Abstract
In prostate cancer (PCa), the functional synergy between androgen receptor (AR) and nuclear factor-κ B (NF-κB) escalates the resistance to therapeutic regimens and promotes aggressive tumor growth. Although the underlying mechanisms are less clear, gene regulatory abilities of coactivators can bridge the transcription functions of AR and NF-κB. The present study shows that MYST1 (MOZ, YBF2 and SAS2, and TIP60 protein 1) costimulates AR and NF-κB functions in PCa cells. We demonstrate that activation of NF-κB promotes deacetylation of MYST1 by sirtuin 1. Further, the mutually exclusive interactions of MYST1 with sirtuin 1 vs AR regulate the acetylation of lysine 16 on histone H4. Notably, in AR-lacking PC3 cells and in AR-depleted LNCaP cells, diminution of MYST1 activates the cleavage of poly(ADP-ribose) polymerase and caspase 3 that leads to apoptosis. In contrast, in AR-transformed PC3 cells (PC3-AR), depletion of MYST1 induces cyclin-dependent kinase (CDK) N1A/p21, which results in G2M arrest. Concomitantly, the levels of phospho-retinoblastoma, E2F1, CDK4, and CDK6 are reduced. Finally, the expression of tumor protein D52 (TPD52) was unequivocally affected in PC3, PC3-AR, and LNCaP cells. Taken together, the results of this study reveal that the functional interactions of MYST1 with AR and NF-κB are critical for PCa progression.
Deregulation of androgen receptor (AR) functions and persistent activation of nuclear factor-κ B (NF-κB) are central to the growth of prostate cancer (PCa) (1–6). PCa remains the second most prevalent malignancy that accounts for a significant number of deaths, which is still rising (7). Despite the fact that during the initial stages most PCas respond to androgen-ablative therapy (5–8), the majority relapse and progress to a stage called castration-resistant PCa. Restoration of AR activity due to amplification or mutation within the AR gene remains one of the hallmarks of castration-resistant PCa (7–9). A growing number of studies propose that restored AR functions in castration-resistant PCa cells disrupt the AR/coactivator balance. The resulting increase in AR signaling perturbs the control of cell cycle regulatory genes, leading to aggressive proliferation of PCa cells (10). Although AR is an androgen-dependent transcription factor, the role of coactivators remains indispensable during androgen-dependent as well as -independent PCa (11–12). Further, the cross talk between AR and NF-κB signaling enhances the proliferative and antiapoptotic effects (11, 13). However, the underlying mechanisms remain obscure. Because transcriptional activities of AR and NF-κB are regulated by coactivators, a common coactivator could boost the synergistic actions of AR and NF-κB in advanced PCa.
The transcriptional function of AR, which controls the G1 to S transition by regulating cyclin-dependent kinase inhibitor 1A (CDKN1A or p21), cyclin-dependent kinases (CDKs), E2F1, and retinoblastoma protein, is governed by its epigenetic posttranslational modifications and its molecular interactions with coactivators (7, 10, 14). A recent study demonstrated cooperation between transcription factors AR and E2F1 for regulating androgen-responsive targets in PCa cells (15), thereby raising speculation that AR and E2F1 functions are controlled by a common regulator. Transcriptional coactivators possessing histone acetyltransferase activity (HAT) modulate the functions of several key transcription factors including p53, NF-κB, and AR (16, 17). HAT coactivators acetylate several lysine residues within the hinge region, particularly the 629RKLKK633 motif of AR, which are required for nuclear localization and transcriptional activation of AR, leading to cellular proliferation (1, 4–6, 18–22). In addition, amplification and overexpression of tumor protein D52 (TPD52) leads to PCa proliferation (23). NF-κB is a vital transcription factor that governs cellular responses to chemotherapeutic agents, infection, and inflammatory and oncogenic signals (24, 25). Activation of one of the NF-κB target genes, intercellular adhesion molecule 1 (ICAM1) leads to cellular invasion and metastasis that prevent apoptosis (26, 27). Overexpression of the p65 subunit of NF-κB in many cancers including head and neck, breast, and prostate strongly demonstrates a direct role of NF-κB in tumor growth (11, 13). A recent study showed that NF-κB enhances the transcriptional activity of AR, which leads to increased secretion of prostate-specific antigen (PSA) and the proliferation of PCa (28). Although the roles of coactivators are established in regulating the individual functions of AR and NF-κB, we report here the role of a coactivator, MYST1, which has the potential to simultaneously control genes, including E2F1, CDKN1A, TPD52, and ICAM1 in PCa cells.
MYST1 (MOZ, YBF2 and SAS2, and TIP60 protein 1; also known as MOF or KAT8) is a well-known member of the MYST family and is composed of a chromodomain, a zinc finger motif, and a HAT domain (29, 30). Acetylation of lysine 16 on histone H4 (H4K16ac) by MYST1 regulates chromatin assembly, transcription activation, and cellular apoptosis upon DNA damage (29–31). In addition, MYST1 undergoes autoacetylation at lysine 274, which is deacetylated by sirtuin 1 (32). However, the biological significance of MYST1 autoacetylation is not fully understood. Down-regulation of MYST1 causes cell cycle defects, reduced gene transcription, a defective response to DNA damage, and embryonic lethality (33–36). These data underline the critical role for MYST1 in a multitude of cellular processes (36, 37). Furthermore, in-depth investigation is required to unravel the functional role of MYST1 in cancers, especially because in primary breast cancer tissue and medulloblastoma, its expression is down-regulated (38, 39). In contrast, MYST1 is overexpressed in non–small cell lung cancer and renal cell carcinoma (39, 40). Given the aberrant expression of MYST1 in cancers, the aim of the present study is to understand the putative role of MYST1 in coregulating the functional links between AR and NF-κB in PCa. We demonstrate that MYST1 is an important coactivator that interacts with AR and NF-κB to promote PCa proliferation.
Materials and Methods
Cell culture
The PCa-derived PC3 cell lines were maintained in DMEM; PC3 cells transformed with AR (PC3-AR) and LNCaP cells were maintained in RPMI 1640 medium. PC3-AR cells were generated as described previously (41). The remaining cell lines were purchased from ATCC. Both the media were supplemented with 10% fetal bovine serum or charcoal-stripped serum and 0.1% antibiotics (penicillin and streptomycin). The cells were maintained in a carbon dioxide incubator with 5% CO2 at 37°C and cells were regularly subcultured by trypsinization.
Transient transfection
PC3, PC3-AR, and LNCaP cells were plated into culture dishes with a density of 1.0 to 1.5 × 106 cells. These cells were transfected with FuGENE (Promega) with plasmids expressing Flag-, hemagglutinin (HA)-tagged MYST1, and V5-tagged MYST1 (wild-type and K274R, the autoacetylation defective mutant). In addition, Flag-Sirt1, Flag-Sirt2, Flag-Sirt3, Flag-Sirt4, Flag-Sirt5, Flag-Sirt6, and Flag-Sirt 7 (Addgene) were used for luciferase and interaction investigations. HA- and myc-tagged p65 were cotransfected with MYST1 or MYST1 and Sirt1 in a triple transfection. After 24 hours of transfection, the cells were harvested and subjected to immunoprecipitation (IP) and immunoblotting (IB) (42–44).
Luciferase assays
LNCaP and PC3 cells were transfected or transduced with luciferase-based PSA promoter or lentivirus containing NF-κB reporter element, which was upstream of a luciferase gene. The quantitation of luciferase activity served as a readout to estimate the activation of NF-κB and PSA reporter elements. To evaluate whether MYST1 enhanced NF-κB activation, MYST1 plasmid was transiently transfected as described above. After 24 hours of transfection, the cells were treated with TNF (25 ng/mL of medium) for 30 minutes or 10 nM dihydrotestosterone (DHT) or methyltrienolone/metribolone, (R1881) for 24 hours; for combinatorial treatments TNF was added for 30 minutes after 24 hours of DHT treatment. After 24 hours, luciferase activity was determined in cell lysates by lysing the transfected cells with passive lysis buffer provided by Promega (42–44).
IP and IB to detect interactions of MYST1
The cells were lysed in an IP lysis buffer (Thermo Scientific) with a protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitors (Sigma-Aldrich). Each cell lysate was incubated at 4°C overnight with different antibodies (MYST1, p65, HA, Myc, V5, and Flag). On the next day, the samples were incubated with agarose beads for 2 hours at 4°C. After incubation, all of the immune complexes were washed and subjected to SDS electrophoresis, and finally IB was performed with antibodies to pan-acetylated lysine (Kac), AR, p65, E2F1, phospho-retinoblastoma protein (p-Rb), TPD52, CDK2, CDK4, CDK6, cleaved poly(ADP-ribose) polymerase (PARP), cleaved caspase 3, and Sirt1 to detect the interactions (43, 44).
Depletion of MYST1 and AR proteins by small interfering RNA (siRNA)
ON-TARGETplus SMART pool MYST1 and AR siRNAs were commercially designed by Dharmacon Inc. AR and MYST1 siRNAs were transfected to PC3-AR/PC3 and LNCaP cells for various experiments. According to the procedure of the kit (Dharmacon Inc), 5 μM siRNA was used and after transfection, the cells were maintained in the incubator for 72 hours. Later, cells were treated with DHT for 24 hours (100 nM) and TNF (25 ng/mL of medium) for 30 minutes. After treatment, cells were harvested and subjected to immunoblotting with AR and MYST1 antibodies for confirming the depletion of AR and MYST1 proteins. In parallel, RNA was also extracted from these cells for quantitative RT-PCR (qRT-PCR).
Immunohistochemistry
For immunohistochemical analysis, after deparaffinization and rehydration, sections of human prostate cancer tissues on slides were treated with 3% hydrogen peroxide (H2O2) to eliminate endogenous peroxidase activity. Slides were then processed for antigen retrieval with 10 mM citrate buffer (pH 6.0) using a pressure chamber (Pascal; Dako Cytomation) for 4 minutes at 125°C, followed by slow cooling. All sections were rinsed with PBS (137 mM NaCl, 2.7 mM potassium chloride, 4.2 mM sodium phosphate, and 1.5 mM potassium phosphate) briefly. The sections were incubated with the MYST1 antibody for 2 hours at room temperature. A Lab Vision UltraVision LP Detection System: HRP Conjugate/DAB Plus Chromogen (Thermo Fisher Scientific) was used as a secondary antibody according to the manufacturer's protocol. Immunoreactivity was visualized with 3,3′-diaminobenzidine, counterstained with hematoxylin, dehydrated, and mounted (45).
qRT-PCR
Total RNA was isolated using an RNeasy Plus Mini Kit (QIAGEN). The isolated total RNA was nondegraded and free from protein and DNA contamination. cDNA synthesis was performed using an AffinityScript cDNA synthesis kit (Agilent Technologies). The resulting cDNA was used as a template for comparative qRT-PCR (Brilliant II FAST SYBR Green qPCR master mix; Agilent Technologies) with validated real-time gene-specific primers (RealTimePrimers.com) for the following genes: E2F1, TPD52, ICAM1, CDKN1A, and TNF.com). qRT-PCR data were analyzed, and Ct values were normalized with a housekeeping gene (GAPDH) and calculated using the 2−ΔΔCt method. Results are expressed in the form of fold expression (46). Data were analyzed and are expressed as the means ± SEM of 3 independent experiments (n = 3). Statistical analysis was performed by using 2-tailed t tests, and further P values were determined for highlighting the significance of the differences in gene expression between (TNF+DHT) and (MYSTi TNF+DHT) conditions (46).
5-Bromo-2′-deoxyuridine (BrdU) ELISA
Parental and MYST1-depleted PC3, PC3-AR, and LNCaP cells were treated with TNF, DHT, and TNF and DHT together and then were incubated with BrdU for 16 hours. Subsequently, the cells were fixed and an ELISA was performed according to the manufacturer's instructions.
Annexin staining and cell cycle analysis by flow cytometry
Wild-type and MYST1-depleted PC3, PC3-AR, and LNCaP cells were treated with TNF and DHT, trypsinized, and washed with PBS. The harvested cells were incubated either with annexin and propidium iodide (PI) together or with PI and RNase and then were subjected to flow cytometry analysis (42–44). For annexin staining, the cells were not fixed as for the cell cycle analysis.
Results
MYST1 augments transcriptional activation of NF-κB in PCa cells
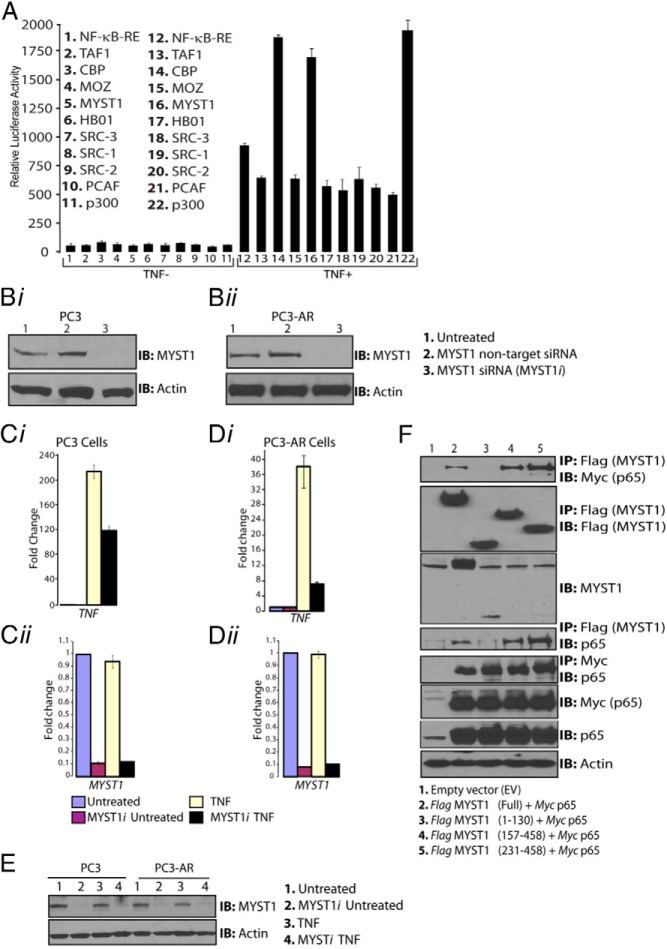
To identify a coactivator that could regulate both AR and NF-κB, HAT-containing coactivators were initially screened for their capacity to potentiate NF-κB activation. The rationale for selecting HAT-containing coactivators stems from their ability to acetylate lysine residues as well as their multidomain structures that enable molecular interactions between chromatin and transcription factors (16, 47). To this end, PC3 cells were cotransfected with plasmids, which contained the NF-κB reporter element (NF-κB-RE) in tandem with a luciferase gene and one of the HAT-containing coactivators (TAF1, CBP, MOZ, MYST1, HB01, SRC-3, SRC-2, SRC-1, PCAF, or p300) (16, 47). As expected, the known coactivators p300 and CBP potentiated TNF-mediated activation of NF-κB-RE by 3.0-fold, and, importantly, MYST1 potentiated NF-κB activity by 2.5-fold (Figure 1A).
Figure 1.
MYST1 is a potential coactivator of NF-κB transcriptional activity. A, PC3 cells were cotransfected with a battery of HAT-containing coactivators and tested by a luciferase-based reporter assay for activation of NF-κB-RE. After 24 hours of transfection, cells were treated with 25 ng of TNF/mL of medium for 30 minutes. After cell lysis and determination of luciferase activity, results showed that the activation of NF-κB-RE was augmented by MYST1, CBP, and p300. Bi and Bii, IB analysis confirmed the down-regulation of MYST1 expression by siRNA (MYST1i) in parental PC3 and PC3-AR cells without affecting the expression of the housekeeping protein actin. Ci and Di, qRT-PCR data showed an increased level of TNF transcript, which was down-regulated by 50% as a result of the knockdown of MYST1 in parental PC3 cells and was reduced by 80% in PC3-AR cells. Cii, Dii, and E, No changes were observed in the levels of MYST1 mRNA (Cii and Dii) and protein (E) in PC3 and PC3-AR cells. F, After 24 hours, cells were harvested and lysed, and IP was performed with equal quantities of cell lysates by incubation with anti-Flag antibody. The IB analysis revealed that p65 interacted with the 157–458 and 231–458 regions of MYST1, which represents the HAT domain.
TNF is a major activator as well as a downstream target of transcriptionally active NF-κB (48). To test the biological significance of MYST1 in NF-κB activation, first, the expression of MYST1 was depleted by siRNA (MYST1i) in PC3 and PC3-AR cells (Figure 1, Bi and Bii); then, the expression of TNF was determined after treatment with TNF (Figure 1C). In PC3 cells, the expression of TNF was enhanced by 200-fold, whereas in PC3-AR, the expression of TNF was enhanced by 40-fold compared with that in untreated cells (Figure 1, Ci and Di). Notably, MYST1 depletion after siRNA transfection reduced TNF expression by 50% in PC3 cells, whereas in PC3-AR cells, TNF expression was reduced by 80%. Thus, in the presence of AR, down-regulation of MYST1 negated NF-κB activation to a greater extent. The levels of MYST1 mRNA and protein remained consistently the same in PC3 and PC3-AR cells (Figure 1, Cii, Dii, and E). As shown in Supplemental Figure 1A, expression levels of p65 in PC3 (lanes 1–4) or PC3-AR (lanes 5–8) cells remained unaltered, and TNF treatment led to its nuclear localization (41). Taken together, these data indicate a greater ability of MYST1 to potentiate NF-κB in the presence of AR.
Molecular interactions between coactivators with transcription factors are pivotal during gene regulation (49, 50). To test whether p65, which is the major subunit of NF-κB, interacts with MYST1, PC3 cells were cotransfected with either wild-type Flag-MYST1 or truncated constructs of Flag-MYST1 and Myc-p65. IP with anti-Flag followed by IB with anti-p65 antibodies did not show any binding between the N-terminal of MYST1 (1–130 amino acids) and p65. However, the overlapping constructs, which expressed the HAT domain of MYST1 (157–458 and 231–458) clearly interacted with p65 (Figure 1F). Notably, anti-MYST1 antibody reacted with the N-terminal of MYST1. Overall, these observations suggested a role of MYST1 as a coactivator of NF-κB in PCa cells.
Deacetylation of MYST1 is essential for NF-κB activation in PCa cells
Previously it was demonstrated that lysine 274 on MYST1 is the major site of autoacetylation in 293T cells (30). To confirm whether lysine 274 of MYST1 remains a major autoacetylation site in PC3 cells (30), wild-type V5-MYST1 and an autoacetylation defective mutant, V5-MYST1K274R, were transfected into PC3 cells. IP with anti-V5 followed by IB with Kac antibodies revealed that the wild-type MYST1 undergoes a marked acetylation compared with the autoacetylation defective mutant, MYST1K274R (Figure 2A), indicating that lysine 274 remained the key autoacetylation site in PCa cells too (30).
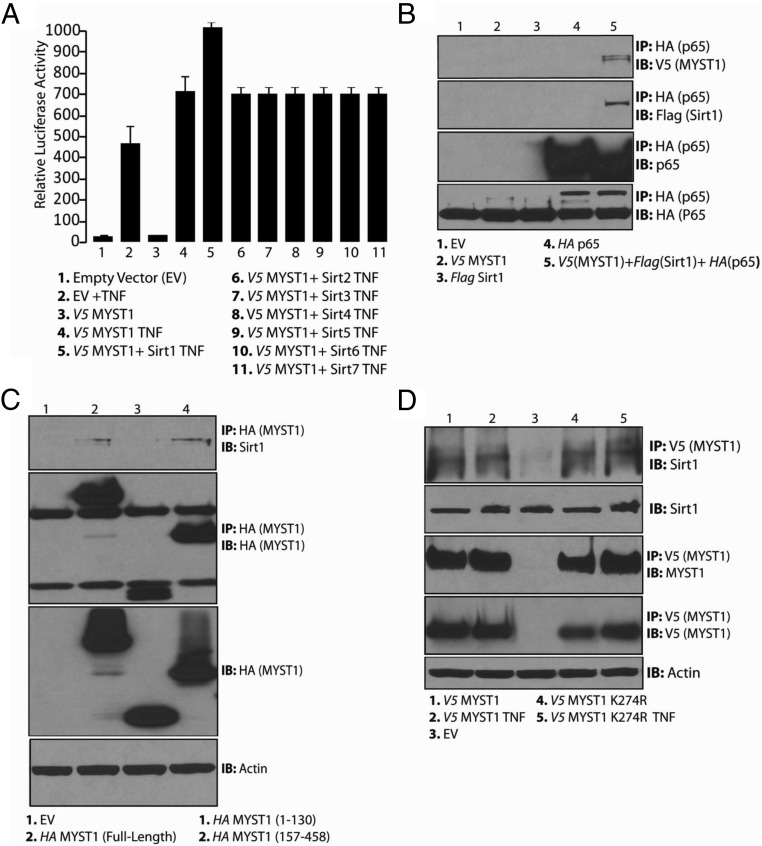
Figure 2.
Deacetylation of MYST1 activates NF-κB functions. A, After 24 hours of transfection, the cells were harvested and lysed, and, subsequently, respective cell lysates were subjected to IP with anti-V5 followed by IB with Kac antibodies. Autoacetylation of V5-tagged wild-type MYST1 was dramatically abrogated upon the mutagenesis of lysine 274 to arginine (MYST1K274R). B, qRT-PCR analysis revealed that MYST1K274R was more potent than wild-type MYST1 in activating the TNF expression in PC3 cells after treatment with TNF. C, Similarly, qRT-PCR analysis revealed that MYST1K274R was more potent in activating the TNF expression in PC3-AR cells after treatment with TNF. D, V5-MYST1 in untreated PC3 cells showed autoacetylation and interaction with p65, which was completely lost upon treatment with TNF.
To examine the role of MYST1 autoacetylation in NF-κB activation, we sought to compare the abilities of wild-type MYST1 or MYST1K274R to activate TNF expression after treatment with TNF. Both PC3 and PC3-AR cells were transfected with wild-type MYST1, MYST1K274R, and empty vector. In PC3 cells, the ability of MYST1K274R to activate the expression of TNF was ∼0.5 times greater than that of the wild-type MYST1. However, in PC3-AR cells, the MYST1K274R was ∼2.0 times more effective than the wild-type MYST1 in activating the expression of TNF (Figure 2B). Notably, in PC3-AR cells, MYST1K274R activated TNF expression 6.0 times greater than the control, whereas in PC3 cells, this activation was ∼2.0 times (Figure 2B). Taken together, these findings suggest a greater ability of the deacetylated MYST1 to augment NF-κB activation in AR-expressing cells (Figure 2, B and C).
To further confirm the effects of TNF treatment on MYST1 autoacetylation and its association with p65, PC3 cells transfected with wild-type V5-MYST1 and mutant V5-MYST1K274R were treated with TNF (Supplemental Figure 2A). Equal quantities of the respective proteins were subjected to IP with anti-V5 and IB with anti-Kac and anti-p65 antibodies. The results demonstrated that autoacetylated MYST1 interacted strongly with p65 in untreated cells compared with that in the TNF-treated cells. TNF deacetylated MYST1 and reduced MYST1 interactions with p65 (Figure 2D). A mild interaction between autoacetylation defective MYST1K274R and p65 in untreated cells was observed, which was enhanced after TNF treatment. These results indicated that the functional interaction between MYST1K274R and p65 could activate transcription, which in part explained the enhancement of TNF expression by MYST1K274R as shown in Figure 2, B and C. Moreover, the fact that MYST1K274R activated TNF directed the next task of identifying the enzyme that deacetylates MYST1 in PCa cells.
Sirt1-mediated MYST1 deacetylation during proinflammatory conditions
It had been earlier reported that Sirt1 deacetylates autoacetylated MYST1 (32). Nevertheless, the biological basis of Sirt1-mediated MYST1 deacetylation remains less understood. To address this, PC3 cells were cotransfected with an NF-κB-RE and wild-type MYST1 together with one of the Sirt-expressing plasmids (ie, Flag-Sirt1, Flag-Sirt2, Flag-Sirt3, Flag-Sirt4, Flag-Sirt5, Flag-Sirt6, or Flag-Sirt7). The expression levels of all of the Sirts are shown in Supplemental Figure 2B. After treatment with TNF, the luciferase data revealed that the cells coexpressing Sirt1 and MYST1, showed greater activation of NF-κB-RE than the cells expressing only MYST1. Hence, Sirt1-mediated MYST1 deacetylation may potentiate NF-κB activation during proinflammatory conditions (Figure 3A).
Figure 3.
Sirt1 regulates autoacetylation of MYST1. A, A luciferase assay was performed to confirm that Sirt1 potentiated the MYST1-mediated activation of NF-κB after TNF treatment of PC3 cells that were cotransfected with MYST1, Sirt1, and NF-κB RE. B, Molecular interaction of MYST1, p65, and Sirt1 in PC3 cells transfected with V5-MYST1, HA-p65, and Flag-Sirt1 after IP with HA followed by IB with V5 and Flag antibodies. C, In PC3 cells, the endogenous Sirt1 showed interaction with the full-length and C terminus of MYST1 (157–458). D, Endogenous Sirt1 interacted with both wild-type and mutant MYST1K274R independent of TNF treatment of PC3 cells.
The above findings suggested that MYST1, p65, and Sirt1 could interact in untreated cells. To verify this possibility, PC3 cells were transfected with plasmids expressing Flag-Sirt1, HA-p65, and V5-MYST1. Subsequently, IP with HA antibody (p65) revealed that Sirt1 (Flag) and MYST1 (V5) are associated with p65 in untreated PC3 cells (Figure 3B). The relative expression levels of the respective proteins are shown in Supplemental Figure 2C.
To determine the mode of interaction between MYST1 and endogenous Sirt1, HA-tagged full-length and truncated MYST1-expressing plasmids were transfected into PC3 cells. IP with HA followed by IB with Sirt1 antibodies revealed that the Sirt1 binds to the HAT domain of MYST1 (Figure 3C). These results suggested that the deacetylation of MYST1 by Sirt1 during proinflammatory conditions could be facilitating the activation of NF-κB in PCa cells. The interaction between MYST1 and Sirt1 raised the question of whether Sirt1 binds to wild-type MYST1 or autoacetylation defective mutant MYST1K274R during proinflammatory conditions. Next, PC3 cells were transfected with empty vector, wild-type MYST1, and MYST1K274R. Subsequently, after TNF treatment, the respective cell lysates were subjected to IP with V5 and IB Sirt1 antibodies (Figure 3D). Taken together, the data show that both wild-type MYST1 and MYST1K274R interacted with Sirt1, independent of TNF treatment and acetylation status.
AR competes with Sirt1 for binding to MYST1 in PCa cells
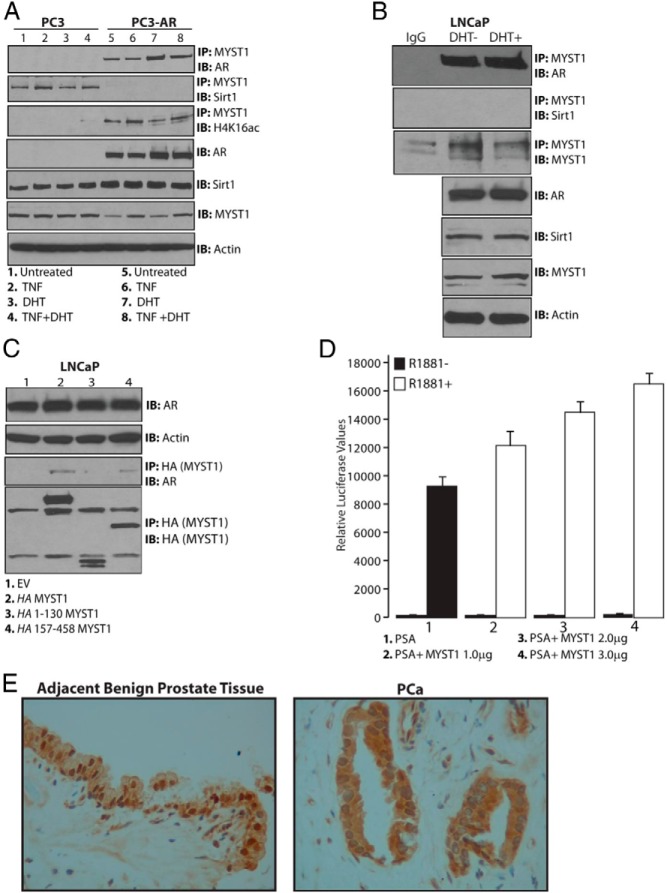
Because MYST1, p65, and Sirt1 showed functional interaction (Figure 3), it was essential to examine the presence of AR as well as the effect of TNF and DHT treatments on this interaction. Both PC3 and PC3-AR cells were treated with TNF and DHT alone or in combination. The endogenous MYST1 protein was immunoprecipitated, and, subsequently, IB analyses were performed with Sirt1 and AR antibodies. These data revealed that MYST1 was associated with Sirt1 in PC3 cells; however, in PC3-AR cells, MYST1 interacted with AR, but not with Sirt1. Notably, the expression levels of endogenous Sirt1 and MYST1 in both the cell lines remain comparable. The interactions of MYST1 with p65 remained unaltered in both PC3 and PC3-AR cells. In particular, the association of MYST1 with H4K16ac occurred mainly in PC3-AR cells. These results suggested the distinct roles of MYST1-AR-p65 in PC3-AR cells and MYST1-Sirt1-p65 in PC3 cells (Figure 4A). To further confirm the interaction of MYST1 with endogenous AR, LNCaP cells were used. The results after IP and IB showed that, as in PC3-AR cells, MYST1 interacted with AR in LNCaP cells rather than with Sirt1 (Figure 4B).
Figure 4.
Mutually exclusive interactions of MYST1 with Sirt1 or AR in PCa cells. A and B, Endogenous MYST1 interacted with Sirt1 in PC3 cells but not in PC3-AR and LNCaP cells wherein MYST1 interacts with AR. C, Endogenous AR interacted with the full-length and the 157–458 region of MYST1. D, Induction of PSA luciferase activity in LNCaP cells by increasing amounts of MYST1-expressing plasmid upon treatment with 10 nM R1881 treatment confirms the role of MYST1 as a coactivator of AR. E, Immunohistochemical analysis with anti-MYST1 antibodies of formalin-fixed human prostate cancer tissues (n = 5) revealed that MYST1 was nuclear in the benign prostate epithelium adjacent to PCa cells, in which MYST1 was cytoplasmic as well as nuclear. Original magnification, ×200.
To delineate the motif on MYST1 that interacts with AR, LNCaP cells were transfected with HA-tagged full-length and truncated MYST1 plasmids. IP with anti-HA antibodies followed by IB with anti-AR antibodies showed that in addition to the full-length MYST1, AR interacted with the 157–458 motif, which constitutes the HAT domain of MYST1 (Figure 4C).
To determine whether MYST1 could serve as a putative coactivator of AR transcriptional functions, LNCaP cells were transfected with a luciferase reporter–based PSA promoter in the presence or absence of MYST1 and synthetic androgen R1881. The luciferase data revealed that increasing levels of MYST1 expression augmented the activation of the PSA promoter in the presence of AR and R1881 (Figure 4D); 3 μg of MYST1-expressing plasmid enhanced PSA activation by at least 2-fold.
To validate the presence of MYST1 in human prostate tissues, 5 independent (n = 5) formalin-fixed PCa tissues (Gleason grades 6 and 7) were subjected to immunohistochemical analysis with anti-MYST1 antibodies. The data revealed that MYST1 was mainly nuclear in the benign prostate epithelial cells adjacent to PCa cells. However, in PCa cells, MYST1 was nuclear as well as cytoplasmic (Figure 4E). Although the mechanisms are not completely clear as yet, the major goal of our next studies is to elucidate the dynamics of MYST1 acetylation during activation of AR vs NF-κB in PCa cells.
MYST1 modulates the genes controlling cellular proliferation
Our next aim was to clarify whether MYST1 is a coactivator of NF-κB and AR and may affect the expression of their target genes with the potential to regulate proliferation of PCa cells. To test this conjecture, MYST1 protein was depleted by siRNA (MYST1i) in PC3, PC3-AR, and LNCaP cells (Figure 5). After treatments with TNF and DHT, the expression levels of PSA, E2F1 (15), CDKN1A or p21 (14), TPD52 (23, 51), and ICAM1 (27) were analyzed. The data demonstrated that DHT-activated PSA was diminished in MYST1-depleted LNCaP cells. As expected, PC3 and PC3-AR cells showed no activation, because they do not express PSA (Figure 5, A–C).
Figure 5.
MYST1 depletion affects the expression of cell cycle regulatory genes. PC3 and PC3-AR cells were treated either alone with 25 ng/mL TNF for 30 minutes or 100 nM DHT for 24 hours and in combination, in which TNF was added after 24 hours of DHT treatment. A, B, and C, MYST1 was depleted (MYST1i) by siRNA in PC3, PC3-AR, and LNCaP cells. Then, the parental and MYST1i cells were examined for the expression of E2F1, PSA, CDKN1A, TPD52, and ICAM1. ***, No PSA was detected in PC3 and PC3-AR cells. The fold changes between DHT+TNF and DHT+TNF+MYSTi are expressed as the means ± SEM (n = 3): #, P < .05; ##, P < .01.
Furthermore, we noted that independent of TNF or DHT treatments, the expression level of E2F1 was reduced by 2.5-fold in PC3-AR and LNCaP cells compared with that in PC3 cells (∼ 2.0-fold), as a result of the down-regulation of MYST1 expression (Figure 5, A–C). In contrast, reduced E2F1 expression was concomitant with up-regulation of CDKN1A/p21 by 2.0-fold in PC3-AR but not in LNCaP and PC3 cells. However, alterations in E2F1 and CDKNI1A levels were independent of DHT and TNF treatments (Figure 5, A–C).
Recently, a study demonstrated that down-regulation of TPD52 expression attenuates the proliferation of PCa cells (51). The gene expression data revealed that TPD52 was induced by 2- to 3-fold upon TNF and DHT treatments in PC3, PC3-AR, and LNCaP cells. However, the activation was completely lost in MYST1-depleted PC3, PC3-AR, and LNCaP cells, despite TNF and DHT treatments (Figure 5, A–C).
ICAM1, one of the key targets of NF-κB, has been shown to promote tumor metastases (52, 53). The data reveal that the expression of ICAM1 in PC3, PC3-AR, and LNCaP cells was induced between 3.0- and 3.5-fold after DHT and TNF treatments (Figure 5, A and B). In contrast, after DHT and TNF treatments, ICAM1 expression was decreased more in PC3-AR and LNCaP cells (3.0- to 3.5-fold) than in PC3 cells (1.4-fold) as a result of the depletion of MYST1 expression (Figure 5, A and B).
Finally, to examine the functional impact of MYST1 in PC3, PC3-AR, and LNCaP cells, statistical analysis was performed by comparing the difference between DHT+TNF and MYST1i DHT+TNF conditions. The data revealed that the ICAM1 expression level was affected at P < .01 in MYST1-depleted cells despite DHT and TNF treatments. The effects of DHT and TNF treatments on TPD52 expression due to MYST1 depletion was affected at P < .01 in LNCaP and PC3 cells but at P < .05 in PC3 cells. The induction of CDKN1A in PC3-AR cells was P < .01 compared with P < .05 in LNCaP cells). However, PC3 cells did not show any significant difference between DHT and TNF treatments in the presence or on depletion of MYST1. Similarly, the inhibition of E2F1 due to MYST1 depletion in PC3-AR and LNCaP cells was affected at P < .01 with no major difference noted in PC3 cells. Taken together, these data confirm that MYST1 is a potential regulator of AR and NF-κB transcriptional targets, which are involved in triggering the proliferation of PCa cells.
Effect of MYST1 down-regulation on cell cycle regulatory proteins
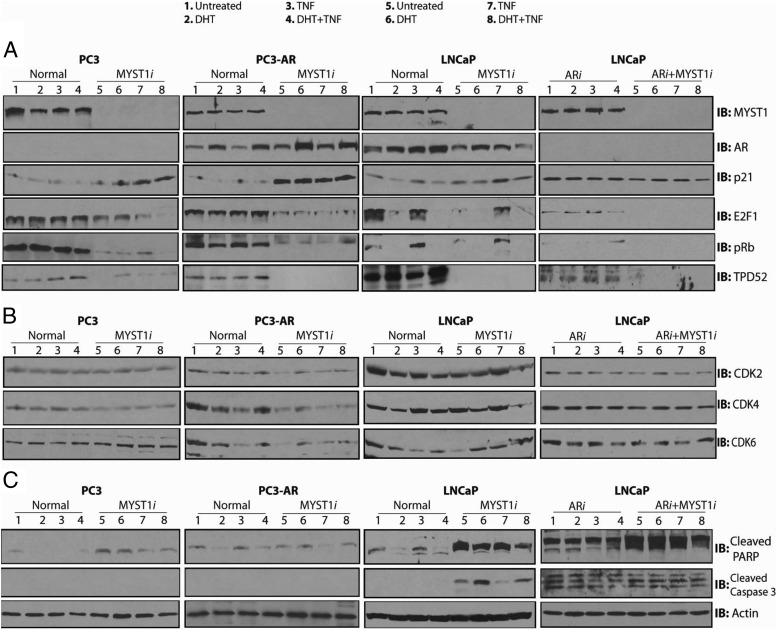
The biochemical significance of MYST1 was investigated by depleting its expression using siRNA (MYST1i) in PC3, PC3-AR, and LNCaP cells (Figure 6A, top panels, lanes 1–4 vs lanes 5–8). In addition, the expression levels of AR (ARi) alone and AR plus MYST1 were depleted in LNCaP cells. After 72 hours of siRNA treatment, the cell lines were treated with DHT alone (lanes 2 and 6), TNF alone (lanes 3 and 7), and DHT and TNF combined (lanes 4 and 8). Clearly, 100 nM DHT enhanced the level of AR in PC3-AR and LNCaP cells. As expected, DHT-mediated induction was not observed in AR-depleted LNCaP cells. Although DHT and TNF treatments enhanced AR expression in PC3-AR cells, AR expression levels in parental LNCaP cells were reduced (Figure 6A, panel 2).
Figure 6.
MYST1 differentially regulates the level of proteins that regulate cell cycle and apoptosis. A, All the IB analyses were performed on the same batch of biological samples. Panel 1 demonstrates effective depletion of MYST1 (MYST1i), ARi, and ARi+MYST1i proteins in PC3, PC3-AR, and LNCaP cells compared with that in the parental lines. Panel 2 shows the levels of AR in all the parental and siRNA-treated cell lines. Panels 3, 4, and 5 show that down-regulation of MYST1 in PC3 and PC3-AR cells enhanced the level of p21 (panel 3), which was concomitant with the dramatic decline in the levels of E2F1 (panel 3) and p-Rb (panel 4). Note that the dilution of anti-p21 antibody used for PC3-AR cells was 1:2000, which did not detect any p21 protein in PC3 cells until a 1:500 dilution was used to probe the respective membrane. Slight increases in p21 levels could be observed in parental LNCaP cells as a result of the MYST1 depletion that was not seen in AR alone (ARi)– or ARi+MYSTi–depleted LNCaP cells. Panel 6 shows that the levels of TPD52 are negatively affected in all of the respective cell lines because of the down-regulation of MYST1. B, No significant alterations in the levels of CDK2 (panel 1), CDK4 (panel 2), and CDK6 (panel 3) were observed in parental and MYST1i PC3 cells. However, the PC3-AR cells showed an overall reduction in the levels of CDK2, CDK4, and CDK6 upon MYST1 down-regulation. Although the levels of CDK2, CDK4, and CDK6 are detectable in MYST1-depleted PC3-AR cells after treatments, their levels are still lower than those of the parental PC3-AR cells. The LNCaP cells showed a decline in the level of CDK6, depending on the expression of MYST1. However, the levels of CDK2, CDK4, and CDK6 remained unchanged in ARi and ARi+MYST1i LNCaP cells. C, Although PC3 and LNCaP cells showed substantial increases in the levels of cleaved PARP (panel 1) due to MYST1 depletion, cleaved caspase 3 (panel 2) was only detectable in MYST1i, ARi, and ARi+MYST1i LNCaP cells.
Based on the qRT-PCR data shown in Figure 5, we probed levels of p21 in these cells. IB revealed that depleting MYST1 in PC3 and PC3-AR cells enhanced the p21 level independent of treatments. However, p21 was detected in PC3-AR cells at an antibody dilution as low as 1:2000, whereas in PC3 cells p21 was detected at a higher 1:500 dilution. In LNCaP cells, regardless of MYST1 status, or in AR alone– and AR and MYST1–depleted cells, no substantial alterations in the levels of p21 were observed (Figure 6A, panel 3).
In PC3 and PC3-AR cells, MYST1 depletion independent of any treatments reduced the levels of E2F1 and p-Rb. In parental LNCaP cells, DHT alone and together with TNF reduced the levels of E2F1 and p-Rb. In LNCaP cells in which only AR was depleted, lower amounts of E2F1 without any significant levels of p-Rb were detected. However, in AR plus MYST1-depleted cells, neither E2F1 nor p-Rb was detected (Figure 6A, panels 4 and 5).
Although DHT and TNF treatments slightly elevated the levels of TPD52 expression in parental PC3, PC3-AR, and LNCaP cells, MYST1 depletion markedly reduced its expression (Figure 6A, panel 6). Similar findings were noted in ARi and ARi plus MYST1i LNCaP cells.
Alterations in p21, E2F1, and p-Rb levels in different PCa cell lines prompted us to examine the levels of CDK2, CDK4, and CDK6 in PC3, PC3-AR, and LNCaP cells. Surprisingly, no changes in the levels of CDK2, CDK4, and CDK6 were observed in PC3 cells. However, compared with that in control cells (Figure 6B, lane 1), in PC3-AR cells, MYST1 depletion reduced the expression of CDK2, CDK4, and CDK6 (Figure 6B, compare lanes 1 and 5). Notably, in MYST1-depleted PC3-AR cells, treatments with DHT and TNF alone and together elevated the levels of these CDKs to some extent; however, their levels remained lower than those of the controls. In LNCaP cells, a decline in the level of CDK6 due to MYST1 depletion was noted. In addition, the levels of CDK2 and CDK4 were reduced in MYST1-depleted LNCaP cells after treatments with DHT and TNF. Finally, no changes in the levels of CDK2, CDK4, and CDK6 were observed in ARi and ARi plus MYST1i LNCaP cells (Figure 6B).
The finding of no major alterations in the CDKs of PC3 and LNCaP cells raised the question of the impact of MYST1 depletion on proapoptotic proteins. We observed that MYST1 depletion led to a dramatic increase in the cleaved PARP levels in PC3 and LNCaP cells (Figure 6C, panel 1). These results were further verified by reprobing the membranes with anti–cleaved caspase 3 antibody. Although cleaved caspase 3 was undetectable in PC3, the level of cleaved caspase 3 was increased in untreated MYST1-depleted cells and those treated with DHT but not TNF (Figure 6C, panel 2). Finally, AR alone– and AR and MYST1-depleted LNCaP cells showed cleavage of PARP and caspase 3; however, their levels are dramatically higher in double knocked-down cells. Taken together, these data reveal a dichotomy in MYST1 functions. In PC3-AR cells, MYST1 regulated cell cycle genes; however, in PC3 and LNCaP cells, it regulated apoptotic pathway genes. Importantly, these data underline the fact that targeting MYST1 could be therapeutically beneficial regardless of AR status.
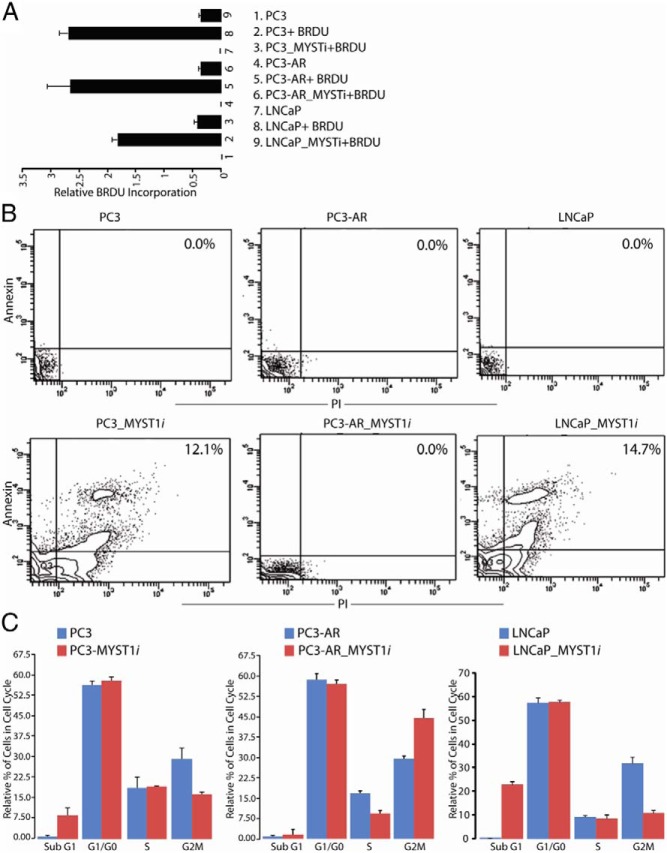
MYST1 depletion induces apoptosis in PC3 and LNCaP cells but G2M arrest in PC3-AR cells
Next, we sought to corroborate the cellular consequences of cleaved PARP, caspase 3, and p21 on the cell cycle profiles of PC3, PC3-AR, and LNCaP cells. To this end, BrdU incorporation, annexin/PI staining, and cell cycle analysis were performed in parental and MYST1-depleted PC3, PC3-AR, and LNCaP cells.
The data from the BrdU assay revealed that MYST1-depleted PC3, PC3-AR, and LNCaP cells exhibited lesser incorporation of BrdU than the parental cells, which indicated that MYST1 had a role in cellular proliferation (Figure 7A). However, decreased BrdU incorporation may not indicate whether the cells are undergoing apoptosis or growth arrest. Therefore, MYST1-depleted and parental PC3, PC3-AR, and LNCaP cells were subjected to staining with annexin and PI. The flow cytometry data revealed that PC3-AR cells did not show any double-positive cells, which represent apoptotic cells. In contrast, PC3 (12.1%) and LNCaP (14.7%) cells undergo apoptosis (Figure 7B). To confirm the above findings and explain the lesser incorporation of BrdU and annexin/PI staining in PC3-AR cells, these cells were stained with PI for cell cycle analysis. Compared with parental PC3 and LNCaP cells, the MYST1-depleted PC3 and LNCaP cells exhibited 5- and 20-fold increases, respectively, in the percentage of cells during the sub-G1 phase (Figure 7C). Further, compared with their parental cells, the MYST1-depleted PC3-AR cells showed a 1.5-fold increased accumulation of cells in the G2M phase, which was accompanied by a 2-fold decline of cells in the S phase (Figure 7C). No effect of DHT or TNF on the cell cycle profiles of PC3, LNCaP, and PC3-AR cells was noted (data not shown). Taken together, these results demonstrate that MYST1 down-regulation activates PARP and apoptosis in AR-null PC3 cells, whereas in PC3-AR cells it activates p21 and cell cycle arrest.
Figure 7.
MYST1 mediates apoptosis or G2M arrest in PC3 and PC3-AR cells. A, BrdU assay reveals that MYST1-depleted cells exhibited reduced incorporation of BrdU compared with that in the parental PC3, PC3-AR, and LNCaP cells. B, Annexin/PI staining by flow cytometry revealed that the lower BrdU incorporation in PC3 and LNCaP cells could be due to the loss of cells by apoptosis. The values have been added to the quadrant that represents double-positive cells, which indicates that these cells demonstrated annexin as well as PI positivity. These panels show a representative of 3 experiments performed independently. C, Apoptosis and the cell cycle were analyzed by flow cytometry after staining of the cells with PI and RNase treatment. Depletion of MYST1 in PC3 and LNCaP cells led to accumulation of cells in the sub-G1 phase, which confirms apoptosis. In PC3-AR cells, MYST1 down-regulation that activates p21 leads to G2M arrest. Means and SD were calculated from the data acquired from 3 independent experiments, which were performed in triplicate with each cell type. The percentage of cells in each phase of the cell cycle was calculated from the total number of gated cells, which was set to 100%.
In summary, we propose that TNF-mediated NF-κB activation causes MYST1 deacetylation by Sirt1 (Figure 8). The mutually exclusive MYST1 interaction with Sirt1 or AR controls H4K16ac. Notably, MYST1 depletion in PC3 and LNCaP cells activates apoptosis, whereas in PC3-AR cells it augments G2M arrest. Thus, MYST1 could be a potential therapeutic target for blocking the growth of PCa, regardless of the AR status.
Figure 8.

A model depicting the functional and molecular interplay of MYST1 with AR, NF-κB, and Sirt1 in PCa. MYST1 interacts with the key transcription factors and also plays a critical role in the expression of key cell cycle regulatory proteins. The model shows that the proinflammatory milieu, such as TNF treatment, causes deacetylation of MYST1. The green ball represents the acetyl moiety, which is removed by Sirt1 after TNF treatment. Further, we advocate that because of the mutually exclusive nature of MYST1 interactions, the MYST1–p65-Sirt1 complex act as a repressor complex and the MYST1–p65-AR complex functions as an activator complex, which together enhance proliferation and inhibit growth arrest or apoptosis of PCa cells.
Discussion
For more than 70 years, AR signaling has served as the most critical clinical target during PCa progression (7). Therefore, therapeutic opportunities as well as challenges in PCa are also centered on controlling AR functions (10, 54). Although AR is defined as an androgen-dependent transcription factor, evidence indicates that in advanced, castration-resistant PCa, AR signaling still controls disease progression despite androgen ablation therapy. The mechanisms supporting this persistent AR signaling are complex and remain to be fully deciphered. Coactivators display the potential not only to activate AR but also to liaise with NF-κB to promote PCa growth (10, 54). Moreover, coactivators are critical because of their need during both the androgen-dependent and -independent functions of AR (2, 7, 55). Thus, coactivators remain an attractive target for therapeutic development in castration-resistant disease.
At least 3 postulated mechanisms explain the resistance to androgen ablation therapy: first, amplification or alteration in the AR gene; second, enhanced signaling by MAPK that activates AR independent of its natural androgen; and third, activation of alternative pathways that supersede AR signaling to promote antiapoptotic mechanisms (10). Further, androgen-independent AR reactivation has the potential to disrupt AR transcriptional functions by altering the pools of coregulators (2, 7, 55). MYST1, a HAT that is conserved among higher eukaryotes, was identified in Drosophila as a part of the dosage compensation complex (56). In mammalian cells, MYST1 is associated with either MSL1/MSL2/MSL3 or as MSLv1 complexes (29, 56). The major substrate of MYST1 HAT activity is H4K16ac (29–31). In addition, MYST1 is associated with Sirt1, which regulates its autoacetylation on lysine 274 (30). Our findings provide some of the first evidence that highlights the biological significance of MYST1-Sirt1-p65 and MYST1-AR-p65 complexes during PCa proliferation. The association between H4K16ac and AR could provide a distinct sign to identify AR target genes in castration-resistant PCa.
In the present study, various human PCa cell lines were studied to investigate the role of MYST1. PC3 cells represent a cellular model of advanced PCa and do not express AR and PSA. PC3-AR cells are PC3 cells transformed with AR, and LNCaP cells express endogenous AR with a point mutation in its ligand-binding domain (7). We identified MYST1 as a coactivator of NF-κB during proinflammatory conditions. PC3 and PC3-AR cells showed similar levels of p65 expression. However, we observed differences in the levels of TNF expression in these cells, which indicated that the NF-κB activity was greater in PC3 cells than in PC3-AR cells. Although the underlying reasons for this differential activation of NF-κB are not fully clear, it was possible that because of genetic loss of AR, PCa cells became addicted to the NF-κB pathway (57). However, depletion of MYST1 expression reduced NF-κB activation comparatively more in PC3-AR than in PC3 cells, which suggested that MYST1 could be functionally linked to AR and NF-κB signaling pathways. In addition, AR expression in PC3-AR cells does not inhibit the expression of p65 or vice versa. To further explain the biochemical basis of MYST1-mediated activation of NF-κB, we performed IP using truncated MYST1, which revealed that p65 binds to the HAT domain of MYST1. Further, we demonstrated for the first time that autoacetylated MYST1 was deacetylated by Sirt1 upon TNF-mediated NF-κB activation. However, because of persistent activation of NF-κB in PCa cells, the coactivator role of MYST1 needs to be further characterized in macrophages and cellular models of other cancers. Our data also demonstrated that MYST1 interacted with AR or Sirt1 in a mutually exclusive manner. Although most transcriptional coactivators are mostly nuclear, in human PCa tissues, MYST1 was nuclear as well as cytoplasmic, which might enable MYST1 to synergistically activate the functions of AR and p65 effectively. Although the mechanisms are still unclear, these results suggested that the nuclear and deacetylated MYST1 (MYST1K274R) could possibly serve as a coactivator of p65 during proinflammatory conditions. The unavailability of a site-specific autoacetylated antibody posed a challenge for full unraveling of the functional dichotomy between acetylated and deacetylated forms of MYST1. Notably, we found that down-regulation of MYST1 expression in PC3, PC3-AR, and LNCaP cells reduced the expression levels of TPD52 that could possibly contribute to the activation of PARP or p21, which could direct the cells for apoptosis or cell cycle arrest (23, 26, 27, 51). However, these findings suggested that TPD52 could be a potential downstream target of MYST1 in PCa cells. Given that TPD52 was enhanced by DHT and TNF treatments, most likely it could be regulated by MYST1, AR, and NF-κB. The expression of ICAM1 is affected more in PC3-AR cells than in PC3 cells, indicating that the concurrent presence of AR and NF-κB might aggressively promote cellular proliferation and indicate a bad prognosis for PCa treatment. Our findings are further substantiated by reports highlighting the fact that signaling via the NF-κB pathway is more aggressive in the presence of AR in PCa (13). In PC3-AR cells, MYST1 depletion leads to a reduction in the number of cells in the S phase leading to G2M arrest that was concomitant with activation of p21 and a decline in the levels of p-Rb, E2F1, and CDKs. The LNCaP cells, which express a mutated AR in contrast to a wild-type AR in transformed PC3-AR cells, undergo apoptosis by the activation of PARP and caspase 3. Although AR is known to regulate cell cycle proteins including CDKs, E2F1, and p-Rb (7, 10), the disparity in the activation of p21 in PC3-AR and LNCaP cells could be attributed to cell type specificity and mutation within the AR gene.
Intercepting the androgen-AR axis transiently abrogates PCa growth. However, PCa relapses due to reactivation of AR, genetic loss of cell cycle regulatory proteins such as p53, and persistent activation of NF-κB (2, 8, 10, 54, 55). Our data revealed that MYST1 is indeed a critical cofactor for the cellular proliferation of PCa cells regardless of their AR status. Interestingly, there is a clear dichotomy in the MYST1 functions to control apoptotic vs cell cycle genes. It is likely that MYST1-p65-AR is serving as a dysregulated activator complex, which is repressing p21 in PC3-AR cells, whereas MYST1-p65-Sirt1 acts as a repressor complex of apoptotic pathway in PC3 cells. Although this study explains the significance of MYST1 in PCa cells, these findings may help to unravel mechanisms in other cancers such as renal cell carcinoma, in which MYST1 has been shown to be up-regulated (40). Finally, the data on apoptosis and cell growth from annexin/PI staining and BrdU incorporation experiments, respectively, strongly support the observation that MYST1 regulated PCa proliferation by blocking either apoptotic or cell cycle regulatory genes.
Accumulating evidence indicates that NF-κB expression in PCa cells drives AR-regulated gene expression and cellular proliferation (11). A recent study suggested that the weak adrenal androgen dehydroepiandrosterone stimulates NF-κB activation (58). Interestingly, AR and NF-κB activation could be facilitated by similar coactivators including CBP, p300, TIF2, and SRC-1. However, mechanisms that bridge AR and NF-κB are not completely understood. The present study provides evidence that MYST1 integrates the functions of AR and NF-κB and, hence, could be a potential therapeutic target for PCa treatment. Finally, our future goals are to develop a highly specific antibody to the MYST1 autoacetylation site for unraveling the dynamics of MYST1 acetylation and the structural and molecular basis of MYST1 selectivity for AR and NF-κB genes and determining the significance of H4K16ac in its effects on the progression of PCa.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank Robert Fisher, Steven B. McMahon, Yali Dou, and Ronen Marmostein for sharing antibodies to CDKs, V5-MYST1 and V5-MYST1K274R, and MYST1 constructs; Jigneshkumar Patel for technical assistance; and Aisha M. Williams from the Writing Center of Medgar Evers College at the City University of New York and Sharmila Sridharan for help during the preparation of this article.
S.M. was supported by the National Cancer Institute, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- androgen receptor
- BrdU
- 5-bromo-2′-deoxyuridine
- CDK
- cyclin-dependent kinase
- CDKN1A
- cyclin-dependent kinase inhibitor 1A
- DHT
- dihydrotestosterone
- H4K16ac
- acetylation of lysine 16 on histone H4
- HA
- hemagglutinin
- HAT
- histone acetyltransferase activity
- IB
- immunoblotting
- ICAM1
- intercellular adhesion molecule 1
- IP
- immunoprecipitation
- Kac
- pan-acetylated lysine
- NK-κB
- nuclear factor-κB
- p-Rb
- phospho-retinoblastoma protein
- PARP
- poly(ADP-ribose) polymerase
- PCa
- prostate cancer
- PI
- propidium iodide
- PSA
- prostate-specific antigen
- qRT-PCR
- quantitative RT-PCR
- siRNA
- small interfering RNA
- Sirt1
- sirtuin 1
- TPD52
- tumor protein D52.
References
- 1. Shiota M, Yokomizo A, Masubuchi D, et al. Tip60 promotes prostate cancer cell proliferation by translocation of androgen receptor into the nucleus. Prostate. 2010;70:540–554. [DOI] [PubMed] [Google Scholar]
- 2. Cronauer MV, Culig Z. Molecular aspects of prostate cancer. World J Urol. 2012;30:277–278. [DOI] [PubMed] [Google Scholar]
- 3. Culig Z, Santer FR. Androgen receptor co-activators in the regulation of cellular events in prostate cancer. World J Urol. 2012;30:297–302. [DOI] [PubMed] [Google Scholar]
- 4. Gaughan L, Logan IR, Cook S, Neal DE, Robson CN. Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor. J Biol Chem. 2002;277:25904–25913. [DOI] [PubMed] [Google Scholar]
- 5. Halkidou K, Gnanapragasam VJ, Mehta PB, et al. Expression of Tip60, an androgen receptor coactivator, and its role in prostate cancer development. Oncogene. 2003;22:2466–2477. [DOI] [PubMed] [Google Scholar]
- 6. Brady ME, Ozanne DM, Gaughan L, et al. Tip60 is a nuclear hormone receptor coactivator. J Biol Chem. 1999;274:17599–17604. [DOI] [PubMed] [Google Scholar]
- 7. Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal. 2008;6:e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Basu S, Tindall DJ. Androgen action in prostate cancer. Horm Cancer. 2010;1:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang H, Tindall DJ. The role of the androgen receptor in prostate cancer. Crit Rev Eukaryot Gene Expr. 2002;12:193–207. [DOI] [PubMed] [Google Scholar]
- 10. Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. [DOI] [PubMed] [Google Scholar]
- 11. Nadiminty N, Lou W, Sun M, et al. Aberrant activation of the androgen receptor by NF-κB2/p52 in prostate cancer cells. Cancer Res. 2010;70:3309–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelius T, Filleur S, Yemelyanov A, et al. Androgen receptor targets NFκB and TSP1 to suppress prostate tumor growth in vivo. Int J Cancer. 2007;121:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nadiminty N, Dutt S, Tepper C, Gao AC. Microarray analysis reveals potential target genes of NF-κB2/p52 in LNCaP prostate cancer cells. Prostate. 2010;70:276–287. [DOI] [PubMed] [Google Scholar]
- 14. Kadowaki Y, Chari NS, Teo AE, Hashi A, Spurgers KB, McDonnell TJ. PI3 kinase inhibition on TRAIL-induced apoptosis correlates with androgen-sensitivity and p21 expression in prostate cancer cells. Apoptosis. 2011;16:627–635. [DOI] [PubMed] [Google Scholar]
- 15. Altintas DM, Shukla MS, Goutte-Gattat D, et al. Direct cooperation between androgen receptor and E2F1 reveals a common regulation mechanism for androgen-responsive genes in prostate cells. Mol Endocrinol. 2012;26:1531–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen H, Tini M, Evans RM. HATs on and beyond chromatin. Curr Opin Cell Biol. 2001;13:218–224. [DOI] [PubMed] [Google Scholar]
- 17. Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. [DOI] [PubMed] [Google Scholar]
- 18. Fu M, Wang C, Zhang X, Pestell RG. Acetylation of nuclear receptors in cellular growth and apoptosis. Biochem Pharmacol. 2004;68:1199–1208. [DOI] [PubMed] [Google Scholar]
- 19. Fu M, Liu M, Sauve AA, et al. Hormonal control of androgen receptor function through SIRT1. Mol Cell Biol. 2006;26:8122–8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fu M, Rao M, Wang C, et al. Acetylation of androgen receptor enhances coactivator binding and promotes prostate cancer cell growth. Mol Cell Biol. 2003;23:8563–8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu M, Rao M, Wu K, et al. The androgen receptor acetylation site regulates cAMP and AKT but not ERK-induced activity. J Biol Chem. 2004;279:29436–29449. [DOI] [PubMed] [Google Scholar]
- 22. Fu M, Wang C, Reutens AT, et al. p300 and p300/cAMP-response element-binding protein-associated factor acetylate the androgen receptor at sites governing hormone-dependent transactivation. J Biol Chem. 2000;275:20853–20860. [DOI] [PubMed] [Google Scholar]
- 23. Rubin MA, Varambally S, Beroukhim R, et al. Overexpression, amplification, and androgen regulation of TPD52 in prostate cancer. Cancer Res. 2004;64:3814–3822. [DOI] [PubMed] [Google Scholar]
- 24. Karin M, Cao Y, Greten FR, Li ZW. NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. [DOI] [PubMed] [Google Scholar]
- 25. Natoli G, Ghisletti S, Barozzi I. The genomic landscapes of inflammation. Genes Dev. 2011;25:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. [DOI] [PubMed] [Google Scholar]
- 27. Chen PC, Lin TH, Cheng HC, Tang CH. CCN3 increases cell motility and ICAM-1 expression in prostate cancer cells. Carcinogenesis. 2012;33:937–945. [DOI] [PubMed] [Google Scholar]
- 28. Zhang L, Altuwaijri S, Deng F, et al. NF-κB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mellert HS, McMahon SB. hMOF, a KAT(8) with many lives. Mol Cell. 2009;36:174–175. [DOI] [PubMed] [Google Scholar]
- 30. Yuan H, Rossetto D, Mellert H, et al. MYST protein acetyltransferase activity requires active site lysine autoacetylation. EMBO J. 2012;31:58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sykes SM, Mellert HS, Holbert MA, et al. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol Cell. 2006;24:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu L, Li L, Lv X, Wu XS, Liu DP, Liang CC. Modulations of hMOF autoacetylation by SIRT1 regulate hMOF recruitment and activities on the chromatin. Cell Res. 2011;21:1182–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharma GG, So S, Gupta A, et al. MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol Cell Biol. 2010;30:3582–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carrozza MJ, Utley RT, Workman JL, Côté J. The diverse functions of histone acetyltransferase complexes. Trends Genet. 2003;19:321–329. [DOI] [PubMed] [Google Scholar]
- 35. Gupta A, Guerin-Peyrou TG, Sharma GG, et al. The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Mol Cell Biol. 2008;28:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith ER, Cayrou C, Huang R, Lane WS, Côté J, Lucchesi JC. A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16. Mol Cell Biol. 2005;25:9175–9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mendjan S, Taipale M, Kind J, et al. Nuclear pore components are involved in the transcriptional regulation of dosage compensation in Drosophila. Mol Cell. 2006;21:811–823. [DOI] [PubMed] [Google Scholar]
- 38. Pfister S, Rea S, Taipale M, et al. The histone acetyltransferase hMOF is frequently downregulated in primary breast carcinoma and medulloblastoma and constitutes a biomarker for clinical outcome in medulloblastoma. Int J Cancer. 2008;122:1207–1213. [DOI] [PubMed] [Google Scholar]
- 39. Song JS, Chun SM, Lee JY, Kim DK, Kim YH, Jang SJ. The histone acetyltransferase hMOF is overexpressed in non-small cell lung carcinoma. Korean J Pathol. 2011;45:386–396. [Google Scholar]
- 40. Wang Y, Zhang R, Wu D, et al. Epigenetic change in kidney tumor: downregulation of histone acetyltransferase MYST1 in human renal cell carcinoma. J Exp Clin Cancer Res. 2013;32:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dai JL, Maiorino CA, Gkonos PJ, Burnstein KL. Androgenic up-regulation of androgen receptor cDNA expression in androgen-independent prostate cancer cells. Steroids. 1996;61:531–539. [DOI] [PubMed] [Google Scholar]
- 42. Mujtaba S, He Y, Zeng L, et al. Structural basis of lysine-acetylated HIV-1 Tat recognition by PCAF bromodomain. Mol Cell. 2002;9:575–586. [DOI] [PubMed] [Google Scholar]
- 43. Mujtaba S, He Y, Zeng L, et al. Structural mechanism of the bromodomain of the coactivator CBP in p53 transcriptional activation. Mol Cell. 2004;13:251–263. [DOI] [PubMed] [Google Scholar]
- 44. Mujtaba S, Manzur KL, Gurnon JR, Kang M, Van Etten JL, Zhou MM. Epigenetic transcriptional repression of cellular genes by a viral SET protein. Nat Cell Biol. 2008;10:1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hechtman JF, Xiao GQ, Unger PD, Kinoshita Y, Godbold JH, Burstein DE. Anti-glutamate receptor 2 as a new potential diagnostic probe for prostatic adenocarcinoma: a pilot immunohistochemical study. Appl Immunohistochem Mol Morphol. 2012;20:344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mujtaba S, Winer BY, Jaganathan A, et al. Anthrax SET protein: a potential virulence determinant that epigenetically represses NF-κB activation in infected macrophages. J Biol Chem. 2013;288:23458–23472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Allis CD, Berger SL, Cote J, et al. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. [DOI] [PubMed] [Google Scholar]
- 48. Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol. 2011;12:715–723. [DOI] [PubMed] [Google Scholar]
- 49. Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. [DOI] [PubMed] [Google Scholar]
- 50. Patel J, Pathak RR, Mujtaba S. The biology of lysine acetylation integrates transcriptional programming and metabolism. Nutr Metab (Lond). 2011;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ummanni R, Teller S, Junker H, et al. Altered expression of tumor protein D52 regulates apoptosis and migration of prostate cancer cells. FEBS J. 2008;275:5703–5713. [DOI] [PubMed] [Google Scholar]
- 52. Huang WC, Chan ST, Yang TL, Tzeng CC, Chen CC. Inhibition of ICAM-1 gene expression, monocyte adhesion and cancer cell invasion by targeting IKK complex: molecular and functional study of novel α-methylene-γ-butyrolactone derivatives. Carcinogenesis. 2004;25:1925–1934. [DOI] [PubMed] [Google Scholar]
- 53. Chen YC, Lu PH, Hsu JL, Yu CC, Guh JH. ICAM-1 and AMPK regulate cell detachment and apoptosis by N-methyl-N′-nitro-N-nitrosoguanidine, a widely spread environmental chemical, in human hormone-refractory prostate cancers. Toxicol Appl Pharmacol. 2011;257:412–419. [DOI] [PubMed] [Google Scholar]
- 54. Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Culig Z, Klocker H, Bartsch G, Hobisch A. Androgen receptors in prostate cancer. Endocr Relat Cancer. 2002;9:155–170. [DOI] [PubMed] [Google Scholar]
- 56. Li X, Wu L, Corsa CA, Kunkel S, Dou Y. Two mammalian MOF complexes regulate transcription activation by distinct mechanisms. Mol Cell. 2009;36:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lindholm PF, Bub J, Kaul S, Shidham VB, Kajdacsy-Balla A. The role of constitutive NF-κB activity in PC-3 human prostate cancer cell invasive behavior. Clin Exp Metastasis. 2000;18:471–479. [DOI] [PubMed] [Google Scholar]
- 58. Sun HZ, Yang TW, Zang WJ, Wu SF. Dehydroepiandrosterone-induced proliferation of prostatic epithelial cell is mediated by NFKB via PI3K/AKT signaling pathway. J Endocrinol. 2010;204:311–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.