Abstract
Platelet-derived growth factor BB and its receptor (PDGFRβ) play a pivotal role in the development of renal glomerular mesangial cells. Their roles in increased mesangial cell proliferation during mesangioproliferative glomerulonephritis have long been noted, but the operating logic of signaling mechanisms regulating these changes remains poorly understood. We examined the role of a recently identified MAPK, Erk5, in this process. PDGF increased the activating phosphorylation of Erk5 and tyrosine phosphorylation of proteins in a time-dependent manner. A pharmacologic inhibitor of Erk5, XMD8-92, abrogated PDGF-induced DNA synthesis and mesangial cell proliferation. Similarly, expression of dominant negative Erk5 or siRNAs against Erk5 blocked PDGF-stimulated DNA synthesis and proliferation. Inhibition of Erk5 attenuated expression of cyclin D1 mRNA and protein, resulting in suppression of CDK4-mediated phosphorylation of the tumor suppressor protein pRb. Expression of cyclin D1 or CDK4 prevented the dominant negative Erk5- or siErk5-mediated inhibition of DNA synthesis and mesangial cell proliferation induced by PDGF. We have previously shown that phosphatidylinositol 3-kinase (PI3-kinase) contributes to PDGF-induced proliferation of mesangial cells. Inhibition of PI3-kinase blocked PDGF-induced phosphorylation of Erk5. Since PI3-kinase acts through Akt, we determined the role of Erk5 on Akt phosphorylation. XMD8-92, dominant negative Erk5, and siErk5 inhibited phosphorylation of Akt by PDGF. Interestingly, we found inhibition of PDGF-induced Erk5 phosphorylation by a pharmacological inhibitor of Akt kinase and kinase dead Akt in mesangial cells. Thus our data unfold the presence of a positive feedback microcircuit between Erk5 and Akt downstream of PI3-kinase nodal point for PDGF-induced mesangial cell proliferation.
Keywords: Akt kinase, cell proliferation, MAP kinase, receptor tyrosine kinase, renal physiology
four different polypeptide chains are identified for platelet-derived growth factors (PDGFs): A, B, C, and D. These platelet-derived growth factor subunits form five different isoforms by homo- or heterodimerization via disulfide bonds. The classical PDGF-A and -B chains are secreted as homo- and heterodimers, while the PDGF-C and -D are secreted as latent forms and require further processing to produce mature homodimers (19). All these isoforms bind to two PDGF receptors (PDGFRα and PDGFRβ) with distinct affinity and specificity. The PDGF BB homodimer or AB heterodimer binds to both receptors. However, PDGF-C binds only to the PDGFRα, while PDGF-D predominantly interacts with homodimeric PDGFRββ and by low affinity with PDGFRαβ (21).
Mesangial cells are considered vascular pericytes that constitute one-third of the cell type of glomeruli, the filtering units of the kidney. They respond to injury and contribute to the hypercellularity observed in number of glomerular diseases (1). IgA nephropathy, membranoproliferative glomerulonephritis, and lupus nephritis are characterized by proliferation of mesangial cells (25). Although many other growth factors stimulate proliferation of mesangial cells, PDGF, especially the BB isoform, is the most potent mitogen for these cells (1, 25). In fact, most cytokine-induced proliferation of mesangial cells is caused by autocrine stimulation of PDGF BB. PDGFRβ is the predominant isoform expressed in renal mesangial cells while PDGFRα is expressed at lower abundance (9, 40). PDGFRβ knockout mice display defect in glomerular mesangial cell development. A similar phenotype was observed with PDGF-B chain deletion in mice (37).
PDGFRs are composed of a large extracellular segment containing IgG-like domains followed by juxtamembrane domain and a split COOH-terminal part with kinase domains separated by an interkinase stretch (22). Binding of PDGFs to the extracellular domain of the receptor induces a conformational change to relieve an autoinhibitory constraint at the juxtamembrane domain on the intrinsic tyrosine kinase activity of the receptor (4, 6). Dimeric receptors then undergo transphosphorylation at multiple tyrosine residues, which act as docking sites for many signaling proteins. Two important enzymes, c-Src and phosphatidylinositol (PI) 3 kinase bind, through their SH-2 domains to specific phosphotyrosine residues and act in tandem to increase proliferation of mesangial cells (11). Another signaling protein Grb2 binds to the interkinase domain at phosphotyrosine-716 to activate the Ras/MEK/Erk1/2 MAPK signaling, which also contributes to mesangial cell proliferation (10).
MAPKs exist as a conserved family in a three-tiered hierarchical module. In this cascade, the proximal kinases respond to distinct extracellular stimuli to propagate signal to the terminal kinases, which undergo dual phosphorylation at the Thr-X-Tyr motifs present in the activation loop (43). There are four family members of these kinases, Erk1/2, JNK, p38, and the most recent addition, Erk5 (30, 31). Erk5 was originally identified as BMK (big MAPK)-1. The NH2 terminus of Erk5 is required for its cytoplasmic targeting. The following kinase domain shares 66% sequence identity with that of Erk2 (44). Furthermore, Erk5 contains a long COOH-terminal domain, which gives it twice the size compared with other MAPKs (46). Although it was originally identified as a stress-activated MAPK, many growth factors and cytokines such as EGF, VEGF, FGF-2, PDGF, IL-6, and trophic factors like NGF and BDNF are known to activate this kinase (31). Erk5 is abundantly expressed in many tissues including the heart (46). Similarly abundant expression of Erk5 has been reported in renal glomeruli including glomerular mesangial cells (38). Since PDGF plays an important role in inducing proliferation of mesangial cells in glomerular injury, we investigated the role of Erk5 in PDGF-induced proliferative response. Our results show that PDGF-stimulated Erk5 activation is necessary for proliferation of mesangial cells. We also demonstrate that Erk5 regulates cyclin D1-dependent inactivation of pRb to drive mesangial cell proliferation. We show that phosphatidylinositol 3-kinase (PI3-kinase) and its downstream target Akt kinase regulate PDGF-induced Erk5 phosphorylation. Furthermore, we provide evidence that a positive feedback loop involving Akt and Erk5 contributes to PDGF-induced mesangial cell proliferation.
MATERIALS AND METHODS
Materials.
Recombinant PDGF BB (described as PDGF in results) was purchased from R&D Systems. Tissue culture materials and first-strand cDNA synthesis kit were obtained from Invitrogen. The PI3-kinase inhibitor Ly294002 and Akt inhibitor MK2206 were purchased from Calbiochem and Selleck Chemicals, respectively. The following antibodies were used: phospho-Erk5 (Thr-218/Tyr-220), anti-phosphotyrosine, phospho-Erk1/2 (Thr-202/Tyr-204), phospho-pRb (Ser-795), phospho-Akt (Ser-473), phospho-Akt (Thr-308), phospho-GSK3β (Ser-9), Akt, GSK3β, and Erk5 (Cell Signaling Technologies); phospho-PDGFRβ (Tyr-857) and PDGFRβ (Upstate Biotechnology); anti-actin and anti-FLAG (Sigma); pRb (Santa Cruz Biotechnology); and anti-HA (Covance). TRI reagent, phenylmethylsulfonylfluoride, Na3VO4, and Nonidet P-40 were obtained from Sigma. XMD8-92 was purchased from Tocris Bioscience. FuGENE HD was purchased from Promega Dominant negative FLAG-Erk5, and constitutively active HA-MEK5 DD was kindly provided by Dr. Cathy Tournier (University of Manchester, Manchester, UK) and Dr. Melanie H. Cobb (University of Texas Southwestern Medical Center at Dallas), respectively (17, 32). Expression plasmids for HA-cyclin D1, HA-CDK4, HA-PTEN, HA-Akt K179M (dominant negative Akt), and HA-Myr-Akt (constitutively active Akt) have been described previously (3, 7). Two independent siRNAs targeted against Erk5 (siErk5A and siErk5B) were purchased from Sigma.
Cell culture.
Rat glomerular mesangial cells were grown in DMEM in the presence of 17% fetal bovine serum as described previously (7, 14). Cells were serum starved for 24 h to make them quiescent before incubation with 20 ng/ml PDGF for the indicated periods of time. In some experiments, the cells were treated with different inhibitors for 1 h before incubation with PDGF.
DNA synthesis and proliferation assays.
DNA synthesis was determined using [3H]thymidine incorporation assay as described previously (3, 7). For measurement of cell proliferation, PDGF-treated mesangial cells were trypsinized at the indicated time periods and counted using a hemocytometer as previously described (3, 16).
Cell lysis and immunoblotting.
After treatment, the cell monolayer was washed with PBS. The cells were then lysed with radioimmunoprecipitation assay buffer (20 mM Tris·HCl pH 7.5, 5 mM EDTA, 150 mM NaCl, 1 mM Na3VO4, 1 mM PMSF, 0.1% protease inhibitor cocktail, and 1% Nonidet-P40) at 4°C for 30 min. The crude extract was centrifuged at 12,000 g at 4°C for 30 min, the supernatant was collected, and protein was estimated. Equal amounts of proteins were separated by SDS-PAGE. The separated proteins were transferred to the PVDF membrane. Immunoblotting was performed using indicated antibodies. The protein signals were developed by horseradish peroxidase-conjugated secondary antibody using the ECL reagent as previously described (14, 15).
RNA isolation and quantitative real-time RT-PCR.
Total RNA was prepared from mesangial cells using the TRI reagent according to manufacturer's protocol. Expression of cyclin D1 was determined by quantitative real-time RT-PCR. One microgram of total RNA was used to make cDNAs using oligo-dT and MMLV reverse transcriptase reagents from Invitrogen. The cDNA was amplified and quantified in a 96-well plate using cyclin D1 primers (forward: 5′-CATCCGCAAACATGCACAGACCTT-3′ and reverse: 5′-TTTCTCTTCGCGGATGCCACTACT-3′) in 7500 real-time PCR machine (Applied Biosystems) using PCR conditions of 94°C for 10 min followed by 45 cycles at 94°C for 30 s, 58°C for 30 s and 72°C for 30s. The level of mRNA was normalized by GAPDH in the same sample. Data were analyzed by comparative Ct method as described previously (14).
Transfection.
Mesangial cells were transfected with plasmid vectors or siRNAs targeted against Erk5 using FuGENE HD essentially as described previously (14, 15). For siRNA transfection, scrambled RNA was used as control.
Statistics.
The significance of the data was analyzed by ANOVA followed by Student-Newman-Keuls analysis (11, 14, 15).
RESULTS
Erk5 regulates PDGF-induced mesangial cell proliferation.
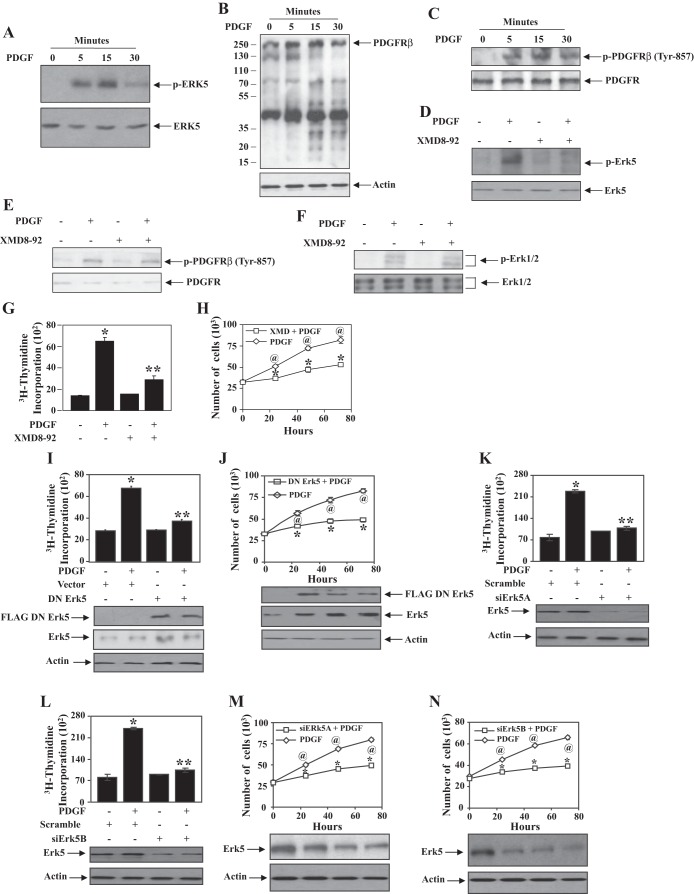
PDGF increases signal transduction pathways to initiate mitogenesis leading to proliferation of glomerular mesangial cells (1). Multiple kinases have been shown to contribute to PDGF-induced cell proliferation (10, 37); however, the role of Erk5 in this process has not been investigated. We examined the effect of PDGF on phosphorylation/activation of Erk5 in mesangial cells. The results showed that PDGF significantly increased phosphorylation of Erk5 in a time-dependent manner (Fig. 1A). This increase in phosphorylation was concomitant with increased tyrosine phosphorylation of multiple proteins including PDGFR at Tyr-857 induced by PDGF (Fig. 1, B and C). To determine the involvement of Erk5 in mesangial cell proliferation, we used a specific inhibitor of this kinase, XMD8-92 (45). Incubation of mesangial cells with XMD8-92 significantly inhibited phosphorylation of Erk5 in response to PDGF (Fig. 1D). XMD8-92 did not have any effect on PDGF-induced tyrosine phosphorylation of PDGFR and phosphorylation of Erk1/2 (Fig. 1, E and 1F). These results indicate that XMD8-92 specifically blocks Erk5 without affecting Erk1/2 and PDGFR tyrosine kinase activity. To determine the role of Erk5 in mesangial cell proliferation, the effect of XMD8-92 on DNA synthesis and cell proliferation was measured. As expected, PDGF increased DNA synthesis that was significantly inhibited by XMD8-92 (Fig. 1G). Similarly, XMD8-92 significantly attenuated PDGF-induced proliferation assessed by cell counting (Fig. 1H).
Fig. 1.
Platelet-derived growth factor (PDGF) activates Erk5 to increase proliferation of mesangial cells. A–C: quiescent mesangial cells were incubated with 20 ng/ml PDGF for indicated periods of time. The cell lysates were immunoblotted with phospho-Erk5, Erk5 (A), anti-phosphotyrosine, actin (B), and phospho-PDGF receptor (PDGFR), PDGFR (C) antibodies. D–F: quiescent mesangial cells were treated with XMD8-92 for 1 h before incubation with PDGF for 5 min. The cell lysates were immunoblotted with indicated antibodies. A–F: a representative of 3 independent experiments is shown. G and H: mesangial cells were treated with XMD8-92 and PDGF as described above. [3H]thymidine incorporation and cell counts were determined as described in materials and methods. In G, means ± SE of 6 measurements are shown. *P < 0.0001 vs. control; **P < 0.0001 vs. PDGF treated. A representative of 3 independent experiments is shown. In H, means ± SE of triplicate measurements are shown. @P < 0.001 vs. control; *P < 0.003 vs. PDGF alone at corresponding time points. A representative of 4 independent experiments is shown. I–N: mesangial cells were transfected with dominant negative (DN) Erk5 (I and J) or siRNAs against Erk5 (siErk5A or siErk5B; K–N). Vector or scrambled RNA was used as control. [3H]thymidine incorporation (I, K, and L) and cell counts (J, M, and N) were determined as described in materials and methods. Bottom: expression of FLAG-tagged dominant negative Erk5, Erk5, and actin in parallel samples. In I, K, and L, means ± SE of 6 measurements are shown. *P < 0.01 vs. control; **P < 0.001 vs. PDGF treated. In J, M, and N, means ± SE of triplicate measurements are shown. @P < 0.006 vs. control; *P < 0.006 vs. PDGF alone at corresponding time points. For I, K, and L, a representative of 4 independent experiments is shown. For J, M, and N, a representative of 3 independent experiments is shown.
To further examine the specificity of Erk5 in mesangial cell proliferation, we employed a vector expressing dominant negative Erk5. The results showed that expression of dominant negative Erk5 significantly inhibited PDGF-induced DNA synthesis (Fig. 1I). Similarly, dominant negative Erk5 markedly blocked PDGF-induced mesangial cell proliferation assessed by cell counts (Fig. 1J). These observations were validated using siRNAs targeted against Erk5 (siErk5A and siErk5B). Expression of siErk5A significantly prevented DNA synthesis and proliferation of mesangial cells in response to PDGF (Figs. 1, K and M). A second siRNA targeting Erk5 (siErk5B) produced similar results (Fig. 1, L and N). Collectively, these data demonstrate that PDGF activates Erk5, which is required for proliferation of mesangial cells.
Erk5 controls cell cycle regulatory proteins.
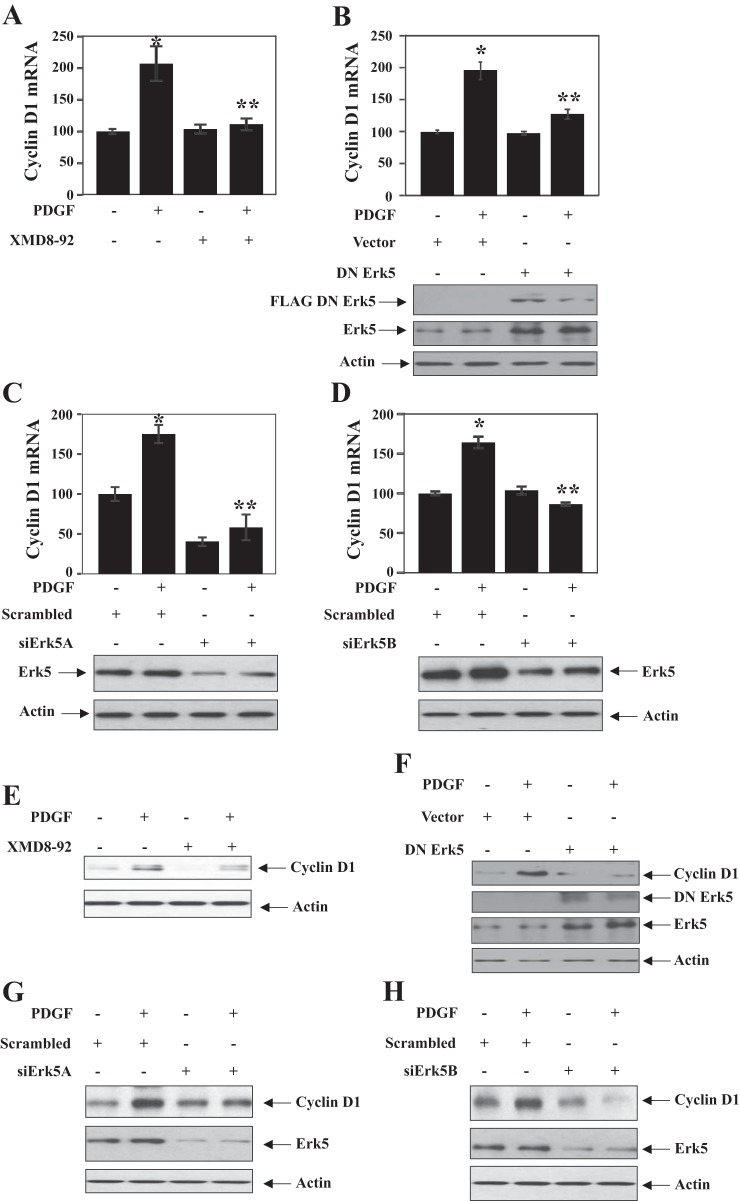
We have previously shown that PDGF regulates cell cycle proteins to promote mesangial cell proliferation (7, 11). Cyclin D1 promotes S-phase entry to initiate DNA synthesis. Therefore, we determined the role of Erk5 in cyclin D1 expression. Incubation of mesangial cells with PDGF increased the expression of cyclin D1 mRNA (Fig. 2). Inhibition of Erk5 using XDM8-92 blocked PDGF-induced cyclin D1 mRNA expression (Fig. 2A). Expression of dominant negative Erk5 also inhibited PDGF-stimulated expression of cyclin D1 mRNA (Fig. 2B). To confirm this observation, we used siRNAs targeted against Erk5. The Expression of two different siRNAs (siErk5A and siErk5B) abrogated cyclin D1 mRNA expression in response to PDGF (Fig. 2, C and D). In similar experiments, we examined cyclin D1 protein expression. PDGF increased cyclin D1 protein levels in mesangial cells (Fig. 2, E–H). Inhibition of Erk5 by XDM8-92 or dominant negative mutant abolished PDGF-stimulated cyclin D1 protein levels (Fig. 2, E and F). Similarly, the two independent siRNAs targeting Erk5 inhibited PDGF-induced expression of cyclin D1 protein (Fig. 2, G and H).
Fig. 2.
Inhibition of Erk5 blocks PDGF-induced cyclin D1 expression. Mesangial cells were treated with XMD8-92 (A and E) or transfected with either dominant negative Erk5 (B and F) or siERK5A (C and G) or siErk5B (D and H) before incubation with PDGF for 24 h. Expression of cyclin D1 mRNA was determined as described in materials and methods (A–D). Means ± SE of quadruplicate measurements are shown. *P < 0.001 vs. control; **P < 0.001 vs. PDGF-treated. B–D, bottom: expression of dominant negative Erk5, total Erk5 (B), and downregulation of Erk5 by siErk5A (C) and siErk5B (D). A–D: a representative of 3 independent experiments is shown. E–H: cyclin D1 protein expression was determined by immunoblotting. A representative of 4 independent experiments is shown.
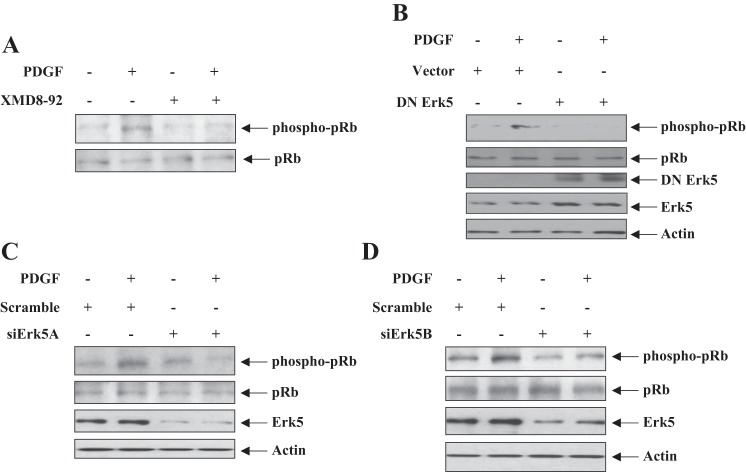
Cyclin D1 regulates activation of CDK4/6, the G1 cyclin-dependent kinases. These kinases phosphorylate the tumor suppressor pRb at Ser-795, resulting in its inactivation leading to cell cycle regulation. Thus we were interested in the involvement of Erk5 in this event. PDGF increased phosphorylation of pRb at Ser-795 (Fig. 3). Inhibition of Erk5 by XDM8-92 or kinase dead mutant significantly attenuated the PDGF-induced phosphorylation of pRb (Fig. 3, A and B). Also, expression of siErk5A and siErk5B targeting Erk5 significantly inhibited pRb phosphorylation by PDGF (Fig. 3, C and D). These results together demonstrate a role for Erk5 in regulating cyclin D1/CDK4/6/pRb in mesangial cells.
Fig. 3.
Erk5 regulates phosphorylation of pRb. A: mesangial cells were treated with XMD8-92 before incubation with PDGF for 24 h. B–D: mesangial cells were transfected with dominant negative Erk5 (B) or siErk5A (C) or siErk5B (D). The cells were incubated with PDGF for 24 h. The cell lysates were immunoblotted with indicated antibodies. A representative of 4 independent experiments is shown.
Cyclin D1/CDK4 downstream of Erk5 regulates proliferation of mesangial cells.
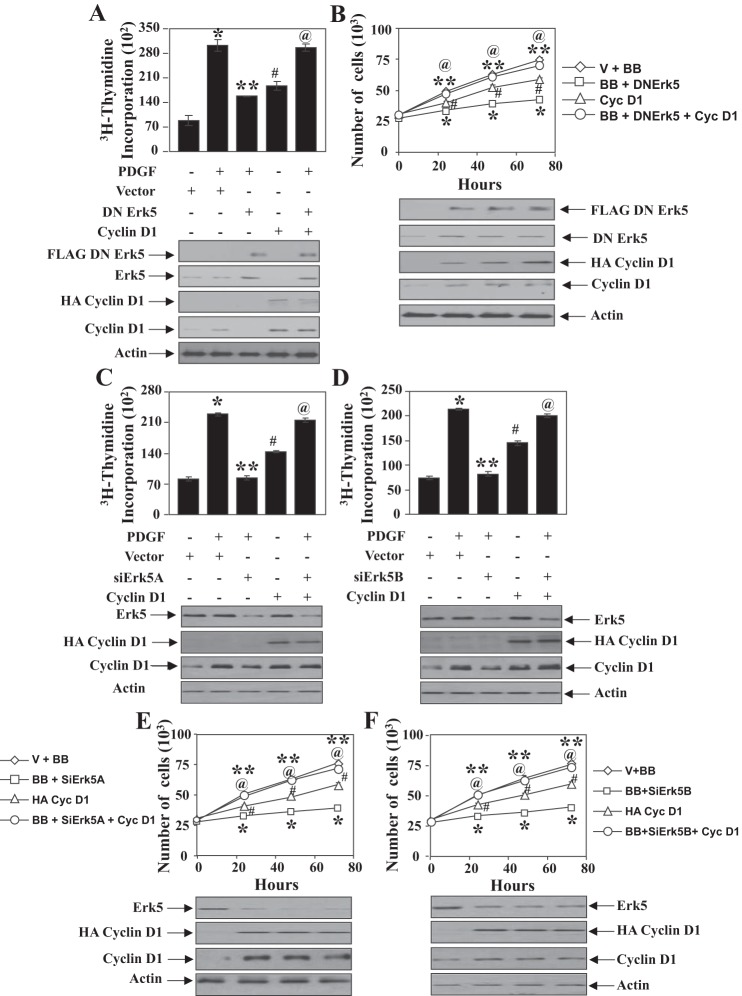
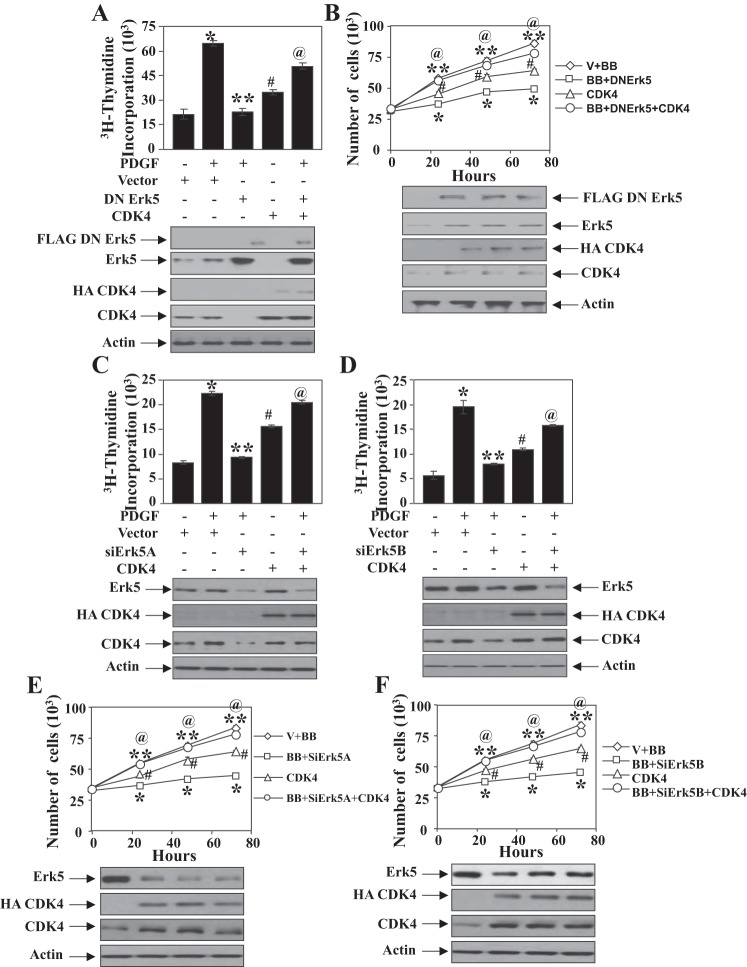
G1 cyclins and their assisted kinases regulate S-phase entry of cells. We have shown that Erk5 regulates proliferation of mesangial cells (Fig. 1). Because cyclin D1 is regulated by Erk5, we first examined the contribution of this cyclin in Erk5-mediated DNA synthesis. As expected, expression of dominant negative Erk5 inhibited PDGF-induced DNA synthesis (Fig. 4A). However, expression of cyclin D1 increased the DNA synthesis and significantly prevented the dominant negative Erk5-mediated inhibition of PDGF-stimulated DNA synthesis (Fig. 4A). Similarly, cyclin D1 enhanced proliferation and abrogated the inhibitory effect of dominant negative Erk5 on proliferation of mesangial cells induced by PDGF (Fig. 4B). Expression of cyclin D1 produced identical effects on two different siErk5-induced suppression of PDGF-induced DNA synthesis and cell proliferation (Fig. 4, C–F).
Fig. 4.
PDGF-stimulated Erk5-mediated mesangial cell proliferation is regulated by cyclin D1. Mesangial cells were transfected with dominant negative Erk5 and cyclin D1 (A and B) or siErk5A and cyclin D1 (C and E) or siErk5B plus cyclin D1 (D and F). PDGF-induced DNA synthesis (A, C, and D) and proliferation (B, E, and F) were determined as described in materials and methods. Bottom: expression of indicated proteins from a parallel experiment. In A, C, and D, means ± SE of triplicate measurements are shown. *P < 0.001 vs. control; **P < 0.001 vs. PDGF-treated; #P < 0.001 vs. control; @P < 0.001 vs. PDGF plus dominant negative Erk5 or PDGF plus siErk5A or siErk5B. In B, E, and F, means ± SE of triplicate measurements are shown. @P < 0.002 vs. control; *P < 0.0002 vs. PDGF alone; #P < 0.01 vs. control; **P < 0.0002 vs. PDGF plus DN Erk5 or PDGF plus siErk5A or PDGF plus siErk5B. For A, a representative of 4 independent experiments is shown. For B–F, representatives of 3 independent experiments are shown.
One of the downstream targets of cyclin D1 is CDK4. Phosphorylation of pRb by CDK4 results in its inactivation and induces S-phase entry. We have shown above that Erk5 regulates phosphorylation of pRb (Fig. 3). Therefore, we tested the involvement of CDK4 in Erk5-mediated mesangial cell proliferation. Expression of wild-type CDK4 increased DNA synthesis and proliferation and significantly reversed the dominant negative Erk5-mediated inhibition of PDGF-stimulated DNA synthesis and cell proliferation (Fig. 5, A and B). Similarly, CDK4 restored the suppression of PDGF-mediated DNA synthesis and mesangial cell proliferation induced by expression of siErk5A and siErk5B targeting Erk5 (Fig. 5, C–F). Collectively, these results demonstrate a role for Erk5 in regulating cyclin D1-dependent CDK4-mediated mesangial cell proliferation in response to PDGF.
Fig. 5.
PDGF-stimulated Erk5-mediated mesangial cell proliferation is regulated by CDK4. Mesangial cells were transfected with dominant negative Erk5 and CDK4 (A and B) or siErk5A and CDK4 (C and E) or siErk5B plus CDK4 (D and F). PDGF-induced DNA synthesis (A, C, and D) and proliferation (B, E, and F) were determined as described in materials and methods. Bottom: expression of indicated proteins from a parallel experiment. In A, C, and D, means ± SE of triplicate measurements are shown. *P < 0. 001 vs. control; **P < 0.001 vs. PDGF alone; #P < 0.01 vs. control; @P < 0.001 vs. PDGF plus DN Erk5 or PDGF plus siErk5. In B, E, and F, means ± SE of triplicate measurements are shown. @P < 0.003 vs. control; *P < 0.003 vs. PDGF alone; #P < 0.001 vs. control; **P < 0.003 vs. PDGF plus DN Erk5 or PDGF plus siErk5. For A, a representative of 4 independent experiments is shown. For B–F, representatives of 3 independent experiments are shown.
PI3-kinase regulates PDGF-induced Erk5 phosphorylation.
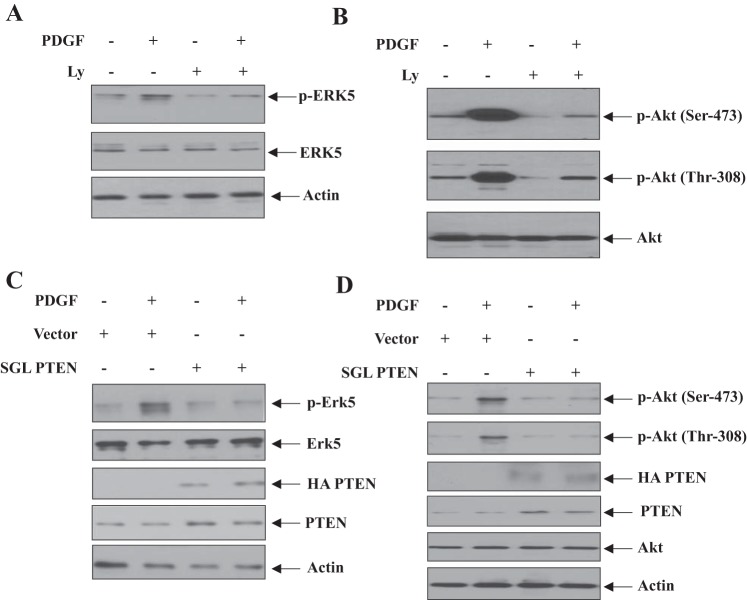
We have previously shown that PI3-kinase regulates mesangial cell proliferation (8, 10). Also, we reported that PI3-kinase controls cell cycle regulatory proteins in response to PDGF in mesangial cells (11). Since our above results demonstrate that Erk5 contributes to these processes, we tested the hypothesis that PI3-kinase is involved in Erk5 phosphorylation. We used the PI3-kinase inhibitor Ly294002. Incubation of mesangial cells with Ly294002 significantly inhibited PDGF-induced phosphorylation of Erk5 concomitant with attenuation of phosphorylation of Akt, a measure of PI3-kinase inhibition (Fig. 6, A and B) (7). Because Ly294002 is a nonspecific inhibitor of PI3-kinase activity, we used a deletion mutant of the p85 regulatory subunit of PI3-kinase (Δp85) that renders dominant negative effect on this kinase (20). The expression of dominant negative PI3-kinase markedly blocked phosphorylation of Erk5 (data not shown). To confirm this observation, we used, PTEN, which acts as an endogenous inhibitor of PI3-kinase signaling (28). Similar to dominant negative PI3-kinase, expression of PTEN prevented Erk5 and Akt phosphorylation induced by PDGF (Fig. 6, C and D).
Fig. 6.
Phosphatidylinositol 3-kinase (PI3-kinase) regulates PDGF-stimulated phosphorylation of Erk5. A and B: mesangial cells were treated with 25 μM Ly294002 for 1 h before incubation with PDGF for 5 min. The cell lysates were immunoblotted with indicated antibodies. C and D: mesangial cells were transfected with HA-tagged PTEN expression vector. The cells were incubated with PDGF for 5 min. The cell lysates were immunoblotted with indicated antibodies. Representatives of 3 independent experiments are shown.
Erk5 regulates PDGF-induced Akt activation.
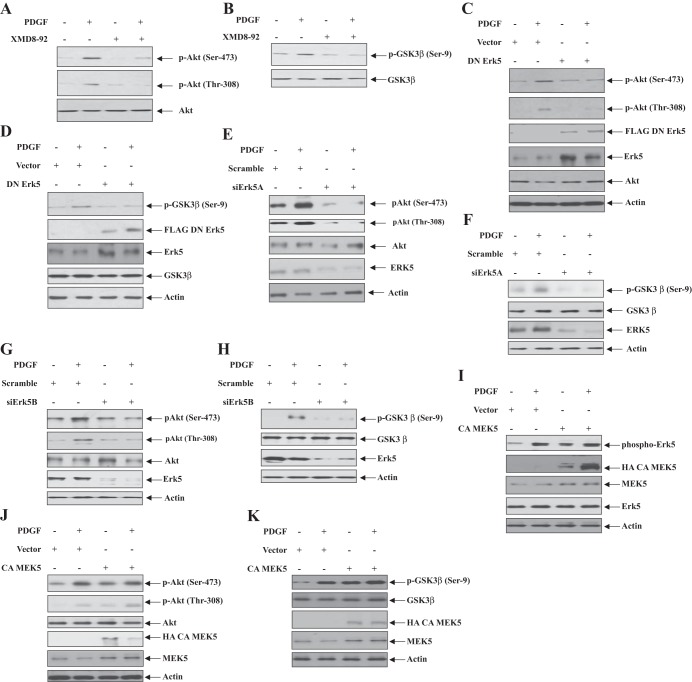
We have shown previously that the PI3-kinase target Akt regulates PDGF-induced proliferation of mesangial cells (7, 11). Because Erk5 also contributes to PDGF-stimulated proliferation of these cells (Fig. 1), we tested whether Erk5 regulates Akt activation. The results showed that the Erk5 inhibitor XMD8-92 attenuated PDGF-induced Akt phosphorylation at Thr-308 and Ser-473 (Fig. 7A). Because phosphorylation at both these sites of Akt increases its activity, we examined the phosphorylation of one of its endogenous substrates GSK3β. XMD8-92 abrogated PDGF-stimulated phosphorylation of GSK3β (Fig. 7B). Similarly, expression of dominant negative mutant of Erk5 significantly inhibited phosphorylation of Akt and GSK3β (Fig. 7, C and D), which was verified by siRNAs targeting Erk5 (Fig. 7, E–H).
Fig. 7.
Erk5 regulates PDGF-stimulated Akt activity. A and B: mesangial cells were treated with XMD8-92 before incubation with PDGF for 5 min. C–K: mesangial cells were transfected with dominant negative Erk5 (C and D) or siErk5A (E and F) or siErk5B (G and H) or constitutively active (CA) MEK5 (I–K). The cells were then incubated with PDGF for 5 min. The cell lysates were immunoblotted with indicated antibodies. Representatives of 3 independent experiments are shown for A–I and K. For J, a representative of 4 independent experiments is shown.
Erk5 is activated by its upstream kinase, MEK5. Thus we examined the role of MEK5 in activation of Akt using a constitutively active mutant of MEK5. As expected, expression of constitutively active MEK5 increased phosphorylation of Erk5 similar to that observed with PDGF (Fig. 7I). Similarly, MEK5 increased phosphorylation of Akt at Ser-473 and Thr-308, resulting in its activation as indicated by the phosphorylation of GSK3β (Fig. 7, J and K). These results demonstrate that MEK5/Erk5 signaling acts upstream of Akt kinase in mesangial cells.
Akt kinase regulates Erk5 phosphorylation.
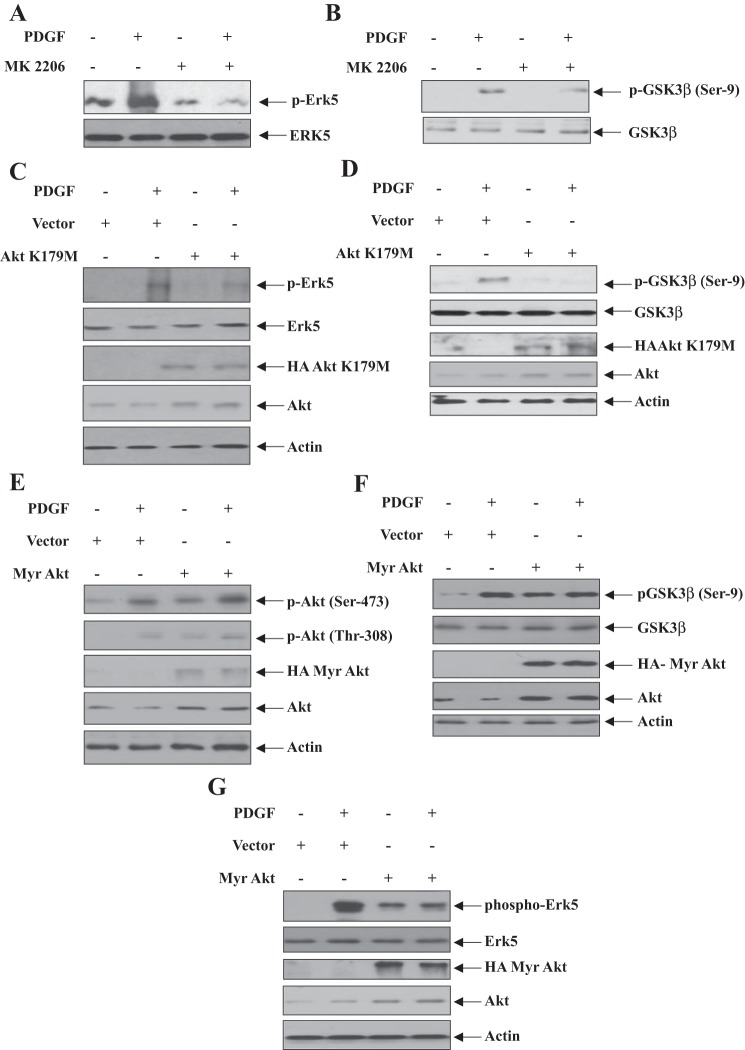
Akt is a downstream target of PI3-kinase that regulates PDGF-induced mesangial cell proliferation (7). Because Erk5 also contributes to mesangial cell proliferation, we postulated that Akt regulates Erk5 activation. We showed that an Akt kinase inhibitor MK2206 significantly inhibited PDGF-induced phosphorylation of Erk5 (Fig. 8A). MK2206 also inhibited phosphorylation of the endogenous Akt substrate GSK3β (Fig. 8B), demonstrating successful inhibition of Akt kinase activity. To confirm these results, a dominant negative mutant of Akt (Akt K179M) was transfected into mesangial cells. Expression of this Akt mutant blocked phosphorylation of Erk5 and GSK3β in response to PDGF (Fig. 8, C and D). To further confirm these data, a constitutively active mutant, Myr-Akt, was used. Expression of Myr-Akt increased phosphorylation and activation of Akt as judged by phosphorylation of GSK3β, similar to PDGF treatment of mesangial cells (Fig. 8, E and F). Myr-Akt mimicked the effect of PDGF on Erk5 phosphorylation (Fig. 8G). Collectively, these results conclusively show the presence of a positive feedback loop between Erk5 and Akt that drives PDGF-induced mesangial cell proliferation.
Fig. 8.
Akt kinase regulates PDGF-induced phosphorylation of Erk5. Mesangial cells were treated with MK2206 before incubation with PDGF for 5 min (A and B). Mesangial cells were transfected with dominant negative Akt K179M (C and D) or Myr Akt (E–G). The cells were incubated with PDGF for 5 min. The cell lysate were immunoblotted with indicated antibodies. Representatives of 3 independent experiments for A–F and 4 independent experiments for G, respectively, are shown.
DISCUSSION
In the present study, we show activation of Erk5 in glomerular mesangial cells by PDGF, the mitogenic factor for these cells. We demonstrate that Erk5 is essential for PDGF-induced mesangial cell proliferation. Furthermore, Erk5 utilizes the classical cyclin D1/CDK4 pathway to increase pRb phosphorylation and mesangial cell proliferation. Interestingly, we report that PI3-kinase/Akt signal transduction regulates Erk5 phosphorylation by PDGF. Finally, we provide the first evidence for the presence of a positive feedback loop between Erk5 and Akt in PDGFR signaling (Fig. 9).
Fig. 9.
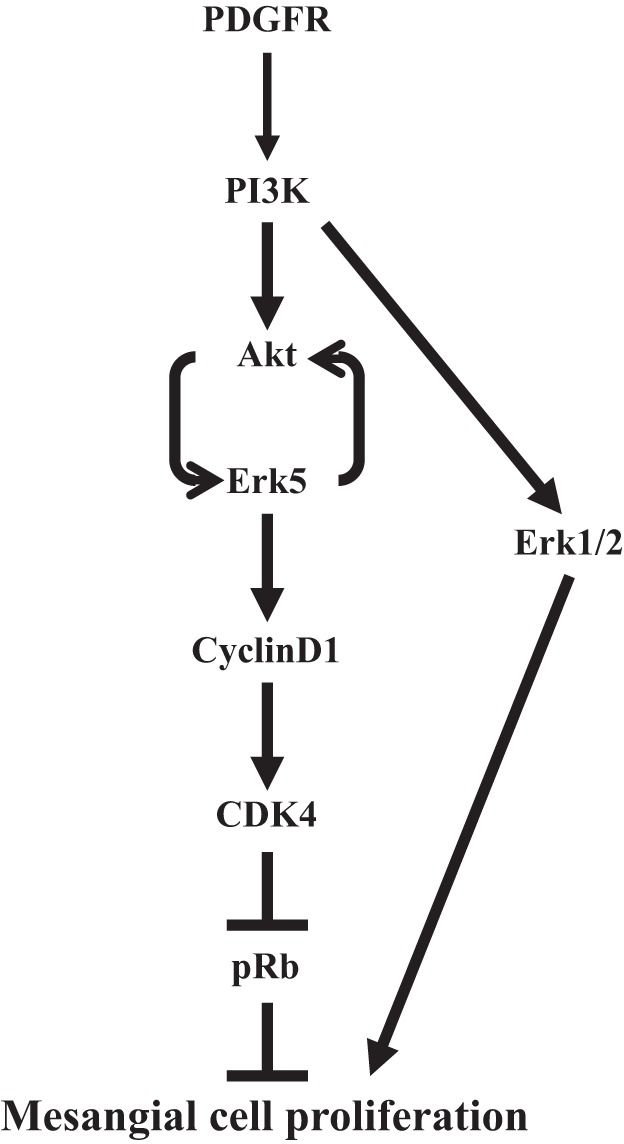
Cartoon summarizes the results demonstrating the positive feedback loop involving Akt and Ekr5, which enhance proliferation of mesangial cells after PDGFR activation.
Proliferation of mesangial cells is a major pathologic feature in proliferative and inflammatory glomerular diseases in human and in experimental animals (1, 24, 25). Neutralization of PDGFRβ prevents mesangioproliferative glomerulonephritis in rat (25). These studies establish the importance of PDGFR signal transduction in proliferation of mesangial cells under pathological conditions (1). PDGF induces pleotrophic effects including activation of phospholipase C, phospholipase A2, Erk1/2, PI3-kinase, and c-Src in mesangial cell (10, 11, 22). Interestingly, it was previously shown that in vivo inhibition of Erk1/2 partially blocked increase in glomerular cell number observed in anti-thy-1-induced glomerulonephritis in rat (5). It should be noted that in this study the activation of Erk1/2 was investigated as early changes during the course of disease progression. A more recent study using a rat model of combined uninephrectomy and anti-thy-1-induced mesangioproliferative glomerulonephritis demonstrated increased levels of phosphorylation of Erk5 in a prolonged fashion that coincided with enhanced extracellular matrix protein expression (38). In this study, the in vitro viability of mesangial cells and collagen expression was shown to be dependent on Erk5. However, in the anti-Thy-1-induced proliferative glomerulonephritis, a significant role of PDGF has been established (1, 24, 25). We have previously reported that in mesangial cells PDGF activates Erk1/2 MAPK downstream of PI3-kinase (10). Furthermore, we showed that Erk1/2 partially contributes to the proliferation of mesangial cells induced by PDGF (7) (Fig. 9). This observation supports the findings that Erk1/2 inhibition in anti-thy-1-induced glomerulonephritis only partially ameliorates increase in glomerular cell number (5). Importantly, we established a significant contribution of PI3-kinase/Akt signaling in PDGF-stimulated mesangial cell proliferation (7, 10). In the present study, we for the first time demonstrate that PDGF, which contributes to the pathogenesis of mesangioproliferative glomerulonephritis, increases phosphorylation of Erk5 in mesangial cells (Fig. 1). Importantly, our data show that Erk5 contributes to the proliferation of mesangial cells in response to PDGF (Fig. 1).
An increased level of cyclin D1 is often associated with enhanced cell proliferation. Overexpression of cyclin D1 in mice increases cell proliferation (27, 41). Also, PDGF significantly increases the expression of cyclin D1 in mesangial cells and is necessary for their proliferation (26). Previously, it was shown that PI3-kinase regulates cyclin D1 expression in response to PDGF in smooth muscle cells (29). However, Erk1/2 but not Erk5 was responsible for cyclin D1 expression in fibrosis (36). In contrast to these studies, our results demonstrate contribution of Erk5 to PDGF-induced cyclin D1 in mesangial cell proliferation (Fig. 2).
Cyclin D1 and CDK4 act cooperatively to drive cell cycle entry. Cyclin D1/CDK4 complex phosphorylates pRb, resulting in its inactivation. Microinjection of cells with cyclin D1/CDK4 complex phosphorylates pRb to induce cell cycle entry (12). Furthermore, introduction of in vitro phosphorylated pRb by cyclin D1/CDK4 complex into cells increases DNA synthesis (13). We found that in mesangial cells PDGF-stimulated cyclin D1 increased the phosphorylation of pRb at the CDK4 site Ser-795. Interestingly, this phosphorylation is prevented by inhibition of Erk5 (Fig. 3). In fact, expression of cyclin D1 or CDK4 reverses the reduction in PDGF-stimulated DNA synthesis and cell proliferation that results from Erk5 inhibition (Figs. 4 and 5). Collectively, these results demonstrate a significant role of Erk5 in PDGF-induced cell cycle progression and proliferation of mesangial cells.
The role of class IA PI3-kinase in proliferation and survival of cells is established. We have previously shown that PDGF-stimulated PI3-kinase activity is essential for proliferation in mesangial cells and metanephric mesenchymal cells, a subpopulation of which differentiate into mesangial cells (2, 10, 11, 34). In the present study, we identified Erk5 as an effector of PI3-kinase signaling that contributes to proliferation of mesangial cells using a pharmacological inhibitor, dominant negative PI3-kinase, and an endogenous inhibitor of PI3-kinase, PTEN (Fig. 6 and data not shown). One of the downstream targets of PI3-kinase is Akt. This kinase is activated by variety of extracellular stimuli including PDGF (18). We showed previously that PDGF activates Akt in mesangial cells, resulting in regulation of cell cycle proteins (7, 11).
Recently, a role of Erk5 in activation of Akt kinase was reported by osmotic stress, NGF and VEGF in regulating cell survival (17, 23, 33, 35, 42). More recently, Erk5 was shown to increase Akt phosphorylation in dermal microvascular endothelial cells in response to VEGF. However, this activation increased tubular morphogenesis without having an effect on proliferation of these cells (35). In the present study, we find that both Erk5 and its upstream kinase MEK5 regulate phosphorylation of Akt at both Thr-308 and Ser-473 in mesangial cells, resulting in their proliferation (Figs. 1 and 7). Surprisingly, we found that inhibition of Akt significantly blocked phosphorylation of Erk5 in response to PDGF (Fig. 8). To our knowledge, this is the first demonstration for the presence of a positive feedback loop between Akt and Erk5 in the PDGFR signaling (Fig. 9). These results corroborate our previous studies demonstrating a role of Akt kinase in mesangial cell proliferation (7, 11). Further studies will be necessary to delineate the mechanism of this feedback loop. Many Akt kinase inhibitors have been developed; however, they display severe toxicity. As our results demonstrate Erk5 as a potent mediator in the signaling circuit of PDGF leading to mesangial cell proliferation, development of safe inhibitors targeting this kinase will be beneficial for mesangioproliferative glomerulonephritis.
GRANTS
The results described in this manuscript are the work supported by Veterans Affairs Service Merit Review Grant 5I01BX000926 and National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-50190 (to G. G. Choudhury). G. G. Choudhury is a recipient of Veterans Affairs Senior Research Career Scientist Award. B. S. Kasinath, Y. Gorin, and H. E. Abboud are supported by National Institutes of Health and Veterans Affairs Research Service grants (B. S. Kasinath and H. E. Abboud). N. Ghosh-Choudhury is supported by Veterans Affairs Research Service Merit Review grant and a pilot grant from Cancer Therapy and Research Center at San Antonio.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.B., F.D., X.L., and S.P. performed experiments; A.B., F.D., N.G.-C., and G.G.C. analyzed data; A.B., F.D., N.G.-C., X.L., S.P., Y.G., B.S.K., H.E.A., and G.G.C. approved final version of manuscript; N.G.-C. interpreted results of experiments; Y.G., B.S.K., and H.E.A. edited and revised manuscript; G.G.C. conception and design of research; G.G.C. prepared figures; G.G.C. drafted manuscript.
ACKNOWLEDGMENTS
We thank Jeffrey L. Barnes for critically reading the manuscript.
Present address of S. Pal: Amrita University, Kollam, Kerala, India.
REFERENCES
- 1.Abboud HE. Mesangial cell biology. Exp Cell Res 318: 979–985, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Arar M, Xu YC, Elshihabi I, Barnes JL, Choudhury GG, Abboud HE. Platelet-derived growth factor receptor beta regulates migration and DNA synthesis in metanephric mesenchymal cells. J Biol Chem 275: 9527–9533, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bera A, Ghosh-Choudhury N, Dey N, Das F, Kasinath BS, Abboud HE, Choudhury GG. NFkappaB-mediated cyclin D1 expression by microRNA-21 influences renal cancer cell proliferation. Cell Signal 25: 2575–2586, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature 411: 355–365, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bokemeyer D, Panek D, Kramer HJ, Lindemann M, Kitahara M, Boor P, Kerjaschki D, Trzaskos JM, Floege J, Ostendorf T. In vivo identification of the mitogen-activated protein kinase cascade as a central pathogenic pathway in experimental mesangioproliferative glomerulonephritis. J Am Soc Nephrol 13: 1473–1480, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Chiara F, Bishayee S, Heldin CH, Demoulin JB. Autoinhibition of the platelet-derived growth factor beta-receptor tyrosine kinase by its C-terminal tail. J Biol Chem 279: 19732–19738, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Choudhury GG. Akt serine threonine kinase regulates platelet-derived growth factor-induced DNA synthesis in glomerular mesangial cells: regulation of c-fos AND p27(kip1) gene expression. J Biol Chem 276: 35636–35643, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Choudhury GG, Biswas P, Grandaliano G, Fouqueray B, Harvey SA, Abboud HE. PDGF-mediated activation of phosphatidylinositol 3 kinase in human mesangial cells. Kidney Int 46: 37–47, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Choudhury GG, Grandaliano G, Jin DC, Katz MS, Abboud HE. Activation of PLC and PI 3 kinase by PDGF receptor alpha is not sufficient for mitogenesis and migration in mesangial cells. Kidney Int 57: 908–917, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Choudhury GG, Karamitsos C, Hernandez J, Gentilini A, Bardgette J, Abboud HE. PI-3-kinase and MAPK regulate mesangial cell proliferation and migration in response to PDGF. Am J Physiol Renal Physiol 273: F931–F938, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Choudhury GG, Mahimainathan L, Das F, Venkatesan B, Ghosh-Choudhury N. c-Src couples PI 3 kinase/Akt and MAPK signaling to PDGF-induced DNA synthesis in mesangial cells. Cell Signal 18: 1854–1864, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Connell-Crowley L, Elledge SJ, Harper JW. G1 cyclin-dependent kinases are sufficient to initiate DNA synthesis in quiescent human fibroblasts. Curr Biol 8: 65–68, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Connell-Crowley L, Harper JW, Goodrich DW. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol Biol Cell 8: 287–301, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das F, Ghosh-Choudhury N, Bera A, Kasinath BS, Choudhury GG. TGFbeta-induced PI 3 kinase-dependent Mnk-1 activation is necessary for Ser-209 phosphorylation of eIF4E and mesangial cell hypertrophy. J Cell Physiol 228: 1617–1626, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das F, Ghosh-Choudhury N, Dey N, Mandal CC, Mahimainathan L, Kasinath BS, Abboud HE, Choudhury GG. Unrestrained mammalian target of rapamycin complexes 1 and 2 increase expression of phosphatase and tensin homolog deleted on chromosome 10 to regulate phosphorylation of Akt kinase. J Biol Chem 287: 3808–3822, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey N, Das F, Ghosh-Choudhury N, Mandal CC, Parekh DJ, Block K, Kasinath BS, Abboud HE, Choudhury GG. microRNA-21 governs TORC1 activation in renal cancer cell proliferation and invasion. PLoS One 7: e37366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finegan KG, Wang X, Lee EJ, Robinson AC, Tournier C. Regulation of neuronal survival by the extracellular signal-regulated protein kinase 5. Cell Death Differ 16: 674–683, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81: 727–736, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev 15: 197–204, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ghosh-Choudhury N, Abboud SL, Nishimura R, Celeste A, Mahimainathan L, Choudhury GG. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem 277: 33361–33368, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Heldin CH. Autocrine PDGF stimulation in malignancies. Ups J Med Sci 117: 83–91, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79: 1283–1316, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal 19: 2003–2012, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Iida H, Seifert R, Alpers CE, Gronwald RG, Phillips PE, Pritzl P, Gordon K, Gown AM, Ross R, Bowen-Pope DF, Johnson RJ. Platelet-derived growth factor (PDGF) and PDGF receptor are induced in mesangial proliferative nephritis in the rat. Proc Natl Acad Sci USA 88: 6560–6564, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jefferson JA, Johnson RJ. Experimental mesangial proliferative glomerulonephritis (the anti-Thy-1.1 model). J Nephrol 12: 297–307, 1999 [PubMed] [Google Scholar]
- 26.Lang S, Hartner A, Sterzel RB, Schocklmann HO. Requirement of cyclin D1 in mesangial cell mitogenesis. J Am Soc Nephrol 11: 1398–1408, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Lovec H, Grzeschiczek A, Kowalski MB, Moroy T. Cyclin D1/bcl-1 cooperates with myc genes in the generation of B-cell lymphoma in transgenic mice. EMBO J 13: 3487–3495, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Page K, Li J, Wang Y, Kartha S, Pestell RG, Hershenson MB. Regulation of cyclin D(1) expression and DNA synthesis by phosphatidylinositol 3-kinase in airway smooth muscle cells. Am J Respir Cell Mol Biol 23: 436–443, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22: 153–183, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene 26: 3100–3112, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Ranganathan A, Pearson GW, Chrestensen CA, Sturgill TW, Cobb MH. The MAP kinase ERK5 binds to and phosphorylates p90 RSK. Arch Biochem Biophys 449: 8–16, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Razumovskaya E, Sun J, Ronnstrand L. Inhibition of MEK5 by BIX02188 induces apoptosis in cells expressing the oncogenic mutant FLT3-ITD. Biochem Biophys Res Commun 412: 307–312, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Ricono JM, Arar M, Choudhury GG, Abboud HE. Effect of platelet-derived growth factor isoforms in rat metanephric mesenchymal cells. Am J Physiol Renal Physiol 282: F211–F219, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Roberts OL, Holmes K, Muller J, Cross DA, Cross MJ. ERK5 is required for VEGF-mediated survival and tubular morphogenesis of primary human microvascular endothelial cells. J Cell Sci 123: 3189–3200, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Squires MS, Nixon PM, Cook SJ. Cell-cycle arrest by PD184352 requires inhibition of extracellular signal-regulated kinases (ERK) 1/2 but not ERK5/BMK1. Biochem J 366: 673–680, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev 15: 205–213, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Urushihara M, Takamatsu M, Shimizu M, Kondo S, Kinoshita Y, Suga K, Kitamura A, Matsuura S, Yoshizumi M, Tamaki T, Kawachi H, Kagami S. ERK5 activation enhances mesangial cell viability and collagen matrix accumulation in rat progressive glomerulonephritis. Am J Physiol Renal Physiol 298: F167–F176, 2010 [DOI] [PubMed] [Google Scholar]
- 40.van Roeyen CR, Ostendorf T, Floege J. The platelet-derived growth factor system in renal disease: an emerging role of endogenous inhibitors. Eur J Cell Biol 91: 542–551, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369: 669–671, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Finegan KG, Robinson AC, Knowles L, Khosravi-Far R, Hinchliffe KA, Boot-Handford RP, Tournier C. Activation of extracellular signal-regulated protein kinase 5 downregulates FasL upon osmotic stress. Cell Death Differ 13: 2099–2108, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 79: 143–180, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Yan C, Luo H, Lee JD, Abe J, Berk BC. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J Biol Chem 276: 10870–10878, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Yang Q, Deng X, Lu B, Cameron M, Fearns C, Patricelli MP, Yates JR, 3rd, Gray NS, Lee JD. Pharmacological inhibition of BMK1 suppresses tumor growth through promyelocytic leukemia protein. Cancer Cell 18: 258–267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou G, Bao ZQ, Dixon JE. Components of a new human protein kinase signal transduction pathway. J Biol Chem 270: 12665–12669, 1995 [DOI] [PubMed] [Google Scholar]