Abstract
An elevated heat-shock protein (HSP) content protects cells and tissues, including skeletal muscles, from certain stressors. We determined if heat stress and the elevated HSP content that results is correlated with protection of contractile characteristics of isolated fast and slow skeletal muscles when contracting at elevated temperatures. To elevate muscle HSP content, one hindlimb of Sprague–Dawley rats (21–28 days old, 70–90 g) was subjected to a 15 min 42 °C heat-stress. Twenty-four hours later, both extensor digitorum longus (EDL) and soleus muscles were removed, mounted in either 20 °C or 42 °C Krebs-Ringer solution, and electrically stimulated. Controls consisted of the same muscles from the contra-lateral (non-stressed) hindlimbs as well as muscles from other (unstressed) animals. Isolated muscles were twitched and brought to tetanus every 5 min for 30 min. As expected, HSP content was elevated in muscles from the heat-stressed limbs when compared with controls. Regardless of prior treatment, both EDL and soleus twitch tensions were lower at 42 °C when compared with 20 °C. In addition, when incubated at 42 °C, both muscles showed a drop in twitch tension between 5 and 30 min. For tetanic tension, both muscles also showed an increase in tension between 5 and 30 min when stimulated at 20 °C regardless of treatment but when stimulated at 42 °C no change was observed. No protective effect of an elevated HSP content was observed for either muscle. In conclusion, although heat stress caused an elevation in HSP content, no protective effects were conferred to isolated contracting muscles.
Keywords: Heat stress, Hsp25, Hsp72, Skeletal muscle, Contraction, Rat, In vitro
Introduction
The heat released during skeletal muscle contraction can increase both body and tissue temperatures such that muscle temperatures of 44 °C have been reported in trained rats run to exhaustion (Brooks et al. 1971). Not surprisingly, elevated temperatures (>40 °C) are known to result in an early onset of fatigue (for review, see Allen 2009). Elevated temperatures are also known to cause protein unfolding, denaturation, and aggregation which can impair muscle enzyme or contractile protein function. In response to temperature elevations and other protein damaging stressors, cells respond by rapidly synthesizing protective proteins known as “heat stress” or “heat shock” proteins (HSPs; Welch 1987; Welch 1992). Cells and tissues with an elevated HSP content are better able to tolerate stressful conditions (Li et al. 1983; Li et al. 1991), and this is thought to be the result of HSPs acting as molecular chaperones. It follows that an elevated HSP content might be beneficial to skeletal muscle during contractions at elevated temperatures.
The protective effects of heat stress and HSPs in cardiac muscle have been extensively studied (for reviews, see Latchman 2001; Noble et al. 2008), and there is considerable support to suggest an elevated HSP content by heat stress or other means confers a cross-tolerance protection to cardiac tissue against ischemic stressors (Karmazyn et al. 1990; Currie et al. 1993; Kawana et al. 2000). While a considerable number of studies have investigated heat stress and HSPs in cardiac tissue, less attention has focused on heat-stressed skeletal muscle (Garramone et al. 1994; Lepore et al. 2000). More importantly, very few studies have provided any evidence for an HSP-mediated protection to skeletal muscle during contraction. In the seminal paper, Thomas and Noble (1999) examined the effects of a prior heat stress and elevation of HSPs on the contractility of the rat plantaris muscle and observed no improvement in post fatigue force recovery. Other studies have also showed no protection to contracting skeletal muscles (Nosek et al. 2000). However, more recent studies suggest that HSPs may be beneficial to skeletal muscle under certain conditions of stress (McArdle et al. 2004; Kayani et al. 2008; Touchberry et al. 2012; Liu et al. 2013). For example, a heat-shock pretreatment and/or the ensuing Hsp72 enhanced total protein and aided protection against muscle damage (Touchberry et al. 2012). Studies using transgenic overexpression of Hsp72 have also suggested HSPs to be beneficial to contracting skeletal muscle (Liu et al. 2013).
In view of the fact that elevated muscle temperatures have detrimental effects on skeletal muscle contractile properties, coupled with the knowledge that HSPs provide a cross-tolerance effect to cardiac and skeletal muscle, it was of interest to determine if elevated skeletal muscle HSP content would aid skeletal muscles contracting at an elevated temperatures. Thus, the purpose of this study was to determine whether a prior heat stress and the resultant increase in HSP content altered the contractile characteristics of isolated fast (extensor digitorum longus) and slow (soleus) muscles when stimulated to contract at 42 °C. It was hypothesized that muscles with an increased HSP content would show better contractile characteristics at 42 °C when compared with control muscles stimulated at the same temperature.
Methods
Animals and heat stress
Male juvenile Sprague–Dawley rats (n = 40), 21–28 days old, (70–84 g) were used in this study. Animals were maintained on a 12 h dark/light cycle, housed at 20 ± 1 °C, 50 % relative humidity and provided with food and water ad libitum. The Animal Care Committee of the University of Toronto approved all experimental procedures. Animals were split into a one limb heat stress group (1LHS) and a control (C) group. One hindlimb from each animal in the one-limb heat-stress group was subjected to a heat stress (42 °C for 15 min), while the contra-lateral (1LC; contra-lateral control) limb served as a control. Prior to heat stress, the animals were anesthetized with sodium pentobarbital (65 mg/kg, ip) and rectal and intramuscular temperatures (both the heat stressed and the contra-lateral control limbs) monitored using a TST202A rectal probe (Biopac Systems Inc., Goleta, Ca, USA) and MT-23/3 needle thermisters (Physiotemp, Clinton, NJ, USA), respectively. Once anesthetized, animals were placed in a prone position on a temperature-controlled heating unit and the right hindlimb placed in a heating bath (model no. 165050, Radnoti LLC, Mondrovia, Ca, USA) maintained at 45 °C to begin the heat stress. Heat stress began once intramuscular temperature reached 42 °C and was maintained for 15 min. Following the heat stress, animals were allowed to recover at room temperature for 24 h. Isolated muscles from the two groups were equally divided into a 20 °C (C20, n = 10; 1LC20, n = 10; 1LHS20, n = 10) group and a 42 °C (C42, n = 10; 1LC42, n = 10; 1LHS42, n = 10) group.
In vitro tissue preparation and muscle stimulation
The soleus and extensor digitorum longus (EDL) muscles were removed in random order from both [heat-stressed and non-heat-stressed (contra-lateral)] limbs of the one-limb heat-stressed groups, and from one limb from animals from the control group. Prior to removal, ligatures were tied to the tendons at both ends of the muscle. After a muscle had been removed from a limb, the incision was covered and periodically moistened with 0.85 % w/v saline solution. Each muscle was mounted in an organ bath (Radnoti, model no. 165050) and equilibrated for 1–2 min at room temperature in a Krebs–Ringer bicarbonate solution (137 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1 mM NaH2PO4, and 24 mM NaHCO3) continuously perfused with 95 % O2–5 % CO2 gas and thermostatically maintained at 20 °C or 42 °C. A ligature was tied to a fixed point at the bottom of the bath and to a force transducer (Biopac, TSD105A) above the bath.
The muscle was stimulated directly by an electrical impulse traveling between two platinum electrodes (Radnoti, model no. 158814), using a 0.2 ms square wave pulse of supra-maximal voltage. Following the determination of optimal muscle length (Lo), peak twitch tension (Pt), half force relaxation time (FRT1/2), maximum rate of tension development (dP/dt), and maximum tetanic tension (Po) were measured at 5, 10, 15, 20, 25, and 30 min post-equilibration using a Biopac Data Acquisition system and Acknowledge software. The stimulation frequencies used to elicit Po for this experiment were as used by Segal and Faulkner (1985). Following the completion of the stimulation protocol, muscles were removed from the bath and freeze-dried. Measurements involving the force of contraction were normalized to the dry weight of the muscle.
Polyacrylamide gel electrophoresis and Western blotting
The soleus and EDL from five animals subjected to the heat stress were removed after 24 h of recovery and frozen. They were homogenized in 600 mM NaCl, 15 mM Tris, pH 7.5, and protein concentration was determined by the method of Lowry et al. (1951). One-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed according to the method described by Laemmli (1970), except that the separating gel (0.15 × 4.5 × 8 cm) consisted of a 5–15 % polyacrylamide gradient. Following electrophoretic separation, proteins were transferred to nitrocellulose membranes (0.22 um pore size, Bio-Rad Laboratories) using the Bio-Rad mini-protean II gel transfer system as described by Towbin et al. (1979). Following protein transfer, blots were reacted with a polyclonal antibody specific for Hsp72 (ADI-SPA-812, ENZO, USA) and Hsp25 ADI-SPP-715, ENZO, USA), diluted 1:1,000 in TTBS with 2 % non-fat dried milk as previously described (Locke 2008). To determine non-specific binding, duplicate gels were run, transferred, and reacted, but the primary antibody was omitted. Immunoblots were scanned using an Agfa Arcus II scanner and quantification of bands performed by Kodak 1D Image Analysis Software (Kodak Scientific Imaging Systems, New Haven, CT). Standard curves were constructed to assure linearity.
Statistics
Data from the muscle stimulation protocol for each muscle type from the six groups were evaluated using a two-way ANOVA with a Tukey’s post hoc test, at each time period. Data from the Western blot analysis for the two groups were evaluated using a two-sample t test. A significance level of p < 0.05 was applied for all statistical comparisons.
Results
Physical characteristics and temperature responses
Mean body mass ranged from 72.5–84.4 g, with no significant difference between any of the groups of animals studied. No significant differences were observed between any of the muscle groups for either wet mass or dry mass (data not shown). Rectal temperature of heat-stressed animals was maintained at 36.0 ± 0.4 °C while intra-muscular temperature of the heat-stressed limb was raised to 42.5 ± 0.1 °C (during the heat stress treatment while the contra-lateral limb remained at 34.6 ± 0.4 °C.
HSP expression following heat stress
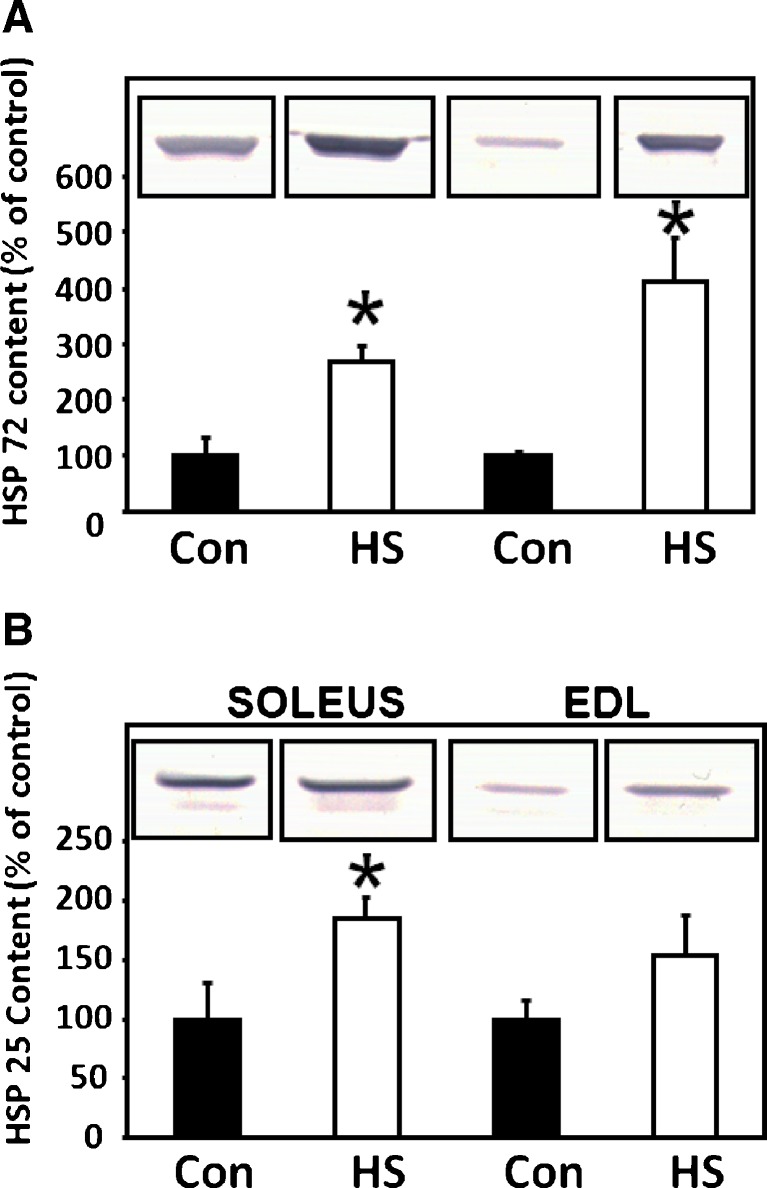
To determine if the one-limb heat stress significantly increased levels of HSP content in the soleus and EDL muscles, Hsp72 and Hsp25 content in the soleus and EDL muscles from both the heat-stressed and contra-lateral control limbs was assessed in a separate subset of animals (n = 5) by Western blot analyses. Following densitometric scanning and quantification of bands on the blots, a significant (p < 0.05) increase in Hsp72 content was observed in both the heat-stressed soleus and EDL when compared with contra-lateral control muscles. However, Hsp25 was significantly (p < 0.05) increased only in the soleus following heat stress and not in the EDL. Figure 1 shows representative Western blots as well as the quantification of Hsp72 and Hsp25 bands from the EDL and soleus muscles, respectively.
Fig. 1.
a Heat stress increases Hsp72 in soleus and EDL muscles: Hsp72 content is presented graphically with a representative picture of a portion of a Western blot superimposed on the graph. Solid bars are from controls while open bars are from muscles 24 h after a 15-min 42 °C heat stress. Statistical analysis revealed that one-limb heat stress significantly increased Hsp72 content in the soleus and EDL. b Heat stress increases Hsp25 in soleus muscles: Hsp25 content is presented graphically with a representative picture of a portion of a Western blot superimposed on the graph. Solid bars are from controls while open bars are from muscles 24 h after a 15-min 42°C heat stress. Statistical analysis revealed that one-limb heat stress significantly increased Hsp25 content in the soleus and but not the EDL. Error bars denote SEM. *Denotes significant difference (p < 0.05) compared with control
Twitch tension and rate of tension development
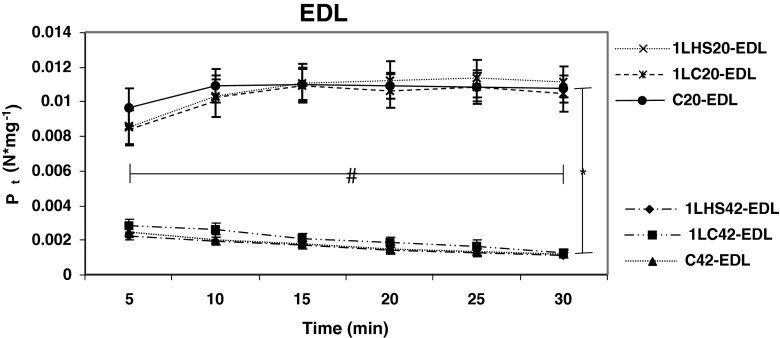
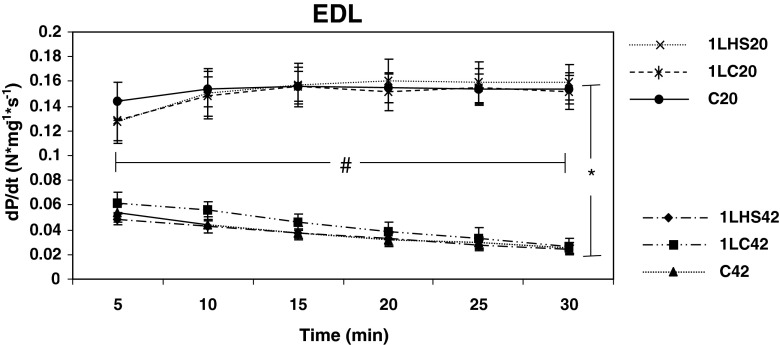
Twitches from muscles stimulated at 5-min intervals were analyzed to determine peak twitch tension (Pt), and maximum rate of tension development (dP/dt) for 30 min. EDL muscles stimulated at 20 °C showed a significantly (p < 0.05) higher Pt (Fig. 2) and dP/dt (Fig. 3) than muscles stimulated at 42 °C at all time points examined. EDL muscles stimulated at 20 °C also showed significantly (p < 0.05) higher Pt measurements at 30 min when compared with 5 min, while EDL muscles stimulated at 42 °C were significantly (p < 0.05) lower at 30 min than at 5 min. Similar results were observed with dP/dt, except for the C20 group where no significant difference was observed between 5 and 30 min.
Fig. 2.
Peak EDL twitch tension over 30 min at 20 °C and 42 °C. Peak twitch tension (P t) values were obtained from muscles twitched at 5-min intervals for 30 min. Significant differences (denoted by asterisk) were observed between groups stimulated at 20 °C and 42 °C. Statistical differences (denoted by number sign) were also observed between the 5- and 30-min values in all groups. Significant differences were also observed between temperatures for all time points. Error bars denote SEM
Fig. 3.
Rates of tension development for EDL over 30 min at 20 °C and 42 °C. Rates of tension development (dP/dt) were calculated from EDL muscles twitched at 5-min intervals. Significant differences were observed between groups stimulated at 20 °C and 42 °C. Statistical differences were also observed between the 5- and 30-min values in all groups (except C20) with the 20 group showing an increase and the 42 °C groups showing a decrease. No significant difference was detected between 5 and 30 min for the C20 group. Error bars denote SEM. An asterisk denotes a significant difference (p < 0.05) between temperature groups while number sign denotes differences between 5 and 30 min with groups
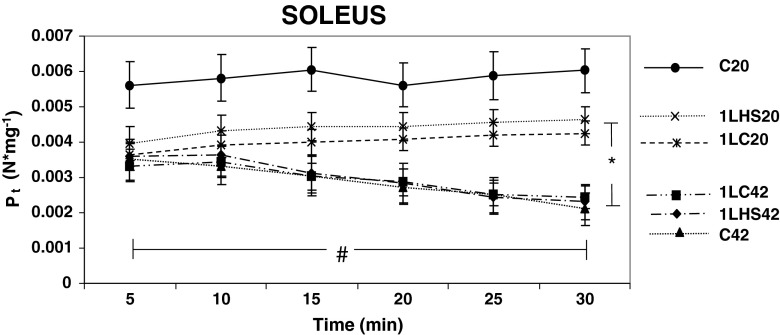
Soleus muscles simulated at 20 °C or 42 °C (Fig. 4) showed that all groups, except the C20 group, demonstrated significant (p < 0.05) differences between the 5 and 30 min times. When compared with the values at 5 min 1LC42, 1LHS42, and C42 exhibited a significantly (p < 0.05) decreased Pt at 30 min, while 1LHS20 and 1LC20 showed a significantly increased Pt at 30 min. The dP/dt for soleus muscles showed no significant differences between any groups at any time during the first 30 min of the protocol at both temperatures (Fig. 5). However, when 5 and 30 min were compared, all groups stimulated at 42 °C showed a significantly (p < 0.05) decreased dP/dt at 30 min while 1LHS20 showed an increased dP/dt at 30 min.
Fig. 4.
Peak soleus twitch tensions between 5 and 30 min at 20 °C and 42 °C. Twitch tension (P t) values were obtained from muscles twitched at 5-min intervals. Significant differences were observed for all groups between 5 and 30 min except for the C20 group. At 30 min, significant differences were detected between muscles stimulated at 20 °C or 42 °C temperatures. Error bars denote SEM. An asterisk denotes significant difference (p < 0.05) between temperature groups while number sign denotes differences between 5 and 30 min with groups
Fig. 5.
Soleus muscles rates of tension development at 20 °C and 42 °C. Rates of tension development (dP/dt) were calculated at 5-min intervals from soleus twitch tensions. At 5 min, no significant differences were observed between groups stimulated at 20 °C and 42 °C, but by 30 min significant decreases were observed between the 5 and 30 min values in all groups stimulated at 42 °C. Error bars denote SEM. A number sign denotes significant difference (p < 0.05) between 5 and 30 min with groups
Relaxation time
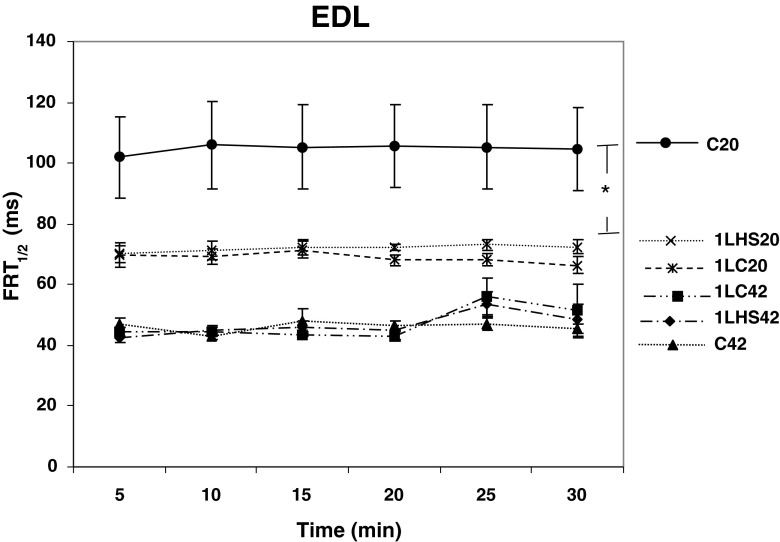
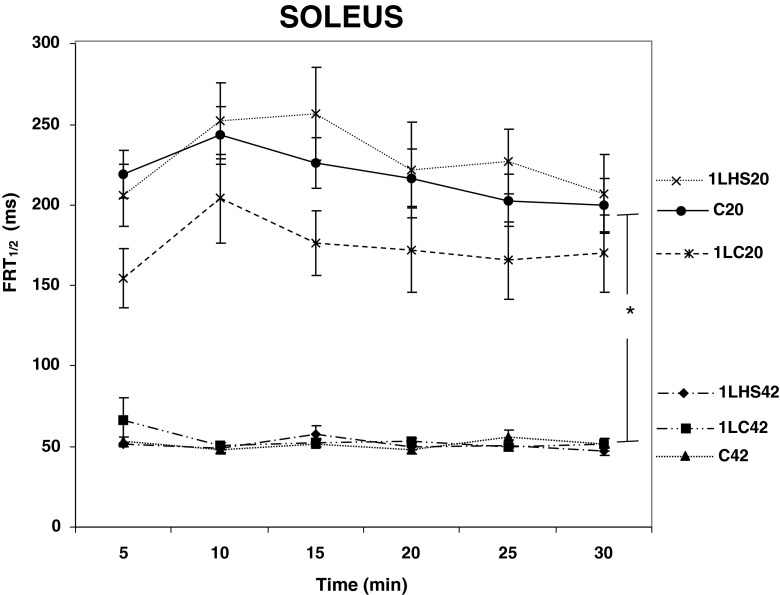
In order to study the effects of a prior heat stress on the Ca2+ re-uptake ability, half force relaxation time (FRT1/2) was measured. One-way ANOVA analyses showed no significant differences between the EDL muscles stimulated at 42 °C (Fig. 6). Interestingly, significant (p < 0.05) differences between EDL C20 and all other EDL groups were detected at all time points. In contrast, all soleus muscle groups stimulated at 20 °C had significantly (p < 0.05) greater times for FRT1/2 when compared with the groups stimulated at 42 °C (Fig. 7). There were no significant differences in FRT1/2 between 5 and 30 min for any of the soleus muscles groups examined.
Fig. 6.
Half force relaxation time for EDL at 20 °C and 42 °C. Half force relaxation time (FRT 1/2) was calculated at 5-min intervals from twitch tensions. At 5 min and thereafter, no significant differences were observed between groups stimulated at either 20 °C or 42 °C with the exception of the C20 group which was significantly different from all other groups at all time points. Error bars denote SEM. An asterisk denotes significant difference (p < 0.05) between temperature groups
Fig. 7.
Half force relaxation time for soleus muscles at 20 °C and 42 °C. Half force relaxation time (FRT 1/2) was calculated at 5-min intervals from twitch tensions. At 5 min and thereafter, significant differences were observed between groups stimulated at 20 °C and 42 °C. These differences remained throughout the 30 min. No significant differences between 5 and 30 min were detected for any group. Error bars denote SEM. An asterisk denotes significant difference (p < 0.05) between temperature groups
Tetanic tension
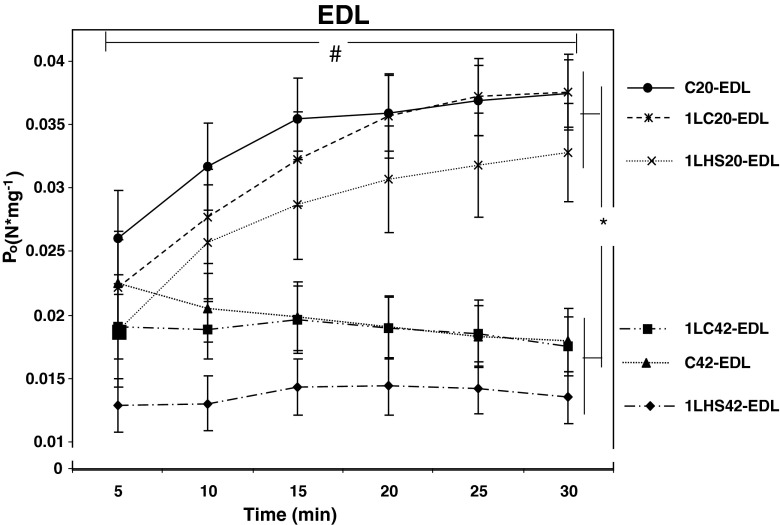
Following each twitch assessment (5-min intervals), muscles were also subjected to a maximum tetanic contraction (Po). At 5 min, there were no significant differences in Po between the EDL muscle groups (Fig. 8). However, as time increased, the EDL muscle groups stimulated at 20 °C gradually increased Po such that, at 30 min, all 20 °C stimulated muscles were significantly (p < 0.05) greater from all 42 °C stimulated EDL muscles. In addition, a significant (p < 0.05) increase in EDL Po between 5 and 30 min was also detected for the all groups stimulated at 20 °C. In contrast, Po for the EDL muscle groups stimulated at 42 °C only maintained their values (Fig. 8).
Fig. 8.
EDL muscle tetanic tension at 20 °C and 42 °C. Tetanic tension (P o) values were obtained at 5-min intervals. No significant differences were observed between groups stimulated at 20 °C and 42 °C at 5 min, but by 30 min, statistical differences were observed between the 5 and 30 min values and between groups stimulated at either 20 °C or 42 °C temperatures. Error bars denote SEM. An asterisk denotes a significant difference (p < 0.05) between temperature groups while number sign denotes differences between 5 and 30 min with groups
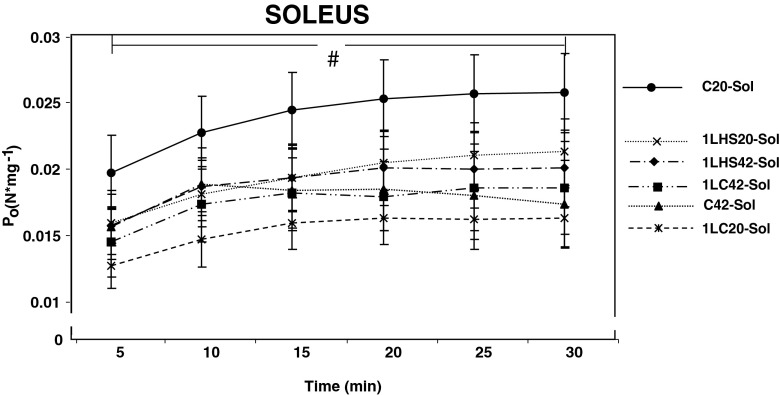
For soleus muscles, there were no significant differences between groups for Po during the first 30 min of the stimulation protocol (Fig. 9). However, statistically significant (p < 0.05) differences in Po were observed between 5 and 30 min in all muscle groups stimulated at 20 °C, but not for any muscle group stimulated at 42 °C.
Fig. 9.
Soleus muscle tetanic tension at 20 °C and 42 °C. Tetanic tension (P o) values were obtained at 5-min intervals. No significant differences were observed between groups stimulated at 20 °C and 42 °C at 5 min. At 30 min, statistical differences were observed from the five values for all groups stimulated at 20 °C. Groups stimulated at 42 °C remained unchanged. Error bars denote SEM. A number sign denotes significant difference (p < 0.05) between 5 and 30 min with groups
Discussion
An elevated skeletal muscle HSP content by heat stress or other means has been shown to be beneficial to certain cells and tissues during a subsequent exposure to certain stressors (Li et al. 1983; Currie et al. 1993; Garramone et al. 1994; Kawana et al. 2000). The purpose of this paper was to investigate whether a single-limb heat stress, 24 h prior, would alter the contractile characteristics of isolated fast and slow skeletal muscles stimulated at two temperatures. It was hypothesized that an increased HSP content following the heat stress would aid muscles during contraction at 42 °C. In the rat, a temperature elevation to 42 °C for 15 min has been shown to elicit a significant heat stress response in skeletal muscle (Locke and Tanguay 1996, Lepore et al. 2000; Bryantsev et al. 2002; Touchberry et al. 2012). Our one-limb heat stress also increased Hsp72 and Hsp25 content in the soleus muscle and Hsp72 in the EDL muscle when compared with contra-lateral controls. Despite these observed increases in muscle HSP content while being exposed to the same hormonal milieu, no protection or beneficial effects on muscle contractile properties were observed for either muscle at 20 °C or 42 °C. The exact reason for the lack of protection remains unknown and is likely related to several factors.
HSPs are thought to act as molecular chaperones that allow proteostasis to be maintained during exposures to protein damaging stressors. However, the exact mechanism(s) of HSP protection remain to be clarified. Since each cell type expresses a unique profile of proteins that can vary in function and location, it may be that some stressors result in protein damage at specific cell locations. For example, in striated muscle, the sarcolemma or cytoskeleton may be damaged by one type of stressor, while other stressors may specifically damage contractile proteins and metabolic enzymes, or impair protein synthesis. Although the common component for activation of the cellular stress response is thought to be damaged or denatured proteins, the specific locations and functions of susceptible proteins and their specific stressors have not yet been determined. Given that the stressors to the isolated muscle in the present study were temperature and/or metabolic, it suggests that an elevated Hsp25 and −72 may not confer protection against these stressors. In support of this, most studies demonstrating striated muscle HSP-mediated protection have used models of cross-tolerance where exposure to “stressor A” is used to protect against “stressor B” and not against the original “stressor A.” For example, an elevated HSP content from heat stress/shock appears to protect cardiac and skeletal muscle against an ischemic stress. However, there are no studies examining striated muscle protection against a temperature stress, suggesting HSPs may not provide protection against all stressors. In agreement with this, Nosek et al. (2000) used transgenic mice that over-expressed Hsp72 and reported no protection against intermittent fatiguing stimulation for EDL and soleus muscles. As mentioned previously, Thomas and Noble (1999) used heat shock and also found no support for any HSP-mediated protective effect for force recovery following fatigue. Whether an HSP-mediated protection works primarily via cross-tolerance or confers protection against only certain stressors remains to be determined.
In the present study, the length of recovery (24 h) following heat stress and the temperatures selected for muscle stimulation may also have been important factors for the lack of protection observed. While the initial heat stress clearly elevated HSP content, the function and location of the HSPs in muscle remains unknown. The newly synthesized HSPs from the initial heat stress might still be occupied within the muscle fibres and thus unavailable to provide protection during the subsequent isolated muscle stimulations. Larkins et al. (2012)) showed HSPs are present at much lower density in rat EDL and soleus muscles than their potential binding sites and that heat stress caused a tight, yet differential, HSP binding to membranous and cytoskeletal targets. Thus, while an increased HSP content was observed, the amount of “available” versus “non-available” HSP, as well as the intra-cellular location of the HSPs remains unknown. In retrospect, a longer recovery time might also have allowed the HSPs to complete their heat stress-related repair functions and become available allowing the muscle to cope with the subsequent contraction-related stressors.
Temperature can drastically alter contractile properties (Ranatunga and Wylie 1983; Harrison and Bers 1989; Coupland and Ranatunga 2003) and the level of stress (42 °C) used in the present study may have been too severe. All muscles stimulated at 42 °C experienced a decrease in Pt over the 30 min. In contrast, most EDL and soleus groups stimulated at 20 °C showed a statistically significant increase in Pt or no difference over this time period. The effects of temperature on Po also differed. Although Po measured at 5 min for all groups was similar between the two temperatures, the muscles stimulated at 20 °C showed an increase in force production while the muscles stimulated at 42 °C showed no change in force production over the 30 min. Using a similar model, Segal and Faulkner (1985) suggested that, at elevated temperatures, muscle fibers in the core rely on anaerobic glycolysis to a greater extent. This was likely due to the fibers on the perimeter acting as an oxygen sink preventing sufficient oxygen diffusion to the inner fibers. This greater reliance on anaerobic glycolysis would result in more lactate and H+ ions, resulting in an earlier fatigue (Myers and Ashley 1997). This was likely a factor during the stimulation at 42 °C.
Ca2+ re-uptake into the sarcoplasmic reticulum (SR) has been acknowledged as the rate-limiting event for FRT1/2 (Stein et al. 1982). Relaxation velocity depends on Ca2+ reuptake from the cytosol to the SR via phospholamban and the sarco(endo)plasmic reticulum Ca2+ATPase (SERCA). In the present study, all groups stimulated at 42 °C had a lower FRT1/2 compared with the groups stimulated at 20 °C. This could be due to the lower Pt of the muscles stimulated at 42 °C, since these muscles would require less absolute relaxation to reach their half way point than muscles that had a greater Pt. Alternatively, O’Brien et al. (1997)) have shown that a prior heat shock resulted in an enhancement of maximal Ca2 + −pumping activity. Interestingly, Fu and Tupling (2009) used co-transfections of Hsp70 and the fast fiber-specific SERCA in HEK-293 cells and showed Hsp70 forms a protective interaction with the SERCA2a during heat stress, thereby allowing maximal activity by reducing oxidation and nitrosylation. Other studies have also suggested a role for and HSP-mediated protection of the SR (Tupling et al. 2004, 2008). The data reported herein suggest this may not occur in intact or isolated skeletal muscles.
At 20 °C, temperature was not likely a stress to the muscles, and therefore, both muscles performed well when exposed to the 20 °C temperature, whereas at 42 °C the muscles were stressed with the soleus exhibiting a greater tolerance than the EDL. In the rat, the EDL is composed of 56 % fast glycolytic, 42 % fast oxidative glycolytic, and 2 % slow oxidative fibers, while the soleus is composed of 13 % fast oxidative glycolytic and 87 % slow oxidative fibers (Armstrong and Phelps 1984). Thus, these muscles differ considerably in physiological and biochemical characteristics, including twitch force, speed of contraction, fatigue resistance, SR Ca2+ release and uptake, and cross-sectional area (Gollnick and Hodgson 1986). Another difference between these two muscles is the constitutive expression of Hsp72 (Locke and Tanguay 1996). The soleus contains a rather high Hsp72 during unstressed conditions while the EDL shows a more typical pattern of low Hsp72 during unstressed conditions. It is tempting to suggest the greater temperature tolerance of the soleus might be due to the increased basal level of HSPs; however, since there was no observed effect of an increased Hsp25 and −72 content on any of the contractile properties, it suggests these HSPs may not be involved. It remains possible that the soleus has an increased tolerance to elevated temperatures due to other inherent factors. The temperature of 42 °C likely presents a considerable stress to the muscles, and therefore, the greater oxidative capacity of the soleus conferred a greater tolerance to the stressors than the EDL. In view of this, perhaps it is not surprising that these muscles behaved differently under the two conditions. Kössler and Küchler (1987)) have shown that the soleus produces the greatest Pt at temperatures between 37 °C and 41 °C, whereas the EDL produces the greatest Pt at temperatures between 16 °C and 22 °C.
In conclusion, a prior heat stress does not appear to benefit the contractile properties of the EDL and soleus muscles when contracting at temperatures of 20 °C or 42 °C. Significant differences were detected between muscles (EDL vs soleus) and the effect of temperature on contractile properties. Performance of the EDL at 42 °C was immediately reduced when compared with 20 °C, while the soleus showed no difference in response to the early stages of the protocol at the two temperatures. These data suggest that prior heat stress, and possibly certain HSPs, may not be beneficial to muscle contraction at 42 °C.
References
- Allen DG. Fatigue in working muscles. J Appl Physiol. 2009;106(2):358–359. doi: 10.1152/japplphysiol.91599.2008. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171(3):259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Hittleman KJ, Faulkner JA, Beyer RE. Tissue temperature and whole-animal oxygen consumption after exercise. Am J Physiol. 1971;221:427–431. doi: 10.1152/ajplegacy.1971.221.2.427. [DOI] [PubMed] [Google Scholar]
- Bryantsev AL, Loktionova SA, Ilyinskaya OP, Tararak EM, Kampinga HH, Kabakov AE. Distribution, phosphorylation, and activities of HSP25 in heat-stressed H9c2 myoblasts: a functional link to cytoprotection. Cell Stress Chaperones. 2002;7:146–155. doi: 10.1379/1466-1268(2002)007<0146:DPAAOH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland ME, Ranatunga KW. Force generation induced by rapid temperature jumps in intact mammalian (rat) skeletal muscle fibres. J Physiol. 2003;548(2):439–449. doi: 10.1113/jphysiol.2002.037143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie RW, Tanguay RM, Kingma JG., Jr Heat-shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation. 1993;87:963–971. doi: 10.1161/01.CIR.87.3.963. [DOI] [PubMed] [Google Scholar]
- Fu MH, Tupling AR. Protective effects of Hsp70 on the structure and function of SERCA2a expressed in HEK-293 cells during heat stress. Am J Physiol Heart Circ Physiol. 2009;296(4):H1175–H1183. doi: 10.1152/ajpheart.01276.2008. [DOI] [PubMed] [Google Scholar]
- Garramone RR, Jr, Winters RM, Das DK, Deckers PJ. Reduction of skeletal muscle injury through stress conditioning using the heat-shock response. Plast Reconstr Surg. 1994;93(6):1242–1247. doi: 10.1097/00006534-199405000-00021. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Hodgson DR. The identification of fiber types in skeletal muscle: a continual dilemma. Exerc Sport Sci Rev. 1986;14:81–104. doi: 10.1249/00003677-198600140-00006. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Bers DM. Influence of temperature on the calcium sensitivity of the myofilaments of skinned ventricular muscle from the rabbit. J Gen Physiol. 1989;93(3):411–428. doi: 10.1085/jgp.93.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmazyn M, Mailer K, Currie WR. Acquisition and decay of heat-shock-enhanced postischemic ventricular recovery. Am J Physiol. 1990;259:H424–H431. doi: 10.1152/ajpheart.1990.259.2.H424. [DOI] [PubMed] [Google Scholar]
- Kawana K, Miyamoto Y, Tanonaka K, Han-no Y, Yoshida H, Takahashi M, Takeo S. Cytoprotective mechanism of heat shock protein 70 against hypoxia/reoxygenation injury. J Mol Cell Cardiol. 2000;32(12):2229–2237. doi: 10.1006/jmcc.2000.1250. [DOI] [PubMed] [Google Scholar]
- Kayani AC, Close GL, Broome CS, Jackson MJ, McArdle A. Enhanced recovery from contraction-induced damage in skeletal muscles of old mice following treatment with the heat shock protein inducer 17-(allylamino)-17-demethoxygeldanamycin. Rejuvenation Res. 2008;11(6):1021–1030. doi: 10.1089/rej.2008.0795. [DOI] [PubMed] [Google Scholar]
- Kössler F, Küchler G. Contractile properties of fast and slow twitch muscles of the rat at temperatures between 6 and 42 degrees C. Biomed Biochim Acta. 1987;46(11):815–822. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins NT, Murphy RM, Lamb GD. Absolute amounts and diffusibility of HSP72, HSP25, and αB-crystallin in fast- and slow-twitch skeletal muscle fibers of rat. Am J Physiol Cell Physiol. 2012;302:C228–C239. doi: 10.1152/ajpcell.00266.2011. [DOI] [PubMed] [Google Scholar]
- Latchman DS. Heat shock proteins and cardiac protection. Cardiovasc Res. 2001;51(4):637–646. doi: 10.1016/S0008-6363(01)00354-6. [DOI] [PubMed] [Google Scholar]
- Lepore DA, Hurley JV, Stewart AG, Morrison WA, Anderson RL. Prior heat stress improves survival of ischemic-reperfused skeletal muscle in vivo. Muscle Nerve. 2000;23:1847–1855. doi: 10.1002/1097-4598(200012)23:12<1847::AID-MUS8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Li GC, Meyer JL, Mak JY, Hahn GM. Heat-induced protection of mice against thermal death. Cancer Res. 1983;43:5758–5760. [PubMed] [Google Scholar]
- Li GC, Li LG, Liu YK, Mak JY, Chen LL, Lee WM. Thermal response of rat fibroblasts stabily transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci. 1991;88:1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Lin CH, Lin CY, Lee CC, Lin MT, Wen HC. Transgenic overexpression of heat shock protein 72 in mouse muscle protects against exhaustive exercise-induced skeletal muscle damage. J Formos Med Assoc. 2013;112:24–30. doi: 10.1016/j.jfma.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Locke M. Hsp accumulation and Hsf activation in rat skeletal muscle during compensatory hypertrophy. Acta Physiol. 2008;192(3):403–411. doi: 10.1111/j.1748-1716.2007.01764.x. [DOI] [PubMed] [Google Scholar]
- Locke M, Tanguay RM. Increased HSF activation in muscles with a high constitutive HSP 70 expression. Cell Stress and Chaperones. 1996;1:189–196. doi: 10.1379/1466-1268(1996)001<0189:IHAIMW>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2004;18:355–357. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- Myers J, Ashley E. Dangerous curves. A perspective on exercise, lactate, and the anaerobic threshold. Chest. 1997;111(3):787–795. doi: 10.1378/chest.111.3.787. [DOI] [PubMed] [Google Scholar]
- Noble EG, Milne KJ, Melling CWJ. Heat shock proteins and exercise: a primer. Applied physiology, nutrition, and metabolism. 2008;33(5):1050–1065. doi: 10.1139/H08-069. [DOI] [PubMed] [Google Scholar]
- Nosek TM, Brotto MA, Essig DA, Mestril R, Conover RC, Dillmann WH, Kolbeck RC. Functional properties of skeletal muscle from transgenic animals with upregulated heat shock protein 70. Physiol Genomics. 2000;4(1):25–33. doi: 10.1152/physiolgenomics.2000.4.1.25. [DOI] [PubMed] [Google Scholar]
- O’Brien PJ, Li GO, Locke M, Klabunde RE, Ianuzzo CD. Compensatory up-regulation of cardiac SR Ca2 + −pump by heat-shock counteracts SR Ca2 + −channel activation by ischemia/reperfusion. Mol Cell Biochem. 1997;173(1–2):135–143. doi: 10.1023/A:1006840013439. [DOI] [PubMed] [Google Scholar]
- Ranatunga KW, Wylie SR. Temperature-dependent transitions in isometric contractions of rat muscle. J Physiol. 1983;339:87–95. doi: 10.1113/jphysiol.1983.sp014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol. 1985;248(3):C265–C270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- Stein RB, Gordon T, Shriver J. Temperature dependence of mammalian muscle contractions and ATPase activities. Biophys J. 1982;40(2):97–107. doi: 10.1016/S0006-3495(82)84464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Noble EG. Heat shock does note attenuate low-frequency fatigue. Can J Physiol Pharmacol. 1999;77(1):64–70. doi: 10.1139/y99-011. [DOI] [PubMed] [Google Scholar]
- Touchberry CD, Gupte AA, Bomhoff GL, Graham ZA, Geiger PC, Gallagher PM. Acute heat stress prior to downhill running may enhance skeletal muscle remodeling. Cell Stress and Chaperones. 2012;17(6):693–705. doi: 10.1007/s12192-012-0343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupling AR, Gramolini AO, Duhamel TA, Kondo H, Asahi M, Tsuchiya SC, Borrelli MJ, Lepock JR, Otsu K, Hori M, MacLennan DH, Green HJ. HSP70 binds to the fast-twitch skeletal muscle sarco(endo)plasmic reticulum Ca2+ − ATPase (SERCA1a) and prevents thermal inactivation. J Biol Chem. 2004;279(50):52382–52389. doi: 10.1074/jbc.M409336200. [DOI] [PubMed] [Google Scholar]
- Tupling AR, Bombardier E, Vigna C, Quadrilatero J, Fu M. Interaction between Hsp70 and the SR Ca2+ pump: a potential mechanism for cytoprotection in heart and skeletal muscle. Applied physiology, nutrition, and metabolism. 2008;33(5):1023–1032. doi: 10.1139/H08-067. [DOI] [PubMed] [Google Scholar]
- Welch WJ. The mammalian heat shock (or stress) response: a cellular defense mechanism. Adv Exp Med Biol. 1987;225:287–304. doi: 10.1007/978-1-4684-5442-0_26. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]