Abstract
SAM(g2) is an automated analysis that transforms the MEG data into a functional image of spike-like activity, giving the source waveforms for those locations. Since the source waveforms estimated by SAM have higher signal-to-noise ratio (SNR) than does the raw MEG data, it is possible to automatically mark the location and timing of each spike for comparisons with dipole fit procedures. Both SAM(g2) and equivalent current dipole (ECD) fits were used to analyze MEG interictal spike recordings in 10 patients with cortical dysplasias and medial temporal lobe epilepsy. The ECD fit locations obtained by manual spike classification and latency marking were compared with those found by automated SAM(g2) procedures. When the SNR of interictal activity was high (compared to the background) with a clear single focus, there was excellent agreement between the ECD cluster location and the SAM(g2) maximum. However, when the SNR of spikes was low, manual single ECD location scatter was larger than SAM(g2) reconstructions. When multiple independent interictal spike loci were present, there was some disagreement between SAM(g2) and ECD scatter in the cases of low SNR spikes. When SAM(g2) indicated multiple coupled spike loci, the residual variance for the dipole fit was high and its scatter unacceptably large – even for multiple dipole models. This study demonstrates that SAM(g2) is equivalent to ECD fit for localizing interictal spikes when there is a single locus and good SNR. Further studies are required to validate cases in which there are multiple spike loci or poor SNR.
Keywords: MEG, Epilepsy, Interictal spike, Localization, Minimum-variance beamformer, Dipole fit
INTRODUCTION
The traditional approach to MEG interictal spike localization is the equivalent current dipole fit. However, that procedure is difficult to automate, and generally requires manual identification of spikes. Furthermore, the model order (number of dipoles to fit) may vary among spikes. SAM(g2) was developed to automate epilepsy data analysis, freeing it from model-order considerations [Robinson, 2002]. SAM(g2) represents the MEG data in two forms: a three-dimensional functional image of g2 (excess kurtosis – a measure of “spikiness”NN), and SAM virtual sensors estimating the source waveforms at the coordinates identified in the functional image. Because the signal-to-noise ratio of the spikes is greater in the virtual sensors than in the raw MEG data, the spikes are easily marked by adaptive threshold detection, and classified by source location. Thus, dipole fit can be automated following SAM(g2). In this study, we compared the localization of interictal spikes by SAM(g2) and by dipole fit. The objective of this comparison was two-fold: 1) to determine if SAM(g2) and dipole fit are equivalent for cases in which there appears to be a single spike locus, and 2) to determine how they differ when multiple spike loci are present.
METHODS
MEG epilepsy data recordings from 10 patients with diagnoses of cortical dysplasia and medial temporal lobe epilepsy were analyzed using SAM(g2) and automated dipole fit. This was a retrospective study, as data from all patients had previously been subjected to manual spike marking and dipole fit. Several minutes of data were recorded from each patient in a passband of dc – 75 Hz (300 Hz sample rate) using a CTF 275-channel whole cortex MEG helmet. These data were divided into one-minute segments for our analysis.
The SAM(g2) algorithm begins with computing SAM coefficients for estimating the source waveforms at coordinates within the region of interest (ROI). The source waveform for any coordinate θ is simply the weighted sum of measurements (referred to as a “virtual sensor”):
where
C is the covariance matrix of MEG measurements M, and Bθ is the lead field (forward solution for a unit current dipole at θ). Excess kurtosis (g2) is used as a measure of spikiness at each coordinate. It is computed from each source waveforms as:
where σ is the standard deviation and T is the number of time samples. A functional image is made up from the g2 values at each coordinate.
The SAM(g2) images were computed for a ROI of x = {−10.0, 10.0 cm}, y = {−9.0, 9.0 cm}, z = {0.0, 14 cm}, relative to the head frame, in 5 mm steps; this ROI enclosed the entire head, in all cases. Epileptic activity was imaged by SAM(g2) for the 20 to 70 Hz frequency range which provided optimal image contrast for interictal spike activity. A list of the local maxima in the SAM(g2) images was saved, and SAM virtual sensors were computed for each location for viewing source waveforms in the dc – 75 Hz range. The same list of maxima was used to generate virtual sensors for the 20 – 70 Hz activity. The peak of each spike in the 20 – 70 Hz source time series was marked using an adaptive threshold-crossing algorithm, and a best-fit current dipole computed for ± 2 samples around each marker (5 samples). Dipoles having greater than 10% residual variance were rejected. In cases for which spikes appeared correlated among several loci, a two dipole fit was applied in addition to the single dipole fit. The SAM(g2) image and dipole markers were then co-registered with the corresponding MRI of each patient. The presence and number of interictal spikes was confirmed by viewing the dc – 75 Hz virtual sensors. We then compared the relative location of the SAM(g2) maxima to the centroid of the dipole scattergram. Spikes were also marked manually for subsequent dipole fit. These results were compared with the fits from automated dipole marking.
RESULTS
These 10 patients exhibited a variety of spike patterns. The automated SAM(g2) and dipole fit methods were successful, with no user intervention. In 7 out of 10 patients, excellent correlation was found between the SAM(g2) results and prior manual spike localization. In the remaining 3 cases, manual data analysis failed to localize MEG abnormalities (including one patient where magnetic dental work interfered with standard dipole analysis). The interictal spike loci found by SAM(g2) for these 3 patients was concordant with the other clinical findings.
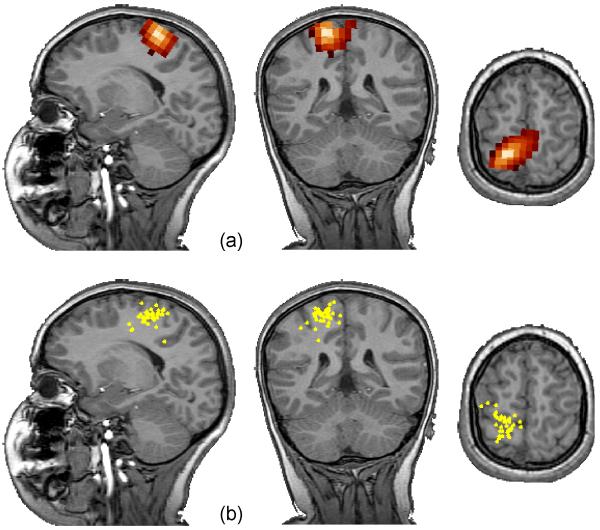
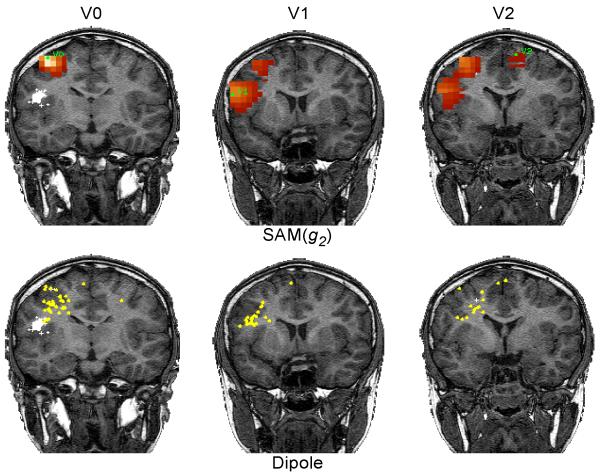
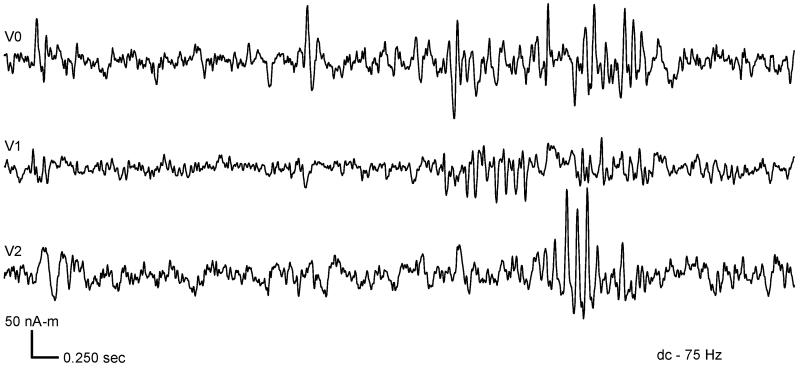
One example of a patient with a single interictal spike locus is shown in Fig. 1. A single maximum was found in the SAM(g2) image near the left central sulcus (Fig. 1a). There is little difference between the two methods other than that the corresponding dipole fit scattergram (Fig. 1b) indicates a slightly deeper source. Another patient presented with multiple spike loci. The coronal views are shown in Fig. 2. In the top row, each of three spike loci are shown – labeled V0 and V1 in the left hemisphere, and V2 in the right hemisphere. The virtual sensor waveforms for these sites are shown in Fig. 3. These appear to be independent regions of interictal spiking. That is, the source waveforms have little correlation with one another, although several spikes are coincident in time. The scattergram for single dipole fits to markers derived from each of the three source waveforms is shown in the bottom row of Fig. 2. All fits shown have less than 10% residual variance. Although the residual variance was reduced when fitting this data to two dipoles, this had little effect on the scattergram. The dipole fits appear in the same region indicated by SAM(g2), but were unable to discern three distinct loci from these plots. The automated spike markers were usually placed earlier than the corresponding manually placed markers. Dipole scatter was greater for the manually-marked data than for the SAM(g2) analysis.
Figure 1.
A single interictal spike locus is seen in both SAM(g2) (a) and dipole fit (b). The dipole fit scatter is small (for dipoles with < 10 % residual variance). However, its centroid is slightly deeper than that indicated by SAM(g2).
Figure 2.
In one of the patients, SAM(g2) found three spike loci, labelled V0, V1, and V2 (top row). Spikes were marked in each source waveform for these loci. Single dipole fits were then computed around each spike. The scatterplots (residual variance < 10 %) for spikes corresponding to each source are shown in the bottom row. The dipole scatterplots are unable to resolve the different loci.
Figure 3.
Three SAM virtual sensors, corresponding to the three maxima in the SAM(g2) image, show independent source waveforms with some spike correlation.
DISCUSSION
This study demonstrates that SAM(g2) is equivalent to the ECD fit for localizing MEG interictal spikes when there is a single spike locus and good SNR. We were able to obtain good quality ECD fits using SAM(g2) for automated spike detection. When multiple independent interictal spike loci were present, there was some disagreement between SAM(g2) and ECD scatter in the cases of low SNR spikes. When SAM(g2) indicated multiple coupled spike loci, the residual variance for dipole fit was higher and its scatter larger – even for multiple dipole models. The difference between dipole scatter for the automated and manually-placed spike markers is attributable to the SAM virtual sensors emphasizing the earliest portion of the spike, before excitation has spread. The placement of manual markers were based upon the MEG sensor waveforms, in which the spike peaks appear later than those seen in the virtual sensors. The increased scatter of dipole fits to manual markers results from the spread of excitation as the spike progresses. The automated spike marking procedure, using the SAM virtual sensors is not only faster than manual marking, it is also more accurate in that it shows where the spikes have originated and not where they have spread.
The multifocal example (Fig. 2 and 3) suggests that selection of model order for dipole fit could vary from spike to spike. Some spikes were independent while others were correlated. Had we examined the spectrum of singular values, it would have explained these data by a three source model. This is precisely what was found using SAM(g2). However, the three-source model does not necessarily apply to all spikes, as not all sources are active simultaneously. Hence, model order needs to be determined for each individual spike (or perhaps for each sample). This could be done by coincidence of the SAM(g2) automated markers.
Examination of these results calls to question whether dipole fit is truly necessary. SAM(g2) localization compared favorably with dipole fit in cases with a single interictal spike locus. In multi-focal cases, SAM(g2) consistently yielded a sharper representation of each site of spike generation. SAM(g2) has the advantage of being automated as well. Nonetheless, further testing will be necessary to determine the sensitivity and specificity of SAM(g2) analysis in single and multiple focus cases.
REFERENCES
- Robinson SE, Vrba J, Otsubo H, Ishii R. In: Nowak H, Haueisein J, Giessler F, Huonker R, editors. Finding epileptic loci by nonlinear parameterization of source waveforms; Proceedings of the 13th International Conference on Biomagnetism; Jena. 2002.Aug, pp. 220–222. [Google Scholar]