Abstract
Nutritional medical treatment is the first step to achieve adequate glycemic control and prevent diabetic complications. Lifestyle changes include moderate weight loss (7%) and regular physical activity (150 min/week). The appropriate diet composition is < 30% total fat, < 10% saturated fats, > 15 g/1000 kcal fiber, half soluble, 45–60% of carbohydrates with amoderate intake of sugar (50 g/day) and protein intake of 15–20% of the total calories a day. Patients need to limit the intake of saturated fats to < 7% of the daily calorie intake. Monounsaturated fatty acids such as olive oil and other vegetable oils are recommended. L-carnitine, α-lipoic acid, berberine and ω-3 fatty acids can be useful supplements.
Keywords: berberine, diabetes, diet, L-carnitine, ω-3
Introduction
Type 2 diabetes mellitus is one of the most widespread endocrinological diseases in the general population and especially among hospitalized patients. It was estimated to affect 2.8% of the worldwide population in the year 2000, and it is expected to affect 4.4% in 2030 due to the population aging and aconstant increase in obesity; this situation is reaching the classification point of an “epidemic” phenomenon [1].
Type 2 diabetes involves every aspect of the patient's life; it is associated with an increase of cardiovascular risk, and can cause diabetic nephropathy, retinopathy, and diabetic neuropathy. In particular, autonomic neuropathy can cause asignificant increase in P-wave duration and dispersion, which might be responsible for the recurrence of atrial fibrillation [2]. Type 2 diabetes is closely associated with obesity, and it is known that adipose tissue serves as an important active endocrine organ that produces anumber of hormone-like compounds that can increase insulin resistance [3].
In recent years, several studies have shown that obesity may cause cardiovascular disease through different mechanisms such as sub-clinical inflammation, endothelial dysfunction, increase in sympathetic tone, atherogenic lipid profile, thrombogenic factors and obstructive sleep apnea [4].
Excess visceral fat, together with central obesity, plays an important role in causing insulin resistance, hypertriglyceridemia and changes in the particle sizes of low density lipoproteins (LDL) and in giving low concentrations of high-density lipoproteins (HDL). The mechanisms by which excess fat causes insulin resistance are complex: they involve different pathophysiological routes and are mediated by cytokines and other inflammatory mediators, such as elevated levels of leptin. Insulin resistance causes type 2 diabetes mellitus, which by itself may start or accelerate the atherogenic process through several additional mechanisms, such as hyperglycemia [4, 5].
In pathological conditions such as obesity and diabetes, there is adeterioration of the adiposity buffer, which determines alower uptake of free fatty acids by the adipocyte, and therefore agreater cardiolipotoxicity. Also, inflammation transcription factors are activated in the adipocyte such as the nuclear factor kappa β (NF-κB) that induces the production of inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin-1 and -6 (IL-1 and IL-6), visfatin and C-reactive protein (CRP), that, once released by the epicardial adipocyte, can be transported by the vasa vasorum, reaching the coronary arteries in which they exert their pro-atherogenic effects. Also, the presence of inflammatory cells in the epicardial fatty tissue may occur in response to plaque instability, through apoptosis and neovascularization processes [6].
It is important to highlight that the volume of the epicardial fatty tissue is an independent factor for the presence of total occlusion of coronary arteries. Relating these parameters to the clinical and biochemical parameters of the metabolic syndrome, it was found that patients with type 2 diabetes mellitus have agreater volume of epicardial fatty tissue compared with non-diabetic controls (166.1 ±60.6 cm 3 vs. 123.4 ±41.8 cm 3, p < 0.0001). Moreover, this volume was associated with the components of the metabolic syndrome and with greater severity of coronary atherosclerosis [7, 8].
For all these reasons, multi-targeted intervention is very important: better control of blood glucose reduces the risk of retinopathy and maybe neuropathy; blood pressure control significantly reduces cerebrovascular events, heart failure, loss of vision and microalbuminuria [9]. Multi-targeted intervention may also improve renal function and reduce serum uric acid levels within 6 months [10, 11].
The nutritional management of the diabetic patient goes beyond being only asource of nutrients; it is the first step of diabetes general treatment. Abalanced diet is not only involved in glycemic control (amount, type and time of food intake, insulin therapy, etc.), but it also affects the entire metabolism, preventing the progression of diabetes and concomitant complications [12].
Each diabetic patient has aspecific calorie requirement according to age, weight, sex, exercise, etc.; the distribution of macronutrients depends on the lipid scheme and renal function, the schedule of intake, lifestyle and hypoglycemic drugs administered. Also, above all, personal, familiar and cultural preferences need to be considered.
It is essential to offer the diabetic patient adequate educational support, keeping the treatment as simple as possible, and reinforcing the efforts at each visit. Support with educational courses, especially for patients having problems reaching their therapeutic goals, can contribute to improvement of patients’ health conditions [12–16]. There are different published recommendations about how macronutrients should be distributed in the diet [13, 14, 17], and how to early detect complications linked to particular diseases [18]. However, all the guidelines agree in saying that the calculation of the total energetic expense should be 30 kcal/kg for aperson with normal weight, 20–25 kcal/kg for overweight people and 35 kcal/kg for people with lower weight. Obviously, to lose weight, amoderate caloric restriction is recommended (250 to 500 kcal less than the daily average intake calculated in the meal plan), along with low aerobic exercise under medical prescription with previous cardiovascular assessment. Moderate weight loss in obese patients is 5 kg to 9 kg, regardless of the initial weight; the aim is reduce the risk of hyperglycemia, hypercholesterolemia and hypertension. Diets with an extremely low energetic content should only be provided in hospital, under the observation of aphysician.
Carbohydrates
Carbohydrates are the energetic substrate related to the greatest impact on glycemia levels. The total amount of carbohydrates is the main factor responsible for the post-prandial response, but there are other variables, such as type of carbohydrate, richness in fiber, the way of cooking, degree of maturity, etc., that can play arole. Moreover, there are other factors that can also influence post-prandial glycemia such as pre-prandial glycemia, macronutrient distribution of the whole meal (fats and proteins) and the hypoglycemic treatment administered: oral tablets or insulin.
Most scientific societies recommend the individualization of carbohydrate contribution, agreeing with the fact that the diet should provide carbohydrates in the form of fruits, cereals, pasta, legumes, vegetables and tubers (Table I).
Table I.
Different scientific societies’ recommendations on diet nutritional composition in diabetes
| Parameter | European Diabetes Association, 2004 | American Diabetes and Nutritionist Associations, 2008 | Canadian Diabetes Association, 2008 |
|---|---|---|---|
| Proteins | 10–20% ICT | 15–50% ICT | 15–20% ICT (as general population) |
| Carbohydrates | 45–60% (personalized) | > 130 g/day (personalized) | 45–60% ICT |
| Low glycemic index | Yes | Modest benefit | Yes |
| Sugar | 50 g/day, if there is good control | No limits within total content | < 10% ICT |
| Fiber | > 40 g/day | 14 g/1000 kcal (as general population) | 25–50 g/day |
| Total fats | < 35% ICT; < 30% if overweight | Personalized | < 35% ICT |
| Saturated fats | < 10% ICT; < 8% if high LDL | < 7% ICT | < 7% ICT |
| Polyunsaturated fats | < 7% ICT | > 2 fish/week | < 10% ICT |
| Monounsaturated fats | 10–20% ICT | No reference | Use instead of saturated |
| Cholesterol | < 300 mg/day (less if high LDL) | < 200 mg/day | |
| Omega-3 fatty acids | 2–3 servings of fish aweek | > 2 servings of fish aweek | Consume as much fish as plants |
| Alcohol | < 10 g (women); < 20 g (men) | 1 glass/day (women); 2 glasses/day (men) | Limit to 1/day (women) and 2/day (men), with risk of late hypoglycemia |
ICT – total calorie intake, LDL – low-density lipoproteins
Although there are no long-term studies, it seems that eating starches of legumes has apositive effect on glycemia, because of the persistent effect on post-prandial glycemia, with no sudden increases; it may prevent both post-prandial hyperglycemia and late hypoglycemia [13, 14, 17].
Fats
The main objective of ahealthy diet is to reduce the contribution of saturated fats and cholesterol. Monounsaturated fatty acids such as olive oil and other vegetable oils such as omega-3 fatty acids (ω-3 PUFA) are recommended (the consumption of fish is essential) for the effect they have on hypertriglyceridemia and cardiovascular events. In this sense, we need to remember that the consumption of fried fish is not recommended, due to the extra caloric contribution [13, 14, 17].
Proteins
In general, it is recommended that the diet should provide the same amount of proteins as ahealthy diet, individualized according to the characteristics and nutritional state of the patient. In developed countries, there is atrend towards high consumption of proteins.
The contribution of 15–20% of the total daily calories in the form of proteins (as recommended for the general population) widely covers the needs, even in situations of requirement increase, as in the case of adiabetic patient with hyperglycemia. On the other hand, there is no evidence that in diabetic patients with normal renal function the protein intake recommendation should be modified. Even in the case of nephropathies, when there is microalbuminuria, protein intake of 0.8–1 g/kg of body weight is recommended, and if there is proteinuria, the intake should reach 0.8 g/kg aday [13, 14, 17].
Fiber
Foods rich in fiber, such as fruits and vegetables, are still recommended; special mention is made of whole cereals. Although the protective effect of fibers against some chronic diseases is well established [19, 20], the effectiveness of fibers in lipid and glycemic metabolism remains uncertain. For the general population, an intake of 26 g/day and 38 g/day is recommended, for women and men, respectively. There is no reason to increase the fiber dose in diabetic patients [13, 14, 17].
Sweeteners
The consumption of sugar is forbidden in the diet of adiabetic patient. The use of other caloric sweeteners, such as fructose and polyalcohols, does not seem to offer additional advantages. In the case of fructose, its consumption is advised as acomponent of natural foods, but not as asweetener, due to its damaging effect on plasma glucose. Polyalcohols offer 2 kcal/g, making them interesting in low-calorie diets, although they may cause side effects such as diarrhea at high doses. In relation to non-caloric sweeteners, there is aconsensus that they are not detrimental to health, but there is no evidence that they improve glycemic control in the long-term [13, 14, 17].
Nutraceutics use in type 2 diabetes mellitus
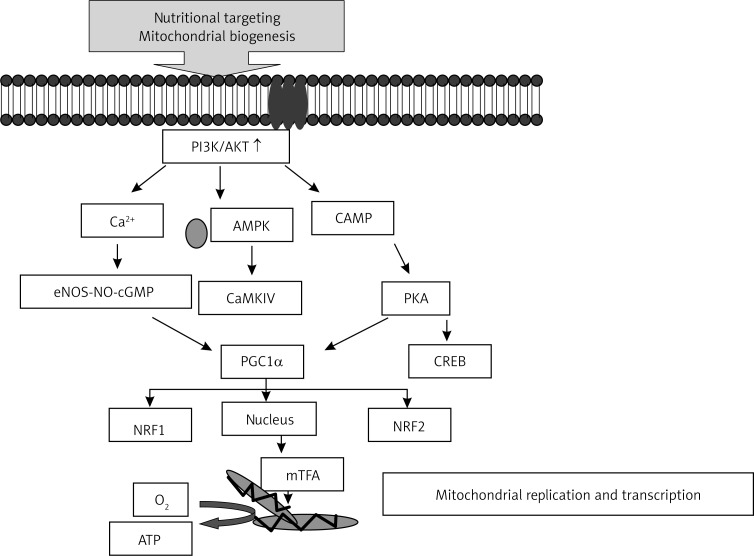
Insulin resistance is an important feature of type 2 diabetes mellitus and obesity. The underlying mechanisms of insulin resistance are still unclear. Adipose tissue, skeletal muscle and liver are major organs involved in glucose metabolism and, therefore, play important roles in insulin resistance. Oxidative stress has been suggested to be involved in the pathology of insulin resistance. Studies have shown that insulin resistance is associated with mitochondrial dysfunction, such as reduced mitochondrial number and ATP production. In diabetic patients, the expression of genes involved in oxidative phosphorylation is significantly reduced in the skeletal muscle. Mitochondria are the major site of reactive oxygen species production in the body. If the efficiency of oxidative phosphorylation is reduced, more O2 – is generated at the expense of ATP; therefore, reducing oxidative damage by improving mitochondrial function seems arational way to prevent and treat insulin resistance [21] (Figure 1).
Figure 1.
Possible mechanisms of the protective effects afforded by mitochondria-targeting nutrients/compounds through the PGC-1α-mediated increase in mitochondrial biogenesis
AMPK – AMP-activated protein kinase, NRF – nuclear respiratory factor, NO – nitric oxide, NOS – nitric oxide synthase, CaMKIV – calcium/calmodulin-dependent protein kinase IV, mTFA – mitochondrial transcription factor A, PKA – protein kinase A, CREB – cAMP-responsive element binding protein, PPARs – peroxisome proliferators-activated receptors, PGC-1α – PPAR-γ coactivator-1α
In the following paragraphs, we will describe some kind of supplements (Table II) that have been shown to be good co-adjuvants along with diet and drug treatment in improving chronic degenerative disease such as type 2 diabetes mellitus [21].
Table II.
Nutraceutical supplements
| Nutraceutics | Action |
|---|---|
| L-carnitine | Promotion of insulin sensitivity and hypolipidemic actions |
| α-Lipoic acid | Treatment of diabetic neuropathy and degenerative neuronal disease |
| Berberine | Hypoglycemic and hypolipidemic actions |
| ω-3 | Anti-arrhythmic effect and decrease of triglycerides |
L-carnitine
L-carnitine (β-hydroxy-γ-trimethylaminobutyrate), anatural vitamin-like compound, is anubiquitous constituent of mammalian plasma and tissues, mainly distributed among skeletal and cardiac muscles. L-carnitine is supplied through dietary sources (e.g., meat, dairy products), and by biosynthesis from lysine and methionine. Supplementation studies have shown that L-carnitine promotes insulin sensitivity and has lipid-lowering actions. L-carnitine performs anumber of essential intracellular and metabolic functions, such as fatty acid transport across the inner mitochondrial membrane into the matrix for β-oxidation, detoxification of potentially toxic metabolites, regulation of the mitochondrial acyl-Co A/CoA ratio, and stabilization of cell membranes. L-carnitine facilitates the elimination of short- and medium-chain fatty acids accumulating in mitochondria as aresult of normal or abnormal metabolism. L-carnitine also has effects on oxidative metabolism of glucose in tissues.
Rajasekar et al. [22] demonstrated that L-carnitine could improve insulin action in the fructose-fed rat model of insulin resistance. Skeletal muscle is an insulin-sensitive tissue, which is also asite of insulin resistance in the fructose-fed rat and it is vulnerable to oxidative damage. Considering this, these authors evaluated the role of L-carnitine in mitigating oxidative stress and lipid accumulation in the insulin sensitive skeletal muscle in awell-characterized model of insulin resistance. The effects of L-carnitine in this model suggest that its supplementation may have some benefits in patients suffering from insulin resistance.
Calò et al. [23] evaluated the effect of L-carnitine on the gene and protein expression of oxidative stress related proteins heme oxygenase-1 (HO-1) and endothelial nitric oxide synthase (ecNOS) in the absence and presence of oxidative stress induced by H2O2 in cultured human endothelial cells.
The authors found that L-carnitine can be an effective anti-oxidant in different in vitro assays when compared to standard anti-oxidant compounds such as α-tocopherol, anatural anti-oxidant, and Trolox, which is awater-soluble analogue of tocopherol. This is the first report that has utilized amolecular biological approach to demonstrate adirect stimulatory effect of carnitine on gene and protein expression of the oxidative stress related markers HO-1 and ecNOS.
This is important, because, according to Dayanandan et al. [24], lipid peroxides are the first step in the development of atherosclerosis. In their study L-carnitine treatment in Wistar rats (300 mg/kg body weight/day) for 7 and 14 days caused asignificant reduction in the tissue lipid peroxidation and amarked improvement in the antioxidant status. In this way carnitine maintains the normal function of the cells [24].
Lee et al. [25] investigated the lipolytic effects of L-carnitine in 3T3-L1 adipocytes. L-carnitine at 10–100 nM suppressed lipid accumulation. The results suggest an anti-obesity action of L-carnitine. L-carnitine may modulate lipid metabolism by stimulation of lipolysis and β-oxidation accompanied by corresponding changes in gene expression and suppression of adipogenic gene expression [25].
This was confirmed in type 2 diabetic patients by Derosa et al. [26]: these authors showed that, after 3 and 6 months, L-carnitine 1-g tablet, twice aday, significantly lowered plasma Lp(a) level compared with placebo in selected hypercholesterolemic patients with newly diagnosed type 2 diabetes mellitus.
Regarding nonalcoholic steatohepatitis (NASH), it is the most common cause of chronic liver disease in western countries. The prevalence is between 10% and 24% in the general population and reaches 75% in the obese groups. The pathogenesis of NASH is associated with disorders of energy metabolism, including obesity, insulin resistance, and dyslipidemia. The real mechanisms leading to NASH are still unclear, but nutritional, metabolic, genetic, viral, and other factors cause or contribute to fatty liver disease. The existing model that explains the pathogenesis of NASH is the “two-hit” hypothesis, first proposed by Day and James. According to this hypothesis, steatosis represents the “first hit” which increases the vulnerability of the liver to various “second hits” that, in turn, lead to inflammation, fibrosis, and cellular death. Several factors have been suggested to constitute the second hits, such as oxidative stress, pro-inflammatory cytokines, and gut-derived bacterial endotoxin. It was observed that NASH is associated with amore atherogenic lipid profile, including hypertriglyceridemia, ahigher plasma concentration of very low-density lipoprotein (VLDL) and LDL that are larger in size, and with lower levels of high-density lipoprotein.
Fatty acids are asource of oxidative stress and damage of mitochondria with increased β-oxidation and raising levels of reactive oxygen species. Recently, it has been hypothesized that L-carnitine could improve the outcome of NASH, because it reduces lipid levels, limits oxidative stress, and modulates inflammatory responses.
In this regard, Malaguarnera et al. [27] evaluated whether L-carnitine treatment could determine histological changes at liver biopsy and modify humoral parameters. The authors randomly dispensed one 1-g of L-carnitine twice aday in addition to the diet or diet alone for 24 weeks in patients with NASH. This study suggested that L-carnitine supplementation to the diet is useful for reducing TNF-α and CRP, and for improving liver function, glucose plasma level, lipid profile, HOMA-IR, and histological manifestations of NASH [27].
As already described above, L-carnitine serves as an obligatory cofactor for mitochondrial β-fatty acid oxidation to facilitate the transport of long-chain fatty acids across the mitochondrial membrane, thus resulting in more efficient mitochondrial oxidative phosphorylation and glucose use. This improves mitochondrial function, consequently improving both endothelial cells and smooth muscle cell insulin-stimulated nitric oxide bioavailability and glucose use, and ultimately improves endothelial function and systemic insulin sensitivity [28].
This was confirmed by Derosa et al. [29, 30], who compared orlistat plus L-carnitine versus orlistat alone in order to observe effects on body weight, glycemic and lipid control, and inflammatory parameters in obese type 2 diabetic patients. Two hundred and fifty-eight patients with uncontrolled type 2 diabetes mellitus (HbA1c > 8.0%) in therapy with different oral hypoglycemic agents or insulin were randomized to take orlistat 120 mg three times aday plus L-carnitine 2 γ once aday or orlistat 120 mg three times aday. They observed abetter decrease in body weight, glycemic profile, HOMA-IR, LDL-C, and adiponectin (ADN) and afaster improvement in fasting plasma insulin, total cholesterol, triglycerides, leptin, tumor necrosis factor (TNF-α) and hs-CRP with orlistat plus L-carnitine compared to orlistat alone. They also recorded an improvement in vaspin with orlistat plus L-carnitine, not reached with orlistat alone. The same authors reported faster improvement of lipid profile, insulin resistance parameters, glycemic control, and body weight with sibutramine plus L-carnitine compared to sibutramine alone in type 2 diabetic patients [31, 32].
α-Lipoic acid
α-Lipoic acid (1,2-dithiolane-3-pentanoic acid) is synthesized in most prokaryotic and eukaryotic cells. In humans, α-lipoic acid exists in the body as aportion of several multi-enzyme complexes involved in energy formation and is an essential component of mitochondrial respiratory enzymes. α-Lipoic acid is clinically used in the treatment of diabetic neuropathy and is also effective in degenerative neuronal disease, atherosclerosis and other abnormalities [33].
The development of insulin resistance has been shown to be an early step in the development of cardiovascular diseases in diabetic patients. Oxidative stress may be important in the development of coronary artery disease. Fructose loaded rats, which show the characteristic features of insulin resistance, also display an imbalance between the peroxidation process and the anti-oxidant system. α-Lipoic acid is known for its potent anti-oxidant effects. In a study conducted by Thirunavukkarasu et al. [34], male Wistar rats received acontrol diet containing starch and water ad libitum, or a fructose-enriched diet (> 60% of total calories), or fructose-enriched diet + α-lipoic acid or a control diet + α-lipoic acid. After the 20-day treatment period, the insulin sensitivity index in terms of HOMA was assessed. The levels of lipid peroxidation markers and the enzymatic and non-enzymatic anti-oxidant status in the heart tissue were measured. Plasma and heart tissue lipids were also analyzed. Fructose rats showed decreased insulin sensitivity as reflected by high values of HOMA, increased peroxidation, impaired anti-oxidant status and lipid abnormalities in the cardiac tissue. These abnormalities were attenuated and the anti-oxidant levels were enhanced by α-lipoic acid. The reduction in HOMA values suggests that α-lipoic acid improves insulin sensitivity. Improvement of insulin sensitivity and enhancement of cardiac anti-oxidant status suggest that α-lipoic acid may be useful as acardioprotective agent in insulin-resistant states [34].
Kim et al. [35] reported that α-lipoic acid reduces the activity of the kinase protein activated by the AMP (AMPK) and works as asensor in the cell activated when cell energy is reduced. The activation of hypothalamic AMPK reverts the effects of α-lipoic acid in the intake of food and the release of energy. Hyperphagia induced by 2 deoxyglucose is reverted by the AMPK inhibition. Hypothalamic AMPK is important in the central regulation of food intake and energy release and α-lipoic acid excerpts anti-obesity effects through the suspension of such activity in the hypothalamic AMPK [35].
Berberine
Berberine (molecular formula C20H18NO4 and molecular weight of 336.36) is the main active component of the ancient Chinese herb Coptis chinensis Franch, which has been used to treat diabetes for thousands of years. Berberine is an over-the-counter drug, which is used to treat gastrointestinal infections in China. Berberine hydrochloride (B·HCl·n H2O), the most popular form of berberine, has been used in several studies [36–40].
The chemical structures of berberine and related isoquinoline alkaloids are quite different from the commonly used hypoglycemic agents. Significant decreases in HbA1c (from 9.5 ±0.5% to 7.5 ±0.4%, p < 0.01), fasting plasma glucose (from 10.6 ±0.9 mmol/l to 6.9 ±0.5 mmol/l, p < 0.01), post-prandial glucose (from 19.8 ±1.7 mmol/l to 11.1 ±0.9 mmol/l, p < 0.01) and plasma triglycerides (from 1.13 ±0.13 mmol/l to 0.89 ±0.03 mmol/l, p < 0.05) were observed by Jun Yin et al. [36] when berberine was given to both newly diagnosed diabetic patients and poorly controlled diabetic patients for 3 months.
Berberine increases insulin receptor expression and improves glucose utility both in vitro and in animal models. Berberine increased insulin receptor messenger RNA and protein expression in avariety of human cell lines, including CEM, HCT-116, SW1990, HT1080, 293T, and hepatitis B virus-transfected human liver cells.
In the clinical study by Zhang et al. [37], berberine significantly lowered fasting plasma glucose, HbA1c, triglycerides, and insulin levels in patients with type 2 diabetes mellitus. The decrease of fasting plasma glucose and HbA1c obtained with berberine was similar to those reached with metformin and rosiglitazone. In the berberine treated patients, the percentages of peripheral blood lymphocytes that express insulin receptor were significantly elevated after therapy. Berberine also effectively lowered fasting plasma glucose in chronic hepatitis B and hepatitis C patients with type 2 diabetes mellitus. Liver function was improved greatly in these patients, as shown by the reduction of liver enzymes. Their results confirmed the activity of berberine on the insulin receptor in humans and its relationship with the glucose-lowering effect. Berberine could be an ideal medicine for type 2 diabetes mellitus with amechanism different from metformin and rosiglitazone.
Flora Affuso et al. [38] used anutraceutical product with berberine, policosanol and red yeast rice in patients with metabolic syndrome. There were significant reductions in blood glucose and insulin, and asignificant reduction in arterial systolic blood pressure. Part of the anti-hyperglycemic activity of berberine was due to adecrease in the availability of glucose after ameal. In particular, berberine suppresses intestinal disaccharidases (sucrase and maltase), reducing the intestinal absorption of glucose. This latter effect is very interesting and may explain the slight, but significant reduction in post-prandial glycemia observed in the treated group.
Yifei Zhang et al. [39] described similar results when they treated type 2 diabetic and dyslipidemic patients with berberine. Berberine had arobust glucose lowering effect by significantly reducing fasting plasma glucose and post-prandial glucose by 1.4 mmol/l and 3.1 mmol/l, respectively, at 3 months and HbA1c by 0.9% from the initial levels of 7.5%. The decline in HbA1c achieved with berberine was fully comparable with that with existing pharmacologic products used in treatment of type 2 diabetes mellitus. However, the HOMA values, and serum fasting and post-prandial insulin concentrations were not significantly different between the berberine and placebo groups. An 18% reduction of serum cholesterol, 35.9% of triglycerides and 21% of LDL-C were achieved in type 2 diabetes mellitus after 3-month treatment. In addition, the authors observed that systolic (SBP) and diastolic (DBP) blood pressures were significantly reduced with berberine by 7 mm Hg and 5 mm Hg, respectively (p < 0.001 and p < 0.005, respectively). Modest weight loss was also demonstrated.
Obviously, berberine cannot be an adequate single drug therapy for all diabetic patients; however, it may be at least useful as an adjuvant to standard therapy. With regard to safety, berberine does not have any toxicity.
Omega-3
Omega-3 polyunsaturated fatty acid (ω-3 PUFA) therapy continues to show great promise in primary and, particularly in secondary prevention of cardiovascular diseases. The most compelling evidence for cardiovascular benefits of ω-3 PUFA comes from the GISSI trial [41], showing that the early administration of low-dose (1 g/day) ω-3 PUFA reduces total mortality and sudden death, suggesting an anti-arrhythmic effect of this drug. Further evidence come from the GISSI-HF trial [42], where ω-3 PUFA (1 g/day) provided asmall benefit in terms of mortality and admission to hospital for cardiovascular reasons in patients with heart failure in the context of usual care.
Derosa et al. [43] evaluated the effect of standardized dietary supplementation with ω-3 PUFAs on the level of some markers of vascular remodeling in patients with combined dyslipidemia. They determined body mass index, glycemic profile, blood pressure, lipid profile, lipoprotein(a), plasminogen activator inhibitor-1, homocysteine, fibrinogen, high-sensitivity C-reactive protein, ADN. They also evaluated metalloproteinase (MMP)-2 and MMP-9, and tissue inhibitors of MMP-1 and -2, involved in cardiovascular disease and diabetes [44]. Omega-3 PUFAs gave abetter lipid profile and improvement of coagulation, fibrinolytic and inflammatory parameters than placebo [43]. The treatment with ω-3 PUFA not only improved lipids in abaseline situation, but it also improved all insulin resistance parameters in apost-prandial situation simulated with an oral fat load. This is another important action of ω-3 PUFA, which can increase their utility in clinical practice [45, 46]. Moreover, ω-3 PUFA long-term supplementation proved to be associated with asignificant reduction in SBP, DBP, pulse pressure, and basal heart rate in hypertriglyceridemic patients with normal-high blood pressure [47]. Based on this evidence, the target ω-3 PUFA consumption should be at least 800 mg/day to 1,000 mg/day for individuals with known coronary heart disease and heart failure to obtain the anti-arrhythmic effect, and at least 2,500–3,000 mg/day to decrease triglycerides.
In conclusion, nutritional medical treatment is avery important part of the medical surveillance of the diabetic patient; the diet needs to provide adequate energy to achieve areasonable weight, as well as adequate growth and development.
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Bissinger A, Grycewicz T, Grabowicz W, Lubinski A. The effect of diabetic autonomic neuropathy on P-wave duration, dispersion and atrial fibrillation. Arch Med Sci. 2011;7:806–12. doi: 10.5114/aoms.2011.25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson MB, Ahima RS. Neuroendocrine and metabolic effects of adipocyte-derived hormones. Clin Sci (Lond) 2006;110:143–52. doi: 10.1042/CS20050243. [DOI] [PubMed] [Google Scholar]
- 4.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 5.Sierra-Johnson J, Romero-Corral A, Somers VK, et al. IGF-I/IGFBP-3 ratio: amechanistic insight into the metabolic syndrome. Clin Sci (Lond) 2009;116:507–12. doi: 10.1042/CS20080382. [DOI] [PubMed] [Google Scholar]
- 6.Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res. 2008;40:442–5. doi: 10.1055/s-2008-1062724. [DOI] [PubMed] [Google Scholar]
- 7.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is asource of inflammatory mediators. Circulation. 2003;108:2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 8.Ueno K, Anzai T, Jinzaki M, et al. Increased epicardial fat volume quantified by 64-multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circ J. 2009;73:1927–33. doi: 10.1253/circj.cj-09-0266. [DOI] [PubMed] [Google Scholar]
- 9.Ješić M, Sajić S, Ješić M, et al. Microalbuminuria in relation to metabolic control and blood pressure in adolescents with type 1 diabetes. Arch Med Sci. 2011;7:1037–41. doi: 10.5114/aoms.2011.26617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konishi M, Sugiyama S, Sugamura K, et al. Association of pericardial fat accumulation rather than abdominal obesity with coronary atherosclerotic plaque formation in patients with suspected coronary artery disease. Atherosclerosis. 2010;209:573–8. doi: 10.1016/j.atherosclerosis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Athyros VG, Hatzitolios AI, Karagiannis A, IMPERATIVE Collaborative Group IMproving the imPlemEntation of cuRrent guidelines for the mAnagement of major coronary hearT disease rIsk factors by multifactorial interVEntion. The IMPERATIVE renal analysis. Arch Med Sci. 2011;7:984–92. doi: 10.5114/aoms.2011.26610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyes Ramírez MP, Morales González JA, Madrigal Santillán EO. Diabetes. Tratamiento nutricional. Med Int Mex. 2009;25:454–60. [Google Scholar]
- 13.American Diabetes Association. Bantle JP, Wylie-Rosett J, Albright AL. Nutrition recommendations and interventions for diabetes: aposition statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl. 1):S61–78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 14.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008. Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada: Nutrition Therapy. Can J Diabetes. 2008;32(Suppl. 1):S40–5. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Franz MJ, Boucher JL, Green-Pastors J, Powers MA. Evidence-based nutrition practice guidelines for diabetes and scope and standards of practice. J Am Diet Assoc. 2008;108(4 Suppl. 1):S52–8. doi: 10.1016/j.jada.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Lerman I, López-Ponce A, Villa AR, et al. Pilot study of two different strategies to reinforce self care behaviors and treatment compliance among type 2 diabetes patients from low income strata. Gac Med Mex. 2009;145:15–9. [PubMed] [Google Scholar]
- 17.Mann JI, De Leeuw I, Hermansen K, Diabetes and Nutrition Study Group (DNSG) of the European Association Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis. 2004;14:373–94. doi: 10.1016/s0939-4753(04)80028-0. [DOI] [PubMed] [Google Scholar]
- 18.Koura HM, Abdallah Ismail N, Kamel AF, Ahmed AM, Saad-Hussein A, Effat LK. Along-term study of bone mineral density in patients with phenylketonuria under diet therapy. Arch Med Sci. 2011;7:493–500. doi: 10.5114/aoms.2011.23417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada Y, Hosoya S, Nishimura S, et al. Effect of bread containing resistant starch on postprandial blood glucose levels in humans. Biosci Biotechnol Biochem. 2005;69:559–66. doi: 10.1271/bbb.69.559. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JA. Resistant starch: metabolic effects and potential health benefits. J AOAC Int. 2004;87:761–8. [PubMed] [Google Scholar]
- 21.Liu J, Shen W, Zhao B, et al. Targeting mitochondrial biogenesis for preventing and treating insulin resistance in diabetes and obesity: hope from natural mitochondrial nutrients. Adv Drug Deliv Rev. 2009;61:1343–52. doi: 10.1016/j.addr.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Rajasekar P, Anuradha CV. Effect of L-carnitine on skeletal muscle lipids and oxidative stress in rats fed high-fructose diet. Exp Diabetes Res. 2007;2007:72741. doi: 10.1155/2007/72741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calò LA, Pagnin E, Davis PA, et al. Antioxidant effect of L-carnitine and its short chain esters: relevance for the protection from oxidative stress related cardiovascular damage. Int J Cardiol. 2006;107:54–60. doi: 10.1016/j.ijcard.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 24.Dayanandan A, Kumar P, Panneerselvam C. Protective role of L-carnitine on liver and heart lipid peroxidation in atherosclerotic rats. J Nutr Biochem. 2001;12:254–7. doi: 10.1016/s0955-2863(00)00151-0. [DOI] [PubMed] [Google Scholar]
- 25.Lee MS, Lee HJ, Lee HS, Kim Y. L-carnitine stimulates lipolysis via induction of the lipolytic gene expression and suppression of the adipogenic gene expression in 3T3-L1 adipocytes. J Med Food. 2006;9:468–73. doi: 10.1089/jmf.2006.9.468. [DOI] [PubMed] [Google Scholar]
- 26.Derosa G, Cicero AF, Gaddi A, Mugellini A, Ciccarelli L, Fogari R. The effect of L-carnitine on plasma lipoprotein(a) levels in hypercholesterolemic patients with type 2 diabetes mellitus. Clin Ther. 2003;25:1429–39. doi: 10.1016/s0149-2918(03)80130-3. [DOI] [PubMed] [Google Scholar]
- 27.Malaguarnera M, Gargante MP, Russo C, et al. L-carnitine supplementation to diet: anew tool in treatment of nonalcoholic steatohepatitis. Arandomized and controlled clinical trial. Am J Gastroenterol. 2010;105:1338–45. doi: 10.1038/ajg.2009.719. [DOI] [PubMed] [Google Scholar]
- 28.Whaley-Connell A, Sowers JR. Hypertension and insulin resistance. Hypertension. 2009;54:462–4. doi: 10.1161/HYPERTENSIONAHA.109.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derosa G, Maffioli P, Ferrari I, et al. Comparison between orlistat plus L-carnitine and orlistat alone on inflammation parameters in obese diabetic patients. Fundam Clin Pharmacol. 2011;25:642–51. doi: 10.1111/j.1472-8206.2010.00888.x. [DOI] [PubMed] [Google Scholar]
- 30.Derosa G, Maffioli P, Ferrari I, et al. Orlistat and L-carnitine compared to orlistat alone on insulin resistance in obese diabetic patients. Endocr J. 2010;57:777–86. doi: 10.1507/endocrj.k10e-049. [DOI] [PubMed] [Google Scholar]
- 31.Derosa G, Maffioli P, Salvadeo SA, et al. Sibutramine and L-carnitine compared to sibutramine alone on insulin resistance in diabetic patients. Intern Med. 2010;49:1717–25. doi: 10.2169/internalmedicine.49.3401. [DOI] [PubMed] [Google Scholar]
- 32.Derosa G, Maffioli P, Salvadeo SA, et al. Effects of combination of sibutramine and L-carnitine compared with sibutramine monotherapy on inflammatory parameters in diabetic patients. Metabolism. 2011;60:421–9. doi: 10.1016/j.metabol.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Booker SJ. Unraveling the pathway of lipoic acid biosynthesis. Chem Biol. 2004;11:10–2. doi: 10.1016/j.chembiol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Thirunavukkarasu V, Anitha Nandhini AT, Anuradha CV. Cardiac lipids and antioxidant status in high fructose rats and the effect of alpha-lipoic acid. Nutr Metab Cardiovasc Dis. 2004;14:351–7. doi: 10.1016/s0939-4753(04)80025-5. [DOI] [PubMed] [Google Scholar]
- 35.Kim MS, Park JY, Namkoong C, et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–33. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 36.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–7. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Wei J, Xue R, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59:285–92. doi: 10.1016/j.metabol.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 38.Affuso F, Mercurio V, Ruvolo A, et al. Anutraceutical combination improves insulin sensitivity in patients with metabolic syndrome. World J Cardiol. 2012;4:77–83. doi: 10.4330/wjc.v4.i3.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Li X, Zou D, et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559–65. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 40.Derosa G, Maffioli P, Cicero AF. Berberine on metabolic and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. Expert Opin Biol Ther. 2012;12:1113–24. doi: 10.1517/14712598.2012.704014. [DOI] [PubMed] [Google Scholar]
- 41.Marchioli R, Barzi F, Bomba E, GISSI-Prevenzione Investigators Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 42.Gissi-HF Investigators. Tavazzi L, Maggioni AP. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): arandomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–30. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 43.Derosa G, Maffioli P, D'Angelo A, et al. Effects of long chain omega-3 fatty acids on metalloproteinases and their inhibitors in combined dyslipidemia patients. Expert Opin Pharmacother. 2009;10:1239–47. doi: 10.1517/14656560902865601. [DOI] [PubMed] [Google Scholar]
- 44.Lewandowski KC, Banach E, Bienkiewicz M, Lewinski A. Matrix metalloproteinases in type 2 diabetes and non-diabetic controls: effects of short-term and chronic hyperglycaemia. Arch Med Sci. 2011;7:294–303. doi: 10.5114/aoms.2011.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derosa G, Cicero AFG, Fogari E, D'Angelo A, Bonaventura A, Maffioli P. Effects of n-3 PUFAs on insulin resistance after an oral fat load. Eur J Lipid Sci Technol. 2011;113:950–60. [Google Scholar]
- 46.Derosa G, Cicero AFG, Fogari E, et al. n-3 PUFAs effects on post-prandial variation of metalloproteinases, inflammatory and insulin resistance parameters in dyslipidemic patients: evaluation with euglycemic clamp and oral fat load. J Clin Lipidol. 2012;6:553–64. doi: 10.1016/j.jacl.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Cicero AF, Derosa G, Di Gregori V, Bove M, Gaddi AV, Borghi C. Omega 3 polyunsaturated fatty acids supplementation and blood pressure levels in hypertriglyceridemic patients with untreated normal-high blood pressure and with or without metabolic syndrome: aretrospective study. Clin Exp Hypertens. 2010;32:137–44. doi: 10.3109/10641960903254448. [DOI] [PubMed] [Google Scholar]