Abstract
Mechanical stimulation of osteoblasts activates many cellular mechanisms including the release of ATP. Binding of ATP to purinergic receptors is key to load-induced osteogenesis. Osteoblasts also respond to fluid shear stress (FSS) with increased actin stress fiber formation (ASFF) that we postulate is in response to activation of the P2Y2 receptor (P2Y2R). Furthermore, we predict that ASFF increases cell stiffness and reduces the sensitivity to further mechanical stimulation. We found that small interfering RNA (siRNA) suppression of P2Y2R attenuated ASFF in response to FSS and ATP treatment. In addition, RhoA GTPase was activated within 15 min after the onset of FSS or ATP treatment and mediated ASFF following P2Y2R activation via the Rho kinase (ROCK)1/LIM kinase 2/cofilin pathway. We also observed that ASFF in response to FSS or ATP treatment increased the cell stiffness and was prevented by knocking down P2Y2R. Finally, we confirmed that the enhanced cell stiffness and ASFF in response to RhoA GTPase activation during FSS drastically reduced the mechanosensitivity of the osteoblasts based on the intracellular Ca2+ concentration ([Ca2+]i) response to consecutive bouts of FSS. These data suggest that osteoblasts can regulate their mechanosensitivity to continued load through P2Y2R activation of the RhoA GTPase signaling cascade, leading to ASFF and increased cell stiffness.
Keywords: P2Y2 receptor, osteoblast mechanosensitivity, cytoskeleton, Rho GTPases, fluid flow
dynamic loading of bone subjects osteoblasts to various forms of mechanical stimuli that induces osteogenesis and maintains bone homeostasis (5, 47). In vitro studies have been able to mimic these different types of mechanical stimuli that osteoblasts experience during dynamic loading of bone, such as substrate strain, hydraulic pressure, and interstitial fluid flow-induced shear stress (FSS) (17). Among the cellular changes that occur in response to FSS, reorganization of the actin cytoskeleton is thought to regulate cellular mechanosensitivity and mechanical behavior (4, 41). Moreover, changes in the actin cytoskeleton can also attenuate the anabolic response of osteoblasts during consecutive bouts of mechanical stimuli, although the cellular mechanism is unclear (14).
Restructuring of the actin cytoskeleton is largely dependent on purinergic signaling that has emerged as an essential component in osteoblast mechanotransduction (15, 24, 40). At the onset of FSS, osteoblasts release ATP and UTP stored in vesicles due to an influx of extracellular calcium through activation of mechanosensitive and voltage-sensitive calcium channels (11, 15, 45). This release of nucleotides activates purinergic receptors (P2), initiates several downstream markers of osteogenesis, such as c-Fos, ERK1/2, cyclooxygenase-2 (COX-2) expression, and PGE2 release (3, 31). P2 receptors are classified as G protein-coupled receptors P2Y (P2Y1,2,4,6,11,12,13,14) or nonselective, ligand-gated ion channels P2X (P2X1–7) (39). The P2Y2R and P2Y13R knockout mice have both been shown to increase trabecular and cortical bone formation to dynamic loading, which suggests a negative regulation by the P2Y receptor in bone formation (40, 56). The negative feedback associated with the P2Y13R is thought to be mediated by excessive ATP release (56). In contrast, the mechanism through which P2Y2R downregulates the mechanosensitivity of bone is unknown, but we predict this receptor has a crucial function in regulating mechanotransduction.
Purinergic signaling has been associated with actin stress fiber formation (ASFF) and changes in cell stiffness, although the specific pathway that mediates ASFF in response to mechanical stimuli is poorly understood (14). FSS induces a rapid switch between actin filament polymerization and depolymerization that results in the reorganization of the actin cytoskeleton to form new stress fibers (1, 12, 32). The structural changes in the actin cytoskeleton contribute to the cell stiffness and can dictate the sensitivity of the cell to mechanical stimuli (7, 41, 57). As G protein-coupled receptors, P2Y receptors have the potential to modulate ASFF through their ability to activate the RhoA GTPase pathway (49). Upon RhoA GTPase activation, the downstream Rho kinase (ROCK) has been shown to phosphorylate LIM kinase-2 (LIMK-2) at Thr505 (1, 32, 49). Activated LIMK-2 mediates actin cytoskeleton reorganization in various cell types by phosphorylating, thereby inactivating, the actin-severing protein, cofilin (16, 37, 52, 53). Cofilin binding to actin promotes depolymerization of the actin filaments while phosphorylation of this protein enables actin polymerization and stress fiber formation (16, 37, 53). Overall, evidence suggests that the RhoA GTPase-activated ROCK/LIMK/cofilin pathway can regulate the actin cytoskeletal dynamics via a P2Y receptor in osteoblasts during mechanotransduction.
Several reports suggest that cells alter their apparent stiffness to enhance or protect themselves to mechanical stimuli (18, 25, 35). The loss of mechanosensitivity during continuous loading occurs at the cellular level in osteoblasts, as well as at the tissue level (9, 27). Adaptation in cell stiffness may also enhance the ability of cells to cope or even survive potentially injurious forces. Of the different cytoskeletal components, the F-actin and intermediate filaments appear to contribute the most to cell stiffness (20, 22). The ability of osteoblasts to alter their mechanosensitivity through actin cytoskeleton reorganization could have a large influence on the anabolic response (41, 57). While these data point to the cytoskeleton in defining the mechanosensitivity of the cell, the underlying mechanisms through which osteoblasts regulate their mechanosensitivity are not clearly understood. The purpose of our study was to identify the specific signaling mechanisms through which osteoblasts regulate their mechanosensitivity. The ASFF in response to FSS was hypothesized to be by P2Y2R-mediated activation of the RhoA GTPase and its downstream signaling pathway that, in turn, attenuates osteoblast mechanosensitivity.
MATERIALS AND METHODS
Cell culture.
MC3T3-E1 osteoblast-like cells (Clone 14, ATCC, Bethesda, MD) were cultured in normal medium, which consisted of α-MEM, 10% FBS (GIBCO, New York, NY), 100 U/ml penicillin G, and 100 μg/ml streptomycin and buffered to pH 7.35 with 26 mM NaHCO3. Cells were maintained in a humidified incubator at 37°C with 5% CO2-95% air and subcultured every 72 h. All cell culture media and antibiotics were purchased from Sigma Chemical, St. Louis, MO unless otherwise noted. For testing, cells were seeded at 1 × 103 cells/cm2 on glass slides coated with rat tail type I collagen (50 μg/ml in 0.02 N acetic acid, BD, Franklin Lakes, NJ), and experiments were conducted when the cells reached 80–90% confluency.
FSS condition.
Cells were exposed to laminar fluid flow inside a parallel plate flow chamber using a gravity-driven closed flow loop that applied a shear stress of 12 dyn/cm2 FSS as previously described (13). The apparatus was maintained at 37°C and the medium was aerated with 5% CO2-95% air during experiments. Cells were treated with 5 U/ml of apyrase (Sigma) in culture media before and during FSS to hydrolyze extracellular ATP and inhibit its downstream action. To determine whether RhoA GTPase mediated events during FSS, cells were pretreated with 10 μM ROCK inhibitor Y-27623 (35) (EMD Biosciences, San Diego, CA) for 60 min before stimulation with FSS.
Suppression of P2Y2R siRNA.
To determine the role of P2Y2R in osteoblast mechanotransduction, we used a small interfering RNA (siRNA) strategy to suppress this receptor. Briefly, MC3T3-E1 cells were cultured in normal medium without antibiotics and grown to 60–70% confluency before transfection. A transfection mixture containing the transfection reagent (sc-29528, Santa Cruz Biotechnology, Santa Cruz, CA) and 0.5 μg siRNA duplex targeting P2Y2R (sc-42580, Santa Cruz Biotechnology) or scrambled siRNA (C-siRNA) sequence was prepared in transfection media (sc-36868, Santa Cruz Biotechnology) and incubated for 15 min at room temperature. After being washed with transfection media, the cells were incubated with the transfection mixture for 5 h at 37°C. Subsequently, the transfection mixture was replaced with normal media and the cells were allowed to grow.
To determine the extent of P2Y2R suppression, cells were collected at 24, 48, 72, and 96 h and lysed in 80 μl of lysis buffer consisting of the following (in mM): 5 HEPES, 150 NaCl, 26% glycerol, 1.5 MgCl2, 0.2 EDTA, 0.5 dithiothreitol, and 0.5 phenylmethylsulfonyl fluoride. Protein concentrations were determined and equal protein content was loaded onto a SDS-PAGE. The samples were electrophoretically transferred to a nitrocellulose membrane and blocked with Tris-buffered saline containing 5% nonfat dry milk and 0.05% Tween-20 (TBST) for 1 h. The membrane was incubated overnight at 4°C with 1 μg/ml rabbit antibodies against P2Y2R (Santa Cruz Biotechnology). After washes with TBST, the membrane was incubated with IgG anti-rabbit antibody conjugated with horseradish peroxidase (HRP, Santa Cruz Biotechnology) for an hour. Detection of P2Y2R was carried out using an Enhanced Chemiluminescence kit (NEN Life Science Products, Boston, MA). Rabbit anti-GAPDH (1 μg/ml) was used for loading control. Functional studies of RhoA GTPase activation, ASFF, or changes in cell stiffness in response to various treatments were performed during maximum suppression of P2Y2R.
RhoA GTPase activation.
The activation of RhoA GTPase in MC3T3-E1 cells was measured using a G-LISA kit (BK124, Cytoskeleton). Following specified treatments, we collected cell lysates in the lysis buffer provided in the kit and immediately stored in liquid nitrogen for later use. To determine RhoA GTPase activation, 50-μl aliquots of each sample with a protein concentration of 1 mg/ml were added, in triplicate, to a 96-well plate. Anti-RhoA GTPase antibody was added to each well, followed by HRP-labeled secondary antibody and the detection reagent. The optical density (OD) at 490 nm was measured to determine the magnitude of active RhoA GTPase in each sample. The degree of activation of RhoA GTPase was reported as a fold increase compared with static controls, which were not subjected to any form of treatment or mechanical stimuli. As a positive control for RhoA activation, cells were treated with 10 μM lysophosphatidic acid (LPA) for 30 min.
Ca2+ imaging with fluid flow.
MC3T3-E1 cells were grown for 3–4 days on type I collagen-coated (50 μg/ml in 0.02 N acetic acid) quartz slides to achieve 40–50% confluency. Before each experiment, cells were treated with siRNA to knock down P2Y2R expression. For flow experiments, cells were rinsed three times with Hanks' balanced saline solution (HBSS) and then loaded with 3 μM Fura-2/AM (Molecular Probes, Eugene, OR), a fluorescent Ca2+ probe, in HBSS for 30 min at 37°C. Loaded cells were rinsed three times with HBSS and incubated for an additional 30 min with HBSS alone to ensure complete deesterification of the fluorescent molecule, yet minimize intracellular compartmentalization.
A parallel-plate flow chamber with a uniform flow channel height of 250 μm was used to apply a fluid shear stress of 12 dyn/cm2 to the cells, as previously described (21, 57). Laminar flow was introduced to the chamber through a syringe mounted on a Harvard Syringe Pump (PHD Programmable; Harvard Apparatus, Holliston, MA) that controlled the flow rate. To establish a fluid-flow intracellular Ca2+ concentration ([Ca2+]i) baseline, cells were exposed to fluid shear of 1 dyn/cm2 for 3 min that was then increased to 12 dyn/cm2 for 10–15 min. Flow was then stopped for several min to allow the cells to recover, after which the 12 dyn/cm2 FSS was reintroduced. To determine the role of RhoA GTPase in this response, MC3T3-E1 cells were treated with 10 μM Y-27632 or 10 μM LPA while loading with Fura-2. Corresponding flow rates for each of the FSS levels were 1 and 15 ml/min, respectively. A ratiometric video-image analysis system (Intracellular Imaging, Cincinnati, OH) was used to record changes in [Ca2+]i. Fura-2 fluorescence was visualized with a Nikon inverted microscope using a Nikon ×30 fluor objective. The cells were illuminated with a Xenon lamp equipped with quartz collector lenses. A shutter and filter changer containing the two different interference filters (340 and 380 nm) was computer controlled. In this system, emitted light was passed through a 430-nm dichroic mirror, filtered at 510 nm, and imaged with an integrating CCD video camera. The ratio of light emitted at 340-nm and 380-nm excitation was determined (F340/F380) from consecutive frames, and the [Ca2+]i for each cell was calculated from this ratio by comparison with fura-2 free acid standards. Computer-generated individual Ca2+ traces were population means derived from simultaneous recording of Ca2+ in the 10–15 single cells in the field of view. In response to both bouts of FSS, the [Ca2+]i of MC3T3-E1 cells treated with Y-27623 (10 μM for 60 min) or siRNA knockdown of P2Y2R were measured and compared with cells treated without drug treatment or the C-siRNA.
Immunocytochemistry.
All immunofluorescence was carried out on cells fixed with 2% paraformaldehyde (Electron Microscopy Sciences) in PBS containing 0.1% Triton X-100 (Sigma) for 30 min on ice. Cells were then blocked with 3% BSA (Sigma) at room temperature for an hour and incubated with the respective primary antibodies. Rabbit polyclonal anti-phospho (Ser3) cofilin (Abcam), rabbit polyclonal anti-phospho (Thr505) LIMK-2 (Abcam) was used to detect phosphorylation of cofilin (P-cofilin) and LIMK2 (P-LIMK-2), respectively. AF-488 phalloidin (Molecular Probes Biostatus) was used to label F-actin and DRAQ5 (Alexis) was used as a nuclear stain. Slides were mounted using the Slow Fade Antifade kit and images were obtained using a Zeiss LSM510 laser scanning confocal microscope.
Cellular stiffness.
The apparent stiffness of individual cells was measured using an Atomic Force Microscope (AFM) (BioScope II, Veeco) mounted on an inverted optical microscope. Soft microlever probes (MLCT-AUNM, Veeco) with a conical tip and a spring constant of 0.01 N/m were first calibrated using a thermal fluctuation method in fluid. Individual cells with normal morphology were then identified under the optical microscope and positioned under the probe. An area of 30 μm × 30 μm was first scanned at a speed of ∼3 μm/s to generate a topographic map of an individual cell. Seven to ten points were selected over the cell body at the nuclear and perinuclear regions for indentation measurements. The peripheral region of the cell was avoided due to its relatively thin cell height and influence of the rigid substrate. At each selected point over the cell body, the cantilever tip indented the cell membrane at a speed of ∼2.5 μm/s until a force of 100 pN was reached, which typically resulted in an indentation depth of <70 nm (10% of the cell height). The elastic modulus at each point was estimated from the recorded force-deflection curve using a Hertz-based model (42) as defined by the following:
| (1) |
where E is the apparent stiffness, F is the cantilever force measured by the AFM, υ is the Poisson ratio of the cytoplasm (υ = 0.4) (33), ϕ is the opening angle of the conical cantilever tip (ϕ = 35°). The indentation depth (δ) was calculated by subtracting the cantilever deflection from the piezo displacement of the probe. The apparent stiffness for a given cell was defined by the average elastic modulus across each point measured. A total of five to six cells were measured following a given treatment, and each experiment was then repeated thrice. The average cell stiffness for each treatment was compared with static controls and reported as a fold increase. Static controls were not subjected to any form of treatment or mechanical stimuli.
Statistical analysis.
The change in cell stiffness and RhoA GTPase activation were reported as a fold increase (means ± SD) relative to static controls. Significance of all experiments was determined using one-way or two-way analysis of variance (ANOVA) with a Tukey post hoc test to determine significance when multiple comparisons in the study were made. Significance was defined by a P value < 0.05. Data for a given outcome measurement were pooled together in the case that no significant difference was found for a given treatment between different passages of cells.
RESULTS
ASFF with application of FSS is due to P2Y2R.
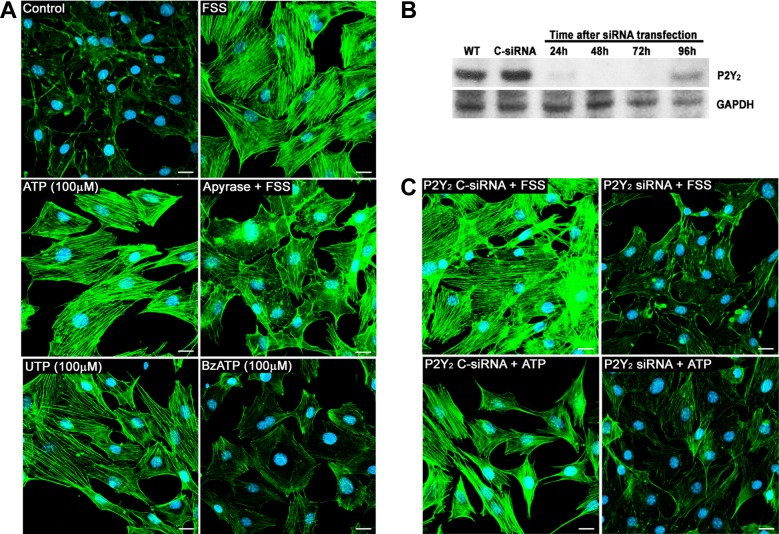
We have previously demonstrated that in response to FSS, osteoblasts exhibit an increase in ASFF, which is abolished by hydrolysis of extracellular ATP with apyrase (14). In a dose-response study, we found that 100 μM of ATP or UTP contribute the most to ASFF compared with BzATP, which primarily activates P2X (Fig. 1A). The increased ASFF in response to FSS was also completely eliminated in the presence of apyrase (Fig. 1A). Based on these results, we hypothesized that the G protein-coupled receptor P2Y2 is responsible for ASFF in osteoblasts. To delineate its role in mechanotransduction, we suppressed P2Y2R in MC3T3 cells using siRNA. A transient suppression was successfully obtained, with significant knockdown occurring 48 h after transfection, followed by a full recovery to basal levels of protein synthesis 96 h after transfection as shown by Western blot (Fig. 1B). Hence, all the knockdown experiments were conducted 48 h posttransfection.
Fig. 1.
P2Y2 receptor (P2Y2R) is required for the fluid shear stress (FSS)-induced actin stress fiber formation (ASFF) in osteoblasts. A: MC3T3-E1 cells were stained for F-actin (green) and nucleus (blue) to determine the effects of FSS, exogenous nucleotide treatment, and nucleotide release on ASFF. Stress fiber formation in MC3T3-E1 cells is clearly evident 60 min after the onset of FSS (12 dyn/cm2). In static conditions cells treated with 100 μM ATP for 30 min also showed an increase in ASFF. The treatment with apyrase (0.5 U/ml) to hydrolyze extracellular ATP released during FSS attenuated ASFF. At a concentration of 100 μM, ATP and UTP exhibited the largest degree of ASFF, whereas BzATP at the same concentration had minimal to no effect on ASFF. B: protein levels of P2Y2R in MC3T3-E1 cells were analyzed by Western blot at 24, 48, 72, and 96 h following P2Y2 small interfering RNA (siRNA) transfection. Peak suppression of P2Y2R was observed at 48 h. A scrambled sequence was used as negative control siRNA (C-siRNA) and did not alter P2Y2R expression 48 h after transfection. C: suppression of P2Y2R greatly reduced stress fiber formation in response to 30 min treatment with either ATP or FSS. Bar indicates 20 μm.
The downregulation of P2Y2 receptors greatly reduced the formation of actin stress fibers to FSS applied for 60 min. Similar results were observed when these cells were treated with 100 μM ATP treatment for 30 min (Fig. 1C), indicating that the activation of the P2Y2 receptor is required for FSS-induced stress fiber formation. To ensure the transfection reagents used in the process did not alter the normal function of the cell, a C-siRNA was used. No significant difference was found on ASFF in response to FSS or ATP in cells treated with C-siRNA compared with nontransfected cells.
P2Y2R is required for the activation of RhoA GTPase during FSS to form stress fibers.
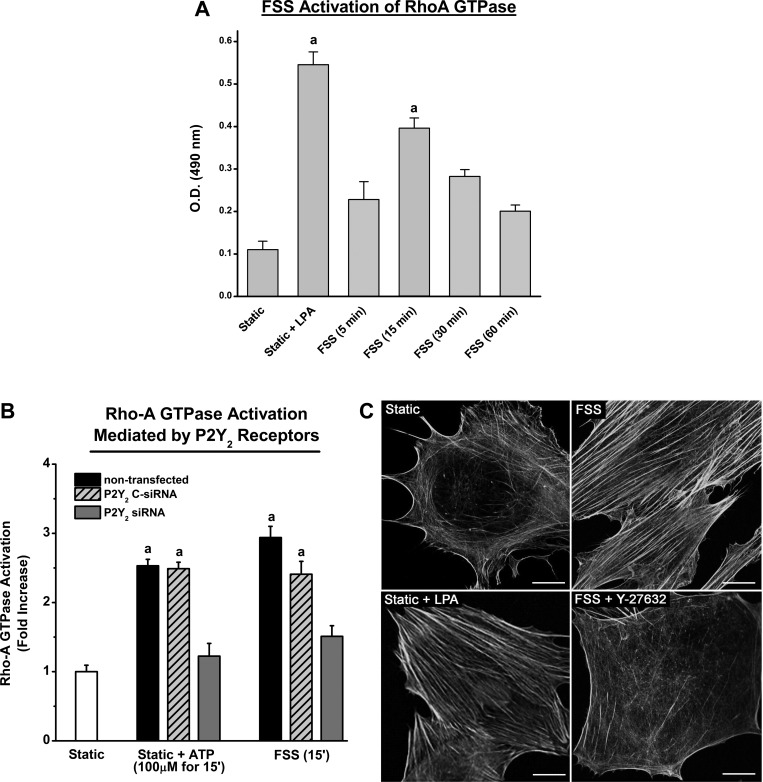
Shear stress initiates numerous cellular events in osteoblasts that eventually lead to protein expression required for bone formation. Among the early cellular events is the rapid reorganization of the actin cytoskeleton and formation of stress fibers. We postulate that this active reorganization will increase cell stiffness, making MC3T3-E1 cells less pliable, and therefore less responsive, to continued mechanical stimulation. The formation of actin stress fibers was one of the initial functions attributed to the small GTPase RhoA but has also been found to be involved in a variety of signaling process. Using the G-LISA kit, we found that FSS increased the active form or GTP-bound RhoA in MC3T3-E1 cells, as measured by an increase in absorbance at 490 nm. The graph in Fig. 2A shows that RhoA GTPase is activated within 5 min of the onset of FSS with peak activation (∼4-fold increase) observed at 15 min. This increase was similar to the approximately sixfold increase achieved by treatment with 10 μM LPA, a potent activator of RhoA GTPase.
Fig. 2.
RhoA GTPase is activated during FSS to form stress fibers due to the activation of P2Y2R. A: RhoA GTPase was measured in MC3T3 cells at different time points following the onset of FSS (12 dyn/cm2). Treatment with 1 μM lysophosphatidic acid (LPA) under static conditions elicited a maximal activation of RhoA GTPase (∼6-fold). The maximum increase in RhoA GTPase activation during FSS occurred 15 min after the onset of stimuli and was approximaely four times greater than compared with static controls (a indicates P value <0.05 compared with static control). B: G-LISA assay was used to measure RhoA GTPase activation in response to ATP treatment and FSS in nontransfected cells and cells transfected with P2Y2R siRNA. The peak RhoA GTPase activation occurred 15 min after the onset of either ATP treatment or FSS in nontransfected cells. This peak was nearly abolished with the suppression of P2Y2R. Cells transfected with C-siRNA did not exhibit any significant differences compared with nontransfected cells. aP value <0.05 compared with static control. C: MC3T3-E1 cells, stained for F-actin, exhibited a diffuse organization of actin under static conditions compared with cells exposed to FSS (12 dyn/cm2) for 60 min. LPA treatment (10 μM for 30 min), which activates RhoA GTPase, significantly increased the ASFF. Inhibition of ROCK with 10 μM Y27632 during FSS significantly inhibits ASFF. Bar indicates 20 μm.
We further demonstrated that the purinergic receptor P2Y2 is required for FSS-induced RhoA GTPase activation in MC3T3-E1 cells (Fig. 2B). The fold change in active RhoA GTPase after 60 min of FSS or 30 min of 100 μM ATP stimulation in P2Y2R knockdown cells was significantly attenuated compared with nontransfected or C-siRNA cells. To determine whether RhoA GTPase activation via the P2Y2R is essential for the formation of stress fibers, the downstream effector of RhoA GTPase ROCK was blocked with Y-27632 before FSS. AF-488 phallodin staining of actin stress fibers showed that FSS-induced ASFF was completely prevented by inhibiting ROCK, whereas activation of RhoA GTPase by LPA caused a pronounced increase in ASFF (Fig. 2C).
RhoA GTPase activation during FSS initiates the ROCK/LIMK/cofilin pathway.
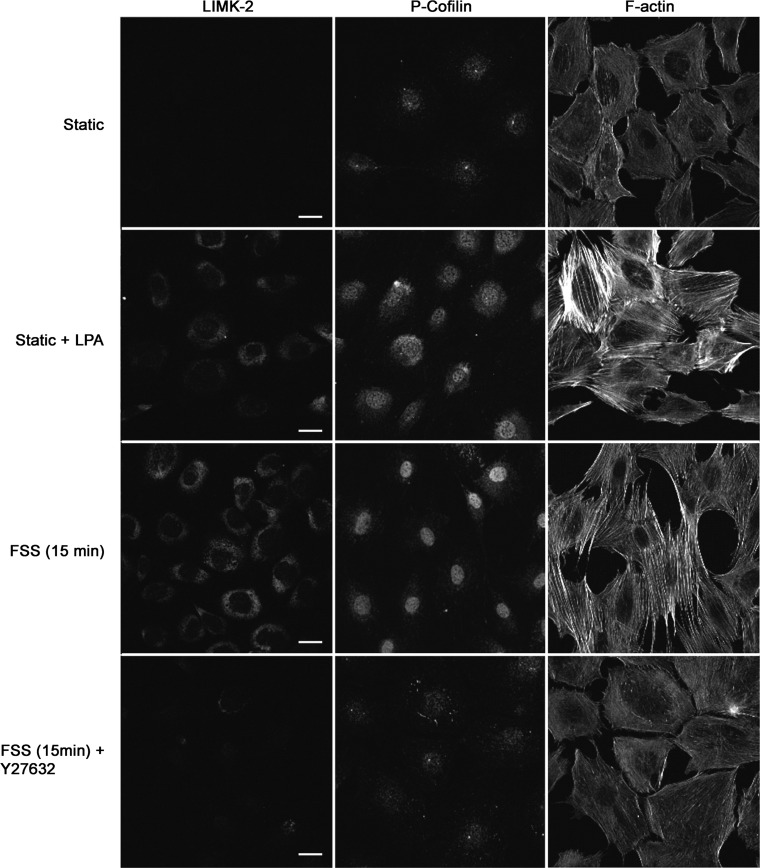
Numerous studies have shown that the ROCK/LIMK/Cofilin pathway is strongly affiliated with stress fiber formation in many cell types. A serine-threonine protein kinase LIM-K is activated by several proteins including ROCK, and cofilin is one of the primary substrates of this enzyme. Upon phosphorylation, cofilin is rendered inactive and hence unable to bind and perform its function of severing actin filaments, thereby stabilizing actin stress fibers. Immunofluorescence data indicates that LIMK-2 is phosphorylated at Thr505 within 15 min of the onset of FSS, and this phosphorylation directly correlates with an increase in P-cofilin (Fig. 3). Similar results were achieved when RhoA GTPase was activated using LPA. However, only faint staining of P-cofilin was observed under static conditions or when the ROCK inhibitor Y-27632 was used before FSS. These photomicrographs indicate that the ASFF induced by FSS in osteoblasts is mediated by the activation of the ROCK/LIMK/Cofilin pathway by RhoA GTPase as illustrated in Fig. 6.
Fig. 3.
Activation of the Rho kinase (ROCK)/Lim kinase (LIMK)/cofilin pathway during FSS to form ASFF in osteoblasts. The presence of phosphorylated cofilin (P-cofilin) (right) was increased after 15 min treatment with 1 μM LPA under static conditions, as well as after 15 min of FSS (12 dyn/cm2). Inhibition of ROCK during FSS with 10 μM Y27632 attenuated the presence of P-cofilin. A similar response was observed in LIMK-2 phosphorylation under the same conditions (left). Bar indicates 20 μm.
Fig. 6.
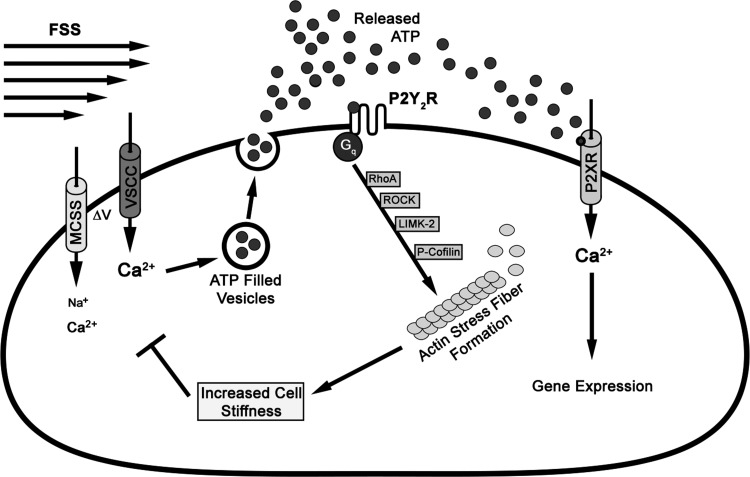
Proposed model for the loss of mechanosensitivity in osteoblasts due to ASFF. The application of FSS triggers the opening up of mechano- and voltage-sensitive calcium channels. The influx of Ca2+ through these channels leads to vesicular release of the autocrine-paracrine signaling molecule ATP. Once in the extracellular space, ATP activates both the P2Y2 and the P2X receptors. In this study we demonstrate that RhoA GTPase is activated by P2Y2R, which initiates a cascade of events via the ROCK/LIMK-2/cofilin pathway to culminate in actin filament reorganization to form stress fibers. This increases the cell stiffness and prevents the activation of calcium channels to the second bout of FSS.
P2Y2R regulates changes in cell stiffness to mechanical stimuli in osteoblasts.
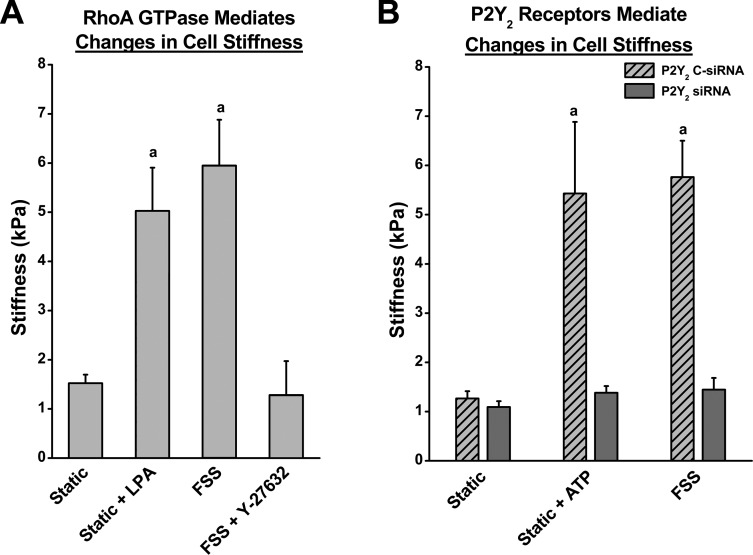
The mechanosensitivity of a cell is largely dependent on the structural integrity of its actin cytoskeleton. Increase in actin stress fibers is known to elevate the overall cell stiffness and thereby thought to potentially decrease the mechanosensitivity (19, 20). Here we demonstrate that the purinergic receptor P2Y2 regulates ASFF and, thereby, osteoblast cell stiffness by activating RhoA GTPase. Osteoblasts subjected to 15 min of FSS exhibited an approximately sixfold increase in cell stiffness as measured by AFM. To determine whether RhoA GTPase is involved in this increase in stiffness, cells were first treated with 10 μM LPA for 15 min to activate RhoA GTPase. LPA treatment produced an approximately fivefold increase in cell stiffness (Fig. 4A). The inhibition of ROCK during FSS with 10 μM Y27632 significantly blocked any changes in cell stiffness, implicating RhoA GTPase in the regulation of cell stiffness during FSS.
Fig. 4.
P2Y2R regulate ATP and FSS-induced changes cell stiffness in osteoblasts. Atomic Force Microscopy was used to determine the apparent stiffness of osteoblasts exposed to various treatments. A: under static conditions, the average cell stiffness was 1.06 kPa. Cell stiffness significantly increased by approximately fivefold in response to 1 μM LPA treatment and by approximately sixfold after 15 min of FSS (12 dyn/cm2). Inhibition of ROCK activation with 10 μM Y27632 treatment prevented changes in cell stiffness due to FSS. B: FSS-induced increase in cell stiffness was also dependent on activation of P2Y2R. The rise in cell stiffness induced by ATP treatment and FSS were suppressed by siRNA-mediated knockdown of the P2Y2R in MC3T3-E1 cells. In nontransfected ATP-treated cells, the apparent stiffness was approximately fourfold greater than compared with static control cells. aP value <0.05 compared with static control.
Given the role P2Y2R has in mediating ASFF through RhoA, we suppressed P2Y2R expression with siRNA that significantly reduced the increase in cell stiffness following 15 min of FSS or 30 min of treatment with ATP (Fig. 4B). Overall, these results provide a cogent argument that P2Y2R-mediated activation of RhoA GTPase is critical for the increase in cellular stiffness and potentially, regulation of mechanosensitivity.
Increase in cellular stiffness due to ASFF during FSS reduces the mechanosensitivity of osteoblasts to consecutive stimulation.
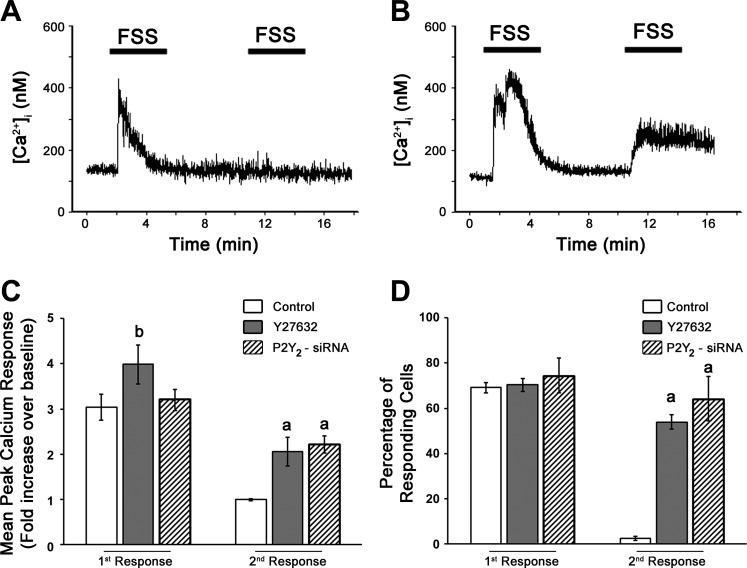
Osteoblast adaptation to mechanical stimulation was determined based on the [Ca2+]i response to successive bouts of FSS. The peak [Ca2+]i response of untreated cells to the initial bout of FSS was approximately three times greater than basal [Ca2+]i levels, with over 80% of the cells responding with a 0.5-fold or greater increase in [Ca2+]i (Fig. 5, C and D). The [Ca2+]i response to a second bout of FSS was significantly smaller in untreated cells, with peak [Ca2+]i just ∼1.5 times greater than basal [Ca2+]i levels. Furthermore, less than 5% of cells responded to this second bout of shear (Fig. 5, C and D). These data are in accordance with the physiology of the bone and the law of diminishing returns to mechanical load and provides evidence for the desensitization of osteoblasts to physical stimuli (5). We found that inhibition of ROCK with Y27632 and siRNA knockdown of P2Y2R significantly increased the peak [Ca2+]i during the first response to FSS, although it did not affect the number of cells responding (Fig. 5, C and D). Furthermore, both ROCK inhibition and siRNA knockdown of P2Y2R also significantly increased the peak [Ca2+]i and number of responding cells compared with untreated controls during the second bout of FSS (Fig. 5, C and D). Based on these results, ROCK mediates ASFF via the P2Y2 receptor activation during FSS. Hence, the activation of ROCK through RhoA GTPase is able to attenuate the [Ca2+]i response in osteoblasts to successive bouts of FSS, providing strong evidence for its function in regulating osteoblast mechanosensitivity. Although ASFF mediated by RhoA GTPase does not become visually evident until 30 min after the onset of FSS, the loss of mechanosensitivity was apparent at 15 min, as observed by the lack of [Ca2+]i response to subsequent bouts of FSS. The rapid loss of sensitivity to shear can be, at least in part, attributed to the initial formation of stress fibers and increase in cell stiffness.
Fig. 5.
Osteoblasts mechanosensitivity during FSS is retained by blocking RhoA GTPase signaling and siRNA knockdown of P2Y2R. The intracellular Ca2+ ([Ca2+]i) in MC3T3-E1 cells was measured in response to two consecutive sessions of FSS. A: representative trace of the Ca2+ response to FSS showing the peak [Ca2+]i increased within 1 min after the onset of the first bout of FSS (12 dyn/cm2), but no rise was observed during the second bout after a 5-min rest period. B: representative [Ca2+]i trace indicating that when cells were pretreated with 10 μM Y27632 before FSS, two distinct [Ca2+]i spikes were observed during both loading bouts of FSS (12 dyn/cm2). C: bar graph showing that Y27632 inhibition of ROCK and siRNA knockdown of P2Y2R increased the peak [Ca2+]i during the first and second FSS compared with the control cells first and second peak values, respectively. D: similar to the magnitude of response, the percentage of cells responding to the second application of FSS was significantly higher when treated with 10 μM Y27632 or siRNA knockdown of P2Y2R. aP < 0.05 compared with control cells second response, bP < 0.05 compared with control cells during first response.
DISCUSSION
We have demonstrated that activation of the P2Y2R receptor is required for the actin cytoskeletal rearrangement in response to FSS in vitro. Our findings show that RhoA GTPase was activated in response to FSS and is downstream of P2Y2R activation. Our data indicate that changes in cell stiffness to physical stimuli in osteoblast are dependent on the P2Y2R and RhoA GTPase signaling pathway. In this study, we suggest a novel mechanism by which purinergic signaling can regulate osteoblast mechanosensitivity in vitro.
The mechanical forces generated in bone during dynamic loading stimulates purinergic signaling in osteoblasts and is a crucial to osteogenesis (39). The vesicular release of ATP is highly regulated by the influx of intracellular calcium through mechanosensitive and voltage-sensitive channels (11, 15, 45, 46). Extracellular ATP released during mechanical stimulation then activates purinergic receptors (P2) in an autocrine/paracrine fashion. Purinergic signaling then results in the immediate release of intracellular calcium from the endothelium reticulum (23), and upregulation of anabolic predictors such as c-Fos, ERK1/2 phosphorylation, COX-2 production, and PGE2 release (15, 31, 41). Extracellular ATP has also been shown to enhance alkaline phosphatase production and increases mineralization required for new bone formation (2, 36).
Purinergic signaling has also emerged as an essential component of mechanotransduction in osteoblasts. We, and others, have shown that the P2X7 receptor enhances proliferation, ERK1/2 phosphorylation, and COX-2 production in osteoblasts (24, 31), and that knockout of this receptor significantly attenuates load-induced bone formation in vivo (29). In contrast, P2Y2R and P2Y13R knockout mice exhibit excessive bone formation in response to mechanical loading (40, 56), demonstrating that these receptors inhibit the anabolic response of bone. Based on our results, the increased bone formation in the P2Y2R knockout mice under dynamic loading (40) may be explained by the increased mechanosensitivity due to a lack of ASFF to downregulate the osteoblasts sensitivity to mechanical stimuli. How the P2Y13R influences the mechanosensitivity of bone exactly is unclear (56) but is worth investigating if it downregulates osteoblast mechanosensitivity similar to the P2Y2R given its potential to activate RhoA GTPases (34).
One of the early responses to mechanical loading in osteoblasts is the reorganization of actin into stress fibers (38, 41) that has been associated with an increase in cell stiffness (19). The ability of osteoblasts to adapt to a mechanical force and modify their mechanosensitivity may provide a means for the cell to increase its sensitivity to low magnitudes of mechanical stimulation or, conversely, provide a protective mechanism against excessive or continuous loads. We have shown that the release of nucleotides in response to FSS and cyclic hydraulic pressure can regulate actin cytoskeletal reorganization that coincides with changes in cell stiffness (14). Depolymerization of the actin-cytoskeleton in osteoblasts decreases cell stiffness, which subsequently increases their mechanosensitivity by increasing the deformation of the plasma membrane under strain (7, 54, 57). We have previously postulated that altering cell stiffness may be one mechanism through which parathyroid hormone treatment can enhance mechanically stimulated bone formation (48, 57).
Our data indicate that the P2Y2R facilitates changes in the mechanical behavior of osteoblasts through increasing ASFF and cell stiffness. The P2Y2R is a G protein-coupled receptor that can be activated by ATP or UTP (40). In this study, we show that the P2Y2R activates RhoA GTPase, probably through G protein activation of a Rho guanine exchange factor. We also show that activation of RhoA GTPase regulates the response of osteoblasts to FSS, similar to other cell types that experience various forms of mechanical stimuli (6, 12, 55). In response to FSS, RhoA GTPase activation in osteoblasts was evident as early as 5 min after the onset of stimuli and reached peak activation at 15 min. The activation of RhoA GTPase within 5 min of the onset of FSS coincides with the extracellular concentration of ATP being at its highest level based on previous studies (15).
We show that actin is rapidly reorganized into stress fibers in response to FSS and that this occurs through stimulation of the RhoA GTPase pathway. Other cell types have also been shown to regulate cytoskeleton organization through activation of RhoA GTPase (12, 32, 52, 53). Stress fiber formation is permitted once cofilin is phosphorylated and dissociates from actin filaments to allow actin polymerization. Phosphorylation of cofilin can occur through activation of the LIM kinase family, which consists of LIMK-1 and LIMK-2, by phosphorylation at Thr508 or Thy505, respectively (26, 30, 53). LIMK-1 and LIMK-2 are closely related serine-threonine kinases, whose structures contain two NH2-terminal LIM domains, an internal PDZ domain, and a COOH-terminal protein kinase domain (1, 16, 50). However, LIMK-2 is found more readily in embryonic and adult tissue compared with LIMK-1 (51). Similar to previous studies, our results demonstrated that LIMK-2 is activated downstream of RhoA GTPase and phosphorylated at Thr505 (1, 52). Phosphorylation of LIMK-2 has been shown to be an essential component of actin cytoskeleton dynamics in many cell types, and in this study we have shown it to be associated with ASFF during mechanical stimuli of osteoblasts.
The rapid activation of RhoA GTPase following 5 min of FSS was sufficient to downregulate the sensitivity of osteoblasts to a second bout of FSS 10 min after the first stimulation. Although ASFF is not visually evident until 30 min after the onset of FSS, the mechanosensitivity was reduced much earlier, based on the lack of a [Ca2+]i response to a second bout of FSS. One possibility for this temporal discrepancy is that RhoA GTP is geranylgeranylated to the plasma membrane; thus cortical actin found beneath the plasma membrane would be a probable early target of RhoA GTPase activation. This cortical actin regulates membrane stiffness and anchors many membrane proteins, including ion channels that would alter Ca2+ signaling (43). Another study has reported that an initial bout of FSS, lasting only 2 min, did not affect the subsequent [Ca2+]i response to a second bout of FSS (9). The ability of osteoblasts to retain their mechanosensitivity following only 2 min of FSS may be due to a lack of time for the cell to respond with activation of RhoA GTPase. Given the results from our study, osteoblasts appear to exhibit a threshold for prolonged mechanical stimuli that is between 2 and 5 min, after which they begin to downregulate their mechanosensitivity.
The temporal aspects of mechanical loading are known to have a large influence on the anabolic response of bone at the tissue and cellular levels (5, 51). Despite several advances in understanding mechanotransduction in osteoblasts, little is known about the cellular mechanism involved in regulating mechanosensitivity of osteoblasts. In this study, we provide new insights into a possible regulatory mechanism to physical stimuli. Figure 6 illustrates the cascade of events when FSS is applied to osteoblasts. The physical stimulus triggers the opening of the mechanosensitive cation channel (MSCC), which subsequently opens L-type voltage-gated calcium channels (L-VSCC) (8, 10, 28, 48). This increase in [Ca2+]i induces the vesicular release of ATP into the extracellular space, which then activates purinergic receptors, specifically the P2Y2R (15, 31, 46). Based on our results, the P2Y2R then activates RhoA GTPase and consequently activates ROCK, which culminates in the actin reorganization to form stress fibers via the LIM-K/cofilin pathway. In parallel with the RhoA/ROCK/LIM-K/cofilin pathway, P2Y2R activation has also been shown to initiate intercellular calcium release from the ER that is linked with ASFF as well (8, 23). Increased ASFF stiffens the cells, consequently attenuating the ability of osteoblasts to detect further mechanical stimulus and arresting the downstream signaling that would lead to osteogenesis. Robling et al. (44) have reported that mechanically induced osteogenesis can be enhanced with a break in between loading bouts, suggesting that the ability of the cells responding to physical stimuli is only temporarily attenuated. We propose that formation of ASFF via P2Y2R activation is a way to desensitize osteoblasts, which can potentially regain its sensitivity over time. Based on our findings, we predict that the increased bone formation observed in the P2Y2R knockout mice can be partly attributed to the continuous signaling to the physical stimuli (40).
In summary, our findings suggest a novel mechanism by which the purinergic receptor P2Y2R can regulate osteoblast mechanosensitivity. Furthermore, we directly implicate P2Y2R to ASFF in response to FSS through a RhoA GTPase-dependent mechanism that, in turn, activates the ROCK/LIMK/cofilin pathway. Additionally, our data indicate that the increase in cell stiffness in response to FSS is also mediated by the P2Y2R receptors. Finally, our findings suggest that by blocking ROCK and the eventual ASFF during FSS, the loss in mechanosensitivity of the osteoblasts can be averted.
GRANTS
This work was funded by National Institutes of Health Grants National Institute of Arthritis and Musculoskeletal and Skin Diseases R01AR051901 and National Institute of Diabetes and Digestive and Kidney Diseases R01 DK-058246.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.G., W.Y., G.R.M., A.K., V.G., E.A., and K.C. performed experiments; J.G., W.Y., G.R.M., A.K., V.G., E.A., K.C., and R.L.D. analyzed data; J.G., W.Y., G.R.M., A.K., E.A., K.C., and R.L.D. interpreted results of experiments; J.G., W.Y., G.R.M., A.K., and V.G. prepared figures; J.G., W.Y., and V.G. drafted manuscript; J.G., V.G., and R.L.D. edited and revised manuscript; W.Y., E.A., K.C., and R.L.D. conception and design of research; R.L.D. approved final version of manuscript.
REFERENCES
- 1.Amano T, Tanabe K, Eto T, Narumiya S, Mizuno K. LIM-kinase 2 induces formation of stress fibres, focal adhesions and membrane blebs, dependent on its activation by Rho-associated kinase-catalysed phosphorylation at threonine-505. Biochem J 354: 149–159, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boskey AL, Doty SB, Binderman I. Adenosine 5′-triphosphate promotes mineralization in differentiating chick limb-bud mesenchymal cell cultures. Microsc Res Tech 28: 492–504, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Bowler WB, Dixon CJ, Halleux C, Maier R, Bilbe G, Fraser WD, Gallagher JA, Hipskind RA. Signaling in human osteoblasts by extracellular nucleotides. Their weak induction of the c-fos proto-oncogene via Ca2+ mobilization is strongly potentiated by a parathyroid hormone/cAMP-dependent protein kinase pathway independently of mitogen-activated protein kinase. J Biol Chem 274: 14315–14324, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Buckley MJ, Banes AJ, Levin LG, Sumpio BE, Sato M, Jordan R, Gilbert J, Link GW, Tran Son Tay R. Osteoblasts increase their rate of division and align in response to cyclic, mechanical tension in vitro. Bone Miner 4: 225–236, 1988 [PubMed] [Google Scholar]
- 5.Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone 30: 781–786, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell 116: 167–179, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Charras GT, Horton MA. Single cell mechanotransduction and its modulation analyzed by atomic force microscope indentation. Biophys J 82: 2970–2981, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen NX, Ryder KD, Pavalko FM, Turner CH, Burr DB, Qiu JY, Duncan RL. Ca2+ regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am J Physiol Cell Physiol 278: C989–C997, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Donahue SW, Donahue HJ, Jacobs CR. Osteoblastic cells have refractory periods for fluid-flow-induced intracellular calcium oscillations for short bouts of flow and display multiple low-magnitude oscillations during long-term flow. J Biomech 36: 35–43, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Duncan R, Misler S. Voltage-activated and stretch-activated Ba2+ conducting channels in an osteoblast-like cell line (UMR 106). FEBS Lett 251: 17–21, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Duncan RL, Akanbi KA, Farach-Carson MC. Calcium signals and calcium channels in osteoblastic cells. Semin Nephrol 18: 178–190, 1998 [PubMed] [Google Scholar]
- 12.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 420: 629–635, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng 32: 1053–1060, 1988 [DOI] [PubMed] [Google Scholar]
- 14.Gardinier JD, Majumdar S, Duncan RL, Wang L. Cyclic hydraulic pressure and fluid flow differentially modulate cytoskeleton re-organization in MC3T3 osteoblasts. Cell Mol Bioeng 2: 133–143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3–E1 osteoblasts. J Bone Miner Res 20: 41–49, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal P, Pandey D, Siess W. Phosphorylation-dependent regulation of unique nuclear and nucleolar localization signals of LIM kinase 2 in endothelial cells. J Biol Chem 281: 25223–25230, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gurkan UA, Akkus O. The mechanical environment of bone marrow: a review. Ann Biomed Eng 36: 1978–1991, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Jaasma MJ, Jackson WM, Keaveny TM. The effects of morphology, confluency, and phenotype on whole-cell mechanical behavior. Ann Biomed Eng 34: 759–768, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Jaasma MJ, Jackson WM, Tang RY, Keaveny TM. Adaptation of cellular mechanical behavior to mechanical loading for osteoblastic cells. J Biomech 40: 1938–1945, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Jackson WM, Jaasma MJ, Baik AD, Keaveny TM. Over-expression of alpha-actinin with a GFP fusion protein is sufficient to increase whole-cell stiffness in human osteoblasts. Ann Biomed Eng 36: 1605–1614, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech 31: 969–976, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janmey PA, Euteneuer U, Traub P, Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol 113: 155–160, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorgensen NR, Henriksen Z, Brot C, Eriksen EF, Sorensen OH, Civitelli R, Steinberg TH. Human osteoblastic cells propagate intercellular calcium signals by two different mechanisms. J Bone Miner Res 15: 1024–1032, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WS, Dixon SJ, Sims SM, Thompson DD. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol 17: 1356–1367, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Ko KS, McCulloch CA. Partners in protection: interdependence of cytoskeleton and plasma membrane in adaptations to applied forces. J Membr Biol 174: 85–95, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Koga T, Koga T, Awai M, Tsutsui J, Yue BY, Tanihara H. Rho-associated protein kinase inhibitor, Y-27632, induces alterations in adhesion, contraction and motility in cultured human trabecular meshwork cells. Exp Eye Res 82: 362–370, 2006 [DOI] [PubMed] [Google Scholar]
- 27.LaMothe JM, Zernicke RF. Rest insertion combined with high-frequency loading enhances osteogenesis. J Appl Physiol 96: 1788–1793, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Li J, Duncan RL, Burr DB, Turner CH. L-type calcium channels mediate mechanically induced bone formation in vivo. J Bone Miner Res 17: 1795–1800, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem 280: 42952–42959, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Lin T, Zeng L, Liu Y, DeFea K, Schwartz MA, Chien S, Shyy JY. Rho-ROCK-LIMK-cofilin pathway regulates shear stress activation of sterol regulatory element binding proteins. Circ Res 92: 1296–1304, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Genetos DC, Shao Y, Geist DJ, Li J, Ke HZ, Turner CH, Duncan RL. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca(2+)- and ATP-dependent in MC3T3–E1 osteoblasts. Bone 42: 644–652, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285: 895–898, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Mak AF, Lai WM, Mow VC. Biphasic indentation of articular cartilage: I. Theoretical analysis. J Biomech 20: 703–714, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Malaval C, Laffargue M, Barbaras R, Rolland C, Peres C, Champagne E, Perret B, Terce F, Collet X, Martinez LO. RhoA/ROCK I signalling downstream of the P2Y(13) ADP-receptor controls HDL endocytosis in human hepatocytes. Cell Signal 21: 120–127, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci 119: 508–518, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Nakano Y, Addison WN, Kaartinen MT. ATP-mediated mineralization of MC3T3–E1 osteoblast cultures. Bone 41: 549–561, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Nebl G, Meuer SC, Samstag Y. Dephosphorylation of serine 3 regulates nuclear translocation of cofilin. J Biol Chem 271: 26276–26280, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Norvell SM, Ponik SM, Bowen DK, Gerard R, Pavalko FM. Fluid shear stress induction of COX-2 protein and prostaglandin release in cultured MC3T3–E1 osteoblasts does not require intact microfilaments or microtubules. J Appl Physiol 96: 957–966, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Orriss IR, Burnstock G, Arnett TR. Purinergic signalling and bone remodelling. Curr Opin Pharmacol 10: 322–330 [DOI] [PubMed] [Google Scholar]
- 40.Orriss IR, Utting JC, Brandao-Burch A, Colston K, Grubb BR, Burnstock G, Arnett TR. Extracellular nucleotides block bone mineralization in vitro: evidence for dual inhibitory mechanisms involving both P2Y2 receptors and pyrophosphate. Endocrinology 148: 4208–4216, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Pavalko FM, Chen NX, Turner CH, Burr DB, Atkinson S, Hsieh YF, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in MC3T3–E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol Cell Physiol 275: C1591–C1601, 1998 [PubMed] [Google Scholar]
- 42.Radmacher M, Fritz M, Kacher CM, Cleveland JP, Hansma PK. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys J 70: 556–567, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Razinia Z, Makela T, Ylanne J, Calderwood D. Filamins in mechanosensing and signaling. Annu Rev Biophys 41: 227–246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robling AG, Burr DB, Turner CH. Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading. J Bone Miner Res 15: 1596–1602, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Romanello M, Codognotto A, Bicego M, Pines A, Tell G, D'Andrea P. Autocrine/paracrine stimulation of purinergic receptors in osteoblasts: Contribution of vesicular ATP release. Biochem Biophys Res Commun 331: 1429–1438, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Romanello M, Pani B, Bicego M, D'Andrea P. Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun 289: 1275–1281, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am 66: 397–402, 1984 [PubMed] [Google Scholar]
- 48.Ryder KD, Duncan RL. Parathyroid hormone enhances fluid shear-induced [Ca2+]i signaling in osteoblastic cells through activation of mechanosensitive and voltage-sensitive Ca2+ channels. J Bone Miner Res 16: 240–248, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Sah VP, Seasholtz TM, Sagi SA, Brown JH. The role of Rho in G protein-coupled receptor signal transduction. Annu Rev Pharmacol Toxicol 40: 459–489, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Scott RW, Olson MF. LIM kinases: function, regulation and association with human disease. J Mol Med 85: 555–568, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res 17: 1613–1620, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem 276: 670–676, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Sumi T, Matsumoto K, Takai Y, Nakamura T. Cofilin phosphorylation and actin cytoskeletal dynamics regulated by rho- and Cdc42-activated LIM-kinase 2. J Cell Biol 147: 1519–1532, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takai E, Costa KD, Shaheen A, Hung CT, Guo XE. Osteoblast elastic modulus measured by atomic force microscopy is substrate dependent. Ann Biomed Eng 33: 963–971, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res 98: 176–185, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Wang N, Rumney RMH, Yang L, Robaye B, Boeynaems JM, Skerry TM, Gartland A. The P2Y13 receptor regulates extracellular ATP metabolism and the osteogenic response to mechanical loading. J Bone Miner Res 28: 1446–1456, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Ryder KD, Bethel JA, Ramirez R, Duncan RL. PTH-induced actin depolymerization increases mechanosensitive channel activity to enhance mechanically stimulated Ca2+ signaling in osteoblasts. J Bone Miner Res 21: 1729–1737, 2006 [DOI] [PubMed] [Google Scholar]