Abstract
Introduction
The issue of whether various drug-eluting stents (DES) provide similar benefit in diabetic patients with coronary artery disease remains unclear. The purpose of the study is to assess the clinical utility of the second-generation and first-generation DES in patients with diabetes mellitus by a meta-analysis.
Material and methods
A systematic literature search of PubMed, EMBASE, and Cochrane databases was conducted. We included randomized trials involving head-to-head comparison of clinical outcomes of second- versus first-generation DES in patients with a diagnosis of diabetes with at least 6-month follow-up data. Summary statistics were calculated using random-effects models.
Results
A total of 10 trials with 4503 patients were available for analysis. The pooled analyses showed that the second-generation everolimus-eluting stent (EES) significantly lowered all-cause mortality (risk ratio (RR) = 0.58, 95% CI: 0.37–0.90; p = 0.01) and the risk of stent thrombosis (RR = 0.46, 95% CI: 0.22–0.95; p = 0.03) compared with the first-generation sirolimus-eluting stents (SES) and the overall first-generation DES, respectively. Moreover, the EES showed a tendency toward reducing the incidence of recurrent myocardial infarction when compared with paclitaxel-eluting stents (PES) (RR = 0.58, p = 0.08). In contrast, the second-generation zotarolimus-eluting stents (ZES) were associated with increased rates of stent thrombosis and risk of target lesion revascularization in comparison with the SES (both p < 0.05) or the overall first-generation DES (both p < 0.05).
Conclusions
The second-generation EES are highly effective in reducing the risk of major cardiac events in diabetic patients with coronary artery disease.
Keywords: everolimus-eluting stents, zotarolimus-eluting stents, diabetes, meta-analysis
Introduction
Drug-eluting stents (DES) have become the most widely used coronary stents in clinical practice [1]. A number of clinical trials have verified the benefits of DES in reducing the rates of in-stent restenosis or target lesion revascularization (TLR) compared with bare metal stents in unselected patients with coronary artery diseases [2–4]. Patients with diabetes are especially prone to restenosis after stenting, making DES preferable to bare-metal stents in this patient population [5]. Currently, diabetic patients make up approximately 25% of those treated with DES [6]. A pooled analysis from the SPIRIT and COMPARE trials showed an interaction between diabetes mellitus and stent type on clinical outcomes [7]. Several meta-analyses have previously reported the efficacy and safety of the first-generation sirolimus-eluting stents (SES) versus bare-metal stents [8] or the first-generation paclitaxel-eluting stents (PES) [9] in patients with diabetes mellitus. However, it remains unclear whether the second-generation DES (e.g. everolimus-eluting stents (EES) or zotarolimus-eluting stents (ZES)) and the first-generation DES are able to provide a similarly beneficial effect in these specific subjects.
Therefore, here we performed a meta-analysis of randomized controlled trials (RCTs) comparing the second- versus first-generation DES to elucidate the clinical utility of various DES in patients with diabetes mellitus.
Material and methods
Search strategy
Eligible studies were identified through a computerized literature search of PubMed, EMBASE, and Cochrane databases until July 2013. Complex search strategies were formulated using the following text words: everolimus-eluting stent, zotarolimus-eluting stent, second-generation eluting stent, sirolimus-eluting stent, paclitaxel-eluting stent, first-generation eluting stent, diabetes, diabetic, human, random. An extensive search of the ISI Web of Science database using cross-references from the eligible articles and relevant reviews was also conducted. The search was restricted to English-language literature.
Selection criteria
Randomized controlled trials involving head-to-head comparison of clinical outcomes of second-generation DES (EES or ZES) versus first-generation DES (SES or PES) in patients with a diagnosis of diabetes were eligible for the meta-analysis. Moreover, more than 6-month follow-up data were required to be reported. We excluded studies that compared clinical utility of DES with BMS, and post-hoc analyses of RCTs were also excluded.
Study enrollment and data extraction
Two investigators independently reviewed all citations to identify the eligible studies and used a standardized form to extract the data including characteristics of study, participant, and procedure characteristics as well as follow-up duration from each study. Clinical outcomes of all-cause death, stent-thrombosis, reinfarction, and TLR were also recorded. The reviewers resolved differences through consensus, and the principal investigators resolved any disagreements. Quality of eligible articles was evaluated with a quality scale (a 5-point scale) by Jadad et al. [10].
Statistical analysis
The Mantel-Haenszel method for random effects was used to investigate the combined results of clinical endpoints in individual studies. Risk ratios (RR) with 95% confidence intervals (CI) for all results were computed as summary estimates.
Statistical heterogeneities across studies were quantified using the I2 statistic [11]. To investigate the clinical factors impacting clinical outcomes, we stratified and analyzed data on TLR and stent thrombosis according to the type of DES, stent length, dual antiplatelet therapy (DAPT) duration, and follow-up period. Sensitivity analyses were conducted to examine the robustness of the effect by alternatively using a fixed-effect model. We qualitatively assessed publication bias using the funnel plot method. The significance level was set at p < 0.05. Analyses were performed with the RevMan 5.1 software (The Cochrane Collaboration, Copenhagen, Denmark). The meta-analysis was prepared according to the PRISMA guidelines [12].
Results
Selected studies and characteristics
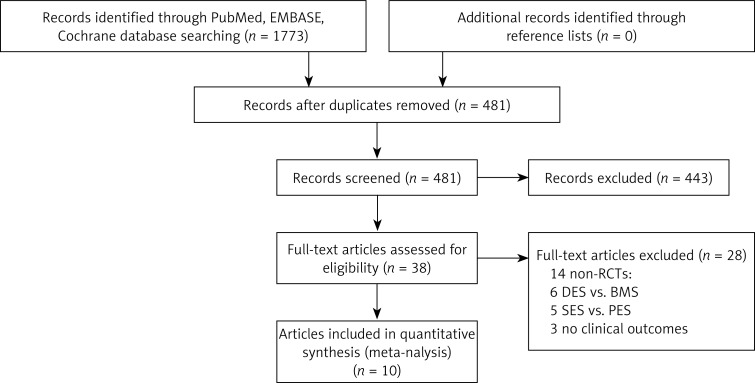
The initial electronic database search identified 1773 items. Of them 10 articles [13–22] were eligible for inclusion in the analysis, and no additional relevant study was identified from the references and citations of the eligible articles and review articles (Figure 1).
Figure 1.
Flowchart of selection of studies for inclusion
BMS – bare-metal stents, DES – drug-eluting stents, PES – paclitaxel-eluting stents, RCTs – randomized controlled trials, SES – sirolimus-eluting stent.
Table I summarizes the design features of the individual studies. A total of 4503 diabetic patients, 2350 being randomly allocated to the second-generation DES implantation group and 2153 to the first-generation DES implantation group, were included for analysis. They received DAPT for no less than 6 to 12 months according to current practice guidelines or study design protocol. The mean age of patients ranged from 62.9 to 68.1 years, and the percentage of males was from 57% to 74.5%. Total stent length per patient was less than 30.0 mm except for the ZEST Diabetic study (37.6 mm) [20] and the mean diameter of reference vessels ranged from 2.67 mm to 3.20 mm. Among the 10 included trials, 7 and 8 reported a lower percentage of insulin use [13, 14, 16–18, 21, 22] and glycoprotein IIb/IIIa inhibitor use [13–15, 17–21], respectively. In addition, of these trials, one reported 10-month follow-up data [17]; 4 reported 12-month data [13, 15, 16, 21]; 2 reported 18-month results [14, 19]; and 3 reported ≥ 24-month findings [18, 20, 22]. The level of evidence for each article was graded with a score of 3 to 4 according to the Jadad quality score (Table I).
Table I.
Baseline characteristics of randomized controlled trials included in the meta-analysis
| Study name, year | Comparisons | No. enrolled | Mean age [years] | Male (%) | Current smoker (%) | Insulin use (%) | ACS (%) | Target vessel, LAD/LCX/RCA (%) | Reference diameter [mm] | Stent length[mm] | DAPT duration[m] | Use of GP IIb/IIIa inhibitors (%) | Follow-up [m] | Jaded score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESSENCE-DIABETES, 2011 | EES vs. SES | 149/151 | 63.3 | 59 | 24 | 15 | 41.7 | 60/15.3/24.7 | 2.77 | 28.7 | 12 | 3 | 12 | 4 |
| ISAR-TEST-4 diabetic, 2013 | EES vs. SES | 184/193 | 68.1 | 74.5 | 14.2 | 32.5 | 40.3 | 45/27/28 | – | – | 6 | – | 36 | 4 |
| SORT OUT IV, 2012 | EES vs. SES | 194/196 | 63.6 | 74.4 | 26 | 32.1 | 34.4 | 38.5/25.2/33.3 | 3.2 | 29.6 | 12 | 17.1 | 18 | 3 |
| SPIRIT IV, 2010 | EES vs. PES | 786/399 | 63.4 | 63.3 | 18.2 | – | 29.1 | 39.6/26.9/33.6 | 2.74 | 21.75 | 12 | 18.3 | 12 | 4 |
| SPIRIT V Diabetic, 2012 | EES vs. PES | 218/106 | 65.5 | 69 | 16.3 | 28.5 | 52.4 | 42.7/29.3/28 | – | – | 6 | – | 12 | 3 |
| Naples-Diabetes, 2011 | ZES vs. SES vs. PES | 75/76/75 | 64 | 57 | 18.6 | 27 | 14.1 | 61.5/23.2/21.4 | 2.84 | 25 | 6–12 | 31.7 | 36 | 3 |
| ZEST Diabetic, 2012 | ZES vs. SES vs. PES | 268/247/245 | 62.9 | 60.8 | 24.9 | – | 52.9 | 49.4/21.6/29 | – | 37.6 | 12 | 2.2 | 24 | 3 |
| SORT OUT III, 2011 | ZES vs. SES | 169/168 | 66 | 71 | 29 | – | 44.5 | 40/26/31 | – | 24 | 12 | 22 | 18 | 4 |
| DiabeDES III, 2011 | ZES vs. SES | 66/61 | 63.2 | 72.8 | 34.5 | 27.3 | 38.6 | 41/15.5/43.5 | 2.86 | 21.4 | 12 | 23.6 | 10 | 4 |
| ENDEAVOR IV, 2009 | ZES vs. PES | 241/236 | 64 | 60.6 | – | 43.2 | 52.5 | 39.5/29/31.5 | 2.67 | 20.8 | 12 | 24.5 | 12 | 4 |
ACS – acute coronary syndrome, DAPT – dual antiplatelet therapy, EES – everolimus-eluting stents, LAD – left anterior descending artery, LCX – left circumflex artery, NA – not available, PES – paclitaxel-eluting stents, RCA – right coronary artery, STEMI – ST-segment elevation myocardial infarction, TIMI – thrombolysis in myocardial infarction, SES – sirolimus-eluting stents, ZES – zotarolimus-eluting stent
Meta-analysis for stent thrombosis
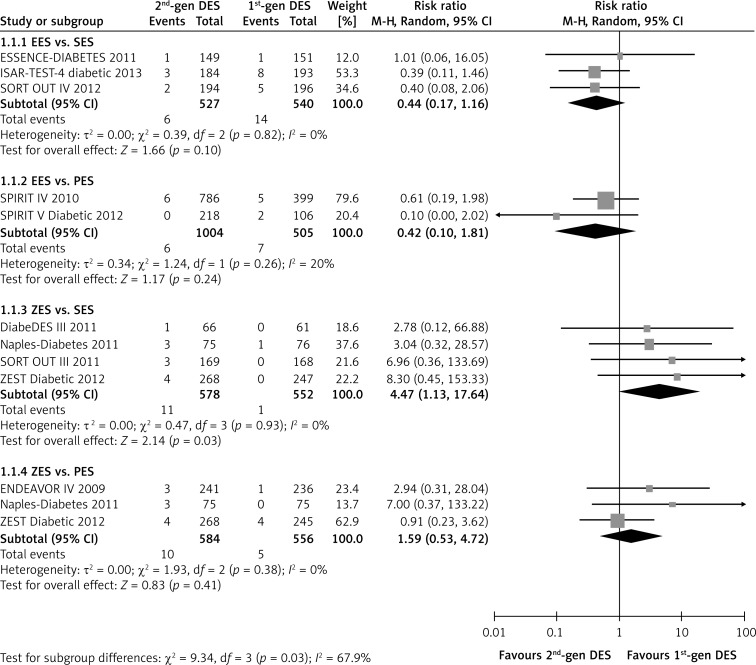
The pooling analyses showed that there was no significant difference in the risk of probable/definite stent thrombosis between the second-generation EES and the first-generation SES or PES (EES vs. SES: RR = 0.44, p = 0.10, I2 =0%; EES vs. PES: RR = 0.42, p = 0.24, I2 = 20%), whereas EES presented a significant benefit compared with the overall first-generation DES (RR = 0.46; p = 0.03; Table II). In contrast, the second-generation ZES markedly increased the incidence of adverse outcome compared with the first-generation SES (RR = 4.47, 95% CI: 1.13–17.64; p = 0.03; I2 = 0%, Figure 2), and the unfavorable effect of ZES was also observed when compared with the overall first-generation DES (RR = 2.91, 95% CI: 1.14–7.43, p = 0.03, Table II). In addition, the neutral inter-group effect was observed consistently in other subgroups, regardless of implanted stent length, DAPT duration, and follow-up duration (all p > 0.10, Table II).
Table II.
Subgroup analyses based on the data on TLR and stent thrombosis
| Factors | TLR | Stent thrombosis | ||||
|---|---|---|---|---|---|---|
| No. of studies | RR (95% CI) | Value of p | No. of studies | RR (95% CI) | Value of p | |
| EES implantation | 5 | 0.84 (0.52, 1.34) | 0.46 | 5 | 0.46 (0.22, 0.9) | 0.03 |
| ZES implantation | 5 | 2.37 (1.24, 4.52) | 0.009 | 5 | 2.91 (1.14, 7.43) | 0.03 |
| Stent length < 25.0 mm | 4 | 1.86 (0.69, 5.00) | 0.22 | 4 | 1.40 (0.45, 4.35) | 0.56 |
| Stent length ≥ 25.0 mm | 4 | 1.07 (0.39, 2.98) | 0.90 | 4 | 1.34 (0.45, 3.97) | 0.60 |
| DAPT duration < 12 months | 3 | 1.93 (0.86, 4.34) | 0.11 | 3 | 0.89 (0.17, 4.63) | 0.89 |
| DAPT duration = 12 months | 7 | 1.21 (0.62, 2.34) | 0.57 | 7 | 1.08 (0.55, 2.12) | 0.83 |
| 10-month follow-up | 1 | 2.78 (0.12, 66.88) | 0.52 | 1 | 2.78 (0.12, 66.88) | 0.53 |
| ≤ 12-month follow-up | 5 | 1.10 (0.72, 1.68) | 0.65 | 5 | 0.79 (0.32, 1.93) | 0.61 |
| > 12-month follow-up | 5 | 1.93 (0.91, 4.08) | 0.09 | 5 | 1.27 (0.49, 3.29) | 0.62 |
CI – confidence interval, DAPT – dual antiplatelet therapy, EES – everolimus-eluting stents, RR – risk ratio, TLR – target lesion revascularization, ZES – zotarolimus-eluting stent
Figure 2.
Forest plot of risk ratios in stent thrombosis in patients treated with second-generation DES compared with first-generation DES
CI – confidence intervals, DES – drug-eluting stents, MH – Mantel-Haenszel method.
Meta-analysis for target lesion revascularization
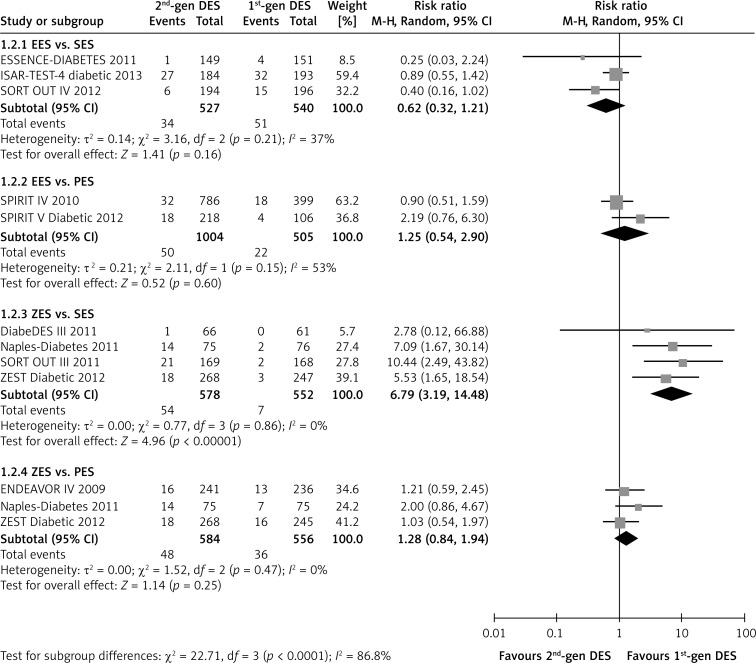
A benefit associated with EES implantation on reducing the incidence of TLR was not observed (RR = 0.62, p = 0.16; I2 = 37%, Figure 3). However, the use of ZES significantly increased the need for repeat revascularization in comparison with the SES (RR = 6.79, 95% CI: 3.19–14.48; p < 0.01; I2 = 0%, Figure 3) or the overall first-generation DES (RR = 2.37, 95% CI: 1.24–4.52; p = 0.009, Table II). Additionally, the inferiority of the second-generation DES seemed to be marked in diabetic patients with follow-up duration of more than 12 months (p = 0.09, Table II). Nevertheless, compared with the first generation PES, both EES and ZES did not show a significant difference in the rate of TLR (both p > 0.10, Figure 3).
Figure 3.
Forest plot of risk ratios in target lesion revascularization in patients treated with second-generation DES compared with first-generation DES. Abbreviations as in Figure 2
Meta-analysis for recurrent myocardial infarction and all-cause death
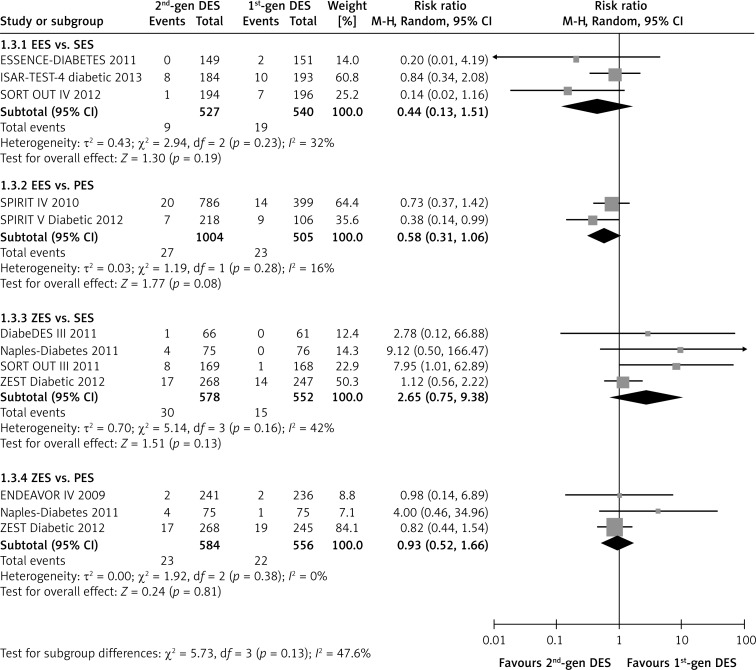
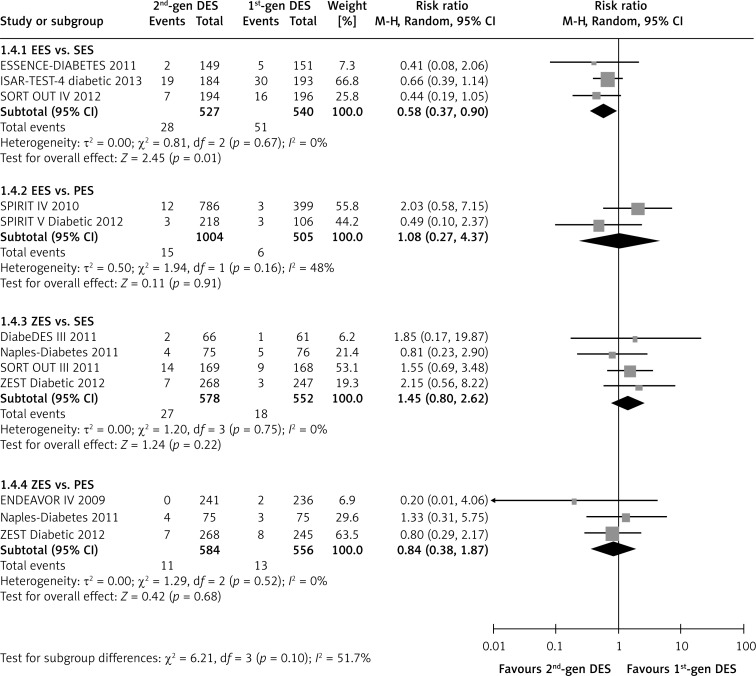
The second-generation EES did not present a benefit in reducing the risk of recurrent myocardial infarction compared with the first-generation SES (RR = 0.44, p = 0.19; I2 = 32%), but showed a beneficial tendency compared with PES (RR = 0.58, 95% CI 0.31–1.06; p = 0.08; I2 = 16%, Figure 4). However, there was no statistically significant difference between ZES and SES or PES (ZES vs. SES: RR = 2.65, p = 0.13; I2 = 42%; ZES vs. PES: RR = 0.93, p = 0.81; I2 = 16%; Figure 4). In addition, the use of EES was associated with reduced incidence of all-cause death compared with SES in patients with diabetes (RR = 0.58, 95% CI: 0.37–0.90; p = 0.01; I2 = 0%; Figure 5). Except for this, no significant inter-group differences were found (all p > 0.10, Figure 5).
Figure 4.
Forest plot of risk ratios in reinfarction in patients treated with second-generation DES compared with first-generation DES. Abbreviations as in Figure 2
Figure 5.
Forest plot of risk ratios in all-cause death in patients treated with second-generation DES compared with first-generation DES. Abbreviations as in Figure 2
Sensitivity analysis and publication bias
In the sensitivity analyses, after alternatively using the fixed-effect model, the pooled estimate of ZES versus SES on recurrent myocardial infarction became statistically significant (RR = 1.82, p = 0.04, I2 = 42%). Except for the process, other sensitivity analyses did not show any relevant influence on the overall results, which further confirmed in direction and magnitude all the findings in the present study. Funnel plots were performed for all outcomes, and essential symmetries regarding overall stent thrombosis, TLR, recurrent myocardial infarction, and all-cause death were found, suggesting no publication bias in the meta-analysis.
Discussion
The meta-analysis revealed that the second-generation EES significantly lowered the incidence of all-cause death compared with the first-generation SES and showed a tendency toward reducing recurrent myocardial infarction when compared with the PES. Moreover, the EES seemed likely to be more beneficial in lowering stent thrombosis than the overall first-generation DES. In contrast, the second-generation ZES were associated with an increased rate of stent thrombosis and TLR in comparison with the SES or the overall first-generation DES. Additionally, there were no significant differences in these outcomes between other comparisons of various DES.
Diabetes was a major predictor of restenosis secondary to exaggerated intimal hyperplasia in patients undergoing percutaneous coronary intervention (PCI) [23]. Previous clinical studies and meta-analyses demonstrated the benefit of the first-generation DES (SES or PES) in reducing late luminal loss and the need for repeat revascularization in patients with diabetes mellitus compared with BMS. The newer second-generation DES, especially EES, appeared to be likely to improve further the clinical outcomes in unclassified coronary artery diseases [24–26]. However, the clinical value of the second-generation DES in diabetic patients remains unclear. A study by Sakata et al. indicated that diabetes mellitus and non-diabetes mellitus lesions showed a similar in-stent vessel response, which was detected using 3D intravascular ultrasound technique, regardless of the DES type [27]. Of note, the drug used in the eluting stent, such as paclitaxel, could significantly attenuate the release of soluble vasoconstrictors (e.g. serotonin or thromboxane B2) that contribute to microvascular impairment. Such acute downstream vascular paralysis was beneficial in preventing the no-reflow phenomenon in patients undergoing stenting [28]. Recently a mixed treatment comparison analysis demonstrated that among the currently used DES, EES was the most efficacious and safe in diabetic patients in terms of reducing the need for repeat revascularization and the incidence of stent thrombosis [29]. The results support the overall clinical outcomes in the current study, which found lower rates of stent thrombosis, recurrent myocardial infarction, or all-cause death in patients treated with the EES.
There were relative differences among the DES in terms of efficacy and safety. In our analyses, the second-generation ZES were associated with higher rates of TLR and stent thrombosis compared with the SES or the overall first-generation DES. The SORT OUT III diabetes study indicated that treatment with ZES compared to SES resulted in a higher major adverse cardiac event rate in diabetic and nondiabetic patients [19]. The inter-group difference was mainly driven by a higher rate of TLR owing to increased intima hyperplasia in the ZES group [17]. Patients with diabetes develop a diffuse and rapidly progressive form of atherosclerosis, which increases the likelihood of requiring revascularization procedures [30, 31]. Moreover, diabetes promotes endothelial dysfunction and abnormalities in platelet activity and blood coagulation as well as increasing the risk of coronary thrombosis [32]. However, in these specific subsets of patients with high risk of restenosis and stent thrombosis, the second-generation ZES showed inferiority in lowering these clinical outcomes to the first-generation DES, especially the SES. The finding was consistent with the results from a large-scale network meta-analysis on unselected coronary artery diseases [33].
Methodologically, the use of a random-effect model, no publication bias, and relatively low statistical heterogeneities among the included trials might ensure the robustness of conclusions from the current study. Due to the limited study number and sample size, the results of the subgroup analyses were not solid enough, so they should be interpreted with caution. Therefore, more large-scale studies are required to further verify the findings and conclusions in the subgroup analyses of the current study.
In conclusion, this study has evaluated the current evidence from RCTs comparing clinical outcomes of the second- versus first-generation DES in patients with diabetes mellitus. Compared with the first-generation SES, the second-generation EES resulted in a significant decrease in all-cause mortality. The EES also showed a beneficial effect on reducing the incidence of reinfarction and stent thrombosis in comparison with the PES and the overall first-generation DES, respectively. Conversely, the use of the second-generation ZES was associated with increased risk of TLR and stent thrombosis compared with the first-generation DES, especially the SES. On the basis of these observations, the EES should be preferentially recommended in patients with diabetes mellitus while undergoing PCI.
References
- 1.Derkacz A, Protasiewicz M, Poręba R, et al. Plasma asymmetric dimethylarginine predicts restenosis after coronary angioplasty. Arch Med Sci. 2011;7:444–8. doi: 10.5114/aoms.2011.23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone GW, Ellis SG, Cannon L, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294:1215–23. doi: 10.1001/jama.294.10.1215. [DOI] [PubMed] [Google Scholar]
- 3.Sabate M, Cequier A, Iniguez A, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482–90. doi: 10.1016/S0140-6736(12)61223-9. [DOI] [PubMed] [Google Scholar]
- 4.Veselka J, Čadová P, Adla T, et al. Dual-source computed tomography angiography and intravascular ultrasound assessment of restenosis in patients after coronary stenting for bifurcation left main stenosis: a pilot study. Arch Med Sci. 2012;8:455–61. doi: 10.5114/aoms.2012.29220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeng M, Jensen LO, Kaltoft A, et al. Comparison of stent thrombosis, myocardial infarction, and mortality following drug-eluting versus bare-metal stent coronary intervention in patients with diabetes mellitus. Am J Cardiol. 2008;102:165–72. doi: 10.1016/j.amjcard.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 6.Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes. Part II: recent advances in coronary revascularization. J Am Coll Cardiol. 2007;49:643–56. doi: 10.1016/j.jacc.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 7.Stone GW, Kedhi E, Kereiakes DJ, et al. Differential clinical responses to everolimus-eluting and Paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124:893–900. doi: 10.1161/CIRCULATIONAHA.111.031070. [DOI] [PubMed] [Google Scholar]
- 8.de Waha A, Dibra A, Kufner S, et al. Long-term outcome after sirolimus-eluting stents versus bare metal stents in patients with diabetes mellitus: a patient-level meta-analysis of randomized trials. Clin Res Cardiol. 2011;100:561–70. doi: 10.1007/s00392-010-0278-8. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F, Dong L, Ge J. Meta-analysis of five randomized clinical trials comparing sirolimus- versus paclitaxel-eluting stents in patients with diabetes mellitus. Am J Cardiol. 2010;105:64–8. doi: 10.1016/j.amjcard.2009.08.652. [DOI] [PubMed] [Google Scholar]
- 10.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 11.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–6. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim WJ, Lee SW, Park SW, et al. Randomized comparison of everolimus-eluting stent versus sirolimus-eluting stent implantation for de novo coronary artery disease in patients with diabetes mellitus (ESSENCE-DIABETES): results from the ESSENCE-DIABETES trial. Circulation. 2011;124:886–92. doi: 10.1161/CIRCULATIONAHA.110.015453. [DOI] [PubMed] [Google Scholar]
- 14.Jensen LO, Thayssen P, Junker A, et al. Comparison of outcomes in patients with versus without diabetes mellitus after revascularization with everolimus- and sirolimus-eluting stents (from the SORT OUT IV trial) Am J Cardiol. 2012;110:1585–91. doi: 10.1016/j.amjcard.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Kereiakes DJ, Cutlip DE, Applegate RJ, et al. Outcomes in diabetic and nondiabetic patients treated with everolimus- or paclitaxel-eluting stents: results from the SPIRIT IV clinical trial (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) J Am Coll Cardiol. 2010;56:2084–9. doi: 10.1016/j.jacc.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Grube E, Chevalier B, Guagliumi G, et al. The SPIRIT V diabetic study: a randomized clinical evaluation of the XIENCE V everolimus-eluting stent vs the TAXUS Liberte paclitaxel-eluting stent in diabetic patients with de novo coronary artery lesions. Am Heart J. 2012;163:867–75e1. doi: 10.1016/j.ahj.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Jensen LO, Maeng M, Thayssen P, et al. Late lumen loss and intima hyperplasia after sirolimus-eluting and zotarolimus-eluting stent implantation in diabetic patients: the diabetes and drug-eluting stent (DiabeDES III) angiography and intravascular ultrasound trial. EuroIntervention. 2011;7:323–31. doi: 10.4244/EIJV7I3A56. [DOI] [PubMed] [Google Scholar]
- 18.Briguori C, Airoldi F, Visconti G, et al. Novel approaches for preventing or limiting events in diabetic patients (Naples-diabetes) trial: a randomized comparison of 3 drug-eluting stents in diabetic patients. Circ Cardiovasc Interv. 2011;4:121–9. doi: 10.1161/CIRCINTERVENTIONS.110.959924. [DOI] [PubMed] [Google Scholar]
- 19.Maeng M, Jensen LO, Tilsted HH, et al. Outcome of sirolimus-eluting versus zotarolimus-eluting coronary stent implantation in patients with and without diabetes mellitus (a SORT OUT III Substudy) Am J Cardiol. 2011;108:1232–7. doi: 10.1016/j.amjcard.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 20.Jang SJ, Park DW, Kim WJ, et al. Differential long-term outcomes of zotarolimus-eluting stents compared with sirolimus-eluting and paclitaxel-eluting stents in diabetic and nondiabetic patients: two-year subgroup analysis of the ZEST randomized trial. Catheter Cardiovasc Interv. 2013;81:1106–14. doi: 10.1002/ccd.24603. [DOI] [PubMed] [Google Scholar]
- 21.Kirtane AJ, Patel R, O'Shaughnessy C, et al. Clinical and angiographic outcomes in diabetics from the ENDEAVORIV trial: randomized comparison of zotarolimus- and paclitaxel-eluting stents in patients with coronary artery disease. JACC Cardiovasc Interv. 2009;2:967–76. doi: 10.1016/j.jcin.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Kufner S, Byrne RA, Mehilli J, et al. Second-versus first-generation “Limus”-eluting stents in diabetic patients with coronary artery disease: A randomized comparison in setting of ISAR-TEST-4 trial. Catheter Cardiovasc Interv. 2013;82:E769–76. doi: 10.1002/ccd.24741. [DOI] [PubMed] [Google Scholar]
- 23.Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA. 2005;293:1501–8. doi: 10.1001/jama.293.12.1501. [DOI] [PubMed] [Google Scholar]
- 24.Kedhi E, Joesoef KS, McFadden E, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375:201–9. doi: 10.1016/S0140-6736(09)62127-9. [DOI] [PubMed] [Google Scholar]
- 25.Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010;362:1663–74. doi: 10.1056/NEJMoa0910496. [DOI] [PubMed] [Google Scholar]
- 26.Park DW, Kim YH, Yun SC, et al. Comparison of zotarolimus-eluting stents with sirolimus- and paclitaxel-eluting stents for coronary revascularization: the ZEST (comparison of the efficacy and safety of zotarolimus-eluting stent with sirolimus-eluting and paclitaxel-eluting stent for coronary lesions) randomized trial. J Am Coll Cardiol. 2010;56:1187–95. doi: 10.1016/j.jacc.2010.03.086. [DOI] [PubMed] [Google Scholar]
- 27.Sakata K, Waseda K, Kume T, et al. Impact of diabetes mellitus on vessel response in the drug-eluting stent era: pooled volumetric intravascular ultrasound analyses. Circ Cardiovasc Interv. 2012;5:763–71. doi: 10.1161/CIRCINTERVENTIONS.111.962878. [DOI] [PubMed] [Google Scholar]
- 28.Kleinbongard P, Böse D, Konorza T, et al. Acute vasomotor paralysis and potential downstream effects of paclitaxel from stents implanted for saphenous vein aorto-coronary bypass stenosis. Basic Res Cardiol. 2011;106:681–9. doi: 10.1007/s00395-011-0177-9. [DOI] [PubMed] [Google Scholar]
- 29.Bangalore S, Kumar S, Fusaro M, et al. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: mixed treatment comparison analysis of 22,844 patient years of follow-up from randomised trials. BMJ. 2012;345:e5170. doi: 10.1136/bmj.e5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Circulation. 2003;108:1655–61. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 31.Goraya TY, Leibson CL, Palumbo PJ, et al. Coronary atherosclerosis in diabetes mellitus: a population-based autopsy study. J Am Coll Cardiol. 2002;40:946–53. doi: 10.1016/s0735-1097(02)02065-x. [DOI] [PubMed] [Google Scholar]
- 32.Hammoud T, Tanguay JF, Bourassa MG. Management of coronary artery disease: therapeutic options in patients with diabetes. J Am Coll Cardiol. 2000;36:355–65. doi: 10.1016/s0735-1097(00)00732-4. [DOI] [PubMed] [Google Scholar]
- 33.Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]