INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is estimated to affect approximately 1% to 5% of children between the ages of 5 and 13 years.1–4 OSAS has been shown to contribute to neurobehavioral and cognitive morbidity in children, including poor school performance, impaired language skills, inattention, hyperactivity, and reduced quality of life.5–10 OSAS is also associated with significant physiologic consequences, including insulin resistance, elevated markers of inflammation, systemic hypertension, and, in severe cases, left ventricular dysfunction and cor pulmonale.11–17
Pathophysiologic mechanisms for the development of OSAS can be broadly divided into anatomic factors that reduce the caliber of the upper airway, such as craniofacial abnormalities, obesity, and adenotonsillar hypertrophy, and factors that increase upper-airway collapsibility, such as neurologically based alterations in upper airway muscle tone.18–20 Deficits in central control of ventilation (eg, Chiari malformation) may also play an etiologic role in some cases.
INDICATIONS FOR POSITIVE AIRWAY PRESSURE
The primary risk factors for OSAS in children are distinct from those in the adult population, with adenotonsillar hypertrophy being implicated in most childhood cases.21–23 Adenotonsillectomy is therefore considered to be the first-line treatment for otherwise healthy children with OSAS.1 A recent large, randomized controlled trial demonstrated the efficacy of early adenotonsillectomy in improving behavior, quality of life, and polysomnographic features of childhood OSAS.10 Although most patients with OSAS will undergo surgery, not all pediatric patients with OSAS are appropriate surgical candidates. In addition, some patients continue to be symptomatic and have polysomnographic evidence of residual OSAS after adenotonsillectomy; risk factors include severe preoperative OSAS, obesity, neuromuscular disorders characterized by hypotonia, and craniofacial abnormalities.24–26 These patients may be candidates for positive airway pressure (PAP) therapy.27
MECHANISM OF ACTION OF PAP
PAP is a noninvasive method of treating OSAS, and is the most common treatment modality in adults. The basic mechanism involves it delivering intraluminal airway pressure that is above the critical closing pressure of the airway to overcome dynamic obstruction by stenting the airway open.28,29 This positive pressure is generated by an air compressor in the continuous PAP (CPAP) machine, and is transmitted to the patient’s airway through a single conduit that connects with the patient via a variety of interfaces, including nasal pillows, nasal masks (Fig. 1), and full-face masks. The device continually adjusts its output to the patient’s breathing pattern to maintain a constant pressure. A fixed leak valve is positioned near the mask to prevent rebreathing of carbon dioxide. Fig. 1 shows a typical PAP apparatus.
Fig. 1.
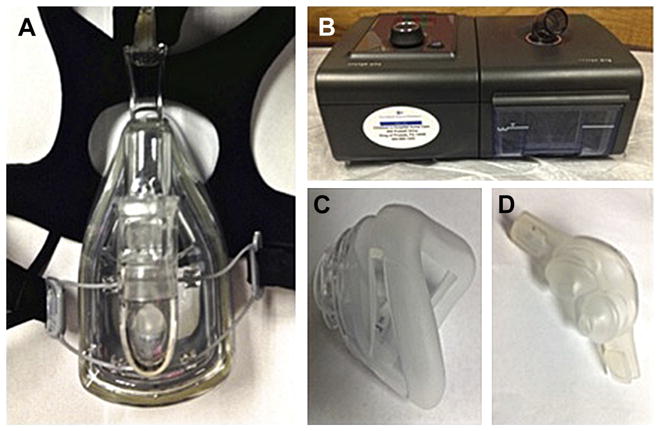
(A) A full-face CPAP mask with headgear attached to a forehead spacer. (B) A CPAP air compressor machine with humidifier. (C) A nasal CPAP mask. (D) A nasal CPAP device with nasal pillows.
It must be noted that it is not the movement of air but rather the pressure generated and maintained through the airway that prevents obstructive events. Selection of an appropriately sized mask that provides a snug fit devoid of air leaks is central to maintenance of positive pressure throughout the system. Mask straps, cushions around the interface, and forehead spacers facilitate mask fit and comfort.
Contemporary CPAP devices have evolved in both size and function, with devices becoming more portable and technologically advanced. Most CPAP machines available today are equipped with data-storage capabilities, digitally recording the number of apnea events and air leaks over time. Data can be downloaded from the machine during follow-up, and serve as an objective measure of CPAP usage to provide feedback to the patient and family to aid in improving adherence. In addition, the incorporation of a humidifier in PAP devices has been demonstrated in adults to decrease drying of the nasal mucosa that can lead to nasal congestion, dryness, and epistaxis, and to increase adherence.30,31
EFFICACY OF PAP FOR THE TREATMENT OF CHILDHOOD OSAS
Although it should be noted that the use of home CPAP for children weighing less than 40 lb (18 kg) or younger than 7 years has not been approved by the US Food and Drug Administration, extensive clinical experience and numerous studies have demonstrated CPAP to be safe and efficacious in children of all ages, including infants.32–34 Marcus and colleagues27 demonstrated PAP therapy (both CPAP and bilevel PAP [BPAP]) to be effective in improving respiratory parameters in pediatric patients aged 2 to 16 years with OSAS, with highly significant improvements in both the apneahypopnea index (AHI; number of apneas and hypopneas per hour of sleep) (P = .003) and oxyhemoglobin saturation nadir (P = .001) 6 months after initiating PAP therapy.27 These results were accompanied by significant improvements in subjective parental assessment of sleepiness, snoring, and difficulty breathing during sleep.
CHALLENGES OF PAP ADHERENCE
Benefit from CPAP therapy is predicated on a snugly fit mask that can maintain positive pressure to stent the upper airway. Adherence with the device can be difficult, especially for children with developmental delays, anxiety, or other behavioral problems. These children often verbally and physically resist the caregiver’s efforts to put on the mask. Consequently, they develop conditioned anxiety because of poorly fitting equipment and repeated association of the sight, sound, and sensation of CPAP with discomfort from the mask, physiologic arousal from struggling, or both.35
When treatment failure occurs in the course of OSAS treatment with CPAP, adherence is the major cause, accounting for up to 92% in one study.32 Earlier studies on the efficacy of CPAP in childhood OSAS used subjective self-report and parent-report measures of CPAP usage, and reported a high rate of adherence.32,36 More recently, usage recordings by the CPAP machine, which can be digitally downloaded, have enabled objective assessments of adherence and have demonstrated CPAP adherence to be frequently suboptimal. Table 1 summarizes studies investigating CPAP adherence in children.
Table 1.
Summary of select studies investigating CPAP adherence in children
| Authors,Ref. Year | Population | Age | OSAS Characteristics | Associated Conditions | Measure of Adherence | Findings |
|---|---|---|---|---|---|---|
| DiFeo et al,43 2012 | N = 56 children and their parents 68% male 59% African American 36% Caucasian |
2–16 y | AHI 19 ± 16/h Naïve to CPAP |
71% obese 23% with neurodevelopmental disabilities 20% with genetic syndromes |
Usage data from machine (at 1 and 3 mo) | Average use 3 ± 3 h per night after first month, 2.8 ± 2.7 h on third month Greatest predictor of use was maternal education Older, typically developing African American youth with low social support had poor adherence |
| Simon et al,44 2012 | N = 51 children and their parents 51% male 51% non-Hispanic Caucasian 37% African American 64% had Medicaid |
8–17 y | Average AHI 17/h Average CPAP use of 22.9 mo |
73.5% overweight/ obese | Usage data from machine | Poor adherence with average use of 3.35 h per night Questionnaire developed was able to identify specific barriers to CPAP |
| Marcus et al,41 2006 | N = 29 children 72% male 51% African American |
2–16 y | Newly diagnosed OSAS | 65% obese 10% craniofacial abnormalities 34% systolic hypertension |
Parental report and usage data from machine for 6 mo | Average use 3.8 ± 3.3 h per night 9 dropouts Parental report overestimated actual use No difference in adherence between CPAP and BPAP |
| O’Donnell et al,37 2006 | N = 50 children | Mean 10 ± 5.1 y 66% male |
Median AHI = 11.3 | 78% with comorbidity | Usage data from machine | Average use 6.3 h per night |
| Koontz et al,35 2003 | N = 20 children 55% African American 30% Caucasian |
1–17 y | Nonadherent children referred by physicians | 45% with some degree of developmental delay | Usage data from machine | 3 groups:
|
| Massa et al,36 2002 | N = 66 children 59% male |
Infant to 19 y | All moderate to severe OSAS (AHI >5 per hour) | 35% craniosynostosis syndromes 9.1% isolated facial defects 6.1% obese 3% trisomy 21 3% cerebral palsy |
Parental report | 67.7% report good adherence (uses every night and all night long) CPAP tolerated by 86% |
| Marcus et al,17 1994 | N = 94 children 64% male |
2 wk to 19 y | — | 27% obese 25% craniofacial abnormalities 13% trisomy 21 |
Parental report | 12.7% with inadequate adherence |
In a randomized double-blind trial comparing the efficacy and adherence of CPAP and BPAP in PAP-naïve children with OSAS, intention-to-treat analysis that included 8 of 29 patients who failed to return for CPAP usage downloads found adherence for all participants to be suboptimal, at an average usage of 3.8 ± 3.3 hours per night.27 Another study by O’Donnell and colleagues37 reported similar findings of suboptimal adherence, with mean usage of 4.7 hours per night. These results are similar to the findings of a group of Australian investigators who also found a mean CPAP use of 4.7 hours in their study evaluating patterns of CPAP adherence during the first 3 months of treatment in children.38 However, it should be noted that although these numbers are close to or higher than the adult criteria for satisfactory CPAP adherence39 (ie, at least 4 hours/night on 70% of nights), children have a longer sleep duration than adults, and thus thresholds for “adequate” levels of adherence may actually be higher. Furthermore, a study by Marcus and colleagues40 suggested that longer duration of CPAP use (mean minutes used per night) correlates inversely with Epworth Sleepiness Scale scores.
It should also be noted that data collected in the context and controlled setting of a study may not reflect typical levels of adherence in practice settings, in which monitoring, feedback, and support levels are likely to be less intense. This aspect represents a particular challenge for clinicians caring for children with OSAS in clinical practice. Even in an experimental setting, one study reported a dropout rate of 24% (7 of 29) during the course of an investigation that provided free equipment and comprehensive social and technical support.41 It can be theorized that the reluctance to continue with CPAP treatment in the clinical setting, where resources may be limited, is even more prevalent.
FACTORS INFLUENCING CPAP ADHERENCE
Literature from studies on adult CPAP adherence identified categories of factors that influence or predict adherence to CPAP use: (1) disease characteristics; (2) patient characteristics; (3) treatment titration procedures; (4) technological device factors and side effects; and (5) psychological and social factors. A summary of these factors and their relationship to the course of CPAP treatment is summarized in Table 2.42
Table 2.
Factors that influence on CPAP adherence in adults
| Factor | Relationship to Course of Treatment | ||
|---|---|---|---|
| Pre-CPAP Exposure | Initial CPAP Exposure | Home CPAP Treatment | |
| Disease and patient characteristics | Disease severity Sleepiness Upper airway patency Race Socioeconomic status |
Depression Mood Personality type |
Depression Mood Personality type |
| Treatment titration procedure | — | Autotitrating CPAP | — |
| Technological device factors and side effects | Claustrophobia | Heated humidification | Heated humidification |
| Psychological and social factors | Disease- and treatment-specific knowledge | Disease- and treatment- specific knowledge | Disease- and treatment- specific knowledge Decisional balance Active coping style Disease-specific risk perception |
Abbreviation: CPAP, continuous positive airway pressure.
Adapted from Sawyer AM, Gooneratne NS, Marcus CL, et al. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev 2011;15(6):345; with permission.
Although these categories provide a framework to better understand patients’ decisions to adhere to CPAP treatment, one cannot simply extrapolate and assume that these factors are important in the pediatric population. Several retrospective and observational studies also implicate some of these factors in poor CPAP adherence in the pediatric population. However, although only a few prospective studies,43,44 discussed herein, are available, these seem to demonstrate a different profile of factors from those shown to predict poor adherence in adults.
Psychosocial Factors
One prospective study collected data potentially pertaining to CPAP adherence and correlated these with objective CPAP adherence data. In their cohort of 56 children aged 2 to16 years, DiFeo and colleagues43 evaluated patient characteristics (obesity, race, gender), disease characteristics (severity of OSAS as evidence by AHI), child characteristics (presence/absence of developmental delay and attention-deficit/hyperactivity disorder), CPAP-related factors (CPAP pressure and nasal symptoms), and psychosocial factors (maternal education, social support). Financial/insurance barriers were controlled for by providing all of the participants with free equipment and treatment.43 Their analysis revealed that the greatest predictor of CPAP use was maternal education (r = 0.290, P = .033 for mean hours used per night). Disease-specific factors such as polysomnographic measures and symptoms of excessive daytime sleepiness, which predict CPAP adherence in the adult population, did not significantly correlate with poor CPAP use, nor was there predictive value found in clinical factors such as CPAP pressure and nasal symptoms. The investigators postulated that higher maternal education provided better parental understanding of the consequences of OSAS and the importance of treatment adherence.
DiFeo and colleagues43 also found the Medical Outcomes Study Social Support (MOSS) questionnaire, a measure of family social support, to be somewhat predictive of CPAP adherence, though not as strongly as maternal education. This finding suggests that a combination of caregiver (in this case mothers) knowledge and social support are both important to adherence in children. Several empirical investigations have found that patients’ level of adherence is positively associated with their motivation to achieve or maintain good health. Cobb and Jones45 suggested that social support encompasses: (1) the supportive behavior of individuals family and friends; (2) the nature of the social network surrounding individuals; and (3) the individual’s perceptions of the support provided by their family and friends. However, social support may also exert a stressful influence on the patient when the level of support is perceived as threatening to the patient’s autonomy.46 This influence is likely to be of greatest importance with adolescent patients.
Patients’ Characteristics
In the aforementioned prospective study that evaluated risk factors for pediatric CPAP adherence, DiFeo and colleagues43 also found that certain patient characteristics predicted poor CPAP adherence. African American children, independent of socioeconomic status, were less likely to be adherent to CPAP than children of other races. This finding mirrors observations found in adult CPAP adherence literature.47 Older children, particularly adolescents, were also less likely to adhere to CPAP therapy, which is consistent with data from studies of adherence to other pediatric medical regimens (see later discussion).48–50
Device Factors
Most studies have shown that the type of CPAP device used does not affect adherence, suggesting that adherence is affected by general factors more than by device-specific or disease-specific factors. However, one pediatric study showed that full-face masks were associated with lower adherence than nasal masks.37 Studies in both children and adults comparing different types of pressure delivery, such as BPAP or C-Flex/BiFlex (which deliver a slight decrease in pressure during early expiration and/or inspiration), have had mixed results, but have generally not shown an improvement in adherence in comparison with standard CPAP.41,51,52
Factors Affecting Adherence in Adolescents
Studies on adherence to other medical regimens for the treatment of asthma, epilepsy, and diabetes have shown poor adherence in adolescents.53,54 In their review of factors influencing adherence to medical treatments, Fotheringham and Sawyer46 observed lower adherence in adolescents than in adults. These investigators argue that poor adherence may be due to the struggle for greater autonomy at home, and reflects rebellion against the regimens’ control over patients’ lives.
There is a paucity of data describing CPAP adherence in adolescents. Prashad and colleagues55 investigated CPAP adherence in 21 adolescents using qualitative semistructured interviews that explored issues of stress, parenting style, family structure and organization, behavioral and emotional problems, knowledge of OSAS, and benefits of CPAP, combined with usage data from the CPAP machine. Their qualitative results found that patients/families with high CPAP usage had stable family structure, high knowledge of CPAP, free communication between parent and adolescent, motivation from a desire to please the caregiver, and an “authoritative parenting style”56 whereby children are encouraged to inquire about rules to fully understand them. In this context, parents explained the importance of CPAP use and gave adolescents the choice to use CPAP or not. Adherence was also higher in patients who were prescribed CPAP before adolescence. This finding was postulated to be related to the longer opportunity for CPAP to become a part of the patient’s routine when it is prescribed at a younger age, instead of in adolescence when issues of rebellion and emerging autonomy may prove challenging. It was concluded that health education and family involvement are important components in promoting CPAP adherence, and that support strategies should be tailored to the individual and take into account the developmental process of adolescence.
OVERCOMING BARRIERS TO ADHERENCE
Child/Parent Engagement
Successful promotion of CPAP adherence infers addressing child/parent engagement in the initial acceptance of CPAP. Some barriers to this include preconceived notions about CPAP, and outcome expectations incongruent to that of the treating physician and staff.57 Determination of the child’s and parent’s preexisting attitudes toward CPAP is an important first step, and functions to dispel disbeliefs and tailor patient education on outcome expectations. Central to this process of engaging the child and the parent is maintenance of a positive and supportive approach by everyone involved. Such an approach is especially important in children with complex medical problems, for whom gradual CPAP exposure in a supportive setting and anticipatory guidance for troubleshooting may be particularly important.42
Identification of Specific Barriers
To improve adherence, it is vital to identify specific barriers to adherence that both families and patients themselves experience. A significant portion of the literature on adherence has focused predominantly on the concerns of the patients’ family alone.58 To address this gap, Simon and colleagues44 constructed a psychometric questionnaire to evaluate child and family barriers to CPAP adherence. The Adherence Barriers to CPAP Questionnaire (ABCQ) included 31 questions developed across several conceptually derived domains including side effects, time management, health care provider relationships, equipment concerns, and family support, to name a few. The ABCQ demonstrated excellent reliability and validity, and correlated with objective adherence rates. Having a greater amount of barriers identified on ABCQ was associated with poorer rates of adherence for both parent (r = −0.44, P = .002) and the child (r = −0.44, P = .002). Table 3 summarizes the most frequently endorsed barriers to CPAP reported. The ABCQ represents the first tool specifically to assess specific barriers to CPAP, but further investigation is needed to determine its utility as a measure of treatment outcomes.
Table 3.
Most frequently endorsed barriers to CPAP reported on the ABCQ
| Parent-Report Item (% Agree/Strongly Agree) | Child-Report Item (% Agree/Strongly Agree) | ||
|---|---|---|---|
| Does not use when away from home | 45.1 | Does not use when away from home | 47.0 |
| Child not feeling well | 44.0 | Just want to forget about OSAS | 43.1 |
| Forgets | 39.2 | Not feeling well | 42.0 |
| Child does not feel like using CPAP | 30.0 | Forgets | 39.2 |
| Child just wants to forget about OSAS | 23.6 | No one helps to use CPAP at night | 31.4 |
| Child embarrassed about using CPAP | 22.0 | Embarrassed about using CPAP | 29.4 |
Abbreviations: ABCQ, adherence barriers to CPAP questionnaire; CPAP, continuous positive airway pressure; OSAS, obstructive sleep apnea syndrome.
Adapted from Simon SL, Duncan CL, Janicke DM, et al. Barriers to treatment of paediatric obstructive sleep apnoea: development of the adherence barriers to continuous positive airway pressure (CPAP) questionnaire. Sleep Med 2012;13(2):172–7; with permission.
Initial Acceptance
An unpleasant initial exposure to CPAP has been observed to lead to a prolonged period of treatment rejection from both child and caregiver.57 Patients with tactile aversions (eg, children with developmental delays) are particularly challenging because they are not typically accepting of the mask at the time of initial presentation. Families and medical practitioners must be prepared to invest substantial time and patience toward successfully initiating and maintaining CPAP therapy.
Overcoming the child’s aversion to CPAP may require desensitization procedures that allow the child to slowly habituate by gradual exposure to the mask and machine (ie, operant conditioning). An example would be to divide the procedures into daytime and nighttime practices in an attempt to establish CPAP as part of the child’s routine activities.59 This approach is further detailed in Table 4. Each practice session should start with an enjoyable, calming activity so the child begins to associate CPAP with positive experiences. The child should be offered small rewards such as stickers or a prize each time he or she is successful with the practice session, in addition to a verbal praise when the child complies with the parental request. Any negative behaviors such as crying or yelling are ignored. For older children or adolescents, a token economy can be implemented, designed to increase desirable behavior and decrease undesirable behavior with the use of tokens, such as stickers or check marks. The child should receive a sticker immediately after displaying desirable behavior (eg, complying with a request to put the CPAP mask on for designated amount of time). The stickers are collected (or counted up on a chart) and later exchanged for a meaningful object or privilege. Emphasis is placed on practice, patience, and persistence for the overall implementation of CPAP. The goal is to avoid struggles and negative interactions around CPAP, and to implement CPAP in the least stressful way possible for children and parents.
Table 4.
Desensitization procedure for infants and school-aged children
| Daytime Practice | Nighttime Practice |
|---|---|
Introducing the mask
|
Adjusting to the sounds at night
|
Turning on the air
Wearing the mask with airflow while lying down
|
Making PAP part of your child’s bedtime routine
|
Abbreviation: PAP, positive airway pressure.
Adapted from The Children’s Hospital of Philadelphia. CPAP education: noninvasive ventilation for infants and children. 2013. Available at: http://www.chop.edu/service/sleep-center/cpap-education/non-invasive-ventilation-for-infants-and-children.html. Accessed November 25, 2013; with permission.
The location where CPAP initiation occurs has also been observed to have an effect on adherence to therapy. Ramirez and colleagues60 evaluated long-term adherence to CPAP among 62 children with OSAS who were followed in a pediatric CPAP/noninvasive unit. A high level of adherence was observed in patients who initiated CPAP and followed up in the unit. The investigators postulated that the high level of adherence may have been due to the availability of a dedicated team that provided positive and continuous support for the patient and family from CPAP initiation through follow-up. However, most patients can successfully undergo CPAP initiation in the outpatient setting.
Behavioral Intervention
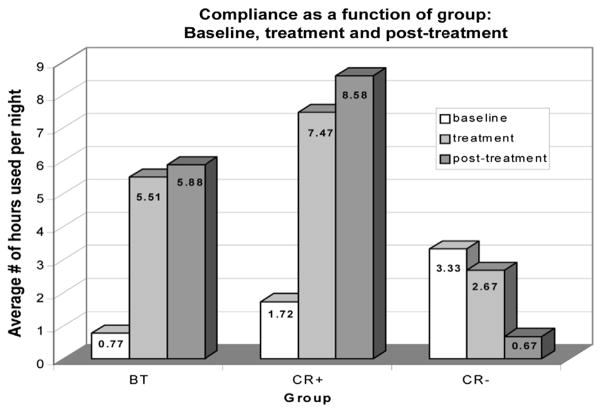
Behavioral conditioning may be a useful tool to help achieve CPAP acceptance. Koontz and colleagues35 studied 20 children with OSAS, aged 1 to 17 years, who were referred from their respective primary physicians for CPAP nonadherence. Patients and their guardians were then allowed to self-select into 1 of 3 treatment arms: (1) behavior therapy; (2) behavior consultation and recommendation uptake; (3) behavior consultation without recommendation uptake. Before assignments into their respective treatment arms, all children and their guardians attended a behavior consultation with trained staff. This consultation sought to identify individual information about the child’s preferences and dislikes to generate recommendations. Results of the study showed that patients in both behavior therapy (75%) and behavior consultation with recommendation uptake (100%) had a statistically significantly heavier CPAP use than those in the behavior consultation group without recommendation uptake (0%). A graphic representation of the results is shown in Fig. 2. This retrospective and descriptive interventional study suggests that both brief behavior consultations and extensive behavioral therapy may offer effective and relatively inexpensive interventions for CPAP adherence.
Fig. 2.
Results of CPAP adherence strategies in a group of children referred for behavioral counseling for poor CPAP adherence. Subjects were divided into 3 groups: BT received consultation and recommendations plus a course of behavior therapy; CR+ received a 1.5-hour consultation and recommendation session; CR− underwent a consultation and behavior therapy was recommended, but the family did not follow up. The y-axis shows the average number of hours per night of CPAP use. The hours of use are further subdivided into baseline, during treatment, and posttreatment values. Seventy-five percent of children who received behavior intervention (CR+ and BT groups) tolerated CPAP successfully (P<.5 for CR+ vs CR− group; other comparisons were not significant). (Adapted from Koontz KL, Slifer KJ, Cataldo MD, et al. Improving pediatric compliance with positive airway pressure therapy: the impact of behavioral intervention. Sleep 2003;26(8):1012; with permission.)
Side Effects
Similar to any form of treatment, CPAP has associated side effects that may have a negative impact on adherence if unaddressed. Nasal symptoms are the most common side effect, caused by the drying effects of CPAP by altering nasal mucosa and impeding mucociliary clearance.30,61 This symptom can be ameliorated with a heated humidifier, maintenance of clean equipment, and, in some cases, intranasal steroids, saline, antihistamines, montelukast, or other treatments.31,62 Skin irritation and even ulceration can occur from a tight-fitting mask or accumulation of skin oils and debris from poor mask maintenance; rarely, allergies to the mask may occur.57 A properly fitted mask and daily cleaning of the equipment is thus integral to CPAP adherence. Central apnea at high pressures can be a complication of CPAP use in some children because of an active Hering-Breuer reflex.63 Switching from CPAP to BPAP with a backup rate addresses this problem.
AN EXAMPLE OF A CLINICAL APPROACH
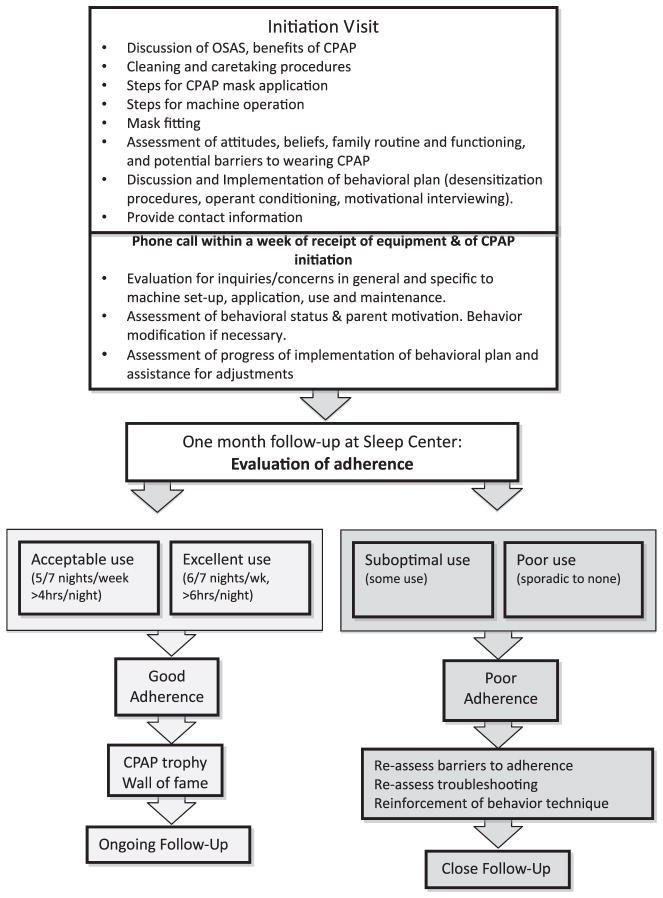
The Sleep Center at The Children’s Hospital of Philadelphia (CHOP) is a tertiary referral center for children with a broad range of sleep disorders (Fig. 3, see Table 4). The center comprises a multi-disciplinary team of sleep physicians, behavioral psychologists, respiratory therapists, and nurses, collaborating to provide comprehensive evaluation and management of patients with various sleep disorders, including OSAS. A general description of the CHOP PAP protocol as a model of care follows.
Fig. 3.
Example of a CPAP adherence algorithm for patients with obstructive sleep apnea.
The sleep physician’s role in the initial CPAP visit is to educate the family about OSAS, its pathophysiology, complications, and the importance of treating it. The basic mechanism of how CPAP works and side effects that might be encountered are also discussed. The respiratory therapist and nurses present different types of mask interfaces from which the family/patient can choose. The patient is encouraged to try on several masks to determine which is the most comfortable (Fig. 4). Having the patient and family involved in choosing the mask offers them an opportunity to increase a sense of control in a situation where they may feel a loss of control. The psychologist assesses the patient’s and caregiver’s attitudes and beliefs about CPAP, potential barriers to using CPAP, in addition to family functioning and patient and family routines. He or she then discusses behavioral techniques (eg, mask desensitization, operant conditioning) to help improve adherence, as well as strategies to implement CPAP into the specific patient’s daily routine. For example, for a family whose household is chaotic or whose child does not have a “typical routine,” the psychologist and family may together develop a specific evening and bedtime routine that ends with putting on the CPAP.
Fig. 4.
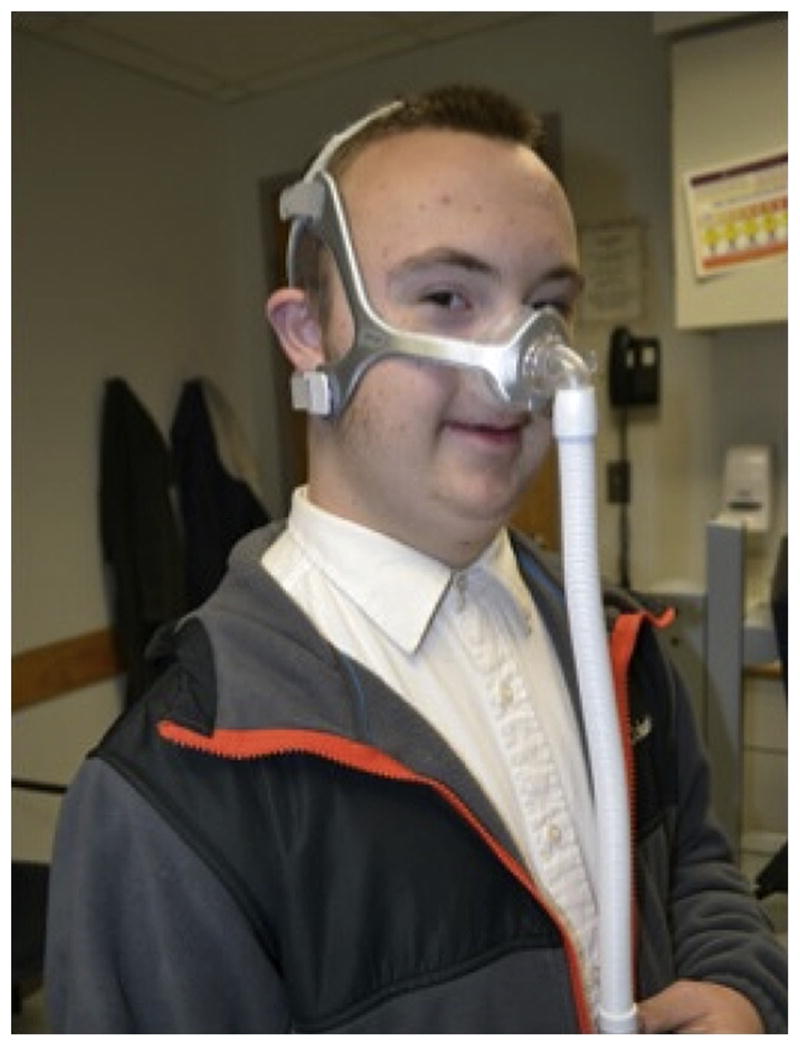
A patient with trisomy 21 trying on a nasal mask during a CPAP initiation visit.
For an adolescent, it may be beneficial to discuss a division of responsibilities between the adolescent and caregiver to reduce feelings in both parties of being overburdened. A caregiver may agree to remind the adolescent to get his or her CPAP ready before bed and to help with the deeper weekly cleaning of the equipment, while the adolescent agrees to be responsible for putting the CPAP on each night and participate in the daily care of the equipment (eg, pouring water out of the humidifier, rinsing the humidifier, tubing, and mask each day, and setting out to air dry in the morning). This strategy aims to respect and improve the autonomy and responsibility of the adolescent while providing the necessary support he or she likely needs in adhering to a medical intervention without a sense of being nagged.
A durable medical equipment representative also meets with the family to discuss machine operation, maintenance, and troubleshooting, and to provide equipment for the family to take home that day. At the end of the visit, it is verified that the family is given a network of support by providing contact information for them to call in case of further inquiry. This process is spearheaded by the CPAP coordinator (a position split between a respiratory therapist and psychologist) to provide an organized channel for subsequent clinic follow-up. A CPAP titration polysomnogram is typically not performed until the child is tolerating CPAP set at a low pressure at home.
Within a week, a phone call is made by a CPAP coordinator to check adherence and to troubleshoot any problems such as device issues or CPAP side effects, as well as to assess behavioral implementation concerns or barriers. If behavioral concerns exist or persist, the psychologist follows up by phone regularly. The respiratory therapist and nurses also provide phone support for technical or side effects and medical concerns as needed. Patients are encouraged to follow up at the clinic after 1 month, at which time adherence is assessed. The CPAP machine’s usage data is downloaded to provide an objective measure of the patient’s adherence. The results are then discussed with the patient and family, who are shown a visual representation of the usage (Fig. 5). Those who have good adherence are given an incentive CPAP trophy (when developmentally appropriate) (Fig. 6) and have their picture posted on the “CPAP Hall of Fame.” Patients with poor adherence are reassessed by the team for specific barriers to adherence such as troubleshooting machine problems, issues with the mask fit or putting it on correctly, or behavioral barriers. Adolescents may need help troubleshooting simple issues, such as finding an electrical outlet near their bed, developing a plan to take their machine with them when staying overnight at relatives’ or friends’ homes, or setting an alert on their phone to remind them to put on their mask at night. For all patients, technical support and behavioral reinforcement is provided, with close follow-up thereafter. Children are seen on a regular basis, with the frequency of their clinic visits depending on their age, severity of medical condition, and CPAP adherence.
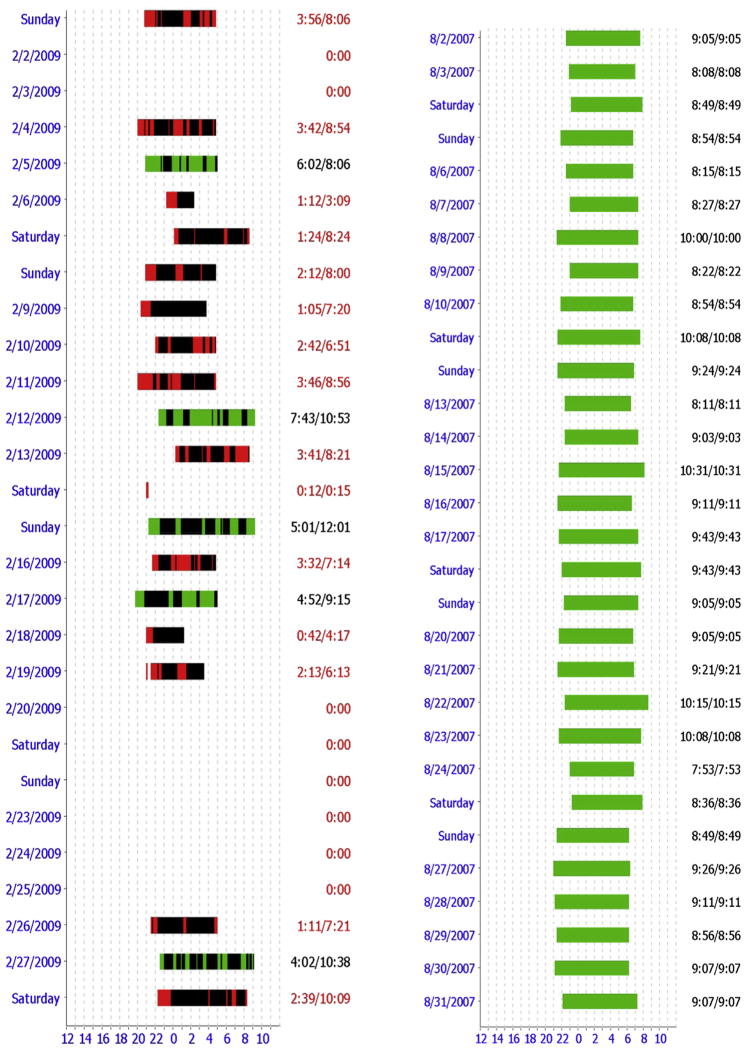
Fig. 5.
CPAP data downloads. The column on the left shows usage data from a nonadherent patient, and the column on the right is from an adherent patient. The x-axis shows 24-hour clock time and the left y-axis shows the date. CPAP use for 4 hours or more is shown in green, less than 4 hours in red; air leak is shown in black. Corresponding numbers are shown on the right y-axis. Air leak may be due to the mask being damaged, fitting poorly, or being off the face when the air compressor is turned on.
Fig. 6.

A patient receiving a CPAP champion trophy for good adherence.
In almost all cases, CPAP is implemented in an outpatient setting. Occasionally a family (typically with an infant, toddler, or developmentally delayed child) is unable to implement a desensitization program, and a short 2- to 4-week intervention with home nursing is implemented. This approach often breaks the cycle of CPAP refusal, anxiety, and frustration, and demonstrates to the family that the child can tolerate CPAP. Typically parents are able to continue implementing CPAP at the end of the nursing intervention. Rarely, patients are admitted to an inpatient unit when all other measures fail.
SUMMARY
CPAP is a safe and effective treatment for children with OSAS that has many beneficial effects. The major limiting factor for successful treatment with CPAP is adherence. Psychosocial factors and patient characteristics are the main predictors of poor adherence, with low maternal education being the strongest. Managing suboptimal adherence entails engaging and educating the child and parent about CPAP. This approach involves discussing preexisting attitudes, outcome expectations, and side effects, in addition to providing constant support from a dedicated team. More intensive cognitive-behavioral interventions may prove useful in overcoming poor CPAP adherence in complex cases.
KEY POINTS.
Obstructive sleep apnea syndrome (OSAS) is a relatively common disorder affecting approximately 1% to 5% of children aged 5 to 13 years, and is associated with adverse neurobehavioral, cognitive, and physiologic consequences.
Adenotonsillectomy is considered to be the first-line treatment; however, not all patients are surgical candidates and some patients will continue to have symptoms after surgery.
Continuous positive airway pressure (CPAP) is a safe and effective treatment for children with OSAS who do not improve with surgery or who are not candidates for surgery. The major limiting factor for successful treatment with CPAP is adherence.
Psychosocial factors and patient characteristics are the main predictors of poor adherence, with low maternal education being the strongest.
Managing suboptimal adherence entails engaging and educating the child and parent about CPAP; this involves discussing preexisting attitudes, outcome expectations, and side effects, in addition to providing constant support from a dedicated team.
More intensive cognitive behavioral interventions may prove useful in overcoming poor CPAP adherence in complex cases.
Acknowledgments
The authors would like to thank the Children’s Hospital of Philadelphia CPAP team for their dedication to patients and their families.
Footnotes
Disclosures: C.L. Marcus has received funding from Philips Respironics and Ventus, and was funded, in part, by NIH RO1 HL58585. NIHMS-ID: 571573.
References
- 1.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 2.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li AM, So HK, Au CT, et al. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax. 2010;65(11):991–7. doi: 10.1136/thx.2010.134858. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien LM, Holbrook CR, Mervis CB, et al. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics. 2003;111(3):554–63. doi: 10.1542/peds.111.3.554. [DOI] [PubMed] [Google Scholar]
- 5.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117(4):e769–78. doi: 10.1542/peds.2005-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beebe DW, Ris MD, Kramer ME, et al. The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep. 2010;33(11):1447–56. doi: 10.1093/sleep/33.11.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beebe DW, Byars KC. Adolescents with obstructive sleep apnea adhere poorly to positive airway pressure (PAP), but PAP users show improved attention and school performance. PLoS One. 2011;6(3):e16924. doi: 10.1371/journal.pone.0016924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurnatowski P, Putyński L, Lapienis M, et al. Neurocognitive abilities in children with adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 2006;70(3):419–24. doi: 10.1016/j.ijporl.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Barnes ME, Gozal D, Molfese DL. Attention in children with obstructive sleep apnoea: an event-related potentials study. Sleep Med. 2012;13(4):368–77. doi: 10.1016/j.sleep.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1098–103. doi: 10.1164/ajrccm.157.4.9704080. [DOI] [PubMed] [Google Scholar]
- 12.Amin RS, Kimball TR, Bean JA, et al. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(10):1395–9. doi: 10.1164/rccm.2105118. [DOI] [PubMed] [Google Scholar]
- 13.Duman D, Naiboglu B, Esen HS, et al. Impaired right ventricular function in adenotonsillar hypertrophy. Int J Cardiovasc Imaging. 2008;24(3):261–7. doi: 10.1007/s10554-007-9265-1. [DOI] [PubMed] [Google Scholar]
- 14.Hunt CE, Brouillette RT. Abnormalities of breathing control and airway maintenance in infants and children as a cause of cor pulmonale. Pediatr Cardiol. 1982;3(3):249–56. doi: 10.1007/BF02240461. [DOI] [PubMed] [Google Scholar]
- 15.Kelly A, Dougherty S, Cucchiara A, et al. Catecholamines, adiponectin, and insulin resistance as measured by HOMA in children with obstructive sleep apnea. Sleep. 2010;33(9):1185–91. doi: 10.1093/sleep/33.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deboer MD, Mendoza JP, Liu L, et al. Increased systemic inflammation overnight correlates with insulin resistance among children evaluated for obstructive sleep apnea. Sleep Breath. 2012;16(2):349–54. doi: 10.1007/s11325-011-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus CL, Carroll JL, Koerner CB, et al. Determinants of growth in children with the obstructive sleep apnea syndrome. J Pediatr. 1994;125(4):556–62. doi: 10.1016/s0022-3476(94)70007-9. [DOI] [PubMed] [Google Scholar]
- 18.Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med. 1996;153(6 Pt 1):1880–7. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- 19.Kulnis R, Nelson S, Strohl K, et al. Cephalometric assessment of snoring and nonsnoring children. Chest. 2000;118(3):596–603. doi: 10.1378/chest.118.3.596. [DOI] [PubMed] [Google Scholar]
- 20.Brooks LJ, Stephens BM, Bacevice AM. Adenoid size is related to severity but not the number of episodes of obstructive apnea in children. J Pediatr. 1998;132(4):682–6. doi: 10.1016/s0022-3476(98)70360-9. [DOI] [PubMed] [Google Scholar]
- 21.Shintani T, Asakura K, Kataura A. Evaluation of the role of adenotonsillar hypertrophy and facial morphology in children with obstructive sleep apnea. ORL J Otorhinolaryngol Relat Spec. 1997;59(5):286–91. doi: 10.1159/000276955. [DOI] [PubMed] [Google Scholar]
- 22.Marcus CL. Obstructive sleep apnea syndrome: differences between children and adults. Sleep. 2000;23(Suppl 4):S140–1. [PubMed] [Google Scholar]
- 23.Arens R, McDonough JM, Costarino AT, et al. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164(4):698–703. doi: 10.1164/ajrccm.164.4.2101127. [DOI] [PubMed] [Google Scholar]
- 24.Smith DF, Benke JR, Yaster S, et al. A pilot staging system to predict persistent obstructive sleep apnea in children following adenotonsillectomy. Laryngoscope. 2013;123(7):1817–22. doi: 10.1002/lary.23925. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell RB, Kelly J. Outcome of adenotonsillectomy for obstructive sleep apnea in obese and normal-weight children. Otolaryngol Head Neck Surg. 2007;137(1):43–8. doi: 10.1016/j.otohns.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Suen JS, Arnold JE, Brooks LJ. Adenotonsillectomy for treatment of obstructive sleep apnea in children. Arch Otolaryngol Head Neck Surg. 1995;121(5):525–30. doi: 10.1001/archotol.1995.01890050023005. [DOI] [PubMed] [Google Scholar]
- 27.Marcus CL, Beck SE, Traylor J, et al. Randomized, double-blind clinical trial of two different modes of positive airway pressure therapy on adherence and efficacy in children. J Clin Sleep Med. 2012;8(1):37–42. doi: 10.5664/jcsm.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcus CL, McColley SA, Carroll JL, et al. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol (1985) 1994;77(2):918–24. doi: 10.1152/jappl.1994.77.2.918. [DOI] [PubMed] [Google Scholar]
- 29.Smith PL, Wise RA, Gold AR, et al. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol (1985) 1988;64(2):789–95. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 30.Pepin JL, Leger P, Veale D, et al. Side effects of nasal continuous positive airway pressure in sleep apnea syndrome. Study of 193 patients in two French sleep centers. Chest. 1995;107(2):375–81. doi: 10.1378/chest.107.2.375. [DOI] [PubMed] [Google Scholar]
- 31.Massie CA, Hart RW, Peralez K, et al. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest. 1999;116(2):403–8. doi: 10.1378/chest.116.2.403. [DOI] [PubMed] [Google Scholar]
- 32.Marcus CL, Ward SL, Mallory GB, et al. Use of nasal continuous positive airway pressure as treatment of childhood obstructive sleep apnea. J Pediatr. 1995;127(1):88–94. doi: 10.1016/s0022-3476(95)70262-8. [DOI] [PubMed] [Google Scholar]
- 33.McNamara F, Sullivan CE. Obstructive sleep apnea in infants and its management with nasal continuous positive airway pressure. Chest. 1999;116(1):10–6. doi: 10.1378/chest.116.1.10. [DOI] [PubMed] [Google Scholar]
- 34.Downey R, 3rd, Perkin RM, MacQuarrie J. Nasal continuous positive airway pressure use in children with obstructive sleep apnea younger than 2 years of age. Chest. 2000;117(6):1608–12. doi: 10.1378/chest.117.6.1608. [DOI] [PubMed] [Google Scholar]
- 35.Koontz KL, Slifer KJ, Cataldo MD, et al. Improving pediatric compliance with positive airway pressure therapy: the impact of behavioral intervention. Sleep. 2003;26(8):1010–5. doi: 10.1093/sleep/26.8.1010. [DOI] [PubMed] [Google Scholar]
- 36.Massa F, Gonsalez S, Laverty A, et al. The use of nasal continuous positive airway pressure to treat obstructive sleep apnoea. Arch Dis Child. 2002;87(5):438–43. doi: 10.1136/adc.87.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Donnell AR, Bjornson CL, Bohn SG, et al. Compliance rates in children using noninvasive continuous positive airway pressure. Sleep. 2006;29(5):651–8. [PubMed] [Google Scholar]
- 38.Nixon GM, Mihai R, Verginis N, et al. Patterns of continuous positive airway pressure adherence during the first 3 months of treatment in children. J Pediatr. 2011;159(5):802–7. doi: 10.1016/j.jpeds.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Pepin JL, Krieger J, Rodenstein D, et al. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Am J Respir Crit Care Med. 1999;160(4):1124–9. doi: 10.1164/ajrccm.160.4.9802027. [DOI] [PubMed] [Google Scholar]
- 40.Marcus CL, Radcliffe J, Konstantinopoulou S, et al. Effects of positive airway pressure therapy on neurobehavioral outcomes in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185(9):998–1003. doi: 10.1164/rccm.201112-2167OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117(3):e442–51. doi: 10.1542/peds.2005-1634. [DOI] [PubMed] [Google Scholar]
- 42.Sawyer AM, Gooneratne NS, Marcus CL, et al. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–56. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DiFeo N, Meltzer LJ, Beck SE, et al. Predictors of positive airway pressure therapy adherence in children: a prospective study. J Clin Sleep Med. 2012;8(3):279–86. doi: 10.5664/jcsm.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon SL, Duncan CL, Janicke DM, et al. Barriers to treatment of paediatric obstructive sleep apnoea: development of the adherence barriers to continuous positive airway pressure (CPAP) questionnaire. Sleep Med. 2012;13(2):172–7. doi: 10.1016/j.sleep.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 45.Cobb S, Jones JM. Social support, support groups and marital relationships. In: Duck S, editor. Personal relationships. Vol. 5. London: Academic; 1984. pp. 47–66. [Google Scholar]
- 46.Fotheringham MJ, Sawyer MG. Adherence to recommended medical regimens in childhood and adolescence. J Paediatr Child Health. 1995;31(2):72–8. doi: 10.1111/j.1440-1754.1995.tb00750.x. [DOI] [PubMed] [Google Scholar]
- 47.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strunk RC, Bender B, Young DA, et al. Predictors of protocol adherence in a pediatric asthma clinical trial. J Allergy Clin Immunol. 2002;110(4):596–602. doi: 10.1067/mai.2002.128803. [DOI] [PubMed] [Google Scholar]
- 49.Smith BA, Shuchman M. Problem of nonadherence in chronically ill adolescents: strategies for assessment and intervention. Curr Opin Pediatr. 2005;17(5):613–8. doi: 10.1097/01.mop.0000176443.26872.6e. [DOI] [PubMed] [Google Scholar]
- 50.Khan M, Song X, Williams K, et al. Evaluating adherence to medication in children and adolescents with HIV. Arch Dis Child. 2009;94(12):970–3. doi: 10.1136/adc.2008.156232. [DOI] [PubMed] [Google Scholar]
- 51.Chihara Y, Tsuboi T, Hitomi T, et al. Flexible positive airway pressure improves treatment adherence compared with auto-adjusting PAP. Sleep. 2013;36(2):229–36. doi: 10.5665/sleep.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakker J, Campbell A, Neill A. Randomized controlled trial comparing flexible and continuous positive airway pressure delivery: effects on compliance, objective and subjective sleepiness and vigilance. Sleep. 2010;33(4):523–9. doi: 10.1093/sleep/33.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chigier E. Compliance in adolescents with epilepsy or diabetes. J Adolesc Health. 1992;13(5):375–9. doi: 10.1016/1054-139x(92)90032-7. [DOI] [PubMed] [Google Scholar]
- 54.Orrell-Valente JK, Jarlsberg LG, Hill LG, et al. At what age do children start taking daily asthma medicines on their own? Pediatrics. 2008;122(6):e1186–92. doi: 10.1542/peds.2008-0292. [DOI] [PubMed] [Google Scholar]
- 55.Prashad PS, Marcus CL, Maggs J, et al. Investigating reasons for CPAP adherence in adolescents: a qualitative approach. J Clin Sleep Med. 2013;9(12):1303–13. doi: 10.5664/jcsm.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monaghan M, Horn I, Alvarez V, et al. Authoritative parenting, parenting stress, and self-care in pre-adolescents with Type 1 diabetes. J Clin Psychol Med S. 2012;19:255–61. doi: 10.1007/s10880-011-9284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirk VG, O’Donnell AR. Continuous positive airway pressure for children: a discussion on how to maximize compliance. Sleep Med Rev. 2006;10(2):119–27. doi: 10.1016/j.smrv.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 58.DiMatteo MR, Giordani PJ, Lepper HS, et al. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 59.The Children’s Hospital of Philadelphia. [Accessed November 25, 2013];CPAP education: non-invasive ventilation for infants and children. 2013 Available at: http://www.chop.edu/service/sleep-center/cpap-education/non-invasive-ventilation-for-infants-and-children.html.
- 60.Ramirez A, Khirani S, Aloui S, et al. Continuous positive airway pressure and noninvasive ventilation adherence in children. Sleep Med. 2013;14(12):1290–4. doi: 10.1016/j.sleep.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 61.Constantinidis J, Knobber D, Steinhart H, et al. Fine-structural investigations of the effect of nCPAP-mask application on the nasal mucosa. Acta Otolaryngol. 2000;120(3):432–7. doi: 10.1080/000164800750000694. [DOI] [PubMed] [Google Scholar]
- 62.Qureshi A, Ballard RD. Obstructive sleep apnea. J Allergy Clin Immunol. 2003;112(4):643–51. doi: 10.1016/j.jaci.2003.08.031. quiz: 652. [DOI] [PubMed] [Google Scholar]
- 63.Waters KA, Everett FM, Bruderer JW, et al. Obstructive sleep apnea: the use of nasal CPAP in 80 children. Am J Respir Crit Care Med. 1995;152(2):780–5. doi: 10.1164/ajrccm.152.2.7633742. [DOI] [PubMed] [Google Scholar]