Abstract
Dietary restriction (DR) has been shown to increase life span and reduce disease incidence across a variety of species. Recent research suggests that chronic adult DR may also alter age-related cognitive decline. The purpose of this study was twofold: (1) to examine the potential deficits in spatial learning ability in the aged F344 X BN hybrid F1 rat with specific attention to the contributory effects of motoric impairments and (2) to determine the influence of chronic adult DR on any such impairments. The Morris water maze (MWM) task was employed with a 1.8 m diameter tank, 10 cm2 escape platform, 28°C water, and an automated collapsing central starting platform. Spatial learning impairments in the aged rats were evident on all dependent measures during training and the probe test. Motoric function, as reflected in measures of strength and locomotion demonstrated profound age-related performance impairments that were attenuated by chronic adult DR. The present data also replicate previous reports, indicating that DR attenuates the age-related impairments of performance in the MWM as indexed by the latency measure in acquisition, but critically was dissociated from any DR effect on measures of preference and, more critically, accuracy in the probe test. Collectively, the most parsimonious interpretation of DR effects on MWM performance would appear to be the preservation of motoric, and not cognitive, function.
Keywords: Cognition, Motoric function, Morris water maze
One of the more disconcerting age-related changes across a variety of species is a decline in learning and memory. Aging rodents have been shown to be a promising animal model for studying memory deficits. In particular, tasks of spatial memory have been employed [1-8] with clearly demonstrated deficits in aged rats as a model associated with aging humans [9-11]. Deficits that accompany the decline of spatial learning and memory in aged rats include a loss of visual acuity [12], thermoregulatory control [3], impairments in sensorimotor behavior [6, 8, 13], and the development of lesions and diseases [14]. These age-related deficits present a challenge to the accurate evaluation of spatial memory performance and have to be clearly distinguished from cognitive impairments in spatial memory performance.
The most commonly used rat strain in aging research is the Fischer 344 (F344), due to its extensive age-related database and the absence of genetic variability [15]. Genomic homogeneity, which is a feature of F344 rats due to inbreeding, results in reduced genetic variance [13, 16]. However, the specific tumor susceptibilities of this rat model and its functional life span of 26-month limits its utility. The National Institute of Aging (NIA) recently developed a new rat model, the Fischer 344 X Brown Norway (F344 X BN) hybrid F1 rat strain, which is heterozygous at every locus, but provides the genetic uniformity of inbred strains [13]. The enriched genetic combination in the hybrid F1 rats makes that strain more susceptible to environmental factors and provides a more robust rodent model for the study of aging in humans [13, 17]. Furthermore, F344 X BN hybrid F1 rats are especially recommended for aging research, due to lower tumor susceptibility and an increased life span of 39-months of age [15, 18]. Thus, the availability of F344 X BN hybrid F1 rats provides an opportunity to examine the process of aging with the advantage of the F344 stock, but without several important limitations.
Dietary restriction (DR) is the only known manipulation that can alter the aging process in rodents. Our understanding of the nature of DR and how it regulates the onset and rate of aging processes remains to be established. There is an impressive body of evidence showing that DR increases rats’ maximum life span and reduces diseases [14, 19, 20]. The generality of this phenomenon across a number of species suggests that DR may be used as an important model in studying aging-related processes [1, 7, 8].
Although DR effects on longevity are well established, an understanding of its effects on age-related motoric and cognitive deficits is still unclear and merits further investigation. There are some recent studies reporting that chronic adult DR alters age-related cognitive decline [6, 7, 21]. One early study [22] using an eight-arm radial maze showed that DR decreased age-related spatial deficits in 16-month old F344 rats, suggesting that DR had a beneficial effect on cognitive processes involved in spatial memory and learning. The majority of studies focusing on spatial memory and learning use the Morris water maze [23] and more specifically report a preservation of age-related cognitive deterioration by chronic adult DR in spatial reference and working memory [6, 7]. However, there are several studies with contrary results [1, 20, 24], suggesting that effects of DR on age-related processes in cognition seems to be much more constrained than its effects on longevity. Some literature even indicates that chronic calorie restriction may damage brain regions important to learning and memory [25-27].
One constraint on DR studies is the issue of genetic variance of the animals [13]. No favorable effect of chronic adult DR was detected on cognition in 24-month old F344 [24] and 30-month old BN rats, but chronic adult DR showed a beneficial effect on cognition in 30-month old F344 X BN hybrid F1 rats [13]. The authors argued that the heterogeneity of the hybrid F1 animals may be the critical factor required for the animals to exhibit susceptibility to beneficial effects of chronic adult DR on cognition. Thus, the reported effects of chronic adult DR on age-related learning impairments and its beneficial effects on cognitive function may be strain dependent. In light of the relatively limited knowledge about F344 X BN hybrid F1 rats and their advantages regarding genetic heterogeneity, it was considered important to examine the potential beneficial effects of DR on cognition in this strain.
A second constraint in studies examining effects of DR on cognition is the fact that studies commonly investigate old rats rather than aged rats near the end of their life span. Given that DR should alter the rate of aging the greatest difference between DR rats and rats fed ad libitum ought to be found near the end of their life span. Accordingly, an examination of the effects of chronic adult DR in aged rats near the end of their life span should be most informative.
A third constraint in DR studies is the capability of distinguishing between measures of cognitive and non-cognitive functions, such as motoric abilities. Although behavioral measures of cognitive function may never be completely pure or free of demands on motor systems [28] drawing inferences about effects of chronic adult DR on cognition will be facilitated with the use of multiple dependent measures. Thus, multiple dependent measures are important and necessary to increase the reliability and validity of assessing cognitive function. Accordingly, the use of multiple measures should specifically address the question of whether chronic adult DR has any robust effects on preservation of cognitive function.
The present study examined aged F344 X BN hybrid F1 rats near the end of their life span by assessing cognitive and motoric function with multiple measures. The purpose of this study was twofold: (1) to examine the potential deficits in spatial learning ability in 36-month old F344 X BN hybrid F1 rats with specific attention regarding contributory effects of motoric impairments and (2) to determine the influence of chronic adult DR on any such impairments.
Experimental Procedures
Subjects
F344 X BN hybrid F1 male rats (N = 36) were ordered from the colony at the National Center for Toxicology Research (NCTR) in Jefferson, Arkansas and used as subjects. At the NCTR all rats were fed a standard diet ad libitum (AL) (National Institute of Health (NIH)-31 standard diet). Beginning at 14 weeks of age the rats were either maintained on an AL diet or kept on a hypocaloric diet. The dietary restricted (DR) rats received a gradually reduced (over 2 weeks) daily food portion until their food intake was reduced to an amount of 60% of that received by the AL rats. For DR rats, the diet was reformulated (NIH-31 vitamin fortified) to include 1.67 x additional supplementation of all vitamins. Mineral concentrations were the same for both formulations. At NCTR the rats were housed individually with a light/dark cycle set so that the light was on from 0600 to 1800 h. The three groups of rats (ns = 12) received from the NCTR colony were: (1) a young 3-month old AL group, (2) an aged 36-month old population that were fed an AL diet throughout life, and (3) an aged 36-month old DR group. The animals were received two weeks prior their target age from NCTR. To minimize any stress associated with changing housing environment the animals were maintained in the same single housing environment as they had been at the NCTR. Single housing also precludes competition between rats for their daily food ration. Animals were housed according to NIH guidelines in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Specifically, the animal facility was maintained at 21 ± 2°C, 50 ± 10% relative humidity, and had a 12-h cycle with lights on at 0700 h (EST). The research protocol was approved by the Institutional Animal Care and Use Committee (IACUC).
Apparatus
The Morris water maze used during the experiment was an aluminum tank, painted flat black, measuring 1.8 m in diameter by 0.60 m high and was filled to a water depth of 0.40 m. The water temperature was maintained at 28 ± 1°C and no group differences in time of floating were noted. The escape platform measured 10 X 10 cm and was located 2.5 cm below the surface of the water level on a radius of 45 cm in one of the four locations within the North, South, East, or West arbitrarily designated quadrants. Black non-toxic tempura paint was added to the water to render it opaque. The tank, in a 2.40 X 2.40-m room, was surrounded by two sets of cues. One set consisted of four distinct background curtains, composing the walls of the environment: solid navy in the northern direction, solid white in the southern direction, vertical navy and white stripes (7.6 cm wide) in the eastern direction, and horizontal navy and white stripes (7.6 cm wide) in the western direction; the second set of cues consisted of 12 objects outside the tank perimeter. There were four hanging objects suspended from the ceiling ranging in size from a 20 cm coffee can to a 60 cm mailbox and eight objects on the curtains, including circles and quadrangles. An elevator, a centrally located hydraulic lift, served as the starting point for all animals. An black opaque bucket with a diameter of 15 cm and a height of 10 cm sat on top of the hydraulic lift. The bottom of the bucket sat 1 cm out of the water. The hydraulic lift collapsed to a height of 20 cm below the water surface with the initiation of a trial. A closed circuit video system, recessed mounted in the ceiling above the center of the pool, recorded the swimming behavior of the animals.
Procedures
Design
A mixed-model analysis of variance (ANOVA) was employed with group (young AL rats, aged AL rats, and aged DR rats) as the independent variable. The experiment was divided into four phases consisting of acquisition training on day 1, 2, and 3, and a probe test on day 3. In addition, an activity test was conducted a week before starting the water maze test, and a climbing and a vision test were conducted on day 4, testing physical functions. Two consecutive days prior to the start of the experiment each animal was handled for five minutes to gentle the animals so that they could be more easily handled for the experiment.
Locomotor activity test
Locomotor activity was assessed using an automated apparatus (San Diego Instrument, Inc.) that contained 8 pairs of infrared photocell beams 1.5 cm from the floor. Animals were placed individually into one of four 16 cm diameter round steel chambers under low levels of illumination and in the presence 70dB white noise. Locomotor activity was assessed over a 60 min test session and number of infrared photocell interruptions was the dependent variable.
Acquisition training
All animals received 20 training trials across 3 days: eight trials on day 1 and 2 followed by four training trials on day 3. The platform was located in a fixed location for each subject during training; however, platform location (North, South, East, or West) was balanced across subjects. A training trial began with the rat being placed into the pool in the open elevator located in the center of the pool, initially above the water surface. After a time period of 10 sec the elevator was lowered into the water and the animal’s swimming behavior was recorded for 60 sec. If the animal failed to find the platform within 60 sec, the trial ended and the rat was directed to the platform. The animal was allowed to remain on the platform for 15 sec before being removed and returned to a holding cage for a 5 min intertrial interval. The dependent variables were obtained using chromotrack software version 4.0 (San Diego Instruments, San Diego, CA). The dependent variables included escape response latency to the platform location, travel distance to the target location, and mean swim speed in cm per second. Because results for escape latency and travel distance were similar and highly correlated we focus specifically on latency to the platform for the more detailed evaluation of performance. The most important reason for including escape latency, within the design, as the dependent variable derived from the fact that many of the studies that assess chronic adult DR effects in spatial memory processes, rely primarily on latency measures [6, 13, 20, 24, 30].
Probe test
Immediately following the last acquisition trial on day 3 the platform was removed from the pool; the visual-spatial environment was left unaltered. The probe test was conducted like the acquisition training trials with the same starting position in the center of the pool. The animal’s swimming behavior was recorded for 60 sec. At the end of the 60 sec period the platform was placed in the pool and the animal permitted to find and remain on it for 15 sec. The dependent variables included swim speed, quadrant preference, i.e., the relative distribution of swimming time in each of the quadrants, and platform crossing, i.e., the number of crossings through the conceptual location of the platform in each of the quadrants, were included as dependent variables. Both measures were analyzed taking the quadrant containing the prior target platform into account relative to the opposite quadrant, to reflect the accuracy with which the animals are able to precisely locate the previous platform location (discrimination variable) [29, 31]. In addition, the centroid [9] is reported, which is a proximity index that records the mean distance between the animal and the platform location computed 18x/sec throughout the trial duration. It provides information about the accuracy of the rat’s entire search performance and identifies the rat’s position with respect to the exact platform location. Lower scores indicate a better performance. Mean swim speed in cm per second was also recorded.
Climbing test
The animal’s ability to climb onto an elevated platform on day 4 was used as an index of motor strength. A two tiered platform was used in this test. The bottom tier was 14 × 14 cm, and the top tier was 10 × 10 cm both black in color and were separated by a white 10 × 10 × 5 cm styrofoam block. Due to the black colored maze the white styrofoam block was clearly visible. Each rat was placed in the water with their two front feet resting on the edge of the lower tier. The latency for the animals to climb onto the top platform with all four limbs was recorded and used as an indicator of motoric strength. The maximum latency permitted was 30 sec.
Vision test
A final test was conducted to determine whether age-related impairments in the visual system contribute to difficulties in solving the spatial learning task. The platform was made visible by using a 5 cm high styrofoam block affixed to the top of the platform hat created a contrast to the black tank and water. The vision test included two trials, using the platform location in the adjacent quadrants of acquisition training. In trial one, the platform was randomly placed in one of the two adjacent quadrant locations of the pool unfamiliar to the animal and the latency to swim to the platform was recorded. In trial two, the alternative quadrant location was used with an otherwise identical procedure. The maximum latency permitted was 30 sec for each trial and the average of both trials was used.
Data analysis
All data were analyzed by an ANOVA using SPSS Statistical Software, Release 14 (2005) and SYSTAT Statistical Software, Release 11.2.01 (2004). For each task a mixed-model ANOVA comparing performance of the three groups was performed [32]. In addition, specific planned contrasts were employed to evaluate potential group and trial dependent effects. For example, planned contrasts were employed to access age effects (young AL vs. aged AL rats), DR effects (aged AL vs. aged DR rats), and the magnitude of DR effects (young AL vs. aged DR rats). Orthogonal decomposition analyses were conducted for each group to ascertain the nature of their learning function. One-sample t-tests and non-parametric tests were conducted as appropriate. Chi-Square tests were conducted to test for distributional group differences on the motor and cognitive measures. An alpha level of p ≤ 0.05 was considered significant for all statistical tests used. Violations of compound symmetry were treated preferentially with use of orthogonal decompositions, or, if necessary with the Greenhouse-Geisser degrees of freedom correction factor [33].
Results
Health and body weight
All young 3-month old AL and aged 36-month old DR rats completed the behavioral testing sessions and were without obvious health problems. In the aged 36-month old AL group, consistent with the animals being tested at the terminal phase of life, two rats died before the completion of the study. Four rats with large tumors were excluded from the study after day 1 of testing when it became obvious that the tumors interfered with their movement. The mean (±S.E.M.) body weight of the three rat groups, young AL, aged AL, and aged DR rats were 238 grams (±2.7), 521 grams (±4.2), and 298 (±2.8), respectively. A one-way ANOVA indicated a significant effect of group, F(2, 27) = 1749.9, p ≤ 0.001. Planned contrasts revealed an age effect between the aged AL rats and their 3-month old counterparts [F(1, 27) = 3411.9, p ≤ 0.001], a significant DR effect within the aged rat population [F(1, 27) = 2109.1, p ≤ 0.001], and a significant difference between the young AL and aged DR rats [F(1, 27) = 233.9, p ≤ 0.001]. Collectively, these results indicate that chronic DR modulates the large weight increase during aging with an intermediate position for aged adult DR animals relative to young AL and aged AL rats.
Acquisition training
Escape latencies
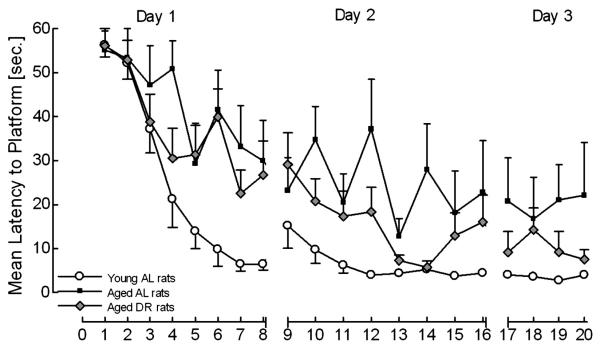
Escape latencies during acquisition training are shown in Figure 1 as a function of age and DR across the 20 training trials. A mixed-model ANOVA was conducted with additional orthogonal component analyses when necessary. Results of the ANOVA are summarized in Table 1. Planned comparisons revealed a significant age effect between young AL and aged AL rats [F(1, 27) = 19.3, p ≤ 0.001], a DR effect between aged AL and aged DR rats [F(1, 27) = 9.3, p ≤ 0.01], and no significant difference between young AL and aged DR rats. These results suggest a beneficial effect of chronic adult DR on response latency with the magnitude of the DR effect permitting the aged DR group to approximate the asymptotic performance level of the young AL group.
Figure 1.
Mean (±S.E.M.) escape response latency is presented by group as a function of training trials for the 3 days of acquisition training.
Table 1.
Results from ANOVAs on the multiple measures assessed in acquisition training and the probe test.
| Training | Dependent measures |
Group | Trial | Trial x Group |
|---|---|---|---|---|
| Acquisition (20 trials) |
Escape latency (sec) | F(2, 27) = 11.19*** | F(19, 513) = 20.40*** | F(2, 27) = 7.6**, q |
| Travel distance (cm) | F(2, 27) = 8.82** | F(19, 513) =17.42*** | F(2, 27) = 26.0***, q | |
| Speed (cm/sec) | F(2, 27) = 0.53 | F(19, 513) =3.33* | F(38, 513) = 1.54 | |
| Probe Test | Quadrant preference (sec) | F(2, 27) = 2.01 | F(3, 81) = 40.58*** | F(6, 81) = 8.05*** |
| Platform crossing (no.) | F(2, 27) = 10.10*** | F(3, 81) = 58.92*** | F(6, 81) = 11.44*** | |
| Centroid (cm) | F(2, 27) = 11.31*** | |||
| Speed (cm/sec) | F(2, 27) = 0.77 |
Note: p ≤ 0.001 (2-tailed),
p ≤ 0.01 (2-tailed),
p ≤ 0.05 (2-tailed);
q = quadratic component, no. = number of crossings
For day 1, planned comparisons revealed an age effect between young AL and aged AL animals [F(1, 27) = 9.5, p ≤ 0.01], but no significant difference between aged AL and aged DR animals, suggesting no DR effect. For day 2, planned comparisons revealed a significant age effect in performance between young AL and aged AL rats [F(1, 27) = 18.3, p ≤ 0.001] and a DR effect between aged AL and aged DR rats [F(1, 27) = 4.3, p ≤ 0.05]. However, a difference between young AL and aged DR rats [F(1, 27) = 7.3, p ≤ 0.05] indicated that the magnitude of the DR effect did not permit the performance of the DR group to attain the level of the young AL group. For day 3, planned comparisons revealed an age effect between young AL and aged AL animals [F(1, 27) = 13.7, p ≤ 0.001], and a DR effect between aged AL and aged DR animals [F(1, 27) = 5.1, p ≤ 0.05]. The failure to detect a significant difference between the young AL and the aged DR animals further suggested a beneficial effect of chronic adult DR as asymptotic performance levels were attained.
Overall, age-related deficits in escape response latency were apparent across all three days of acquisition training. Escape latency decreased systematically for all groups until asymptotic performance on the third and last testing day with most superior performance for the young AL rats and an intermediate position for the aged DR rats. Chronic adult DR significantly improved performance in the aged population of rats, specifically after repeated testing at day 2 and day 3, as indexed by escape latency.
Travel distance
Because analyses of escape latency and travel distance during training were found to be significantly highly correlated, both across (r = .91) and within groups (r = .81 - .96), results for travel distance are only reported in Table 1.
Swim speed
Statistics for swim speed during training revealed no significant group differences and are only reported in Table 1. The mean ± S.E.M. speed (cm/sec) across the 20 training trials was 23.6 ± 1.1 for the young AL rats, 25.7 ± 2.9 for the aged AL animals, and 24.2 ± 0.6 for the aged DR group.
Probe test
Quadrant preference
Figure 2a illustrates quadrant preference in the probe test, with the amount of time spent in each of the four quadrants when no platform was present. A one-way ANOVA was conducted on the discrimination variable; i.e., the time spent in the quadrant containing the target platform relative to the time spent in the opposite quadrant. A significant effect of group was noted [F(2, 27) = 8.5, p ≤ 0.01; ω2 = 0.33; 1-β = 0.99] with large effect size and high power. Planned contrasts demonstrated a significant discrimination effect between young AL rats and their aged AL counterparts [F(1, 27) = 15.3, p ≤ 0.001], suggesting an age effect. Young AL rats spent significantly more time in the prior target quadrant compared to the opposite quadrant relative to the aged AL rats. No significant difference was noted between the aged AL and the aged DR, suggesting that DR did not alter the age-related impairment in task performance. The lack of a DR effect was strongly supported by a significant difference between the young AL rats and the aged DR rats [F(1, 27) = 8.0, p ≤ 0.01]. Nevertheless, some minimal effect of DR on preference was suggested by the fact that time spend in the target quadrant by the aged DR rats was above chance performance [t(11) = 5.5, p ≤ 0.001].
Figure 2.
(a) Mean (±S.E.M.) time spent in the platform, opposite, right, and left quadrants during the probe test (chance performance is 15 sec/quadrant). (b) Mean (±S.E.M.) platform crossing in each quadrant by group during the probe test., ***Discrimination effect, p ≤ 0.001.
Overall, the quadrant preference data revealed superior performance for young AL rats relative to aged AL rats, indicating an age effect. Although, some DR effect was suggested by the time spent in the target quadrant per se relative to chance performance, this effect is not readily interpretable because no DR effect was found when comparing that group to the others on the discrimination variable, indicating that chronic adult DR did not have a beneficial effect on age-related impairments in relative quadrant preference.
Platform crossing
Figure 2b depicts the mean platform crossing of the potential target areas for each of the quadrants, reflecting the accuracy with which the animals were able to locate precisely the previous platform location during the probe test. A one-way ANOVA was conducted on the discrimination variable; i.e., the number of crossings in the quadrant containing the prior target platform relative to the number of crossings in the opposite quadrant. A significant effect of group was noted [F(2, 27) = 12.0, p ≤ 0.001; ω2 = 0.42; 1-β = 0.99] with large effect size and high power. Planned contrasts demonstrated a significant discrimination effect between young AL and aged AL rats [F(1, 27) = 20.3, p ≤ 0.001], indicating an age effect. Young AL rats crossed the prior target platform location compared to the opposite platform location significantly more often relative to aged AL rats. Further, no significant effect was noted between aged AL and aged DR rats, suggesting that DR did not alter age-related deficits in accuracy of navigation. The lack of a DR effect was strongly supported by a significant difference between the young AL rats and the aged DR rats [F(1, 27) = 12.9, p ≤ 0.001].
Overall, the platform crossing data revealed superior performance for young AL rats relative to aged AL rats, indicating a pronounced age effect. However, no statistically significant DR effect was found, suggesting that DR did not affect age-related deficits in accuracy in locating the prior platform location.
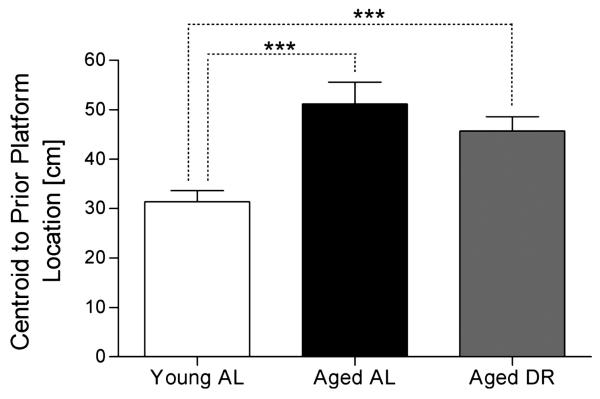
The centroid
The centroid performance for each group on the probe test is shown in Figure 3. An ANOVA revealed a significant group effect [F(2, 27) = 11.3, p ≤ 0.001]. Planned contrasts revealed a significant difference between young AL and aged AL [F(1,27) = 17.8, p ≤ 0.001], but no significant difference between aged DR and the aged AL, indicating that DR did not affect age-related performance deficits, suggesting that DR had no detectable effect on the age-related decline in accuracy of spatial navigation. The lack of a DR effect was strongly supported by the profound and significant difference between the young AL rats and the aged DR rats [F(1, 27) = 13.9, p ≤ 0.001].
Figure 3.
Mean (±S.E.M.) responses during probe test lasting 60 sec by group for the centroid., ***p ≤ 0.001
Swim speed
Statistics for swim speed during the probe test revealed no significant group differences (see Table 1).
Motor-behavioral tasks
Climbing test
Latency data from the climbing test, as an index of motor strength, are illustrated in Figure 4a. Because of marked heterogeneity of error variance with no error variance in one of the groups, the Kruskal-Wallis non-parametric test was used and revealed a significant group effect (χ2 = 25.17, p ≤ 0.001). A Mann-Whitney U test, comparing two independent samples, revealed a significant age effect between young AL and aged AL rats (p ≤ 0.001), a DR effect between aged AL and aged DR rats (p ≤ 0.001), and a difference between young AL and aged DR rats (p ≤ 0.001), indicating that although a DR effect permitted great improvement in performance, the magnitude of the DR effect did not permit the aged rats to approximate the performance level of the young AL rats.
Figure 4.
(a) Mean (±S.E.M.) latency assessed in the climbing test as an index of motor strength (b) Infrared (IR) photocell interruption (±S.E.M.) assessed in the activity test as an index of motor function. (c) Mean (±S.E.M.) latency assessed in the vision test as an index of visual ability., **p ≤ 0.01, ***p ≤ 0.001
Locomotor activity test
Locomotor activity as an index of motor function is illustrated in Figure 4b. The ANOVA revealed a significant effect of group [F(2, 27) = 17.6, p ≤ 0.001]. Planned contrasts revealed a significant age effect [young AL vs. aged AL rats, F(1, 27) = 34.6, p ≤ 0.001], a DR effect [aged AL vs. aged DR rats, F(1, 27) = 11.5, p ≤ 0.01] and a significant difference between young AL and aged DR was also noted [F(1, 27) = 9.3, p ≤ 0.01]. Thus, even though the magnitude of the DR effect promoted greater activity than aged AL rats, it was insufficient to restore activity levels to that of young adults.
Relationship between cognitive and motor-behavior measures
The individual data scores in Figures 5a and b clearly show that the aged AL rats are different from the aged DR and young AL rats on the motor dimension of locomotor activity with near non-overlapping distributions (Fisher Exact Probability Test: p ≤ 0.01 and p ≤ 0.001, respectively). Chronic adult DR clearly permitted the aged DR rats to increase their level of locomotor activity, although not to the level of performance of the young AL group. However, the effects of DR on the potential and various cognitive measures on the MWM probe test were markedly different. On the latency measure collected during the probe test (to the prior platform location) (Figure 5a) DR significantly improved performance in the aged population and permitted the aged DR rats to approximate performance of the young AL group as shown by their greatly overlapping distributions. In contrast, on the probe test centroid measure (Figure 5b), not only was no difference noted between the aged DR group and the aged AL rats, but the aged DR rats remained significantly impaired relative to the young AL rats (p ≤ 0.01), suggesting that although DR is able to improve escape response latency it failed to increase accuracy of searching as shown with the centroid measure.
Figure 5.
Individual animal scores on the putative cognitive measures of the probe test as a function of scores on tests within the motoric domain. Locomotor activity and (a) probe test ‘escape’ latency or (b) the probe test centroid. Motor strength and (c) probe test ‘escape’ latency or (d) the probe test centroid. The dotted lines represent the distributional division criteria.
For the second motor dimension, reflecting motor strength (see Figure 5c and d), chronic adult DR permitted the aged animals to increase their performance level in the direction of the distribution for the young AL rats, however, they remained non-overlapping. With respect to the cognitive measures, there were again disparate effects on latency and the centroid measure. For the latency measure (Figure 5c), DR significantly improved performance in the aged rat population and further suggested that the magnitude of the DR effect permitted the ages DR group to approach the performance level of the young AL group. For the centroid measure (Figure 5d), aged DR rats displayed no significant difference from the aged AL rats as shown by their overlapping distributions and they remained significantly impaired relative to the young AL rats (p ≤ 0.01). Overall, these results suggest compelling beneficial effects of DR on the dimensions of motoric function. Within the cognitive domain, a beneficial effect of DR was seen on the latency measure permitting the aged DR group to approach the performance level of the young AL group. However, no beneficial DR effect was observed on the probe test centroid, the cumulative measure of accuracy of spatial navigation.
Vision test
Latency data from the vision test are illustrated in Figure 4c. The ANOVA revealed no significant effect of group. All animals promptly found the visible platform, suggesting that no profound age-related visual impairments were apparent in the training and the probe test.
Discussion
The major finding of the present study was that chronic adult dietary restriction (DR) in F344 X BN hybrid F1 rats attenuated the age-related impairment of performance in the Morris water maze, as indexed by escape response latency in acquisition training, but little, if any, support for DR was found across other primary measures of quadrant preference and, more critically, of accuracy in the probe test, including platform crossings and the centroid. These results suggest that the beneficial effect of DR on escape response latency is more an index of the preservation of motoric, and not cognitive, function. This is a key issue as it addresses the question of whether cognitive function is affected by DR, and thus whether it is possible to alter the temporal progression of cognitive aging without altering motoric function. One remaining caveat, however, of this study is the possibility that chronic adult DR might alter the rate of age-related cognitive decline.
Three issues contributed to the goal of evaluating DR effects on cognitive and motoric function in aged F344 X BN hybrid F1 rats.
First, the terminal phase of a rat’s life span appeared to provide an important time at which to examine effects of DR on the functional life span and motoric function. The functional life span was extended with chronic adult DR when referring to survival rate and demonstrated the expected robust effects of DR. Thus, DR was beneficial to increase survival rate and longevity of F344 X BN hybrid F1 rats. Motoric function demonstrated age-related performance impairments. The data indicate that DR attenuates age-related losses in motoric function in F344 X BN hybrid F1 rats, when assessed near the end of their life span. Clearly, the effects of DR on motoric function, assessed both within and across tasks, gave evidence that DR had beneficial effects on motor systems. Previous studies have similarly reported beneficial effects of DR on age-related impairments in motor performance of rodents [6, 35].
Second, cognitive function demonstrated prominent age-related performance deficits. Robust spatial learning impairments in the aged AL rats were evident on all dependent measures during training and the probe test. These results are consistent with much previous research, demonstrating age-related deficits in cognitive function [4, 8, 36].
Evidence of preservation of cognitive function for aging by chronic adult DR was dependent on the phase of the task and dependent measures. During acquisition training results on escape response latency demonstrated significant age differences that were attenuated when aged AL rats were restricted by diet. Clearly, these results suggest a beneficial effect of DR on spatial learning in acquisition training and replicate previous reports, indicating that DR attenuates the age-related impairments of performance in the MWM as indexed by the latency measure [6, 20, 30]. The individual data scores in Figures 5a-d clearly indicate a beneficial effect of DR on the motor domain, reflected by locomotor activity and motor strength, and the dimension of cognitive function reflected by escape response latency. Although travel distance may be a preferable relative to escape latency as a measure of acquisition performance in the Morris water maze [28], our data did not show any crucial differences between escape latency and travel distance, with no difference in swim speed for the three groups. The finding that swim speed did not differ for the three groups is not an uncommon finding [3, 6], and may reflect configuration of the task to minimize thermal stress by using water with a temperature of 28°C. Preventing hypothermia by warming 23 month rats between trials in the MWM task has been shown to significantly improve performance [3]. Thus, the use of tepid water temperature in the present experiment may have been a factor in the outcome that swim speed did not differ between groups. The presence of deficits in climbing and locomotion but the absence of swim speed differences suggests that swim speed might be controlled by different mechanisms than climbing and locomotion.
In contrast, results of the probe test revealed little, if any, DR effects on cognitive function, as indexed by measures of preference and accuracy. The multiple measures used in the probe test, such as quadrant preference, platform crossing, and the centroid, consistently failed to show a beneficial DR effect. Consistent with other researchers [34] the centroid appears to be a powerful measure which utilizes computer tracking to index the rat’s implicit knowledge of the exact hidden platform location throughout the trial. Although there are no pure measures of cognitive function in the MWM [28] all of the measures of probe test performance, suggested the inference that DR had no beneficial effect on the cognitive processes involved in probe test performance. It is important to note that this conclusion is not readily dismissed as a failure of the DR manipulation per se, as there were prominent DR effects on survival, body weight, and motor function. An explanation for DR effects on acquisition performance in the absence of exhibiting superior probe performance is that DR might be effective in enhancing either learning or working memory process (acquisition training), without affecting long term or spatial memory (probe test). The differences that emerged in acquisition training on day 1 between aged DR rats and aged AL animals might be a reflection of differences in learning strategies used.
Third, by testing F344 X BN hybrid F1 rats at 36-month of age, the present study took advantage of their enriched genetic combination and suggested that genetic heterogeneity does not necessarily foster beneficial effects of DR on cognition when assessed near the end of their life span. Recently reported differences in DR effects on different strains [13, 24] argued that heterogeneity might be a factor that suggests a beneficial effect of DR on cognition. However, one of the limitations in these studies was the age when both of the inbred strains were tested. Whereas the F344 and BN rats were tested near the end of their life span, at 24-month and 30-month of age, respectively, the F344 X BN hybrid F1 rats were tested as old but not as aged rats, at 30 months of age. The reported beneficial effect of DR on cognitive aging found in the F344 X BN hybrid F1 generation of male rats [13] might be more a result of testing the animals earlier in their life span than their inbred strain counterparts. The absence of a beneficial effect of DR on cognition in F344 X BN hybrid F1 rats is supported by the fact that the authors did not find any beneficial effects in 9 month and 18 month old hybrid F1 rats [13]. Thus, it appears that DR does not have a beneficial effect on cognition; particularly not in F344 X BN hybrid F1 rats near the end of their life span.
In summary, the present study gives clear evidence for beneficial DR effects in 36-month old F344 X BN hybrid F1 rats near the end of their life span on (1) survival rate and (2) motoric function. Evidence for preservation of cognitive function by DR was primarily limited to escape response latency in acquisition training. In contrast, the measures of preference and, more critically, accuracy (platform crossing and centroid) provided no compelling evidence for a protective effect of chronic adult DR, suggesting that the age-related cognitive impairments are not altered by near lifelong DR. Thus, the most parsimonious interpretation of the beneficial DR effect in escape response latency in aged F344 X BN hybrid F1 rats near the end of their life span would appear to be the preservation of motoric, and not cognitive, function.
Acknowledgements
This study was supported, in part, by R01 grants from the NIH to RMB and CFM (DA013712, DA09160, HD043680, and DA013965) as well as a K02 award (DA014401).
References
- [1].Bellush LL, Wright AM, Walker JP, Kopchick J, Colvin RA. Caloric restriction and spatial learning in old mice. Physiol. Behav. 1996;60(2):541–547. doi: 10.1016/s0031-9384(96)80029-1. [DOI] [PubMed] [Google Scholar]
- [2].Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol. Aging. 1995;16(2):149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- [3].Lindner MD, Gribkoff VK. Relationship between performance in the Morris water task, visual acuity, and thermoregulatory function in aged F-344 rats. Behav. Brain Res. 1991;45:45–55. doi: 10.1016/s0166-4328(05)80179-2. [DOI] [PubMed] [Google Scholar]
- [4].Magnusson KR, Scruggs B, Aniya J, Wright KC, Ontl T, Xing Y, Bai L. Age-related deficits in mice performing working memory tasks in a water maze. Behav. Neurosci. 2003;117(3):485–495. doi: 10.1037/0735-7044.117.3.485. [DOI] [PubMed] [Google Scholar]
- [5].Mizumori SJY, Kalyani A. Age and experience-dependent representational reorganization during spatial learning. Neurobiol. Aging. 1997;18(6):651–659. doi: 10.1016/s0197-4580(97)00150-4. [DOI] [PubMed] [Google Scholar]
- [6].Pitsikas N, Carli M, Fidecka S, Algeri S. Effects of life-long hypocaloric diet on age-related changes in motor and cognitive behavior in a rat population. Neurobiol. Aging. 1990;11(4):417–423. doi: 10.1016/0197-4580(90)90008-n. [DOI] [PubMed] [Google Scholar]
- [7].Pitsikas N, Algeri S. Deterioration of spatial and nonspatial reference and working memory in aged rats: protective effect of life-long calorie restriction. Neurobiol. Aging. 1992;13(3):369–373. doi: 10.1016/0197-4580(92)90110-j. [DOI] [PubMed] [Google Scholar]
- [8].Shukitt-Hale B, Mouzakis G, Joseph JA. Psychomotor and spatial memory performance in aging male Fischer-344 rats. Exp. Gerontol. 1998;33(6):615–624. doi: 10.1016/s0531-5565(98)00024-2. [DOI] [PubMed] [Google Scholar]
- [9].Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairments in aging: Development of a learning index for performance in the Morris water maze. Behav. Neurosci. 1993;107(4):618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- [10].Gallagher M, Nicolle MM. Animal models of normal aging: relationship between cognitive decline and markers in hippocampal circuitry. Behav. Brain Res. 1993;57(2):155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- [11].Ingram DK, Spangler EL, Iijima S, Ikari H, Kuo H, Greig NH, London ED. Rodent models of memory dysfunction in Alzheimer’s disease and normal aging: moving beyond the cholinergic hypothesis. Life Sci. 1994;55(25-26):2037–2049. doi: 10.1016/0024-3205(94)00384-x. [DOI] [PubMed] [Google Scholar]
- [12].O’Steen WK, Spencer RL, Bare DJ, McEwen BS. Analysis of severe photoreceptor loss and Morris water-maze performance in aged rats. Behav. Brain Res. 1995;68:151–158. doi: 10.1016/0166-4328(94)00168-f. [DOI] [PubMed] [Google Scholar]
- [13].Markowska AL, Savonenko A. Retardation of cognitive aging by life-long diet restriction: implications for genetic variance. Neurobiol. Aging. 2002;23:75–86. doi: 10.1016/s0197-4580(01)00249-4. [DOI] [PubMed] [Google Scholar]
- [14].Lipman RD, Dallal GE, Bronson RT. Effects of genotype and diet on age-related lesions in ad libitum fed and calorie-restricted F-344, BN, and BNF3F1 rats. J. Gerontol. 1999;54A(11):B478–491. doi: 10.1093/gerona/54.11.b478. [DOI] [PubMed] [Google Scholar]
- [15].Sprott RL. Development of animal models of aging at the national institute on aging. Neurobiol. Aging. 1991;12(6):635–638. doi: 10.1016/0197-4580(91)90113-x. [DOI] [PubMed] [Google Scholar]
- [16].Sprott RL, Ramirez I. Animal models of aging research: Current inbred and hybrid rat and mouse models. ILAR Journal. 1997;V38(3) doi: 10.1093/ilar.38.3.104. [DOI] [PubMed] [Google Scholar]
- [17].Lindner MD, Schallert T. Aging and atropine effects on spatial navigation in the Morris water task. Behav. Neurosci. 1988;102(5):621–634. doi: 10.1037//0735-7044.102.5.621. [DOI] [PubMed] [Google Scholar]
- [18].Van der Staay FJ, Blokland A. Behavioral Differences between outbred Wistar, inbred Fischer-344, Brown Norway, and hybrid Firscher-344 X Brown Norway rats. Physiol. Behav. 1996;60(1):97–109. doi: 10.1016/0031-9384(95)02274-0. [DOI] [PubMed] [Google Scholar]
- [19].Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. C.C. Thomas; Springfield, Ill.: 1988. [Google Scholar]
- [20].Yanai S, Okaichi Y, Okaichi H. Long-term dietary restriction causes negative effects on cognitive functions in rats. Neurobiol. Aging. 2004;25:325–332. doi: 10.1016/S0197-4580(03)00115-5. [DOI] [PubMed] [Google Scholar]
- [21].Gould TJ, Bowenkamp KE, Larson G, Zahniser NR, Bickford PC. Effects of dietary restriction on motor learning and cerebellar noradrenergic dysfunction in aged F-344 rats. Brain Res. 1995;684:150–158. doi: 10.1016/0006-8993(95)00407-h. [DOI] [PubMed] [Google Scholar]
- [22].Stewart J, Mitchell J, Kalant N. The effects of life-long food restriction on spatial memory in young and aged Fischer 344 rats measured in the eight-arm radial and the Morris water mazes. Neurobiol. Aging. 1989;10(6):669–675. doi: 10.1016/0197-4580(89)90003-1. [DOI] [PubMed] [Google Scholar]
- [23].Morris RGM. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981;12:239–260. [Google Scholar]
- [24].Markowska AL. Life-long diet restriction failed to retard cognitive aging in Fischer-344 rats. Neurobiol. Aging. 1999;20(2):177–189. doi: 10.1016/s0197-4580(99)00031-7. [DOI] [PubMed] [Google Scholar]
- [25].Lee J, Herman JP, Mattson MP. Dietary restriction selectively decreases glucocorticoid receptor expression in the hippocampus and cerebral cortex of rats. Exp. Neurol. 2000;166(2):435–441. doi: 10.1006/exnr.2000.7512. [DOI] [PubMed] [Google Scholar]
- [26].Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4(2):117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- [27].Sapolsky RM. Do the salutary effects of food restriction occur because of or despite of the accompanying hyperadrenocorticism? Neurobiol. Aging. 1995;16(5):849–850. doi: 10.1016/0197-4580(95)00068-p. [DOI] [PubMed] [Google Scholar]
- [28].Lindner MD. Reliability, distribution, and validity of age-related cognitive deficits in the Morris water maze. Neurobiol. Learn. Mem. 1997;68:203–220. doi: 10.1006/nlme.1997.3782. [DOI] [PubMed] [Google Scholar]
- [29].Carman HM, Mactutus CF. Ontogeny of spatial navigation in rats: A role for response requirements? Behav. Neurosci. 2001;115(4):870–879. [PubMed] [Google Scholar]
- [30].Algeri S, Biagini L, Manfridi A, Pitsikas N. Age-related ability of rats kept on a life-long hypocaloric diet in a spatial memory test. Longitudinal observations. Neurobiol. Aging. 1991;12:277–282. doi: 10.1016/0197-4580(91)90003-3. [DOI] [PubMed] [Google Scholar]
- [31].Carman HM, Mactutus CF. Proximal versus distal cue utilization in spatial navigation: the role of visual acuity? Neurobiol. Learn. Mem. 2002;78(2):332–346. doi: 10.1006/nlme.2002.4062. [DOI] [PubMed] [Google Scholar]
- [32].Winer BJ. Statistical principles in experimental design. 2nd ed McGraw-Hill; New York: 1971. [Google Scholar]
- [33].Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- [34].Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav. Neurosci. 1993;107(4):618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- [35].Ingram DK, Weindruch R, Spanger EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J. Gerontol. 1987;42(1):78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- [36].Wyss JM, Chambless BD, Kadish I, van Groen T. Age-related decline in water maze learning and memory in rats: strain differences. Neurobiol. Aging. 2000;21:671–681. doi: 10.1016/s0197-4580(00)00132-9. [DOI] [PubMed] [Google Scholar]