Abstract
In the United States, one-third of infected individuals contracted Human Immunodeficiency Virus-1 (HIV-1) via injecting drugs with contaminated needles or through risky behaviors associated with drug use. Research demonstrates concomitant administration of psychostimulants and HIV-1-proteins damage neurons to a greater extent than viral proteins or the drug alone. To model the onset of HIV-1-infection in relation to a history of drug use, the current research compared behavior and extracellular dopamine and metabolite levels following Tat1–86 infusions in animals with and without a history of cocaine (Coc) experience (10 mg/kg; i.p.; 1 injection/day × 9 days). Animals receiving a behaviorally sensitizing regimen of Coc demonstrated a decrease in extracellular dopamine concentration in the nucleus accumbens, consistent with evidence describing up-regulation of dopamine transporter uptake. Contrary to this effect, Tat1–86 microinfusion into the nucleus accumbens following the sensitizing regimen of Coc caused a significant increase in extracellular dopamine levels (nM) within 48 h with no difference in percent of baseline response to Coc. After 72 h, Tat + Coc treated animals demonstrated a blunted effect on potassium-stimulated extracellular dopamine release (percent of baseline) with a corresponding decrease in expression of behavioral sensitization to Coc challenge. A persistent decrease in extracellular dopamine metabolite levels was found across all time-points in Tat-treated animals, regardless of experience with Coc. The current study provides evidence for divergent neurochemical and behavioral outcomes following Tat-treatment; contingent upon experience with Coc.
Keywords: cocaine, dopamine, dopamine transporter, HIV-1, sensitization, Tat
Psychostimulant drug use, including that of cocaine (Coc), is a serious public health concern (National Institute on Drug Abuse 2009). This is attributed, in part, to plasticity and toxicity to the dopaminergic neural systems originating in the brainstem and extending to limbic structures in the brain (Ferris et al. 2008; Yamamoto and Raudensky 2008; Buttner et al. 2005). In addition, drug abuse and high-risk behavior is an established vehicle for the propagation of Human Immunodeficiency Virus-1 (HIV-1) (Baggaley et al. 2006; Hudgens et al. 2002; Kaplan and Heimer 1992). Drug abuse disorders and consequent neuronal adaptations will typically precede HIV-1 infection. Therefore, the acquisition of HIV-1 in relation to drug use suggests that basic research designed to administer the virus/proteins concomitant with an acute drug administration may not fully model the newly HIV-1-infected, injection drug user. Indeed, in vivo research on the interactions of Coc use and HIV-1 infection is lacking, and the vast majority of extant work does not attempt to model the progression of drug intake in relation to a later onset of HIV-1-infection. Therefore, the current research is designed to address the lack of basic research regarding HIV-1-induced neurotoxicity in established models of drug abuse/addiction.
An additional impetus for this research originates from the convergence of both HIV-1 protein, Tat1–86, and Coc actions on mesolimbic dopamine (DA) systems in the brain, including DA nerve terminals in the ventral striatum (Chang et al. 2008; Silvers et al. 2006; Aylward et al. 1993). For example, Tat has been recently demonstrated to inhibit the DA transporter (DAT) (Zhu et al. 2009a; Ferris et al. 2009a; Aksenov et al. 2008). Similarly, Coc is known to alter DA nerve terminal morphology (Horne et al. 2008), function, and neurochemistry (Lack et al. 2008) through interactions with the DAT protein. These Coc-induced alterations include regulation of DAT trafficking (Zahniser and Sorkin 2009; Crits-Christoph et al. 2008), DAT conformational states (Schmitt et al. 2008; Mateo et al. 2005), DA uptake (Lack et al. 2008; Mateo et al. 2005), and altered DA release (Argilli et al. 2008). Given Coc-induced DA system plasticity – especially plasticity of established loci of HIV-1 protein action – we hypothesized that Tat-induced toxicity at the DA nerve terminal is differentiated between drug-experienced and drug-naïve populations. Indeed, others have outlined the clinical variants of HIV-1-induced pathology, which includes discriminating the drug abusing and general populations (Everall et al. 2005) in terms of disease progression and pathology (Nath, 2010).
There are many basic research models of drug abuse, and ‘sensitization’ is often used to assess neuroplasticity following repeated Coc exposure. Drug sensitization – whereby a rodent becomes increasingly sensitive to the effects (e.g., behavioral) of drug challenge after repeated, intermittent drug administration – is a model hypothesized to describe the transition from recreational drug use to addiction. The corresponding changes in neurochemistry and nerve-terminal morphology have been extensively documented (Lack et al. 2008; Nader et al. 2006) and correlate well with behavioral alterations in this model (Marin et al. 2009; Sabeti et al. 2003).
In the present study, we seek to understand HIV-1 protein Tat-induced alterations in nerve terminal function and behavior within both Coc-sensitized and drug-naïve animals. An advantage of this design is that it mimics the onset of HIV-1 exposure in relation to abuse of drugs over time, providing an understanding of how HIV-1 proteins interact with a DA system that has changed as a consequence of repeated Coc exposure. Thus, Coc-induced plasticity may be important for the drug abuse variant of HIV-1 neurological pathology, which may specifically target the dopaminergic system.
Materials and methods
Animals
Forty male Sprague-Dawley rats (300–350 g; Harlan Laboratories, Birmingham, AL, USA) were double-housed on a 12-h light/dark cycle with food and water available ad libitum. All animals were handled and habituated daily for at least 1 week prior to surgery. After surgery, the animals were single-housed. All animals were maintained according to the National Institutes of Health (NIH) guidelines in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of South Carolina, Columbia (assurance number A-3049-01).
Surgery
All animals were anesthetized prior to surgery using sevoflurane gas, induced using 7% inhalant and maintained at 3% inhalant for the duration of the surgery. All animals received one microdialysis guide cannula (Bioanalytical Systems, Inc., West Lafayette, IN, USA; BAS) in either the left or the right nucleus accumbens (NAc) using stereotaxic coordinates AP +1.2 mm, L ±2.0 mm, DV −5.0 mm relative to Bregma, midline, and skull surface, respectively. Guide cannulas were fixed to the skull with metal screws and dental acrylic/cement. Immediately following surgery, animals were given one subcutaneous injection of buprenorphin for pain (0.1 mg/kg). Animals were allowed 3–5 days to recover following surgery and before the first microdialysis session and commencement of the injection schedule.
Drug injection and microdialysis schedule
The experiment consisted of 14 days of once-daily injections of either Coc (10 mg/kg/mL) or saline (Sal; 1 mL/kg), three microdialysis sessions (on Days 1, 9, and 11), and 3 days of locomotor activity assessment (on Days 12, 13, and 14). The injection, microdialysis, and locomotor schedules are described herein, and specifics of the microdialysis and locomotor assessment procedures are described in subsequent sections.
On the morning of the first microdialysis session (Day 1), all animals were randomly assigned to either the Sal or Coc treatment group. All animals received their first intraperitoneal (i.p.) injection of either Sal (1 mL/kg) or Coc (10 mg/kg/mL) within the testing chambers, so that the neurochemical response to acute injection of Sal/Coc could be monitored. Following the first injection in the microdialysis chambers, animals then received one daily injection of either Sal or Coc in their home cages for seven consecutive days (i.e., Days 2–8).
Immediately preceding the home cage i.p. injection on Day 7, awake freely-moving rats were administered an intra-accumbal infusion of the recombinant HIV-1 protein Tat1–86 (LAI/Bru strain of HIV-1 clade B, GenBank accession no. K02013; Diatheva, Fano, Italy) or vehicle (Veh) control, depending on randomly assigned group membership; splitting the Sal and Coc treated groups into four groups (Veh + Sal, Veh + Coc, Tat + Sal, Tat + Coc). Specifically, stylets were removed from the guide cannulas and replaced with an infusion probe (BAS). This probe was used to infuse either 20 µg of Tat1–86 in 5 µL (4 µg/µL) of artificial CSF (aCSF), or the aCSF (5 µL) as control. The concentration of Tat1–86 was chosen because it is well within the range of in vivo concentrations utilized in studies demonstrating striatal neurotoxicity (cf, Bansal et al. 2000; Cass et al. 2003; Theodore et al. 2006), it has been shown to be neurotoxic in our model (Bansal et al. 2000), and it is consistent with earlier microdialysis experiments performed in our laboratory (Ferris et al. 2009a).
A second microdialysis session was performed 2 days (48 h) following the micro-infusion of Veh/Tat1–86 (i.e., on Day 9) to monitor the neurochemical response to i.p. Coc or Sal injection. Following this session, all animals received one daily i.p. injection of Sal (1 mL/kg) in their home cages for two consecutive days (Days 10–11). A third microdialysis session was performed on Day 11 to monitor the local neurochemical response to 100 mM of potassium (KCl) perfused through the inlet line.
In the days that followed (Days 12–13), all animals continued to receive one daily i.p. injection of Sal (1 mL/kg), but were immediately placed in a locomotor activity chamber for 60 min to habituate to the locomotor testing apparatus. Finally, on the last day (Day 14), all animals were given a challenge i.p. injection of Coc (10 mg/kg/mL) and placed immediately in locomotor activity chambers for 60 min. Immediately following the last day of locomotor testing, animals were killed and brains were extracted and frozen for histological assessment of probe placement.
Microdialysis
On the morning of each microdialysis session, stylets were removed from the guide cannulas and replaced with a microdialysis probe (BAS) composed of a semipermeable polyacrylonitrile membrane which extended 2.0 mm beyond the ventral tip of the guide cannulas. The probes were continuously perfused at 2.0 µL/min with aCSF: NaCl 150 mM, KCl 3.0 mM, CaCl2 1.7 mM, MgCl2 0.9 mM, d-glucose 4.9 mM; pH = 6.5.
The procedure for Session 1 (Day 1) and Session 2 (Day 9) was identical. Collection of dialysates in 15 min fractions from the probes began 2 h following probe insertion. Dialysates were collected in vials with 5.0 µL of a solution containing perchloric acid (0.05 N), sodium bisulfite (200 mM) and EDTA (1.0 mM) to minimize spontaneous oxidation of DA. After the third baseline collection, animals were given an i.p. injection of either Coc (10 mg/kg/mL) or Sal (1 mL/kg) within the testing bowls according to group membership established on Day 1. All injections were timed and administered within 1 min following the third baseline collection. These sessions ended after collecting five fractions following the i.p. injection, for a total of eight collected fractions.
Session 3 (Day 11) was the same as Sessions 1 and 2, with a single exception. Instead of receiving an i.p. injection in the testing chamber, the probe inlet line from each animal was switched to aCSF with a higher concentration of KCl (100 mM), which is often used to evoke DA release from local surrounding tissue (Ferris et al. 2009b; Cass et al. 2003). This solution was perfused for a single fraction (Fraction #4), after which the probe inlet line was switched back to the original aCSF. This session ended after collecting four fractions following the switch back to original aCSF for a total of eight fractions collected.
Locomotor activity
The activity monitors were square (40 × 40 cm) locomotor activity chambers (Hamilton-Kinder Inc., Poway, CA, USA) that detected free movement of animals. This equipment used an infrared photocell grid (32 emitter/detector pairs) to measure locomotor activity by infrared photocell interruptions. The chambers were converted into round (~ 40 cm diameter) compartments by adding clear Plexiglas inserts in order to prevent animals from resting in corners; and photocell emitter/detector pairs were tuned by the manufacturer to handle the extra perspex width. Research from our laboratory demonstrates that circular chambers may facilitate the detection of behavioral sensitization (Harrod et al. 2005). All activity monitors were located in a room isolated from the rat colony and microdialysis chambers.
The procedure for locomotor activity habituation and assessment was identical on Days 12 and 13. All animals were injected with Sal (i.p.; 1 mL/kg) and immediately placed into the center of the locomotor activity chambers for 60 min to record activity. The procedure for locomotor activity during Coc challenge (Day 14) was the same as that used on Days 12 and 13, with a single exception. Instead of receiving Sal, all animals were injected with Coc (i.p.; 10 mg/kg/mL) and immediately placed into the center of the locomotor activity chambers for 60 min to record activity.
Multiple automated measures of activity – including total activity, distance traveled in the center and periphery (cm), nose pokes into the periphery and center, entries into center and periphery, and time spent in center and periphery (s) – were investigated. Total activity represents all detectable movements (photocell interruptions) that occur in the chamber. ‘Center’ dependent measures refer to a circular region located in the center most portion of the compartment. ‘Peripheral’ dependent measures refer to a circular region surrounding the center located in the outer-most portion of the compartment. In order to determine the activity in center versus periphery, Hamilton-Kinder, Inc. software was used to impose a circular region (~ 25% of total area) in the center of the compartment during the data reduction phase (i.e., following completion of the activity session) of the experiment, yielding the activity data in the center. Time course data for all automated measures of activity are presented in 5-min blocks of a 60-min session, or collapsed across the whole 60-min session.
HPLC
All dialysates were analyzed by liquid chromatography with electrochemical detection. Separation of DA from metabolites was achieved by injecting 15 µL of each sample onto a C-18 analytical column (100 × 1 mm; 3 µm; BAS) using a mobile phase (pH 3.4) containing 14.5 mM NaH2PO4, 30 mM sodium citrate, 27 µM disodium EDTA, 10 mM diethylamine HCl, 2.2 mM 1-octane sulfonic acid, 4% acetonitrile and 1% tetrahydrofuran at a flow rate of 100 µL/min. DA was detected by oxidation at a glassy carbon electrode with an applied potential of +650 mV versus an Ag/AgCl reference electrode. Peaks corresponding to DA were quantified by comparison with a three point external standard curve bounding the expected range of DA values.
Results
Acute Coc response
As expected, Session 1 DA levels (concentration and % of baseline) significantly increased, while 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) levels (% of baseline) significantly decreased following Coc administration relative to baseline and Sal-treated controls. There were no baseline differences in DA or metabolite levels between groups.
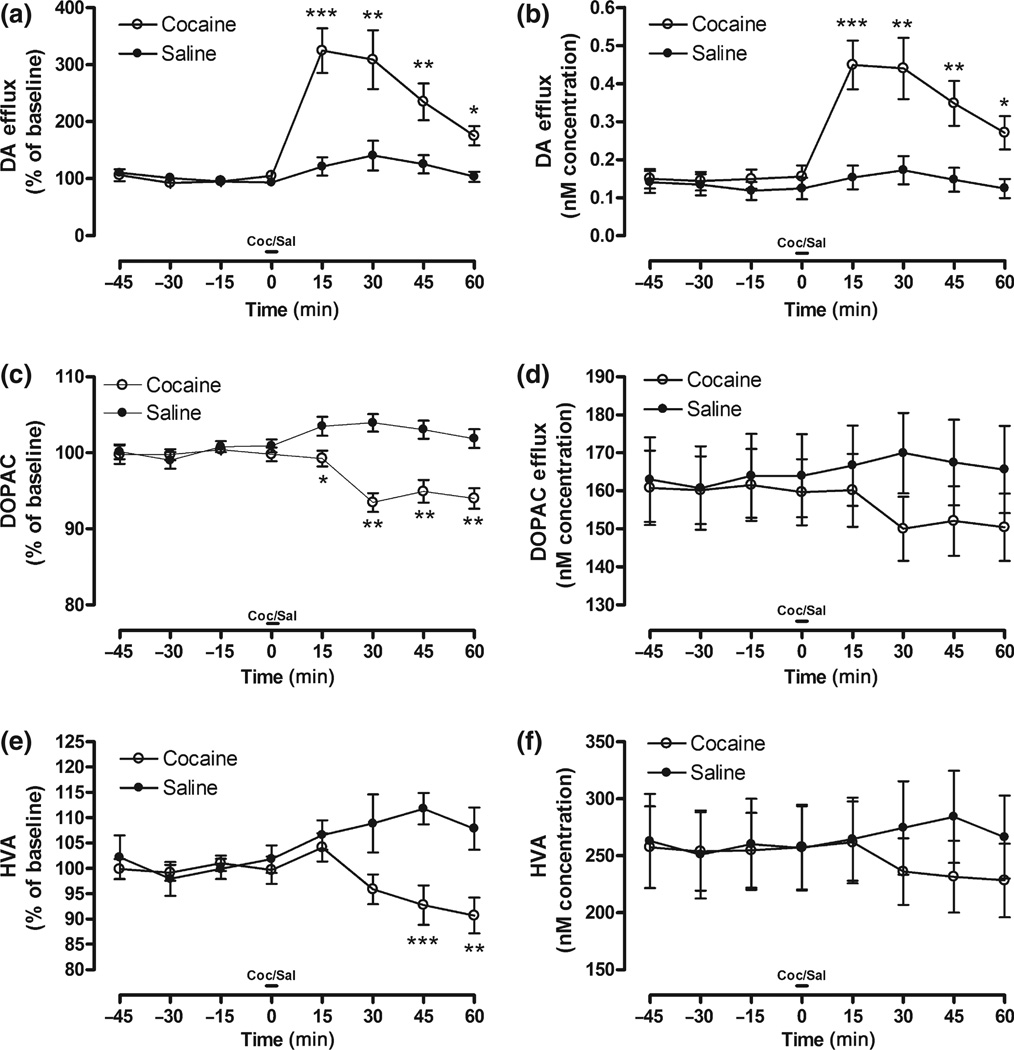
With respect to DA levels, the 8 (Time; within) × 2 (Coc vs. Sal) mixed-model anova using DA recovery (% of baseline) as the dependent variable indicated a main effect of Coc, F(1, 28) = 12.78, p < 0.001, and a Coc × Time interaction, F(7, 196) = 17.45, p < 0.0001. Raw data demonstrated a similar finding with a main effect of Coc, F(1, 28) = 6.33, p < 0.01, and a Coc × Time interaction, F(7, 196) = 11.37, p < 0.0001. To further specify these effects, planned comparisons were performed on time-points as indicated in Fig. 1(a and b). These results indicate that Coc significantly increased DA overflow in the NAc.
Fig. 1.
DA (a,b), DOPAC (c,d), and HVA (e,f) overflow in response to acute injection of Coc or Sal prior to Tat or Veh infusion represented as percent of baseline (left column) and concentration (nM; right column) across time. Note: Significant planned comparisons following anova noted (*p < 0.05, **p < 0.01, ***p < 0.001) at each time-point.
Dopamine metabolites decreased following acute Coc injection. There was a main effect of Coc on DOPAC levels (%), F(1, 36) = 29.95, p < 0.0001, and a Coc × Time interaction, F(7, 252) = 9.32, p < 0.0001. Planned comparisons demonstrated significantly lower DOPAC levels at various time-points in Fig. 1(c). Raw DOPAC demonstrated a Coc × Time interaction, F(7, 252) = 3.04, p < 0.01. Planned comparisons were not performed on raw data time-points, as there was no main effect of Coc in the anova. For HVA levels (%), there was a main effect of Coc, F(1, 34) = 8.047, p < 0.01, and a Coc × Time interaction, F(7, 238) = 7.05, p < 0.001. Planned comparisons demonstrated significantly lower HVA levels at various time-points in Fig. 1(e). Raw HVA demonstrated a Coc × Time interaction, F(7, 238) = 4.97, p < 0.0001. Planned comparisons were not performed on raw data time points, as there was no main effect of Coc in the anova.
Cocaine response 48 h post-Tat infusion
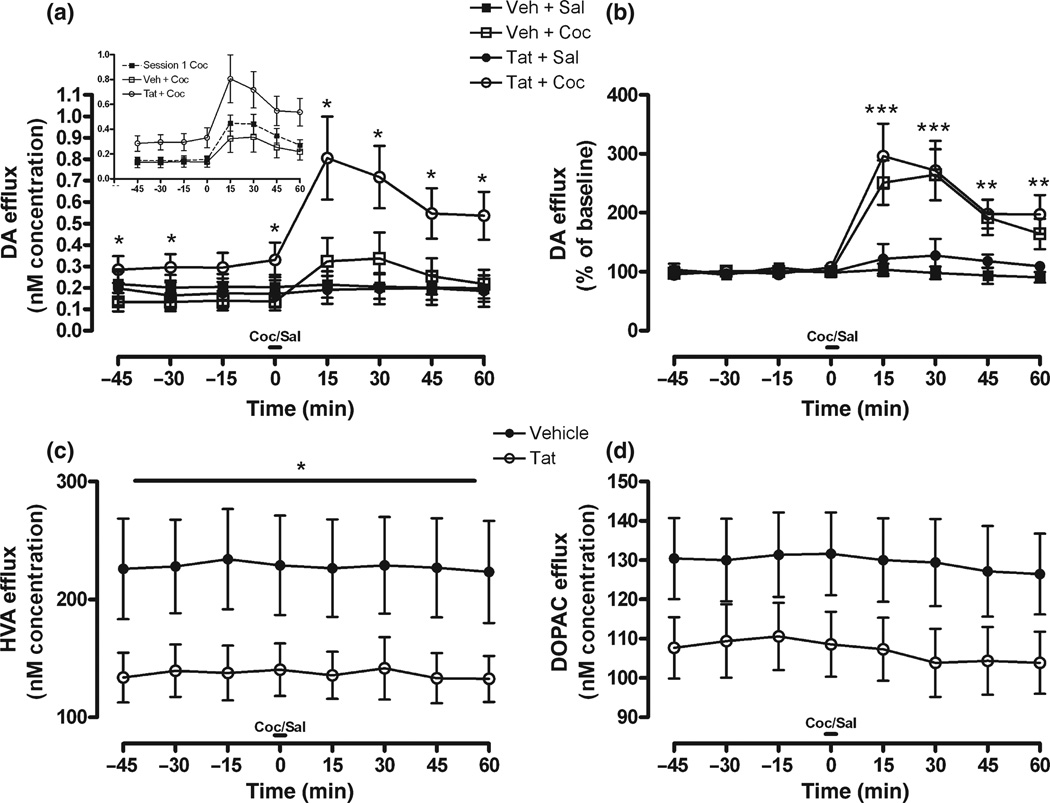
The response to 10 mg/kg Coc challenge was monitored 8 days into repeated Coc administration and 48 h following intra-accumbal Tat/Veh infusion. For raw data, a Coc × Time interaction indicated that Coc, but not Sal injection increased DA levels above their respective baselines, F(7, 182) = 9.54, p < 0.0001. However, because Tat/ Veh-infusion affected basal DA tone in an opposite manner, these groups averaged to indicate no main effect of Coc relative to Sal levels at any given time-point. When groups were analyzed individually (Fig. 2a), these raw data showed that Tat + Coc treated animals had significantly higher basal and Coc-evoked DA levels relative to all groups. Contrary to this, the Veh + Coc group had lower basal DA levels, which contributed to the inability of DA levels to elevate past Sal-treated animals, despite a significant response to Coc. Planned comparisons shown in Fig. 2(a) demonstrated specific time points in which these two groups diverged. In addition, Tat / Veh infusion differentially affected DA tone relative to Session 1 DA levels (Fig. 2a; inset), as the groups’ Coc response diverged from Session 1 levels in opposite directions. Given that raw DA concentration increased following Coc-challenge for both Tat and Veh-treated animals, and the fact there were significant differences in basal extracellular DA levels, data were expressed as percent of baseline in order to more specifically examine any difference in the DA systems response to Coc. When data were plotted as percent of baseline (Fig. 2b), DA levels in both Tat + Coc and Veh + Coc treated animals increased significantly, F(1, 26) = 16.59, p < 0.0001, as was the case in Session 1. Planned comparisons demonstrated significant effects for all time-points following Coc injection.
Fig. 2.
DA (a,b), HVA (c), and DOPAC (d) overflow in response to Coc or Sal challenge 48 h following Tat or Veh infusion; represented as concentration (nM; a,c,d) and percent of baseline (b) across time. In addition, DA overflow in response to Coc challenge in both Tat- and Veh-treated animals is shown relative to acute Coc response from Session 1 (a; inset). For metabolites (c,d), Coc-pretreatment had no effect on Tat-induced reduction of DOPAC or HVA levels, therefore Sal/Coc factor was collapsed to show the main effect of Tat-treatment, irrespective of Coc-treatment. Metabolite response to Coc-challenge, expressed as percent of baseline, was no different than Session 1; thus was not presented. Note: Significant planned comparisons following anova noted (*p < 0.05, **p < 0.01, ***p < 0.001) at each time-point.
With respect to metabolites, Tat infusion significantly reduced raw HVA levels, as shown in Fig. 2(c), F(1, 33) = 3.94, p < 0.05, while raw DOPAC levels (Fig. 2d) demonstrated a strong, albeit non-significant attenuation. Percent of baseline data indicated Coc × Time interactions for both HVA, F(7, 231) = 2.02, p < 0.05, and DOPAC, F(7, 245) = 2.04, p < 0.05. Post-injection metabolite levels for Coc-treated animals were reduced by 5–10%, irrespective of Tat-treatment (data not shown); similar to the response to Coc in Session 1.
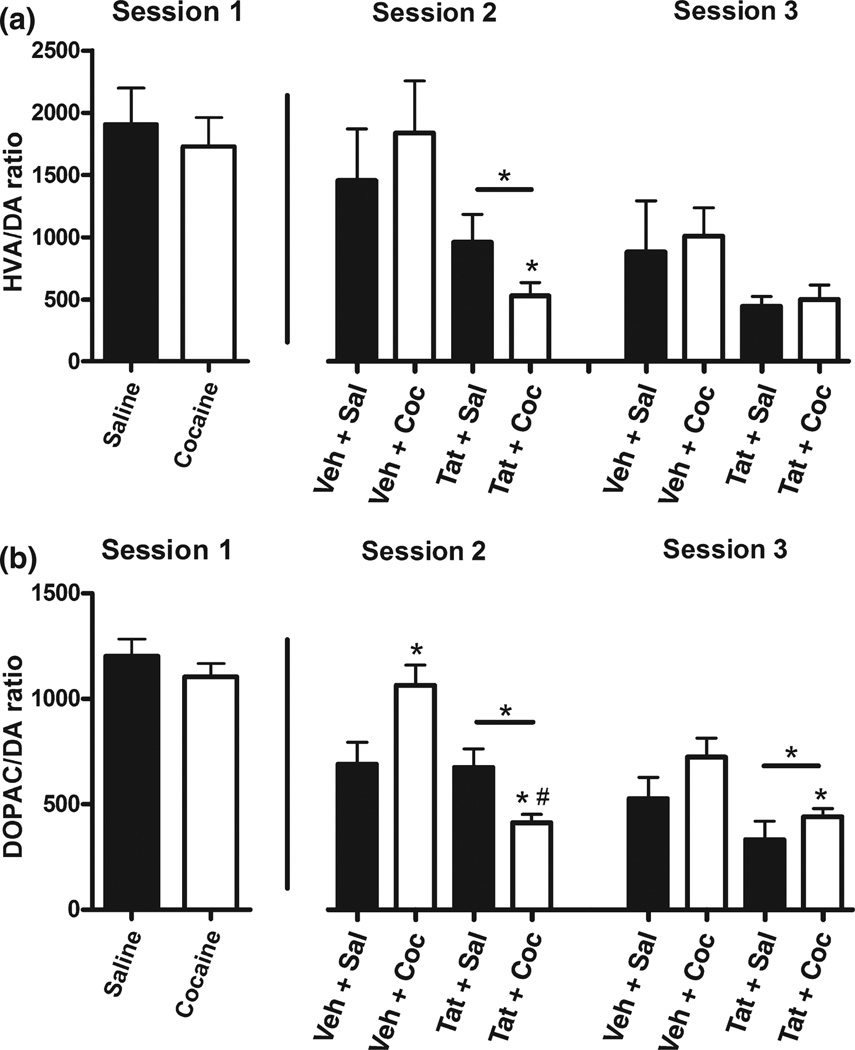
Metabolite/DA ratios (Fig. 3) for each session were derived from each session’s respective baseline concentrations that were collected prior to Coc or Sal injection. In other words, baseline is the average concentration at time-points −45 to −15, calculated independently for each session and group, and utilized exclusively for ratios that correspond to the session in which it was derived. Note that ratios are not derived from tissue content, but rather from extracellular concentrations. Nevertheless, given that DA and metabolites oscillate synchronously (Castaneda et al. 2004), alterations in the extracellular ratio likely indicate aberrant DA turnover and DA system function. For Session 2, Tat-infusion significantly decreased HVA/DA ratios, F(1, 29) = 7.61, p < 0.01; and Fig. 3(a) shows that Tat + Coc-treated animals were particularly affected. DOPAC/DA (Fig. 3b) ratios were similar to HVA/DA ratios with a main effect of Tat, F(1, 31) = 15.19, p < 0.0001, in addition to a Tat × Coc interaction, F(1, 31)= 13.65, p < 0.001. Planned comparisons shown in Fig. 3 (Session 2 column) demonstrated that Tat + Coc ratios were significantly lower than for all other groups, and that Coc treatment increased ratios in Veh-infused animals.
Fig. 3.
HVA/DA (a) and DOPAC/DA (b) ratios during each microdialysis session. Ratios for each session were calculated from each session’s respective baseline concentrations, which correspond to time-points prior to Coc or Sal challenge (i.e., −45 to −15 min). Tat/ Veh infusion occurred before Session 2, so it is not represented in Session 1. Note: Horizontal bar represents significant main effect of Tat-treatment, *p < .05 for Tat + Coc vs. Veh + Coc; #p < 0.05 for Tat + Coc vs. Tat + Sal.
Potassium-stimulated dopamine release
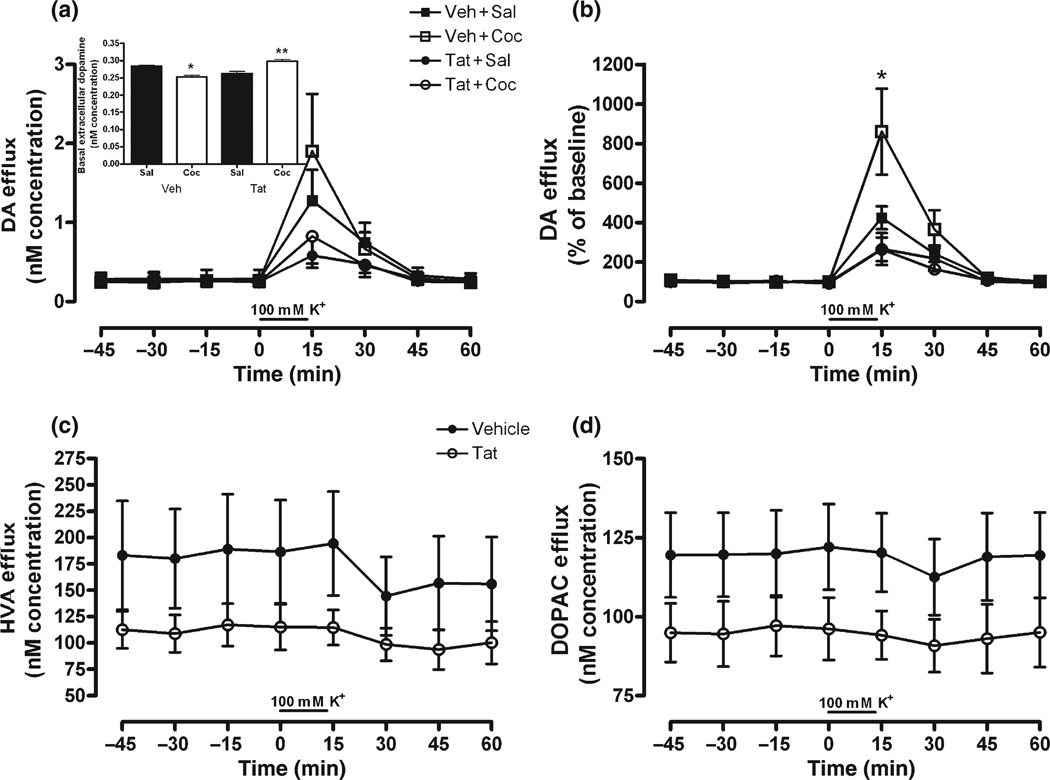
The DA response to perfusion of KCl was monitored two days following response to Coc. For raw DA levels (Fig. 4a), there was a Tat × Time interaction, F(7, 126) = 3.50, p < 0.01. Similar to Session 2, Fig. 4(a) (inset) demonstrated a main effect of treatment on basal extracellular DA levels, F(3, 11) = 14.84, p < 0.001, with Bonferonni comparisons indicating significantly lower basal DA levels for Veh + Coc relative to Veh + Sal (p < 0.05), and significantly higher basal DA levels for Tat + Coc relative to every other group (p < 0.01). The higher variability in raw extracellular DA levels that followed higher KCl levels precluded significant effects for time-points following KCl perfusion (i.e., 0–60 min). Similar to Session 2, and given basal differences in extracellular DA levels, the data were also expressed as percent of baseline. Percent of baseline data demonstrated that repeated Coc-treatment augmented KCl-stimulated DA release, and that DA release was attenuated by Tat-treatment irrespective of Coc-treatment. An 8 × 2 × 2 anova using percent of baseline DA response indicated a main effect of Tat, F(1, 18) = 9.81, p < 0.001; and Tat × Time, F(7, 126) = 6.59, p < 0.0001, Coc × Time, F(7, 126) = 2.14, p < 0.05, and Tat × Coc × Time, F(7, 126) = 2.37, p < 0.05, interactions. Veh + Coc treated animals displayed significantly greater DA response to KCl-stimulation than all other groups (Fig. 4b), indicating the repeated Coc-mediated neurochemical sensitized response to potassium. However, there were no Session 3 differences between groups in raw HVA or DOPAC levels, despite strong Tat-induced declining trends for both metabolites (Fig. 4c and d).
Fig. 4.
DA (a,b), HVA (c), and DOPAC (d) overflow in response to potassium perfusion 72 h following Tat or Veh infusion; represented as concentration (nM; a,c,d) and percent of baseline (b) across time. In addition, significant differences in basal extracellular DA levels are similar to Session 2 differences (a; inset). For metabolites (c,d), Coc-pretreatment had no effect on Tat-induced reduction of DOPAC or HVA levels, therefore Sal/Coc factor was collapsed to show the main effect of Tat-treatment, irrespective of Coc-treatment. Metabolite response to Coc-challenge, expressed as percent of baseline, was no different than Session 1; thus was not presented. Note: Significant planned comparisons following anova noted (*p < 0.05) at each time-point; and for inset, *p < 0.05 and **p < 0.01 for Veh + Coc vs. Veh + Sal and Tat + Coc vs. every other group represented.
3,4-Dihydroxyphenylacetic acid/DA ratios demonstrated a main effect of Tat, F(1, 18) = 7.76, p < 0.05 (Fig. 3b; Session 3 column). There were no main effects in HVA/DA ratios, despite similar trends to both Session 2 and DOPAC/ DA ratios (Fig. 3a; Session 3 column).
Locomotor activity
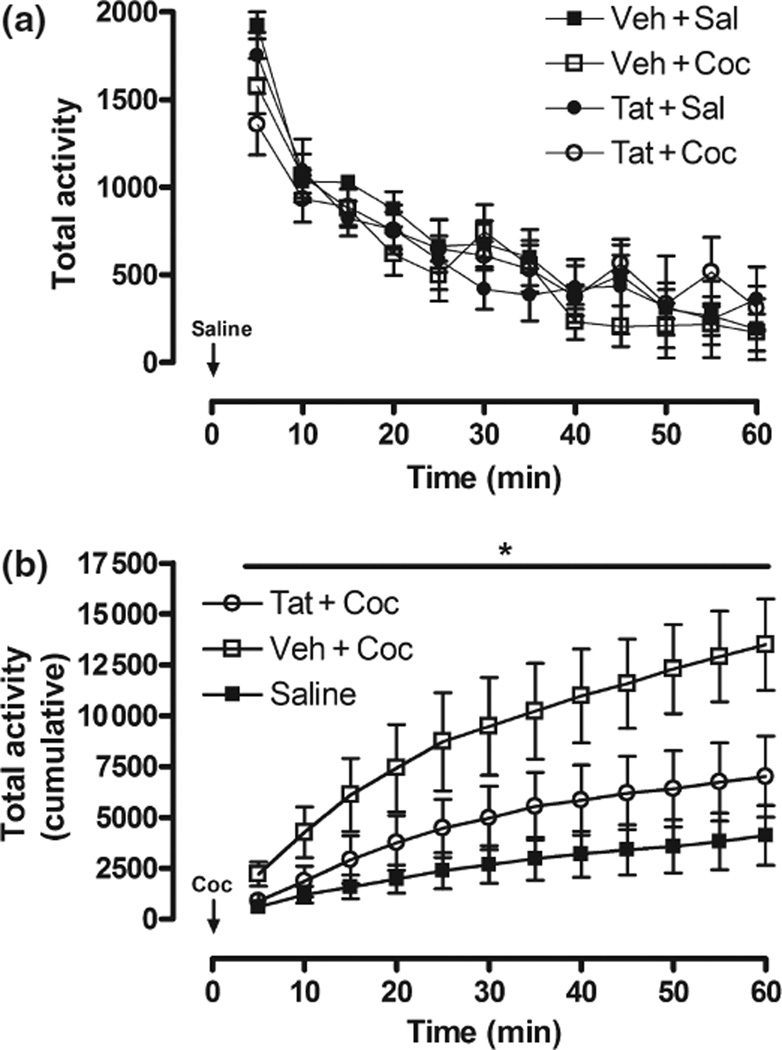
anova [12 (5-min time intervals, within) × 2 (Tat vs. Veh) × 2 (repeated Coc vs. Sal)], using total activity as the dependent measure, revealed a main effect of time within Sessions 1 and 2 (p < 0.001), indicating that all animals decreased locomotor activity as time progressed (Fig. 5a).
Fig. 5.
Total locomotor activity (cm) following Sal habituation session (a) and Coc challenge (b). Values are represented as total activity in each 5-min bin for the Sal session to highlight habituation across time (a), and cumulative activity every 5 min to demonstrate the differences between groups following Coc challenge (b). There was no significant difference between groups in response to Sal (a) and significant main effect of Veh + Coc treated rats across all time points in response to Coc-challenge *p < 0.05; (b). The increase of activity in Veh + Coc animals, but not Tat + Coc animals, is indicative of sensitization to the locomotor stimulating effects of Coc, which is attenuated by Tat treatment. Veh + Sal and Tat + Sal animals’ response to Coc challenge did not differ so these groups were combined for clarity.
During the Coc challenge session, the 12 × 2 × 2 mixed anova, using total activity as the dependent measure, indicated a main effect of repeated pre-treatment of Coc, F(1, 18) = 5.05, p < 0.05, and interactions of Tat × Time, F(11, 198) = 2.39, p < 0.05, and Tat × Coc × Time, F(11, 198) = 3.63, p < 0.01 (Fig. 5b). Planned comparisons indicated that Veh + Coc-treated, but not Tat + Coc-treated, animals had significantly greater total activity than Veh + Sal-treated and Tat + Sal-treated animals in response to Coc challenge, and when compared to their own baseline from Session 2 (all comparisons, p < 0.05). Taken together, the main effect of repeated pre-treatment of Coc indicates Coc-mediated behavioral sensitization, while the interactions highlighted the fact that Tat altered this Coc-induced sensitized response. Notably, the Tat-induced attenuation of sensitization was maintained even when the data were reduced to the first half of the session (30 min), when Coc challenge was most efficacious.
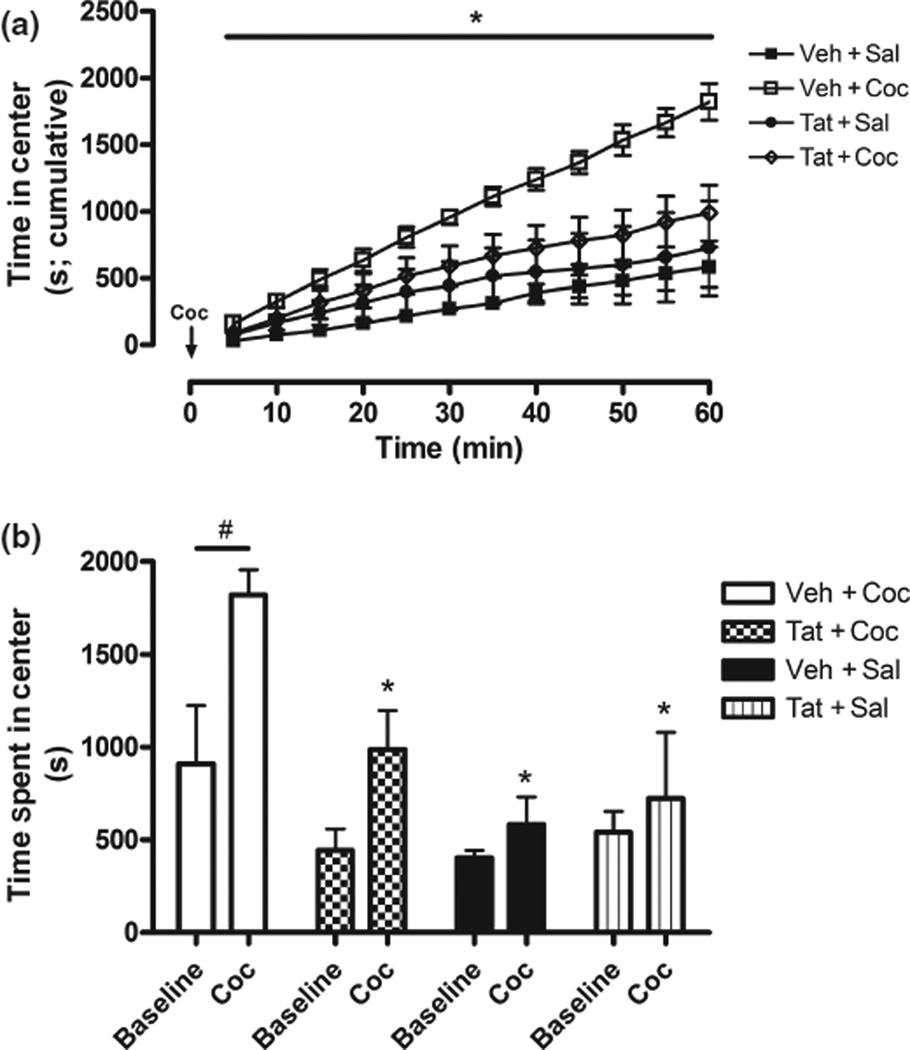
Prior research demonstrates that centrally-directed activity, relative to total activity, may be a more sensitive indicator of behavioral sensitization (see Harrod et al. 2005, 2008). Therefore, to further characterize behavior, four dependent measures were analyzed in order to assess activity in the center of the chambers, including total time in center (s; Fig. 6), distance traveled in center (cm), nose/body pokes from center into periphery, and full entry from center into periphery. These measures were largely redundant, as each demonstrated a main effect of repeated Coc and Coc × Time interactions [e.g., Time spend in center, F(1, 18) = 7.76, p < 0.01 for main effect and F(11, 198) = 2.20, p < 0.05 for interaction (Fig. 6a and b)]. Selecting only animals that received repeated Coc injections (i.e., Tat + Coc and Veh + Coc), Tat × Time interactions and main effects of Tat within this group were maintained for all the dependent measures. The centrally-directed analyses replicated the total field analysis, and indicated two effects: (i) behavioral sensitization in animals receiving Veh + Coc, and (ii) the sensitized response is markedly reduced in animals receiving Tat + Coc. Finally, time spent in the center of the testing chamber was found to be significantly correlated with KCl-stimulated DA release from microdialysis Session 3 (r = 0.50, p < 0.05), suggesting Tat-induced DA system vulnerability may predict attenuation of the Coc-induced sensitized response.
Fig. 6.
Amount of time spent in the center of locomotor testing chamber following Coc challenge, expressed as cumulative record across 5-min intervals (a) and as total time in center during habituation/baseline session and Coc challenge session (b). Veh + Coc-treated rats, but not Tat + Coc-treated animals, spent significantly more time in the center of the chamber, relative to all groups (a,b) and baseline (b) indicating repeated Coc injections sensitize centrally-directed behavior; an affect abrogated by Tat-treatment. Note: Horizontal bar (#p < 0.05) indicates main effect of Coc challenge relative to baseline; *p < 0.05 for significant decrease of time in center compared to Veh + Coc group following Coc challenge.
Discussion
The current investigation demonstrated that repeated Coc injections, Tat infusion, and the combination of the two uniquely alter both neurochemical and behavioral outcomes. Namely, we have shown for the first time several key findings that inform pharmacotherapy for drug users who contract HIV-1. These findings were: (i) a hyperdopaminergic tone in the NAc of animals exposed to both Tat and Coc, but not following Coc or Tat alone, (ii) Coc did not alter the Tat-induced decline in DA metabolite levels, but did interact with Tat exposure to alter DA turnover, (iii) Tat infusion markedly attenuated Coc-induced behavioral and neurochemical sensitization. These novel findings are consistent with the current literature and have implications for neuroAIDS therapeutics, as discussed below.
Apropos the first finding, animals that received repeated Coc injections + Tat infusion demonstrated elevated basal DA levels in Sessions 1 and 2, as well as post-Coc injection DA levels. Percent of baseline DA response revealed that the response of the DA system to Coc-challenge appears indistinguishable between Tat- and Veh-treated animals, and that the discrepancy from raw DA levels following Coc-challenge is attributable to differences in pre-Coc, basal tone. Thus, while both groups’ DA reached 300% of baseline following Coc-challenge, this translates to roughly 400% greater extracellular DA levels for Tat + Coc animals relative to Veh + Sal animals, and roughly 150% greater extracellular DA levels for Veh + Coc animals relative to Veh + Sal animals. Previous work has demonstrated several neurochemical and behavioral findings that are congruent with the increased DA tone demonstrated here. First, in behavioral experiments we previously demonstrated that Tat-treated rats have an increased locomotor response to acute Coc injection (Harrod et al. 2008), as one would expect to accompany higher DA levels in the NAc. Second, in neurochemical experiments, we have demonstrated that Tat rapidly inhibits DAT function (Ferris et al. 2009a; Zhu et al. 2009a; Aksenova et al. 2006; Wallace et al. 2006). The decrease in DAT function likely precedes the generalized loss of nerve terminal function (Ferris et al. 2009a), is dose-dependent (Zhu et al. 2009a), and occurs as a consequence of direct Tat-DAT protein interactions as determined via surface plasmon resonance / fluorescence resonance energy transfer analysis (Zhu et al. 2009a). The specific molecular interactions between Tat and Coc appear to involve an allosteric regulation of the DAT (Zhu et al. 2009b), although additional work is needed to model the DAT regulatory mechanisms.
Although our Tat + Coc findings concur with previous work, as one would expect higher DA tone following a decrease in uptake, an interesting caveat is that Tat + Sal-treated animals did not demonstrate increased basal DA tone during Session 2. Notably, a trend toward increased DA tone in Tat + Sal-treated animals did manifest after injection of Sal. The discrepancy in DA tone between Tat + Coc- and Tat + Sal-treated animals may be explained by Tat’s interaction with nerve terminal plasticity associated with repeated Coc injections. For example, Veh + Coc-treated animals demonstrated decreased DA tone in the current design. This decrease is well-supported in the drug use literature, and corresponds to an increase in DA uptake efficiency via DAT trafficking to the cell surface (Zahniser and Sorkin 2009; Crits-Christoph et al. 2008; Lack et al. 2008). Some researchers extrapolate that this increase in DAT uptake efficiency is a compensatory mechanism to offset aberrantly high extracellular DA levels following repeated exposure to Coc. The decrease in DA tone of the Veh + Coc group is a key finding, confirming that DA nerve terminals have been altered by the repeated drug treatment in the current design. Therefore, while Tat may have attenuated uptake of DA in both Tat + Coc and Tat + Sal groups, the discrepancy in DA tone between these groups may be attributed to reduced DAT uptake interacting with documented Coc-induced plasticity of additional nerve-terminal proteins. Moreover, if Coc-induced plasticity is a factor in determining outcome of Tat-infusion, then clinically demonstrated variants of HIV-1 neuropathology occur prior to viral infection, via baseline differences in nerve terminal function that stem from drug experience.
Given evidence for DAT alterations, an agent that specifically competes with Tat at the DAT while not interacting with transport of DA would be an ideal candidate, yet currently does not exist. In addition, agents that increase DA tone have been shown to potentiate HIV-1-induced cognitive decline, and the current work demonstrates HIV-1-infected Coc abusers may be pre-disposed to DA increases independent of direct pharmacological action at the DAT. It is not clear whether the increased DA potentiates neurotoxicity in the current study, especially considering the fact that some DA uptake inhibitors have been shown to be neuroprotective (Hinkin et al. 2001). If DA is involved, then D2 agonists at doses appropriate for regulating pre-synaptic function may indirectly offset increased DA tone. Detailed time-course studies that document the extent to which such drugs could mitigate both DA levels and the severity and progression of neurodegenerative markers in Coc-experienced animals are necessary.
A second finding from the current study is that DA metabolites, DOPAC and HVA, are persistently decreased following Tat-infusion regardless of previous experience with Coc. These data match the extant literature (Larsson et al. 1991), as a reduction in HVA has been noted in CSF of HIV-1-infected patients, and this correlates with neuropsychological impairment (Di Rocco et al. 2000). DOPAC systems are less variable in the course of HIV-1 infection (Obermann et al. 2009), and documented HIV-1-induced DOPAC alterations are rare. DOPAC reductions in the current data but not in humans may reflect established species differences in dopamine metabolism and metabolite levels (Garrick and Murphy 1980).
One possible explanation for reduced metabolites in the current data involves DAT activity. It is well established that monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT) are located in DA nerve-terminals, and that DA uptake via the DAT is a major route whereby these enzymes gain access to, and metabolize, DA that is not repackaged and recycled. Under normal conditions metabolite levels synchronously oscillate with DA levels and with activity of the DA system (Castaneda et al. 2004). In an intact system exposed to a DAT inhibitor such as Coc, DA levels increase whereas metabolite levels transiently decrease. Indeed, in the current investigation metabolite levels decreased around 10% following acute Coc injection (Session 1). This decrease has been established previously (Kalivas and Duffy, 1990; Hurd and Ungerstedt, 1989) and is thought to be mediated by the reduced access of MAO/ COMT to DA in the nerve terminal following Coc-induced blocking of the DAT (Hurd and Ungerstedt 1989; Westerink and Van Putten 1987; Zetterström et al. 1986). Thus, reduced metabolite levels in Tat-treated animals may, in part, be explained by Tat-induced reductions in DAT function.
Indeed, ratios from the current study may be contingent upon plasticity of several key proteins, including the DAT, MAO, and COMT. This is especially true considering that ratios in the current study are derived from extracellular concentrations, not tissue content. Given that metabolite levels were the same for Tat-treated groups, the significantly lower ratio in Tat + Coc-treated animals relative to Tat + Sal-treated animals, suggests that metabolism is insufficient to offset increasing DA levels in Session 2. In addition, this ratio suggests that the difference between Coc and Sal treatment reside in altered DA uptake and not differences in metabolism, whereas altered metabolism explains the main effect of Tat vs. Veh treatment ratios.
The combination of increased DA levels and decreased metabolite levels in Tat + Coc-treated animals suggest that pharmacotherapies that metabolize DA may effectively normalize aberrant DA tone and decrease the progression and severity that stems from the possibility of increased DA interacting with HIV-1 proteins. Agents that block MAO function have been shown to increase the severity of Simian Immunodeficiency Virus-induced cognitive decline (Czub et al. 2004), and facilitation of DA metabolism in the drug abusing population may offset high DA levels without incurring as many unwanted side-effects as D2 agonists.
A third finding from the current study is that Tat-infusion into the NAc disrupted both neurochemical release- and behavioral locomotor-sensitization. While basal extracellular DA levels remained significantly higher for Tat + Coc animals and significantly lower for Veh + Coc animals, variability in response to higher KCl levels precluded significant differences in raw values following perfusion of KCl. It is common to express data as percent of baseline, as was done in Session 2, when differences in basal levels persist. In general, data expressed as percent of baseline during Session 3 replicated earlier work demonstrating that Tat attenuated KCl-stimulated DA release in the NAc (Ferris et al. 2009b) and diminished the induction of Coc-induced behavioral sensitization (Harrod et al. 2008). The current data also indicated that although DA release was sensitized following repeated Coc-injections, Tat completely abolished this Coc-sensitized DA neurochemical response. In a corollary manner, repeated Coc injections sensitized the locomotor response to Coc challenge, which was significantly attenuated in Tat-treated animals. As with previous reports, altered DA transmission and locomotor activity were related (Lack et al. 2008). The current data demonstrated that neurochemical release of DA in Session 3 was correlated with locomotor sensitization (r = 0.50, p < 0.05). Thus, the Veh + Coc control group demonstrated that the current design/treatment regimen induced neurochemical and behavioral sensitization, and that Tat infusion in the NAc effectively prevented expression of this sensitization. As noted, previous work in our laboratory demonstrated that an early treatment of Tat could subsequently prevent the induction of behavioral sensitization (Harrod et al. 2008). However, it remained unclear whether Tat could disrupt the expression of sensitization, once it had been induced. This is important because it is likely that the drug abusing population possesses altered neurochemistry at the time of HIV-1 -infection and exposure to Tat. The current study makes it clear that Tat effectively disrupts DA nerve terminal function in both naïve and Coc-exposed animals, and that this disruption is profound enough to interfere with expression of behavioral and neurochemical sensitization to Coc. In addition, the course and magnitude of Tat-induced disruption is altered by Coc experience.
Collectively, the current behavioral and neurochemical data support clinical data demonstrating subcortical DA vulnerability (Obermann et al. 2009; Paul et al. 2002) in HIV-1 infected patients. Additionally, our data suggest that Tat can interact negatively with the neuroplasticity associated with chronic drug use. Namely, the HIV-1 protein, Tat, interacts with Coc to alter DA systems differently than DA alterations associated with Tat or Coc treatment alone. The hyperdopaminergic state demonstrated in animals receiving both Tat and Coc treatment may be one mediator of increased neurotoxicity observed following both HIV-1-protein + Coc administration.
Indeed, DA has been shown to be neurotoxic (Quinton and Yamamoto 2006) and clinical studies have demonstrated that agents which increase DA levels (Czub et al. 2001, 2004: Factor et al. 1994; Hriso et al. 1991), including MAO inhibitors (Czub et al. 2001), can exacerbate clinical manifestations of HIV-1 associated neurological impairment. Nevertheless, it is important to note that these demonstrations have been in the absence of DA uptake inhibitors, and that DA uptake inhibitors have also been shown to be neuroprotective (e.g., Hinkin et al. 2001). Therefore, future experimentation is required to elucidate how Tat interacts with the neuroplasticity associated with repeated Coc injections to produce the increased DA tone, and whether the DA overflow participates in neurotoxicity. One possibility is that the molecular alterations involve direct regulatory interactions between Tat and repeated Coc exposure on the DAT protein (Zhu et al. 2009b).
In conclusion, the current investigation provides data to support the possibility that neurobiological complications in HIV-1 infected drug users may possess a different time-course and outcome compared to the drug-naïve population. Our design demonstrates that basic HIV-1 research using chronic drug abuse models should be included in future experimentation, as drug-induced plasticity may alter HIV-1 protein interaction with DA nerve terminals, with resultant unique pathological outcomes. These unique outcomes, in turn, require unique pharmacotherapy for this population that may include offsetting high DA tone.
Acknowledgements
The present research was supported by grants DA014401, DA013137 (RMB) and HD043680 (CFM) from the National Institutes of Health. There are no conflicts or competing interests associated with the present research.
Abbreviations used
- aCSF
artificial CSF
- Coc
cocaine
- COMT
catechol-O-methyltransferase
- DA
dopamine
- DAT
DA transporter
- DOPAC
3,4-dihydroxyphenylacetic acid
- HIV-1
Human Immunodeficiency Virus-1
- HVA
homovanillic acid
- MAO
monoamine oxidase
- NAc
nucleus accumbens
- Sal
saline
- Veh
vehicle
References
- Aksenov MY, Aksenova MV, Silvers JM, Mactutus CF, Booze RM. Different effects of selective dopamine uptake inhibitors, GBR 12909 and WIN 35428, on HIV-1 Tat toxicity in rat fetal midbrain neurons. Neurotoxicology. 2008;29:971–977. doi: 10.1016/j.neuro.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray RD, Mactutus CR, Booze RM. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci. Lett. 2006;395:235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J. Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Henderer JD, Mcarthur JC, Brettschneider RD, Harris GJ, Barta RE, Pearlson GD. Reduced basal ganglia volume in HIV-1 associated dementia - results from quantitative neuroimaging. Neurology. 1993;43:2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- Baggaley RR, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS. 2006;20:805–812. doi: 10.1097/01.aids.0000218543.46963.6d. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CR, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Buttner A, Kroehling C, Mall G, Penning R, Weis S. Alterations of the vascular basal lamina in the cerebral cortex in drug abuse: a combined morphometric and immunohistochemical investigation. Drug Alcohol Depend. 2005;79:63–70. doi: 10.1016/j.drugalcdep.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Cass WA, Harned ME, Peters LE, Nath A, Maragos WF. HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Res. 2003;984:133–142. doi: 10.1016/s0006-8993(03)03122-6. [DOI] [PubMed] [Google Scholar]
- Castaneda TR, Prado BM, Prieto D, Mora F. circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J. Pineal Res. 2004;36:177–185. doi: 10.1046/j.1600-079x.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crits-Christoph P, Newberg A, Wintering N, Ploessl K, Gibbons MBC, Ring-Kurtz S, Gallop R, Present J. Dopamine transporter levels in cocaine dependent subjects. Drug Alcohol Depend. 2008;98:70–76. doi: 10.1016/j.drugalcdep.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czub S, Koutsilieri E, Sopper S, et al. Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta Neuropathol. 2001;101:85–91. doi: 10.1007/s004010000313. [DOI] [PubMed] [Google Scholar]
- Czub S, Czub M, Koutsilieri E, Sopper S, Villinger R, Muller JG, Stahl-Hennig C, Riederer P, ter Meulen V, Gosztonyi G. Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta Neuropathol. 2004;107:216–226. doi: 10.1007/s00401-003-0801-3. [DOI] [PubMed] [Google Scholar]
- Di Rocco A, Bottiglieri T, Dorfman D, Werner R, Morrison C, Simpson D. Decreased homovanilic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clin. Neuropharmacol. 2000;23:190–194. doi: 10.1097/00002826-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Everall IR, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotox. Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- Factor SA, Podskalny GD, Barron KD. Persistent neuroleptic-induced rigidity and dystonia in aids dementia complex – a clinicopathological case-report. J. Neurol. Set. 1994;127:114–120. doi: 10.1016/0022-510x(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci. Biobehav. Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. The human immunodeficiency virus-1-associated protein, tat(l-86), impairs dopamine transporters and interacts with cocaine to reduce nerve terminal function: a no-net-flux microdialysis study. Neuroscience. 2009a;159:1292–1299. doi: 10.1016/j.neuroscience.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. In vivo microdialysis in awake, freely moving rats demonstrates HIV-1 Tat-induced alterations in dopamine transmission. Synapse. 2009b;63:181–185. doi: 10.1002/syn.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick NA, Murphy DL. Species-differences in the deamination of dopamine and other substrates for monoamine-oxidase in brain. Psychopharmacology. 1980;72:27–33. doi: 10.1007/BF00433804. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Booze RM, Welch M, Browning CE, Mactutus CF. Acute and repeated intravenous cocaine-induced locomotor activity is altered as a function of sex and gonadectomy. Pharmacol. Biochem. Behav. 2005;82:170–181. doi: 10.1016/j.pbb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Mactutus CR, Fitting S, Hasselrot U, Booze RM. Intra-accumbal Tat(l-72) alters acute and sensitized responses to cocaine. Pharmacol. Biochem. Behav. 2008;90:723–729. doi: 10.1016/j.pbb.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Hardy DJ, Farinpour R, Newton T, Singer E. Methylphenidate improves HIV-1-associated cognitive slowing. J. Neuropsychiatry Clin. Neurosci. 2001;13:248–254. doi: 10.1176/jnp.13.2.248. [DOI] [PubMed] [Google Scholar]
- Horne MK, Lee J, Chen F, Lanning K, Tomas D, Lawrence AJ. Long-term administration of cocaine or serotonin reuptake inhibitors results in anatomical and neurochemical changes in noradrenergic, dopaminergic, and serotonin pathways. J. Neurochem. 2008;106:1731–1744. doi: 10.1111/j.1471-4159.2008.05534.x. [DOI] [PubMed] [Google Scholar]
- Hriso E, Kuhn T, Masdeu JC, Grundman M. Extrapyramidal symptoms due to dopamine-blocking agents in patients with AIDS encephalopathy. Am. J. Psychiatry. 1991;148:1558–1561. doi: 10.1176/ajp.148.11.1558. [DOI] [PubMed] [Google Scholar]
- Hudgens MG, Longini IM, Vanichseni S, Hu DJ, Kitayaporn D, Mock PA, Halloran ME, Satten GA, Choopanya K, Mastro TD. Subtype-specific transmission probabilities for human immunodeficiency virus type 1 among injecting drug users in Bangkok, Thailand. Am. J. Epidemiol. 2002;155:159–168. doi: 10.1093/aje/155.2.159. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Ungerstedt U. Cocaine: an in vivo microdialysis evaluation of its acute action on dopamine transmission in rat striatum. Synapse. 1989;3:48–54. doi: 10.1002/syn.890030107. [DOI] [PubMed] [Google Scholar]
- Kaplan EH, Heimer R. A model-based estimate of HIV infectivity via needle sharing. J. Acquir Immune Defic. Syndr Hum. Retrovirol. 1992;5:1116–1118. [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- Lack CM, Jones SR, Roberts DCS. Increased breakpoints on a progressive ratio schedule reinforced by IV cocaine are associated with reduced locomotor activation and reduced dopamine efflux in nucleus accumbens shell in rats. Psychopharmacology. 2008;195:517–525. doi: 10.1007/s00213-007-0919-4. [DOI] [PubMed] [Google Scholar]
- Larsson M, Hagberg L, Forsman A, Norkrans G. Cerebrospinal-fluid catecholamine metabolites in HIV-infected patients. J. Neurosci. Res. 1991;28:406–409. doi: 10.1002/jnr.490280313. [DOI] [PubMed] [Google Scholar]
- Marin MT, Berkow A, Golden SA, Koya E, Planeta CS, Hope BT. Context-specific modulation of cocaine-induced locomotor sensitization and ERK and CREB phosphorylation in the rat nucleus accumbens. Eur. J. Neurosci. 2009;30:1931–1940. doi: 10.1111/j.1460-9568.2009.06982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DCS, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat. Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nath A. Human Immunodeficiency Virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann. N Y. Acad. Sci. 2010;1187:122–128. doi: 10.1111/j.1749-6632.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. NIDA Infofacts: Crack and Cocaine. 2009 http://www.drugabuse.gov/InfoFacts/cocaine.html.
- Obermann M, Kuper M, Kastrup O, Yaldizli O, Esser S, Thiermann J, Koutsilieri E, Arendt G, Diener HC, Maschke M. Substantia nigra hyperechogenicity and CSF dopamine depletion in HIV. J. Neurol. 2009;256:948–953. doi: 10.1007/s00415-009-5052-3. [DOI] [PubMed] [Google Scholar]
- Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci. Biobehav. Rev. 2002;26:353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. Aaps J. 2006;8:E337–E347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J. Pharmacol. Ex. Ther. 2003;305:180–190. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- Schmitt KC, Zhen J, Kharkar P, Mishra M, Chen N, Dutta AK, Reith MEA. Interaction of cocaine-, benztropine-, and GBR12909-like compounds with wild-type and mutant human dopamine transporters: molecular features that differentially determine antagonist-binding properties. J. Neurochem. 2008;107:928–940. doi: 10.1111/j.1471-4159.2008.05667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JM, Aksenov MY, Aksenova MV, Beckley J, Olton P, Mactutus CF, Booze RM. Dopaminergic marker proteins in the substantia nigra of human immunodeficiency virus type 1-infected brains. J. Neurovirol. 2006;12:140–145. doi: 10.1080/13550280600724319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Maragos WF. Methamphetamine and human immunodeficiency virus protein Tat synergize to destroy dopaminergic terminals in the rat striatum. Neuroscience. 2006;137:925–935. doi: 10.1016/j.neuroscience.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120-and tat(l-72)-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Van Putten FM. Simultaneous determination of the rates of synthesis and metabolism of dopamine in various areas of the rat brain: application to the effects of (+)-amphetamine. Eur. J. Pharmacol. 1987;133:103–110. doi: 10.1016/0014-2999(87)90211-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J. Neuroimmune Pharmacol. 2008;3:203–217. doi: 10.1007/s11481-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Trafficking of dopamine transporters in psychostimulant actions. Semin. Cell Dev. Biol. 2009;20:411–417. doi: 10.1016/j.semcdb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterstrom X, Sharp T, Ungerstedt U. Further evaluation of the mechanisms by which amphetamine reduces striatal dopamine metabolism – A brain dialysis study. Eur. J. Pharmacol. 1986;132:1–9. doi: 10.1016/0014-2999(86)90002-6. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mactutus CR, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [H-3]dopamine uptake: dissociation of [H-3]dopamine uptake and [H-3]2 beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J. Pharmacol. Exp. Ther. 2009a;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Mactutus CF, Booze RM. Inhibitory Effects of Recombinant HIV-1 Tat1–86 on Modulation of Dopamine Transporter Function; 2009 Neuroscience Meeting Planner. Society for Neuroscience; Chicago, IL. 2009b. Online. [Google Scholar]