Abstract
OBJECTIVES:
Low levels of vitamin D are associated with a higher mortality in cirrhotic patients, but the role of this deficiency is still unknown. The purpose of this study was to assess the levels of vitamin D in cirrhotic patients with and without bacterial infection.
METHODS:
25-hydroxy (25-OH) vitamin D was assessed by immunoassay in 88 patients hospitalized in our hepatology unit.
RESULTS:
The causes of cirrhosis were mainly alcohol (70%), hepatitis C (10%), or both (9%). Infections (n=38) mainly included bacteriemia (21%), urinary tract infections (24%), and spontaneous bacterial peritonitis (29%). A severe deficiency in vitamin D (<10 ng/ml) was observed in 56.8% of patients. Infections were more frequent in patients with a severe deficiency compared with the others (54 vs. 29%, P=0.02). A severe deficiency in vitamin D was a predictive factor of infection (odds ratio=5.44 (1.35–21.97), P=0.017) independently of the Child–Pugh score (odds ratio=2.09 (1.47–2.97) P=0.00004) and the C-reactive protein level (odds ratio=1.03 (1.002–1.052), P=0.03) in a logistic regression also including the alanine amino transferase (not significant). By a Cox regression analysis, only the presence of an infection was significantly associated with mortality (relative risk=3.24 (1.20–8.76), P=0.02) in a model also associating the Child–Pugh score (not significant) and the presence of a severe deficiency in vitamin D (not significant).
CONCLUSIONS:
Low levels of 25-OH vitamin D were independently associated with bacterial infections in cirrhotic patients. The impact of 25-OH vitamin D supplementation on the infection rate and death of cirrhotic patients should be assessed in randomized trials.
INTRODUCTION
Vitamin D has pleiotropic functions. It is widely recognized to have a central role in calcium metabolism and bone mineralization. A vitamin D deficiency is causally related to rickets in children and osteomalacia in adults, but vitamin D is also physiologically important for the proper function of other organs such as skeletal muscle, heart, brain, and pancreas.1 Vitamin D may also be implicated in innate and acquired immunity.2, 3 Vitamin D could increase innate defense and modulate the activation of lymphocytes implicated in the immune response, leading to a switch toward a T helper 2 response.2, 3
Vitamin D deficiency has been reported in the general population, even in sunny countries, although it is more frequent at high latitudes where seasonal variations in 25-OH (25-hydroxy) vitamin D have been described.1 A low level of 25-OH vitamin D has been associated with increased mortality in the general population in observational studies.4 Prospective trials of supplementation of vitamin D3 with calcium in elderly people showed benefit in terms of reducing mortality.5, 6
The impact of a low level of vitamin D on the risk of bacterial infection has also been suggested recently for patients in intensive care units.7 Historically, the benefit of 25-OH vitamin D on the course of tuberculosis may have contributed to the beneficial effects of sanatoria for patients with tuberculosis in the pre-antimicrobial era. An association between a low level of 25-OH vitamin D and infectious viral diseases has also been reported in children in observational studies, although randomized controlled trials have given conflicting results.8
Recently, a low 25-OH vitamin D level has also been reported to be associated with increased mortality in patients with alcoholic liver disease9 and in patients with cirrhosis,10 but the causal relationship is obscure. As bacterial infections are frequent and are the cause of morbidity and mortality in patients with cirrhosis,11 we hypothesized that the relationship between the lack of vitamin D and the increase in mortality observed in patients with cirrhosis could be because of an increase in bacterial infections. The aim of this study was to compare, in a prospective cohort of hospitalized patients with cirrhosis, the 25-OH vitamin D serum level in patients with and without bacterial infection.
METHODS
Enrollment of patients
Patients were prospectively and randomly enrolled from November 2010 to November 2012 in the liver unit of the University Hospital of Nice, France. Follow-up was conducted until April 2013. Nice is located on the French Riviera and has a Mediterranean climate. Seasonality (summer/autumn vs. winter/spring) has been taken into account in the analysis of data comparing the groups of patients with and without infection. This clinical study was performed according to the declaration of Helsinki, and all participants gave their written informed consent. In the case of encephalopathy, the family was asked for consent, or consent was obtained after resolution of encephalopathy.
The main inclusion criterion was hospitalization with cirrhosis, irrespective of the underlying etiology. The diagnosis of cirrhosis was made from a liver biopsy in compensated cirrhosis or from clinico-biological criteria in patients with decompensated cirrhosis.
For each patient, we collected baseline clinical data including age, gender, body mass index (BMI), an etiology of cirrhosis, the presence and the grade of ascites, and encephalopathy, past medical history, drug history, and noted details of previous hospitalizations within the preceding 2 months. Biological data included alanine amino transferase (ALT), total serum bilirubin, albumin, calcium, phosphorus, creatinine, C-reactive protein (CRP), and the prothrombin index, factor V. All these parameters were measured with standard techniques.
A community-acquired infection was the cause of admission for most of the patients included in the group of infected cirrhotic patients. For any suspicion of infection, an exhaustive work-up was conducted, including full clinical examination, blood culture, urine culture, chest X-ray (sometimes associated with a chest computed tomography-scan), the ascitic white cell count and cultures for all patients with ascites, stool culture if diarrhea present, direct examination and culture of sputum, echocardiography in the case of abnormal heart auscultation, or unexplained fever. In the case of persistent unexplained fever, dental panoramic and sinus X-rays, and chest and abdominal computed tomography-scan with intravenous contrast were performed. Patients with no evidence of infection were prospectively followed for a further 2 months to confirm that no latent infection subsequently became evident.
The diagnosis of spontaneous bacterial peritonitis was made according to the European and the American guidelines, i.e., in the presence of ≥ 250 neutrophilic polymorphonuclear leukocytes/ml. It is clearly outlined in these guidelines that a positive ascitic fluid culture is not necessary for diagnosis.12, 13
Patients who received vitamin D supplementation before admission were excluded. No patients with a previous episode of infection or treatment with an antibiotic in the 2 months before admission were included.
25-OH vitamin D assessment
25-OH vitamin D was measured on admission. Blood was collected in gel separator tubes, cells were separated from serum by centrifugation. The assay was performed within 24 h of sample collection. The Liaison 25-OH vitamin D direct competitive chemiluminescence immunoassay (Diasorin, Stillwater, MN) for quantitative determination of total 25-OH vitamin D2 and 25-OH vitamin D3 in human serum was used (detection range 4–150 ng/ml in 250 μl of serum). The lowest value was 4 ng/ml, which is based on an inter-assay precision that approximates 20% below (functional sensitivity). The observed reference ranged, for the 2.5th to the 97.5th percentile, from 4.8 to 52.8 ng/ml. The following ranges for classification of the 25-OH vitamin D status were as follows: severe deficiency: <10 ng/ml; deficit: 10–20 ng/ml; insufficiency: 20–29 ng/ml; sufficiency: 30–100 ng/ml; and toxicity: >100 ng/ml. (To convert results into SI units: ng/ml × 2.5=nmol/l). The date of collection of blood samples was noted to take the seasonality into account.
Statistics
Comparisons were made between patients with and without a severe deficiency in 25-OH vitamin D (< and ≥10 ng/ml) and between infected and non-infected patients.
Quantitative variables are presented as the median and (25th–75th) interquartile range. Quantitative values between infected and non-infected patients were compared using the Mann–Whitney test or t-test as requested. The χ2-test was used to compare qualitative values. The values of 25-OH vitamin D between the three groups of patients of Child–Pugh A, B and C were compared using the Kruskal–Wallis and Mann–Whitney tests. Logistic regression analysis was performed to determine the independent parameters associated with bacterial infection. The parameters integrated into this model were the presence of a severe deficiency in 25-OH vitamin D, the Child–Pugh score, the CRP, and the ALT level. Using the Child–Pugh score, in this model, the parameters constituting the Child–Pugh score were not included to avoid redundancy. The 25-OH vitamin D level was treated as a categorical variable (with or without a severe deficiency).
Survival studies were performed using Cox regression analyses. The Child–Pugh score (as a continuous variable) and the presence of an infection and of a severe deficiency in 25-OH vitamin D (as categorical variables) were integrated into the Cox analyses. Graphics showing Kaplan–Meier curves were also performed. Kaplan–Meier curves are presented for a 1-year follow-up. All calculations were made using NCSS 2007 (Saugus, MA).
RESULTS
General characteristics of the patients
Inclusion of patients was conducted prospectively by a clinical fellow (M.T.) working only part time (20% of her working time), because her availability depended on external duties without any link to the present study. During the 2 years of the study, patient recruitment by M.T. was performed during her periods of availability, at which time 472 cirrhotic patients were hospitalized in our unit (including the 88 patients reported in our study, i.e., 18.6% of the total number of hospitalized patients). Among the 472 hospitalized patients, 219 patients had an infection as determined by our inclusion criteria (i.e., 46% of the total number of hospitalized patients). This proportion is similar to the data reported in the present study (i.e., 38 on 88: 43%). The gender and mean age were similar between the total number of hospitalized patients and the patients included in our study. Three hundred and thirty-nine men of 58.4±12.0 years and 133 women of 60.3±12.8 years were hospitalized, whereas 58 men of 59.6±11.0 years and 30 women of 60.7±10.6 years were included in the study in the same period. Finally, the proportion of infected patients among the total number of hospitalized patients agreed with that observed in other published cohorts of hospitalized cirrhotic patients.14 Included patients were mainly middle-aged men (Table 1). Most of them (93%) were white Caucasians. The other patients (five men and one woman) originated from North Africa. Causes of cirrhosis were alcohol consumption (71%), hepatitis C (10%), alcohol plus hepatitis C (9%), hepatitis B (3%), non-alcoholic steatohepatitis (NASH; 2%), auto-immune hepatitis (1%), cryptogenic (1%), and others (3%). The median (25th–75th interquartile range) of the 25-OH vitamin D level was 8.8 (5.3–14.1) ng/ml. MELD score was 12.3 (8.7–19.3). Among the 88 patients, 20 (23%) belonged to Child–Pugh class A, 30 (34%) to class B and 38 (43%) to class C. The 25-OH vitamin D level inversely correlated with the Child–Pugh score, which was taken as a continuous variable (r=−0.28, P=0.009) but not with the MELD score. In detail, the 25-OH vitamin D level inversely correlated with the value for albumin (r=0.33, P=0.0016) and tended to correlate negatively with the total bilirubin value (r=−0.2, P=0.055) and the severity of ascitis (r=−0.19, P=0.07), and tended to correlate positively with the prothrombin time (r=0.18, P=0.08). However, no significant association was found between 25-OH vitamin D and the Internationalized Ratio (r=−0.12, P=0.3) or creatininemia (r=0.07, P=0.5). This could explain why the 25-OH vitamin D level was not associated with the MELD. 25-OH vitamin D levels were higher in patients belonging to Child–Pugh class A compared with those belonging to Child–Pugh B and C classes, but were similar between patients belonging to Child–Pugh B and C classes. 25-OH vitamin D levels were also influenced by seasonality, being higher in patients examined during summer/autumn (11.15 (7.8–16.2) ng/ml) compared with during winter/spring (6.1 (4.5–12.7) ng/ml, P=0.001). The repartition of patients according to the Child–Pugh score was similar for the seasonality (blood tests done in summer/autumn: 50% for Child-Pugh A, 40% for Child-Pugh B, and 53% for Child-Pugh C patients, respectively).
Table 1. Characteristics of the studied population.
| Total population (n=88) | Patients with a severe deficiency in 25-OH vitamin D (n=50) | Patients without a severe deficiency in 25-OH vitamin D (n=38) | P | |
|---|---|---|---|---|
| Gender (M/W), n | 58/30 | 36/14 | 22/16 | NS |
| Age (years) median (25th–75th) | 58.5 (51.3–67) | 56.5 (49.8–63) | 63 (56–74.3) | 0.001 |
| Body mass index (kg/m2) | 24.4 (20.8–28.8) | 23.9 (19.8–28.0) | 24.5 (21.2–29.6) | NS |
| Creatininemia (μmol/l) | 66.5 (53.3–85.5) | 64.5 (48.75–83.0) | 69 (56.75–103.75) | NS |
| 25-OH vitamin D (ng/ml) | 8.8 (5.3–14.1) | 5.6 (4.5–7.68) | 15 (12.68–22.15) | <0.0000001 |
| Blood sampling during summer/autumna (%) | 48 | 36 | 63 | 0.01 |
| Child–Pugh A/B/Ca (n) | 20/30/38 | 7/20/23 | 13/10/15 | NS |
M, men; NASH, non-alcoholic steatohepatitis; W, women.
Comparisons were made using the χ2 test. Quantitative variables are presented as the median (interquartile range).
Cirrhotic patients with a severe deficiency in 25-OH vitamin (<10 ng/ml) had more bacterial infections compared with patients with 25-OH vitamin ≥10 ng/ml
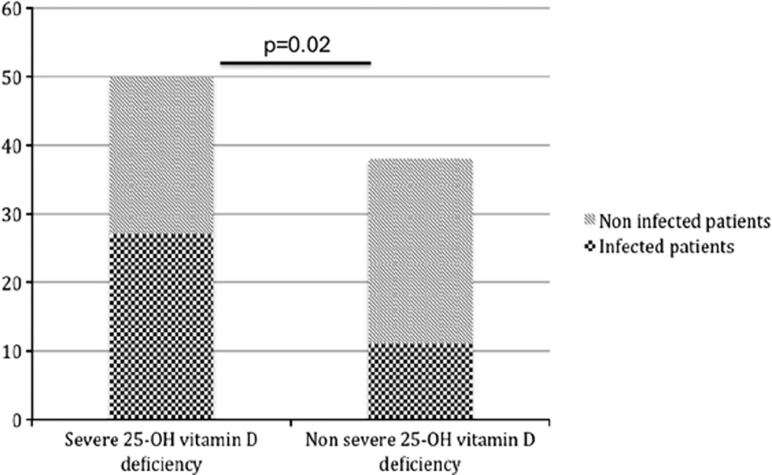
Fifty patients (56.8%) had a severe deficiency in 25-OH vitamin D (<10 ng/ml). Gender, BMI, and the causes of cirrhosis were comparable between patients with and without a severe deficiency in 25-OH vitamin D (Table 1). ALT levels were similar between both groups (43.5 (24.8–72.3) vs. 34 (26.5–57.8) IU/l, P=not significant (NS)). The MELD score was similar between patients with and without a severe deficiency (13.5 (9.0–18.8) vs. 11.6 (8.2–21.0), P=NS). The effect of seasonality was found, as only 36% of patients with a deficit in 25-OH vitamin D, whereas 63.1% of patients without a deficit were sampled during the summer/autumn (P=0.01). A significantly higher number of patients with a severe deficiency were infected (54 vs. 29%, P=0.02) compared with those without a deficit in 25-OH vitamin D (Figure 1). Among the 50 patients with a severe deficiency in 25-OH vitamin D, the proportion of patients with liver insufficiency was significantly unbalanced between infected (Child-Pugh A n=0, Child-Pugh B n=9, and Child-Pugh C n=18) and non-infected patients (Child-Pugh A n=7, Child-Pugh B n=11, and Child-Pugh C n=5; P=0.0008). All the Child-Pugh A patients were in the non-infected group.
Figure 1.
Proportion of infected patients among the cirrhotic patients with and without a severe deficiency in 25-OH vitamin D.
Infected cirrhotic patients more often had severe liver insufficiency and a 25-OH vitamin D deficiency
The main types of infection were urinary tract infections, spontaneous bacterial peritonitis, and bacteremia/septicemia (Table 2). Other causes were pulmonary infections, cutaneous infections, chronic bone infections, and colitis.
Table 2. Bacteriological characteristics of infected patients.
|
Type of bacteria in culture |
|||||||
|---|---|---|---|---|---|---|---|
| Site of infection | Total | Gram-negative bacilli | Gram-positive cocci | C. difficile | Gram-negative cocci | Gram-negative bacilli+Gram-positive cocci | Not documented |
| Bacteriemia | 8 | 3 | 5 | 0 | 0 | 0 | 0 |
| Urinary tract infection | 9 | 6 | 3 | 0 | 0 | 0 | 0 |
| Pulmonary infection | 3 | 0 | 0 | 0 | 2 | 1 | 0 |
| Cutaneous infection | 2 | 0 | 2 | 0 | 0 | 0 | 0 |
| Spontaneous bacterial peritonitis | 11 | 5 | 3 | 0 | 1 | 0 | 2 |
| Chronic bone infection | 2 | 0 | 1 | 0 | 0 | 1 | 0 |
| Other | 3 | 1 | 0 | 2 | 0 | 0 | 0 |
| Total | 15 | 14 | 2 | 3 | 2 | 2 | |
The main bacteria found were Gram-negative bacilli and Gram-positive cocci followed by Gram-negative cocci, including Clostridium difficile. Two patients with spontaneous bacterial peritonitis had a negative ascites culture. Two patients were found to have two different bacteria (Gram-negative bacilli and Gram-positive cocci) in cultures of sputum and from a bone abscess, respectively (Table 2).
Comparisons between infected and non-infected patients were conducted. Gender (25 men (M)/13 women (W) vs. 33 M/17 W, P=NS), age (58.5 (50.8–67.8) vs. 58.5 (51.8–67.5) years, P=NS), BMI (23 (20.6–27.5) vs. 24.8 (21.5–29.5) kg/m2, P=NS), blood sampling during summer/autumn (50 vs. 46%, P=NS), and the causes of cirrhosis were comparable in the infected and non-infected patients. Significantly, more infected patients had a severe deficiency in vitamin D compared with those who are not infected (71 vs. 46%, P=0.019). Similarly, 25-OH vitamin D was significantly lower in infected patients (6.6 (4.8–11.9) vs. 11.2 (6.0–17.0) ng/ml, P=0.01). The MELD score was higher in patients with an infection compared with those without (21.1 (13.5–24.4) vs. 10.4 (8.2–13.1), P=0.00002). Infected patients more often had ascites (84 vs. 50%, P=0.0009) and encephalopathy (39 vs. 10%, P=0.001). Similarly, the other biological parameters included in the definition of the Child–Pugh score were highly different between the two groups. The albumin (27 (22.8–31) vs. 30 (28–37) g/l, P=0.001) and prothrombin index (45.5 (33.3–58.3) vs. 68 (54.5–87) %, P=0.000001) were lower, whereas total bilirubin (84.5 (28.5–177.8) vs. 19 (10.5–35.8), P=0.000006) was higher in infected patients compared with non-infected patients. As a result, the median Child–Pugh score was higher in infected patients (11 (9–13) vs. 7 (5–9), P<0.0001). The repartition of patients into the three classes (A/B/C) of the Child–Pugh score was different between the two groups (0/10/28 vs. 20/20/10, P<0.0000001). CRP was higher in infected patients (27.3 (12.7–52) vs. 8.4 (2.1–14.1) mg/l, P=0.00002). Calcemia and phosphoremia were similar between infected and non-infected patients.
A multivariate logistic regression analysis was performed to determine independent significant parameters associated with infection in cirrhotic patients. All the significant parameters of the univariate analysis were included in the logistic regression model (ALT, Child–Pugh score, CRP, presence of a severe deficiency in 25-OH vitamin D). The presence of a severe deficiency in 25-OH vitamin D was a significant independent factor associated with the presence of an infection (odds ratio=5.44 (1.35–21.97), P=0.017). The severity of the Child–Pugh score (odds ratio=2.09 (1.47–2.97)) and the increase in CRP (odds ratio=1.03 (1.002–1.052) were also independently associated with the presence of an infection in a model also including the ALT level (NS). These results were similar when changing the Child–Pugh score to the MELD score.
The presence of an infection and the severity of the liver insufficiency predicted mortality
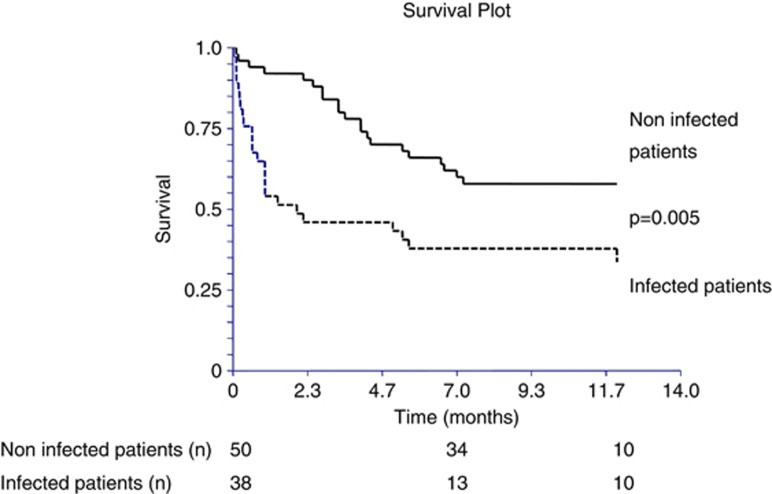
Patients were followed from their inclusion until their death or the last follow-up. Contact by phone with the patient, the family, or the general practitioner was made at the end of the study for all the patients who were alive at the end of hospitalization, to keep informed of their status. The median follow-up period was 212 (66–344) days. During this period, four patients underwent liver transplantation and were alive at the end of the follow-up. For the Cox analyses, the survivals of these four liver-transplanted patients were taken as censored variables (i.e., alive patients). Fourteen patients were lost to follow-up and were considered as missing data for the Cox analysis. Forty-four patients were alive at the end of the follow-up and considered as censored variables. Thirty patients died during the follow-up and were considered as failure variables. Deaths were all liver related. The seasonality was similar between each group (55.5, 47, and 53.3% P=NS, respectively). The Cox regression model included the Child–Pugh score, the presence of an infection, and the presence of a severe deficiency in 25-OH vitamin D. In this model, only the presence of an infection was significantly associated with the mortality (relative risk=3.24 (1.2–8.76), P=0.02).
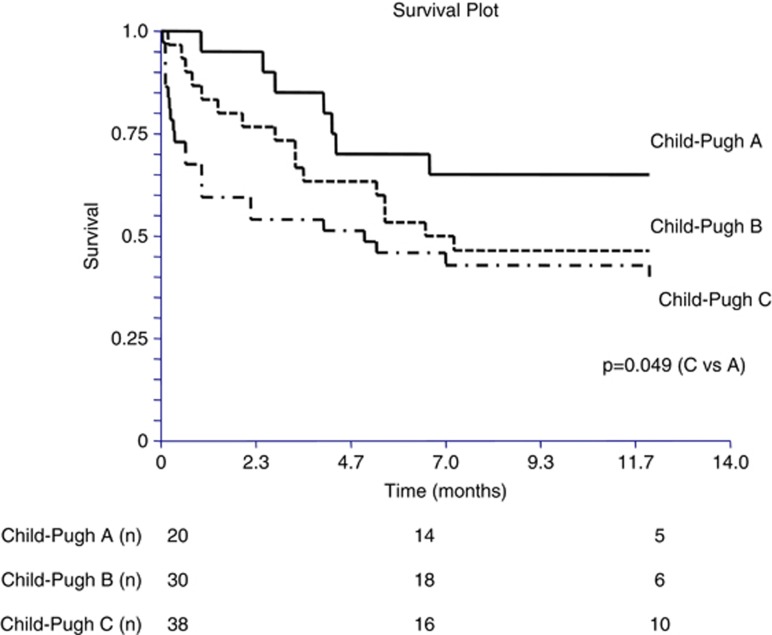
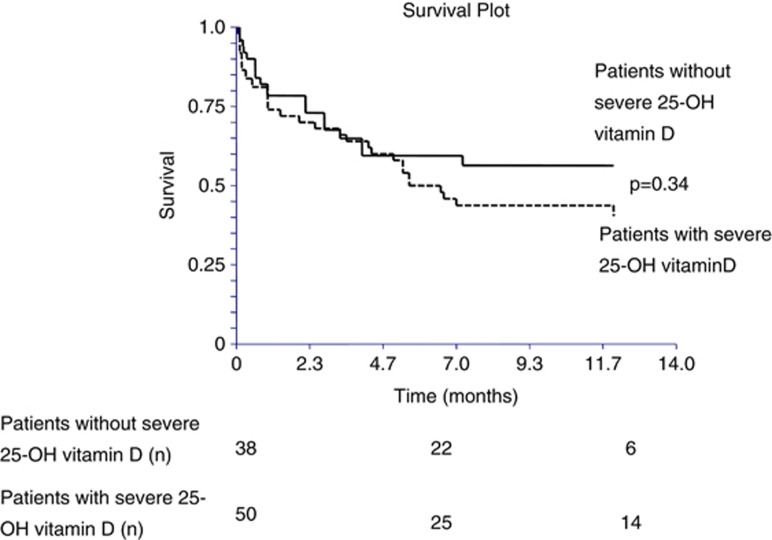
Kaplan–Meier curves are presented in Figures 2, 3 and 4. The presence of infection was significantly associated with increased mortality (log-rank P=0.0063), (Figure 2). Similarly, the higher the Child–Pugh class, the higher the risk of death. Patients in the Child–Pugh C class had a significantly higher risk of death compared with Child–Pugh A class patients (log-rank P=0.049; Figure 3). Although there was a tendency toward a higher mortality in patients with a deficient in vitamin D (25-OH vitamin D <10 ng/ml), it did not reach significance (log-rank P=0.34; Figure 4).
Figure 2.
Kaplan–Meier curves for 1-year survival in infected and non-infected cirrhotic patients.
Figure 3.
Kaplan–Meier curves for 1-year survival in cirrhotic patients according to the Child–Pugh score.
Figure 4.
Kaplan–Meier curves for 1-year survival in patients with and without a severe deficiency in 25-OH vitamin D.
DISCUSSION
Vitamin D is a hormone with pleiotropic effects including stimulation of the innate system and modulation of the immune system.3 As a low level of 25-OH vitamin D has recently been associated with mortality in alcoholic cirrhotic patients,9 and as infection is one of the major causes of death in cirrhotic patients, we hypothesized that the level of 25-OH vitamin D could be low in infected patients. We compared the frequency of a severe deficiency in vitamin D (<10 ng/ml) in a monocentric prospective cohort of infected and non-infected hospitalized cirrhotic patients. A severe deficiency in vitamin D was significantly more frequent in infected patients compared with non-infected patients. Moreover, in a logistic regression analysis, a severe deficiency in 25-OH vitamin D level was associated with the risk of infection, independently of the Child–Pugh score and of the CRP level. The presence of an infection was the only factor predictive of the mortality by Cox regression analysis, which also included the Child–Pugh score and the presence of a severe deficiency in 25-OH vitamin D. These results suggest a potential link between the low level of 25-OH vitamin D and the risk of infection in cirrhotic patients.
The fact that a relevant proportion of patients with a severe deficiency do not develop any infection could be because of several causes. First, most of the cirrhotic patients have an insufficient level of 25-OH vitamin D. A high number has a severe (or at least non severe) 25-OH vitamin D deficiency.1, 10 Second, the Child–Pugh score (i.e., the severity of the liver insufficiency) on its own is an independent risk of infection (in the literature and in our study), some patients with a high Child–Pugh score could be at risk of bacterial infection even without a severe 25-OH vitamin D deficiency. This is illustrated in the Supplementary Table 1 online in which all but one infected patient without a severe deficiency had severe liver insufficiency and a Child–Pugh C score. This suggests, in contrast, that among the cirrhotic patients with a severe deficiency in 25-OH vitamin D, those without severe liver insufficiency could have a smaller risk of infection than patients with severe liver insufficiency. This was verified in our cohort. Hence, among the 50 patients with a severe deficiency in 25-OH vitamin D, the proportion of patients with liver insufficiency was significantly unbalanced between infected (child A n=0, child B n=9, and child C n=18) and non-infected patients (child A n=7, child B n=11, and child C n=5; P=0.0008). Interestingly, all the child A patients were in the non-infected group. Thirdly, the susceptibility to bacterial infection in cirrhotic patients implies other parameters that are not influenced by the vitamin D level, such as episodes of past infection, the frequency of hospitalization, and gastro-intestinal bleeding.14, 15, 16 Finally, because of the limited period of time of observation, we cannot exclude, although it is unlikely, that some of the patients with a severe deficiency could have had an infection after the period of observation.
The role of vitamin D in liver disease has recently been studied, particularly in hepatitis C. Numerous studies around the world have reported that a low 25-OH vitamin D level was associated with more severe fibrosis in patients infected with the hepatitis C virus, irrespective of the viral genotype. Several groups have also demonstrated that a lack of 25-OH vitamin D is associated with a reduced sustained virological response (SVR) rate during treatment with pegylated interferon and ribavirin. Polymorphisms of key enzymes of the metabolism of vitamins in the skin, the liver, or the kidney have also been associated with more fibrosis and with a decrease in SVR.17, 18, 19, 20, 21, 22, 23, 24, 25 Trials of vitamin D supplementation or studies focusing on the impact of the baseline 25-OH vitamin D level have given conflicting results concerning the SVR rate.26, 27
An association between a low 25-OH vitamin D level and the severity of fibrosis has also been reported in patients with non alcoholic fatty liver disease.17, 28, 29 Recently, an inverse correlation between the level of 25-OH vitamin D and the level of hepatitis B virus replication was also shown in chronically infected patients.30 The frequent lack of vitamin D in cirrhotic patients has been known for a long time and could be partly due to liver insufficiency (which could decrease the rate of hydroxylation of cholecalciferol) and cholestasis (which impairs the absorption of fat-soluble vitamins).31 An inverse association between the Child–Pugh score and the level of 25-OH vitamin D has been observed in patients with alcoholic cirrhosis and primary biliary cirrhosis.32
The association of low 25-OH vitamin D and increased mortality has been observed in various populations particularly in the elderly,4, 6 and recently in alcoholic and cirrhotic patients.9, 10 In a Belgian cohort of 324 patients, patients with a severe deficiency in 25-OH vitamin D (level<10 ng/ml) had a significantly higher risk of death compared with those without a deficit.9 The deleterious effect of a low level of vitamin D has also been reported in patients with end-stage liver disease and waiting for a liver transplantation.33, 34, 35, 36 Although there was a tendency toward increased mortality in our patients with a severe deficiency in 25-OH vitamin D, it did not reach significance, possibly because of insufficient power. The reason for the increased mortality in cirrhotic patients with a low level of 25-OH vitamin D is not known. The decrease in liver function could be associated with the decrease in the level of 25-OH vitamin D, as the 25-OH vitamin D is produced from the 25 hydroxylation of cholecalciferol in the liver, and the low level of 25-OH vitamin D could only reflect the hepatocellular insufficiency. In our study, patients belonging to Child–Pugh A group had a slightly higher level of 25-OH vitamin D compared with patients with Child–Pugh B or C. Patients with child B or C had a similar level of 25-OH vitamin D and most of them were deficient, as observed in other studies.10, 31
The fact that Child–Pugh C patients did not have a significantly lower level of 25-OH vitamin D compared with the Child–Pugh B in our study (7.4 (5.2–14.1) vs. 8.4 (4.8–11.7) ng/ml, P=NS) could be because of a lack of power due to an insufficient number of patients in each sub-group. However, it is striking that most of these patients in these groups (60 and 66.6%) were severely deficient in 25-OH vitamin D. This is clinically relevant for the clinician because the supplementation of cirrhotic Child–Pugh C and B patients could be helpful in terms of phospho-calcic and bone metabolism, and also for the potential extra-squeletal vitamin D effects such as the prevention of bacterial infections.
The reason why the 25-OH vitamin D level was not associated with the MELD is not known. However, no significant association was found between 25-OH vitamin D and the Internationalized Ratio (r=−0.12, P=0.3), or creatininemia (r=0.07, P=0.5). This could explain why the 25-OH vitamin D level was not associated with the MELD. Although the Child–Pugh and MELD correlate well, discrepancies between these two scores have been reported37, 38 For example, patients with end-stage liver disease with severe recurrent ascitis or severe encephalopathy can have a relatively low MELD score. These patients could even be considered for liver transplantation with MELD exception. Thus, Child-Pugh and MELD scores are not totally similar for exploring hepatic insufficiency.37, 38
More severe liver disease could be present in patients with a low 25-OH vitamin D owing to more fibrogenesis. A higher degree of fibrosis in patients with hepatitis C and NASH, and with a low level of 25-OH vitamin D has been observed.17, 18, 21, 28, 39 The link between a low 25-OH vitamin D level and mortality in cirrhotic patients could be because of one or several other hormonal effects of vitamin D. Vitamin D has been implicated in the regulation of innate and specific immunity, and could be implicated in the evolution of infections such as tuberculosis or viral respiratory infections.3, 8 Infection is one of the major causes of disease and death in cirrhotic patients.11 Infections could be responsible for a significant part of mortality in previously reported cohorts of cirrhotic patients.9 As expected, in our cohort, mortality in infected patients was higher than in non-infected patients, despite intensive treatment. In our cohort, the site of infection and the identity of the bacteria were similar to those recently reported in another European or US cohorts of cirrhotic patients.15, 16 None of our patients were diagnosed with a fungal or parasitic infection.
A significant lower level of vitamin D has been reported in the group of infected patients coming from a cohort of 3 386 patients hospitalized in intensive care unit.7 These authors reported previously, for a similar population, that the low level of vitamin D was associated with increased mortality.40 In accordance with this, the lower rate of infection and mortality has been noted in peritoneal dialyzed patients treated with vitamin D compared with those who were not.41 Our results are also potentially in accordance with those obtained by Trepo et al.9 who reported a tendency to an increased incidence of spontaneous bacterial peritonitis (15.7 vs. 6.9%, P=0.056) in cirrhotic patients with (n=142) and without (n=112) a severe deficit in 25-OH vitamin D. By contrast, the level of 25-OH vitamin D in the serum and ascites of cirrhotic Chinese patients with (n=19) or without (n=28) spontaneous bacterial peritonitis were also similar.42 In our cohort, spontaneous bacterial peritonitis occurred in 29% of the cases of infection. The impact of 25-OH vitamin D on infections could be different regarding the site of infection in cirrhotic patients.
The mechanisms behind a potential increased risk of infection in cirrhotic patients with a low 25-OH vitamin D level are not yet known. Vitamin D is known to increase innate defences.43 This is partly mediated by antigen-presenting cells such as macrophages and dendritic cells. 25-OH vitamin has to be activated in these cells by 1, 25 hydroxylase, thereby generating the active form of the 1, 25-OH vitamin D, which is able to increase the production of proteins with antibacterial effects such as cathelicidin.2, 3, 44, 45 A lower amount of 25-OH vitamin D could be associated with a lower amount of cathelicidin in cirrhotic patients. In vitro experiments suggested that proteins produced by the innate immune system could be involved in the evolution of hepatitis C.46 Specific polymorphisms in the various enzymes, binding proteins, and the vitamin D receptor (VDR) implicated in the metabolism of vitamin D could explain variations in the effects of low vitamin D in infected patients. Another mechanism that is dependent on vitamin D could be involved such as the modulation of the immune system, which leads to a switch from the Th1 (pro-inflammatory) to the T helper 2 (anti-inflammatory) immune response. The persistent pro-inflammatory Th1 immune response, which is due to a lack of vitamin D, could be deleterious in infected cirrhotic patients.3
Another potential explanation could be linked to the gut microbiota, which recently emerged as an important actor implicated in chronic liver diseases through the gut–liver axis.47 Vitamin D has been implicated in gut permeability, in gut epithelial cell differentiation and in enhanced tight junction formation.48, 49 Gut bacterial overgrowth and increased gut permeability could lead to an increase in endotoxemia through the release of lipopolysaccharide (LPS) in the portal circulation.47 LPS could activate Kuppfer cells and hepatic stellate cells through toll-like receptor 4 signaling. This could promote liver inflammation and fibrosis. Altered metabolism of bile salts because of an imbalance in the gut microbiota could also be implicated in chronic liver diseases. An imbalance in the gut microbiota could be implicated in alcoholic liver diseases and non alcoholic fatty liver disease.47 The metabolism of the vitamin D could also be implicated as the VDR has been recognized to be a bile acid sensor. The activation of VDR by vitamin D in stellate cells could reduce the production of TGFα.50 Moreover, rats fed a vitamin D-deficient “Westernized” high-fat/high-fructose corn syrup diet had significantly worsened steatosis and more lobular inflammation than animals on a low-fat diet with a normal vitamin D content.51
Gut dysbiosis and increased gut permeability could also be implicated in the pathogenesis of cirrhotic patients. Alcoholic cirrhotic patients could be particularly at risk. The more liver insufficiency, the more gut permeability, thereby leading finally to bacterial translocation and infection (including spontaneous bacterial peritonitis but not exclusively).47, 52
The impact of oral supplementation in vitamin D in patients with chronic kidney disease on endotoxemia, and small gut permeability has been tested in a pilot study with inconclusive results.53 However, new prospective studies that include a large number of patients is needed.
Finally, vitamin D could be implicated in the gut–liver axis through the regulation of the digestive immune system, which has a crucial role in the interaction with the gut microbiota. As a modulator of the immune system and as a potential actor of bacterial translocation during bacteremia and spontaneous bacterial peritonitis, vitamin D could be implicated in infections in cirrhotic patients. VDR could also be implicated in gut microbiota instability, in bacterial translocation, and in the action of intestinal biliary salts, leading finally to increased liver inflammation and fibrosis, which has been reviewed recently.54, 55
A potential link between a 25-OH vitamin D deficiency or insufficiency and infection, and mortality in patients suffering from liver diseases, and particularly cirrhotic patients, is of potential interest because of the possibility of providing supplements to patients.56 Current European or American guidelines for the management of cirrhotic patients do not clearly propose a systematic assessment and potential supplementation in vitamin D and calcium. Concerning the risk of osteoporosis, the European Association for the Study of the Liver guidelines for the management of patients suffering from cholestatic liver diseases suggest supplementation with calcium (1,000–1,200 mg/day) and vitamin D (400–800 IU/day), but little clinical data are available to support this.57
Surprisingly, recent prospective studies of supplementation with vitamin D for patients with tuberculosis or exposed to a viral respiratory tract infection did not clearly demonstrate benefit of vitamin D supplementation compared with standard of care.8, 58 Supplementation in vitamin D has several pitfalls. First, the most appropriate form should be chosen, as vitamin D3 would be better absorbed than vitamin D2. Second, the amount and the frequency of administration should be defined while managing observance, which can be low in patients with chronic disease. The efficacy of supplementation as reflected by the increase in the 25-OH vitamin D level in the blood should be checked regularly. Finally, the optimal level is not yet defined (>20/30 ng/ml). The question of the best patients to treat is also an open one. Treating patients with a normal or near normal vitamin D level, before the occurrence of the deficit, could be easier. However, the maximal short-term benefit should be obtained in patients with a severe deficiency or an insufficiency in vitamin D to restore the physiological effects of vitamin D rather than to obtain a “pharmacological” effect in patients with a normal level.
Our study has some limitations and strengths. It is a monocentric study, thus external confirmation should be made before making definitive conclusions and planning strategies for 25-OH vitamin D supplementation in cirrhotic patients. The number of patients was limited and did not allow detection of a difference in mortality between patients with and without a 25-OH vitamin D deficiency. More of the included patients had alcoholic cirrhosis than hepatitis C. This reflects the epidemiological repartition of the causes of cirrhosis in France (such as in Europe) where most cirrhotic patients are alcoholics (http://www.easl.eu/assets/application/files/54ae845caec619f_file.pdf).
Our main objective was to focus on infected vs. non-infected cirrhotic patients. Of course, analysis stratified according to the cause of cirrhosis would have been of interest. However, in this monocentric study, with 2 years of inclusion, not enough patients were included to conduct analyses according to the causes of the cirrhosis. However, focusing mainly of alcoholic cirrhotic patients is of interest, as these patients are more likely to develop a bacterial infection than cirrhotic patients from other causes.14 The strength of the study lies in testing for 25-OH vitamin D with the same high-quality technique that allowed good inter-patient comparability. All infected patients were diagnosed with strict diagnostic criteria of infection, and almost all patients had bacteriological confirmation. All non-infected patients had no infection 2 months before or following 2 months after hospitalization, which permitted good discrimination between the two groups.
In conclusion, 25-OH vitamin D was lower in infected than in non-infected cirrhotic patients. The inverse relationship between 25-OH vitamin D and the risk of infection was independent of the Child–Pugh score, suggesting a unique effect of vitamin D on the risk of infection in these patients. Owing to the severity of the infection in cirrhotic patients, a prospective assessment of 25-OH vitamin D supplementation should be done in randomized clinical trials.
Study Highlights

Acknowledgments
We thank Dr M.C. Brahimi-Horn for editorial correction. In memory of Jean Gabriel Bernard-Cuisinier.
Guarantor of the article: Rodolphe Anty, MD, PhD.
Specific author contributions: R. Anty planned the study, wrote the paper, and approved the final submitted draft; M. Tonohouan collected the data and approved the final submitted draft; P. Ferrari-Panaia performed the assessment of 25-OH vitamin D, corrected and approved the final submitted draft; T. Piche corrected and approved the final submitted draft; A. Pariente participated in the statistical analyses, interpreting the data, drafting the manuscript, and approved the final submitted draft; Q.M. Anstee participated in the statistical analyses, interpreting the data, drafting the manuscript, and approved the final submitted draft; P. Gual corrected and approved the final submitted draft; A. Tran corrected and approved the final submitted draft.
Financial support: This work was supported by grants from the INSERM (France), the University of Nice, and the Programme Hospitalier de Recherche Clinique (Centre Hospitalier Universitaire of Nice). This work was supported by the French Government (National Research agency, ANR) through the “Investments for the future” LABEX SIGNALIFE: program reference no. ANR-11-LABX-0028-01.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Baeke F, Takiishi T, Korf H, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D regulation of immune function. Vitam Horm. 2011;86:1–21. doi: 10.1016/B978-0-12-386960-9.00001-0. [DOI] [PubMed] [Google Scholar]
- Zittermann A, Iodice S, Pilz S, et al. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95:91–100. doi: 10.3945/ajcn.111.014779. [DOI] [PubMed] [Google Scholar]
- Rejnmark L, Avenell A, Masud T, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab. 2012;97:2670–2681. doi: 10.1210/jc.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2011. p. CD007470. [DOI] [PubMed]
- Moromizato T, Litonjua AA, Braun AB, et al. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically III. Crit Care Med. 2013;42:97–107. doi: 10.1097/CCM.0b013e31829eb7af. [DOI] [PubMed] [Google Scholar]
- Yamshchikov AV, Desai NS, Blumberg HM, et al. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15:438–449. doi: 10.4158/EP09101.ORR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepo E, Ouziel R, Pradat P, et al. Marked 25-hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver disease. J Hepatol. 2013;1:00203–1. doi: 10.1016/j.jhep.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Putz-Bankuti C, Pilz S, Stojakovic T, et al. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int. 2012;32:845–851. doi: 10.1111/j.1478-3231.2011.02735.x. [DOI] [PubMed] [Google Scholar]
- Arvaniti V, D'Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Runyon BA. Management of adult patients with ascites due to cirrhosis: update 2012. Hepatology. 2013;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- Fagiuoli S, Colli A, Bruno R, et al. Management of infections in cirrhotic patients: report of a Consensus Conference. Dig Liver Dis. 2014;46:204–212. doi: 10.1016/j.dld.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, O'Leary JG, Reddy KR, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328–2335. doi: 10.1002/hep.25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchetta I, Carotti S, Labbadia G, et al. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012;56:2180–2187. doi: 10.1002/hep.25930. [DOI] [PubMed] [Google Scholar]
- Petta S, Camma C, Scazzone C, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010;51:1158–1167. doi: 10.1002/hep.23489. [DOI] [PubMed] [Google Scholar]
- Petta S, Ferraro D, Camma C, et al. Vitamin D levels and IL28B polymorphisms are related to rapid virological response to standard of care in genotype 1 chronic hepatitis C. Antivir Ther. 2012;17:823–831. doi: 10.3851/IMP2100. [DOI] [PubMed] [Google Scholar]
- Baur K, Mertens JC, Schmitt J, et al. The vitamin D receptor gene bAt (CCA) haplotype impairs the response to pegylated-interferon/ribavirin-based therapy in chronic hepatitis C patients. Antivir Ther. 2012;17:541–547. doi: 10.3851/IMP2018. [DOI] [PubMed] [Google Scholar]
- Baur K, Mertens JC, Schmitt J, et al. Combined effect of 25-OH vitamin D plasma levels and genetic vitamin D receptor (NR 1I1) variants on fibrosis progression rate in HCV patients. Liver Int. 2012;32:635–643. doi: 10.1111/j.1478-3231.2011.02674.x. [DOI] [PubMed] [Google Scholar]
- Lange CM, Bibert S, Kutalik Z, et al. A genetic validation study reveals a role of vitamin D metabolism in the response to interferon-alfa-based therapy of chronic hepatitis C. PLoS One. 2012;7:e40159. doi: 10.1371/journal.pone.0040159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange CM, Bojunga J, Ramos-Lopez E, et al. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J Hepatol. 2011;54:887–893. doi: 10.1016/j.jhep.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Falleti E, Bitetto D, Fabris C, et al. Vitamin D binding protein gene polymorphisms and baseline vitamin D levels as predictors of antiviral response in chronic hepatitis C. Hepatology. 2012;56:1641–1650. doi: 10.1002/hep.25848. [DOI] [PubMed] [Google Scholar]
- Bitetto D, Fattovich G, Fabris C, et al. Complementary role of vitamin D deficiency and the interleukin-28B rs12979860 C/T polymorphism in predicting antiviral response in chronic hepatitis C. Hepatology. 2011;53:1118–1126. doi: 10.1002/hep.24201. [DOI] [PubMed] [Google Scholar]
- Abu-Mouch S, Fireman Z, Jarchovsky J, et al. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naive patients. World J Gastroenterol. 2011;17:5184–5190. doi: 10.3748/wjg.v17.i47.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitson MT, Dore GJ, George J, et al. Vitamin D status does not predict sustained virologic response or fibrosis stage in chronic hepatitis C genotype 1 infection. J Hepatol. 2013;58:467–472. doi: 10.1016/j.jhep.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Scala L, et al. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517–524. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Kwok RM, Torres DM, Harrison SA. Vitamin D and NAFLD: Is it more than just an association. Hepatology. 2013;16:26390. doi: 10.1002/hep.26390. [DOI] [PubMed] [Google Scholar]
- Farnik H, Bojunga J, Berger A, et al. Low vitamin D serum concentration is associated with high levels of hepatitis B virus (HBV) replication in chronically infected patients. Hepatology. 2013;58:1270–1276. doi: 10.1002/hep.26488. [DOI] [PubMed] [Google Scholar]
- Miroliaee A, Nasiri-Toosi M, Khalilzadeh O, et al. Disturbances of parathyroid hormone-vitamin D axis in non-cholestatic chronic liver disease: a cross-sectional study. Hepatol Int. 2010;4:634–640. doi: 10.1007/s12072-010-9194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malham M, Jorgensen SP, Ott P, et al. Vitamin D deficiency in cirrhosis relates to liver dysfunction rather than aetiology. World J Gastroenterol. 2011;17:922–925. doi: 10.3748/wjg.v17.i7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitetto D, Fabris C, Falleti E, et al. Vitamin D and the risk of acute allograft rejection following human liver transplantation. Liver Int. 2010;30:417–444. doi: 10.1111/j.1478-3231.2009.02154.x. [DOI] [PubMed] [Google Scholar]
- Reese PP, Bloom RD, Feldman HI, et al. Changes in vitamin D binding protein and vitamin D concentrations associated with liver transplantation. Liver Int. 2012;32:287–296. doi: 10.1111/j.1478-3231.2011.02638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott-Johnson W, Kerlin P, Clague A, et al. Relationships between blood levels of fat soluble vitamins and disease etiology and severity in adults awaiting liver transplantation. J Gastroenterol Hepatol. 2011;26:1402–1410. doi: 10.1111/j.1440-1746.2011.06746.x. [DOI] [PubMed] [Google Scholar]
- Venu M, Martin E, Saeian K, et al. High prevalence of vitamin A deficiency and vitamin D deficiency in patients evaluated for liver transplantation. Liver Transpl. 2013;14:23646. doi: 10.1002/lt.23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitto S, Lorenzini S, Biselli M, et al. Allocation priority in non-urgent liver transplantation: An overview of proposed scoring systems. Dig Liver Dis. 2009;41:700–706. doi: 10.1016/j.dld.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Cholongitas E, Papatheodoridis GV, Vangeli M, et al. Systematic review: The model for end-stage liver disease—should it replace Child-Pugh's classification for assessing prognosis in cirrhosis. Aliment Pharmacol Ther. 2005;22:1079–1089. doi: 10.1111/j.1365-2036.2005.02691.x. [DOI] [PubMed] [Google Scholar]
- Cholongitas E, Theocharidou E, Goulis J, et al. Review article: the extra-skeletal effects of vitamin D in chronic hepatitis C infection. Aliment Pharmacol Ther. 2012;35:634–646. doi: 10.1111/j.1365-2036.2012.05000.x. [DOI] [PubMed] [Google Scholar]
- Braun A, Chang D, Mahadevappa K, et al. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39:671–677. doi: 10.1097/CCM.0b013e318206ccdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschbaum J, Vychytil A, Lhotta K, et al. Treatment with oral active vitamin D is associated with decreased risk of peritonitis and improved survival in patients on peritoneal dialysis. PLoS One. 2013;8:e67836. doi: 10.1371/journal.pone.0067836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhao L, Ma L, et al. Vitamin D status and expression of vitamin D receptor and LL-37 in patients with spontaneous bacterial peritonitis. Dig Dis Sci. 2012;57:182–188. doi: 10.1007/s10620-011-1824-6. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76:3837–3843. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr AW, Urbanowicz RA, Ball JK. The role of humoral innate immunity in hepatitis C virus infection. Viruses. 2012;4:1–27. doi: 10.3390/v4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Gupta M, Aggarwal R.Gut microbiota and liver disease J Gastroenterol Hepatol 2014. doi: 10.1111/jgh.12556(in press). [DOI] [PubMed]
- Holt PR, Arber N, Halmos B, et al. Colonic epithelial cell proliferation decreases with increasing levels of serum 25-hydroxy vitamin D. Cancer Epidemiol Biomarkers Prev. 2002;11:113–119. [PubMed] [Google Scholar]
- Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–G216. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- Ding N, Yu RT, Subramaniam N, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth CL, Elfers CT, Figlewicz DP, et al. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology. 2012;55:1103–1111. doi: 10.1002/hep.24737. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2013;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponda MP, Breslow JL. Vitamin D3 repletion in chronic kidney disease stage 3: effects on blood endotoxin activity, inflammatory cytokines, and intestinal permeability. Ren Fail. 2013;35:497–503. doi: 10.3109/0886022X.2013.775696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly NP, Litonjua A, Gold DR, et al. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity. J Allergy Clin Immunol. 2011;127:1087–1094. doi: 10.1016/j.jaci.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lade A, Noon LA, Friedman SL. Contributions of metabolic dysregulation and inflammation to nonalcoholic steatohepatitis, hepatic fibrosis, and cancer. Curr Opin Oncol. 2014;26:100–107. doi: 10.1097/CCO.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode A, Fourlanos S, Nicoll A. Oral vitamin D replacement is effective in chronic liver disease. Gastroenterol Clin Biol. 2010;34:618–620. doi: 10.1016/j.gcb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333–1339. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.