Abstract
Obesity is independently associated with left ventricular (LV) hypertrophy and thus may be an important modifier of the hypertrophic cardiomyopathy (HC) phenotype. We examined if obesity modifies the clinical presentation, LV morphology, outflow hemodynamics and exercise tolerance in HC. In this cross-sectional study, 88 obese (body mass index, BMI≥30 kg/m2) and 154 non-obese (BMI<30 kg/m2) patients from the Johns Hopkins HC clinic were compared with respect to a variety of clinical and LV echocardiographic measurements. Obese patients (36.4%) were more likely to report exertional dyspnea (p=0.04) and chest pain (p=0.002), and had higher prevalence of hypertension (p=0.008). LV posterior wall thickness (p=0.01) but not the septal wall (p≥0.21) was significantly higher in obese patients, resulting in an increased LV mass index (p=0.003). No significant differences in LV systolic and diastolic function were observed, but obesity was associated with higher LV stroke volume (p=0.03), inducible LV outflow tract gradients (p=0.045) and chance of developing LV outflow tract obstruction during stress (p=0.035). In multivariate analysis, BMI was associated with increased posterior (but not septal) wall thickness (β=0.15, p=0.02) and LV mass index (β=0.18, p=0.005), particularly in those with hypertension. Obesity was also associated with reduced exercise time and functional capacity, and BMI independently correlated with reduced exercise tolerance. In conclusion, obesity is associated with larger LV mass, worse symptoms, lower exercise tolerance and labile obstructive hemodynamics in HC. The association with increased outflow tract gradients has particular importance as contribution of obesity to the pressure gradients may influence clinical decisions in labile obstructive HC.
Keywords: hypertrophic cardiomyopathy, obesity, hypertension, body mass index, left ventricular mass
INTRODUCTION
Hypertrophic cardiomyopathy (HC) is the most common genetic cardiovascular disease affecting about 1 in 500 persons in the general population, and manifesting as left ventricular (LV) hypertrophy, life-threatening arrhythmias and heart failure1. Obesity has become the most prevalent chronic medical condition in western countries and has a major influence on the development of cardiac disease 2. Despite the large body of evidence indicating an obesity-LV hypertrophy link 3,4, there is a glaring lack of investigation into the potential influence of obesity on LV hypertrophy in HC. Recent guidelines recommend aggressive modification of common cardiovascular risk factors, including obesity, in patients with HC 1. However, beyond the purpose of reducing global cardiovascular risk, a specific rationale for pursuing aggressive weight loss in HC is lacking. A single, intriguing case report wherein substantial weight loss resulted in a decrease in LV wall thickness in an HC patient suggests that some of the LV hypertrophy in HC could be modulated by obesity 5. Wall thickness is not only a cardinal anatomic feature in HC, but may be responsible for LV outflow tract obstruction and heart failure-related symptoms, it counts as a risk factor when assessing a patient’s risk profile for ventricular arrhythmias, and factors into a decision to implant a defibrillator 1. We hypothesized that obesity would be associated with worse LV hypertrophy in HC and thereby potentially affect LV function and exercise capacity. Accordingly, the aims of the study were to analyze the relationship of obesity to clinical presentation, LV structure, function and hemodynamics as determined by echocardiography, and functional capacity as determined by treadmill exercise.
METHODS
This is a cross-sectional analysis of a prospectively generated database of 258 consecutive HC patients at their first visit to the Johns Hopkins HC clinic between February 2005 and July 2012. Data were collected for patients fulfilling the criteria for a diagnosis of HC, defined as unexplained LV hypertrophy with a maximal wall thickness above 15 mm in the absence of other cardiac or systemic condition capable of producing a similar degree of LV hypertrophy 1. Features suggestive of HC such as systolic anterior movement of the mitral valve (SAM), left ventricular outflow tract obstruction and family history of HC were also taken into account for confirming the diagnosis. Patients diagnosed with apical HC (n=10) or with previous septal myectomy or alcohol ablation (n=6) were excluded from the present analysis. This study was approved by the Johns Hopkins Institutional Review Board.
Height and weight were used to calculate body mass index (BMI, kg/m2), and obesity was defined as a BMI ≥30kg/m2. Clinical data including symptoms, comorbidities, medications and family history of HC, sudden cardiac death and coronary artery disease were ascertained by the examining physician. Systolic and diastolic blood pressure was measured during clinical examination. Hypertension was defined as blood pressure ≥130/85 at initial examination or self-reported hypertension with need for antihypertensive medication. Participants who had never smoked >100 cigarettes in their life were considered as non-smokers. Diabetes mellitus was defined as self-reported diabetes or the use of antidiabetic drugs. Dyslipidemia was defined as self-reported dyslipidemia with need for lipid-lowering medication. Self-reported functional capacity was classified according to the New York Heart Association classification.
All echocardiograms were performed using a Vivid 7 cardiac ultrasound machine (GE Healthcare, Milwaukee, Wisconsin) with a 3.5-MHz transducer and following a standardized protocol. The LV linear dimensions were measured from a parasternal long-axis view 6. End-diastolic wall thickness was measured at the basal segment and mid segments of the interventricular septum and at the basal segment of the posterior wall as recommended 6. Maximal septal wall thickness was divided by basal posterior wall thickness to calculate septal to posterior wall thickness ratio, a parameter used to estimate asymmetric septal LV hypertrophy 7. LV end-diastolic and end-systolic volumes were calculated using the biplane Simpson method, and stroke volume as the difference between the two. The monoplane Simpson’s method was used to measure the left atrial volume in the end-systole in apical 4-chamber view, which was then indexed to body surface area 6. Relative wall thickness, LV mass and ejection fraction were calculated with formulas recommended by the American Society of Echocardiography 6.To adjust for the influence of growth without eliminating the impact of excess adiposity, LV mass and volumes were indexed to height in meters to the allometric power of 2.7, as previously suggested 8. From the apical window, pulsed Doppler sample was placed at the mitral valve leaflet tips and the peak velocity of early diastolic mitral inflow (E) and late diastolic mitral inflow (A) were measured, and their ratio (E/A ratio) calculated. Tissue Doppler early diastolic velocity of the septal mitral annulus (Em) was recorded, and the E/Em ratio calculated 9.
Left ventricular outflow tract pressure gradients were measured in the most optimal apical view by continuous-wave Doppler echocardiography under resting conditions and during provocative maneuvers including Valsalva, treadmill exercise and/or amyl nitrite inhalation, to elicit latent obstruction 1. Resting and maximal inducible LV pressure gradients were used to classify HC patients into non-obstructive, labile obstructive or obstructive, according to recent guidelines 1. LV outflow tract diameter was determined at rest at the onset of systole by measuring the minimal distance between the left side of the interventricular septum and the initial echo of the anterior mitral leaflet in the parasternal long-axis view. SAM was evaluated by B- mode echocardiography images and defined as incomplete or complete based on the absence or presence of contact between the mitral leaflet and the septum, respectively 10.
Deformation analysis based on speckle tracking was performed offline using dedicated GE EchoPac software, v. 7.1.1 (GE Ultrasound, Norway) for estimating global longitudinal systolic strain. Three consecutive cardiac cycles with a frame rate of 60–90/s were recorded. The endocardial border of the LV cavity was traced manually from a still frame image and automatically tracked throughout the cardiac cycle by the software. Poorly tracking segments or images that could not be optimized were discarded and not considered for the analysis 11.
Patients underwent treadmill exercise testing unless excluded per the conditions listed below: unable to walk on the treadmill, blood pressure>220/120 mmHg, unstable angina, recent myocardial infarction (<1 week), decompensated heart failure, active atrial arrhythmia (resting heart rate >120 bpm). A standard Bruce protocol was implemented unless patients reported very low exercise tolerance, in which case a modified Bruce protocol was used. Data was collected regarding exercise capacity (expressed as metabolic equivalents, METs, or exercise time in seconds), percentage exercise predicted heart rate (calculated as peak exercise heart rate divided by maximum predicted heart rate and multiplied by 100), systolic and diastolic blood pressure, arrhythmias and symptoms at peak exercise.
All statistical analyses were performed using the SAS package (version 9.3, SAS Institute Inc., Cary, NC). Continuous variables are presented as means ± standard deviation and categorical variables as proportions. Comparisons between obese and non-obese patients were performed by Student’s t tests or chi-square tests as appropriate.Simple correlations were estimated using Spearman’s nonparametric rank correlation coefficient (rs). Univariate and multivariate linear regressions were used to assess the independent association of BMI with functional capacity (METs) and LV structural parameters (maximal septal wall thickness, posterior wall thickness and LV mass index). Hypertension (as a dichotomous variable) and LV outflow tract obstruction (as a categorical variable, where 0=non-obstructive, 1=labile obstructive, 2=obstructive) were included as covariates. Standardized coefficients were also calculated, to facilitate comparison of regression coefficients in the same model. In all models, the interaction between BMI and other covariates was tested. One-way analysis of variance with the post hoc Tukey–Kramer multiple range test was used to compare METs, septal and posterior wall thickness, and LV mass index in patients with and without obesity and hypertension after adjusting for LV outflow tract obstruction. Statistical significance was set at p≤0.05.
RESULTS
The study population consisted of 242 HC patients, and was divided into 2 groups based on a BMI≥30 kg/m2 versus <30 kg/m2. Demographic and clinical characteristics of the two groups are shown in Table 1. Prevalence of obesity in our sample was 36.4%, and 48 obese patients (19.8%) had severe obesity (BMI≥35 kg/m2). There were no significant differences in age, gender, race and family history between the groups. There was a trend for higher prevalence of common cardiovascular risk factors in obese patients, but this was significant only for hypertension. Accordingly, the use of ACE inhibitors was higher in obese patients, but there was no statistical difference in systolic blood pressure. At the time of their initial clinical presentation, obese patients showed higher prevalence of chest pain, dyspnea and limitation in physical activity (New York Heart Association class≥2) and no significant differences in syncope and atrial fibrillation (Table 1).
Table 1.
Demographic and clinical characteristics of the study population.
| Body mass index (kg/m2) | P value |
||
|---|---|---|---|
| <30 (n=154) |
≥30 (n=88) |
||
| Body mass index (Kg/m2, mean and range) | 26±3 (13.8-29.9) |
36±6 (30.1-65) |
<.0001 |
| Age (years) | 53 ±17 | 51 ±15 | 0.39 |
| Men | 64% | 72% | 0.21 |
| Race | |||
| Caucasian | 63% | 64% | 0.92 |
| African American | 21% | 27% | 0.25 |
| Other | 16% | 9% | 0.12 |
| Family history of hypertrophic cardiomyopathy | 24% | 13% | 0.07 |
| Family history of sudden cardiac death | 14% | 12% | 0.72 |
| Family history of coronary artery disease | 22% | 18% | 0.53 |
| Diabetes mellitus | 8% | 15% | 0.08 |
| Dyslipidemia | 45% | 53% | 0.26 |
| Smoker | 21% | 31% | 0.17 |
| Hypertension | 37% | 55% | 0.008 |
| Systolic blood pressure at rest (mmHg) | 130 ±19 | 134 ±18 | 0.12 |
| Diastolic blood pressure at rest (mmHg) | 90 ±28 | 88 ±26 | 0.62 |
| Cardiovascular medications | |||
| Beta blockers | 67% | 68% | 0.94 |
| Calcium antagonists | 25% | 34% | 0.16 |
| Disopyramide | 3% | 3% | 0.86 |
| ACE inhibitors | 16% | 33% | 0.004 |
| Diuretics | 15% | 22% | 0.17 |
| Clinical presentation | |||
| Chest pain | 18% | 38% | 0.002 |
| Dyspnea | 42% | 57% | 0.04 |
| New York Heart Association ≥ 2 | 30% | 49% | 0.003 |
| Syncope | 11% | 13% | 0.62 |
| Paroxysmal atrial fibrillation | 10% | 8% | 0.59 |
| Persistent atrial fibrillation | 2% | 0% | 0.29 |
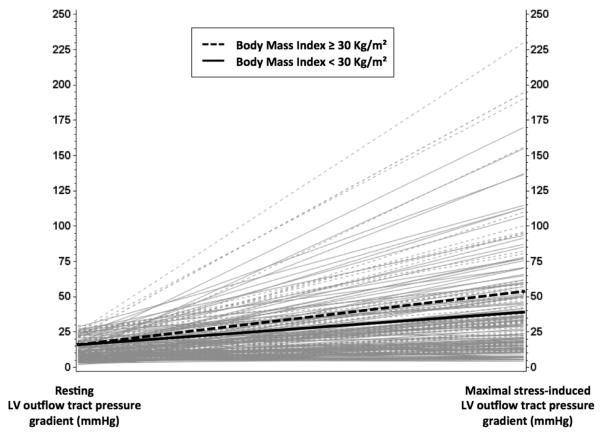
Differences in echocardiographic and exercise parameters between study groups are shown in Table 2. Obese versus non-obese HC patients did not significantly differ with respect to basal and mid septal wall thickness, but a significant difference in posterior wall thickness and LV mass index was observed (Table 2). End-diastolic and end-systolic volumes, and stroke volume index were also significantly higher in obese patients. No significant differences were found in parameters associated with LV remodeling, LV systolic and diastolic function; global longitudinal systolic strain was also similar between the two groups. There was no difference in LV outflow tract diameter and LV pressure gradients at rest (Table 2). However, obese patients showed a trend towards higher stress-induced LV pressure gradients compared to non-obese patients. Non-obstructive, labile obstructive and basal obstructive cases were equally distributed in the whole sample (36%, 37%, and 27%, respectively). When stratified by obesity, no differences were noted in basal obstruction, but the prevalence of labile obstruction appeared relatively higher in obese patients (46% vs. 33%, p=0.06). Upon excluding the 64 patients with obstructive physiology at rest (41 non-obese and 23 obese, with LV pressure gradients of 69±36 vs. 60±33 mmHg, p=0.33, respectively ), obese patients had significantly higher inducible LV pressure gradients (52±53 vs. 38±34 mmHg, p=0.045, respectively, Figure 1) and a significantly higher chance of developing LV outflow tract obstruction during stress (62% vs. 45%, p=0.035) than their non-obese counterparts. Prevalence of SAM was similar in obese and non-obese patients, and so were proportions of complete and incomplete SAM (Table 2).
Table 2.
Echocardiography and Treadmill Exercise Data in the Study Population.
| Body mass index (kg/m2) |
P value |
||
|---|---|---|---|
| <30 (n=154) |
≥30 (n=88) |
||
| Left ventricular morphology | |||
| Septal wall thickness, basal segment (mm) | 18.6±5.1 | 19.4±4.4 | 0.21 |
| Septal wall thickness, mid segment (mm) | 18.7±6.2 | 19.1±6.5 | 0.62 |
| Maximal septal wall thickness (mm) | 20.5±0.6 | 21.4±0.5 | 0.22 |
| Posterior wall thickness (mm) | 10.9±2.7 | 11.9±3.1 | 0.01 |
| Septal/Posterior wall thickness ratio | 1.95±0.6 | 1.89±0.6 | 0.46 |
| LV mass index (g/m2.7) | 58±22 | 67±23 | 0.003 |
| LV end-diastolic volume index (ml/ m2.7) | 23.4±7 | 26.2±8 | 0.007 |
| LV end-systolic volume index (ml/ m2.7) | 8.1±3.5 | 9.2±3.6 | 0.02 |
| Stroke Volume Index (ml/ m2.7) | 15.3±4.9 | 17±6 | 0.03 |
| Relative wall thickness | 2±0.6 | 2±0.6 | 0.68 |
| Left ventricular function | |||
| E/A ratio | 1.32±0.6 | 1.20±0.4 | 0.13 |
| Septal mitral annulus early diastolic velocity (Em, cm/s) | 4.3±1.7 | 4.2±1.5 | 0.70 |
| E/Em ratio | 22.6±12.6 | 22.3±9.8 | 0.87 |
| Left atrial volume index (g/m2) | 38.7±21.7 | 36.5±13.5 | 0.38 |
| LV ejection fraction (%) | 66±9 | 65±10 | 0.24 |
| Global longitudinal systolic strain (%) | −15±3 | −15±3 | 0.41 |
| Left ventricular outflow tract parameters | |||
| LV outflow tract diameter (cm) | 2.1±0.3 | 2.2±0.3 | 0.15 |
| LV outflow tract peak gradient at rest (mmHg) | 27±33 | 25±28 | 0.72 |
| LV outflow tract peak gradient during Valsalva (mmHg) | 30±31 (n=134) |
39±40 (n=83) |
0.05 |
| LV outflow tract peak gradient during treadmill (mmHg) | 53±43 (n=120) |
66±57 (n=64) |
0.08 |
| LV outflow tract peak gradient during amyl nitrite inhalation (mmHg) | 41±41 (n=106) |
53±57 (n=61) |
0.11 |
| Maximal inducible LV outflow tract peak gradient (mmHg) | 55±46 (n=145) |
67±57 (n=86) |
0.09 |
| Dynamic LV outflow tract obstruction | |||
| - Non-obstructive | 40% | 28% | 0.06 |
| - Labile obstruction (stress-induced) | 33% | 46% | 0.06 |
| - Basal obstruction | 27% | 26% | 0.93 |
| Systolic anterior motion (SAM) of the mitral valve | |||
| - No SAM | 18% | 13% | 0.30 |
| - Incomplete SAM | 59% | 64% | 0.59 |
| - Complete SAM | 23% | 23% | 0.95 |
| Treadmill exercise | (n=117) | (n=66) | |
| Metabolic equivalents | 10.6±4.2 | 9±3.5 | 0.007 |
| Percentage exercise predicted heart rate | 86% | 84% | 0.65 |
| Systolic blood pressure at peak exercise (mmHg) | 169±39 | 173±36 | 0.58 |
| Diastolic blood pressure at peak exercise (mmHg) | 82±22 | 80±16 | 0.53 |
| Chest pain | 9% | 20% | 0.04 |
| Shortness of breath | 48% | 49% | 0.90 |
| Arrhythmia | 2% | 0% | 0.57 |
| Exercise time (sec) for Bruce protocols | 603±184 (n=63) |
512±212 (n=34) |
0.03 |
Figure 1. Resting and maximal inducible left ventricular outflow tract pressure gradients in obese and non-obese patients without obstructive physiology at rest.
The figure shows that LV outflow tract pressure gradients generally increased in both obese (dashed lines) and non-obese (solid lines) HC patients under provocative maneuvers, but significantly higher induced LV pressure gradients were observed in those with obesity (p for comparison of maximal inducible LV outflow tract pressure gradients =0.045).
Treadmill exercise was performed by about 75% of patients in each group (117/154 non-obese and 66/88 obese). Functional capacity, expressed as METs, and exercise time (considered only for those who underwent the same Bruce protocol), were significantly lower in obese versus non-obese patients (Table 2), and patients with obesity more frequently reported chest pain during the test. No differences were found for heart rate and blood pressure at peak exercise (Table 2).
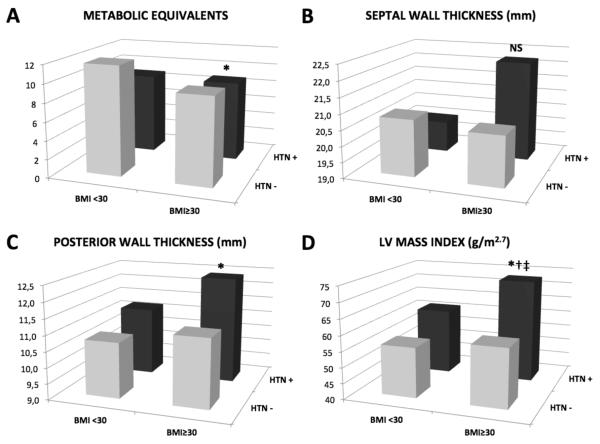
BMI showed a statistically significant negative association with METs, in both univariate and multivariate models (Table 3). Hypertension and LV outflow tract obstruction severity were also responsible for significant reduction of exercise tolerance in our patients (Table 3). When LV outflow tract obstruction severity was substituted with SAM severity or maximal inducible LV pressure gradients results did not significantly change, due to the high correlation between LV outflow tract obstruction severity and these two parameters (rs=0.48, p<.0001; rs=0.85, p<.0001, respectively). A significant interactions was found between BMI and hypertension in the model predicting METs (p=0.04), and multiple comparisons between patients with and without obesity and hypertension revealed lower METs in hypertensive obese patients than in others (Figure 2, A).
Table 3.
Univariate and Multivariate Linear Relationship of Body Mass Index with Functional Capacity and Left Ventricular Structural Parameters.
|
Metabolic
Equivalents (METs) |
Left Ventricular
Septal Wall Thickness (mm) |
Left Ventricular
Posterior Wall Thickness (mm) |
Left Ventricular
Mass Index (g/m 2.7) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| B (SE) |
β | P | B (SE) |
β | P | B (SE) |
β | P | B (SE) |
β | P | |
| Univariate | ||||||||||||
|
| ||||||||||||
| Body Mass Index |
−0.15 (0.05) |
−0.24 | <.001 | 0.093 (0.053) |
0.11 | 0.08 | 0.090 (0.032) |
0.18 | 0.005 | 0.85 (0.25) |
0.22 | <.001 |
|
| ||||||||||||
| Multivariate | ||||||||||||
|
| ||||||||||||
| Body Mass Index |
−0.12 (0.04) |
−0.19 | 0.007 | 0.092 (0.053) |
0.11 | 0.09 | 0.074 (0.031) |
0.15 | 0.02 | 0.70 (0.25) |
0.18 | 0.005 |
| Hypertension | −2.13 (0.56) |
−0.27 | <.001 | 0.162 (0.717) |
0.01 | 0.82 | 0.660 (0.364) |
0.12 | 0.07 | 8.3 (2.8) |
0.19 | 0.003 |
| Left | ||||||||||||
| Ventricular Outflow Tract Obstruction |
−0.73 (0.36) |
−0.14 | 0.046 | −0.334 (0.454) |
−0.05 | 0.46 | 0.736 (0.230) |
0.20 | 0.002 | 5.2 (1.8) |
0.18 | 0.004 |
Values in table are nonstandardized (B) with standard error (SE), standardized correlation coefficients (β), and p values (P).
Figure 2. Contribution of obesity and hypertension to exercise tolerance and left ventricular structure.
Metabolic equivalents were significantly lower, and posterior wall thickness and LV mass index significantly higher in patients with obesity and hypertension, while there was no significant difference between groups in septal wall thickness. HTN= hypertension (+=hypertensive, −=normotensive).* p<.05 vs. non-obese normotensive. † p<.05 vs. obese normotensive. ‡ p<.05 vs. obese normotensive. NS= p>0.19 vs. all groups. Formal testing showed a synergistic interaction of BMI and hypertension on LV mass index (p=0.02).
In univariate analysis, BMI was significantly associated with increased posterior wall thickness and LV mass index (Table 3). After accounting for hypertension and LV outflow tract obstruction, the association between BMI and these parameters remained significant, with comparable standardized coefficients for BMI, hypertension and LV outflow tract obstruction in the model predicting LV mass index (Table 3). No significant association was found between maximal septal wall thickness and BMI or hypertension or LV outflow tract obstruction in multivariate model (Table 3). We found a significant interaction between BMI and hypertension for LV mass index (p for interaction=0.02), and multiple comparisons between patients with and without obesity and hypertension showed that posterior wall thickness and LV mass index were significantly higher in hypertensive and obese patients (Figure 2, C and D), while no significant differences were observed for septal wall thickness (Figure 2, B).
DISCUSSION
We report that obesity independently contributes to LV hypertrophy in HC and negatively impacts symptoms and functional capacity. Our novel findings are that HC patients with obesity are more symptomatic (dyspnea and chest pain more prevalent) and have reduced subjective (higher New York Heart Association class) and objective (lower METs and exercise time) functional capacity when compared to non-obese patients. In multivariate analysis, increased BMI was also independently associated with increased LV mass index by mainly affecting the thickness of the LV posterior wall, and this effect was particularly important in patients with hypertension.
Exertional dyspnea and chest pain are the most common symptoms of HC, and have been related to LV diastolic dysfunction, obstructive hemodynamics, and ischemia from supply-demand mismatch or microvascular disease 1. While we found no significant difference between the study groups in LV pressure gradients at rest, a trend toward an increase in physiologically provoked LV pressure gradients was observed in obese patients, which may in part explain their increased heart failure-related symptoms during day-to-day activities and reduced exercise tolerance. LV pressure gradients increased within each group under stress condition, but significantly higher induced LV pressure gradients were observed in obese patients after excluding those with obstructive physiology at rest (see Figure 1). Notably, the increase of LV pressure gradients in obese patients was consistent throughout different provocative tests (see Table 1), thus reducing the potential for artifacts.
Obesity is associated with increased total blood volume, which in theory should lessen the development of LV outflow tract obstruction. However, this effect could potentially be counteracted by the excess LV hypertrophy seen in our obese patients, which during stress, may reduce the effective LV outflow tract cross sectional area 12. Accordingly, and in contrast with previous reports 13, a hypertensive response to exercise was not noted in our obese patients and no significant differences in systolic blood pressure were observed at peak exercise between the study groups, further supporting an association between obesity and dynamic LV outflow tract obstruction. Further longitudinal studies are needed to confirm the contribution of obesity to LV pressure gradients, as this may influence important clinical decisions in labile obstructive HC. The more frequent use of ACE inhibitors and diuretics in the obese group may have also contributed in part to the elevated gradients. Our data prompt careful consideration and selection of appropriate antihypertensive therapy in HC patients with obesity.
In line with the above results, in multivariate analysis, we confirmed a negative association between LV outflow tract obstruction and reduced exercise tolerance. However, obesity and hypertension appeared to have a predominant influence on exercise capacity in HC, and in multiple comparison analysis, obese hypertensive patients showed lower METs than others (see Figure 2, A). The negative association between BMI and METs has already been described in patients with HC 14,15. Our data not only confirm this association via multivariate analysis but also offer the potential mechanism for this reduced exercise capacity in obese HC patients, namely, higher exercise-induced LV pressure gradients.
We found no differences between study groups in LV systolic and diastolic parameters, even when considering sophisticated measures such as strain and tissue Doppler. We believe that the absence of an additive effect of obesity on LV diastolic function in patients with HC is due to the LV diastolic function already being severely compromised by the primary cardiomyopathy itself (see Table 2). Some studies indicate that LV diastolic dysfunction is a cause of exercise intolerance in both HC 14,15 and obesity 16. In our analysis, LV outflow tract obstruction emerges as a more important determinant of exercise intolerance in HC patients with obesity compared to LV diastolic function.
An independent relationship between BMI and LV mass exists in the general population 3,4 but has not been explored in patients with HC. Asymmetric septal LV hypertrophy (septal to posterior wall thickness ratio ≥1.3 7) is a key diagnostic feature 17,18 and was noted in 86% of our sample. Despite limitations in the application of conventional LV mass formulae in asymmetric LV hypertrophy19, it is often used in HC.20-22 We found LV mass index was significantly associated with increasing BMI, the presence of hypertension and the severity of LV outflow tract obstruction. The latter association has been previously reported 19 and septal reduction reduces LV mass. 20,21,22 Our data highlight the additional interactions of obesity and hypertension with LV mass.
Consensus documents recognize that obesity and hypertension frequently coexist with HC1 but studies have lacked information about their prevalence and potential impact on HC. We found a similar prevalences of obesity (37%) and hypertension (43.4%)in our cohort to that noted in American adults.2,15,19,23,24 Whether hypertensive HC is a distinct clinical entity is unclear 25,26 and studies tend to exclude tout court patients with hypertension.10,18 Our data show a significant interaction between BMI and hypertension on LV mass index, and in secondary analysis the relationship between BMI and LV mass appeared to be significantly stronger in those patients with associated hypertension. Hypertension contributes to cardiovascular risk in the obese 27 and there are additive effects of increasing blood pressure and BMI on LV mass.3,28 Our results suggest similar obesity-hypertension synergism may exist in HC. Specific criteria for the diagnosis of HC in obese and/or hypertensive patients are lacking. Longitudinal studies will help determine whether treating these underlying conditions may prove effective for decreasing the magnitude of LV hypertrophy and possibly improving clinical outcomes of these patients.
In multivariate models, BMI showed a significant association with posterior wall thickness and LV mass index, but not with septal wall thickness. As a result, posterior but not septal wall thickness was significantly higher in obese patients, and multiple comparison analysis showed higher posterior wall thickness mean values in obese patients with associated hypertension. These findings are in line with those previously reported by Karam and colleagues in a series of 78 age- and gender- matched HC patients, in which patients with hypertension had more free wall hypertrophy than those without 25. Recently, a large population based study demonstrated concentric LV hypertrophy in obese subjects compared to age-matched controls 29. These and our data suggest that BMI and hypertension contribute to an element of concentric LV hypertrophy in HC. We hypothesize that the genetic component of the disease is the predominant driver of increasing septal wall thickness 30 that occurs in a setting of concentric LV hypertrophy that may be more influenced by secondary conditions such as obesity and hypertension. An increased myocardial sensitivity to cardiac afterload 25 may coexist in HC patients with obesity, and be more evident in segments that are not usually influenced by genetic factors in HC such as the septum. We recognize and acknowledge that there are “global” variants of HC wherein hypertrophy may extend to the apex and other walls including the posterior wall.
This study had several limitations. Genotyping was available for a minority of patients, but the presence of a combination of key morphologic features of HC such as a septal wall thickness >15 mm, a septal to posterior wall thickness ratio >1.3 and SAM minimize the likelihood of inclusion of non-HC patients in our cohort. Definitive clinical diagnosis of HC does not necessitate a genetic diagnosis since a causative mutation may not be detectable in up to 40-50% of HC patients with a classic HC phenotype. Furthermore, common phenocopy conditions were excluded by testing serum and urine protein electrophoresis, iron profile and serum alpha-galactosidase levels in all patients. Due to the cross-sectional study design we are not able to demonstrate longitudinal changes in wall thickness in HC relative to obesity and consequently infer causality. We do not have cardiac magnetic resonance data, which may provide superior image quality compared to echocardiography particularly in patients with technically challenging images. We did not include any patients whose image quality precluded accurate endocardial border definition. Moreover, all our patients underwent contrast echocardiography wherein the differences between echocardiography and magnetic resonance are less perceptible.
After our paper was accepted for publication, we became aware that a paper entitled “Obesity and its Association to Phenotype and Clinical Course in Hypertrophic Cardiomyopathy” by Olivotto and colleagues was just accepted for publication by the Journal of American College of Cardiology 31. The Authors analyzed the relationship of BMI with LV mass (determined by cardiac magnetic resonance) and heart failure progression in a population of 275 HC patients (mean age 43±14 years, 70% male) with an analogous prevalence of obesity (37%) 31. Interestingly, their findings were very similar to those reported here, thus further strengthening the notion of obesity as a significant modifier of the HC phenotype.
Acknowledgements
We appreciate the assistance provided by the sonographers and nurses of the Johns Hopkins Echocardiography Laboratories and Johns Hopkins HC Clinic. We thank Glenn Lie and Gunnar Hansen of GE Ultrasound for their technical assistance and providing the analysis software.
Source of funding: This work was supported in part by a grant from the National Institutes of Health (HL098046).
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest: None.
REFERENCES
- 1.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212–260. doi: 10.1016/j.jacc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Kumanyika SK, Obarzanek E, Stettler N, Bell R, Field AE, Fortmann SP, Franklin BA, Gillman MW, Lewis CE, Poston WC, 2nd, Stevens J, Hong Y, American Heart Association Council on E. Prevention ICfP Population-based prevention of obesity: the need for comprehensive promotion of healthful eating, physical activity, and energy balance: a scientific statement from American Heart Association Council on Epidemiology and Prevention, Interdisciplinary Committee for Prevention (formerly the expert panel on population and prevention science) Circulation. 2008;118:428–464. doi: 10.1161/CIRCULATIONAHA.108.189702. [DOI] [PubMed] [Google Scholar]
- 3.Avelar E, Cloward TV, Walker JM, Farney RJ, Strong M, Pendleton RC, Segerson N, Adams TD, Gress RE, Hunt SC, Litwin SE. Left ventricular hypertrophy in severe obesity: interactions among blood pressure, nocturnal hypoxemia, and body mass. Hypertension. 2007;49:34–39. doi: 10.1161/01.HYP.0000251711.92482.14. [DOI] [PubMed] [Google Scholar]
- 4.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. Jama. 1991;266:231–236. [PubMed] [Google Scholar]
- 5.Uwaifo GI, Fallon EM, Calis KA, Drinkard B, McDuffie JR, Yanovski JA. Improvement in hypertrophic cardiomyopathy after significant weight loss: case report. South Med J. 2003;96:626–631. doi: 10.1097/01.SMJ.0000053254.23595.14. [DOI] [PubMed] [Google Scholar]
- 6.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Henry WL, Clark CE, Epstein SE. Asymmetric septal hypertrophy. Echocardiographic identification of the pathognomonic anatomic abnormality of IHSS. Circulation. 1973;47:225–233. doi: 10.1161/01.cir.47.2.225. [DOI] [PubMed] [Google Scholar]
- 8.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 9.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Shah JS, Esteban MT, Thaman R, Sharma R, Mist B, Pantazis A, Ward D, Kohli SK, Page SP, Demetrescu C, Sevdalis E, Keren A, Pellerin D, McKenna WJ, Elliott PM. Prevalence of exercise-induced left ventricular outflow tract obstruction in symptomatic patients with non-obstructive hypertrophic cardiomyopathy. Heart. 2008;94:1288–1294. doi: 10.1136/hrt.2007.126003. [DOI] [PubMed] [Google Scholar]
- 11.Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007;116:2597–2609. doi: 10.1161/CIRCULATIONAHA.106.647172. [DOI] [PubMed] [Google Scholar]
- 12.Harrison MR, Grigsby CG, Souther SK, Smith MD, DeMaria AN. Midventricular obstruction associated with chronic systemic hypertension and severe left ventricular hypertrophy. Am J Cardiol. 1991;68:761–765. doi: 10.1016/0002-9149(91)90650-a. [DOI] [PubMed] [Google Scholar]
- 13.Burger JP, Serne EH, Nolte F, Smulders YM. Blood pressure response to moderate physical activity is increased in obesity. Neth J Med. 2009;67:342–346. [PubMed] [Google Scholar]
- 14.Choi EY, Ha JW, Rim SJ, Kim SA, Yoon SJ, Shim CY, Kim JM, Jang Y, Chung N, Cho SY. Incremental value of left ventricular diastolic function reserve index for predicting exercise capacity in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2008;21:487–492. doi: 10.1016/j.echo.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Kjaergaard J, Johnson BD, Pellikka PA, Cha SS, Oh JK, Ommen SR. Left atrial index is a predictor of exercise capacity in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2005;18:1373–1380. doi: 10.1016/j.echo.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Turzyniecka M, Wild SH, Krentz AJ, Chipperfield AJ, Clough GF, Byrne CD. Diastolic function is strongly and independently associated with cardiorespiratory fitness in central obesity. J Appl Physiol. 2010;108:1568–1574. doi: 10.1152/japplphysiol.00023.2010. [DOI] [PubMed] [Google Scholar]
- 17.Klues HG, Schiffers A, Maron BJ. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients. J Am Coll Cardiol. 1995;26:1699–1708. doi: 10.1016/0735-1097(95)00390-8. [DOI] [PubMed] [Google Scholar]
- 18.Maron BJ, Gottdiener JS, Epstein SE. Patterns and significance of distribution of left ventricular hypertrophy in hypertrophic cardiomyopathy. A wide angle, two dimensional echocardiographic study of 125 patients. Am J Cardiol. 1981;48:418–428. doi: 10.1016/0002-9149(81)90068-0. [DOI] [PubMed] [Google Scholar]
- 19.Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, De Santis M, Quarta G, Nistri S, Cecchi F, Salton CJ, Udelson JE, Manning WJ, Maron BJ. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:559–566. doi: 10.1016/j.jacc.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 20.Mazur W, Nagueh SF, Lakkis NM, Middleton KJ, Killip D, Roberts R, Spencer WH., 3rd Regression of left ventricular hypertrophy after nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy. Circulation. 2001;103:1492–1496. doi: 10.1161/01.cir.103.11.1492. [DOI] [PubMed] [Google Scholar]
- 21.Soliman OI, Geleijnse ML, Michels M, Dijkmans PA, Nemes A, van Dalen BM, Vletter WB, Serruys PW, ten Cate FJ. Effect of successful alcohol septal ablation on microvascular function in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2008;101:1321–1327. doi: 10.1016/j.amjcard.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 22.Deb SJ, Schaff HV, Dearani JA, Nishimura RA, Ommen SR. Septal myectomy results in regression of left ventricular hypertrophy in patients with hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 2004;78:2118–2122. doi: 10.1016/j.athoracsur.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 23.Florian A, Masci PG, De Buck S, Aquaro GD, Claus P, Todiere G, Van Cleemput J, Lombardi M, Bogaert J. Geometric assessment of asymmetric septal hypertrophic cardiomyopathy by CMR. J Am Coll Cardiol. 2012;5:702–711. doi: 10.1016/j.jcmg.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Aslam F, Haque A, Foody J, Shirani J. The frequency and functional impact of overlapping hypertension on hypertrophic cardiomyopathy: a single-center experience. J Clinl Hypert. 2010;12:240–245. doi: 10.1111/j.1751-7176.2009.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karam R, Lever HM, Healy BP. Hypertensive hypertrophic cardiomyopathy or hypertrophic cardiomyopathy with hypertension? A study of 78 patients. J Am Coll Cardiol. 1989;13:580–584. doi: 10.1016/0735-1097(89)90596-2. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro LM. Hypertrophic cardiomyopathy in the elderly. British Heart Journal. 1990;63:265–266. doi: 10.1136/hrt.63.5.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, Ernst ND, Horan M. Body mass index and the prevalence of hypertension and dyslipidemia. Obesity Research. 2000;8:605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- 28.Lauer MS, Anderson KM, Levy D. Separate and joint influences of obesity and mild hypertension on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol. 1992;19:130–134. doi: 10.1016/0735-1097(92)90063-s. [DOI] [PubMed] [Google Scholar]
- 29.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JA, Bluemke DA. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2010;3:266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binder J, Ommen SR, Gersh BJ, Van Driest SL, Tajik AJ, Nishimura RA, Ackerman MJ. Echocardiography-guided genetic testing in hypertrophic cardiomyopathy: septal morphological features predict the presence of myofilament mutations. Mayo Clinic Proc. 2006;81:459–467. doi: 10.4065/81.4.459. [DOI] [PubMed] [Google Scholar]
- 31.Olivotto I, Maron BJ, Tomberli B, Appelbaum E, Salton C, Haas TS, Michael Gibson C, Nistri S, Servettini E, Chan RH, Udelson JE, Lesser JR, Cecchi F, Manning WJ, Maron MS. Obesity and its Association to Phenotype and Clinical Course in Hypertrophic Cardiomyopathy. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.03.062. (accepted for publication, doi: 10.1016/j.jacc.2013.03.062) [DOI] [PubMed] [Google Scholar]