Abstract
Background
Siglec-8 is expressed on human eosinophils, where its ligation induces cell death. Paradoxically, Siglec-8-mediated cell death is markedly enhanced by the presence of the activation and survival factor IL-5 and becomes independent of caspase activity.
Objective
In this report we investigate the mechanism of Siglec-8-mediated cell death in activated eosinophils.
Methods
Human peripheral blood eosinophils were treated with agonistic anti-Siglec-8 antibody and IL-5, and cell death was determined by flow cytometry and morphology. Phosphorylation of MAPK was determined by phospho-luminex, flow cytometry, and Western blotting. ROS accumulation was determined by dihydrorhodamine (DHR) fluorescence.
Results
Co-stimulation with anti-Siglec-8 and IL-5 significantly increased the rate and proportion of cells dying by necrosis accompanied by granule release as compared to stimulation with anti-Siglec-8 alone, in which apoptosis predominated. Together with the caspase-independent mode of cell death in co-stimulated cells, these findings suggest the activation of a specific and distinct biochemical pathway of cell death during anti-Siglec-8/IL-5 co-stimulation. Phosphorylation of ERK1/2 and MEK1 was significantly enhanced and sustained in co42 stimulated cells compared to cells stimulated with IL-5 alone; anti-Siglec-8 alone did not cause ERK1/2 phosphorylation. MEK1 inhibitors blocked anti-Siglec-8/IL-5-induced cell death. ROS accumulation was induced by Siglec-8 ligation in a MEK-independent manner. In contrast, ROS inhibitor prevented the anti-Siglec-8/IL-5-induced enhancement of ERK phosphorylation and cell death. Exogenous ROS mimicked stimulation by anti-Siglec-8 and was sufficient to induce enhanced cell death in IL-5-treated cells. Collectively, these data suggest that the enhancement of ERK phosphorylation is downstream of ROS generation.
Conclusions
In activated eosinophils, ligation of Siglec-8 leads to ROS-dependent enhancement of IL-5-induced ERK phosphorylation, which results in a novel mode of biochemically-regulated eosinophil cell death.
Keywords: Eosinophils, cell death, signaling
Introduction
Siglecs are a family of cell surface receptor proteins that are predominantly expressed on hematopoietic cells 1 and bind specific glycans.2 Antibodies specific to individual Siglecs have been used as artificial ligands, with cross-linking inducing Siglec activation. Most Siglecs have cytoplasmic tyrosine residues located within immunoreceptor tyrosine-based inhibitory motifs (ITIM). As ITIMs are known to be involved in inhibitory responses, Siglecs have been considered primarily inhibitory receptors.
Siglec-8 is highly and selectively expressed by human eosinophils, mast cells, and basophils. In contrast to the inhibitory functions observed for other Siglecs, Siglec-8 induces eosinophil cell death in vitro when cross-linked with ligand-coated polymers or anti-Siglec-8 monoclonal antibodies (mAb).3–5 Siglec-F is considered the murine functional paralogue of Siglec-8 based on sharing similar functional properties such as eosinophil-predominant expression, induction of eosinophil cell death, and binding affinity to the same glycan ligand, 6′-sulfated sialyl Lewis X.6–9 Treating mice with agonistic anti-Siglec-F antibody induces eosinophil cell death in vivo and decreases eosinophil levels.7, 10 Moreover, treating allergen-challenged mice with the anti-Siglec-F antibody leads to decreased eosinophilia and improved disease outcomes.11 Notably, allergen-challenged Siglec-F-deficient mice exhibit increased tissue eosinophilia, implicating physiological roles for Siglec-F and Siglec-8 in preventing excessive eosinophil accumulation.12–14
Paradoxically, eosinophil cell death induced by anti-Siglec-8 mAb ligation is enhanced by co-stimulating with cytokines that would normally prolong eosinophil survival, such as IL-5, IL-33 or GM-CSF.15 Consistent with this finding, ex vivo studies showed that eosinophils isolated from the bronchoalveolar fluid of allergen-challenged patients also display enhanced susceptibility to apoptosis when exposed to anti-Siglec-8 antibodies in vitro.16 These results suggest that activated eosinophils, such as those in patients with asthma and allergic diseases may be especially sensitive to pharmacologic approaches that engage Siglec-8. Furthermore, understanding how the mechanism of cell death in activated eosinophils differs from that of steady-state eosinophils may provide therapeutic targets that will be specific to harmful eosinophil functions, leaving innocuous and homeostatic eosinophil functions intact. Mechanistically, in the presence of IL-5, eosinophils become more sensitive to Siglec-8-induced cell death and crosslinking by secondary antibody or protein-A/G is no longer necessary for induction of cell death.17 Furthermore, Siglec-8-mediated cell death is enhanced and, while still ROS dependent, becomes caspase independent.18 The upstream mechanism that leads to this enhancement is not known and is the subject of this study.
In this report, we demonstrate that in activated eosinophils Siglec-8 ligation leads to ROS-dependent enhancement of IL-5-induced ERK phosphorylation and that activation of the MEK/ERK pathway is required for anti-Siglec-8/IL-5 co-stimulation-induced eosinophil cell death.
Methods
Eosinophil purification
Blood eosinophils were purified (to >95% purity) from healthy control subjects, using percoll gradient separation and CD16 magnetic bead negative selection system (Miltenyi) as previously described.19 For each outcome, eosinophils were purified from 3 or more independent donors. Written informed consent was obtained from all blood donors, and the study was approved by the Institutional Review Board of Cincinnati Children’s Hospital.
Eosinophil cultures
Human eosinophils were cultured at 1×106/mL in complete culture media (RPMI 1640 containing 10% FCS) in the presence and absence of recombinant IL-5 (30 ng/mL, Peprotech). Anti-Siglec-8 or its isotype-matched control antibody (Biolegend) was added alone (2.5 μg/mL) or, when cells were cultured in the absence of IL-5, as a premixed solution with an equal amount (10 μg/mL) of the secondary antibody for crosslinking (goat F(ab’)2 anti-mouse IgG1, Southern Biotech). The isotype-matched control antibody did not have an effect on survival or apoptosis/necrosis compared to cells treated with media alone, or in the presence of IL-5 (data not shown). Anti-Siglec-8 monoclonal antibody 2C4 was produced as previously described.5 In some experiments, clone 7C9 anti-Siglec-8 antibody (Biolegend) was used; the results were independent of the anti-Siglec-8 antibody clone used. The MEK1 inhibitors U0126 (EMD-Millipore Chemicals) and PD184352 (Enzo Life Sciences) were pre-incubated for 15 minutes before adding IL-5 ± anti-Siglec-8. When the ROS inhibitor DPI (Sigma-Aldrich) was used, cells were pre-incubated with DPI for 5 minutes and then washed and re-suspended with media.
Determination of eosinophil cell death
Eosinophil apoptosis and death were assessed by flow cytometry with Annexin V and 7AAD staining and a FACS Calibur flow cytometer (Becton Dickinson). To discriminate apoptotic and necrotic cell death by microscopy, cells were cytospun (1–2×105 cells/slide), stained with HEMA 3, and scored by an observer blinded to treatment. Specifically, criteria used for apoptotic cell morphology included picnotic nuclei with condensed chromatin, cells of a normal or decreased size, and an intact plasma membrane. Criteria for necrotic cell morphology included enlarged cells, often of irregular shape, and loss of plasma membrane integrity, often associated with the presence of free extracellular granules.
Immunochemical detection of phosphorylated signaling proteins
Phosphorylated signaling proteins were assessed using three methods: phospholuminex, Western blot, and phospho-flow cytometry. For the phospholuminex assay, an antibody panel kit for 10 phosphoproteins related to human MAP kinase (i.e., phosphorylated MEK1, ERK, MSK1 STAT1, p38, JNK, HSP27, ATF2, p53, and JUN) was employed (Milliplex®, Millipore Corporation). Briefly, eosinophils (1×106/mL) were cultured with or without stimulating factors for the indicated times, washed with cold PBS and lysed with manufacturer-provided lysis buffer (1×107 cells/mL) including a cocktail of protease/phosphatase inhibitors (Thermo Fisher Scientific). Lysates were then filtered with centrifugation in provided filter plates. Total protein concentrations of the lysates were measured and adjusted by diluting with lysis buffer. Lysates were mixed with a cocktail of antibody-specific beads overnight, treated with detection antibody and Streptavidin-PE and analyzed with the Luminex® system. In some experiments, cell lysates were fractionated by NE-PER® nuclear and cytoplasmic extraction kit (Thermo Fisher Scientific), according to manufacturer’s protocols.
For Western blotting, protein lysates were electrophoresed through Bis-Tris 4–15% gradient NuPage® gels (LifeScience-Invitrogen) and transferred to nitrocellulose membranes using the iBlot® dry blot system (LifeScience-Invitrogen) according to manufacturer’s guidelines. Anti-ERK and anti-phospho-ERK (Thr202/Thr204), anti-STAT5, anti-phospho-STAT5 (Tyr694), and secondary (HRP-conjugated anti-rabbit IgG) antibodies were from Cell Signaling Technologies. After blocking with 5% bovine serum albumin (BSA) in TBS-T, membranes were incubated overnight with primary antibodies (1:1000 in TBS-T/1% BSA). Specific binding to these antibodies was detected with HRP-conjugated secondary antibodies using the Luminata Forte® chemiluminescent HRP detection kit (Millipore Corporation). Signal strength of detected bands was measured by densitometry using NIH’s ImageJ software. For phospho-flow cytometry, cells cultured for 2–60 minutes were immediately fixed with 1% paraformaldehyde (PFA) and then permeabilized using 100% ice-cold methanol and overnight incubation at −20°C. Cells were washed with PBS, incubated with phospho-specific antibodies for 1 hour, washed again, incubated with FITC- or Alexa-fluor 647-conjugated secondary antibodies (Jackson ImmunoResearch and Cell Signaling Technologies, respectively) for 30 minutes, and analyzed by flow cytometry.
Reactive oxygen species production measurements
Following stimulation for the indicated time, cells were incubated with 1 μM DHR 123 (Invitrogen) at 37°C for 30 minutes, fixed with 1% PFA, washed with PBS, and subsequently analyzed by flow cytometry. In some experiments, cells were incubated with DHR 123 for 15 minutes prior to the addition of stimulating factors; stimulation was terminated by fixation with 1% PFA.
Measurement of released EPO activity
EPO release was measured as described previously.20 Briefly, following stimulation of eosinophils with indicated stimuli, the substrate O-phenylenediamine (OPD) was added directly to cell suspensions, reaction was stopped by H2SO4 and color intensity measured at 492-nm wavelength. Data are expressed as % release, which is determined by measuring total EPO activity in lysed eosinophils. Cells stimulated with 50ng/mL of phorbol-12-myristate-13-acetate (PMA, Sigma-Aldrich) were used as positive control.
Statistical analysis
Statistical analysis was performed with Prism® software (GraphPad Software). Data from individual experiments were analyzed using the Student’s t test or ANOVA if two or more groups are analyzed, respectively. Data from multiple experiments were analyzed using paired Student’s t test (where each condition is paired with the control from the specific experiment). A p value of less than 0.05 was considered statistically significant. In Figure 1D, where data were from multiple experiments, some of which used samples from the same donor, we employed repeated measures analysis in consultation with the biostatistical core at CCHMC.
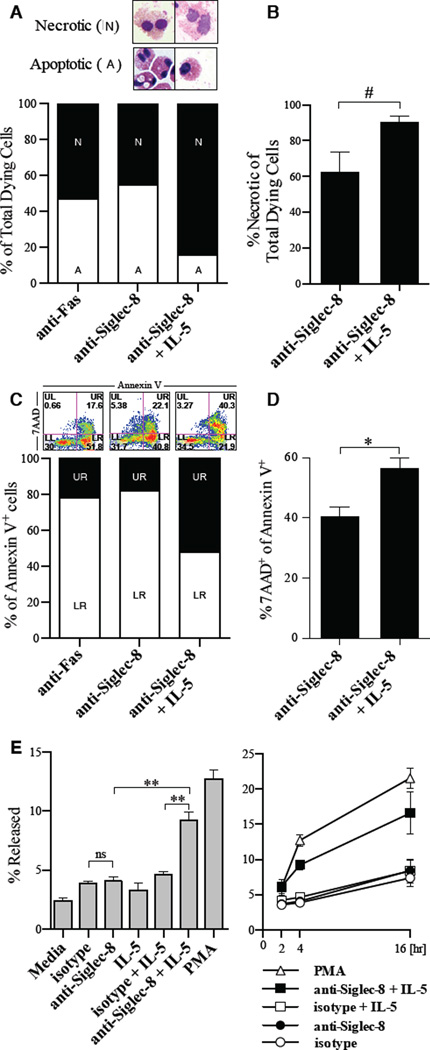
Figure 1. Siglec-8 crosslinking induces a different mode of cell death in activated versus resting eosinophils.
A, Representative morphology and the percentage of dead cells that have apoptotic or necrotic morphology in eosinophils cultured for 24 hours with indicated stimuli (n= 6 experiments and donors). B, Mean percentage of dead cells with necrotic morphology in multiple experiments (n = 6). #p = 0.055. C, Representative Annexin V/7AAD staining and the percent of 7AAD+ and 7AAD−cells among all Annexin V+ cells. D, Mean ratio of 7AAD+ cells among all Annexin V+ cells from multiple experiments (n = 25 experiments with 11 donors; **p <0.01). E, activity of released EPO following incubation with indicated stimuli for 4-hours (left) or over 16 hours (right).
Results
Siglec-8 crosslinking induces a different mode of eosinophil cell death in activated (anti-Siglec-8/IL-5 co-stimulation) versus resting (anti-Siglec-8 stimulation) eosinophils
In order to determine the mode of cell death induced in resting and activated eosinophils, we first examined the morphology of eosinophils (“necrotic” or “apoptotic”) treated with anti-Siglec-8 alone and anti-Siglec-8/IL-5. We found that the morphology of dying cells in anti-Siglec-8/IL-5 co-stimulated cells trended to be more necrotic (p = 0.055, n = 6 independent experiments with 6 independent donors) than that of dying cells treated with anti-Siglec-8 alone (Figure 1A–B). Using an independent approach, we assessed the percentage of 7AAD-positive cells among all Annexin V-positive cells as an indicator of either increased transition of apoptotic cells to secondary necrosis or cells dying primarily by necrosis (example in Figure 1C). Anti-Siglec-8/IL-5 co-stimulated cells had a significantly higher ratio of 7AAD-positive cells compared with cells treated with anti-Siglec-8 alone (Figure 1D, p < 0.001, n = 25 experiments with 11 independent donors). Analysis at early time points (e.g. 8 hours) also showed greater proportion of 7AAD-positive cells in Anti-Siglec-8/IL-5 co-stimulated conditions (data not shown) suggesting direct entry into necrosis. Finally, we could detect greater release of eosinophil peroxidase (EPO) in co-stimulated cells compared with cells treated with IL-5 alone or anti-Siglec-8 alone (Figure 1E). Together with the caspase independence of cell death in IL-5-activated eosinophils, these results suggest that Siglec-8 activation induces different pathways of cell death in activated versus resting eosinophils.
ROS are sufficient for eosinophil cell death and IL-5 enhancement of cell death
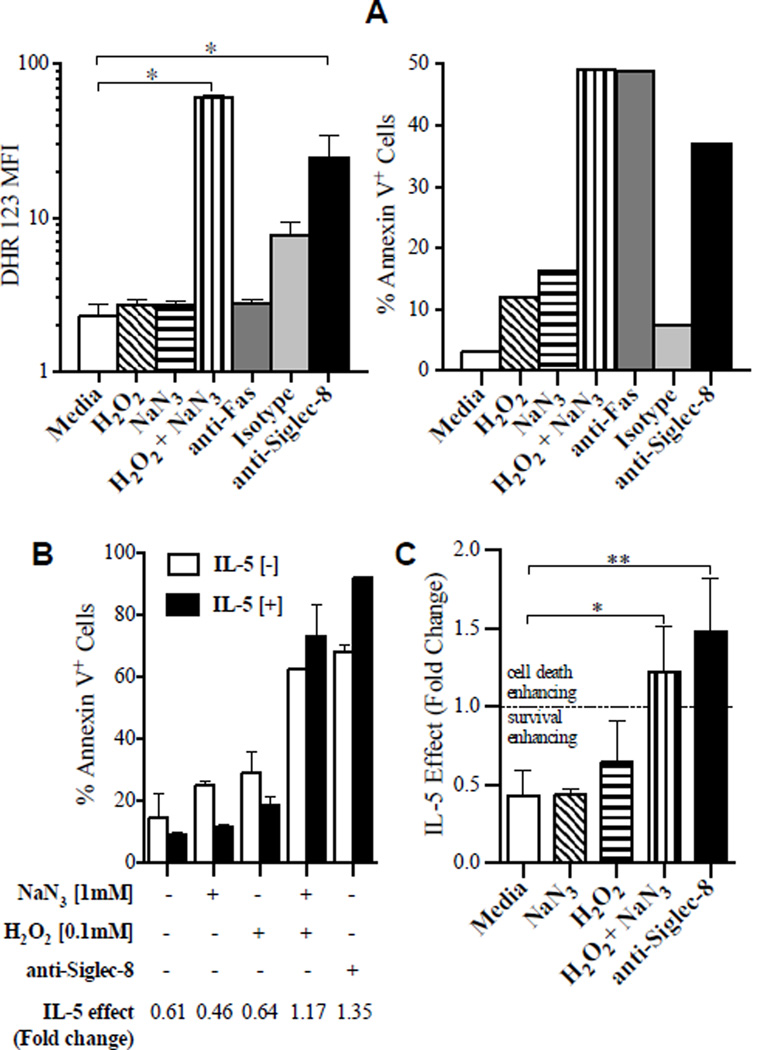
Since ROS were required for anti-Siglec-8-induced cell death and have been implicated in caspase-independent modes of cell death, we hypothesized that ROS are sufficient for the reversal of IL-5’s function from pro-survival to cell death enhancing. Thus, we artificially increased levels of ROS in eosinophils in the presence and absence of IL-5. Treating neutrophils with hydrogen peroxide (H2O2) and sodium azide (NaN3) in combination, but not singly, induced accumulation of intracellular ROS and cell death;21 we observed similar effects in eosinophils (Figure 2A). The amount of ROS accumulation was comparable in H2O2/NaN3 stimulation and anti-Siglec-8 stimulation. Anti-Fas stimulation did not induce accumulation of ROS in eosinophils at the same time point, although the induction of cell death was comparable to anti-Siglec-8 and H2O2/NaN3 stimulation, further supporting the hypothesis that ROS is a mediator, rather than simply a marker, of cell death (Figure 2A). We next examined the effect of IL-5 on this ROS-induced cell death. While IL-5 prevented spontaneous eosinophil cell death, IL-5 enhanced cell death in H2O2/NaN3-treated cells similarly to in anti-Siglec-8-treated cells (see example in Figure 2B). For instance, in a representative experiment, IL-5 decreased the % of AnnexinV+ cells in media, NaN3-treated, and H2O2-treated cells, showing survival enhancement (Figure 2B). In contrast, IL-5 increased the % of Annexin V+ cells in samples treated with H2O2/NaN3 and anti-Siglec-8, showing cell death enhancement. The average of the IL-5 effects (fold change) from 3 experiments are shown in Figure 2C (p < 0.05 and <0.01 for the IL-5-induced effect in H2O2/NaN3- and anti-Siglec-8-treated cells, respectively, compared to control cells). These data suggest that the presence of intracellular ROS is sufficient to induce cell death and to switch IL-5’s function from enhancing survival to enhancing cell death. While IL-5 without intracellular ROS leads to eosinophil survival, IL-5 in the context of intracellular ROS induces cell death above and beyond that induced by ROS alone.
Figure 2. ROS generation is sufficient for eosinophil cell death and IL-5 enhancement of cell death.
A, ROS accumulation induced by exogenous H2O2 ± NaN3, crosslinked anti-Siglec-8 or isotype-matched control antibody was measured following 2 hours of incubation and 30 minutes of loading with DHR. Results are shown as mean fluorescence intensity (MFI) values of DHR (left panel). Aliquots of the same cells were also incubated for 24 hours without DHR in order to determine Annexin V-positive staining (right panel). Representative of 3 experiments.
B, Representative experiment of eosinophils cultured with (closed bar) or without (open bar) IL-5 in conditions with H2O2 ± NaN3 or anti-Siglec-8. The IL-5 effect was determined by dividing the percentage of Annexin V-positive cells in IL-5[+] with IL-5[-] for each condition. Thus, IL-5 effect <1.0 represents survival enhancement, whereas >1.0 implies cell death enhancement.
C, Averaged results from 3 experiments and donors. * p < 0.05, ** p < 0.01.
The MEK/ERK pathway is selectively up-regulated and sustained upon anti-Siglec-8/IL-5 co-stimulation
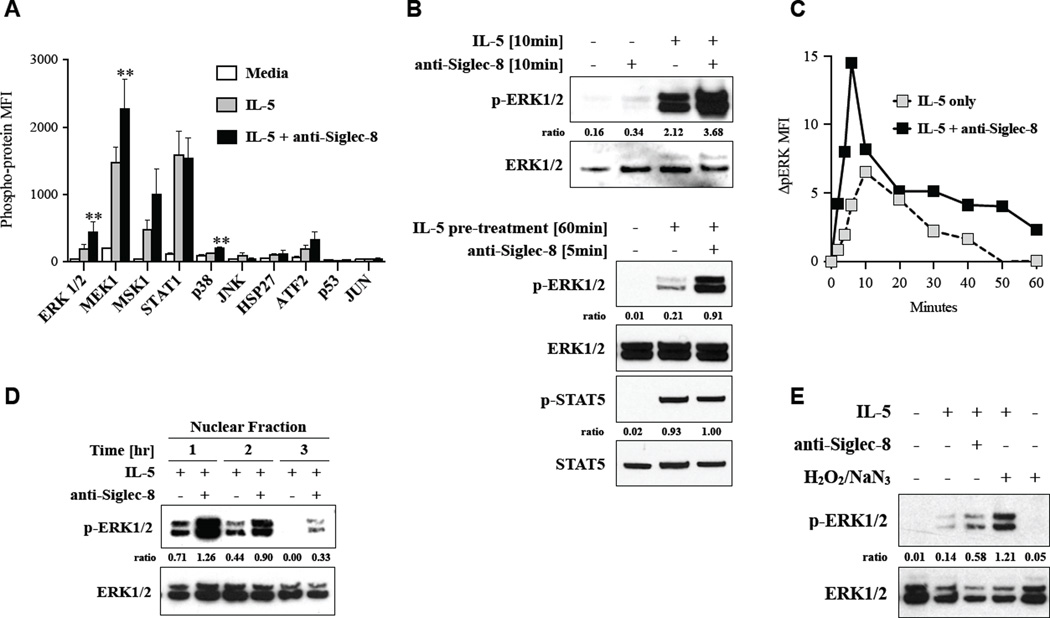
Our data suggested that ROS interact with one of the signaling pathways activated by IL-5. To evaluate possible activation of MAPK signaling, we first employed a comprehensive immunochemical approach with a multiplex bead assay for 10 phosphorylated proteins involved in MAPK signaling. As shown in Figure 3A, ERK and MEK1 were significantly up-regulated in eosinophils co-stimulated with anti-Siglec-8/IL-5 for 10 minutes compared to in eosinophils stimulated with IL-5 alone. Especially, ERK phosphorylation in anti-Siglec-8/IL-5 co-stimulated cells was more than 2-fold higher than in cells stimulated with IL-5 alone (p < 0.01, n = 4 donors). MSK1 phosphorylation was activated by IL-5 and showed a trend to increase with anti-Siglec-8 co-stimulation, but this increase did not reach statistical significance. STAT1 phosphorylation was activated upon IL-5 stimulation as described before 22 but was not significantly enhanced by co-stimulation with anti-Siglec-8. Members of the p38 and JNK pathways, namely p38, JNK, HSP27, ATF2, p53, and JUN, were not robustly induced by IL-5 with or without anti-Siglec-8. On the basis of these findings, we focused on the ERK pathway and confirmed our data with two independent methods. As shown in Figure 3B, the up-regulation of IL-5-induced phospho-ERK by co-stimulation with anti-Siglec-8 was also observed by Western blotting (upper panel). Cells stimulated with anti-Siglec-8 in the absence of IL-5 did not show ERK activation. This up-regulation was even more distinct when anti-Siglec-8 was added 1 hour after pre-treatment of IL-5 (lower panel). This is consistent with previous studies demonstrating IL-5 enhancement occurs with either pretreatment or co-stimulation.18 In contrast to the MEK/ERK pathway, STAT5 was phosphorylated by IL-5 but not enhanced by the co-stimulation with anti-Siglec-8 (lower panel). While ERK is classically associated with cell survival and activation following cytokine stimulation, recent studies have implicated ERK in cell death processes;23 moreover, there is often concomitant ROS accumulation in scenarios where ERK acts as cell death mediator.24, 25 Since the involvement of ERK in survival versus cell death is often determined by spatio-temporal regulation,26 we measured the kinetics of ERK phosphorylation in eosinophils co-stimulated with anti-Siglec-8 and IL-5. Flow cytometry for intracellular ERK phosphorylation demonstrated peak phosphorylation at 5–10 minutes in both IL-5-stimulated and anti-Siglec-8/IL-5 co-stimulated eosinophils (Figure 3C). Moreover, ERK phosphorylation was enhanced and sustained. To measure the duration of the ERK activity, we next examined the nuclear fraction of eosinophil lysates by Western blotting, since ERK is sequestered in the nucleus upon its phosphorylation.27 As shown in Figure 3D, ERK phosphorylation was sustained longer (at least 3 hours) in the nucleus of eosinophils stimulated with IL-5 + anti-Siglec-8 than of eosinophils stimulated with IL-5 alone (Figure 3D). Levels of phospho-ERK were consistently lower in the cytoplasmic fraction compared with the nuclear fraction, but the enhancement and sustained activation in anti-Siglec-8/IL-5 co-stimulated cells compared with IL-5-stimulated cells was consistent with the findings in the nuclear fraction (data not shown). These observations, along with the fact that increases in intracellular ROS can mimic Siglec-8 activation to promote cell death in IL-5-stimulated cells (Figure 2C), suggested that intracellular ROS itself should enhance ERK phosphorylation. To corroborate this, we tested the effect of artificial accumulation of intracellular ROS on ERK phosphorylation. As shown in Figure 3E, addition of H2O2/NaN3 indeed induced prolonged phosphorylation of ERK in IL-5 stimulated cells as is seen with anti-Siglec-8 antibody, whereas cells incubated with H2O2/NaN3 in the absence of IL-5 did not show phosphorylation of ERK. Together, these data demonstrate enhanced and sustained phosphorylation of ERK in co-stimulated cells and raise the possibility that enhanced activation of MEK1/ERK is important for the enhanced eosinophil cell death induced by anti-Siglec-8/IL-5 co-stimulation.
Figure 3. Up-regulation of MEK/ERK pathway following anti-Siglec-8/IL-5 co-stimulation.
A, A phospho-MAPK multiplex bead assay was performed on lysates of eosinophils (n = 4 donors) that were incubated for 10 minutes with indicated stimuli. ** p < 0.01 compared to IL-5.
B, Eosinophils incubated in the indicated conditions were analyzed by Western blotting with specific antibodies for phospho-ERK or total ERK (upper panel) and phospho- and total STAT5. Representative data from 3 independent experiments.
C, Time course of ERK phosphorylation was determined by flow cytometry. Representative of 3 experiments.
D, Western blotting of nuclear fraction from cell lysates of cells treated for 1, 2, or 3 hours. Representative of 3 experiments. Ratio of densitometric value for the band of phospho-kinase to that of total kinase in each lane is indicated.
E, Western blotting of nuclear fraction from cell lysates of cells stimulated for 2 hours with indicated stimuli (representative of 3 experiments).
ERK activation is required for eosinophil cell death induced by anti-Siglec-8/IL-5 co-stimulation
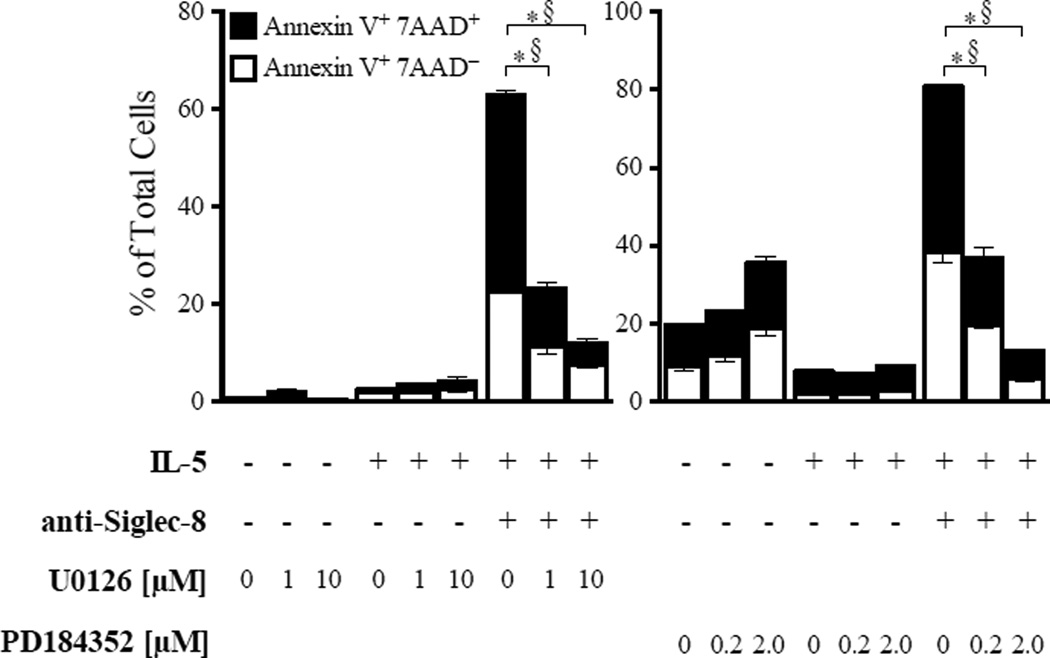
To evaluate the possible role of MEK1/ERK in eosinophil cell death induced by anti-Siglec-8 in IL-5-activated eosinophils, we tested two structurally unrelated inhibitors of MEK1. As shown in Figure 4, pre-incubation with U0126 and PD184352 significantly reduced cell death in eosinophils co-stimulated with IL-5 and anti-Siglec-8 in a dose-dependent manner. Both Annexin V+ 7AAD− and Annexin V+ 7AAD+ cells were decreased in samples treated with MEK1 inhibitors, possibly because IL-5-mediated survival pathways are independent of MAPK activation,28 and thus their action was uncovered by removal of ERK-induced cell death enhancement. Indeed, inhibition of MEK1 did not affect IL-5-afforded survival (Figure 4), consistent with previous reports.29 Together, these data support our hypothesis that the ERK pathway is involved in eosinophil cell death induced by co-stimulation with IL-5 and anti-Siglec-8.
Figure 4. Upregulation of MEK/ERK pathway is required for enhanced cell death induced by anti-Siglec-8/IL-5 co-stimulation.
Eosinophils were pre-incubated with A) U0126 or B) PD184352 at the indicated doses and then cultured in media containing IL-5 ± anti-Siglec-8. Annexin V+ and/or 7AAD+ cells were determined after 24 hours. Shown are representative data from 3 experiments with each inhibitor. *p <0.01 and §p <0.01, compared to % Annexin V+ 7AAD+ and % Annexin V+ 7AAD−, respectively, in IL-5 + anti-Siglec-8 treated cells without inhibitors.
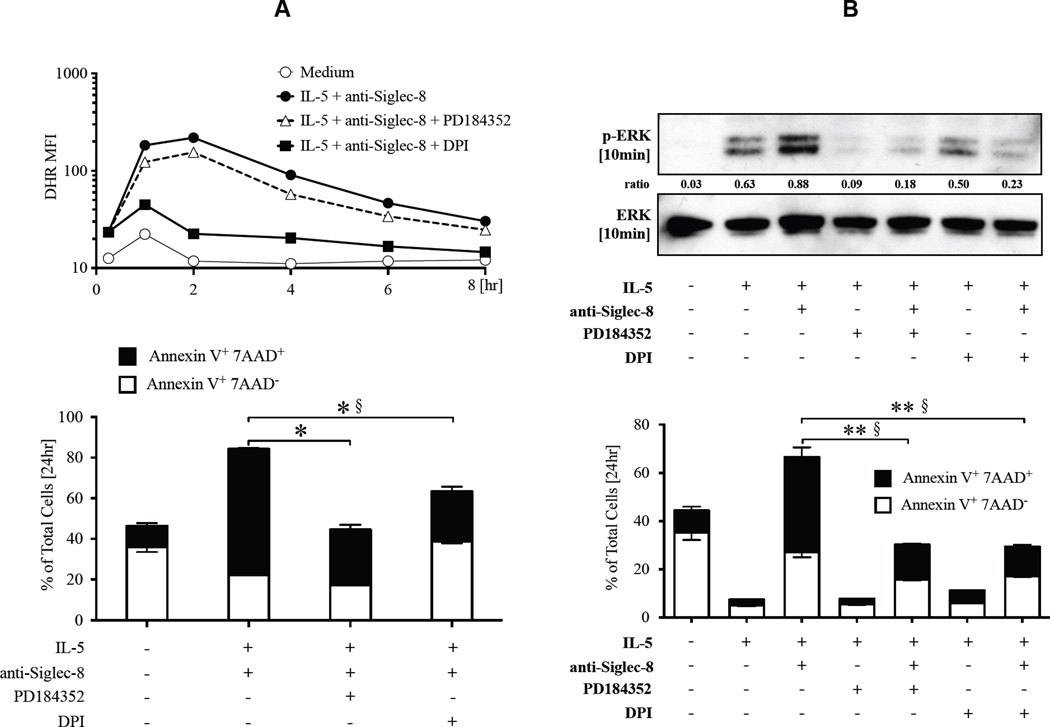
ROS production is required for ERK up-regulation upon anti-Siglec-8/IL-5 co-stimulation
We next examined the hierarchal regulation of ROS and ERK activation. The ROS inhibitor DPI completely suppressed ROS production as expected (Figure 5A). In contrast, inhibition of MEK1 did not significantly affect ROS levels, suggesting ERK is not an upstream mediator of ROS. In contrast, when examining the effects of ROS inhibition on ERK up-regulation, DPI inhibited the up-regulated ERK phosphorylation in eosinophils co-stimulated with anti-Siglec-8 and IL-5, and resulted in inhibition of cell death (Figure 5B). Similarly, inhibition of both ROS production and MEK1 completely inhibited anti-Siglec-8/IL-5-induced eosinophil cell death (data not shown). DPI did not inhibit ERK phosphorylation in the presence of IL-5 alone, whereas PD184352 completely inhibited both IL-5-induced and anti-Siglec-8/IL-5-induced ERK phosphorylation. Together, these results support ROS as an upstream regulator of ERK activation in cells co-stimulated with anti-Siglec-8 and IL-5.
Figure 5. MEK/ERK up-regulation is downstream of the ROS production induced by anti-Siglec-8/IL-5 co-stimulation.
Eosinophils were pre-incubated with or without inhibitors and then cultured in media containing IL-5 and anti-Siglec-8. The lower panel shows Annexin V staining of cells from the same experiment at the 24-hour time point. In A, cells were stained with DHR and ROS level analyzed with flow cytometry (representative of 3 experiments). In B, Western blot of lysates from cells harvested after 10 minutes of stimulation is shown (representative of 3 experiments). Ratio of densitometric value for band of phospho-ERK to that of total ERK in each lane is displayed. *p <0.01 and §p <0.01, compared to % Annexin V+ 7AAD+ and % Annexin V+ 7AAD−, respectively, in IL-5 + anti-Siglec-8 treated cells without inhibitors.
Discussion
In this study, we demonstrate a novel mechanism by which activated eosinophils are more prone to die than resting eosinophils. Specifically, our data support a model wherein engagement of Siglec-8 leads to ROS accumulation in the cell. In a resting eosinophil, which has not had IL-5 exposure, this leads to ROS-dependent and caspase-dependent apoptosis, as has been shown previously.4, 17 In contrast, in an activated eosinophil, in which IL-5 exposure has activated the MEK-1/ERK-1/2 pathway, ROS enhances activation of the MEK/ERK pathway leading to a biochemically and morphologically distinct mode of cell death. This mode of cell death is independent of caspase activation.18 Furthermore, our results indicate that it is dependent on ROS and the consequent MEK/ERK pathway enhancement and resembles necrotic cell death in terms of cell morphology, association with loss of membrane integrity, and lack of exclusion of 7AAD. These findings are consistent with previous reports that have implicated ROS and MAPK pathways in the process of regulated necrosis.30 Notably, stimulation with IL-5 alone leads to enhancement of eosinophil viability.31 Thus, the decision of whether an eosinophil lives or dies by apoptosis or a form of regulated necrosis is integrated at the level of ROS and MAPK signaling. This discovery is reminiscent of the recent appreciation that a single cytokine, such as TNF-α, can induce cell survival, apoptosis, or necroptosis/regulated necrosis depending on the signaling context.32
We demonstrate that co-stimulation of eosinophils with anti-Siglec-8 and IL-5 enhances phosphorylation of multiple proteins in the MEK/ERK pathway and that this pathway is involved in Siglec-8-mediated cell death of IL-5-activated eosinophils. While the studies presented here have focused on IL-5, it is interesting to note that IL-33 also enhances Siglec-8-mediated cell death and that IL-33 also induces ERK activation,15, 33 suggesting that both cytokines may use the same mechanism and the broader significance of this mechanism in cell survival/death decisions. ERK is classically associated with cell survival; however, recent studies have implicated ERK in several cell death mechanisms including apoptosis, autophagic cell death, and programmed necrosis (a.k.a. necroptosis).23, 34–36 The mechanism of ERK-induced cell death, as opposed to cell survival and/or activation, has been hypothesized to involve enhanced levels of phosphorylation, sustained phosphorylation, and/or extended nuclear compartmentalization.26, 37 In our study, we observed enhanced and sustained ERK phosphorylation, supporting that this altered ERK activation and localization may be a decisive event in the cell death of cells co-stimulated with anti-Siglec-8 and classical pro-survival cytokines such as IL-5.
Sustained activation of phospho-ERK has been associated with ROS-induced inactivation of ERK-specific phosphatases.38 For instance, dual-specificity phosphatases (DUSP) are able to dephosphorylate both the threonine and tyrosine residues within the activation loop of MAPK.39, 40 Enzymatic activity of DUSPs requires a catalytic cysteine residue sensitive to oxidation.39, 41 ROS have been shown to inhibit ERK-directed phosphatases, DUSP1 and DUSP6, by oxidation of their catalytic cysteine residues.42, 43 Indeed, in our study we demonstrate that ERK up-regulation and cell death following anti-Siglec-8/IL-5 co-stimulation is ROS dependent. We also show that ROS are sufficient to induce eosinophil cell death (simulating activation by anti- Siglec-8) and that ROS accumulation in cells co-stimulated with IL-5 (simulating co-stimulation with IL-5 and anti-Siglec-8) enhances this cell death, and reverts the effect of IL-5 from pro-survival to pro-cell death. Together, our data implicate ROS-induced enhancement of ERK phosphorylation in Siglec-8-mediated cell death in activated eosinophils.
Our discovery of a new mechanism regulating both cell survival versus cell death and mode of cell death is significant because eosinophil cell death contributes to the pathophysiology of multiple diseases. Ultrastructural and immunohistochemical studies in human tissue from patients with eosinophilic diseases, including allergic disease and hypereosinophilic syndrome (HES)-related endomyocardial disease, demonstrate significant eosinophil cytolysis, which may be a consequence of accidental or biochemically regulated necrosis.44–46 This is often accompanied by release of eosinophil granule proteins in diseased tissue,47, 48 similar to our study showing release of EPO. Importantly, even though the number of eosinophils was decreased in the bronchial tissue of patients with asthma by ~50% after anti-IL-5 treatment, the deposition of extracellular eosinophil granule protein MBP did not differ compared to prior to treatment or compared to patients treated with placebo. 49 Furthermore, intact extracellular eosinophil granules have the ability to function as secretory organelles extracellularly after eosinophil cytolysis.50 Prior studies have focused on targeting eosinophil apoptosis because it is a regulated process and therefore subject to interference, whereas necrosis was viewed as an uncontrollable event until recently. However, studies have now shown that in certain situations, necrosis is biochemically regulated by receptor-interacting protein kinases (RIPK), which can be inhibited by specific inhibitors (necrostatins).51, 52 The ability to inhibit necrosis or redirect cell death from necrosis to apoptosis could lead to improved therapies. Notably, necrostatin treatment significantly improved disease outcomes in animal models of several diseases, such as myocardial infarction, stroke, and systemic inflammatory response syndrome.52–54
In the case of eosinophils, both alive and dead eosinophils can lead to positive and negative outcomes. For instance, eosinophils are homeostatically present in some tissues with no ill effect, presumably because they are restrained in part by signaling through inhibitory receptors, as was shown for SIRP-α and CD22 in mice.55, 56 During eosinophilic inflammatory diseases, eosinophils display an activated phenotype and lead to tissue destruction. Homeostatic or inflammatory eosinophils may die silent deaths by either apoptosis or egress from tissue; however, in many diseases, eosinophils have destructive deaths, as outlined above. Thus, understanding the mechanisms of cell death in eosinophils, especially activated eosinophils, may lead us closer to the goal of preventing destructive forms of eosinophil cell death while not affecting homeostatic functions of eosinophils or silent forms of eosinophil cell death. Furthermore, several pathways that modulate eosinophil survival or cell death are either targets or proposed targets for therapy, including IL-5 and Siglec-8 respectively. Therefore, understanding the biochemical pathways and outcomes that could be affected by such therapy is critical for rational design of specific targets and predicting, preventing, or explaining side effects. Importantly, in our study we demonstrate that resting and activated eosinophils undergo different modes of cell death, apoptosis and a form of regulated necrosis, respectively. Furthermore, our mechanistic studies suggest that ROS and ERK activation are important mediators of the caspase-independent, yet biochemically regulated, anti-Siglec-8-induced cell death of IL-5-activated eosinophils.
Key messages.
Ligation of Siglec-8 in IL-5 activated human eosinophils leads to ROS-dependent enhancement of ERK phosphorylation with subsequent necrotic cell death.
Our findings represent a novel mechanism by which activated eosinophils are more prone to die than resting eosinophils.
Our findings also demonstrate occurrence of biochemically-regulated necrosis in human eosinophils.
Acknowledgements
The authors thank Shawna Hottinger for editorial assistance, and Dr. Mekibib Altaye for assistance with statistical analysis.
Funding sources: This work was supported in part by NIH grants AI088559 (to NZ), P30 DK078392, AI72265 (to BSB) and a grant from the Dana Foundation (to BSB and NZ).
Abbreviations
- IL-5
Interleukin-5
- ROS
Reactive Oxygen Species
- MAPK
Mitogen-activated Protein Kinases
- 7AAD
7-Amino-Actinomycin D
- ERK
Extracellular signal-Regulated Kinases
- MEK1
MAPK/ERK kinase1
- DPI
Diphenyleneiodonium
- DHR 123
Dihydrorhodamine 123
- ITIM
Immunoreceptor Tyrosine-based Inhibitory Motifs
- MSK1
Mitogen-and Stress-activated protein Kinase
- STAT
Signal Transducer and Activator of Transcription
- JNK
c-Jun N-terminal Kinase
- HSP27
Heat shock protein 27
- ATF2
Activating Transcription Factor 2
- FITC
Fluorescein Isothiocyanate
- APC
Allophycocyanin
- PFA
Paraformaldehyde
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Bochner is a co-inventor on existing and pending Siglec-8-related patents. Dr. Bochner may be entitled to a share of royalties received by the University on the potential sales of such products. Dr. Bochner is also a co-founder of, owns stock in, and is on the scientific advisory board of Allakos, Inc., which makes him subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Von Gunten S, Bochner BS. Basic and clinical immunology of Siglecs. Ann N Y Acad Sci. 2008;1143:61–82. doi: 10.1196/annals.1443.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci. 2009;30:240–248. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, et al. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–866. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 4.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 5.Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D'Alessio KJ, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol. 2000;105:1093–1100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- 6.Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39:317–324. doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther. 2012;135:327–336. doi: 10.1016/j.pharmthera.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aizawa H, Zimmermann N, Carrigan PE, Lee JJ, Rothenberg ME, Bochner BS. Molecular analysis of human Siglec-8 orthologs relevant to mouse eosinophils: identification of mouse orthologs of Siglec-5 (mSiglec-F) and Siglec-10 (mSiglec-G) Genomics. 2003;82:521–530. doi: 10.1016/s0888-7543(03)00171-x. [DOI] [PubMed] [Google Scholar]
- 9.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6'-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–1135. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–1163. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, et al. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183:5333–5341. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song DJ, Cho JY, Miller M, Strangman W, Zhang M, Varki A, et al. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin Immunol. 2009;131:157–169. doi: 10.1016/j.clim.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho JY, Song DJ, Pham A, Rosenthal P, Miller M, Dayan S, et al. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 2010;11:154. doi: 10.1186/1465-9921-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–4287. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Na HJ, Hudson SA, Bochner BS. IL-33 enhances Siglec-8 mediated apoptosis of human eosinophils. Cytokine. 2012;57:169–174. doi: 10.1016/j.cyto.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, et al. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. 2007;119:1005–1011. doi: 10.1016/j.jaci.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005;336:918–924. doi: 10.1016/j.bbrc.2005.08.202. [DOI] [PubMed] [Google Scholar]
- 18.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 Priming of Human Eosinophils Alters Siglec-8 Mediated Apoptosis Pathways. Am J Respir Cell Mol Biol. 2008;38:121–124. doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kottyan LC, Collier AR, Cao KH, Niese KA, Hedgebeth M, Radu CG, et al. Eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner. Blood. 2009;114:2774–2782. doi: 10.1182/blood-2009-05-220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adamko DJ, Wu Y, Gleich GJ, Lacy P, Moqbel R. The induction of eosinophil peroxidase release: improved methods of measurement and stimulation. J Immunol Methods. 2004;291:101–108. doi: 10.1016/j.jim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Lundqvist-Gustafsson H, Bengtsson T. Activation of the granule pool of the NADPH oxidase accelerates apoptosis in human neutrophils. J Leukoc Biol. 1999;65:196–204. doi: 10.1002/jlb.65.2.196. [DOI] [PubMed] [Google Scholar]
- 22.Pazdrak K, Justement L, Alam R. Mechanism of inhibition of eosinophil activation by transforming growth factor-beta. Inhibition of Lyn, MAP, Jak2 kinases and STAT1 nuclear factor. J Immunol. 1995;155:4454–4458. [PubMed] [Google Scholar]
- 23.Cagnol S, Chambard J-C. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsunaga Y, Kawai Y, Kohda Y, Gemba M. Involvement of activation of NADPH oxidase and extracellular signal-regulated kinase (ERK) in renal cell injury induced by zinc. J Toxicol Sci. 2005;30:135–144. doi: 10.2131/jts.30.135. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y-J, Cho H-N, Soh J-W, Jhon GJ, Cho C-K, Chung H-Y, et al. Oxidative stress-induced apoptosis is mediated by ERK1/2 phosphorylation. Exp Cell Res. 2003;291:251–266. doi: 10.1016/s0014-4827(03)00391-4. [DOI] [PubMed] [Google Scholar]
- 26.Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci. 2005;118:2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda M, Gotoh I, Adachi M, Gotoh Y, Nishida E. A novel regulatory mechanism in the mitogenactivated protein (MAP) kinase cascade. Role of nuclear export signal of MAP kinase kinase. J Biol Chem. 1997;272:32642–32648. doi: 10.1074/jbc.272.51.32642. [DOI] [PubMed] [Google Scholar]
- 28.Andina N, Didichenko S, Schmidt-Mende J, Dahinden CA, Simon H-U. Proviral integration site for Moloney murine leukemia virus 1, but not phosphatidylinositol-3 kinase, is essential in the antiapoptotic signaling cascade initiated by IL-5 in eosinophils. J Allergy Clin Immunol. 2009;123:603–611. doi: 10.1016/j.jaci.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Kankaanranta H, De Souza PM, Barnes PJ, Salmon M, Giembycz MA, Lindsay MA. SB 203580, an inhibitor of p38 mitogen-activated protein kinase, enhances constitutive apoptosis of cytokine-deprived human eosinophils. J Pharmacol Exp Ther. 1999;290:621–628. [PubMed] [Google Scholar]
- 30.Zhuang S, Kinsey GR, Yan Y, Han J, Schnellmann RG. Extracellular signal-regulated kinase activation mediates mitochondrial dysfunction and necrosis induced by hydrogen peroxide in renal proximal tubular cells. Journal of Pharmacology and Experimental Therapeutics. 2008;325:732–740. doi: 10.1124/jpet.108.136358. [DOI] [PubMed] [Google Scholar]
- 31.Rothenberg ME, Pomerantz JL, Owen WF, Avraham S, Soberman RJ, Austen KF, et al. Characterization of a human eosinophil proteoglycan, and augmentation of its biosynthesis and size by interleukin 3, interleukin 5, and granulocyte/macrophage colony stimulating factor. J Biol Chem. 1988;263:13901–13908. [PubMed] [Google Scholar]
- 32.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Chow JYS, Wong CK, Cheung PFY, Lam CWK. Intracellular signaling mechanisms regulating the activation of human eosinophils by the novel Th2 cytokine IL-33: implications for allergic inflammation. Cell Mol Immunol. 2010;7:26–34. doi: 10.1038/cmi.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devin A, Lin Y, Liu Z-G. The role of the death-domain kinase RIP in tumour-necrosis-factor-induced activation of mitogen-activated protein kinases. EMBO Rep. 2003;4:623–627. doi: 10.1038/sj.embor.embor854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y-T, Tan H-L, Huang Q, Sun X-J, Zhu X, Shen H-M. zVAD-induced necroptosis in L929 cells depends on autocrine production of TNFα mediated by the PKC-MAPKs-AP-1 pathway. Cell Death Differ. 2011;18:26–37. doi: 10.1038/cdd.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramaniam S, Zirrgiebel U, von Bohlen Und Halbach O, Strelau J, Laliberté C, Kaplan D, et al. ERK activation promotes neuronal degeneration predominantly through plasma membrane damage and independently of caspase-3. J Cell Biol. 2004;165:357–369. doi: 10.1083/jcb.200403028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pouysségur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem Pharmacol. 2002;64:755–763. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- 39.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 40.Jeffrey KL, Camps M, Rommel C, Mackay CR. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov. 2007;6:391–403. doi: 10.1038/nrd2289. [DOI] [PubMed] [Google Scholar]
- 41.Kondoh K, Nishida E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim Biophys Acta. 2007;1773:1227–1237. doi: 10.1016/j.bbamcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Levinthal DJ, DeFranco DB. Reversible oxidation of ERK-directed protein phosphatases drives oxidative toxicity in neurons. J Biol Chem. 2005;280:5875–5883. doi: 10.1074/jbc.M410771200. [DOI] [PubMed] [Google Scholar]
- 43.Kamata H, Honda S-I, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 44.Uller L, Anderson M, Greiff L, Persson CGA, Erjefält JS. Occurrence of apoptosis, secondary necrosis, and cytolysis in eosinophilic nasal polyps. Am J Respir Crit Care Med. 2004;170:742–747. doi: 10.1164/rccm.200402-240OC. [DOI] [PubMed] [Google Scholar]
- 45.Armengot M, Garín L, Carda C. Eosinophil degranulation patterns in nasal polyposis: an ultrastructural study. Am J Rhinol Allergy. 2009;23:466–470. doi: 10.2500/ajra.2009.23.3357. [DOI] [PubMed] [Google Scholar]
- 46.Wright BL, Leiferman KM, Gleich GJ. Eosinophil granule protein localization in eosinophilic endomyocardial disease. N Engl J Med. 2011;365:187–188. doi: 10.1056/NEJMc1103005. [DOI] [PubMed] [Google Scholar]
- 47.Protheroe C, Woodruff SA, de Petris G, Mukkada V, Ochkur SI, Janarthanan S, et al. A novel histologic scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:749–755. e11. doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willetts L, Parker K, Wesselius LJ, Protheroe CA, Jaben E, Graziano P, et al. Immunodetection of occult eosinophils in lung tissue biopsies may help predict survival in acute lung injury. Respir Res. 2011;12:116. doi: 10.1186/1465-9921-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 50.Neves JS, Perez SAC, Spencer LA, Melo RCN, Reynolds L, Ghiran I, et al. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci U S A. 2008;105:18478–18483. doi: 10.1073/pnas.0804547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 53.Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 54.Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21:227–233. doi: 10.1007/s10557-007-6035-1. [DOI] [PubMed] [Google Scholar]
- 55.Verjan Garcia N, Umemoto E, Saito Y, Yamasaki M, Hata E, Matozaki T, et al. SIRPα/CD172a regulates eosinophil homeostasis. J Immunol. 2011;187:2268–2277. doi: 10.4049/jimmunol.1101008. [DOI] [PubMed] [Google Scholar]
- 56.Wen T, Mingler MK, Blanchard C, Wahl B, Pabst O, Rothenberg ME. The pan-B cell marker CD22 is expressed on gastrointestinal eosinophils and negatively regulates tissue eosinophilia. J Immunol. 2012;188:1075–1082. doi: 10.4049/jimmunol.1102222. [DOI] [PMC free article] [PubMed] [Google Scholar]