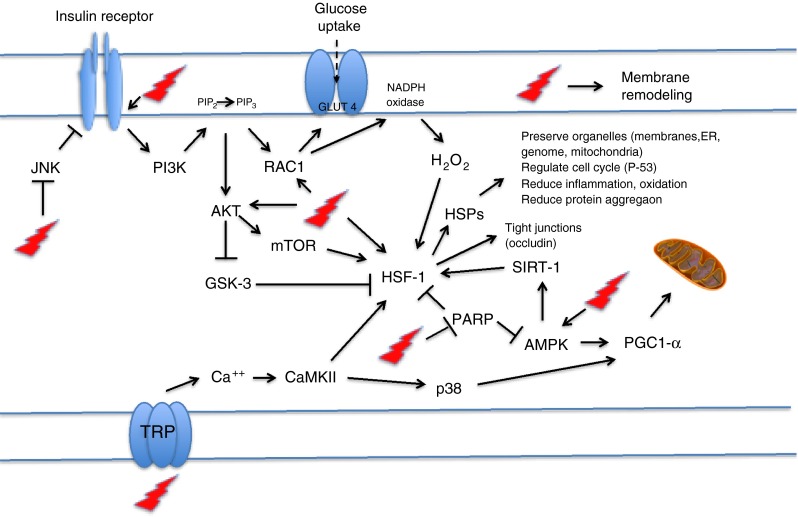
Exercise, heat shock, and the multitarget, membrane-interacting HSP inducers (like hydroximic acid derivatives) can activate many of the same metabolic pathways. These activators increase insulin receptor auto-phosphorylation, block JNK’s inhibitory phosphorylation of insulin receptor, increase Akt phosphorylation, activate mTOR, activate ras-related C3 botulinum toxin substrate 1 (RAC1), increase GLUT4 translocation and increase glucose uptake, increase second messenger H2O2, remodel membranes, increase AMPK, decrease HSF-1 acetylation, deactivates glycogen synthase kinase (GSK) inhibition of HSF-1, increase activation of HSF-1, inhibit poly ADP ribose polymerase (PARP), increase mitochondrial biogenesis and function, increase HSPs that restores stress resilience and organ survival—beta-cell, heart, liver kidney, retina, skin, etc., increase occludin expression and tight junction barrier function, activate the heat sensor transient receptor potential (TRP) that releases calcium as a second messenger to ultimately activate HSF1 and PGC1-α to increase mitochondrial function and synthesis (Dokladny et al. 2008; Crul et al. 2013; Török et al. 2013)
Xenohormetic plant substances
Plants and animals share common cellular survival stress responses, as well as common metabolic energy producing organelles, like mitochondria. Stressed plants synthesize bioactive compounds that can confer stress tolerance and longevity to an animal that consumes them by priming and augmenting the animal’s HSP response. Indeed, many of the xenohormetic substances associated with ancient traditional diets and/or herbal medicines raise HSPs, improve insulin action, and enhance fitness. Perhaps the most effective drug used today in diabetes, metformin, is a xenohormetic plant compound that increases membrane fluidity, raises Hsps, restores metabolic homeostasis, reduces cancer risk, and reduces diabetic mortality (Hooper et al. 2010; Tsuei and Martinus 2012; Nunn et al. 2010; Muller et al. 1997; Wiernsperger 1999; Holman et al. 2008).
Geranylgeranylacetone
Geranylgeranylacetone (GGA) is an antiulcer medication that is widely available in Japan and has been studied as a potential therapeutic agent in many maladies (colitis, ischemia, retinal detachment, infection, etc.). GGA is thought to prolong HSF1 activation (Kavanagh et al. 2011).
Alpha-lipoic acid
Alpha-lipoic acid is a cofactor in oxidative metabolism and has a wide application as an over-the-counter product to aid in weight loss, wound healing, and neuropathy (Gupte et al. 2009b).
Hsp90 inhibitors
Hsp90 represses HSF1 and, therefore, selective Hsp90 inhibitors activate HSF1-dependent transcription. This class of drugs raises Hsp 70 and improves insulin signaling, preserves diabetic islet cells, and reduces diabetic neuropathy. Unfortunately, the inhibition of Hsp 90 increases the toxicity of this class of compounds (Lee et al. 2013; Farmer et al. 2012).
Hemeoxygenase inducers
The Hsp heme oxygenase acts as an antioxidant and apoptosis blocker via its byproducts bilirubin/biliverdin and carbon monoxide. Induction of this Hsp changes the whole phenotype of diabetic animal models with marked reduction in adiposity and increases in adiponectin and insulin sensitivity (Li et al. 2008). The hemeoxygenase inducer L-4F is an apo-lipoprotein A1 mimetic, which reverses the obese mouse phenotype. As an aside, one of the defining criteria of the metabolic syndrome is low apo A1 (the major apo-lipoprotein of HDL). This raises the question: Does a low apo A1 level itself promote a lower HSP state associated with the t2DM (Marino et al. 2012)?
Chemical chaperones
Chemical chaperones are similar to HSPs in their ability to facilitate protein folding. Two have been studied in humans, 4-phenylbuteric acid and tuaroursodeoxycholic acid (a bile salt), which were shown to reduce ER stress, stabilize mitochondria, and improve insulin signaling (Kars et al. 2010; Raciti et al. 2010).
Glucagon-like peptide agonist
A glucagon-like peptide agonist increases ER chaperone BiP (Grp78), reduces ER stress, and improves islets cell culture survival from an FFA challenge (Cunha et al. 2009).
Common metabolic pathways of exercise, heat, and Hsp inducers
The stress of exercise results in a physiological response assuring survival by activating key metabolic events directed at a temporary reduction in anabolism (fat, glycogen, and protein synthesis) and a focus on generation of ATP. Exercise, heat shock, and many of the Hsp inducers on a cellular level activate AMPK, mTOR, PGC1-α, and SIRT1, particularly in skeletal muscle and liver. The net biological effect is increased fat oxidation, oxidative energy production, and mitochondrial biogenesis (Hooper et al. 2010; Reznick and Shulman 2006; Gurd 2011). With exercise conditioning stress tolerance is enhanced, muscle mass is gained, while inflammation and visceral fat mass are reduced. Heat shock and HSP inducers activate the same pathways and can improve exercise tolerance (Panossian et al. 2009).
Hsp induction addresses all of the diverse pathological features associated with t2DM
We view t2DM as a systemic multiorgan inflammatory disease and suggest that we think of it as “systemic diabetes mellitus” (Hooper 2005). The organ systems affected by t2DM are diverse and the disease itself is associated with comorbid diseases like cancer, dementia, and cardiovascular disease. Other seemingly unrelated inflammatory diseases like asthma and rheumatoid arthritis are associated with t2DM (Dandona et al. 2013; Graeber et al. 2013). Certainly, an age-related disease like t2DM that promotes early aging can contribute to major destructive pathologies.
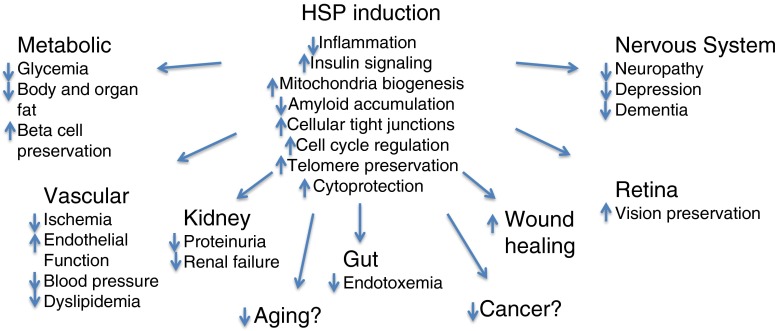
Improving the cellular stress response via heat shock protein induction can play a core role in treating t2DM its complications. By addressing a fundamental defect that is so key to enhancing cellular resilience and survival, Hsp induction is able to promote pleotropic beneficial effects on diverse pathologies associated with t2DM (Fig. 3).
Fig. 3.
HSP induction addresses the diverse pathological complications of t2DM
Reflections
Does the loss of the cellular stress response lie near the core of the pathogenesis of t2DM and the metabolic syndrome? Does a low muscle iHSP state occur years before the metabolic abnormalities appear? Has a sedentary lifestyle with ready access to calories led to an unfit, unconditioned phenotype? Should we begin to think of the syndrome as the “unconditioned syndrome”? We are intrigued that Tobin and coworkers have observed that space flight and zero gravity lab experiments lead to a diabetogenic state with increased inflammation, insulin resistance, and loss of muscle mass (Tobin et al. 2002). Our tissues have evolved over eons to survive a rigorous environment with regular pulses of stress and inflammation and are not prepared to thrive in a sedentary and calorically excessive lifestyle. iHSP induction can ensure that stressors positively influence survival and fitness. This new lifestyle is almost as alien as living in outer space.
New directions
Therapeutic interventions for t2DM focusing on diet and exercise are appropriate. For some individuals, intermittent bouts of intense exercise or fasting may result a better therapeutic impact to recover metabolic homeostasis than lifestyle changes that the body becomes complacent to. Restoration of the cellular stress response via modalities of heat shock and/or medicinal products is warranted. Mimicking exercise opens viable avenues to treat t2DM and its comorbidities.
Acknowledgments
The authors thank Paul Hooper, Annie Hooper, and Chassidy Glaze for proof reading; Alistair Nunn and Michael Tytell for sharing ideas; and Paige Geiger, Anisha Gupte, Dan Kemp, Hirofumi Kai, and Tatsuya Kondo for research efforts.
Abbreviations
- Akt
Protein kinase B
- AMPK
5′ AMP-activated protein kinase
- apo A1
Apo-lipoprotein A1
- ER
Endoplasmic reticulum
- GLUT4
Glucose transporter type 4
- GM3
Monosialodihexosylganglioside
- HSF1
Heat shock factor 1
- HSP
Heat shock protein
- iHSP
Intracellular heat shock proteins
- IRS
Insulin receptor substrate
- mTOR
Mammalian target of rapamycin
- pIKK-β
Inhibitor of nuclear factor kappa-B kinase subunit beta
- pJNK
Phosphorylated c-Jun N-terminal kinase
- PGC1-α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- pps
Pulses per second
- t2DM
Type 2 diabetes mellitus
- V
Volts
Contributor Information
Philip L. Hooper, Email: phoopermd@gmail.com
Gabor Balogh, Email: balogh.eg@gmail.com.
Eric Rivas, Email: EricRivas@texashealth.org.
Kylie Kavanagh, Email: kkavanag@wakehealth.edu.
Laszlo Vigh, Email: vigh@brc.hu.
References
- Adak S, Chowdhury S, Bhattacharyya M. Dynamic and electrokinetic behavior of erythrocyte membrane in diabetes mellitus and diabetic cardiovascular disease. Biochim Biophys Acta. 2008;1780(2):108–115. doi: 10.1016/j.bbagen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Adami A, Pizzinelli P, Bringard A, Capelli C, Malacarne M, Lucini D, et al. Cardiovascular re-adjustments and baroreflex response during clinical reambulation procedure at the end of 35-day bed rest in humans. Appl Physiol Nutr Metab. 2013;38(6):673–680. doi: 10.1139/apnm-2012-0396. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Quesada I, Nadal A (2011) Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol 7(6):346–353. doi:10.1038/nrendo.2011.56 [DOI] [PubMed]
- Apro W, Wang L, Ponten M, Blomstrand E, Sahlin K. Resistance exercise induced mTORC1 signaling is not impaired by subsequent endurance exercise in human skeletal muscle. Am J Physiol Endocrinol Metab. 2013;305(1):E22–E32. doi: 10.1152/ajpendo.00091.2013. [DOI] [PubMed] [Google Scholar]
- Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, et al. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol. 2004;97(2):605–611. doi: 10.1152/japplphysiol.01183.2003. [DOI] [PubMed] [Google Scholar]
- Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, et al. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol (1985) 2004;97(2):605–611. doi: 10.1152/japplphysiol.01183.2003. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanyam M, Adaikalakoteswari A, Monickaraj SF, Mohan V. Telomere shortening & metabolic/vascular diseases. Indian J Med Res. 2007;125(3):441–450. [PubMed] [Google Scholar]
- Balogh G, Peter M, Glatz A, Gombos I, Torok Z, Horvath I, et al. Key role of lipids in heat stress management. FEBS Lett. 2013;587(13):1970–1980. doi: 10.1016/j.febslet.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Hwa Joo C, Jeong TS, Louhelainen J, Cochran AJ, Gibala MJ, et al. Matched work high-intensity interval and continuous running induce similar increases in PGC-1alpha mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J Appl Physiol. 2012;112(7):1135–1143. doi: 10.1152/japplphysiol.01040.2011. [DOI] [PubMed] [Google Scholar]
- Bathaie SZ, Jafarnejad A, Hosseinkhani S, Nakhjavani M. The effect of hot-tub therapy on serum Hsp70 level and its benefit on diabetic rats: a preliminary report. Int J Hyperthermia. 2010;26(6):577–585. doi: 10.3109/02656736.2010.485594. [DOI] [PubMed] [Google Scholar]
- Beever R. The effects of repeated thermal therapy on quality of life in patients with type II diabetes mellitus. J Altern Complement Med. 2010;16(6):677–681. doi: 10.1089/acm.2009.0358. [DOI] [PubMed] [Google Scholar]
- Belotto MF, Magdalon J, Rodrigues HG, Vinolo MA, Curi R, Pithon-Curi TC, et al. Moderate exercise improves leucocyte function and decreases inflammation in diabetes. Clin Exp Immunol. 2010;162(2):237–243. doi: 10.1111/j.1365-2249.2010.04240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky A, Guarente L, Bose A. Acute oxidative stress can reverse insulin resistance by inactivation of cytoplasmic JNK. J Biol Chem. 2010;285(28):21581–21589. doi: 10.1074/jbc.M109.093633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj S, Passi SJ, Misra A. Overview of trans fatty acids: biochemistry and health effects. Diabetes Metab Syndr. 2011;5(3):161–164. doi: 10.1016/j.dsx.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Biro S, Masuda A, Kihara T, Tei C. Clinical implications of thermal therapy in lifestyle-related diseases. Exp Biol Med. 2003;228(10):1245–1249. doi: 10.1177/153537020322801023. [DOI] [PubMed] [Google Scholar]
- Bobkova N, Guzhova I, Margulis B, Nesterova I, Medvinskaya N, Samokhin A, et al. Dynamics of endogenous Hsp70 synthesis in the brain of olfactory bulbectomized mice. Cell Stress Chaperones. 2013;18(1):109–118. doi: 10.1007/s12192-012-0359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brameshuber M, Weghuber J, Ruprecht V, Gombos I, Horvath I, Vigh L, et al. Imaging of mobile long-lived nanoplatforms in the live cell plasma membrane. J Biol Chem. 2010;285(53):41765–41771. doi: 10.1074/jbc.M110.182121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocca L, Cannavino J, Coletto L, Biolo G, Sandri M, Bottinelli R, et al. The time course of the adaptations of human muscle proteome to bed rest and the underlying mechanisms. J Physiol. 2012;590(Pt 20):5211–5230. doi: 10.1113/jphysiol.2012.240267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg Z, Goloubinoff P, Saidi Y, Weiss YG. The membrane-associated transient receptor potential vanilloid channel is the central heat shock receptor controlling the cellular heat shock response in epithelial cells. PloS one. 2013;8(2):e57149. doi: 10.1371/journal.pone.0057149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Bartke A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci. 2012;67(6):652–660. doi: 10.1093/gerona/gls086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52(9):2338–2345. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- Bushell AJ, Klenerman L, Davies H, Grierson I, McArdle A, Jackson MJ. Ischaemic preconditioning of skeletal muscle 2. Investigation of the potential mechanisms involved. J Bone Joint Surg Br Vol. 2002;84(8):1189–1193. doi: 10.1302/0301-620x.84b8.9362. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Greenwood BN, Hansen MK, Moraska A, Higgins K, et al. Habitual physical activity facilitates stress-induced HSP72 induction in brain, peripheral, and immune tissues. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R520–R530. doi: 10.1152/ajpregu.00513.2002. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, et al. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes. 2003;52(9):2205–2212. doi: 10.2337/diabetes.52.9.2205. [DOI] [PubMed] [Google Scholar]
- Chen Y, Voegeli TS, Liu PP, Noble EG, Currie RW. Heat shock paradox and a new role of heat shock proteins and their receptors as anti-inflammation targets. Inflamm Allergy Drug Targets. 2007;6(2):91–100. doi: 10.2174/187152807780832274. [DOI] [PubMed] [Google Scholar]
- Cho S, Choi Y, Park S, Park T. Carvacrol prevents diet-induced obesity by modulating gene expressions involved in adipogenesis and inflammation in mice fed with high-fat diet. J Nutr Biochem. 2012;23(2):192–201. doi: 10.1016/j.jnutbio.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Chou SD, Prince T, Gong J, Calderwood SK. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PloS One. 2012;7(6):e39679. doi: 10.1371/journal.pone.0039679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105(5):1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlee RK, Fisher AG (1979) Skeletal muscle adaptations to growth and exercise. Nurs Pract 4(3):34–35, 55. PubMed PMID: 440641 [PubMed]
- Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55(10):2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crul T, Toth N, Piotto S, Literati-Nagy P, Tory K, Haldimann P, et al. Hydroximic acid derivatives: pleiotropic hsp co-inducers restoring homeostasis and robustness. Curr Pharm Des. 2013;19(3):309–346. doi: 10.2174/138161213804143716. [DOI] [PubMed] [Google Scholar]
- Cunha DA, Ladriere L, Ortis F, Igoillo-Esteve M, Gurzov EN, Lupi R, et al. Glucagon-like peptide-1 agonists protect pancreatic beta-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes. 2009;58(12):2851–2862. doi: 10.2337/db09-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Patel-Chamberlin M, Natarajan R, Todorov I, Ma J, LaPage J, et al. Heat shock protein 27 overexpression mitigates cytokine-induced islet apoptosis and streptozotocin-induced diabetes. Endocrinology. 2009;150(7):3031–3039. doi: 10.1210/en.2008-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P, Ghanim H, Monte SV, Caruana JA, Green K, Abuaysheh S, et al (2013) Increase in the mediators of asthma in obesity and obesity with type 2 diabetes: reduction with weight loss. Obesity. doi:10.1002/oby.20524 [DOI] [PubMed]
- Daugaard JR, Richter EA. Relationship between muscle fibre composition, glucose transporter protein 4 and exercise training: possible consequences in non-insulin-dependent diabetes mellitus. Acta Physiol Scand. 2001;171(3):267–276. doi: 10.1046/j.1365-201x.2001.00829.x. [DOI] [PubMed] [Google Scholar]
- Daugaard JR, Nielsen JN, Kristiansen S, Andersen JL, Hargreaves M, Richter EA. Fiber type-specific expression of GLUT4 in human skeletal muscle: influence of exercise training. Diabetes. 2000;49(7):1092–1095. doi: 10.2337/diabetes.49.7.1092. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillmann WH, Mestril R. Heat shock proteins in myocardial stress. Z Kardiol. 1995;84(Suppl 4):87–90. [PubMed] [Google Scholar]
- Dokladny K, Ye D, Kennedy JC, Moseley PL, Ma TY. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: regulatory role of heat shock factor-1. Am J Pathol. 2008;172(3):659–670. doi: 10.2353/ajpath.2008.070522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol. 1982;53(4):844–850. doi: 10.1152/jappl.1982.53.4.844. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Eriksson JG. Exercise and the treatment of type 2 diabetes mellitus. An update. Sports Med. 1999;27(6):381–391. doi: 10.2165/00007256-199927060-00003. [DOI] [PubMed] [Google Scholar]
- Farmer K, Williams SJ, Novikova L, Ramachandran K, Rawal S, Blagg BS, et al. KU-32, a novel drug for diabetic neuropathy, is safe for human islets and improves in vitro insulin secretion and viability. Exp Diabetes Res. 2012;2012:671673. doi: 10.1155/2012/671673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnfield MM, Breen L, Carey KA, Garnham A, Cameron-Smith D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl Physiol Nutr Metab. 2012;37(1):21–30. doi: 10.1139/h11-132. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Koukoulas I. HSP72 gene expression progressively increases in human skeletal muscle during prolonged, exhaustive exercise. J Appl Physiol (1985) 2000;89(3):1055–1060. doi: 10.1152/jappl.2000.89.3.1055. [DOI] [PubMed] [Google Scholar]
- Figueredo A, Ibarra JL, Rodriguez A, Molino AM, Gomez-delaConcha E, Fernandez-Cruz A, et al. Increased serum levels of IgA antibodies to hsp70 protein in patients with diabetes mellitus: their relationship with vascular complications. Clin Immunol Immunopathol. 1996;79(3):252–255. doi: 10.1006/clin.1996.0076. [DOI] [PubMed] [Google Scholar]
- Fittipaldi S, Dimauro I, Mercatelli N, Caporossi D (2014) Role of exercise-induced reactive oxygen species in the modulation of heat shock protein response. Free Radic Res 48:52–70. PubMed PMID: 23957557 [DOI] [PubMed]
- Frame S, Zheleva D. Targeting glycogen synthase kinase-3 in insulin signalling. Expert Opin Ther Targets. 2006;10(3):429–444. doi: 10.1517/14728222.10.3.429. [DOI] [PubMed] [Google Scholar]
- Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286(3):E411–E417. doi: 10.1152/ajpendo.00317.2003. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Ishimura S, Ota H, Miura T. Lipid chaperones and metabolic inflammation. Int J Inflamm. 2011;2011:642612. doi: 10.4061/2011/642612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Mosser DD, Caron AW, Rits S, Shifrin VI, et al. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J Biol Chem. 1997;272(29):18033–18037. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- Garagnani P, Giuliani C, Pirazzini C, Olivieri F, Bacalini MG, Ostan R, et al. Centenarians as super-controls to assess the biological relevance of genetic risk factors for common age-related diseases: a proof of principle on type 2 diabetes. Aging (Albany NY) 2013;5(5):373–385. doi: 10.18632/aging.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lara JM, Aguilar-Navarro S, Gutierrez-Robledo LM, Avila-Funes JA. The metabolic syndrome, diabetes, and Alzheimer’s disease. Rev Invest Clin. 2010;62(4):343–349. [PubMed] [Google Scholar]
- Gaster M, Staehr P, Beck-Nielsen H, Schroder HD, Handberg A. GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes. 2001;50(6):1324–1329. doi: 10.2337/diabetes.50.6.1324. [DOI] [PubMed] [Google Scholar]
- Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2013;25:1–15. doi: 10.3920/BM2012.0065. [DOI] [PubMed] [Google Scholar]
- Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42(5):861–868. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Armstrong RB, Saubert CW, Piehl K, Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol. 1972;33(3):312–319. doi: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Armstrong RB, Saltin B, Saubert CW, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973;34(1):107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- Gombos I, Crul T, Piotto S, Gungor B, Torok Z, Balogh G et al (2011) Membrane-lipid therapy in operation: the HSP co-inducer BGP-15 activates stress signal transduction pathways by remodeling plasma membrane rafts. PloS One 6(12):e28818. doi:10.1371/journal.pone.0028818 [DOI] [PMC free article] [PubMed]
- Graeber SY, Zhou-Suckow Z, Schatterny J, Hirtz S, Boucher RC, Mall MA (2013) Hypertonic saline is effective in the prevention and treatment of mucus obstruction but not airway inflammation in mice with chronic obstructive lung disease. Am J Respir Cell Mol Biol 49:410–417. PubMed PMID: 23590312 [DOI] [PMC free article] [PubMed]
- Gupta S, Deepti A, Deegan S, Lisbona F, Hetz C, Samali A. HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1alpha-XBP1 signaling through a physical interaction. PLoS Biol. 2010;8(7):e1000410. doi: 10.1371/journal.pbio.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Geiger PC. Age-related differences in skeletal muscle insulin signaling: the role of stress kinases and heat shock proteins. J Appl Physiol (1985) 2008;105(3):839–848. doi: 10.1152/japplphysiol.00148.2008. [DOI] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009;58(3):567–578. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Morris JK, Gorres BK, Geiger PC. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J Appl Physiol. 2009;106(4):1425–1434. doi: 10.1152/japplphysiol.91210.2008. [DOI] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Touchberry CD, Geiger PC. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol. 2011;110(2):451–457. doi: 10.1152/japplphysiol.00849.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd BJ. Deacetylation of PGC-1alpha by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab. 2011;36(5):589–597. doi: 10.1139/h11-070. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Booth FW. Skeletal muscle adaptation to exercise: a century of progress. J Appl Physiol. 2000;88(1):327–331. doi: 10.1152/jappl.2000.88.1.327. [DOI] [PubMed] [Google Scholar]
- Han S, Choi JR, Soon Shin K, Kang SJ. Resveratrol upregulated heat shock proteins and extended the survival of G93A-SOD1 mice. Brain Res. 2012;1483:112–117. doi: 10.1016/j.brainres.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Hara T, Ishida T, Cangara HM, Hirata K. Endothelial cell-selective adhesion molecule regulates albuminuria in diabetic nephropathy. Microvasc Res. 2009;77(3):348–355. doi: 10.1016/j.mvr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, et al. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol. 2012;113(9):1495–1504. doi: 10.1152/japplphysiol.00786.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol. 2008;192(1):127–135. doi: 10.1111/j.1748-1716.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242(9):2278–2282. [PubMed] [Google Scholar]
- Holloszy JO. Adaptation of skeletal muscle to endurance exercise. Med Sci Sports. 1975;7(3):155–164. [PubMed] [Google Scholar]
- Holloszy JO. Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J Physiol Pharmacol Off J Polish Physiol Soc. 2008;59(Suppl 7):5–18. [PubMed] [Google Scholar]
- Holloway GP, Bezaire V, Heigenhauser GJ, Tandon NN, Glatz JF, Luiken JJ, et al. Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J Physiol. 2006;571(Pt 1):201–210. doi: 10.1113/jphysiol.2005.102178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- Holmes B, Dohm GL. Regulation of GLUT4 gene expression during exercise. Med Sci Sports Exerc. 2004;36(7):1202–1206. doi: 10.1249/01.mss.0000132385.34889.fe. [DOI] [PubMed] [Google Scholar]
- Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999;341(12):924–925. doi: 10.1056/NEJM199909163411216. [DOI] [PubMed] [Google Scholar]
- Hooper PL. Systemic diabetes mellitus. Diabetes Technol Ther. 2005;7(2):337. doi: 10.1089/dia.2005.7.337. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper JJ. Loss of defense against stress: diabetes and heat shock proteins. Diabetes Technol Ther. 2005;7(1):204–208. doi: 10.1089/dia.2005.7.204. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. 2009;14(2):113–115. doi: 10.1007/s12192-008-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL, Tytell M, Vigh L. Xenohormesis: health benefits from an eon of plant stress response evolution. Cell Stress Chaperones. 2010;15(6):761–770. doi: 10.1007/s12192-010-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath I, Vigh L. Cell biology: stability in times of stress. Nature. 2010;463(7280):436–438. doi: 10.1038/463436a. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54(Suppl 2):S73–S78. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300(5622):1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care. 2007;30(4):842–847. doi: 10.2337/dc06-2011. [DOI] [PubMed] [Google Scholar]
- Huang HC, Tang D, Xu K, Jiang ZF (2014) Curcumin attenuates amyloid-beta-induced tau hyperphosphorylation in human neuroblastoma SH-SY5Y cells involving PTEN/Akt/GSK-3beta signaling pathway. J Recept Signal Transduct Res (in press). PubMed PMID: 24188406 [DOI] [PubMed]
- Hussey SE, McGee SL, Garnham A, McConell GK, Hargreaves M. Exercise increases skeletal muscle GLUT4 gene expression in patients with type 2 diabetes. Diabetes Obes Metab. 2012;14(8):768–771. doi: 10.1111/j.1463-1326.2012.01585.x. [DOI] [PubMed] [Google Scholar]
- Ito-Nagahata T, Kurihara C, Hasebe M, Ishii A, Yamashita K, Iwabuchi M, et al (2013) Stilbene analogs of resveratrol improve insulin resistance through activation of AMPK. Biosci Biotechnol Biochem 77:1229–1235. PubMed PMID: 23748787 [DOI] [PubMed]
- Joo JI, Kim DH, Choi JW, Yun JW. Proteomic analysis for antiobesity potential of capsaicin on white adipose tissue in rats fed with a high fat diet. J Proteome Res. 2010;9(6):2977–2987. doi: 10.1021/pr901175w. [DOI] [PubMed] [Google Scholar]
- Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, et al. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci U S A. 2007;104(34):13678–13683. doi: 10.1073/pnas.0703650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoglou NP, Iliadis F, Angelopoulou N, Sailer N, Fotiadis G, Voliotis K, et al. Cardiorespiratory capacity is associated with favourable cardiovascular risk profile in patients with type 2 diabetes. J Diabet Complicat. 2009;23(3):160–166. doi: 10.1016/j.jdiacomp.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59(8):1899–1905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Jones KL, Sawyer J, Kelley K, Carr JJ, Wagner JD, et al. Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys. Obesity. 2007;15(7):1675–1684. doi: 10.1038/oby.2007.200. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Zhang L, Wagner JD. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell Stress Chaperones. 2009;14(3):291–299. doi: 10.1007/s12192-008-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011;300(5):E894–E901. doi: 10.1152/ajpendo.00699.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Wylie AT, Chavanne TJ, Jorgensen MJ, Voruganti VS, Comuzzie AG, et al. Aging does not reduce heat shock protein 70 in the absence of chronic insulin resistance. J Gerontol A Biol Sci Med Sci. 2012;67(10):1014–1021. doi: 10.1093/gerona/gls008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khassaf M, Child RB, McArdle A, Brodie DA, Esanu C, Jackson MJ. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J Appl Physiol (1985) 2001;90(3):1031–1035. doi: 10.1152/jappl.2001.90.3.1031. [DOI] [PubMed] [Google Scholar]
- Kiraly MA, Campbell J, Park E, Bates HE, Yue JT, Rao V, et al. Exercise maintains euglycemia in association with decreased activation of c-Jun NH2-terminal kinase and serine phosphorylation of IRS-1 in the liver of ZDF rats. Am J Physiol Endocrinol Metab. 2010;298(3):E671–E682. doi: 10.1152/ajpendo.90575.2008. [DOI] [PubMed] [Google Scholar]
- Kokura S, Adachi S, Manabe E, Mizushima K, Hattori T, Okuda T, et al. Whole body hyperthermia improves obesity-induced insulin resistance in diabetic mice. Int J Hyperth Off J Eur Soc Hyperth Oncol N Am Hyperth Group. 2007;23(3):259–265. doi: 10.1080/02656730601176824. [DOI] [PubMed] [Google Scholar]
- Kondo T, Sasaki K, Matsuyama R, Morino-Koga S, Adachi H, Suico MA, et al. Hyperthermia with mild electrical stimulation protects pancreatic beta-cells from cell stresses and apoptosis. Diabetes. 2012;61(4):838–847. doi: 10.2337/db11-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraniou GN, Cameron-Smith D, Hargreaves M. Acute exercise and GLUT4 expression in human skeletal muscle: influence of exercise intensity. J Appl Physiol. 2006;101(3):934–937. doi: 10.1152/japplphysiol.01489.2005. [DOI] [PubMed] [Google Scholar]
- Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, et al. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51(4):1102–1109. doi: 10.2337/diabetes.51.4.1102. [DOI] [PubMed] [Google Scholar]
- Lash JM, Bohlen HG. Functional adaptations of rat skeletal muscle arterioles to aerobic exercise training. J Appl Physiol. 1992;72(6):2052–2062. doi: 10.1152/jappl.1992.72.6.2052. [DOI] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23(21):7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Lee YJ, Kwon KH. Neuroprotective effects of astaxanthin in oxygen–glucose deprivation in SH-SY5Y cells and global cerebral ischemia in rat. J Clin Biochem Nutr. 2010;47(2):121–129. doi: 10.3164/jcbn.10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Sun C, Zhou Y, Lee J, Gokalp D, Herrema H, et al. p38 MAPK-mediated regulation of Xbp1s is crucial for glucose homeostasis. Nat Med. 2011;17(10):1251–1260. doi: 10.1038/nm.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Gao J, Kosinski PA, Elliman SJ, Hughes TE, Gromada J, et al. Heat shock protein 90 (HSP90) inhibitors activate the heat shock factor 1 (HSF1) stress response pathway and improve glucose regulation in diabetic mice. Biochem Biophys Res Commun. 2013;430(3):1109–1113. doi: 10.1016/j.bbrc.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Lehnen AM, Leguisamo NM, Pinto GH, Markoski M, De Angelis K, Machado UF, et al. Exercise-stimulated GLUT4 expression is similar in normotensive and hypertensive rats. Horm Metab Res = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2011;43(4):231–235. doi: 10.1055/s-0031-1271747. [DOI] [PubMed] [Google Scholar]
- Leite SA, Monk AM, Upham PA, Bergenstal RM. Low cardiorespiratory fitness in people at risk for type 2 diabetes: early marker for insulin resistance. Diabetol Metab Syndr. 2009;1(1):8. doi: 10.1186/1758-5996-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore DA, Hurley JV, Stewart AG, Morrison WA, Anderson RL. Prior heat stress improves survival of ischemic-reperfused skeletal muscle in vivo. Muscle Nerve. 2000;23(12):1847–1855. doi: 10.1002/1097-4598(200012)23:12<1847::aid-mus8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57(6):1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- Liu Y, Steinacker JM (2001) Changes in skeletal muscle heat shock proteins: pathological significance. Front Biosci 6:D12–D25 [DOI] [PubMed]
- Liu Y, Lehmann M, Baur C, Storck M, Sunder-Plassmann L, Steinacker JM. HSP70 expression in skeletal muscle of patients with peripheral arterial occlusive disease. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2002;24(3):269–273. doi: 10.1053/ejvs.2002.1690. [DOI] [PubMed] [Google Scholar]
- Lollo PC, Moura CS, Morato PN, Amaya-Farfan J. Differential response of heat shock proteins to uphill and downhill exercise in heart, skeletal muscle, lung and kidney tissues. J Sports Sci Med. 2013;12(3):461–466. [PMC free article] [PubMed] [Google Scholar]
- Longhurst JC, Kelly AR, Gonyea WJ, Mitchell JH. Chronic training with static and dynamic exercise: cardiovascular adaptation, and response to exercise. Circ Res. 1981;48(6 Pt 2):I171–I178. [PubMed] [Google Scholar]
- Luo Z, Ma L, Zhao Z, He H, Yang D, Feng X, et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1alpha upregulation in mice. Cell Res. 2012;22(3):551–564. doi: 10.1038/cr.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe Y, Gollisch KS, Holton L, Kim YB, Brandauer J, Fuj NL, 2nd, et al (2013) Exercise training-induced adaptations associated with increases in skeletal muscle glycogen content. FEBS J 280:916–926. PubMed PMID: 23206309 [DOI] [PMC free article] [PubMed]
- Maradana MR, Thomas R, O’Sullivan BJ (2013) Targeted delivery of curcumin for treating type 2 diabetes. Mol Nutr Food Res 57:1550–1556. PubMed PMID: 23495213 [DOI] [PubMed]
- Marini M, Abruzzo PM, Bolotta A, Veicsteinas A, Ferreri C. Aerobic training affects fatty acid composition of erythrocyte membranes. Lipids Health Dis. 2011;10:188. doi: 10.1186/1476-511X-10-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino JS, Peterson SJ, Li M, Vanella L, Sodhi K, Hill JW, et al. ApoA-1 mimetic restores adiponectin expression and insulin sensitivity independent of changes in body weight in female obese mice. Nutr Diabetes. 2012;2:e33. doi: 10.1038/nutd.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JP, Hasday JD, He JR, Montain SJ, Cheuvront SN, Sawka MN, et al. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol. 2008;294(1):R185–R191. doi: 10.1152/ajpregu.00532.2007. [DOI] [PubMed] [Google Scholar]
- McMurtry AL, Cho K, Young LJ, Nelson CF, Greenhalgh DG. Expression of HSP70 in healing wounds of diabetic and nondiabetic mice. J Surg Res. 1999;86(1):36–41. doi: 10.1006/jsre.1999.5700. [DOI] [PubMed] [Google Scholar]
- Mestril R, Chi SH, Sayen MR, O’Reilly K, Dillmann WH. Expression of inducible stress protein 70 in rat heart myogenic cells confers protection against simulated ischemia-induced injury. J Clin Investig. 1994;93(2):759–767. doi: 10.1172/JCI117030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestril R, Chi SH, Sayen MR, Dillmann WH. Isolation of a novel inducible rat heat-shock protein (HSP70) gene and its expression during ischaemia/hypoxia and heat shock. Biochem J. 1994;298(Pt 3):561–569. doi: 10.1042/bj2980561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami T, Sumida S, Ishibashi Y, Ohta S. Endurance exercise training inhibits activity of plasma GOT and liver caspase-3 of mice [correction of rats] exposed to stress by induction of heat shock protein 70. J Appl Physiol (1985) 2004;96(5):1776–1781. doi: 10.1152/japplphysiol.00795.2002. [DOI] [PubMed] [Google Scholar]
- Milne KJ, Wolff S, Noble EG. Myocardial accumulation and localization of the inducible 70-kDa heat shock protein, Hsp70, following exercise. J Appl Physiol (1985) 2012;113(6):853–860. doi: 10.1152/japplphysiol.00131.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino S, Kondo T, Sasaki K, Adachi H, Suico MA, Sekimoto E, et al. Mild electrical stimulation with heat shock ameliorates insulin resistance via enhanced insulin signaling. PloS One. 2008;3(12):e4068. doi: 10.1371/journal.pone.0004068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino-Koga S, Yano S, Kondo T, Shimauchi Y, Matsuyama S, Okamoto Y, et al. Insulin receptor activation through its accumulation in lipid rafts by mild electrical stress. J Cell Physiol. 2013;228(2):439–446. doi: 10.1002/jcp.24149. [DOI] [PubMed] [Google Scholar]
- Morton JP, Kayani AC, McArdle A, Drust B (2009) The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med 39(8):643–662. doi:10.2165/00007256-200939080-00003 [DOI] [PubMed]
- Muller S, Denet S, Candiloros H, Barrois R, Wiernsperger N, Donner M, et al. Action of metformin on erythrocyte membrane fluidity in vitro and in vivo. Eur J Pharmacol. 1997;337(1):103–110. doi: 10.1016/s0014-2999(97)01287-9. [DOI] [PubMed] [Google Scholar]
- Murshid A, Eguchi T, Calderwood SK. Stress proteins in aging and life span. Int J Hyperthermia. 2013;29(5):442–447. doi: 10.3109/02656736.2013.798873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Khajeali L, Esteghamati A, Khalilzadeh O, Asgarani F, et al. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones. 2010;15(6):959–964. doi: 10.1007/s12192-010-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhjavani M, Morteza A, Asgarani F, Khalilzadeh O, Ghazizadeh Z, Bathaie SZ, et al. The dual behavior of heat shock protein 70 and asymmetric dimethylarginine in relation to serum CRP levels in type 2 diabetes. Gene. 2012;498(1):107–111. doi: 10.1016/j.gene.2012.01.085. [DOI] [PubMed] [Google Scholar]
- Ndisang JF (2014) The heme oxygenase system selectively modulates proteins implicated in metabolism, oxidative stress and inflammation in spontaneously hypertensive rats. Curr Pharm Des (in press). PubMed PMID: 23978103 [DOI] [PubMed]
- Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, et al. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009;53(3):508–515. doi: 10.1161/HYPERTENSIONAHA.108.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa J, Nakai A, Higashi T, Tanabe M, Nomoto S, Matsuda K, et al. Reperfusion causes significant activation of heat shock transcription factor 1 in ischemic rat heart. Circulation. 1996;94(9):2185–2192. doi: 10.1161/01.cir.94.9.2185. [DOI] [PubMed] [Google Scholar]
- Nomura T, Li XH, Ogata H, Sakai K, Kondo T, Takano Y, et al. Suppressive effects of continuous low-dose-rate gamma irradiation on diabetic nephropathy in type II diabetes mellitus model mice. Radiat Res. 2011;176(3):356–365. doi: 10.1667/rr2559.1. [DOI] [PubMed] [Google Scholar]
- Nunn AV, Guy GW, Brodie JS, Bell JD. Inflammatory modulation of exercise salience: using hormesis to return to a healthy lifestyle. Nutr Metab (Lond) 2010;7:87. doi: 10.1186/1743-7075-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gorman DJ, Karlsson HK, McQuaid S, Yousif O, Rahman Y, Gasparro D, et al. Exercise training increases insulin-stimulated glucose disposal and GLUT4 (SLC2A4) protein content in patients with type 2 diabetes. Diabetologia. 2006;49(12):2983–2992. doi: 10.1007/s00125-006-0457-3. [DOI] [PubMed] [Google Scholar]
- Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA J Am Med Assoc. 2008;299(11):1261–1263. doi: 10.1001/jama.299.11.1259. [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14(11):1112–1120. doi: 10.1016/S1470-2045(13)70366-8. [DOI] [PubMed] [Google Scholar]
- Ostergard T, Andersen JL, Nyholm B, Lund S, Nair KS, Saltin B, et al. Impact of exercise training on insulin sensitivity, physical fitness, and muscle oxidative capacity in first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2006;290(5):E998–E1005. doi: 10.1152/ajpendo.00012.2005. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Pahlavani MA, Harris MD, Moore SA, Weindruch R, Richardson A. The expression of heat shock protein 70 decreases with age in lymphocytes from rats and rhesus monkeys. Exp Cell Res. 1995;218(1):310–318. doi: 10.1006/excr.1995.1160. [DOI] [PubMed] [Google Scholar]
- Pandita TK, Higashikubo R, Hunt CR. HSP70 and genomic stability. Cell Cycle. 2004;3(5):591–592. doi: 10.4161/cc.3.5.863. [DOI] [PubMed] [Google Scholar]
- Panossian A, Wikman G, Kaur P, Asea A. Adaptogens exert a stress-protective effect by modulation of expression of molecular chaperones. Phytomedicine. 2009;16(6–7):617–622. doi: 10.1016/j.phymed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 2001;20(3):446–456. doi: 10.1093/emboj/20.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli JR, Ropelle ER, Cintra DE, De Souza CT, da Silva AS, Moraes JC, et al. Acute exercise reverses aged-induced impairments in insulin signaling in rodent skeletal muscle. Mech Ageing Dev. 2010;131(5):323–329. doi: 10.1016/j.mad.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Perona JS, Vogler O, Sanchez-Dominguez JM, Montero E, Escriba PV, Ruiz-Gutierrez V. Consumption of virgin olive oil influences membrane lipid composition and regulates intracellular signaling in elderly adults with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci. 2007;62(3):256–263. doi: 10.1093/gerona/62.3.256. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol Off J Polish Physiol Soc. 2006;57(Suppl 10):43–51. [PubMed] [Google Scholar]
- Raciti GA, Iadicicco C, Ulianich L, Vind BF, Gaster M, Andreozzi F, et al. Glucosamine-induced endoplasmic reticulum stress affects GLUT4 expression via activating transcription factor 6 in rat and human skeletal muscle cells. Diabetologia. 2010;53(5):955–965. doi: 10.1007/s00125-010-1676-1. [DOI] [PubMed] [Google Scholar]
- Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574(Pt 1):33–39. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93(3):993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- Richter EA, Nielsen JN, Jorgensen SB, Frosig C, Birk JB, Wojtaszewski JF. Exercise signalling to glucose transport in skeletal muscle. Proc Nutr Soc. 2004;63(2):211–216. doi: 10.1079/PNS2004343. [DOI] [PubMed] [Google Scholar]
- Rincon M, Rudin E, Barzilai N. The insulin/IGF-1 signaling in mammals and its relevance to human longevity. Exp Gerontol. 2005;40(11):873–877. doi: 10.1016/j.exger.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Ringholm S, Bienso RS, Kiilerich K, Guadalupe-Grau A, Aachmann-Andersen NJ, Saltin B, et al. Bed rest reduces metabolic protein content and abolishes exercise-induced mRNA responses in human skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301(4):E649–E658. doi: 10.1152/ajpendo.00230.2011. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Krause J, Krause M, O’Hagan C, De Vito G, Boreham C, Murphy C, et al. Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones. 2012;17(3):293–302. doi: 10.1007/s12192-011-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropelle ER, Pauli JR, Prada PO, de Souza CT, Picardi PK, Faria MC et al (2006) Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation. J Physiol 577(Pt 3):997–1007, PubMed PMID: 17008371. Pubmed Central PMCID: 1890392 [DOI] [PMC free article] [PubMed]
- Russell AP, Foletta VC, Snow RJ, Wadley GD (2013) Skeletal muscle mitochondria: a major player in exercise, health and disease. Biochim Biophys Acta. doi:10.1016/j.bbagen.2013.11.016 [DOI] [PubMed]
- Sahin K, Orhan C, Tuzcu Z, Tuzcu M, Sahin N. Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food Chem Toxicol. 2012;50(11):4035–4041. doi: 10.1016/j.fct.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goodyear LJ. Invited review: intracellular signaling in contracting skeletal muscle. J Appl Physiol. 2002;93(1):369–383. doi: 10.1152/japplphysiol.00167.2002. [DOI] [PubMed] [Google Scholar]
- Salo DC, Donovan CM, Davies KJ. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic Biol Med. 1991;11(3):239–246. doi: 10.1016/0891-5849(91)90119-n. [DOI] [PubMed] [Google Scholar]
- Salway KD, Gallagher EJ, Page MM, Stuart JA. Higher levels of heat shock proteins in longer-lived mammals and birds. Mech Ageing Dev. 2011;132(6–7):287–297. doi: 10.1016/j.mad.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Schneider SH, Amorosa LF, Khachadurian AK, Ruderman NB. Studies on the mechanism of improved glucose control during regular exercise in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1984;26(5):355–360. doi: 10.1007/BF00266036. [DOI] [PubMed] [Google Scholar]
- Seo HR, Chung HY, Lee YJ, Bae S, Lee SJ, Lee YS. p27Cip/Kip is involved in hsp25 or inducible hsp70 mediated adaptive response by low dose radiation. J Radiat Res. 2006;47(1):83–90. doi: 10.1269/jrr.47.83. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Bharti S, Ojha S, Bhatia J, Kumar N, Ray R, et al. Up-regulation of PPARgamma, heat shock protein-27 and -72 by naringin attenuates insulin resistance, beta-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br J Nutr. 2011;106(11):1713–1723. doi: 10.1017/S000711451100225X. [DOI] [PubMed] [Google Scholar]
- Sheng T, Yang K (2008) Adiponectin and its association with insulin resistance and type 2 diabetes. J Genet Genom 35(6):321–326. doi:10.1016/S1673-8527(08)60047-8 [DOI] [PubMed]
- Shinohara T, Takahashi N, Ooie T, Hara M, Shigematsu S, Nakagawa M, et al. Phosphatidylinositol 3-kinase-dependent activation of akt, an essential signal for hyperthermia-induced heat-shock protein 72, is attenuated in streptozotocin-induced diabetic heart. Diabetes. 2006;55(5):1307–1315. doi: 10.2337/db05-0266. [DOI] [PubMed] [Google Scholar]
- Silva KC, Rosales MA, Hamassaki DE, Saito KC, Faria AM, Ribeiro PA, et al. Green tea is neuroprotective in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(2):1325–1336. doi: 10.1167/iovs.12-10647. [DOI] [PubMed] [Google Scholar]
- Simar D, Malatesta D, Koechlin C, Cristol JP, Vendrell JP, Caillaud C. Effect of age on Hsp72 expression in leukocytes of healthy active people. Exp Gerontol. 2004;39(10):1467–1474. doi: 10.1016/j.exger.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Simar D, Jacques A, Caillaud C. Heat shock proteins induction reduces stress kinases activation, potentially improving insulin signalling in monocytes from obese subjects. Cell Stress Chaperones. 2012;17(5):615–621. doi: 10.1007/s12192-012-0336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton KD, Wischmeyer PE. Oral glutamine enhances heat shock protein expression and improves survival following hyperthermia. Shock. 2006;25(3):295–299. doi: 10.1097/01.shk.0000196548.10634.02. [DOI] [PubMed] [Google Scholar]
- Song XM, Kawano Y, Krook A, Ryder JW, Efendic S, Roth RA, et al. Muscle fiber type-specific defects in insulin signal transduction to glucose transport in diabetic GK rats. Diabetes. 1999;48(3):664–670. doi: 10.2337/diabetes.48.3.664. [DOI] [PubMed] [Google Scholar]
- Strub GM, Depcrynski A, Elmore LW, Holt SE. Recovery from stress is a function of age and telomere length. Cell Stress Chaperones. 2008;13(4):475–482. doi: 10.1007/s12192-008-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart CA, McCurry MP, Marino A, South MA, Howell ME, Layne AS, et al. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J Clin Endocrinol Metab. 2013;98(5):2027–2036. doi: 10.1210/jc.2012-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Pei Y, Zhou G. When aging-onset diabetes is coming across with Alzheimer disease: comparable pathogenesis and therapy. Exp Gerontol. 2013;48(8):744–750. doi: 10.1016/j.exger.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282(6):E1191–E1196. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- Tanti JF, Ceppo F, Jager J, Berthou F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front Endocrinol. 2012;3:181. doi: 10.3389/fendo.2012.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TeixeiradeLemos E, Reis F, Baptista S, Pinto R, Sepodes B, Vala H, et al. Exercise training decreases proinflammatory profile in Zucker diabetic (type 2) fatty rats. Nutrition. 2009;25(3):330–339. doi: 10.1016/j.nut.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Teixeira-Lemos E, Nunes S, Teixeira F, Reis F. Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc Diabetol. 2011;10:12. doi: 10.1186/1475-2840-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin BW, Uchakin PN, Leeper-Woodford SK. Insulin secretion and sensitivity in space flight: diabetogenic effects. Nutrition. 2002;18(10):842–848. doi: 10.1016/s0899-9007(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Török Z, Tsvetkova NM, Balogh G, Horvath I, Nagy E, Penzes Z, et al. Heat shock protein coinducers with no effect on protein denaturation specifically modulate the membrane lipid phase. Proc Natl Acad Sci U S A. 2003;100(6):3131–3136. doi: 10.1073/pnas.0438003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török Z, Crul T, Maresca B, Schütz GJ, Viana F, Dindia L, Piotto S, Brameshuber M, Balogh G, Péter M, Porta A, Trapani A, Gombos I, Glatz A, Gungor B, Peksel B, Vigh Jr L, Csoboz B, Horváth I, Vijayan MM, Hooper PL, Harwood J, Vigh L (2013) Plasma membranes as heat stress sensors: from lipid-controlled molecular switches to therapeutic applications. Biochim Biophys Acta. doi:10.1016/j.bbamem.2013.12.015 [DOI] [PubMed]
- Touchberry CD, Gupte AA, Bomhoff GL, Graham ZA, Geiger PC, Gallagher PM. Acute heat stress prior to downhill running may enhance skeletal muscle remodeling. Cell Stress Chaperones. 2012;17(6):693–705. doi: 10.1007/s12192-012-0343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuei AC, Martinus RD. Metformin induced expression of Hsp60 in human THP-1 monocyte cells. Cell Stress Chaperones. 2012;17(1):23–28. doi: 10.1007/s12192-011-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanetakou IP, Katsilambros NL, Benetos A, Mikhailidis DP, Perrea DN. “Is obesity linked to aging?”: adipose tissue and the role of telomeres. Ageing Res Rev. 2012;11(2):220–229. doi: 10.1016/j.arr.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Ugurlucan M, Erer D, Karatepe O, Ziyade S, Haholu A, Gungor Ugurlucan F, et al. Glutamine enhances the heat shock protein 70 expression as a cardioprotective mechanism in left heart tissues in the presence of diabetes mellitus. Expert Opin Ther Targets. 2010;14(11):1143–1156. doi: 10.1517/14728222.2010.521500. [DOI] [PubMed] [Google Scholar]
- Vigh L, Torok Z, Balogh G, Glatz A, Piotto S, Horvath I. Membrane-regulated stress response: a theoretical and practical approach. Adv Exp Med Biol. 2007;594:114–131. doi: 10.1007/978-0-387-39975-1_11. [DOI] [PubMed] [Google Scholar]
- Vigh L, Horvath I, Maresca B, Harwood JL. Can the stress protein response be controlled by ‘membrane-lipid therapy’? Trends Biochem Sci. 2007;32(8):357–363. doi: 10.1016/j.tibs.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Vígh L, Literáti PN, Horváth I, Török Z, Balogh G, Glatz A, Kovács E, Boros I, Ferdinándy P, Farkas B, Jaszlits L, Jednákovits A, Korányi L, Maresca B (1997) Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat Med 3:1150–1154 [DOI] [PubMed]
- Volloch V, Gabai VL, Rits S, Force T, Sherman MY. HSP72 can protect cells from heat-induced apoptosis by accelerating the inactivation of stress kinase JNK. Cell Stress Chaperones. 2000;5(2):139–147. doi: 10.1379/1466-1268(2000)005<0139:hcpcfh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rong X, Li W, Yang Y, Yamahara J, Li Y. Rhodiola crenulata root ameliorates derangements of glucose and lipid metabolism in a rat model of the metabolic syndrome and type 2 diabetes. J Ethnopharmacol. 2012;142(3):782–788. doi: 10.1016/j.jep.2012.05.063. [DOI] [PubMed] [Google Scholar]
- Weijers RN. Lipid composition of cell membranes and its relevance in type 2 diabetes mellitus. Curr Diabetes Rev. 2012;8(5):390–400. doi: 10.2174/157339912802083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernstedt P, Sjostedt C, Ekman I, Du H, Thuomas KA, Areskog NH, et al. Adaptation of cardiac morphology and function to endurance and strength training. A comparative study using MR imaging and echocardiography in males and females. Scand J Med Sci Sports. 2002;12(1):17–25. doi: 10.1034/j.1600-0838.2002.120104.x. [DOI] [PubMed] [Google Scholar]
- Whitley D, Goldberg SP, Jordan WD. Heat shock proteins: a review of the molecular chaperones. J Vasc Surg Off Publ Soc Vasc Surg Int Soc Cardiovasc Surg N Am Chapter. 1999;29(4):748–751. doi: 10.1016/s0741-5214(99)70329-0. [DOI] [PubMed] [Google Scholar]
- Wiernsperger NF. Membrane physiology as a basis for the cellular effects of metformin in insulin resistance and diabetes. Diabetes Metab. 1999;25(2):110–127. [PubMed] [Google Scholar]
- Wieten L, van der Zee R, Spiering R, Wagenaar-Hilbers J, van Kooten P, Broere F, et al. A novel heat-shock protein coinducer boosts stress protein Hsp70 to activate T cell regulation of inflammation in autoimmune arthritis. Arthritis Rheum. 2010;62(4):1026–1035. doi: 10.1002/art.27344. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen JN, Richter EA. Invited review: effect of acute exercise on insulin signaling and action in humans. J Appl Physiol. 2002;93(1):384–392. doi: 10.1152/japplphysiol.00043.2002. [DOI] [PubMed] [Google Scholar]
- Xu X, Wang P, Zhao Z, Cao T, He H, Luo Z, et al. Activation of transient receptor potential vanilloid 1 by dietary capsaicin delays the onset of stroke in stroke-prone spontaneously hypertensive rats. Stroke J Cereb Circ. 2011;42(11):3245–3251. doi: 10.1161/STROKEAHA.111.618306. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Fukumoto K, Murakami T, Harada S, Hosono R, Wadhwa R, et al. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett. 2002;516(1–3):53–57. doi: 10.1016/s0014-5793(02)02470-5. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Peng J, Yin K, Wang JH. Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res. 2011;55(1):150–165. doi: 10.1002/mnfr.201000414. [DOI] [PubMed] [Google Scholar]
- Zanuso S, Jimenez A, Pugliese G, Corigliano G, Balducci S. Exercise for the management of type 2 diabetes: a review of the evidence. Acta Diabetol. 2010;47(1):15–22. doi: 10.1007/s00592-009-0126-3. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Luo Z, Ma S, Liu D. TRP channels and their implications in metabolic diseases. Pflugers Arch Eur J Physiol. 2011;461(2):211–223. doi: 10.1007/s00424-010-0902-5. [DOI] [PubMed] [Google Scholar]
- Zierath JR. Invited review: exercise training-induced changes in insulin signaling in skeletal muscle. J Appl Physiol. 2002;93(2):773–781. doi: 10.1152/japplphysiol.00126.2002. [DOI] [PubMed] [Google Scholar]
- Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med. 2000;6(8):924–928. doi: 10.1038/78693. [DOI] [PubMed] [Google Scholar]