Abstract
The current estimate of children (<15 years) living with HIV and AIDS is 2.2 million [UNAIDS/WHO, 2005. AIDS Epidemic Update. UNAIDS, Geneva]. The major source of infection occurs through vertical transmission of the virus from mother to child during delivery [UNAIDS/ WHO, 2005. AIDS Epidemic Update. UNAIDS, Geneva]. Recent studies have shown that timing of HIV-1 infection might be related to the onset and rate of progression of CNS disease [Blanche, S., Mayaux, M.-J., Rouziox, C., Teglas, J.-P., Firtion, G., Monpoux, F., Cicaru-Vigneron, N., Meier, F., Tricoire, J., Courpotin, C., Vilmer, E., Griscelli, C., Delfraissy, J.-F., 1994. Relation of the course of HIV infection in children to the severity of the disease in their mothers at delivery. N. Engl. J. Med. 330 (5), 308–312]. The effects of HIV on the brain are thought to be mediated indirectly through the viral toxins Tat and gp120. This study characterized developmental effects on PPI following intrahippocampal administration of Tat. On postnatal day (P)1, one male and one female pup from each of eight Sprague–Dawley litters were bilaterally injected with 50 µg Tat or saline (1 µl volume). Animals were tested for PPI of the auditory startle response (ASR) (ISIs of 0, 8,40, 80, 120, and 4000 ms, six trial blocks, Latin-square design) on days 30, 60 and 90. Tat altered PPI and the pattern of alterations was different for males and females. For males, a leftward shift was evident in the ISI for maximal inhibition of the response on day 30 and on day 60 (χ2(1) = 4.7,p ≤ .03, and χ2(1) = 5.3, p ≤ .02, respectively), but not on day 90. For females, Tat altered peak ASR latency across PPI trials (8–120 ms) at all days of testing (30, 60, and 90 days of age), as indexed by orthogonal component analyses, indicating less modulation of PPI by ISI. Data collected from a second group that were tested only once at 90 days of age, suggested that the observed adverse Tat effects for males and females early in development were maintained with age. Thus, the diminishing TAT effect on PPI at day 90 in a longitudinal study design was attributed to repeated testing, rather than ‘recovery of function’. Collectively, the data suggested that hippocampal Tat injections in neonatal rats produced alterations in the pre-attentive process of sensorimotor gating, as indexed by PPI.
Keywords: Neonatal rats, Tat, Hippocampus, Prepulse inhibition, Neurobehavioral development
1. Introduction
In 2005 the number of children under 15 years of age living with HIV was estimated to be 2.2 million. More than 700,000 children worldwide under 15 years of age were newly infected with HIV/AIDS, almost all through vertical transmission of the virus from mother to child during pregnancy, delivery and breast-feeding during the first days of life (UNAIDS/WHO, 2005). Since the beginning of the HIV/AIDS epidemic, the course and effects of the disease have been documented and established in adults and the effects of neonatal infections are only beginning to be better understood. Commonly (up to 90%) infants and children with symptomatic HIV infection develop evidence of central nervous system (CNS) dysfunction. HIV-1-associated CNS disease syndrome or HIV-1 encephalopathy appears analogous to HIV-1-associated dementia complex (HAD) in adults (Belman et al., 1988; Belman, 1997; Blanche et al., 1994; Da Cunha et al., 1997; Epstein et al., 1986, 1988). The active and persistent infection of the brain with the retrovirus HIV-1 is a major part of the spectrum of HIV infection in children (Belman et al., 1988; Chearskul et al., 2002; Epstein et al., 1986). The adverse effects of HIV-1 on the developing immune system and nervous system often results in more rapid onset of clinical symptoms and progression to death in infants relative to adults (Belman, 1997). Research indicates that one of the crucial factors of the disease progression in infants is related to the timing of infection (Blanche et al., 1994). The CNS effects of HIV-1 include loss of developmental milestones, progressive motor dysfunction, attentional and cognitive deficits, impaired brain growth, weakness with bilateral pyramidal tract signs, ataxia, expression of HIV antigen in the cerebrospinal fluid, low CD4+ cell count, virus-laden macrophages and multinucleated giant cells, and calcification of the basal ganglia (Belman et al., 1988; Belman, 1997; Blanche et al., 1994; Chearskul et al., 2002; Epstein et al., 1986, 1988; Fauci, 1988). In vivo studies and pathology studies in animal models and humans suggest that the entry of the virus into the CNS causes subsequent damage to the blood–brain barrier leading to alteration of neuronal functions (Annunziata, 2003).
Progress in understanding what components of the virus cross the blood–brain barrier and are responsible for the disease, has mostly been made with examination of in vitro preparations. It has been suggested that two proteins, Tat and gp120, produced by the HIV-1 virus cross the blood–brain barrier and lead to apoptosis and neuropathology (Annunziata, 2003; Bansal et al., 2000; Behnisch et al., 2004; Catani et al., 2003; Cheng et al., 1998; Fauci, 1988; Nath et al., 2000a,b). The viral products are believed to be released into the brain by virus-infected monocyte/macrophage cells (Annunziata, 2003; Bansal et al., 2000; Behnisch et al., 2004; Belman et al., 1988; Brew et al., 1988; Bruce-Keller et al., 2003; Catani et al., 2003; Fauci, 1988; Nath et al., 2000b; Price et al., 1988) and form subtle morphological and metabolic changes in the blood–brain barrier (Annunziata, 2003). Later, after the course of the infiltration of monocytes/macrophages into the CNS, infected microglia form the brain reservoir of the virus. In HIV-encephalopathy, the presence of activated microglia in the brain correlates with astrogliosis, monocyte transmigration and expression of beta-chemokines (Persidsky et al., 1999). Neurotoxicity induced by gp120 has been reported to be mediated primarily by NMDA receptor mechanisms (Barks et al., 1997). In contrast, Tat directly interacts with neurons and produces neurotoxicity via oxidative stress (Aksenov et al., 2003, 2006; Aksenova et al., 2005, 2006) and is a causative agent in HIV-1 neurotoxicity (Nath et al., 2000b). Neuropathology findings indicate that Tat causes neuronal damage, synaptic alterations and glial activation in hippocampal regions, including CA1, CA3/4 areas and dentate gyrus (Behnisch et al., 2004; Bruce-Keller et al., 2003; Cheng et al., 1998; Fitting et al., 2006; Maragos et al., 2003; Nath et al., 2000b).
Cognitive impairments that are linked with HIV-1 encephalopathy include poor attentional abilities, deficits in memory, and reduced alertness (Brew et al., 1988; Nath et al., 2000a; Price et al., 1988). Sensorimotor gating, an early step in the process of attention, may be measured through use of the auditory startle response (ASR) (Hoffman and Ison, 1980). The ASR is a constellation of reflexes elicited by sudden, relatively intense stimuli. It offers many advantages as a behavioral measure of the CNS activity and can be measured in numerous species, including humans, pigeons, rabbits, rats, and mice (Swerdlow et al., 2000; Hoffman and Ison, 1980). ASR demonstrates several forms of behavioral plasticity, such as sensitization, habituation, and prepulse inhibition (PPI) (Jovanovic et al., 2004). Presenting a prepulse that is a relatively weak sensory stimulus, prior to the startle stimulus, induces PPI, a process often termed sensorimotor gating. The mechanism underlying this inhibited response is believed to resemble the process of sensory gating, i.e., filtering incoming sensory stimuli and protecting the mechanism from extraneous stimuli (Jovanovic et al., 2004; Koch and Schnitzler, 1997; Swerdlow et al., 2000). According to previous research PPI is modulated by a complex neuronal circuitry, including the limbic system and frontal cortex, basal ganglia, and pons (Caine et al., 1991, 2001; Fendt et al., 2001; Jovanovic et al., 2004; Swerdlow et al., 2001a,b). An in vivo study demonstrated that intrahippocampal Tat injection in adult male rats produced Tat-adverse effects on sensorimotor gating, indexed by PPI (Fitting et al., 2006). In contrast to adults, pediatric HIV-1 infection and disease occurs in an immature, undeveloped, and incompletely myelinated CNS. Thus, the developmental and maturational stage of the CNS may determine different patterns of HIV-1-associated CNS disease (Belman, 1997).
In order to determine developmental effects of Tat on sensorimotor gating, we injected Tat into the hippocampus on P1. PPI was assessed in young, adolescent and adult male and female rats. At 8 months of age rats were euthanized and morphometric measures were collected from hippocampal cornu ammonus (CA) pyramidal cell fields, the granule cell fields of the superior blade of the dentate gyrus, and the infra hilar area of the dentate gyrus.
The purpose of this study was two-fold: (1) to characterize intrahippocampal Tat effects on PPI of the ASR at different developmental stages, in both male and female rats; (2) to examine effects of Tat on the morphometry of the hippocampal formation.
2. Experimental procedures
2.1. Animals
Sprague-Dawley pregnant dams (N= 8) from Harlan Laboratories, Inc. (Indianapolis, IN) were delivered to the vivarium before gestation day 7 of pregnancy. Dams were housed singly with food (Pro-Lab Rat, Mouse Hamster Chow #3000, NIH diet #31) and water available ad libitum. Females were checked twice daily for the onset of parturition as they approached term. The day pups were found in the cage was designated as P0. On P1, litters were culled to 10 offsprings of equal sexes, if possible. No more than one female and one male per litter were assigned to a single condition. The animal facility was maintained at 21 ± 2 °C, 50 #x000B1; 10% relative humidity and had a 12 h light: 12 h dark cycle with lights on at 07:00 h (EST). The animals were maintained according to the National Institute of Health (NIH) guidelines in AAALAC-accredited facilities. The Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina approved the animal protocol for this research.
2.2. Surgery
Individual pups were gently removed from the dam and cryogenically anesthetized (AVMA, 2001) before being placed in a modified stereotaxic holder for surgery of neonates (Kopf, Inc.). Rubber head bars held the skull in place while bilateral microinjections (1 µl) of saline or (50 µg) of Tat were made directly into hippocampus using the following set of coordinates: right hemisphere −0.3 mm AP, 0.7 mm ML, −3.0 mm DV; left hemisphere −0.3 mm AP, −0.7 mm ML, −3.0 mm DV. The saline and Tat injections were the same volume (1 µl) with Tat being dissolved in saline. The 1 µl injection volume was released over 1 min after a 1-min resting period that allowed the tissue to return to its original conformation. The needle was withdrawn over 2 min to prevent reflux. After the two injections, the piercings in the skin of the head were closed with surgical glue and the pups warmed under a heat lamp (35 °C) before being returned to the dam, where they were closely observed for indications of rejection. No pups were rejected or abused by the dam.
2.3. Experimental design
A randomized block design was employed, with litter as the blocking factor, in which all experimental treatments were represented. More specifically, male and female rats were randomly assigned to one of two conditions, a longitudinally repeated testing group or a single test adult group. All rats in the longitudinally repeated testing group were assessed for PPI of the ASR at 30, 60, and 90 days of age. The animals in the single test adult group were tested only once at 90 days of age, thus serving as a control for repeated testing as well as providing for the relative assessment of recovery of function. Within each group, the two possible treatments were bilateral hippocampal injections of either 1 µl volume saline or 50 µg Tat.
2.4. Apparatus
The startle chamber (SR-Lab Startle Reflex System, San Diego Instruments, Inc.) was enclosed in a 10 cm thick double-walled, 81 cm × 81 cm × 116 cm isolation cabinet (external dimensions) (Industrial Acoustic Company, Inc., Bronx, NY). Each animal was tested individually in the dark with a high-frequency loudspeaker, that produced a background white noise (70 dB(A)) and was mounted inside the chamber 31 cm above the Plexiglas cylinder. The startle chamber consisted of a Plexiglas cylinder 8.75 cm in interval diameter resting on a 12.5 cm × 20 cm Plexiglas stand. The animal’s response to the stimulus produced a deflection of the Plexiglas cylinder, which was converted into an analog signal by a piezoelectric accelerometer. Acoustic stimulus intensities and response sensitivities were calibrated using an SR-LAB Startle Calibration System. Sound levels were measured and calibrated with a sound level meter (Extech Instruments, Waltham, MA) with the microphone placed inside the Plexiglas cylinder. The signals were then digitized (12 bit A to D) and saved to a hard disk on a Pentium class computer.
2.5. Testing procedures
All rats were tested for approximately 20 min. Animals were first exposed to a 5-min acclimation period of 70 dB(A) background of white noise, followed by six single white noise stimuli of 100 dB(A), and 36 PPI trials with 0, 8, 40, 80, 120, and 4000 ms interstimulus intervals (ISIs), assigned by Latin-square design. The stimulus duration was 20 ms. The six single stimuli were defined as adaptation trials and the 0 and 4000 ms PPI trials were the control trials in order to provide the baseline for PPI. The prepulse stimulus intensity was 85 dB(A) with a 20 ms duration as the startle stimulus duration. The ISI interval that we report represents the time from the offset of the prepulse stimulus to the onset of the startle stimulus. For PPI the dependent measures analyzed were peak ASR amplitude, peak ASR latency (from startle stimulus onset to the peak response), and percent PPI. Percent PPI indicates the percent of inhibition in startle amplitude at a prepulse of 100 ms ISI relative to pulse only trials (0 ms ISI). PPI for ISI 100 ms was estimated using the average of PPI trials 80 and 120 ms ISIs. Percent PPI was computed according to the following formula: % PPI = [(0 ms ISI trials – 100 ms ISI trials)/0 ms ISI trials] × 100.
2.6. Histology
At approximately 8 months of age, animals were euthanized (pentobarbital overdose) and the brain was removed from the skull. Cryostat-cut sections (20 µm) through the hippocampal injection sites were collected and Nissl-stained to confirm injection location and pathology. Morphometric measurements of the integrity of the hippocampus were made using a computer-assisted microscopy system (Neurolucida, Microbrightfield, Inc.). Measurement were collected on the cornu ammonus (CA) pyramidal cell field width (CA1 and CA3 region combined), the granule cell field width through the superior blade of the dentate gyrus, and the infra hilar width of the dentate gyrus (µm).
2.7. Statistical analysis
All data were analyzed using analysis of variance (ANOVA) techniques (SPSS, 2005; SYSTAT, 2004; Winer, 1971). For the longitudinally tested animal group three-way mixed-factor ANOVAs, with treatment and sex as between-subjects factors and age as a within-subjects factor were performed on peak ASR amplitude and peak ASR latency in the adaptation trials, control trials and on percent PPI (at 100 ms). A four-way mixed-factor ANOVA, with treatment and sex as between-subjects factors and PPI trials and age as within-subjects factors were performed on peak ASR amplitude and peak ASR latency across PPI trials (8–120 ms). ANOVAs were conducted in order to characterize the ISI function in PPI and to determine whether intrahippocampal administration of Tat on P1 altered PPI across different age groups. ANOVAs for the peak amplitude for response inhibition across PPI trials (8–120 ms ISI) were conducted on log 10 transformed data and log 10 metric for ISI. ANOVAs for the latency response across PPI trials (8–120 ms ISI) were conducted on raw data with the original metric for ISI (8, 40, 80, 120). For the within-subjects terms, potential violations of compound symmetry (Winer, 1971) were preferentially handled by the use of orthogonal decomposition or, if necessary, the use of the Greenhouse–Geisser d.f. correction factor (Greenhouse and Geisser, 1959). Orthogonal component analyses for simple effects of PPI trials separately for each treatment were employed to evaluate the nature of the PPI trial-dependent effects. An added positive use of the orthogonal component analyses is that this statistic also describes the shape of the function by determining its significance (e.g., linear, quadratic, etc. equations). A significant main effect or interaction will thus be described with its significant components. Planned orthogonal component analyses of PPI trials were further analyzed when appropriate. In addition, the ISI at which the maximal inhibition response occurred was recorded across all PPI trials (8–120 ms ISI). The ISI data is categorical in nature, thus, the Pearson chi-square, uncorrected for continuity was applied. To control for repeated testing, the single test adult group, that was tested only once, at 90 days of age, was compared to the longitudinally repeated testing group at 90 days of age with a three-way factorial ANOVA using group, treatment and sex as between-subjects factors. A two-way multivariate analysis of variance (MANOVA), with treatment and sex as between-subjects factors was performed on hippocampal morphometric measurements (CA pyramidal cell fields, the dentate granule cell fields and the infra hilar width of the dentate gyrus) combining left and right hemisphere. The morphometry data of animals tested repeatedly and animals tested only once were combined and analyzed together as no significant group differences were noted on the repeated testing factor. An alpha level of p ≤ .05 (rounded to two decimal places) was considered significant for all statistical tests used.
3. Results
3.1. Adaptation trials
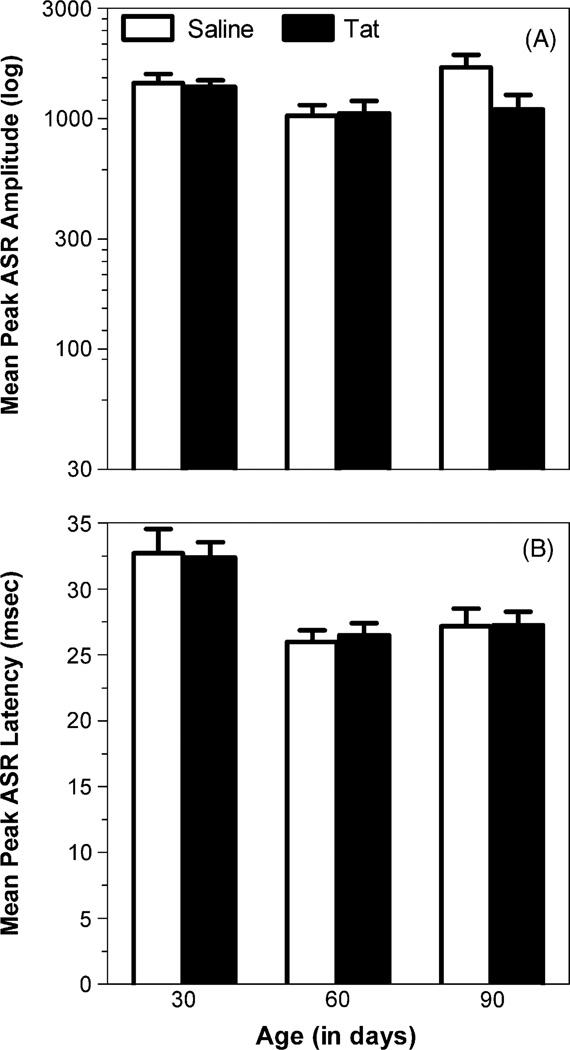
For adaptation trials, a 2(treatment) × 2(sex) × 3(age: 30,60, 90 days old) mixed-model ANOVA conducted on peak ASR amplitude revealed a significant age effect with a quadratic component [F(1, 23) = 18.2, p ≤ .01] and a treatment × age interaction [F(2, 46) = 3.3, p ≤ .05], suggesting an age-dependent alteration in response that became stronger with age. Separate orthogonal component analyses for treatment revealed a significant quadratic component for saline-treated rats [F(1, 11) = 21.9, p ≤ .01]. In contrast, no significant orthogonal component was noted for Tat-treated animals, further supporting a treatment induced alteration of the peak response for adaptation trials across age (Fig. 1A). A mixed-model ANOVA on peak ASR latency revealed a significant age effect with linear and quadratic components [F(1, 23) = 17.7, p < .01 and F(1, 23)= 11.2, p ≤ .01, respectively], illustrating the maturation of the response. No treatment effect and/or interaction were noted on the latency data (Fig. 1B).
Fig. 1.
Data in adaptation trials for the longitudinally tested animal group, tested at 30, 60, and 90 days of age. (A) Mean (±S.E.M.) peak ASR response amplitude with a significant age effect and a significant treatment × age interaction. (B) Mean (±S.E.M.) peak ASR latency with a significant overall age effect, but no treatment × age interaction.
A comparison of the single test adult group, tested only at 90 days of age, and the longitudinally repeated testing group at 90 days of age, by conducting a 2(group) × 2(treatment) × 2(sex) mixed-model ANOVA, revealed no significant effects on peak amplitude or peak latency.
3.2. Prepulse inhibition (PPI) test
One saline-treated male was excluded in PPI assessment at 30 days of age because of procedural error; it did not show any sensitivity to the PPI trials.
3.2.1. Control trials (0 and 4000 ms combined)
For control trials, a 2(treatment) × 2(sex) × 3(age: 30, 60, 90 days old) mixed-model ANOVA conducted on peak ASR amplitude revealed a significant age effect with a prominent quadratic component [F(1, 23) = 37.4, p ≤ .01], indicating an alteration in peak amplitude that declined rapidly after 30 days of age. A mixed-model ANOVA conducted on peak ASR latency revealed a significant age effect with linear and quadratic components [F(1, 23) = 24.3, p ≤ .01 and F(1, 23) = 16.6, p ≤ .01, respectively], also suggesting a decrease in response with age. No treatment effect or/and interaction were noted on peak amplitude and peak latency, indicating no Tat-induced alterations on the baseline ASR.
A comparison of the single test adult group, tested only at 90 days of age, and the longitudinally repeated testing group at 90 days of age, by conducting a 2(group) × 2(treatment) × 2(sex) mixed-model ANOVA, revealed no significant effects on peak amplitude or peak latency.
3.2.2. PPI trials at 8–120 ms ISI
3.2.2.1. Peak ASR amplitude
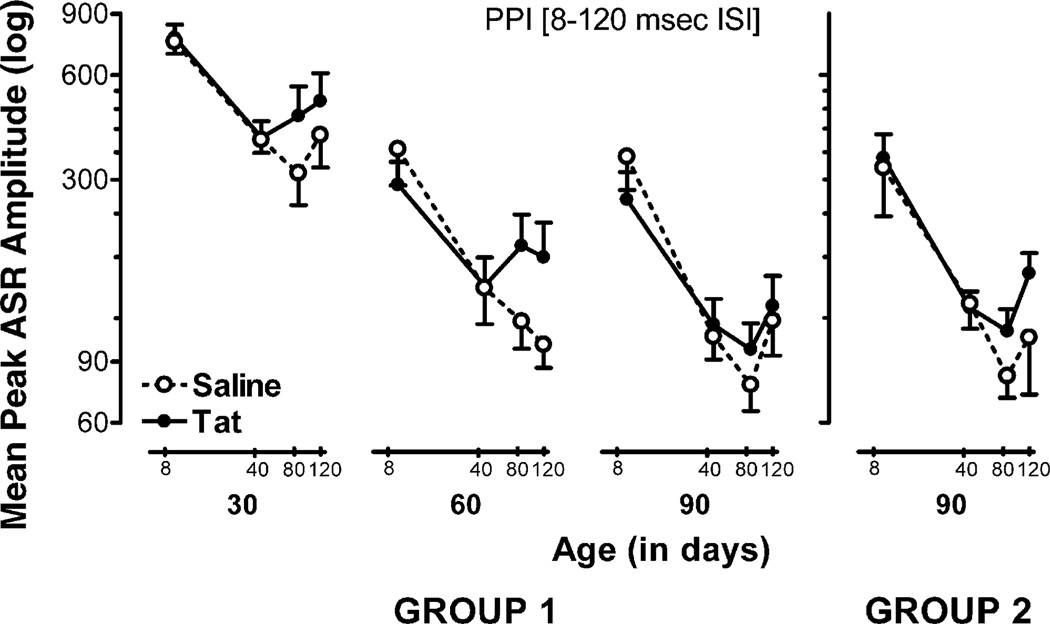
A 2(treatment) × 2(sex) × 3(−3(age: 30, 60, 90 days old) × 4(PPI: 8, 40, 80, 120 ms ISI) mixed-model ANOVA conducted on peak ASR amplitude revealed a significant age effect with a prominent linear component [F(1, 23) = 132.7,p ≤ .01], indicating a continuing decrease in peak response with age. For PPI trials, a significant main effect was noted with a prominent linear component [F(1, 23) = 75.1, p ≤ .01], which was significantly altered by sex, revealing a linear sex × PPI trial interaction [F(1, 23) = 4.6, p ≤ .04], and by age, revealing a linear age × PPI interaction [F(1, 23) = 7.3, p ≤ .01]. To better characterize these alterations in PPI, orthogonal component analyses were conducted separately for treatment and age within each sex. At 30 and 60 days of age saline-treated males revealed prominent linear components [30 days of age: F(1, 6) = 15.2,p ≤ .01; 60 days of age: F(1, 7) = 57.2,p ≤ .01], in contrast to prominent quadratic components for Tat-treated animals [30 days of age: F(1, 7) = 26.5, p ≤ .01; 60 days of age: F(1, 7) = 9.6, p ≤ .01], suggesting a Tat-induced alteration on response inhibition across ISIs in males at 30 and 60 days of age. A leftward peak shift in the inhibition function by Tat treatment across all PPI trials (8–120 ms ISI) was further noted at 30 and 60 days of age [χ2 (1) = 4.7, p ≤ .03, and χ2 (1) = 5.3, p ≤ .02, respectively]. At 90 days of age, both saline and Tat-treated males exhibited prominent linear components [F(1, 7) = 85.6,p ≤ .01 and F(1, 7) = 16.9, p ≤ .01, respectively], indicating that the Tat-induced alterations early in development disappeared at 90 days of age. No inhibition function peak shift was noted at 90 days of age, supporting the diminishing effect across time and repeated testing. For females, orthogonal component analyses revealed no significant differences for treatment and no significant peak shift across ISIs, suggesting that the inhibition function of the peak amplitude response across ISIs was not altered by Tat in female rats at any age.
For the single test adult group, a 2(treatment) × 2(sex) × 4(PPI: 8, 40, 80, 120 ms ISI) mixed-model ANOVA conducted on peak ASR amplitude revealed a prominent linear PPI component [F(1, 23) = 128.0, p ≤ .01], and a significant quadratic sex × PPI effect [F(1, 23) = 5.2,p ≤ .03], supporting the findings of sex-dependent alterations in the peak inhibition function. Within each sex, separate orthogonal component analyses were conducted for the two treatment groups. Saline-treated males revealed a linear component [F(1,5) = 22.4, p ≤ .01], whereas Tat-treated males exhibited in addition to a linear component, a prominent quadratic component [F(1, 7) = 27.8, p ≤ .01], suggesting a Tat-induced alteration on the peak inhibition response across ISIs in males at 90 days of age. These latter observations contrast with the findings of the longitudinally tested male group that indicated the disappearance of the Tat-induced effects at 90 days of age. However, no significant peak shift by Tat across PPI trials (8–120 ms) was noted [χ2(1) = 0.9, p= .34]. For females, orthogonal component and peak shift analyses revealed no significant effects. Fig. 2 illustrates the peak ASR amplitude for males across PPI trials (8– 120 ms ISI) separate for treatment and age.
Fig. 2.
Mean (±S.E.M.) peak ASR amplitude across PPI trials (8–120 ms) for the longitudinally tested males, tested at 30, 60, and 90 days of age (group 1), and males in the single test adult group, tested only at 90 days of age (group 2), illustrated as a function of treatment and age. Group 1: most notable is the leftward peak shift in ISI for maximal inhibition of the response for Tat-injected males at 30 and 60 days of age [χ2 (1) = 4.7, p ≤ .03 and χ2 (1) = 5.3, p ≤ .02, respectively]. Group 2: no peak shift was noted but different response functions were noted for saline and Tat-treated males, as indicated by orthogonal component analyses.
3.2.2.2. Peak ASR latency
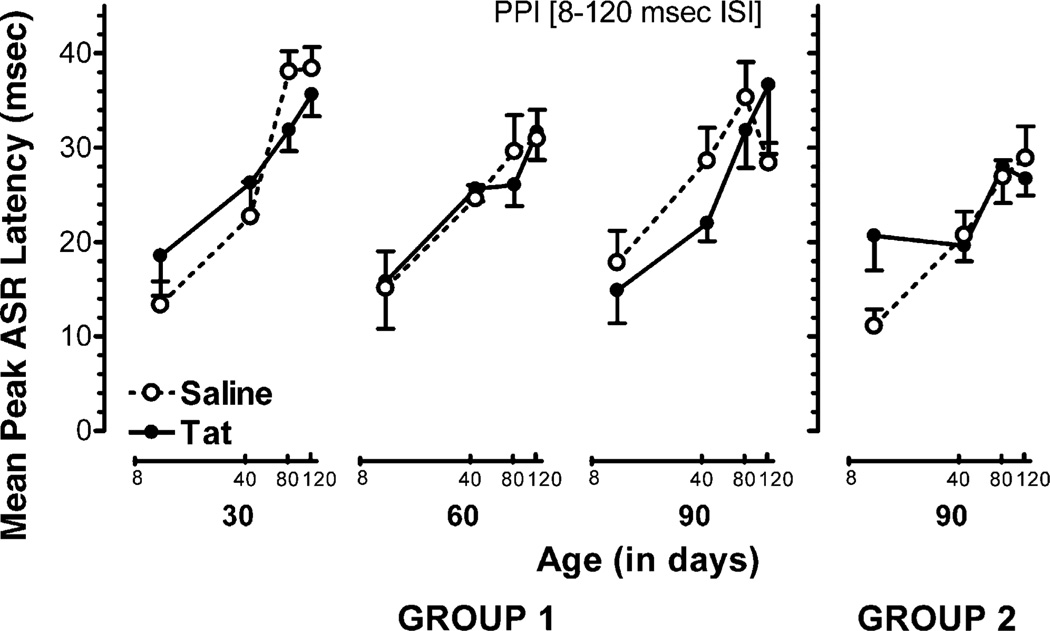
A 2(treatment) × 2(sex) × 3(age: 30, 60, 90 days of age) × 4(PPI: 8, 40, 80, 120 ms ISI) mixed-model ANOVA conducted on peak ASR latency revealed significant main effects for sex [F(1, 23) = 4.2, p ≤ .05], a prominent quadratic age component [F(1, 23) = 15.5,p ≤ .01], and a prominent linear PPI component [F(1, 23) = 375.3, p ≤ .01], indicating that each of the factors, sex, age, and ISIs, significantly altered PPI. The two-way analyses revealed a significant linear PPI × sex interaction [F(1, 23) = 4.7,p ≤ .04] and a linear PPI × age interaction [F(1, 23) = 5.8, p ≤ .03] that was significantly altered by treatment, revealing a significant linear four-way PPI × treatment × sex × age interaction [F(1, 23) = 8.9, p ≤ .01], further indicating that the Tat-induced alteration in the inhibition response was expressed differently with age in females and males. Further specification of these PPI alterations was provided with orthogonal component analyses, conducted separately for both treatment groups within each age and sex. For males, prominent linear components at all three days of age were noted for saline-treated rats [30 days of age: F(1, 7) = 172.8,p ≤ .01; 60 days of age: F(1, 7) = 62.7,p ≤ .01; 90 days of age: F(1, 7) = 17.7, p < .01] as well as for Tat-treated animals [30 days of age: F(1, 7) = 41.0,p ≤ .01; 60 days of age: F(1, 7) = 18.4,p ≤ .01; 90 days of age: F(1, 7) = 20.0,p ≤ .01]. Planned contrasts on the linear components revealed no significant treatment × PPI interactions for any age group [for all three age groups: F(1, 14) < 1.0], suggesting that the inhibition function of the latency response across ISIs was not altered by Tat in male rats at any age. For females, 30- and 60-day-old saline-treated animals revealed in addition to prominent linear components, quadratic components [30 days of age: F(1, 5) = 7.0,p ≤ .05; 60 days of age: F(1, 5) = 10.0,p ≤ .03] and at 90 days of age a prominent quadratic component [F(1, 5) = 22.1, p ≤ .01], but in contrast, Tat-treated females revealed only linear components [30 days of age: F(1, 5) = 32.3,p ≤ .02; 60 days of age: F(1, 5) = 10.9, p ≤ .02; 90 days of age: F(1, 5) = 18.9, p ≤ .01], suggesting a Tat-induced alteration across ISIs in females at all ages. Planned contrasts on the linear components revealed significant treatment × PPI interactions for 30 days old rats [F(1, 10) = 4.9, p ≤ .05], but not for 60 and 90 days old females, supporting Tat-induced alterations that are most profound at 30 days of age. Overall the Tat-induced alterations on the latency inhibition response across ISIs for females contrast the findings reported for male rats that did not reveal significant Tat effects on the latency inhibition response across ISIs at any age.
For the single test adult group, a 2(treatment) × 2(sex) × 4(PPI: 8, 40, 80, 120 ms ISI) mixed-model ANOVA conducted on peak ASR latency revealed a prominent linear PPI component [F(1, 23) = 122.4, p ≤ .01], and a significant sex × PPI interaction [F(3, 69) = 3.5,p < .02], further indicating PPI was expressed differently for females and males. Separate orthogonal component analyses were conducted for treatment within each sex. No orthogonal component differences were noted for males, supporting the lack of a Tat effect for males on the latency inhibition response. Saline-treated females revealed a linear component [F(1, 7) = 25.8, p ≤ .01], in contrast to Tat-treated females that did not show any significant orthogonal components, supporting a Tat-induced alteration across ISIs in females. Fig. 3 illustrates the peak ASR latency for females across PPI trials (8–120 ms ISI) separate for treatment and age.
Fig. 3.
Mean (±S.E.M.) peak ASR latency across PPI trials (8–120 ms) for the longitudinally tested females, tested at 30,60, and 90 days of age (group 1), and females in the single test adult group, tested only at 90 days of age (group 2), illustrated as a function of treatment and age. Group1: most notable is the significant flattening in the inhibition response in Tat-treated females across PPI trials (8–120 ms) at 30 days of age. At 60 and 90 days of age, Tat significantly altered the inhibition response latency curves, as indicated by orthogonal component analyses. Group 2: orthogonal component analyses revealed a significant flattening in the inhibition response in Tat-treated females across PPI trials (8–120 ms).
3.2.2.3. Percent PPI
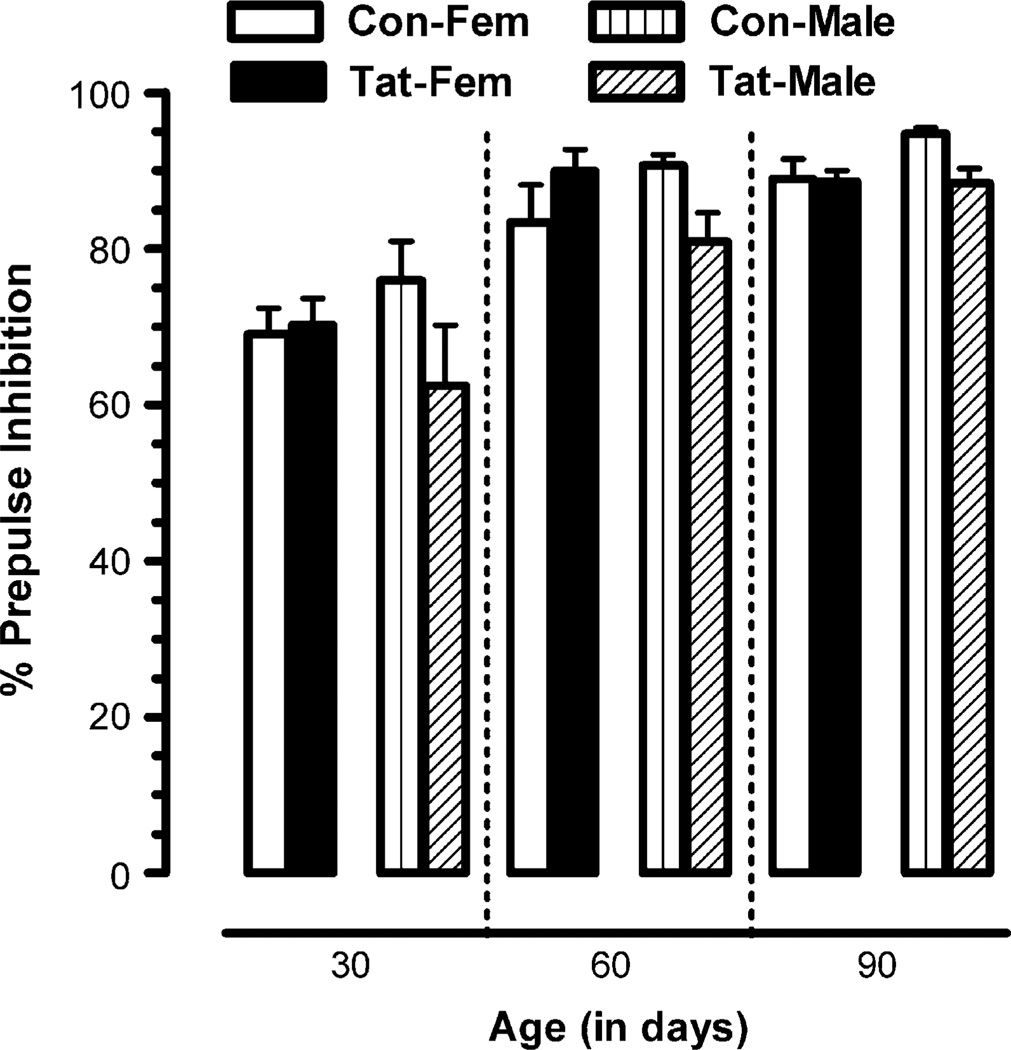
A 2(treatment) × 2(sex) × 3(age: 30, 60, 90 days of age) mixed-model ANOVA conducted on PPI of the startle amplitude for ISI at 100 ms revealed a prominent linear age effect [F(1, 23) = 50.1, p ≤ .01]. A significant treatment × sex interaction was noted [F(1,23) = 4.71,p ≤ .04], that was due to a Tat-induced significant reduction in inhibition for males [F(1, 23) = 6.8, p ≤ .02] but not for females [F(1, 23) ≤ 1.0] (Fig. 4).
Fig. 4.
Percent PPI of the startle amplitude (100 ms ISI) for the longitudinally tested group, tested at 30,60, and 90 days of age. Mean (±S.E.M.) for saline and Tat-treated animals across age, with a significant overall age effect and a significant treatment × sex effect.
A comparison of the single test adult group, tested only at 90 days of age, and the longitudinally repeated testing group at 90 days of age by conducting a 2(group) × 2(treatment) × 2(sex) mixed-model ANOVA revealed no significant group effect or/ and interaction.
3.2.3. Histology
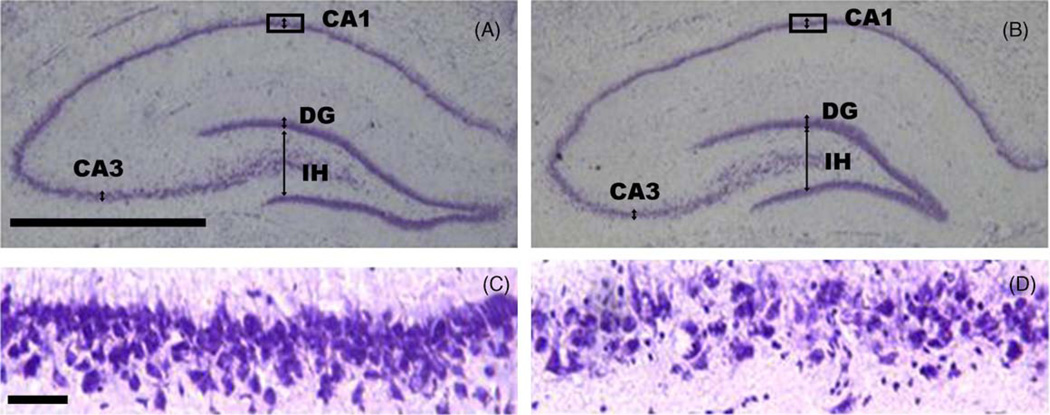
Analysis of Nissl-stained sections through the hippocampus confirmed placement of injection sites into the hippocampal dentate region. Saline-injected animals displayed little pathology, whereas in Tat-injected animals damage to specific regions of the hippocampal cell fields was noted 8 months after surgery (Fig. 5).
Fig. 5.
Nissl-stained hippocampal tissue sections through saline (A and C) and Tat-injected animals (B and D). The rectangles indicate enlargements shown in Panels C and D. Pyramidal cell fields were measured approximately at the arrows, in locations labeled CA1, and CA3. Granule cell fields were measured at the arrow in the superior blade of the dentate gyrus. The infra hilar width of the dentate gyrus was measured at the arrow in the dentate gyrus (IH). CA3, cornu ammonus 3 pyramidal cell fields; CA1, cornu ammonus 1 pyramidal cell fields; DG, granule cell fields of the superior blade of the dentate gyrus. Panels A and B: calibration bar = 1.0 mm; Panels C and D: calibration bar = 0.05 mm).
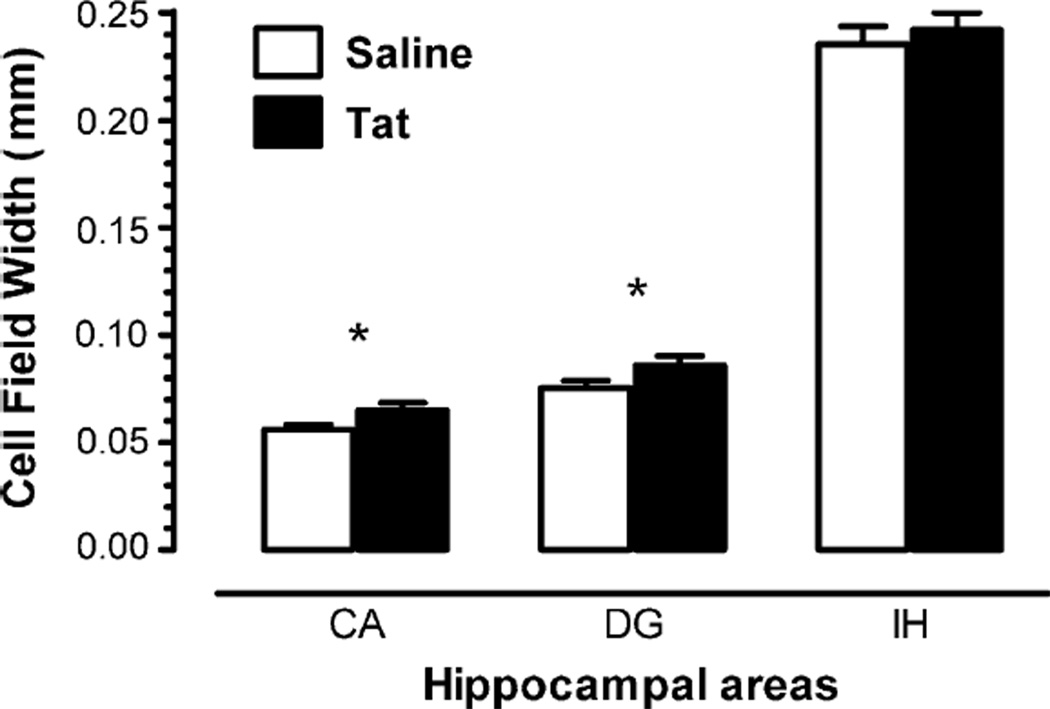
A MANOVA conducted on hippocampal morphometry revealed a significant treatment effect with increased width of cell fields in Tat-treated animals. The CA pyramidal cell fields [F(1, 46) = 4.0, p ≤ .05], and the dentate granule cell fields [F(1, 46) = 3.9, p ≤ .05] were both increased. In addition, the dentate granule cell fields revealed a significant sex effect [F(1, 46) = 6.5, p ≤ .01]. Males showed increased cell field widths compared to females (data not shown). No significant effect was noted for the infra hilar width of the dentate gyrus, indicating that the hippocampal width of the dentate gyrus itself was not affected, but more specifically, the width of the dentate granule cell fields was increased. Fig. 6 illustrates the morphometric measurements on the hippocampus for saline and Tat-treated animals.
Fig. 6.
Mean (±S.E.M.) hippocampal morphometric measurements (CA, CA pyramidal cell fields combining area CA1 and CA3; DG, granule cell fields of the superior blade of the dentate gyrus; IH, infra hilar width of the dentate gyrus (µm) for saline and Tat-treated rats). *p ≤ .05.
4. Discussion
The present experiment investigated the effects of Tat in neonatal male and female rats on sensorimotor gating, as measured by PPI. Tat was injected bilaterally into each hippocampus on P1. Behavioral testing examined PPI using the dependent measures peak ASR amplitude, peak ASR latency, and percent PPI. Tat injections in neonatal rats produced sex-dependent alterations in the pre-attentive processes of sensorimotor gating at 30 and 60 days of age, but only slight alterations at 90 days of age. Data collected from a second adult group that was tested only at 90 days of age, showed evidence for a Tat-induced long-term effect. Results suggest that repeated testing accounts for the diminishing Tat-induced effects on PPI at 90 days of age in the longitudinally tested animal group, rather than ‘recovery of function’ with aging into adulthood.
Focusing on the development of pre-attentive processes, these results indicate an age effect on ASR in both adaptation and control trials with alterations in peak and latency ASR responses. For the prepulse inhibition response, the data revealed a systematic reduction in maximal inhibition, suggesting developmental effects in pre-attentive filtering processes that were significantly altered by Tat.
The Tat-induced alteration in response inhibition was sex-specific. Males seemed to be more sensitive to Tat-induced alterations in peak ASR amplitude for the baseline ASR and the inhibition response. With respect to maximal response inhibition, Tat altered the peak amplitude across ISIs, as indicated by orthogonal component analyses. In addition, a leftward peak shift produced by Tat was evident in the ISI, especially early in development. In contrast, females revealed adverse Tat effects on the response inhibition latency curves across ISIs. There was a significant flattening of the latency curve in Tat-treated females, suggesting less modulation of PPI across ISIs that was most evident when first tested. These sex-dependent alterations on the characteristics of peak and latency response curves may reflect some basic properties of the way the nervous system deals with sensory input (Hoffman and Ison, 1980) and need to be further investigated. Further, a current study reported gender/hormonal effects that play a differential role in effects of drug abuse, HIV infection, and HAD (Kendall et al., 2005). It is speculated that sex-dependent alterations might be due to an interaction between steroids, such as estrogen and Tat, and should be considered in future research. Sex differences in virological and immunological markers of disease progression have been described among HIV-infected adults and children (European Collaborative Study, 2002; Sterling et al., 2001). Overall, the findings suggest that the pre-attentive filtering system of ASR, as indexed by PPI, was altered by Tat for males and females and mediated the ISI function of sensorimotor gating. This finding is supported by a previous study that examined intrahippocampal Tat injections in adult male rats (Fitting et al., 2006). This is especially important, since prepulse inhibition reflects a process that protects the organism from external events, information overload and other threats (Hoffman and Ison, 1980; Jovanovic et al., 2004; Swerdlow et al., 2000). Given the evidence that alterations in the event-related brain potential are among the earliest readily quantifiable alterations observed in the progression to HIV dementia (Castello et al., 1998; Fein et al., 1995), our findings suggest a striking parallel.
In the present study PPI was assessed by peak ASR amplitude and peak ASR latency across a range of ISIs (8–120 ms). The determination of ISI functions rather then percent PPI was employed as a theoretically more sensitive method to study alterations in PPI and may further elucidate the mechanisms underlying pre-attentional processes,as indexed by sensorimotor gating. When using percent PPI only one time point is assessed in a whole process. The usefulness of using a functional approach is the fact that it takes more than just one time point into account and thus, can describe a process much more completely than when using only one time point. In the paper we showed that percent PPI (at 100 ms ISI) did not exhibit any significant Tat-induced effects. The determination of ISI functions (i.e., 8– 120 ms ISIs) appeared more sensitive to Tat-induced alterations in PPI and ought to be considered for future research.
Hippocampal Tat injections also produced an alteration in morphometric measurements of the two examined types of hippocampal cell fields, the pyramidal cell fields in the CA areas and the granule cell fields of the superior blade of the dentate gyrus. The increased field width is most likely a consequence of disaggregation of the pyramidal cells induced by early cell loss following the neonatal Tat exposure. The occurrence and extent of an early hippocampal cell loss needs to be further investigated. Findings indicated that Tat did not alter the integrity of the infra hilar area of the dentate gyrus itself but altered the width of the examined hippocampal cell fields. It is of note that although the Tat injections were targeted to the dentate hilar region, little local damage was produced by the injection. These findings support previous studies indicating that Tat causes neuronal damage, synaptic alterations and glial activation in the hippocampal areas CA1, CA3/4 and the dentate (Behnisch et al., 2004; Bruce-Keller et al., 2003; Cheng et al., 1998; Maragos et al., 2003; Nath et al., 2000b). An in vivo study showed that large cortical neurons, interneurons in the hippocampus, and nigrostriatal fibers were preferentially lost, and thus, selective populations of cells were susceptible to viral protein-induced neurotoxicity (Nath et al., 2000a). The current findings support the concept of selectivity in Tat neurotoxicity in developing animals.
As noted in the introduction, it is well appreciated that HIV-1 infection is predominately an intrapartum event. Thus, from the perspective of an animal model with high face validity, one would employ virotoxin exposure proximal to the time of birth. Our experimental design dictated precisely such a treatment: P1 animals were treated with the virotoxins. However, as one might consider from the perspective of the brain development, the brain growth of P10 rat would more closely match that of human brain growth at birth. Thus it will also be important to ascertain the susceptibility of the developing rat brain at 10 days of age to virotoxin exposure. Which particular view one holds with respect to virotoxin timing as most important—timing the exposure on the basis of “birth” or “brain growth”, will then be directly addressed.
To date, there are no published behavioral studies examining effects of the viral protein gp120 on PPI. Neurotoxicity induced by the HIV protein gp120 has been reported to be mediated primarily by NMDA receptor mechanisms (Barks et al., 1997). Tat appears to be causing neurotoxicity via oxidative stress (Aksenov et al., 2003, 2006; Aksenova et al., 2005, 2006). A previous study examined neurobehavioral developmental effects of gp120 in neonatal rats by daily behavioral observations (Hill et al., 1993). It was reported that the developmental milestones which were unaffected by gp120 (i.e., not delayed) ‘tended to be measures related to physical or sensory development or reflexes, some of which may be mediated by simple reflex circuits’ (Hill et al., 1993). The authors argued that the behaviors which were retarded by gp120 treatment involved more complex motor skills. However, it is important to note that the authors examined the day of onset of these behaviors, rather than the functional integrity of the behavior. Toward this end, further research in our laboratory will examine whether intrahippocampal gp120 injections in neonatal rats cause the same effects in sensorimotor gating as the HIV protein Tat, as indexed by PPI.
Acknowledgements
The authors gratefully thank Dr. Avindra Nath for providing the HIV-1 Tat protein. Further, the authors thank Dr. Heidi M. Carman and Dr. Guanghan Wu for technical assistance. This work was supported by grants from the National Institute on Drug Abuse (DA13137, DA014401) and the National Institute of Child Health and Human Development (HD043680).
Contributor Information
Sylvia Fitting, Email: fitting@sc.edu.
Charles F. Mactutus, Email: mactutus@sc.edu.
References
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27(2):217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Cell culture models of oxidative stress and injury in the central nervous system. Curr. Neurovasc. Res. 2005;2(1):73–89. doi: 10.2174/1567202052773463. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci. Lett. 2006;395(3):235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Annunziata P. Blood-brain barrier changes during invasion of the central nervous system by HIV-1. Old and new insights into the mechanism. J. Neurol. 2003;250:901–906. doi: 10.1007/s00415-003-1159-0. [DOI] [PubMed] [Google Scholar]
- AVMA. Report of the AVMA panel of euthanasia. J. Am. Vet. Med. Assoc. 2001;218:671–696. doi: 10.2460/javma.2001.218.669. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Barks JDE, Liu X-H, Sun R, Silverstein FS. Gp120, a human immunodeficiency virus-1 coat protein, augments excitotoxic hippocampal injury in perinatal rats. Neuroscience. 1997;76(2):397–409. doi: 10.1016/s0306-4522(96)00373-9. [DOI] [PubMed] [Google Scholar]
- Behnisch T, Francesconi W, Sanna PP. HIV secreted protein Tat prevents long-term potentiation in the hippocampal CA1 region. Brain Res. 2004;1012:187–189. doi: 10.1016/j.brainres.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Belman AL, Diamond G, Dickson D, Horoupian D, Llena J, Lantos G, Rubinstein A. Pediatric acquired immunodeficiency syndrome. Am. J. Dis. Child. 1988;142:29–35. doi: 10.1001/archpedi.1988.02150010039017. [DOI] [PubMed] [Google Scholar]
- Belman AL. Infants, children, and adolescents. In: Berger JR, Levy RM, editors. AIDS and the Nervous System. 2nd ed. Lippincott-Raven Publishers; 1997. pp. 223–253. [Google Scholar]
- Blanche S, Mayaux M-J, Rouziox C, Teglas J-P, Firtion G, Monpoux F, Cicaru-Vigneron N, Meier F, Tricoire J, Courpotin C, Vilmer E, Griscelli C, Delfraissy J-F. Relation of the course of HIV infection in children to the severity of the disease in their mothers at delivery. N. Engl. J. Med. 1994;330(5):308–312. doi: 10.1056/NEJM199402033300502. [DOI] [PubMed] [Google Scholar]
- Brew BJ, Rosenblum M, Price RW. AIDS dementia complex and primary HIV brain infection. J. Neuroimmunol. 1988;20:133–140. doi: 10.1016/0165-5728(88)90144-0. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brains. J. Neurosci. 2003;23(23):8417–8442. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Geyer MA, Swerdlow NR. Carbachol infusion into the dentate gyrus disrupts sensorimotor gating of the startle reflex in rats. Psychopharmacology. 1991;105(3):347–354. doi: 10.1007/BF02244429. [DOI] [PubMed] [Google Scholar]
- Caine SB, Humby T, Robbins TW, Everitt BJ. Behavioral effects of psychomotor stimulants in rats with dorsal or ventral subiculum lesions: locomotion, cocaine self-administration, and prepulse inhibition of startle. Behav. Neurosci. 2001;115(4):880–894. doi: 10.1037//0735-7044.115.4.880. [DOI] [PubMed] [Google Scholar]
- Catani MV, Corasaniti MT, Ranalli M, Amantea D, Litovchick A, Lapidot A, Melino G. The Tat antagonist neomycin B hexa-arginine conjugate inhibits gp-120-induced death of human neuroblastoma cells. J. Neurochem. 2003;84:1237–1245. doi: 10.1046/j.1471-4159.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- Chearskul S, Chotpitayasunondh T, Simonds RJ, Wanprapar N, Waranawat N, Punpanich W, Chokephaibulkit K, Mock PA, Neeyapun K, Jetsawang B, Teeraratkul A, Supapol W, Mastro TD, Shaffer N. Survival, disease manifestations, and early predictors of disease progression among children with perinatal human immunodeficiency virus infection in Thailand. Pediatrics. 2002;110(2):e25. doi: 10.1542/peds.110.2.e25. [DOI] [PubMed] [Google Scholar]
- Castello E, Baroni N, Pallestrini E. Neurotological auditory brain stem response findings in human immunodeficiency virus-positive patients without neurologic manifestations Otolol. Ann. Rhinol. Laryngol. 1998;107:1054–1060. doi: 10.1177/000348949810701210. [DOI] [PubMed] [Google Scholar]
- Cheng J, Nath A, Knudsen B, Hochman S, Geiger JD, Ma M, Magnuson DSK. Neuronal excitatory properties of human immunodeficiency virus type 1 TAT protein. Neuroscience. 1998;82(1):97–106. doi: 10.1016/s0306-4522(97)00174-7. [DOI] [PubMed] [Google Scholar]
- Da Cunha A, Mintz M, Eiden LE, Sharer LR. A neuronal and neuroanatomical correlate of HIV-1 encephalopathy relative to HIV-1 encephalitis in HIV-1-infected children. J. Neuropathol. Exp. Neurol. 1997;56(9):974–987. doi: 10.1097/00005072-199709000-00003. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Sharer LR, Oleske JM, Connor EM, Goudsmit J, Bagdon L, Robert-Guroff M, Koenigsberger MR. Neurologic manifestations of human immunodeficiency virus infection in children. Pediatrics. 1986;78:678–687. [PubMed] [Google Scholar]
- Epstein LG, Sharer LR, Goudsmit J. Neurological and neuropathological features of human immunodeficiency virus infection in children. Ann. Neurol. 1988;23(Suppl.):S19–S23. doi: 10.1002/ana.410230709. [DOI] [PubMed] [Google Scholar]
- European Collaborative Study. Level and pattern of HIV-1-RNA viral load over age: differences between girls and boys? AIDS. 2002;16(1):97–104. doi: 10.1097/00002030-200201040-00012. [DOI] [PubMed] [Google Scholar]
- Fauci AS. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fein G, Biggins CA, MacKay S. Delayed latency of the event-related brain potential P3A Component in HIV disease. Progressive effects with increasing cognitive impairment. Arch. Neurol. 1995;52:1109–1118. doi: 10.1001/archneur.1995.00540350103022. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology. 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Intrahippocampal injections of Tat: effects on prepulse inhibition of the auditory startle response in adult rats, submitted for publication. 2006 doi: 10.1016/j.pbb.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;32:95–112. [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: some empirical findings and their implications for how the nervous system processes sensory input. Psychol. Rev. 1980;87(2):175–189. [PubMed] [Google Scholar]
- Hill JM, Mervis RF, Avidor R, Moody TW, Brenneman DE. HIV envelope protein-induced neuronal damage and retardation of behavioral development in rat neonates. Brain Res. 1993;603:222–233. doi: 10.1016/0006-8993(93)91241-j. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Szilagyi S, Chakravorty S, Fiallos AM, Lewison BJ, Parwani A, Schwartz MP, Gonzenbach S, Rotrosen JP, Duncan EJ. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology. 2004;41:401–406. doi: 10.1111/1469-8986.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Kendall SH, Anderson CF, Nath A, Turchan-Cholewo J, Land CL, Mactutus CF, Booze RM. Gonadal steroids differentially modulate neurotoxicity of HIV and cocaine: testosterone and ICI, 182, 780 sensitive mechanism. BioMed Central Neurosci. 2005;6:40–53. doi: 10.1186/1471-2202-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Schnitzler H-U. The acoustic startle response in rats— circuits mediating evocation, inhibition and potentiation. Behav. Brain Res. 1997;89(1–2):35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, Nath A. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience. 2003;117:43–53. doi: 10.1016/s0306-4522(02)00713-3. [DOI] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus CF, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J. Psychopharmacol. 2000a;14(3):222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann. Neurol. 2000b;47:186–194. [PubMed] [Google Scholar]
- Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart LR, Gendelman HE. Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis. Am. J. Pathol. 1999;155:1599–1611. doi: 10.1016/S0002-9440(10)65476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- SPSS. 2005 SPSS 14.0 for Windows 1989–2005 SPSS Inc. [Google Scholar]
- Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N. Engl. J. Med. 2001;344:720–725. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav. Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001a;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hanlon FM, Henning L, Kim YK, Gaudet I, Halim ND. Regulation of sensorimotor gating in rats by hippocampal NMDA: anatomical localization. Brain Res. 2001b;898:195–203. doi: 10.1016/s0006-8993(01)02143-6. [DOI] [PubMed] [Google Scholar]
- SYSTAT. SYSTAT 11 0 for Windows (1989–2004) SYSTAT Inc; 2004. [Google Scholar]
- UNAIDS/WHO. AIDS Epidemic Update. Geneva: UNAIDS; 2005. [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. [Google Scholar]