Summary
Background
The associations of blood pressure with the different manifestations of incident cardiovascular disease in a contemporary population have not been compared. In this study, we aimed to analyse the associations of blood pressure with 12 different presentations of cardiovascular disease.
Methods
We used linked electronic health records from 1997 to 2010 in the CALIBER (CArdiovascular research using LInked Bespoke studies and Electronic health Records) programme to assemble a cohort of 1·25 million patients, 30 years of age or older and initially free from cardiovascular disease, a fifth of whom received blood pressure-lowering treatments. We studied the heterogeneity in the age-specific associations of clinically measured blood pressure with 12 acute and chronic cardiovascular diseases, and estimated the lifetime risks (up to 95 years of age) and cardiovascular disease-free life-years lost adjusted for other risk factors at index ages 30, 60, and 80 years. This study is registered at ClinicalTrials.gov, number NCT01164371.
Findings
During 5·2 years median follow-up, we recorded 83 098 initial cardiovascular disease presentations. In each age group, the lowest risk for cardiovascular disease was in people with systolic blood pressure of 90–114 mm Hg and diastolic blood pressure of 60–74 mm Hg, with no evidence of a J-shaped increased risk at lower blood pressures. The effect of high blood pressure varied by cardiovascular disease endpoint, from strongly positive to no effect. Associations with high systolic blood pressure were strongest for intracerebral haemorrhage (hazard ratio 1·44 [95% CI 1·32–1·58]), subarachnoid haemorrhage (1·43 [1·25–1·63]), and stable angina (1·41 [1·36–1·46]), and weakest for abdominal aortic aneurysm (1·08 [1·00–1·17]). Compared with diastolic blood pressure, raised systolic blood pressure had a greater effect on angina, myocardial infarction, and peripheral arterial disease, whereas raised diastolic blood pressure had a greater effect on abdominal aortic aneurysm than did raised systolic pressure. Pulse pressure associations were inverse for abdominal aortic aneurysm (HR per 10 mm Hg 0·91 [95% CI 0·86–0·98]) and strongest for peripheral arterial disease (1·23 [1·20–1·27]). People with hypertension (blood pressure ≥140/90 mm Hg or those receiving blood pressure-lowering drugs) had a lifetime risk of overall cardiovascular disease at 30 years of age of 63·3% (95% CI 62·9–63·8) compared with 46·1% (45·5–46·8) for those with normal blood pressure, and developed cardiovascular disease 5·0 years earlier (95% CI 4·8–5·2). Stable and unstable angina accounted for most (43%) of the cardiovascular disease-free years of life lost associated with hypertension from index age 30 years, whereas heart failure and stable angina accounted for the largest proportion (19% each) of years of life lost from index age 80 years.
Interpretation
The widely held assumptions that blood pressure has strong associations with the occurrence of all cardiovascular diseases across a wide age range, and that diastolic and systolic associations are concordant, are not supported by the findings of this high-resolution study. Despite modern treatments, the lifetime burden of hypertension is substantial. These findings emphasise the need for new blood pressure-lowering strategies, and will help to inform the design of randomised trials to assess them.
Funding
Medical Research Council, National Institute for Health Research, and Wellcome Trust.
Introduction
High blood pressure was the leading risk factor for the overall global burden of disease in 2010.1 The recent decrease in cardiovascular mortality in high-income countries has been associated with a rise in the numbers of patients living with cardiovascular disease, and the wider use of preventive drugs. Thus, an up-to-date understanding of the associations of blood pressure with different non-fatal and fatal cardiovascular disease outcomes would help to refine strategies for primary prevention and inform the design of future clinical trials.
The Prospective Studies Collaboration meta-analysis of 61 cohorts recruited between 1950 and 1990 reported log-linear associations of systolic and diastolic blood pressure with death from ischaemic heart disease and stroke, with no apparent threshold below which no further reduction in risk is observed, down to a blood pressure of 115/75 mm Hg, in participants aged 40–89 years.2 These findings predated several public health initiatives, including efforts to reduce salt consumption and tobacco use, and the more widespread use of blood pressure-lowering treatments for primary prevention, and did not provide information about major chronic and non-fatal diseases, including heart failure, peripheral arterial disease, abdominal aortic aneurysm, and stable angina. Importantly, no current estimates are available for the lifetime incidence and years of life lost associated with hypertension attributable to specific cardiovascular diseases. Although in previous studies investigators have estimated the associations of cardiovascular disease risk factors with lifetime risks3 or cardiovascular disease-free years of life lost,4 their focus was on total cardiovascular disease, with only one study so far to have analysed the incidence of specific cardiovascular diseases in a competing risks context.5
We sought to address these limitations in a large contemporary cohort, based on linked electronic health records, with high prevalent use of blood pressure-lowering treatments, and with blood pressure measurements done as part of usual clinical practice.
Methods
Data sources
We selected anonymised patients from the CALIBER (CArdiovascular research using LInked Bespoke studies and Electronic health Records) programme.6 This programme was established to provide access to longitudinal data of linked electronic health records through the creation of a common data model with reproducible phenotypes and metadata. Diagnosis codes and endpoints in CALIBER have been validated by independent groups.7 Patients were linked across four clinical data sources: the Clinical Practice Research Datalink (CPRD), the Myocardial Ischaemia National Audit Project (MINAP) registry, Hospital Episodes Statistics (HES), and cause-specific mortality. CPRD provides information about anthropometric measurements, laboratory tests, clinical diagnoses, prescriptions, and medical procedures, coded with the Read clinical coding scheme. The primary care practices in CPRD and the subset of linked practices used in the present analysis are representative of the UK primary care setting8,9 and have been validated for epidemiological research.7 MINAP is a national registry of patients admitted to hospital with acute coronary syndromes. HES provides information about diagnoses (coded with the tenth revision of the International Classification of Diseases [ICD-10]) and medical procedures related to all elective and emergency hospital admissions across all National Health Service hospitals in England.
Study population
We selected patients from 225 primary care practices registered between January, 1997, and March, 2010, who were aged 30 years or older at study entry (the index date), who had been registered with their practices for at least 1 year before the index date and had no previous diagnosis of cardiovascular disease. Appendix p 9 shows the study flow diagram.
Exposure and baseline risk factors
Baseline blood pressure and other cardiovascular disease risk factors were recorded during consultations in primary care. Baseline blood pressure was based on readings taken within 2 years of the index date; these readings were averaged across repeat measurements when available. In sensitivity analyses, we used the blood pressure measurement recorded closest to the index date. Patients were classified as having hypertension if their baseline blood pressure was 140/90 mm Hg or higher, or they had a recorded diagnosis of hypertension, or they received repeat prescriptions (at least two monthly packs) for blood pressure-lowering drugs (including thiazide diuretics, β blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or calcium channel blockers). We distinguished isolated systolic hypertension in which systolic blood pressure is 140 mm Hg or above but diastolic blood pressure is lower than 90 mm Hg, and isolated diastolic hypertension in which systolic blood pressure is lower than 140 mm Hg but diastolic blood pressure is 90 mm Hg or higher. For covariate adjustments, we used the most recent measurement recorded in the year before the index date. We imputed missing covariate values using multiple imputation,10 as implemented in the “mice” algorithm in the statistical package R. We checked whether the imputations were plausible by comparing plots of the distribution of recorded and imputed values of all variables. In the primary analysis, we excluded patients with no baseline blood pressure measurements; in sensitivity analyses we included these patients by imputing their blood pressure at baseline (details in appendix). Phenotyping algorithms combining Read, ICD-10, drug and procedure codes to define risk factors and endpoints are available online.
Endpoints
Endpoints, as previously defined (see appendix pp 1–4),6 were the initial presentation of cardiovascular disease as any of 12 cardiovascular diseases diagnosed in primary care, secondary care, or at death, and total cardiovascular disease (all 12 cardiovascular diseases combined). In analyses of lifetime risks and years of life lost, we combined ischaemic stroke with unclassified stroke because previous studies have shown that 87% of unclassified strokes were ischaemic.11 In sensitivity analyses, we estimated associations with hospital admissions and deaths, or with deaths only. Patients were censored on the date of first cardiovascular disease presentation, death from other causes, deregistration from the practice, or final practice data collection, whichever occurred first.
Study approval was granted by the Independent Scientific Advisory Committee of the Medicines and Healthcare products Regulatory Agency and the MINAP Academic Group. This study is registered at ClinicalTrials.gov, number NCT01164371.
Statistical analysis
Hazard ratios (HRs) were based on disease-specific Cox models with time since study entry as the timescale, adjusted for baseline age (linear and quadratic term) and stratified by sex and primary care practice. We assessed between-practice heterogeneity of these associations by estimating practice-specific associations of blood pressure with each outcome and combining them in a random-effects meta-analysis; this approach allowed us to estimate a heterogeneity-adjusted confidence interval and a prediction interval that expresses uncertainty about the association in a randomly chosen practice.12 We verified the proportional hazards assumption by plotting the Schoenfield residuals. We analysed non-linear associations with blood pressure by modelling blood pressure as a continuous variable (rather than as blood pressure categories) for which we used restricted cubic splines with three knots; this method allows estimation of the HRs in comparison to any single reference value (we chose 115 mm Hg for systolic blood pressure and 75 mm Hg for diastolic blood pressure, since these values are close to the mean for each blood pressure variable).13 We constructed confidence intervals for HRs, setting the α level (probability of type I error) to 0·05 and adjusted for the total number of associations reported in each set of analyses based on the Bonferroni correction. We estimated lifetime risks of each cardiovascular disease and years of life lost associated with hypertension adjusted for the competing risk of other cardiovascular diseases and non-cardiovascular disease mortality based on Cox models with age as the timescale (details in appendix).14,15 To estimate years of life lost attributable to different types of hypertension (isolated systolic, isolated diastolic, or other) in a particular age group, we first estimated the years of life lost for each type of hypertension and computed their weighted average, with weights corresponding to their relative prevalence in people with hypertension in that age group. We estimated confidence intervals for lifetime risks and years of life lost by running the cause-specific Cox models in 200 bootstrap samples. All analyses were done in R version 15.2.
Primary and secondary analyses
In the primary analysis, we report the associations of each outcome with 20/10 mm Hg increase in systolic/diastolic blood pressure across all ages, by age group (30–59, 60–79, and ≥80 years), and for different blood pressure values in each age group, allowing for non-linearity in blood pressure associations, and estimate the lifetime risks and years of life lost associated with hypertension for different index ages (30, 60, and 80 years). In our secondary analysis, we assess the associations of blood pressure with different outcomes after further adjustment for smoking status, diabetes, total cholesterol, and high-density lipoprotein cholesterol; then additional adjustment for body-mass index (BMI); and finally further adjustment for baseline treatment with blood pressure-lowering drugs. Moreover, we study the age-adjusted and sex-adjusted associations of different cardiovascular outcomes with pulse pressure (calculated as systolic blood pressure minus diastolic blood pressure), mean arterial pressure (diastolic blood pressure plus a third of pulse pressure), and mid-blood pressure (0·5 × systolic blood pressure plus 0·5 × diastolic blood pressure). We also report sex-specific blood pressure associations, lifetime risks, and years of life lost, and age-adjusted and sex-adjusted blood pressure associations by whether or not blood pressure-lowering drugs were being used at baseline.
Role of the funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and all authors had final responsibility for the decision to submit for publication.
Results
The summary characteristics of the patient cohort by hypertension status are shown in appendix p 5. Patients with missing baseline blood pressure values were younger, healthier, more likely to be male, more likely to be white, and were less likely to be receiving blood pressure-lowering drugs than were patients whose baseline blood pressure was recorded (appendix p 6). After we excluded the 679 354 patients whose baseline blood pressure was missing (35% of the 1 937 360 patients eligible for inclusion), the primary analysis cohort included 1 258 006 patients (732 657 [58%] of whom were women) who had 83 098 first cardiovascular events during a median follow-up of 5·2 years (IQR 2·1–9·4). In participants with recorded baseline blood pressure, 188 701 (15%) had data missing for smoking status, 691 903 (55%) for BMI, and 1 006 405 (80%) for total cholesterol. A third (431 663) of the cohort had blood pressure of 140/90 mm Hg or higher and a fifth (265 473) received blood pressure-lowering drugs at baseline (median 8 [IQR 2–13] monthly packs per year). Hypertension was recorded in 545 816 (43%) of the patients, 1 094 465 (87%) of whom were classified as having hypertension on the basis of blood pressure of 140/90 mm Hg or higher or physician-coded diagnosis, and the remaining 163 541 (13%) on the basis of medication use only. We noted large variations across different primary care practices in terms of the patients' ethnic backgrounds, socioeconomic status, and uptake of statins and blood pressure-lowering treatments (appendix p 10).
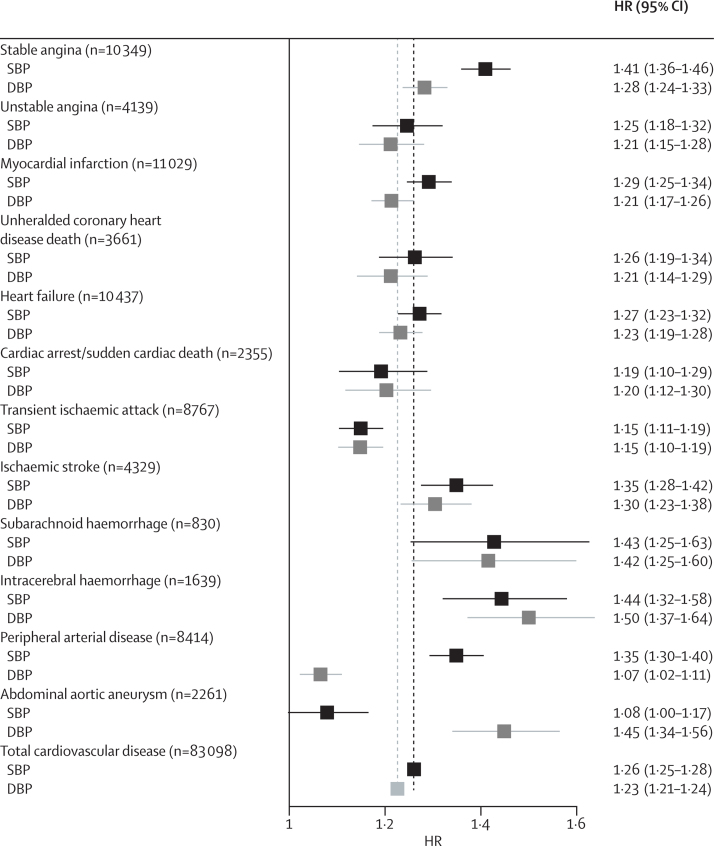
We report associations for 20/10 mm Hg changes in systolic/diastolic blood pressure throughout our analysis, but we note that the equivalence of these increments with respect to cardiovascular disease risks varied with age (overall, a 20 mm Hg change in systolic blood pressure was equivalent in risk to roughly 11 mm Hg increments in diastolic blood pressure). Systolic and diastolic blood pressure showed heterogeneous associations with different cardiovascular outcomes (figure 1). A 20 mm Hg rise in systolic blood pressure showed substantially stronger associations with stable angina (HR 1·41 [95% CI 1·36–1·46]), subarachnoid haemorrhage (1·43 [1·25–1·63]), and intracerebral haemorrhage (1·44 [1·32–1·58]) than with total cardiovascular disease (1·26 [1·25–1·28]). Compared with systolic blood pressure, diastolic blood pressure showed substantially weaker associations with stable angina, peripheral arterial disease, and myocardial infarction and with total cardiovascular disease (figure 1). Abdominal aortic aneurysm had the strongest association of all cardiovascular diseases with diastolic blood pressure (HR 1·45 [95% CI 1·34–1·56]) and mean arterial pressure (HR per 10 mm Hg 1·61 [1·48–1·75]), the weakest association with systolic blood pressure (1·08 [1·00–1·17] per 20 mm Hg), and was the only outcome for which the association with higher pulse pressure was reversed (HR per 10 mm Hg 0·91 [0·86–0·98]) (appendix p 11). Conversely, we noted that peripheral arterial disease had an inverse association with mean arterial pressure (HR 0·90 [95% CI 0·86–0·94]) and the strongest association of all cardiovascular diseases with pulse pressure (1·23 [1·20–1·27]). Associations with mid-blood pressure were strongest for stroke (ischaemic stroke, subarachnoid haemorrhage, and intracerebral haemorrhage), but very similar across other cardiovascular outcomes (appendix p 11).
Figure 1.
Forest plot of HRs (95% CIs) per 20/10 mm Hg increase in systolic (black) or diastolic (grey) blood pressure, adjusted for age and sex
The vertical dashed lines correspond to the associations of SBP (black) or DBP (grey) with total cardiovascular disease. Adjustments include age, quadratic age, and stratification by sex and primary care practice. CIs are Bonferroni corrected (13 endpoints × 2 variables=26 tests). HR=hazard ratio. SBP=systolic blood pressure. DBP=diastolic blood pressure.
Myocardial infarction had a stronger association with systolic blood pressure in women than in men (p<0·0001), but associations with other outcomes were similar in both sexes (appendix p 12). Associations with blood pressure decreased very little after adjustment for cardiovascular disease risk factors, with the largest attenuation in HRs noted for heart failure upon adjustment for BMI (appendix p 13). Further adjustment for blood pressure-lowering drugs attenuated the associations of systolic and diastolic blood pressure with all endpoints by 20–30%, but had no effect on the associations with stroke outcomes (appendix p 13). Similarly, the age-adjusted and sex-adjusted associations with blood pressure were substantially weaker in people who received blood pressure-lowering treatments at baseline than in those who did not, except for the associations with ischaemic stroke and intracerebral haemorrhage, which were similar between treated and untreated patients, and the associations with subarachnoid haemorrhage, which were larger (especially with diastolic blood pressure) in treated than in untreated patients (appendix pp 14–15).
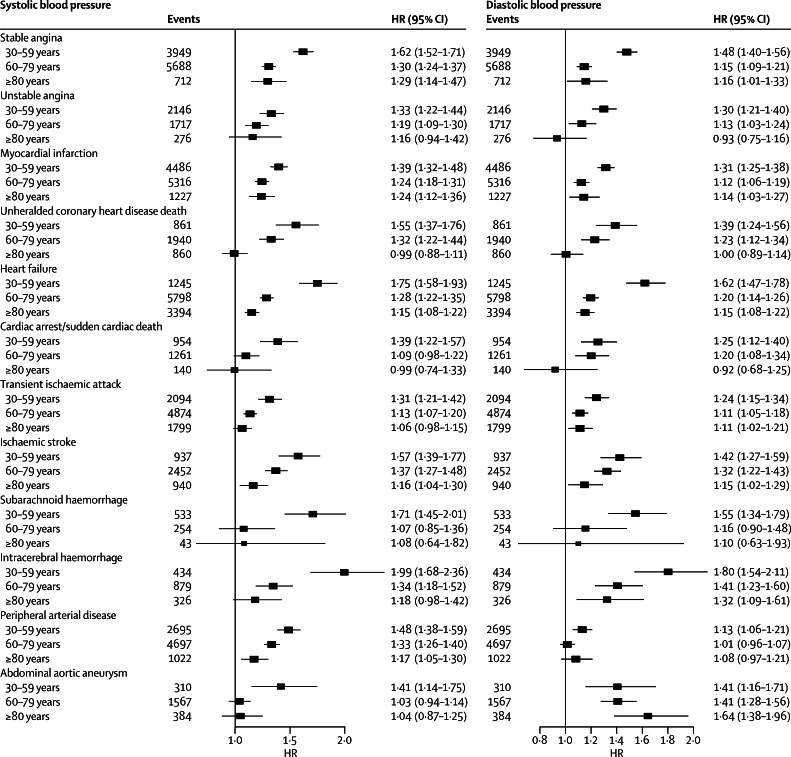
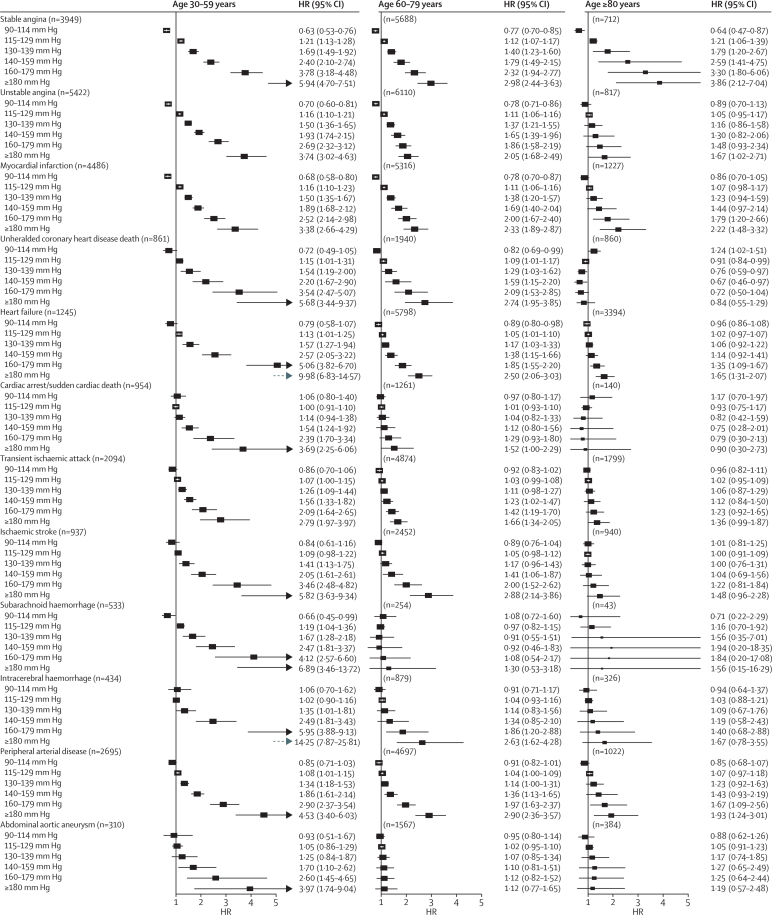
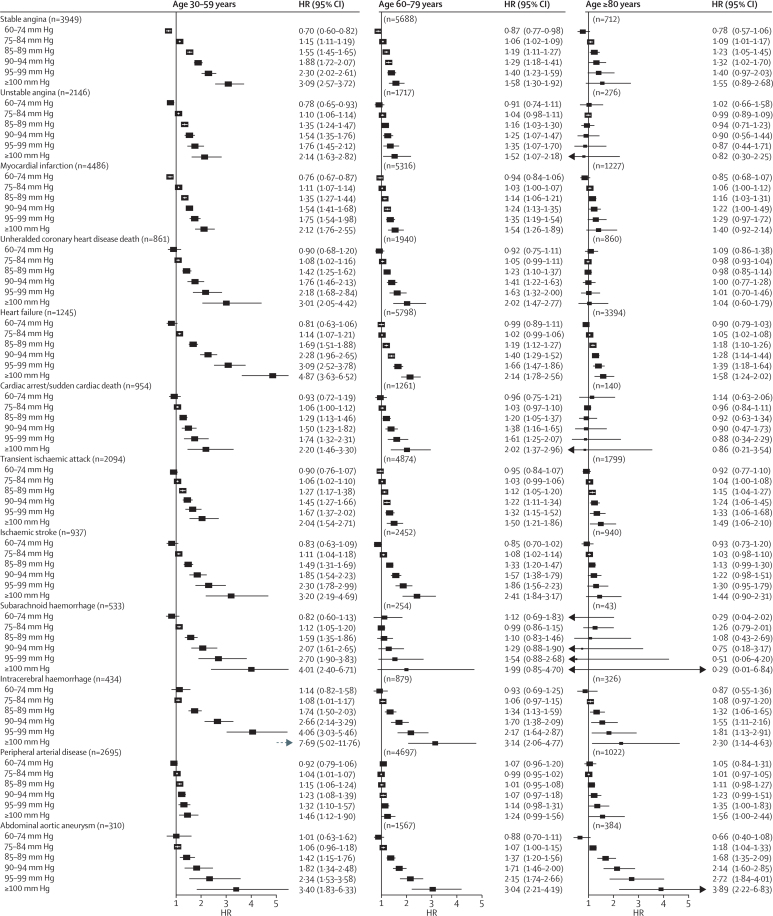
Associations with both systolic and diastolic blood pressure decreased with age for all outcomes at varying rates for different outcomes (figure 2). Across different diseases and ages, the shape of associations with systolic blood pressure (figure 3) and diastolic blood pressure (figure 4) also showed substantial variation. We did not record J-shaped associations with any of the outcomes. Between 30 and 79 years of age, the associations of systolic and diastolic blood pressure with stable and unstable angina, myocardial infarction, unheralded coronary death, and subarachnoid haemorrhage were generally linear, whereas associations with other outcomes were mostly log-linear. For the linear associations, the risk was lowest in people with systolic blood pressure 90–114 mm Hg and was significantly lower than for a higher systolic blood pressure of 115–129 mm Hg). For the log-linear associations, there was little or no further reduction in risk for blood pressure below about 130/85 mm Hg, whereas modest increases in either systolic or diastolic blood pressure above this range were accompanied by large increases in hazard ratios, especially for unheralded coronary death, heart failure, ischaemic stroke, intracerebral haemorrhage, and peripheral arterial disease. In people aged 60 years and older, systolic blood pressure was no longer associated with subarachnoid haemorrhage or with abdominal aortic aneurysm (figure 3). In those aged 80 years and older, the strongest associations for systolic blood pressure were with stable angina, myocardial infarction, intracerebral haemorrhage, and peripheral arterial disease (all linear), and we noted a weak U-shaped association for unheralded coronary death (with systolic blood pressure 140–159 mm Hg having the lowest risk), whereas associations with the remaining outcomes were either not significant or were recorded only with high blood pressure (≥140/90 mm Hg) (figure 3). Similar patterns were recorded in men and women (appendix pp 16–19), and in patients treated or not treated with blood pressure-lowering drugs at baseline (appendix pp 20–23).
Figure 2.
Forest plots of HRs (95% CIs) for 20/10 mm Hg changes in blood pressure in different age groups, adjusted for age and sex
Models included continuous age, age group, interaction between blood pressure and age group (which is the association reported in the forest plot), and stratification by sex and primary care practice. CIs are Bonferroni corrected (3 age groups × 12 endpoints=36 tests). HR=hazard ratio.
Figure 3.
Forest plots of HRs (95% CIs) for different cutoffs of systolic blood pressure (vs reference 115 mm Hg) adjusted for age and sex
Blood pressure was modelled as a continuous variable with splines with three knots. Adjustments include age, quadratic age, and stratification by sex and primary care practice. CIs are Bonferroni corrected (3 age groups × 6 categories × 12 endpoints=216 tests). HR=hazard ratio.
Figure 4.
Forest plot of HRs (95% CIs) for different cutoffs of diastolic blood pressure (vs reference 75 mm Hg) adjusted for age and sex
Blood pressure was modelled as a continuous variable with splines with three knots. Adjustments include age, quadratic age, and stratification by sex and primary care practice. CIs are Bonferroni corrected (3 age groups × 6 categories × 12 endpoints=216 tests). HR=hazard ratio.
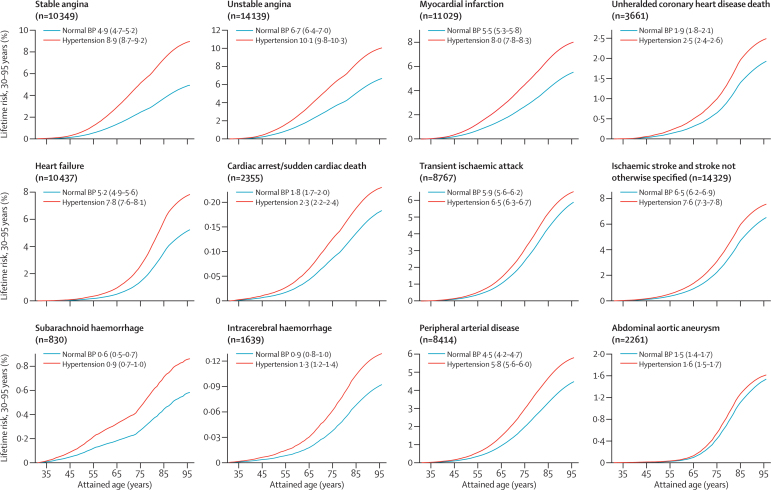
The lifetime risk of total cardiovascular disease at 30 years of age was 63·3% (95% CI 62·9–63·8) in people with hypertension and 46·1% (45·5–46·8) in those with healthy blood pressure (absolute difference 17·2% [95% CI 13·9–20·5]) (figure 5, and see appendix p 24 for estimates by sex). Stable and unstable angina were the most frequent outcomes, with lifetime risks of around 10% in people with hypertension and 6% in those with normal blood pressure. Lifetime risks in people with hypertension were also high for myocardial infarction (8·0% [7·8–8·3]), heart failure (7·8% [7·6–8·1]), and ischaemic stroke(7·6% [7·3–7·8]) (figure 5). However, these risks did not increase by the same proportion in people with hypertension compared with those without the disorder. Thus, ischaemic stroke, transient ischaemic attack, and abdominal aortic aneurysm were only 1·1-times more common in patients with hypertension than in those with normal blood pressure, whereas heart failure was 1·5-times more common and stable angina 1·8-times more common (see appendix p 7 for lifetime risks, risk differences, and lifetime risk ratios for all outcomes and for other index ages).
Figure 5.
Lifetime risk (95% CI) of 12 different cardiovascular diseases in people with hypertension or normal BP from index age 30 years
Hypertension was defined as systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg or use of BP-lowering treatments or physician-recorded diagnosis at baseline. BP=blood pressure.
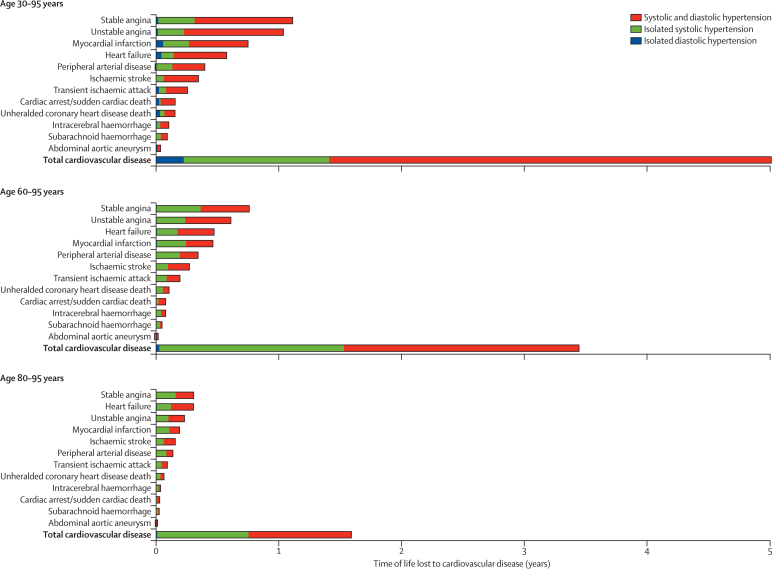
The mean number of cardiovascular disease-free life-years lost associated with hypertension was 5·0 years (95% CI 4·8–5·4) from 30 years of age, 3·4 years (3·3–3·6) from 60 years, and 1·6 years (1·5–1·7) from 80 years (figure 6; appendix p 25 shows estimates by sex). The largest proportions of cardiovascular disease-free years of life lost associated with hypertension at index age 30 years were attributable to stable angina (22%), unstable angina (21%), and myocardial infarction (15%). However, from index age 80 years, stable angina and heart failure accounted for most (19% each) of all cardiovascular disease-free years of life lost, followed by unstable angina (15%), myocardial infarction (12%), and ischaemic stroke (10%; figure 6). Isolated systolic hypertension (prevalent in 35% [109 587/314 574], 60% [113 625/190 300, and 64% [26 274/40 942] of patients with hypertension aged 30–59, 60–79, and ≥80 years, respectively) accounted for a quarter of the cardiovascular disease-free years of life lost associated with hypertension in those aged 30–59 years, and nearly half at older ages. By contrast, isolated diastolic hypertension (prevalent in 10% [31 404/314 574], 1·4% [2761/190 300], and 0·6% [259/40 942] of patients with hypertension aged 30–59, 60–79, and ≥80 years, respectively) accounted for only 0·5% of years of life lost to cardiovascular disease associated with hypertension in those aged 30–59 years, with a negligible contribution (<0·01%) in older age groups (appendix p 7).
Figure 6.
Years of life lost to cardiovascular disease up to 95 years of age associated with hypertension at index ages 30, 60, and 80 years, adjusted for sex, smoking, diabetes, and total and high-density lipoprotein cholesterol
83 098 total cardiovascular disease events occurred.
Our sensitivity analyses showed that general practice variability had no effect on the overall associations, since random effects meta-analysis estimates were almost identical to the fixed effects estimates reported in our main analysis (appendix p 26). The age-adjusted and sex-adjusted associations between blood pressure and endpoints restricted to hospital admissions and death were comparable to the main analysis but were stronger between systolic blood pressure and peripheral arterial disease and between diastolic blood pressure and abdominal aortic aneurysm, and weaker between systolic blood pressure and stable angina (appendix p 27). Patients who had more than one blood pressure measurement (60% of the cohort) had similar associations with endpoints as those based on a single measurement, with the exception of stronger associations with peripheral arterial disease and stable angina (appendix p 28). Similarly, the use of a single measurement closest to the baseline instead of the average blood pressure resulted in smaller but similarly patterned associations (appendix p 29). Associations between blood pressure and cardiovascular outcomes when missing baseline blood pressure was imputed closely matched the main analysis estimates based on complete cases (appendix p 30). The age-adjusted mortality rate was very similar between people with and without baseline blood pressure measurements (HR for mortality in people with missing vs recorded blood pressure 1·01 [95% CI 0·99–1·02]).
Discussion
In this study of 1·25 million patients accruing 83 098 cardiovascular events of 12 different diseases during 5·2 years median follow-up, we showed that the lifetime burden of hypertension remains substantial, despite modern therapy. We show that across different diseases and different ages there was substantial heterogeneity in associations with blood pressure, not only in terms of strength but also in whether an association existed and how it was shaped.
An important advance of this study over previous reports was our ability to compare in one study the associations of blood pressure across a substantially wider range of incident cardiovascular diseases,2,4 across a broader age range (including people younger than 40 years), and a wider range of blood pressure values (including <115/75 mm Hg). This higher resolution approach was enabled by electronic health record linkage and is generalisable to the population rather than those selected individuals who participate in trials or observational research (99% of the UK population are registered with a primary care practice16), and is clinically relevant, focusing on measurements as recorded in clinical care.
For nearly all cardiovascular diseases, we noted that the relative risks decreased with age and no significant deviations from linearity occurred in the associations of either systolic or diastolic blood pressure with different outcomes at any age. The absence of J-shape associations is consistent with previous studies, including the Prospective Studies Collaboration2 and the Framingham study (in which J-shape associations with diastolic blood pressure occurred only when accompanied by systolic blood pressure >140 mm Hg),17 and is supported by trial evidence in elderly people that shows significant and sustained risk reduction for cardiovascular endpoints with lowering of blood pressure.18
Our study reports the lifetime risks of specific cardiovascular outcomes as the first presentation of cardiovascular disease (panel). Unlike previous studies of lifetime risks, we excluded people who had experienced different types of cardiovascular disease before the outcome of interest and therefore our estimates are expected to be substantially lower, especially for outcomes associated strongly with age or pre-existing cardiovascular disease, than those in previous studies. The lifetime risk of heart failure in the Framingham study was 20% overall, and between 11 and 17% (depending on sex) when people with previous myocardial infarction were excluded.19 In our study, we show that the lifetime risk of heart failure after accounting (by competing risks modelling) for patients who experienced another cardiovascular outcome before heart failure is 5%, and is almost twice as high in people with hypertension as in those with normal blood pressure. Similarly, our estimated lifetime risk for stroke (for which we combined ischaemic and haemorrhagic stroke for comparability) was 8–10%, which is substantially lower than the 17–20% reported in the Framingham study.21 This difference could be explained by the late manifestation of ischaemic strokes and because a substantial proportion of them were preceded by a transient ischaemic attack event. Conversely, for subarachnoid haemorrhage, which is more prevalent in younger ages, our lifetime risk estimates (0·6–0·9%) were similar to the previously reported estimates of around 0·5%.22 For composite cardiovascular disease outcomes, our approach is equivalent to other studies, leading to broadly similar estimates allowing for differences in risk factor prevalence, composition of endpoints, and different index ages.3,23,24
Panel. Research in context.
Systematic review
We searched Medline for original research from the past 10 years describing the age-specific associations of blood pressure variables in large observational studies with the initial presentation of a wide range of cardiovascular diseases (in which three or more diseases were studied simultaneously). We used the search terms “first [incident] cardiovascular”, “age-specific”, “blood pressure associations”, “competing risks and hypertension”, “years of life-lost and hypertension”, and “lifetime risks and hypertension” and widened our search for “hypertension” and different causes of death, and used various (three or more) combinations of each endpoint studied as keywords in the same search. We did not restrict our search dates but we only searched for studies published in English. We screened publications by abstract for relevance. The only observational study that fulfilled the criteria for inclusion of several cardiovascular endpoints was the Prospective Studies Collaboration2 which reported the age-specific associations (age 40–89 years, participant recruitment period 1950–90) of blood pressure with death from different types of stroke and coronary heart disease, and the overall (all ages) associations of blood pressure with a wider range of cardiovascular outcomes. One study fulfilled the criteria for analysis of time to first cardiovascular event type in people with hypertension under a competing risks framework.5 Various analyses of the Framingham cohort reported on the associations of blood pressure with incident cardiovascular diseases beyond heart attack and stroke (eg, heart failure19 and peripheral arterial disease20), but hazard ratios were not directly comparable because they were based on different subsets of the Framingham cohort or offspring. Most other studies identified in our searches were small (often <1000 participants) or not relevant because they focused on the associations of blood pressure with cardiovascular disease risk in people who had already developed the different cardiovascular diseases studied here.
Interpretation
Our study confirms the findings from several smaller studies (meta-analysed in the Prospective Studies Collaboration) that investigated individual cardiovascular diseases. Our results extend the previous associations of blood pressure to a wider range (12) of cardiovascular diseases, some of which have rarely been studied as initial presentations, and include non-fatal and chronic presentations of cardiovascular disease (heart failure, peripheral arterial disease, and stable angina); to younger age groups (<40 years); to people with lower blood pressure (<115/75 mm Hg); and to the contemporary population in whom the use of blood pressure-lowering medications is widespread (such drugs were rarely used in the participants of the Prospective Studies Collaboration). Moreover, we report on the associations of a wide range of blood pressure measures for these outcomes, and show that for some cardiovascular diseases, pulse pressure and mean arterial pressure have prognostic value. For the first time, we estimate lifetime risks and years of life lost to different types of cardiovascular disease through the application of competing risks methodology. A better understanding of the different associations of blood pressure with different types of cardiovascular disease and the lifetime risks associated with hypertension at different ages will help to focus guidelines and clinicians to the disease areas in which screening and treatments are more likely to have an effect.
Our findings are also consistent with the pattern of initial cardiovascular disease presentations associated with hypertension reported in 2694 Framingham participants recruited in the 1970s, which to our knowledge is the only previous analysis of cardiovascular disease risks (up to 12 years) with some disaggregation into different outcomes under a competing risks framework.5 Consistent with our findings, the type of cardiovascular outcome in this Framingham subcohort varied by age (with stroke being more common in older hypertensive patients), and sex (with myocardial infarction or fatal coronary heart disease being more frequent in men with hypertension, and stroke more common in women with hypertension).
Substantial debate has surrounded the benefits of treating mild (stage 1) hypertension in young people, especially in those without evidence of target organ damage or calculated to have a low 10-year risk of cardiovascular disease.25 A recent meta-analysis26 showed that during a 5-year follow-up, no significant reduction in cardiovascular events resulted from the administration of blood pressure-lowering treatments to people with mild hypertension, but longer time horizons might be needed to show the benefits of long-term primary prevention in such populations. In the absence of long-term randomised trials, our estimates of lifetime risk and cardiovascular disease-free years of life lost provide epidemiological evidence of substantial morbidity associated with raised blood pressure, irrespective of the starting baseline risk.
Isolated systolic and combined systolic and diastolic hypertension accounted for the largest proportion of cardiovascular disease-free years of life lost, with a negligible contribution from isolated diastolic hypertension, even in those aged 30–59 years in whom the prevalence of isolated diastolic hypertension was highest. Thus, our estimates support the shift in guideline focus in recent years from the importance of diastolic towards the greater importance of systolic pressure in people aged 60 years and older.27
Our data support the possibility that blood pressure functions through different underlying biological mechanisms for different diseases. For example, the strong associations of blood pressure with stable angina and intracerebral haemorrhage might represent the respective causative contributions of left ventricular hypertrophy and vascular rupture to these disorders. Similarly, the arterial rigidity that protects against abdominal aortic aneurysm might account for the weak association with systolic blood pressure and for the stronger association with diastolic blood pressure and mean arterial pressure—findings consistent with previous, smaller, studies of risk factors for abdominal aortic aneurysm.28,29
Our findings have important clinical implications. Recently published clinical practice guidelines from the USA30 and the UK31 agree that the primary purpose of assessment of cardiovascular risk is to provide the basis of a risk discussion with the patient. Our estimates of lifetime risks and years of cardiovascular disease-free life lost to different diseases can be used to extend the existing counselling of patients and decision making, which is based on heart attack and stroke risks alone. Here, we show the importance of other diseases that might be more common; of the 5 years of cardiovascular disease-free life lost associated with hypertension, nearly half were attributable to stable and unstable angina, whereas in the 80 years and older age group, heart failure accounted for nearly a fifth of the years of life lost. Different diseases might also affect patient quality of life differently—for example, patients might value living free from heart failure or peripheral arterial disease differently to living free from a heart attack or stroke. Despite the high burden of heart failure, angina, and peripheral arterial disease in this population, very few large-scale studies have focused on risk factor associations with these outcomes in previously cardiovascular disease-free patients.19,32–34
Our estimates also emphasise the unmet need of existing blood pressure-lowering strategies, as shown by the width of the separation of the lifetime risk curves in people with and without hypertension. Better mitigation of these excess risks could come from better implementation of existing blood pressure-lowering treatments and through better management of other cardiovascular risk factors as part of global risk estimation. An important need also exists for new interventions and our findings have implications for the design of new, and the interpretation of existing, clinical trials. For example, we show that heart failure and peripheral arterial disease are among the most common initial presentations of cardiovascular disease, but are included less frequently in the primary outcome of blood pressure-lowering trials.35 The benefits of treatment of hypertension to reduce the risk of myocardial infarction and stroke are well established36 and although our study has shown less pronounced associations of hypertension with other manifestations of cardiovascular disease, the need for vigorous treatment as part of a package of risk-reduction measures remains undiminished.
Although a major strength of this study is the ability to resolve a wide range of cardiovascular diseases in a validated electronic hospital record linkage setting,7 limitations do exist in the amount of clinical detail presently recorded in national electronic hospital records, such as on imaging results. Our lifetime risks were estimated based on extrapolation of event rates recorded in people entering the cohort at different ages, rather than following up each patient over their residual lifetime. Although we account for age at measurement (by including age as a factor in the model and in the baseline hazard), we have not accounted for changes in blood pressure and other risk factors over time. A more detailed analysis of lifetime risks would take repeated measures into consideration to account for potential changes and would adjust for the time-dependent effect of medications taken long term.
In conclusion, systolic and diastolic blood pressure show heterogeneous associations across a wide range of acute and chronic cardiovascular diseases and at different ages. These findings have implications for the design of new trials and preventive strategies to address the substantial contemporary unmitigated lifetime burden of hypertension.
Acknowledgments
Acknowledgments
This study was done on behalf of the CALIBER programme. This work was supported by the National Institute for Health Research (NIHR) (to AT and HH; grant RP-PG-0407–10314), the Wellcome Trust (to AT, LS, and HH; grant 086091/Z/08/Z), and the Medical Research Council Prognosis Research Strategy (PROGRESS) Partnership (to AT and HH; grant G0902393/99558), and by awards to establish the Farr Institute of Health Informatics Research at UCL Partners (to HH, LS, MJC, JED, SD, and AT), from the Medical Research Council, Arthritis Research UK, British Heart Foundation, Cancer Research UK, Chief Scientist Office, Economic and Social Research Council, Engineering and Physical Sciences Research Council, NIHR, National Institute for Social Care and Health Research, and Wellcome Trust (to AT, LS, and HH; grant MR/K006584/1). AT was supported by Barts and The London NIHR Cardiovascular Biomedical Research Unit, funded by the NIHR. SD is supported by a UCL Provost's Strategic Development Fund Fellowship. LS is supported by a Wellcome Trust Senior Research Fellowship in Clinical Science. JG was funded by an NIHR Doctoral Fellowship (DRF-2009–02–50). ADS is supported by a clinical research training fellowship from the Wellcome Trust (0938/30/Z/10/Z). BW is a NIHR Senior Investigator and his research is supported by the NIHR University College London Hospitals Biomedical Research Centre. MJC is a NIHR Senior Investigator and is supported by the Barts NIHR Cardiovascular Biomedical Research Unit. IRW was supported by the Medical Research Council (unit programme number U105260558).
Contributors
ER analysed and interpreted the data and wrote the report. HH was the principal investigator and had the original research idea. AT contributed to the writing of the report and the interpretation of results. SD prepared the data. ADS coordinated the definition of endpoints and variables. JG did the preliminary analysis and contributed to the definition of endpoints and variables. IRW advised about statistical methodology and the interpretation of results. MP-R wrote the research protocol and obtained approval for the study. AH, ADS, AT, BW, HH, IRW, JED, JG, LS, MJC, MP-R, and SD critically reviewed and commented on the report. All authors saw and approved the final version.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Lim SS, Vos T, Flaxman AD. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, the Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Hippisley-Cox J, Coupland C, Robson J, Brindle P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. BMJ. 2010;341:c6624. doi: 10.1136/bmj.c6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308:1795–1801. doi: 10.1001/jama.2012.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Leip EP, Larson MG, Vasan RS, Levy D. Novel approach to examining first cardiovascular events after hypertension onset. Hypertension. 2005;45:39–45. doi: 10.1161/01.HYP.0000149106.89470.13. [DOI] [PubMed] [Google Scholar]
- 6.Denaxas SC, George J, Herrett E. Data resource profile: cardiovascular disease research using linked bespoke studies and electronic health records (CALIBER) Int J Epidemiol. 2012;41:1625–1638. doi: 10.1093/ije/dys188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrett E, Shah AD, Boggon R. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ. 2013;346:f2350. doi: 10.1136/bmj.f2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher AM, Puri S, van Staa TP. Linkage of the General Practice Research Database (GPRD) with other data sources. Pharmacoepidemiol Drug Saf. 2011;20:S230–S367. [Google Scholar]
- 9.Walley T, Mantgani A. The UK General Practice Research Database. Lancet. 1997;350:1097–1099. doi: 10.1016/S0140-6736(97)04248-7. [DOI] [PubMed] [Google Scholar]
- 10.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley and Sons; New York: 1987. [Google Scholar]
- 11.Go AS, Mozaffarian D, Roger VL, the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–245. [Google Scholar]
- 12.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cadarso-Suarez C, Meira-Machado L, Kneib T, Gude F. Flexible hazard ratio curves for continuous predictors in multi-state models: a P-spline approach. Stat Model. 2010;10:291–314. [Google Scholar]
- 14.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2nd edn. John Wiley and Sons; New York: 2002. [Google Scholar]
- 15.Andersen PK. Decomposition of number of life years lost according to causes of death. Stat Med. 2013;32:5278–5285. doi: 10.1002/sim.5903. [DOI] [PubMed] [Google Scholar]
- 16.Social Exclusion Task Force . Improving the way we meet the primary health care needs of the socially excluded. Joint Health Surveys Unit. The Cabinet Office; Leeds: 2010. [Google Scholar]
- 17.Kannel WB, Wilson PW, Nam BH, D'Agostino RB, Li J. A likely explanation for the J-curve of blood pressure cardiovascular risk. Am J Cardiol. 2004;94:380–384. doi: 10.1016/j.amjcard.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 18.Beckett N, Peters R, Tuomilehto J, the HYVET Study Group Immediate and late benefits of treating very elderly people with hypertension: results from active treatment extension to hypertension in the very elderly randomised controlled trial. BMJ. 2012;344:d7541. doi: 10.1136/bmj.d7541. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd-Jones DM, Larson MG, Leip EP, the Framingham Heart Study Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 20.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J. 2002;143:961–965. doi: 10.1067/mhj.2002.122871. [DOI] [PubMed] [Google Scholar]
- 21.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6:1106–1114. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 22.Vlak MH, Rinkel GJ, Greebe P, Greving JP, Algra A. Lifetime risks for aneurysmal subarachnoid haemorrhage: multivariable risk stratification. J Neurol Neurosurg Psychiatry. 2013;84:619–623. doi: 10.1136/jnnp-2012-303783. [DOI] [PubMed] [Google Scholar]
- 23.Berry JD, Dyer A, Cai X. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wills AK, Lawlor DA, Muniz-Terrera G, the FALCon Study Team Population heterogeneity in trajectories of midlife blood pressure. Epidemiology. 2012;23:203–211. doi: 10.1097/EDE.0b013e3182456567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Clinical Guideline Centre Hypertension: clinical management of primary hypertension in adults. NICE Clinical Guideline 127. August, 2011. http://www.nice.org.uk/nicemedia/live/13561/56008/56008.pdf (accessed Nov 4, 2013).
- 26.Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database Syst Rev. 2012;8 doi: 10.1002/14651858.CD006742.pub2. CD006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams B, Lindholm LH, Sever P. Systolic pressure is all that matters. Lancet. 2008;371:2219–2221. doi: 10.1016/S0140-6736(08)60804-1. [DOI] [PubMed] [Google Scholar]
- 28.Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromsø Study, 1994–2001. Circulation. 2009;119:2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 29.Franks PJ, Edwards RJ, Greenhalgh RM, Powell JT. Risk factors for abdominal aortic aneurysms in smokers. Eur J Vasc Endovasc Surg. 1996;11:487–492. doi: 10.1016/s1078-5884(96)80186-9. [DOI] [PubMed] [Google Scholar]
- 30.Goff DC, Jr, Lloyd-Jones DM, Bennett G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.11.005. published online Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.JBS3 Board Joint British Societies' consensus recommendations for the prevention of cardiovascular disease (JBS3) Heart. 2014;100(suppl 2):ii1–i67. doi: 10.1136/heartjnl-2014-305693. [DOI] [PubMed] [Google Scholar]
- 32.Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimäki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA. 2006;295:1404–1411. doi: 10.1001/jama.295.12.1404. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch AT, Duval S. The global pandemic of peripheral artery disease. Lancet. 2013;382:1312–1314. doi: 10.1016/S0140-6736(13)61576-7. [DOI] [PubMed] [Google Scholar]
- 34.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 35.Turnbull F, the Blood Pressure Lowering Treatment Trialists' Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 36.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.