Abstract
Purpose
Borderline ovarian tumors (BOTs) are more common in young women of reproductive age, and exhibit a better prognosis than malignant ovarian tumors (MOTs). Fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) were compared in their ability to differentiate BOTs from stage I MOTs.
Methods
Among 173 patients who had preoperative FDG PET/CT due to ovarian neoplasms between November 2006 and March 2009, there were 13 patients with BOTs or stage I MOTs. For differential diagnosis of the two tumors, cancer antigen-125 (CA-125) level, the longest diameter of tumors, metabolic indices including maximum standardized uptake value (SUVmax), and volumetric indices including metabolic tumor volume (MTV) were compared, respectively.
Results
The BOT group (n = 7) was comprised of five mucinous and two serous tumors, and the MOT group (n = 6) was comprised of three endometrioid, two clear cell and one mucinous tumors. Among the comparisons between two groups, SUVmax of the BOT group was significantly lower than that of the MOT group (2.9 ± 1.5 vs. 6.6 ± 2.9, p = 0.0223); otherwise, no significant difference was found in age, CA-125, diameter, or MTV. By receiver-operating characteristic curve analysis, SUVmax of 3.7 was the best cutoff value to differentiate BOTs from stage I MOTs, with a sensitivity of 83.3 % and specificity of 85.7, and the area under curve of 0.893 (p = 0.0001, 95 % CI: 0.601∼0.993).
Conclusions
We demonstrated that SUVmax could distinguish BOTs from stage I MOTs, with a high sensitivity and specificity. Metabolic indices determined by FDG PET/CT were more suitable than volumetric indices for differential diagnosis of the two tumors.
Keywords: Borderline ovarian tumor, Stage I malignant ovarian tumor, FDG PET/CT, Standard uptake value (SUV), Metabolic tumor volume (MTV)
Introduction
Borderline ovarian tumors (BOTs) were distinctly categorized by the International Federation of Gynecology and Obstetrics (FIGO) in 1971, and comprise up to 15–20 % of epithelial ovarian neoplasms, which histologically demonstrate cellular proliferation but not destructive stromal invasion [1, 2]. BOTs are more common in young women of reproductive age, and exhibit a better prognosis than malignant ovarian tumors (MOTs) [3]. In regard to the primary treatment of BOTs, conservative management, such as unilateral salpingo-oophorectomy (USO) with comprehensive staging, suffices women wanting to preserve fertility; otherwise, radical management, such as a bilateral salpingo-oophorectomy (BSO) with total abdominal hysterectomy (TAH) including comprehensive staging, is required to reduce tumor recurrence. These therapeutic strategies are worthwhile, considering that BOTs are notoriously resistant to platin-based chemotherapy [4].
MOTs can metastasize to the contralateral ovary, peritoneal cavity, and retroperitoneal lymph nodes, even during early stage disease [1]. Therefore, they should be treated more radically by, for example, BSO with TAH, omentectomy, and complete surgical staging. Furthermore, adjuvant chemotherapy should be administered to reduce tumor recurrence. Accordingly, the differential diagnosis of BOTs from early stage MOTs is important, because this differentiation can influence surgical management, prognosis, and the possibility of fertility-preserving surgery, especially in young patients.
Various diagnostic methods, including tumor markers and imaging modalities, have been used to differentiate BOTs from MOTs. Cancer antigen 125 (CA-125) has been extensively studied as a biomarker for MOTs, but it exhibits poor sensitivity during the early disease stage and low specificity for malignancy [5]. In particular, it has been reported that CA-125 did not increase in one half of early stage MOT cases, and that serum CA-125 levels failed to differentiate BOTs from MOTs [1, 6]. Diagnostic imaging modalities, including computed tomography (CT) and magnetic resonance imaging (MRI), have been performed to assess pelvic masses preoperatively. However, the confident differentiation of BOTs from stage I MOTs was not possible, because these two diseases share morphological features [1].
Positron emission tomography/computed tomography (PET/CT) with F-18 fluorodeoxyglucose (FDG) has been widely used for preoperative staging, predicting prognosis, and detecting the recurrence of ovarian cancers, because it provides a combination of functional and anatomical imaging [7, 8]. However, the clinical role of FDG PET/CT for the differentiation of BOTs from early stage MOTs is doubtful [9]. Thus, we evaluated the diagnostic abilities of FDG PET/CT to differentiate BOTs from early stage (FIGO stage I) MOTs.
Material and Methods
Patients
We retrospectively reviewed preoperative FDG PET/CT and MR images obtained between November 2006 and March 2009, to evaluate adnexal masses suspicious of a primary ovarian neoplasm in 173 patients at our institute. Of these 173 patients, 13 with pathologically proven epithelial ovarian tumors of either early stage MOT (FIGO stage I) or BOT were enrolled in this study. The remainder of the patients had advanced stage ovarian cancer or non-epithelial ovarian tumors, such as mature teratomas. The enrolled patients were allocated to BOT or MOT groups. The BOT group comprised seven patients (48.9 ± 19.6 y; range 14–73 y), and the MOT group comprised six patients (60.0 ± 11.0 y; range 48–77 y). This study was approved by the institutional review board at Seoul National University Hospital, and the requirement for informed consent was waived due to the retrospective design of the study.
Serum CA-125
Serum levels of CA-125 were measured preoperatively in all the patients. Times between preoperative CA-125 measurements before surgery were not significantly different in the two groups (BOT 8.7 ± 4.7 days vs. MOT 11.7 ± 10.2 days, p = 0.8864). Serum CA-125 levels were determined in duplicate using a radioimmunoassay (Fujirebio Diagnostics, Malvern, PA, USA), which had a range up to 500 U/ml. Within-assay and between-assay variation coefficients were < 4.3–8.2 % and < 5.2–8.3 %, respectively.
PET/CT
Whole body PET/CT images were acquired using dedicated PET/CT systems (Biograph 40, Siemens Healthcare, Erlangen, Germany; Gemini, Philips Medical Systems, Andover, USA). Patients fasted at least 4 h prior to PET/CT scanning, furosemide (20 mg) was intravenously administered and a Foley catheter was placed to minimize urine FDG activity. FDG (5.18 MBq/kg) was administered intravenously, and 125 ml of a barium sulfate solution (EZCT [1.5 % weight-volume barium sulfate suspension], Taejoon Pharm, Seoul) was administered orally 1 h prior to scanning. A CT scan (80 mA and 140 kVp) was performed for attenuation correction prior to PET scans. CT scans were obtained using a 5-mm section thickness from the skull base to the mid-thigh; images were reconstructed using a 512 × 512 matrix, and a 50-cm field of view. PET scans were obtained from the mid-thigh to the skull base, and images were reconstructed with a 128 × 128 matrix, using the ordered subset expectation maximum iterative reconstruction algorithm, an 8-mm Gaussian filter, and a 50-cm field of view. In addition, all patients were requested to fill out a questionnaire that contained questions on patients’ information, including fasting time, menstruation period, etc.
MR Technique
MR images were obtained on a 3T or 5T scanner (SIGNA EXCITE, GE Medical Systems, Milwaukee, USA) using a phased array and a body coil for pelvic imaging. Scans were obtained using variable sequences; an axial fast spin-echo T1-weighted sequence; an axial, coronal, and sagittal fast spin-echo T2-weighted sequence; and an axial fat-saturated spoiled gradient-echo T1-weighted sequence. In addition to routine T1- and T2-weighted images of slice thicknesses 5- to 6-mm, axial fast spin echo T1-wighted fat-suppressed (TR/TE 600/13.5) with gadolinium contrast enhanced images were obtained at a slice thickness of 8-mm and a 256 × 128 matrix.
PET Parameter Analyses
Four specific PET parameters, which included both metabolic and volumetric indices, were adopted to differentiate BOTs from early stage MOTs. To acquire PET parameters, in each case, a volume of interest (VOI) was placed on the adnexal mass using a vendor-provided automated contouring program (Volumetric Analysis [version 6.0.14.4], Siemens Healthcare, Erlangen, Germany). The program was set for two different thresholds; a fixed standardized uptake value (SUV) of 1.5, or 50 % of maximum SUV (SUVmax) of the mass. The criteria for the thresholds used here were determined to include all the lesions, since the lowest value of SUVmax was 1.52, or to be based on the fact that 50 % of SUVmax was reasonable by phantom studies [10].
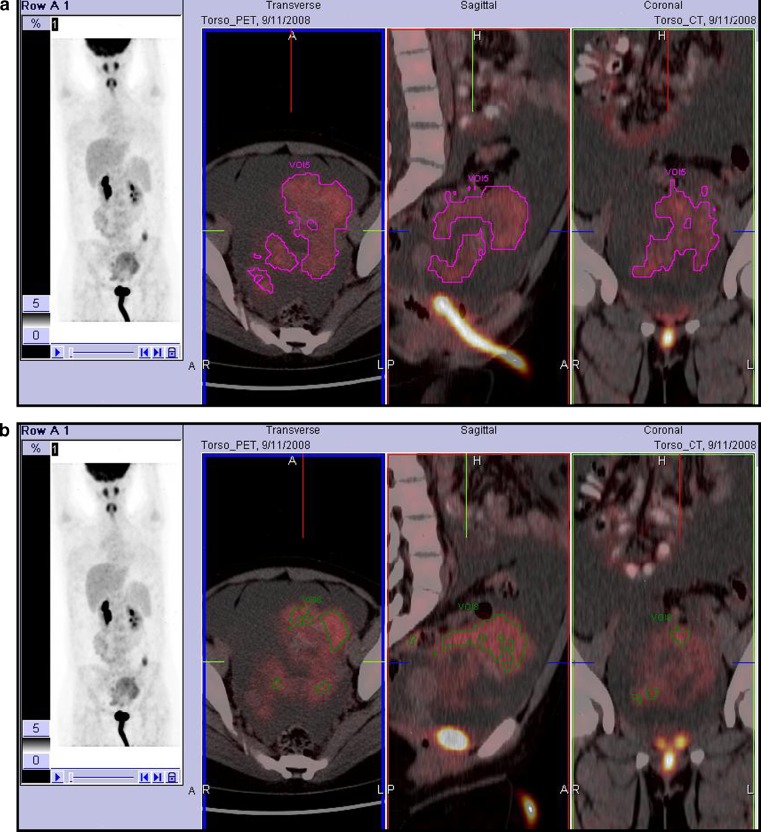
SUVmax, average SUV (SUVavg), metabolic tumor volume (MTV), and total lesional glycolysis (TLG) were measured in each VOI at the two thresholds, respectively. SUVs were calculated as follows: 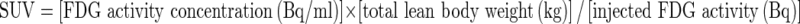 . MTV was measured after converting FDG PET/CT data in Digital Imaging and Communications in Medicine format, as previously described8; voxels presenting SUVs greater than thresholds within a contouring margin were counted when determining MTVs. Lastly, TLG was defined as the product of SUVavg and MTV within the VOI. Figure 1 shows examples of how PET parameters were acquired at the two different thresholds.
. MTV was measured after converting FDG PET/CT data in Digital Imaging and Communications in Medicine format, as previously described8; voxels presenting SUVs greater than thresholds within a contouring margin were counted when determining MTVs. Lastly, TLG was defined as the product of SUVavg and MTV within the VOI. Figure 1 shows examples of how PET parameters were acquired at the two different thresholds.
Fig. 1.
Examples of acquiring PET parameters at the two different thresholds. This patient had mucinous BOT. PET parameters were measured within a VOI placed on the tumor. SUV thresholds were set at 1.5 (a) and 50 % of SUVmax (b). With a fixed SUV threshold of 1.5, MTV and TLG were 322.6 cm3 and 783.9 cm3 (a). With a threshold with 50 % of SUVmax, MTV and TLG were 85.2 cm3 and 291.2 cm3
Statistical Analysis
Statistical analysis was performed using MedCalc software (version 12.0; MedCalc Software, Belgium). Statistical significance was accepted for p values of < 0.05. The Mann-Whitney test for independent samples was used to compare group parameters. Receiver-operating characteristic curve (ROC) analysis with respect to SUVmax was performed to determine the best cutoff value for differentiating BOTs from stage I MOTs. The right-sided Grubb’s test was performed to detect outliers for the comparisons of serum CA-125 levels.
Results
Clinical Characteristics
Thirteen patients diagnosed with an epithelial ovarian tumor, either early stage MOT (FIGO stage I) or BOT were finally enrolled from among the 173 patients initially reviewed. The excluded patients had advanced stage ovarian cancer or a non-epithelial ovarian tumor. Pathological results revealed the BOT group (n = 7) comprised of five mucinous and two serous tumors, and that the MOT group (n = 6) comprised of three endometrioid, two clear cell, and one mucinous tumor. In regard to surgical stages, the BOT patients were all of FIGO stage Ia, and the MOT patients were of FIGO stages 4 Ia, 1 Ib, or 1 Ic.
All patients in the MOT group underwent radical surgery, including TAH with BSO and pelvic lymph node dissection (PLND). On the other hand, patients in the BOT group underwent various types of surgery according to the primary purposes of treatment and clinical conditions. USO or tumorectomy (n = 4), whether combined with TAH or not, was performed on those with incidentally detected BOT during workup for liver transplantation, rectal cancer, or Peutz-Jegher syndrome, or during follow-up for adenomyosis after TAH. Complete surgical staging, including TAH with BSO and PLND, was performed in three patients under clinical suspicion of a malignant ovarian tumor.
No significant difference was found between the clinical and demographic characteristics of the two groups. BOT patients tended to be younger, but this was statistically insignificant (48.9 ± 19.6 vs. 60.0 ± 11.0, p = 0.2949). During the comparative analysis of serum CA-125 levels, one patient in the MOT group was found to have an extremely high level (43,530 U/mL). After excluding this patient as an outlier, mean serum CA-125 levels were not significantly different in the BOT and MOT groups (74.7 ± 115.5 vs. 90.8 ± 131.9, p = 0.6623). Diameters of tumors varied from 3.7 cm to 28.0 cm, but no significant intergroup difference was found (BOT: 13.9 ± 8.6 vs. MOT: 16.1 ± 9.1, p = 0.6171). Table 1 summarizes the demographic and clinical characteristics of the two groups.
Table 1.
Demographic and clinical characteristics
| Characteristics | BOT | MOT |
|---|---|---|
| No. | 7 | 6 |
| Age (y) | 48.9 ± 19.6 | 60.0 ± 11.0 |
| CA-125 (U/ml) | 74.7 ± 115.5 | 90.8 ± 131.9 |
| Diameter (cm) | 13.9 ± 8.6 | 16.1 ± 9.1 |
| Histology | ||
| Mucinous | 5 | 1 |
| Serous | 2 | – |
| Endometrioid | – | 3 |
| Clear cell type | – | 2 |
| FIGO stage | ||
| Ia | 7 | 4 |
| Ib | – | 1 |
| Ic | – | 1 |
BOT Borderline ovarian tumor; MOT malignant ovarian tumor; FIGO International Federation of Gynecology and Obstetrics
Comparisons of Image Findings
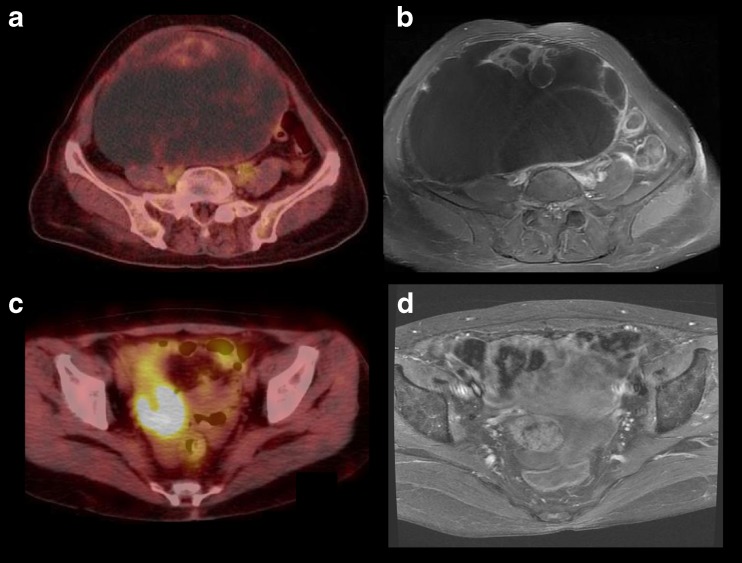
Image characteristics were compared using MR and FDG PET/CT images. The two groups shared anatomical features on MR images. All tumors were septated and multi-loculated with solid elements, including smooth round nodules and papillary projections, with no tendency for any specific feature to predominate in malignancy, such as omental cake, peritoneal nodules, or lymphadenopathy. For both groups, none was a simple unilocular cyst. Ascites was seen in four out of the 13 patients; the others had no definite ascites (n = 8) or a small amount of free fluid (n = 1) in the abdominoperitoneal cavity. On FDG PET/CT images, only mildly increased hypermetabolic activity was seen in the solid portions of BOTs, whereas intense hypermetabolic activity was seen in MOTs. Representative images of both groups are displayed in Fig. 2.
Fig. 2.
Representative images of both groups. BOT is presented as a large cystic mass with hypermetabolic activity in its anterior aspect on FDG PET/CT (a SUVmax of 1.7) and prominent septation on enhanced MR image (b). MOT shows intense hypermetabolic activity in the solid portion of the mass (c SUVmax of 8.8) and intermediate to low signal intensity with good enhancement on MR image (d)
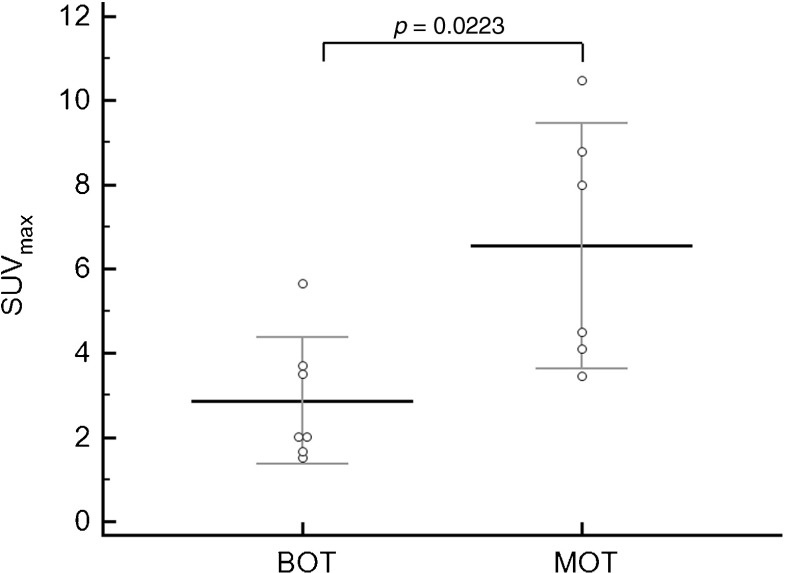
The PET parameters consisted of SUVmax, SUVavg, MTV, and TLG (Table 2). As mentioned above, SUVmax was significantly lower in the BOT group than in the MOT group (2.9 ± 1.5 vs. 6.6 ± 2.9, p = 0.0223). To exclude the potential influence of metabolic variations caused by the menstrual cycle [11, 12], SUVmax were re-compared after excluding three patients who were at mid-cycle of menstruation. According to self-questionnaire answers and the medical record review, nine of the 13 patients were menopausal and four were premenopausal. Of these four patients, two in the BOT group and one in the MOT group were at mid-cycle at the time of FDG PET scanning. Consequently, after excluding three patients who were at the mid-cyle, it was reaffirmed that BOTs had lower SUVmax than those of MOTs (2.5 ± 1.0 vs. 7.0 ± 4.1, p = 0.0317; Fig. 3).
Table 2.
Comparisons of positron emission tomography (PET) parameters
| Variables | BOT | MOT | p |
|---|---|---|---|
| SUVmax | 2.9 ± 1.5 (n = 7) | 6.6 ± 2.9 (n = 6) | 0.0223 |
| SUVmax a | 2.5 ± 1.0 (n = 5) | 7.0 ± 4.1 (n = 5) | 0.0317 |
| Thresholdb | |||
| SUVavg | 1.9 ± 0.3 | 2.6 ± 0.7 | 0.0513 |
| MTV (cm3) | 68.4 ± 122.5 | 364.2 ± 534.6 | 0.0734 |
| TLG (cm3) | 33.2 ± 34.2 | 39.7 ± 35.8 | 0.0734 |
| Thresholdc | |||
| SUVavg | 1.8 ± 0.9 | 4.0 ± 1.8 | 0.0140 |
| MTV (cm3) | 156.4 ± 293.4 | 894.4 ± 1171.3 | 0.6282 |
| TLG (cm3) | 79.1 ± 109.8 | 182.4 ± 230.1 | 0.2343 |
BOT Borderline ovarian tumor; MOT malignant ovarian tumor; SUV max maximum standardized uptake value, SUV avg average standardized uptake value ; MTV metabolic tumor volume; TLG total lesional glycolysis
aExclusion of patients who were in the mid-cycle of menstruation
bFixed threshold with SUV of 1.5
cThreshold with 50 % of SUVmax
Fig. 3.
Comparisons of SUVmax values in the BOT and stage I MOT groups. SUVmax was significantly lower in the BOT groups than in the stage I MOT group (2.9 ± 1.5 vs. 6.6 ± 2.9, p = 0.0223)
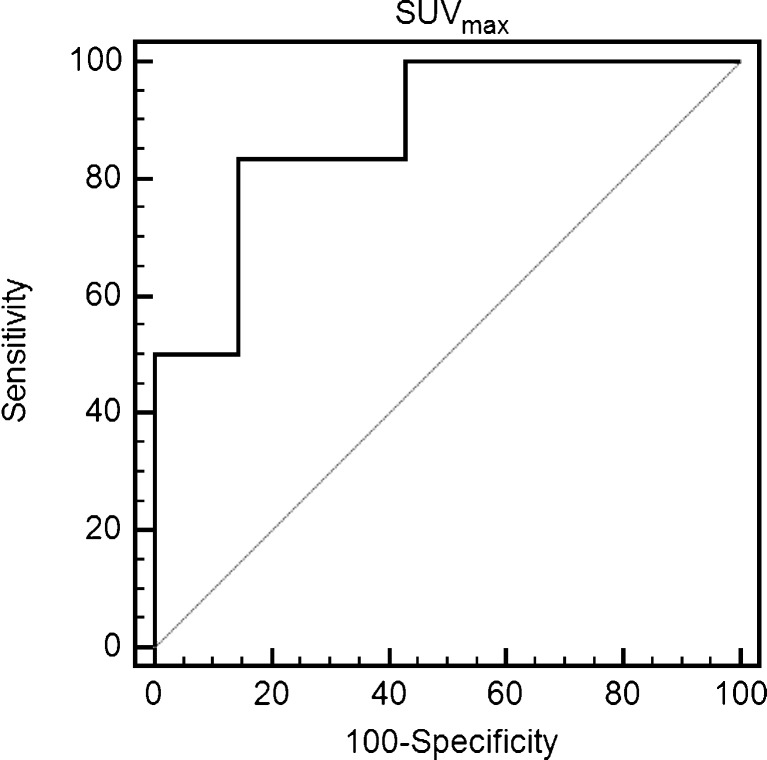
Comparisons of volumetric indices were performed using two different thresholds. At the first threshold (SUVmax of 1.5), SUVavg in the BOT tended to be lower than that in the MOT, but it failed to reach a significant level by a close call (1.9 ± 0.3 vs. 2.6 ± 0.7, p = 0.0513). However, SUVavg in the BOT group was significant lower than in the MOT group (1.8 ± 0.9 vs. 4.0 ± 1.8, p = 0.0140) at the second threshold (50 % of SUVmax). MTV and TLG were not significantly different at either of the two thresholds. Finally, ROC analysis revealed that a SUVmax of 3.7 was the best cutoff value for differentiating BOTs from MOTs with a sensitivity of 83.3 %, a specificity of 85.7 %, and an area under the curve (AUC) of 0.893 (p = 0.0001, 95 % CI: 0.601∼0.993). Data analyses are summarized in Fig. 4.
Fig. 4.
ROC analysis for the determination of the optimal SUVmax cut-off value for differentiating stage I MOT from BOT. The area under the ROC curve for a cutoff value of 3.7 was 0.893 (p = 0.0001, 95 % CI: 0.601∼0.993)
Discussion
In the present study, we investigated the diagnostic abilities of FDG PET/CT to differentiate BOTs from early stage MOTs. Of the various PET parameters examined, SUVmax best differentiated the BOTs and early stage MOTs; SUVmax had a sensitivity of 83.3 % and a specificity of 85.7 % at the optimal cutoff of 3.7. Tumor-volume–based analyses of TLG and MTV revealed these variables could not differentiate the BOTs and early stage MOTs, and anatomical features and tumor markers were not significantly different for the BOTs and early stage MOTs.
It is difficult to differentiate BOTs from early stage MOTs using conventional diagnostic tools, such as anatomical imaging modalities and tumor markers. Furthermore, their MR relaxation characteristics were not useful, since solid components are usually depicted as intermediate signal intensities on both T1-weighted and T2-weighted images of BOTs and early stage MOTs [1]. Contrast MRI enhancement and anatomical features, such as increased septal thickness and solid portion sizes could be helpful, but cannot be used to confidently differentiate these entities [1, 13]. Although CA-125 is a well-known biomarker for the detection of ovarian cancer recurrence, with high accuracy and a high positive predictive value [14], CA-125 levels were only found to be marginally increased in patients with BOT [15].
In the present study, SUVmax was found to be useful for the differential diagnosis of BOTs from early stage MOTs, with high sensitivity and specificity. More specifically, BOTs had lower SUVmax values than early stage MOTs, which can be explained by the physiology of glucose metabolism in these tumors. Glycolysis increases under anaerobic condition (the Warburg effect), and is a characteristic of the malignant state [16], and overexpression of glucose transporters such as glucose transporter 1 (GLUT-1) to cope with facilitated glucose consumption is also commonly observed in various malignant tumors. It was reported that expression level of GLUT-1 in epithelial ovarian tumors increased gradually from borderline to malignant tumors [17]. FDG PET utilizes this fundamental characteristic of malignant cells, and thus the lower SUV of BOT infers a lower malignant potential.
Adding to the malignant potential, SUV values are known to be an important factor in evaluation of treatment response and prognosis prediction in various malignant tumors. It has been reported that GLUT-1 expression, a major determining factor of SUV values, was related to prognosis of malignant tumors [11, 12]. It was suggested that GLUT-1 expression might be an independent prognostic factor in colon cancer [12]. As for malignant ovarian tumors, potential roles of GLUT-1 expression in predicting prognosis remain unclear, but an article recently published by Chung et al. provides a clue to this matter [18]. They reported that the preoperative metabolic tumor burden and its distribution measured by SUV values could predict recurrence in patients with epithelial ovarian cancer. Similarly, it has been reported that the metabolic tumor burden measured by FDG PET could be an independent prognostic factor for tumor recurrence in patients with early stage of cervical cancer [19]. Since metabolic tumor burden generally reflects the total amount of tumor glycolysis, GLUT-1 expression might be related with the prognosis of epithelial ovarian cancer patients.
SUV values were also reported to be associated with histologic grades of epithelial ovarian tumors [17]. Kurokawa et al. reported that SUV values differed by histologic types of epithelial ovarian tumors which was mainly mediated by the expression level of GLUT-1. Among various histologic types of epithelial ovarian tumors, mucinous tumors are typical tumors presenting low SUV values. In this context, considering histologic types of the study population, the difference of SUVmax between the BOT and MOT groups could be explained. In the present study, only one patient had mucinous adenocarcinoma and its SUVmax was 3.5; this was the lowest value in the MOT group. On the contrary, the BOT group mainly consisted with mucinous tumor (n = 5/7), and the average value of SUVmax was 2.0. Mucinous adenocarcinomas account for less than 5 % of all epithelial ovarian cancers, whereas up to 80 % of mucinous tumors are benign or borderline tumors [20]. Thus, it could be useful to measure preoperatively SUV values in predicting histologic type of epithelial ovarian tumors, especially combining with anatomical imaging findings by CT or MR.
SUV provides a measure of the activity within a region of interest, and is the most commonly used PET or PET/CT derived functional biomarker. Furthermore, SUVmax has become a standard parameter in oncology field, since it reflects the most active part of a tumor. However, this unique property of SUVmax causes unexpected problems associated with high intra-tumoral heterogeneity. For example, in response to anti-cancer therapy, tumor cells can exhibit various changes in glucose metabolism and many other biological processes, and thus SUVmax is probably not a ‘proper’ indicator of metabolic change in an entire tumor. To overcome this shortcoming, volumetric indices, such as TLG and MTV, have been utilized as alternatives to SUVs.
TLG and MTV have been actively investigated in the contexts of treatment response evaluation, objective patient monitoring, and treatment planning. MTV is the tumor volume determined by PET using the segmentation technique, and TLG is the product of MTV and SUVavg within a lesion at a certain threshold [21]. Given that TLG provides a measure of tumor global metabolic activity and MTV provides a measure of tumor volume with a FDG uptake greater than a threshold value, TLG and MTV could both provide a better measure of treatment response or tumor burden than SUVs. Indeed, a change in TLG has been shown to be a strong predictor of treatment response in many cancers.
To examine the usefulness of volumetric indices, we applied TLG and MTV to the differential diagnosis of BOTs and early stage MOTs. Two different thresholds were used for volumetric indices; that is, a fixed SUVmax of 1.5 or 50 % of SUVmax. Contrary to our expectations, TLG and MTV did not differentiate BOTs from early stage MOTs, but average SUVs within the thresholds were significantly different in the two groups, which were consistent with the SUVmax analysis results, because SUVavg also reflects metabolism in solid tumor regions. Comparisons of SUVavg values in two groups were more significantly different at a threshold of 50 % of SUVmax (p = 0.0140) than at a threshold of 1.5 (p = 0.0513). In general, the 50 % threshold contained portions of tumors smaller than the 1.5 threshold, because the latter threshold was chosen to include all tumor regions showing low FDG uptake. Thus, SUVavg at a threshold of 50 % was closer to SUVmax than SUVmax at a threshold of 1.5.
The failure of the volumetric indices, TLG and MTV, to significantly differentiate BOTs from early stage MOTs can be explained by the anatomical characteristics of ovarian tumors. Epithelial ovarian tumors are generally cystic, and solid portions are associated with malignancy. Accordingly, volumetric indices tend to be exaggerated by cystic regions, whereas metabolic indices, which provide measures of the metabolic activities of solid tumor portions, are not influenced by the sizes of cystic regions. Accordingly, our results indicate that metabolic indices are more appropriate for the evaluation of tumors containing cystic portions.
The present study is inherently limited by its retrospective design, the small number of patients recruited, and by the fact that it was conducted at a single institution. Usually, patients with adnexal masses strongly suspected to be BOTs by conventional anatomical imaging are not further examined by FDG PET, due to clinical and cost considerations. Actually, only patients with BOTs incidentally found during FDG PET studies for other medical conditions were enrolled in this study. For this reason, mean age in the BOT group was not significantly different from that of the MOT group. Because of the older age of our cohort, the potential influence of menstruation on FDG uptake by ovarian tumors was minimal (only four patients were premenopausal). We also re-compared SUVmax values after excluding three patients in mid menstrual cycle, because ovarian uptake peaks at this time [22, 23], and the result of the comparison confirmed that SUVmax values remained significantly different. A prospective larger-scale study including young patients of reproductive age is suggested to reaffirm diagnostic values of FDG PET/CT in differentiating BOTs from early MOTs.
Conclusions
This study shows that SUVmax values can distinguish BOTs from early stage MOTs, with a high sensitivity and specificity. However, novel PET/CT tumor volume associated indices failed to differentiate the BOTs and early stage MOTs. Accordingly, our results show that metabolic indices determined by PET/CT are more suitable than volumetric indices for the evaluation of ovarian tumors containing cystic portions.
Acknowledgments
Disclosure
The authors declare that they have no vested interest that could be construed to have inappropriately influenced this study.
References
- 1.deSouza NM, O’Neill R, McIndoe GA, Dina R, Soutter WP. Borderline tumors of the ovary: CT and MRI features and tumor markers in differentiation from stage I disease. Am J Roentgenol. 2005;184:999–1003. doi: 10.2214/ajr.184.3.01840999. [DOI] [PubMed] [Google Scholar]
- 2.Gotlieb WH, Chetrit A, Menczer J, Hirsh-Yechezkel G, Lubin F, Friedman E, et al. Demographic and genetic characteristics of patients with borderline ovarian tumors as compared to early stage invasive ovarian cancer. Gynecol Oncol. 2005;97:780–3. doi: 10.1016/j.ygyno.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Manchul LA, Simm J, Levin W, Fyles AW, Dembo AJ, Pringle JF, et al. Borderline epithelial ovarian tumors: a review of 81 cases with an assessment of the impact of treatment. Int J Radiat Oncol Biol Phys. 1992;22:867–74. doi: 10.1016/0360-3016(92)90781-C. [DOI] [PubMed] [Google Scholar]
- 4.Cadron I, Leunen K, Gorp TV, Amant F, Neven P, Vergote I. Management of borderline ovarian neoplasms. J Clin Oncol. 2007;25:2928–37. doi: 10.1200/JCO.2007.10.8076. [DOI] [PubMed] [Google Scholar]
- 5.Bast RC, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15(Suppl. 3):274–81. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- 6.Jacob I, Bast RC., Jr The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4:1–12. doi: 10.1093/humrep/4.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 7.Makhija S, Howden N, Edwards R, Kelley R, Townsend DW, Meltzer CC. Positron emission tomography/computed tomography imaging for the detection of recurrent ovarian and fallopian tube carcinoma: a retrospective review. Gynecol Oncol. 2002;85:53–8. doi: 10.1006/gyno.2002.6606. [DOI] [PubMed] [Google Scholar]
- 8.Chung HH, Kang WJ, Kim JW, Park N-H, Song Y-S, Chung J-K, et al. Role of [18F]FDG PET/CT in the assessment of suspected recurrent ovarian cancer: correlation with clinical or histological findings. Eur J Nucl Med Mol Imaging. 2007;34:480–6. doi: 10.1007/s00259-006-0260-x. [DOI] [PubMed] [Google Scholar]
- 9.Jung DC, Choi HJ, Ju W, Kim SC, Choi K-G. Discordant MRI/FDG-PET imaging for the diagnosis of borderline ovarian tumours. Int J Gynecol Cancer. 2008;18:637–41. doi: 10.1111/j.1525-1438.2007.01116.x. [DOI] [PubMed] [Google Scholar]
- 10.Ciernik IF, Dizendorf E, Baumert BG, Reiner B, Burger C, Davis JB, et al. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys. 2003;57:853–63. doi: 10.1016/S0360-3016(03)00346-8. [DOI] [PubMed] [Google Scholar]
- 11.Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, et al. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015–24. doi: 10.1002/cncr.11159. [DOI] [PubMed] [Google Scholar]
- 12.Haber RS, Rathan A, Weiser KR, Pritsker A, Itzkowitz SH, Bodian C, et al. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998;83(1):3440. doi: 10.1002/(SICI)1097-0142(19980701)83:1<34::AID-CNCR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 13.Takemori M, Nishimura R, Hasegawa K. Clinical evaluation of MRI in the diagnosis of borderline ovarian tumors. Acta Obstet Gynecol Scand. 2002;81:157–61. doi: 10.1034/j.1600-0412.2002.810212.x. [DOI] [PubMed] [Google Scholar]
- 14.Torizuka T, Nobezawa S, Kanno T, Futatsubashi M, Yoshikawa E, Okada H, et al. Ovarian cancer recurrence: role of whole role of whole-body positron emission tomography using 2-[fluorine-18]-fluoro-2-deoxy-d-glucose. Eur J Nucl Med Mol Imaging. 2002;29:797–803. doi: 10.1007/s00259-001-0750-9. [DOI] [PubMed] [Google Scholar]
- 15.van Vierzen PB, Massuger LF, Ruys SH, Barentsz JO. Borderline ovarian malignancy: ultrasound and fast dynamic MR findings. Eur J Radiol. 1998;28:136–42. doi: 10.1016/S0720-048X(97)00122-8. [DOI] [PubMed] [Google Scholar]
- 16.Warburg OPK, Negelein E. Uber den stoffwechsel der carcinommzelle. Biochem Zeitschrift. 1924;152:309–35. [Google Scholar]
- 17.Kurokawa T, Yoshida Y, Kawahara K, Tsuchida T, Okazawa H, Fujibayashi Y, et al. Expression of GLUT-1 glucose transfer, cellular proliferation activity and grade of tumor correlate with [F-18]-fluorodeoxyglucose uptake by positron emission tomography in epithelial tumors of the ovary. Int J Cancer. 2004;109:926–32. doi: 10.1002/ijc.20057. [DOI] [PubMed] [Google Scholar]
- 18.Chung HH, Kwon HW, Kang KW, Kim JW, Park NH, Song YS, et al. Preoperative [18F]FDG PET/CT predicts recurrence in patients with epithelial ovarian cancer. J Gynecol Oncol. 2012;23:28–34. doi: 10.3802/jgo.2012.23.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung SE, Lee JM, Rha SE, Byun JY, Jung JI, Hahn ST. CT and MR imaging of ovarian tumors with emphasis on differential diagnosis. Radiographics. 2002;22:1305–25. doi: 10.1148/rg.226025033. [DOI] [PubMed] [Google Scholar]
- 20.Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2:159–71. doi: 10.1016/S1095-0397(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 21.Kim BS, Kim IJ, Kim S, et al. The prognostic value of the metabolic tumor volume in FIGO stage IA to IIB cervical cancer for tumor recurrence: measured by F-18 FDG PET/CT. Nucl Med Mol Imaging. 2011;45:36–42. doi: 10.1007/s13139-010-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SK, Kang KW, Rho JW, Sim JS, Lee ES, Park SY. Incidental ovarian 18F-FDG accumulation on PET: correlation with the menstrual cycle. Eur J Nucl Med Mol Imaging. 2005;32:757–63. doi: 10.1007/s00259-005-1771-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z, Wang B, Cheng W, Cheng X, Cui R, Huo L, et al. Endometrial and ovarian F-18 FDG uptake in serial PET studies and the value of delayed imaging for differentiation. Clin Nucl Med. 2006;31:781–7. doi: 10.1097/01.rlu.0000247261.82757.ea. [DOI] [PubMed] [Google Scholar]