Highlights
-
•
T cell signaling has a pivotal role in autoimmunity and immunosuppression.
-
•
Immunosuppressive pharmaceuticals often exhibit severe side-effects in patients.
-
•
Gene-encoded peptides have potential as immunosuppressive drug candidates.
-
•
Cyclotides are stable peptides that offer enhanced oral administration properties.
Abstract
The immune system is vital for detecting and evading endogenous and exogenous threats to the body. Failure to regulate this homeostasis leads to autoimmunity, which is often associated with malfunctioning T cell signaling. Several medications are available to suppress over-reactive T lymphocytes, but many of the currently marketed drugs produce severe and life-threatening side-effects. Ribosomally synthesized peptides are gaining recognition from the pharmaceutical industry for their enhanced selectivity and decreased toxicity compared with small molecules; in particular, circular peptides exhibit remarkable stability and increased oral administration properties. For example, plant cyclotides effectively inhibit T lymphocyte proliferation. They are composed of a head-to-tail cyclized backbone and a cystine-knot motif, which confers them with remarkable stability, thus making them attractive pharmaceutical tools.
Introduction
The immune system is responsible for detecting and eliminating foreign pathogens and tumor cells, but avoiding self-recognition. Therefore, tolerance mechanisms are continuously under surveillance to retain this homeostasis. If these immunological tools are over-reactive it can lead to autoimmunity, targeting healthy host cells and organs as well as exogenous and endogenous threats. About 5% of the population in Western countries develops an autoimmune disease during life, and these numbers are constantly increasing [1]. The causes of autoimmunity are still ill-defined, but it is known that there are many parameters involved, such as gender, genetic background, environmental factors and, importantly, malfunctioning lymphocyte development [2]. However, autoimmunity is not only a congenital disease; following an infection, certain bacterial proteins can elicit an ‘unwanted’ immune response. Conversely, there is a link between the decreased appearance of bacterial and parasitic infectious diseases and an increase of allergic reactions, supported by the so-called hygiene hypothesis [3]. Autoimmune diseases can be broadly classified as organ-specific (e.g. multiple sclerosis) or as systemic (e.g. systemic lupus erythematosus). On a molecular level, autoreactive T cells play an important part in disease development and progression [4], and therefore it is a fundamental necessity to take T cell associated pathways into consideration, especially interleukin 2 (IL2) signaling. Gene expression of this T cell growth factor cytokine is induced by the transcription factors NFAT, NFκB or AP-1. IL2 then acts in an autocrine fashion on its own high-affinity cell surface receptor (IL2R) to promote cell proliferation, cell growth and/or inhibition of apoptosis inter alia via mTOR [5,6] (for immunological glossary of terms, see Box 1).
Box 1. Immunological glossary.
| Abbreviation | Term and/or explanation |
|---|---|
| CD | Cluster of differentiation |
| CRAC | Ca2+-release-activated channels |
| IKCa1 | Intermediate conductance calcium-activated potassium channel protein 4 (KCNN4) |
| Kv1.3 | Potassium voltage-gated channel, shaker-related subfamily, member 3 (KCNA3) |
| IP3 | Inositol-1,4,5-triphosphate |
| IP3R | Intramolecular inositol-1,4,5-triphosphate Ca2+-release channel |
| NFAT | Nuclear factor of activated T cells |
| NFκB | Nuclear factor ‘κ-light-chain-enhancer’ of activated B cells |
| AP-1 | Activator protein 1 (dimerization of Fos and Jun) |
| IL2 | Interleukin-2 |
| IL2R | High affinity CD25+ interleukin-2 receptor |
| mTOR | Mammalian target of rapamycin |
| IFNγ | Interferon γ |
| TGFβ | Transforming growth factor β |
Immunosuppressive pharmaceuticals
Various signaling pathways and associated canonical messenger molecules, such as IL2 signaling molecules, offer targets for drug discovery for the treatment of an over-reactive immune response as is the case in autoimmune diseases, allergic reactions and following organ transplantation. Many immunosuppressive agents have already been used in the clinic, demonstrating efficacy while displaying different modes-of-action. Some of these pharmaceuticals target specific kinases and phosphatases, interfere with gene expression, modify DNA via alkylation or inhibit purine or pyrimidine synthesis to block the cell cycle [7].
The most commonly used immunosuppressive drugs and historically one of the oldest are the glucocorticoids. They restrict and inhibit the synthesis and secretion of inflammatory cytokines and support the activation of anti-inflammatory signaling cascades. The best-known member of this class of compounds is cortisone, which interacts with and inhibits, for example, NFκB-dependent signaling, resulting in reduced expression of inflammatory cytokines such as tumor necrosis factor (TNF)α or IL1. Besides, glucocorticoid treatment affects many non-immune related signaling pathways; it interferes in gluconeogenesis, lipolysis, protein catabolism and sodium retention. Consequently, the list of side-effects is long, ranging from Cushing's syndrome, hyperglycemia, myopathia, skin atrophy, osteoporosis, hypertonia, weight gain and immunodeficiency [8].
Vitamin D, a fat-soluble secosteroid, has recently been successfully implemented in immunotherapy although it is not a classical anti-inflammatory agent. The physiologically active form of vitamin D [i.e. calcitriol (1,25-dihydroxycholecalciferol)] shows immunosuppressive properties. Activated T and B cells express the vitamin D receptor on their surface, which can be used to downregulate IL2 signaling. Supplementation therapy of vitamin D has already demonstrated protective effects in the treatment of multiple sclerosis [9]. Other immunosuppressive agents interfere with the cell cycle. For example, the enzyme inhibitors azathioprine, mycophenolate mofetil and methotrexate are non-peptide drugs affecting proliferation by targeting and blocking purine and pyrimidine synthesis [10].
The application of monoclonal antibodies (mAbs), which exhibit high specificity towards their appropriate protein epitopes and hence provide the opportunity to inhibit a target molecule and its resulting effects selectively, is the subject of a tremendous amount of research and constitutes an important branch in the field of immunotherapies. The first mAb approved by the FDA in 1986 was muromonab (Orthoclone® OKT3) directed against human CD3 [11]. It was withdrawn from the market for therapeutic application as a result of high toxicity in 2008, but it still has importance in nonclinical applications [10,12]. Anti-TNF or -TNFR mAbs (e.g. infliximab, adalimumab or golimumab) are widely used in the treatment of rheumatoid arthritis, Bechterew's disease, psoriasis or inflammatory bowel diseases [13]. Blocking different immunological cell surface molecules, such as CD52 (alemtuzumab) [12], CD25 (basiliximab) [14] and CD20 (rituximab), is important in the treatment of autoimmune diseases [15]. Together, mAbs account for over US$40 billion in pharmaceutical sales, and anti-TNF antibodies or protein-based inhibitors remain among the most important therapies for the treatment of many autoimmune disorders [16]. Besides the great therapeutic success of mAbs, adverse effects similar to immunodeficiencies or the formation of anti-drug antibodies are a frequently occurring phenomena [13,17]. Furthermore, the lack of homogeneous tissue distribution, limited half-life and enormous production costs decrease the attractiveness of this epitope-specific passive immunotherapy. In summary, all commonly used immunosuppressive drugs demonstrated great success in the treatment of autoimmune diseases. However, many of them cause unwanted and severe side-effects in patients and therefore there is a high demand for less-toxic immunosuppressive pharmaceuticals.
Immunosuppressive peptides
Bioactive peptides and, in particular, ribosomally synthesized peptides often show reduction in cytotoxicity as compared with small organic compounds – obviously as a result of their inherent targeted molecular action [18–21]. However, it is important to note that drug toxicity is a factor that is compound dependent and there is no panacea that guides whether one compound will or will not be toxic in humans. In the following section we would like to provide an overview of natural peptides (i.e. non-ribosomally and ribosomally synthesized ones) that have not been released on the market yet, but that show promising immunosuppressive properties (Table 1).
Table 1.
Immunosuppressive peptides: molecular targets, side-effects and market status
| Name | Chemical class | Origin | Molecular target(s) | Side-effects | Market status | Refs |
|---|---|---|---|---|---|---|
| Non-ribosomally synthesized peptides | ||||||
| Antamides | Cyclic peptide, cycloamanide peptides | Amanita phalloides spp. (fungus) | Inhibition of mitochondrial permeability transition pores | Induces cell necrosis | In vitro and in vivo studies | [80,81] |
| Collutellin A | Cyclic octapepide | Colletotrichum dematium (fungus) | Reduces IL2 production | Low cytotoxicity reported | In vitro | [20] |
| Cyclosporine A | Cyclic undecapeptide | Tolypocladium inflatum (fungus) | Inhibition of calcineurin by complex with cyclophilins | Hepatotoxicity, nephrotoxicity, neurotoxicity, cytotoxicity | Released: Cicloral®, Ciclosol®, Immunosporin®, Neoimmun®, Sandimmun®, Optoral® | [23,25,82] |
| Didemnin A/B | Cyclic depsipeptide | Trididemnum solidum (tunicate) | Blocks protein and RNA synthesis, binds to eEFA1 and PPT-1 | Antiproliferative, long-term allograft survival were not achieved | In vivo studies (mice), Phase II clinical trials | [22,28,29] |
| FK506 (tracrolimus) Ascomycin (pimecrolimus, SDZ ASM 981) |
Cyclic depsipeptide |
Streptomyces tsukubaensis (bacteria) |
Inhibition of calcineurin by complex with FK binding proteins | Nephrotoxicity, neurotoxicity, higher risk for skin cancer | Released: Advagraf®, Modigraf®, Prograf®, Protopic®; Elidel® | [25] |
| Homophymines | Cyclic depsipeptide | Homophymia spp. (marine sponge) | Antiproliferative, mechanism unknown | Cytotoxic | In vitro | [83,84] |
| Geodiamolides H | Cyclic depsipeptide | Geodia corticostylifera (marine sponge) | Disorganization of actin filaments | Cytotoxic | In vitro | [85] |
| Hymenistatin I | Cyclic octapeptide | Hymeniucidon spp. (marine sponge) | Modulation of the IL2 cell response (comparable with rapamycin) | Not reported | In vivo studies | [86] |
| Jasplakinolide | Cyclic depsipeptide | Jaspis splendens (marine sponge) | Actin stabilization, induces actin polymerization | Cytotoxic | In vitro | [87] |
| Sirolimus (rapamycin), everolimus (and derivatives) |
Cyclic depsipeptide |
Streptomyces hygroscopius (bacteria) |
Inhibition of mTOR |
Hyperlipidemia, thrombocyopenia |
Released: Rapamune®, Certican®, Zortress®, Afinitor® |
[26,88] |
| Ribosomally synthesized and post-translationally modified peptides | ||||||
| Charybdotoxin | Venom peptide | Leiurus quinquestriatus hebraeus (scorpion) | Potassium channel blocker | Less selective blocker | In vitro | [35] |
| Curcacycline B | Orbitide | Jatropha curcas (plant) | PPIase inhbitior | Cytotoxic, carcinogenic | In vitro | [89] |
| Cycloleonurinin | Orbitide | Leonurus heterophyllus (plant) | Not known | Not reported | In vitro | [31,89] |
| Cyclolinopeptide A/B | Orbitide | Linum usitatissimum (plant) | CYPA binding and calcineurin inhibition | Antiapoptotic, nontoxic in vivo | In vivo studies on rats and mice as immune suppressive compound and as stimulatory compound with metathroxate; toxicity studies in mice and rats | [31,51,53] |
| Iberiotoxin | Venom peptide | Buthus tamulus (scorpion) | Potassium channel blocker | Blocker of several BK channels | In vitro and in vivo (rats) | [18] |
| Kalata B1 | Cyclotide | Oldenlandia affinis (plant) | Antiproliferative, IL2-dependent mechanism | No cytotoxicity observed at active dose, cell cycle arrest | In vitro and in vivo | [69,70] |
| Kaliotoxin | Venom peptide | Androctonus mauretanicus (scorpion) | Potassium channel blocker | Not tested | In vitro | [90] |
| Magatoxin | Venom peptide | Centruroides margaritatus (scorpion) | Potassium channel blocker | Cytotoxic up to 20 μm | In vivo (guinea pigs) | [91,92] |
| OSK-1 (alpha-KTx3.7) | Venom peptide | Orthochirus scrobiculosus (scorpion) | Potassium channel blocker | LD50 = 10 μg/kg (mouse), neurotoxic | In vitro and in vivo | [93] |
Non-ribosomally synthesized peptides
Utilization of bioactivity screening led to the identification of a plethora of novel and potentially immunosuppressive compounds in micro- and marine-organisms [22]. Few examples of non-ribosomally synthesized peptides interfering in cytokine signaling are cyclosporine A (CsA), sirolimus and tracrolimus. CsA (Fig. 1a), a cyclic peptide of fungal origin (Tolypocladium inflatum), is widely used in the treatment of autoimmune diseases and to prevent allograft rejection of a transplanted organ. CsA antagonizes the activity of calcineurin, a calcium-dependent serine–threonine phosphatase, which dephosphorylates and activates the transcription factor NFAT to stimulate expression of IL2. Therefore, the dephosphorylation of NFAT is inhibited and consequently the IL2-dependent T cell proliferation repressed. Besides, CsA interferes additionally with p38 and JNK signaling cascades [10,23]. Owing to multiple target pathways, the therapeutic potential of CsA is limited, in particular during long-term treatment, because it has several side-effects, such as hepatotoxicity, nephrotoxicity, neurotoxicity and cytotoxicity [24].
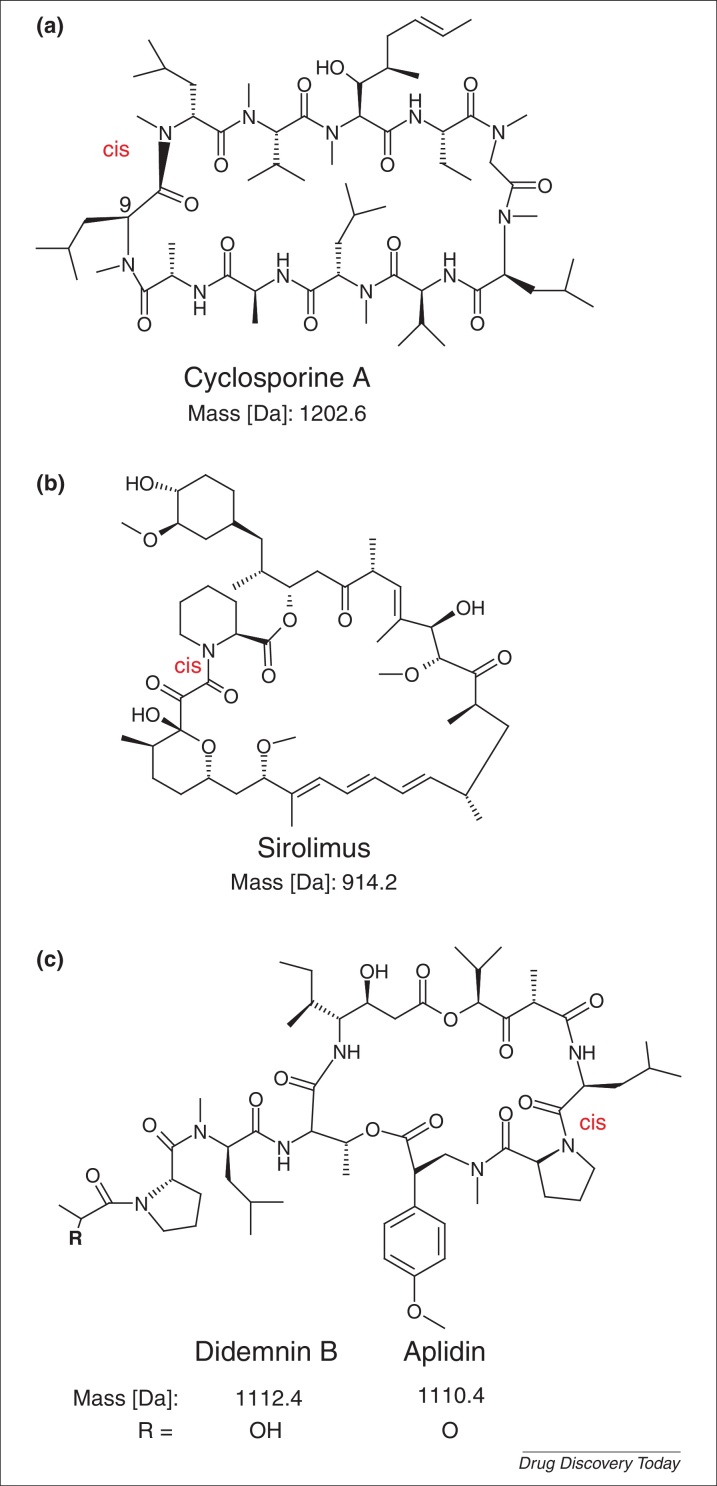
Figure 1.
Non-ribosomally synthesized immunosuppressive peptide compounds. The structures of two of the most frequently used immunosuppressive peptide drugs in the clinic, namely the cyclic undecapeptide cyclosporine A (a) and the depsipeptide sirolimus (rapamycin) (b) are shown. Cyclosporine A has many unique characteristics: it is a highly hydrophobic compound with seven N-methylated amide nitrogens, these modifications are often reported for non-ribosomal peptides from fungi and bacteria. The methylations also favor cis-peptide confirmation and they reduce the number of hydrogen bonds to their surrounding molecules. For example, it is a potent calcineurin blocker, where it forms an inhibitory complex with cyclophilins and members of the peptidyl-prolyl cis–trans-isomerase receptor family. The bacterial cyclic depsipeptide sirolimus binds to other immunophilins (namely the FK506-binding proteins) and this complex binds to mammaliam target of rapamycin (mTOR) and inhibits its serine–threonine kinase activity. Another potent immunosuppressive depsipeptide analog is didemnin B (c), which has been isolated from a marine tunicate (sea squirt). It shares its overall structure (i.e. cyclic backbone, a cis-amide bond and a hydrophobic surface) with cyclosporine A and sirolimus. However, it probably targets the eukaryotic elongation factor 1A and palmitoyl thioesterase 1 to exert its antiproliferative activity. Aplidin, a didemnin B derivative, is currently in Phase III clinical trials and could be released onto the market within the next few years.
FKBP-12 (FK506-binding protein) functions as a chaperone and belongs, together with cyclophilin, to the family of immunophilins. Tacrolimus (FK506), a macrolide lactone first isolated from Streptomyces tsukubaensis interferes with calcineurin-dependent IL2 signaling, by inhibiting FKBP-12 50-fold more potently than CsA [7,25]. Sirolimus (rapamycin; Fig. 1b), a macrolide from Streptomyces hygroscopicus, and everolimus, a more hydrophilic analog of sirolimus, also bind to FKBP-12; they are structurally related to tacrolimus but do not affect calcineurin signaling. Instead, they directly block mTOR signaling, which induces cell cycle arrest [26,27]. Because mTOR is a pivotal protein kinase involved in many physiological processes the list of side-effects of sirolimus and everolimus is long, including leuko- and thrombo-cytopenia, hypercholesterinemia, anorexia and gastroenterocolitis [27].
Didemnins are depsipeptides from the marine sponge Trididemnum solidum. In particular, didemnin B (Fig. 1C) was characterized for its immunosuppressive and antitumor activities [22]. Didemnin B inhibits lymphocyte activation at concentrations of 10 pg/ml (IC50), which corresponds to a 100-fold increased potency compared with CsA. However, didemnin B had no influence on IL2 or IL4 cytokine levels; observations of a delayed cell cycle progression led to suggestions about a nonspecific effect on the basis of RNA and/or protein inhibition and cell toxicity rather than a targeted effect as observed for CsA. A detailed study comparing naive lymphocytes and lymphoblasts provided evidence that several didemnins exhibited a cytotoxic, but also a targeted mode-of-action [28]. More recently, target deconvolution studies revealed that didemnin B binds to palmitoyl protein thioesterase 1 and eukaryotic elongation factor 1α. It was found that the calmodulin/Ca2+-dependent phosphorylation of the latter was inhibited competitively, similar to that reported for CsA [29]. These cellular targets might explain the reduced protein synthesis of cells by didemnin B and give evidence for a target mechanism [28]. Uptake of didemnin B by strongly proliferating cells was significantly higher and therefore this compound appeared as an ideal drug in cancer treatment or to suppress activation of the immune system. But several clinical studies surprisingly failed during Phase I or II because of unexpected in vivo toxicity in humans and also because of a strong administration dependency caused by a very short half-life [22]. Nevertheless, didemnins are very well investigated and for human welfare they still possess an interesting repertoire of antiproliferative and immunosuppressive peptide drugs. In fact this was exemplified for the compound aplidin, a didemnin-derived cyclic peptide that is in Phase II and Phase III clinical trials for anticancer studies of solid and hematological malignant neoplasm, T cell lymphoma, myelofibrosis and multiple myeloma [22,30].
Ribosomally synthesized and post-translationally modified peptides
Ribosomally synthesized and post-translationally modified small peptides (RiPPs) occur in many taxa and are gene-encoded synthesized as precursor proteins. These precursors consist of several domains each with specific functions; detailed information about RiPPs and their biosynthesis was recently reviewed [31,32] (Fig. 2a). Multifaceted enzyme machineries enable the post-translational modifications (e.g. cyclization, oxidative folding, hydrolysis, ligations, phosphorylation) necessary for their biosynthesis. The manifold of chemical modifications reported for RiPPs reflect nature's biosynthetic potential and the high diversity of RiPPs to serve as peptide libraries for drug discovery.
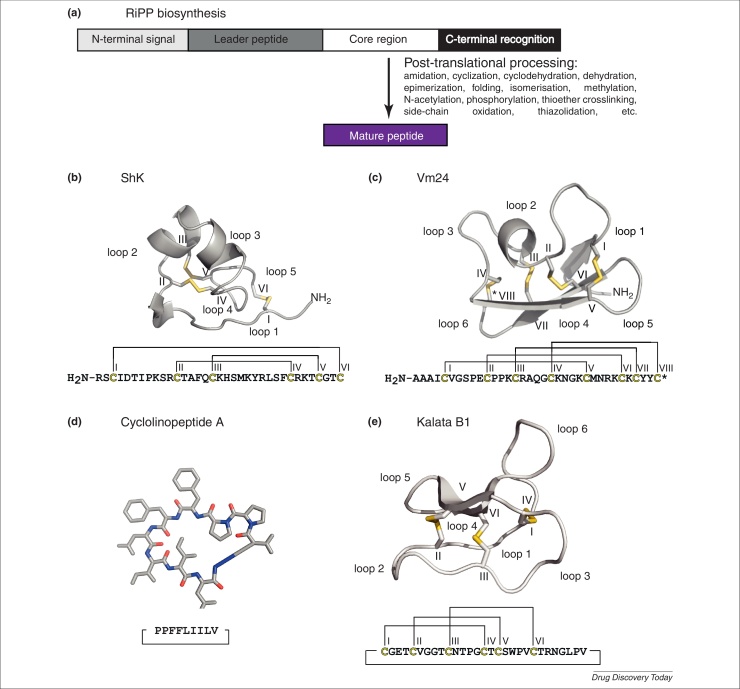
Figure 2.
Ribosomally synthesized immunosuppressive peptides. In contrast to small non-ribosomally synthesized peptides that were mainly isolated from microorganisms, fungi and marine sources, there are a number of gene-encoded bioactive peptides with immunosuppressive activity from higher organisms, such as plants and animals. They are all synthesized via a common mechanism from a larger precursor protein consisting of a signal peptide, an N-terminal leader sequence followed by a core region and a short C-terminal recognition sequence (a). The precursor will be post-translationally modified to form and release the mature peptide from the core region. The leader sequence and C-terminal recognition sequence are important for excision, cyclization and post-translational processing of the mature domain. The N-terminal endoplasmic reticulum (ER) signal is important for cellular translocation to secretory compartments such as the ER [31]. Exemplarily, the structural cartoons (stick-mode or ribbon cartoon) of four immunosuppressive gene-encoded peptides are shown (b–e). The primary structures are illustrated below the cartoon and head-to tail cyclization of the backbone is indicated, where applicable. The cysteine residues are numbered with Roman numerals and the backbone loops labeled, where applicable. The structure of the ShK toxin from the sea anemone Stichodactyla helianthus is presented (b). The cysteine connectivity is highlighted in yellow. The peptide is a 35-mer with a molecular weight of 4061 Da. The secondary structure consists of two short α-helices between residues 14–19 and 21–24 and a pair of 310-helices resembling turns from residue 9 to 13. The structure of the αKTX-type scorpion venom toxin Vm24 (c) from Vaejovis mexicanus smithi is shown and the four disulfide bonds are highlighted in yellow. The C terminus of this peptide is amidated (as indicated by an asterisk). The molecular weight of this 36-mer peptide is 3864 Da. The secondary structure consists of a small α-helix between residues 13 and 16 and an extended triple-stranded antiparallel β-sheet. Cyclolinopeptide A (d) from Linum usitatissimum is known as a promising immunosuppressive compound. The heptapeptide has a molecular mass of 1040 Da and it is shown in a ribbon cartoon with backbone atoms in grey, oxygens in blue and nitrogens in red. The secondary structure is a β-turn and its X-Pro amide bond is in cis conformation. The cyclotide kalata B1 (e) is illustrated as a ribbon cartoon. The 29-mer has a molecular mass of 2890 Da. The head-to-tail cyclized peptide is stabilized by its unique cystine-knot motif. Characteristic secondary structure elements of cyclotides include a β-hairpin involving loop 4 and loop 5. A third strand within loop 1 is associated with the hairpin to form a triple-stranded β-sheet as indicated. Several intramolecular hydrogen bonds further stabilize the topology of the peptide.
Venom toxins. Venom toxins provide an enormous library of natural products for drug development [21]. There is high variability among all venom toxins isolated from sea anemones, snakes, scorpions, cone snails, jelly fish and other animals. Most of them are linear peptides and, like the conopeptides and certain scorpion venom peptides, are rich in cysteines to stabilize their active conformation [31]. The most common mode-of-action for many bioactive venom toxins with clinical potential is the blockage of ion channels [18]. Currently, there is one synthetic conopeptide on the market, namely ziconotide (Prialt®) derived from the ω-conotoxin MVIIA of Conus magnus to treat patients with severe chronic pain [19]. Nevertheless, the therapeutic application of ziconotide is limited by its toxicity [33], but improvements in the administration regime provided protection against severe side-effects [34].
Ion channels, often expressed by specific cell types, are generally found to be promising targets for new drugs, on the basis of their important influence on cell signaling, cell communication and regulation of the cell membrane potential [35]. In particular, calcium (Ca2+) and potassium (K+) ion channels play an important part during T cell activation, and are therefore attractive for future drug development [36]. After T cell stimulation, intracellular Ca2+ is released from the endoplasmic reticulum into the cytosol via inositol-1,4,5-trisphosphate (IP3). Furthermore, Ca2+ release-activated channels (CRAC) promote the influx of extracellular Ca2+ into the cytosol. This sustained elevated cytosolic Ca2+ concentration triggers IL2 gene expression. To maintain the membrane potential of the activated T cell and to ensure constant influx of Ca2+ ions during the activation period, which depends on a homeostatic intramolecular ion level and membrane potential, IKCa and Kv1.3 channels play an important part by pumping out K+ [37,38]. Importantly, several of these ion channels are found to be highly upregulated in T cells from patients with autoimmune diseases such as multiple sclerosis. Therefore, a blockage of these channels would be an efficient pharmacological strategy [39]. There are several reports of peptide-based potassium channel blockers [40,41], but they often lack specificity as a result of the fact that the heteromeric members of a channel family frequently consist of the same subunits. The ShK-type K+-channel blocker from the sea anemone Stichodactyla heliathus is a well-characterized representative for a potential immunosuppressive venom toxin. In comparison to other K+-channel blockers from scorpions, ShK is different and, for example, lacks a β-sheet, which is replaced by two α-helices instead [40] (Fig. 2b). ShK blocks the T cell specific Kv1.3 channel with an IC50 of 16 pM, as well as the Kv1.1 channel and several other ion channels with high affinities. Intriguingly, the lack of specificity was demonstrated because the D-allo-ShK was still able to block several K+ ion channels with high affinity [40,42]. However, recently it was suggested that the D-ShK analog has ∼50,000-fold lower affinity for the Kv1.3 channel (IC50 = 22 μm) as compared with the L-ShK analog (IC50 = 250 pM) [43]. The SAR for the 35-mer peptide was studied intensively and several modifications (ShK-Dap22, ShK-amide or ShK-K-amide) enhanced its selectivity, IC50 and serum stability. The example of the ShK-K-amide peptide demonstrates the high potential of peptide engineering: the amide derivative had increased selectivity to block the Kv1.3 over the Kv1.1 potassium channels [41]. Owing to the general importance of peptide toxins for drug discovery, toxin analogs were characterized extensively in vitro and in vivo in mouse models for autoimmune diseases such as multiple sclerosis, delayed-type hypersensitivity, rheumatoid arthritis and for chronic-relapsing, as well as acute adoptive autoimmune encephalomyelitis [40,44].
The K+-channel blocker Vm24 (α-KTx 23.1) which was recently isolated from the venom of the Mexican scorpion Vaejovis mexicanus smithi is a potent and selective Kv1.3-channel blocker (Kd = 0.5–2.9 pM) with a 1500-fold higher affinity for Kv1.3 compared to ten other channels (Fig. 2c) [45,46]. This promising lead peptide was intensively studied with respect to its SAR revealing two pharmacophores, namely the variable residues before the α-helix at position 13–16 and the conserved C-terminal β-hairpin, which are crucial for channel specificity and channel inhibition, respectively [45]. The Kv1.3-channel inhibition leads to an antiproliferative effect on activated T cells or, to be more precise, it induces an IL2R downregulation and a disturbance in mobilization. Furthermore, it was shown that the proliferation of effector memory T cells was reduced, whereas naive and central memory T cells sustained their proliferation by a compensatory upregulation of the IKCa1 channel. Treatment of mice with 10 mg/kg of the venom peptide did not show any toxicity, suggesting little off-target effects [44,46]. The immunosuppressive activity was confirmed in vivo, using a delayed-type hypersensitivity autoimmune mouse model, demonstrating a significant reduction of symptoms of disease compared with the control group [45].
In summary, venom toxins are promising tools to inhibit specific ion channel families effectively with picomolar or nanomolar affinities. However, low channel selectivity and insufficient serum stability require future optimization. Many venom toxins interfere with IL2 cytokine signaling and their molecular targets are different to calcineurin inhibitors (e.g. CsA), which offers possibilities for co-administration therapy. One big step toward the development of suitable immunosuppressive drugs in the near future is to engineer peptides with increased stability for maintaining or improving oral administration properties and a promising tool to achieve this is peptide cyclization.
Plant orbitides. Orbitides are ribosomally synthesized N-to-C cyclized plant peptides with five to 12 amino acids lacking cysteines. Their distribution within the plant kingdom as well as their primary sequences are very heterogeneous and were summarized by a recent review [31]. Several members of the orbitide family (i.e. cyclolinopeptides, cycloleonurinins, hymenistatins and curcacyclines) exhibit potential immunosuppressive activity. For example, cyclolinopeptides A and B (Fig. 2d) were characterized as T cell antiproliferative peptides comparable to the well-known immunosuppressive agent CsA [47]. Many orbitides bind to cyclophilin A (and other cyclophilins) and subsequently inhibit calcineurin-dependent T cell activation. Cyclolinopeptide A exhibits a tenfold lower affinity for calcineurin than CsA and was found to be nontoxic [48–51]. Meanwhile, several cyclolinopeptide A analogs have been synthesized successfully to enhance their bioactivity further [52,53].
The immunosuppressive activity of orbitides is characterized by weak affinity to their molecular target calcineurin. Additionally, several peptide drugs are already on the market targeting the same cellular receptor and exhibiting similar modes-of-action. Nevertheless, this peptide family appears to be of interest as a result of their absence of toxicity, hopefully promising a lack of unwanted side-effects in future applications.
Plant cyclotides. Cyclotides are possibly the largest class of gene-encoded cyclic peptides [54]. They were initially discovered from the African plant Oldenlandia affinis (Rubiaceae) that has been traditionally used to speed up labor and to avoid postpartum complications [55]. Recently the molecular principle of this uterotonic activity was elucidated: the oxytocic cyclotide kalata B7 was found to modulate the oxytocin and vasopressin V1a receptors, members of the G-protein-coupled receptor family, inducing strong contractility in human uterine smooth muscle cells [56,57].
Extensive screening efforts for the discovery of novel cyclotide-containing plants has led to the identification of these peptides in the plant families of Rubiaceae, Violaceae, Cucurbitaceae, Fabaceae, Solanaceae and Poaceae, summarized in [58]. Cyclotides are approximately 30 amino acids in size and common structural features of all cyclotides are the cyclic cystine-knot motif, two short antiparallel β-sheets and a few conserved residues that are important for structural stability, folding and enzymatic processing [59–61].
The biological function of cyclotides appears to be a defense against herbivores, based on observations that cyclotides modulate the growth and development of several invertebrate animals, such as insects [62,63], molluscs [64] and nematodes [65]. Besides, cyclotides exhibit a range of bioactivities such as anti-HIV [66], anticancer [67] and antibacterial [68], and they are capable of inhibiting the proliferation of immune cells [69].
Kalata B1 (Fig. 2e) was identified as an active compound in a herbal extract of O. affinis using a bioactivity-guided screening protocol for cell proliferation inhibition of human peripheral blood mononuclear cells. Interestingly, the antiproliferative activity was not caused by cytotoxicity but the lymphocytes were kept in cell cycle arrest [69]. Following the discovery of a dose-dependent immunosuppressive activity of kalata B1, the cellular mechanisms and structure–activity studies were performed. Several amino acid mutants of the cyclotide kalata B1, including an all-d-cyclotide enantiomer, were synthesized and analyzed, revealing that the inherent immunosuppressive activity of cyclotides was compound-specific, stereospecific and directly related to T cell biology.
The IL2 cytokine release and the expression of the high affinity IL2R of activated T cells were significantly reduced upon treatment with an active lysine mutant [T20K]kalata B1. The effect was reversible upon exogenous administration of IL2 [70]. The cyclotide [T20K]kalata B1 exhibited similar IL2-dependent inhibitory effects compared to CsA, but it is clear that cyclotides suppress T cell polyfunctionality and arrest the proliferation of immune-competent cells through inhibiting IL2 biology at more than one site. Furthermore, the effector cytokines IFNγ and TNFα had a reduced expression level upon [T20K]kalata B1 treatment [70]. Using a different approach, myelin oligodendrocyte-glycoprotein-derived peptide fragments were grafted onto a cyclotide scaffold, which did not only improve their stability but one engineered molecule displayed potent ability to prevent disease development in a mouse model of experimental autoimmune encephalomyelitis [71].
Cyclotides are frequently referred to as potential drug templates for medicinal and therapeutic applications, taking advantage of their unique three-dimensional topology [72], which makes them remarkably stable against chemical, thermal or enzymatic degradation compared with linear peptides or peptides missing a well-defined three-dimensional conformation [73]. In particular, the cyclotide framework is further stabilized by three disulfide bonds and the resulting cystine-knot motif [72]. Hence they have been extensively investigated in peptide-grafting applications (i.e. grafting of bioactive amino acid sequences onto the cyclotide scaffold) summarized in [72] and exemplified in [74]. For instance, this resulted in the synthesis of an orally bioavailable and active bradykinin B1 receptor antagonist for successful pain therapy in mice [75]. Improvements in bioavailability after oral administration are certainly one of the most promising aspects for cyclic peptides as therapeutic agents. Furthermore, cyclic peptides in general are considered to have enhanced selectivity over their linear counterparts by conferring conformational constraint [76,77]. This represents the great market potential of cyclotides as effective peptide-based and presumably orally bioavailable drugs [78].
Concluding remarks
There are a few immunosuppressive peptide drugs already on the market or in the clinic (e.g. cyclosporine, sirolimus, tacrolimus), but lack of specificity causes severe or even life-threatening side-effects. One key requirement for modern pharmaceuticals is a specific mode-of-action with little or no side-effects in vivo. Additional important points are their stability, bioavailability and efficacy. Owing to today's knowledge and technology, scientists have access to huge libraries of bioactive peptides, present in all kingdoms of life around the world. Therefore, a major aim in this field is to find novel natural peptides with highly specific immune inhibitory or modulatory activity profiles. Some examples of such promising future peptide drugs that were discussed in this review include animal peptide toxins (e.g. ShK and Vm24) and circular plant peptides (e.g. orbitides and cyclotides). Owing to natural selection and evolutionary pressure, these natural peptides were optimized for specific targets and functions (i.e. predator defense, catching prey, intraspecies messenger), fine-tuned over millions of years and, hence, offer great advantages in drug development over synthetic-derived compounds [79]. In addition, peptides can easily be engineered, functionalized and modified to enhance their bioavailability, stability and specificity and efficiency. Lastly, lead products can be produced on a large-scale by chemical synthesis or by recombinant technologies to fulfill the manifold requirements of the pharmaceutical industry [21]. In summary, these immunosuppressive peptides derived from natural sources could occupy a niche of compounds in the molecular mass range between 500 and 5000 Da, which is currently lacking, to drive the field of peptide pharmaceuticals ahead into a bright future [21].
Acknowledgement
Research on immunosuppressive peptides in our laboratory has been financially supported by the Austrian Science Fund FWF (P24743-B21).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Davidson A., Diamond B. Autoimmune diseases. N. Engl. J. Med. 2001;345:340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 2.Ermann J., Fathman C.G. Autoimmune diseases: genes, bugs and failed regulation. Nat. Immunol. 2001;2:759–761. doi: 10.1038/ni0901-759. [DOI] [PubMed] [Google Scholar]
- 3.Hasseldam H. Immunomodulatory effects of helminths and protozoa in multiple sclerosis and experimental autoimmune encephalomyelitis. Parasite Immunol. 2013;35:103–108. doi: 10.1111/pim.12023. [DOI] [PubMed] [Google Scholar]
- 4.Yin Y. Structural basis for self-recognition by autoimmune T-cell receptors. Immunol. Rev. 2012;250:32–48. doi: 10.1111/imr.12002. [DOI] [PubMed] [Google Scholar]
- 5.Lin J.X., Leonard W.J. Signaling from the IL-2 receptor to the nucleus. Cytokine Growth Factor Rev. 1997;8:313–332. doi: 10.1016/s1359-6101(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 6.Malek T.R., Bayer A.L. Tolerance, not immunity, crucially depends on IL-2. Nat. Rev. Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 7.Allison A.C. Immunosuppressive drugs: the first 50 years and a glance forward. Immunopharmacology. 2000;47:63–83. doi: 10.1016/s0162-3109(00)00186-7. [DOI] [PubMed] [Google Scholar]
- 8.Rhen T., Cidlowski J.A. Antiinflammatory action of glucocorticoids – new mechanisms for old drugs. N. Engl. J. Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 9.Antico A. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun. Rev. 2012;12:127–136. doi: 10.1016/j.autrev.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Getts D.R. Current landscape for T-cell targeting in autoimmunity and transplantation. Immunotherapy. 2011;3:853–870. doi: 10.2217/imt.11.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao S. Advances in targeting cell surface signalling molecules for immune modulation. Nat. Rev. Drug Discov. 2013;12:130–146. doi: 10.1038/nrd3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown J.W., Coles A.J. Alemtuzumab: evidence for its potential in relapsing–remitting multiple sclerosis. Drug Des. Dev. Ther. 2013;7:131–138. doi: 10.2147/DDDT.S32687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Schouwenburg P.A. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat. Rev. Rheumatol. 2013;9:164–172. doi: 10.1038/nrrheum.2013.4. [DOI] [PubMed] [Google Scholar]
- 14.Scherer H.U. Targeting activated T cells: successful use of anti-CD25 monoclonal antibody basiliximab in a patient with systemic sclerosis. Ann. Rheum. Dis. 2006;65:1245–1247. doi: 10.1136/ard.2005.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lunardon L., Payne A.S. Rituximab for autoimmune blistering diseases: recent studies, new insights. G. Ital. Dermatol. Venereol. 2012;147:269–276. [PMC free article] [PubMed] [Google Scholar]
- 16.Elvin J.G. Therapeutic antibodies: market considerations, disease targets and bioprocessing. Int. J. Pharm. 2013;440:83–98. doi: 10.1016/j.ijpharm.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Marodi L., Casanova J.L. Primary immunodeficiencies may reveal potential infectious diseases associated with immune-targeting mAb treatments. J. Allergy Clin. Immunol. 2010;126:910–917. doi: 10.1016/j.jaci.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Bergeron Z.L., Bingham J.P. Scorpion toxins specific for potassium (K+) channels: a historical overview of peptide bioengineering. Toxins. 2012;4:1082–1119. doi: 10.3390/toxins4111082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Essack M. Conotoxins that confer therapeutic possibilities. Mar. Drugs. 2012;10:1244–1265. doi: 10.3390/md10061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren Y. Colutellin A, an immunosuppressive peptide from Colletotrichum dematium. Microbiology. 2008;154:1973–1979. doi: 10.1099/mic.0.2008/017954-0. [DOI] [PubMed] [Google Scholar]
- 21.Craik D.J. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013;81:136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 22.Lee J. Didemnins, tamandarins and related natural products. Nat. Prod. Rep. 2012;29:404–424. doi: 10.1039/c2np00065b. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda S., Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47:119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 24.de Mattos A.M. Nephrotoxicity of immunosuppressive drugs: long-term consequences and challenges for the future. Am. J. Kidney Dis. 2000;35:333–346. doi: 10.1016/s0272-6386(00)70348-9. [DOI] [PubMed] [Google Scholar]
- 25.Ho S. The mechanism of action of cyclosporin A and FK506. Clin. Immunol. Immnopathol. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 26.Inoki K. Signaling by target of rapamycin proteins in cell growth control. Microbiol. Mol. Biol. Rev. 2005;69:79–100. doi: 10.1128/MMBR.69.1.79-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahan B.D. Individuality: the barrier to optimal immunosuppression. Nat. Rev. Immunol. 2003;3:831–838. doi: 10.1038/nri1204. [DOI] [PubMed] [Google Scholar]
- 28.Vera M.D., Joullie M.M. Natural products as probes of cell biology: 20 years of didemnin research. Med. Res. Rev. 2002;22:102–145. doi: 10.1002/med.10003. [DOI] [PubMed] [Google Scholar]
- 29.Marco E. Structural basis for the binding of didemnins to human elongation factor eEF1A and rationale for the potent antitumor activity of these marine natural products. J. Med. Chem. 2004;47:4439–4452. doi: 10.1021/jm0306428. [DOI] [PubMed] [Google Scholar]
- 30.Rawat D.S. Marine peptides and related compounds in clinical trial. Anticancer Agents Med. Chem. 2006;6:33–40. doi: 10.2174/187152006774755519. [DOI] [PubMed] [Google Scholar]
- 31.Arnison P.G. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013;30:108–160. doi: 10.1039/c2np20085f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X., van der Donk W.A. Ribosomally synthesized and post-translationally modified peptide natural products: new insights into the role of leader and core peptides during biosynthesis. Chemistry. 2013;19:7662–7677. doi: 10.1002/chem.201300401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webster L.R. Open-label, multicenter study of combined intrathecal morphine and ziconotide: addition of morphine in patients receiving ziconotide for severe chronic pain. Pain Med. 2008;9:282–290. doi: 10.1111/j.1526-4637.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell A.A. Administering ziconotide and monitoring patients treated with ziconotide: expert opinions. Pain Manag. Nurs. 2013;14:e84–e94. doi: 10.1016/j.pmn.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Bagal S.K. Ion channels as therapeutic targets: a drug discovery perspective. J. Med. Chem. 2013;56:593–624. doi: 10.1021/jm3011433. [DOI] [PubMed] [Google Scholar]
- 36.Varga Z. Ion channels in T lymphocytes: an update on facts, mechanisms and therapeutic targeting in autoimmune diseases. Immunol. Lett. 2010;130:19–25. doi: 10.1016/j.imlet.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Feske S. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 2012;12:532–547. doi: 10.1038/nri3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh-hora M. Calcium signaling in the development and function of T-lineage cells. Immunol. Rev. 2009;231:210–224. doi: 10.1111/j.1600-065X.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- 39.Panyi G. Ion channels and lymphocyte activation. Immunol. Lett. 2004;92:55–66. doi: 10.1016/j.imlet.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 40.Beeton C. Analogs of the sea anemone potassium channel blocker ShK for the treatment of autoimmune diseases. Allergy Drug Targets. 2011;10:313–321. doi: 10.2174/187152811797200641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pennington M.W. A C-terminally amidated analogue of ShK is a potent and selective blocker of the voltage-gated potassium channel Kv1.3. FEBS Lett. 2012;586:3996–4001. doi: 10.1016/j.febslet.2012.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beeton C. The d-diastereomer of ShK toxin selectively blocks voltage-gated K+ channels and inhibits T lymphocyte proliferation. J. Biol. Chem. 2008;283:988–997. doi: 10.1074/jbc.M706008200. [DOI] [PubMed] [Google Scholar]
- 43.Dang B. Native chemical ligation at Asx-Cys, Glx-Cys: chemical synthesis and high-resolution X-ray structure of ShK toxin by racemic protein crystallography. J. Am. Chem. Soc. 2013;135:11911–11919. doi: 10.1021/ja4046795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beeton C. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol. Pharmacol. 2005;67:1369–1381. doi: 10.1124/mol.104.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurrola G.B. Structure, function, and chemical synthesis of Vaejovis mexicanus peptide 24: a novel potent blocker of Kv1.3 potassium channels of human T lymphocytes. Biochemistry. 2012;51:4049–4061. doi: 10.1021/bi300060n. [DOI] [PubMed] [Google Scholar]
- 46.Varga Z. Vm24, a natural immunosuppressive peptide, potently and selectively blocks Kv1.3 potassium channels of human T cells. Mol. Pharmacol. 2012;82:372–382. doi: 10.1124/mol.112.078006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benedetti E., Pedone C. Cyclolinopeptide A: inhibitor, immunosuppressor or other? J. Pept. Sci. 2005;11:268–272. doi: 10.1002/psc.674. [DOI] [PubMed] [Google Scholar]
- 48.Gaymes T.J. Cyclolinopeptide A (CLA) mediates its immunosuppressive activity through cyclophilin-dependent calcineurin inactivation. FEBS Lett. 1997;418:224–227. doi: 10.1016/s0014-5793(97)01345-8. [DOI] [PubMed] [Google Scholar]
- 49.Ruchala P. Synthesis, conformation, and immunosuppressive activity of CLX and its analogues. Biopolymers. 2003;70:497–511. doi: 10.1002/bip.10422. [DOI] [PubMed] [Google Scholar]
- 50.Wieczorek Z. Immunosuppressive activity of cyclolinopeptide A. Pept. Res. 1991;4:275–283. [PubMed] [Google Scholar]
- 51.Siemion I.Z. Cyclolinopeptides and their analogs – a new family of peptide immunosuppressants affecting the calcineurin system. Arch. Immunol. Ther. Exp. (Warsz.) 1999;47:143–153. [PubMed] [Google Scholar]
- 52.Kaczmarek K. Synthesis, conformational analysis and immunological activity of beta3Phe-substituted cyclolinopeptide A analogues. J. Pept. Sci. 2009;15:166–174. doi: 10.1002/psc.1099. [DOI] [PubMed] [Google Scholar]
- 53.Katarzynska J. Cyclolinopeptide derivatives modify methotrexate-induced suppression of the humoral immune response in mice. Eur. J. Med. Chem. 2011;46:4608–4617. doi: 10.1016/j.ejmech.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 54.Gruber C.W. Distribution and evolution of circular miniproteins in flowering plants. Plant Cell. 2008;20:2471–2483. doi: 10.1105/tpc.108.062331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gran L. On the effect of a polypeptide isolated from ‘Kalata-Kalata’ (Oldenlandia affinis DC) on the oestrogen dominated uterus. Acta Pharmacol. Toxicol. (Copenh.) 1973;33:400–408. doi: 10.1111/j.1600-0773.1973.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 56.Koehbach J. Insights into the molecular evolution of oxytocin receptor ligand binding. Biochem. Soc. Trans. 2013;41:197–204. doi: 10.1042/BST20120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koehbach J. Oxytocic plant cyclotides as templates for peptide G protein-coupled receptor ligand design. Proc. Natl. Acad. Sci. U. S. A. 2013 doi: 10.1073/pnas.1311183110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koehbach J. Cyclotide discovery in Gentianales revisited-identification and characterization of cyclic cystine-knot peptides and their phylogenetic distribution in Rubiaceae plants. Biopolymers. 2013;100:438–452. doi: 10.1002/bip.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goransson U. The conserved glu in the cyclotide cycloviolacin O2 has a key structural role. Chembiochem. 2009;10:2354–2360. doi: 10.1002/cbic.200900342. [DOI] [PubMed] [Google Scholar]
- 60.Gruber C.W. A novel plant protein-disulfide isomerase involved in the oxidative folding of cystine knot defense proteins. J. Biol. Chem. 2007;282:20435–20446. doi: 10.1074/jbc.M700018200. [DOI] [PubMed] [Google Scholar]
- 61.Saska I. An asparaginyl endopeptidase mediates in vivo protein backbone cyclization. J. Biol. Chem. 2007;282:29721–29728. doi: 10.1074/jbc.M705185200. [DOI] [PubMed] [Google Scholar]
- 62.Barbeta B.L. Plant cyclotides disrupt epithelial cells in the midgut of lepidopteran larvae. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1221–1225. doi: 10.1073/pnas.0710338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gruber C.W. Insecticidal plant cyclotides and related cystine knot toxins. Toxicon. 2007;49:561–575. doi: 10.1016/j.toxicon.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 64.Plan M.R. Backbone cyclised peptides from plants show molluscicidal activity against the rice pest Pomacea canaliculata (golden apple snail) J. Agric. Food Chem. 2008;56:5237–5241. doi: 10.1021/jf800302f. [DOI] [PubMed] [Google Scholar]
- 65.Colgrave M.L. The anthelmintic activity of the cyclotides: natural variants with enhanced activity. Chembiochem. 2008;9:1939–1945. doi: 10.1002/cbic.200800174. [DOI] [PubMed] [Google Scholar]
- 66.Gustafson K.R. Anti-HIV cyclotides. Curr. Protein Pept. Sci. 2004;5:331–340. doi: 10.2174/1389203043379468. [DOI] [PubMed] [Google Scholar]
- 67.Gerlach S.L. Anticancer and chemosensitizing abilities of cycloviolacin 02 from Viola odorata and psyle cyclotides from Psychotria leptothyrsa. Biopolymers. 2010;94:617–625. doi: 10.1002/bip.21435. [DOI] [PubMed] [Google Scholar]
- 68.Pranting M. The cyclotide cycloviolacin O2 from Viola odorata has potent bactericidal activity against Gram-negative bacteria. J. Antimicrob. Chemother. 2010;65:1964–1971. doi: 10.1093/jac/dkq220. [DOI] [PubMed] [Google Scholar]
- 69.Grundemann C. Do plant cyclotides have potential as immunosuppressant peptides? J. Nat. Prod. 2012;75:167–174. doi: 10.1021/np200722w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grundemann C. Cyclotides suppress human T-lymphocyte proliferation by an interleukin 2-dependent mechanism. PLoS ONE. 2013;8:e68016. doi: 10.1371/journal.pone.0068016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang C.K. Molecular grafting onto a stable framework yields novel cyclic peptides for the treatment of multiple sclerosis. ACS Chem. Biol. 2013 doi: 10.1021/cb400548s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Craik D.J. Cyclotides as a basis for drug design. Exp. Opin. Drug Discov. 2012;7:179–194. doi: 10.1517/17460441.2012.661554. [DOI] [PubMed] [Google Scholar]
- 73.Colgrave M.L., Craik D.J. Thermal, chemical, and enzymatic stability of the cyclotide kalata B1: the importance of the cyclic cystine knot. Biochemistry. 2004;43:5965–5975. doi: 10.1021/bi049711q. [DOI] [PubMed] [Google Scholar]
- 74.Ji Y. In vivo activation of the p53 tumor suppressor pathway by an engineered cyclotide. J. Am. Chem. Soc. 2013;135:11623–11633. doi: 10.1021/ja405108p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong C.T. Orally active peptidic bradykinin B1 receptor antagonists engineered from a cyclotide scaffold for inflammatory pain treatment. Angew. Chem. Int. Ed. Engl. 2012;51:5620–5624. doi: 10.1002/anie.201200984. [DOI] [PubMed] [Google Scholar]
- 76.Craik D.J. Chemistry. Seamless proteins tie up their loose ends. Science. 2006;311:1563–1564. doi: 10.1126/science.1125248. [DOI] [PubMed] [Google Scholar]
- 77.Gilon C. Backbone cyclization: a new method for conferring conformational constraint on peptides. Biopolymers. 1991;31:745–750. doi: 10.1002/bip.360310619. [DOI] [PubMed] [Google Scholar]
- 78.Smith A.B. Cyclotides: a patent review. Expert Opin. Ther. Pat. 2011;21:1657–1672. doi: 10.1517/13543776.2011.620606. [DOI] [PubMed] [Google Scholar]
- 79.Gruber C.W. Ligand-based peptide design and combinatorial peptide libraries to target G protein-coupled receptors. Curr. Pharm. Des. 2010;16:3071–3088. doi: 10.2174/138161210793292474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Azzolin L. Antamanide, a derivative of Amanita phalloides, is a novel inhibitor of the mitochondrial permeability transition pore. PLoS ONE. 2011;6:e16280. doi: 10.1371/journal.pone.0016280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wieczorek Z. Immunosuppressive activity in the series of cycloamanide peptides from mushrooms. Peptides. 1993;14:1–5. doi: 10.1016/0196-9781(93)90003-y. [DOI] [PubMed] [Google Scholar]
- 82.Jorgensen K.A. Calcineurin phosphatase activity and immunosuppression. A review on the role of calcineurin phosphatase activity and the immunosuppressive effect of cyclosporin A and tacrolimus. Scand. J. Immunol. 2003;57:93–98. doi: 10.1046/j.1365-3083.2003.01221.x. [DOI] [PubMed] [Google Scholar]
- 83.Andavan G.S., Lemmens-Gruber R. Cyclodepsipeptides from marine sponges: natural agents for drug research. Mar. Drugs. 2010;8:810–834. doi: 10.3390/md8030810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zampella A. Homophymines B-E and A1-E1, a family of bioactive cyclodepsipeptides from the sponge Homophymia sp. Org. Biomol. Chem. 2009;7:4037–4044. doi: 10.1039/b910015f. [DOI] [PubMed] [Google Scholar]
- 85.Rangel M. Cytoskeleton alterations induced by Geodia corticostylifera depsipeptides in breast cancer cells. Peptides. 2006;27:2047–2057. doi: 10.1016/j.peptides.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 86.Cebrat M. Immunosuppressive activity of hymenistatin I. Peptides. 1996;17:191–196. doi: 10.1016/0196-9781(95)02123-x. [DOI] [PubMed] [Google Scholar]
- 87.Sawitzky H. The anti-proliferative agent jasplakinolide rearranges the actin cytoskeleton of plant cells. Eur. J. Cell Biol. 1999;78:424–433. doi: 10.1016/S0171-9335(99)80085-5. [DOI] [PubMed] [Google Scholar]
- 88.Yang H. mTOR kinase structure, mechanism and regulation. Nature. 2013;497:217–223. doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morita H. Solution state conformation of an immunosuppressive cyclic dodecapeptide, cycloleonurinin. Tetrahedron. 1997;53:7469–7478. [Google Scholar]
- 90.Liebau S. Selective blockage of Kv1.3 and Kv3.1 channels increases neural progenitor cell proliferation. J. Neurochem. 2006;99:426–437. doi: 10.1111/j.1471-4159.2006.03967.x. [DOI] [PubMed] [Google Scholar]
- 91.Jang S.H. Anti-proliferative effect of Kv1.3 blockers in A549 human lung adenocarcinoma in vitro and in vivo. Eur. J. Pharmacol. 2011;651:26–32. doi: 10.1016/j.ejphar.2010.10.066. [DOI] [PubMed] [Google Scholar]
- 92.Suarez-Kurtz G. Peptidyl inhibitors of shaker-type Kv1 channels elicit twitches in guinea pig ileum by blocking Kv1.1 at enteric nervous system and enhancing acetylcholine release. J. Pharmacol. Exp. Ther. 1999;289:1517–1522. [PubMed] [Google Scholar]
- 93.Mouhat S. K+ channel types targeted by synthetic OSK1, a toxin from Orthochirus scrobiculosus scorpion venom. Biochem. J. 2005;385:95–104. doi: 10.1042/BJ20041379. [DOI] [PMC free article] [PubMed] [Google Scholar]