Abstract
Neuronostatin is a recently described peptide hormone encoded by the somatostatin gene. We previously showed that intraperitoneal injection of neuronostatin into mice resulted in c-Jun accumulation in pancreatic islets in a pattern consistent with the activation of glucagon-producing α-cells. We therefore hypothesized that neuronostatin could influence glucose homeostasis via a direct effect on the α-cell. Neuronostatin enhanced low-glucose-induced glucagon release in isolated rat islets and in the immortalized α-cell line αTC1-9. Furthermore, incubation with neuronostatin led to an increase in transcription of glucagon mRNA, as determined by RT-PCR. Neuronostatin also inhibited glucose-stimulated insulin secretion from isolated islets. However, neuronostatin did not alter insulin release from the β-cell line INS 832/13, indicating that the effect of neuronostatin on insulin secretion may be secondary to a direct action on the α-cell. In agreement with our in vitro data, intra-arterial infusion of neuronostatin in male rats delayed glucose disposal and inhibited insulin release during a glucose challenge. These studies suggest that neuronostatin participates in maintaining glucose homeostasis through cell-cell interactions between α-cells and β-cells in the endocrine pancreas, leading to attenuation in insulin secretion.
Keywords: neuronostatin, glucose homeostasis, islet function, glucagon
somatostatin was originally described as an inhibitor of growth hormone release from the anterior pituitary gland (1). It is now known that somatostatin exerts its actions in a variety of tissues, including the endocrine pancreas, via five related G protein-coupled receptors. Somatostatin is produced by the δ-cells of the pancreatic islet and acts in a paracrine fashion to reduce secretion of both insulin and glucagon from β-cells and α-cells, respectively (19, 20).
The somatostatin preprohormone is 116 amino acids in length, including the 24-amino acid signal peptide, somatostatin-14 and the NH2-terminally extended form of somatostatin, somatostatin-28, both at the COOH terminus of the prohormone. For many years it was postulated that the somatostatin preprohormone contained another biologically active peptide. Based on bioinformatic analyses of conserved sequences and dibasic cleavage sites, an additional peptide in the somatostatin preprohormone was identified, which we named neuronostatin (17).
Like somatostatin, neuronostatin influences a variety of biological systems. When injected centrally, neuronostatin inhibits food and water intake (17, 25) and increases mean arterial pressure (17, 24). In the periphery, neuronostatin exerts direct inhibitory effects on the contractility of isolated cardiomyocytes (6, 21) and has been demonstrated to stimulate c-Fos expression in the human gastric tumor cell line KATOIII (17). Because neuronstatin is produced in the same tissues as somatostatin, including the endocrine pancreas, and specifically in pancreatic δ-cells, we hypothesized that neuronostatin would alter endocrine pancreatic function. Indeed, intraperitoneal injection of neuronostatin into male mice led to an increase in the expression of c-Jun in pancreatic islets in a pattern consistent with induction in the α-cells (17). We therefore sought to determine whether neuronostatin could influence glucose homeostasis through an action on the pancreatic α-cell.
MATERIALS AND METHODS
Materials and Animals
Sprague-Dawley rats were purchased from Harlan (Indianapolis, IN). All protocols and procedures were approved by the Saint Louis University Animal Care and Use Committee. INS 832/13 (rat insulinoma) cells were obtained from the Washington University Tissue Culture Support Center (St. Louis, MO). αTC-1 clone 9 (αTC1-9) cells were obtained from ATCC (Manassas, VA). DMEM and RPMI 1640 containing l-glutamine, CMRL-1066, and DMEM tissue culture media, l-glutamine, penicillin, and streptomycin were from GIBCO-BRL (Grand Island, NY). FCS was obtained from Hyclone Laboratories (Logan, UT). All other reagents were from commercially available resources.
In Vitro Experiments
Islet isolation.
Islets were isolated from 250- to 300-g male Sprague-Dawley rats by collagenase digestion, as previously described (9), and cultured overnight in complete CMRL-1066 (containing 2 mM l-glutamine, 10% heat-inactivated FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin) at 37°C under atmosphere of 95% air-5% CO2 before experimentation.
Glucose-stimulated hormone secretion.
Islets were washed three times with Krebs-Ringer bicarbonate (KRB) buffer (25 mM HEPES, 115 mM NaCl, 24 mM NaHCO3, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, pH 7.4) containing 3 mM d-glucose and 0.1% BSA. Groups of 15 islets were aliquoted into 10 × 75 mm borosilicate tubes and preincubated for 30 min at 37°C with shaking in 200 μl of KRB containing either 3 or 20 mM glucose with or with out the indicated concentrations of neuronostatin. The preincubation solution was removed, and glucose-stimulated hormone secretion was initiated by the addition of 200 μl of either 3, 10, or 20 mM d-glucose with or with out the indicated concentrations of neuronostatin or somatostatin. Following a 30-min incubation, the KRB was removed, and insulin or glucagon content was determined by RIA at the Diabetes Research and Training Center, Washington University School of Medicine (St. Louis, MO).
For studies examining hormone secretion from cell lines, INS 832/13 or αTC1-9 cells were plated in 96- or 24-well plates at a density of 0.25 × 105 cells/well or 1.0 × 105 cells/well in complete medium [RPMI 1640 (INS832/13) or DMEM (αTC1-9) supplemented with 10% FCS, l-glutamine, and antibiotics]. The day of the experiment, cells were washed in PBS and allowed to preincubate in low- or high-glucose KRB buffer for 1 h in the presence or absence of the indicated peptides. Hormone secretion was performed using a 2-h static incubation under the conditions indicated in the figure legends. Supernatants were collected, and insulin or glucagon content was determined by RIA at Washington University (St. Louis, MO).
IP3 assay.
For IP3 generation, αTC1-9 cells were plated at a density of 25,000 cells/100 μl in a 96-well plate one day prior to the experiment. On the day of the experiment the medium was removed, and cells were washed with PBS and allowed to incubate in KRB buffer containing 25 mM d-glucose and 0.1% BSA at 37°C under atmosphere of 95% air-5% CO2 for 30 min. Cells were treated as indicated in the figure legends. Following treatment, cells were lysed in 100 μl of 100% TCA (ice cold), transferred to a microfuge tube, and centrifuged at 1,000 g at 4°C for 10 min. TCA was removed from the extract using a TCTFE (1,1,2-trichloro-1,2,2-trifluoroethane)-trioctylamine solution. IP3 levels were measured by the inositol-1,4,5-triphosphate [3H] Radioreceptor Assay kit (PerkinElmer Life Sciences, Boston, MA) according to the manufacturer's protocols.
cAMP determination.
For cAMP generation, cells were plated at a density of 25,000 cells/100 μl in a 96-well plate 1 day prior to the experiment. On the day of the experiment, the medium was removed, and cells washed with PBS and allowed to incubate in KRB containing 3 or 20 mM d-glucose and 0.1% BSA at 37°C under atmosphere of 95% air-5% CO2 for 30 min. For islet studies, individual islets were counted into groups of 15 islets and transferred into 10 × 75 mm borosilicate tubes and preincubated for 30 min at 37°C with shaking in 200 μl of KRB containing 3 mM glucose. Cells or islets were treated as indicated in the figure legends. Following treatment, 100 μl of ice-cold ethanol was added, and samples were dried in a rotary evaporator. Sample pellets were resuspended in appropriate assay buffers, and cAMP was measured by radioimmunoassay (PerkinElmer), BioTrak enzyme immunoassay (Amersham, Piscataway, NJ), or Bridge-It fluorometric assay (Mediomics, St. Louis, MO) according to the manufacturers' protocols.
Electrophoresis and western blot analysis.
Cellular proteins (100 pg total protein/sample) were separated by SDS-PAGE (4–20% polyacrylamide gradient), transferred to PVDF membranes, and imaged with a chemiluminescent detection system per the manufacturer's instructions (Bio-Rad, Hercules, CA). All primary and secondary antibody dilutions were at 1:1,000 (Cell Signaling, Beverly MA).
PCR and real-time PCR.
Total RNA was isolated using the RNeasy RNA isolation kit, (Qiagen, Valencia, CA). First-strand cDNA synthesis was performed using oligo(dT) and reverse transcriptase, and standard PCR was performed, or real-time PCR (RT-PCR) was performed using SYBR Green reagent (Qiagen) and the Research DNA Engine Opticon System with continuous fluorescence detection (MJ Research) according to the manufacturers' protocols or as previously described (2). The fold change in expression of target genes was determined using the following mathematical equations based on threshold:
where q refers to sample and cb refers to the calibrator internal control GAPDH or actin. Primer sequences have been previously described (11).
In Vivo Experiments
Anesthesia and cannulations.
Ketamine (Ketaset; Fort Dodge Animal Health, Fort Dodge, IA)-xylazine (TranquiVed, Vedco, St. Joseph, MO) (60 mg ketamine/8 mg xylazine/ml, 0.1 ml/100 g body wt ip) anesthesia was employed for implantation of jugular (4) and carotid (24) cannulae as previously described (17). Postanesthetic analgesia was provided by injection of buprenorphine 0.05 mg/kg sc. Postanesthetic fluid replacement included subcutaneous sterile saline (0.9% NaCl) to balance anticipated fluid loss (3:1). Postsurgical weight loss of greater than 10% excluded animals from the testing protocol.
Glucose challenge.
Cannulated animals were moved to a quiet testing room immediately after lights on (0600) on the day after surgery, where they remained undisturbed for 2 h. Then, extension tubing (45 cm, PE50, filled with heparinized saline) was connected to the carotid and jugular catheters. The rats were left undisturbed for an additional 30 min. After removal of 0.025 ml of blood from the jugular vein for glucose determinations (Contour II Monitor; Bayer, glucose oxidase method), the animals received a bolus injection via the carotid artery of 0.5 ml of saline vehicle or vehicle containing 10 μg of neuronostatin. Immediately thereafter, the carotid line was attached to an infusion pump (flow rate 0.05 ml/min), and saline or neuronostatin (1 μg/min) was infused for 35 min. A jugular blood sample was taken 5 min later for glucose determination, after which all animals received an intra-arterial bolus injection of 1 g/kg body wt dextrose in 0.1 ml of saline. Additional jugular blood samples were taken 1, 5, 10, 15, and 30 min after glucose administration. Blood samples were taken similarly for glucose determinations 5 min before and immediately prior to glucose administration and 1, 10, and 30 min later. At the same sampling time points, an additional 0.2 ml of whole blood was removed via the jugular vein catheter into heparinized syringes for subsequent separation of plasma and determination of insulin content by ELISA at Washington University.
Statistical analysis.
Data were analyzed using ANOVA with Tukey's multiple comparison test (for all hormone level data), two-way ANOVA with Bonferroni post hoc analysis, or Mann-Whitney U-test (for AUC data). The nonparametric U-test was used for the AUC data, as the data were transformed to be normalized to baseline blood glucose or insulin values.
RESULTS
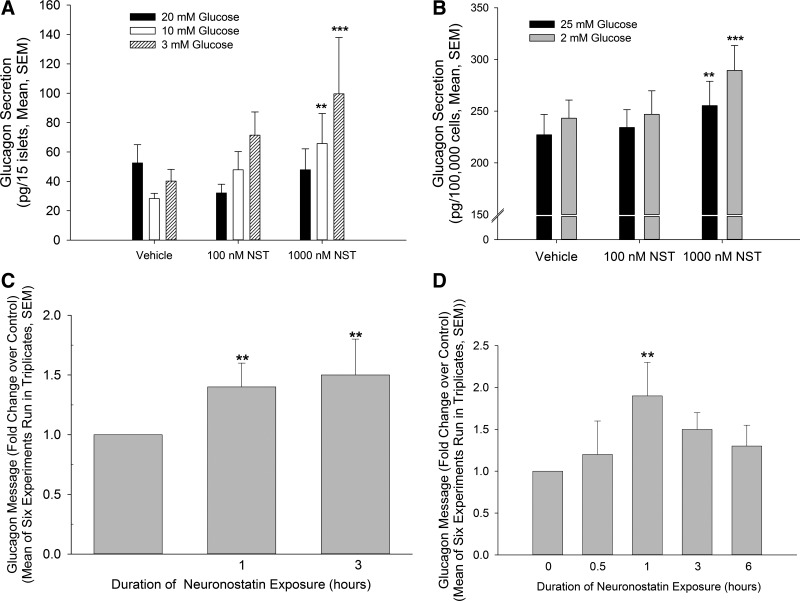
Since intraperitoneal injection of neuronostatin led to an accumulation of c-Jun in pancreatic islets in mice in a pattern consistent with activation of α-cells, the effect of neuronostatin on glucagon release by isolated rat islets and by the immortalized αTC1-9 cells was examined. Treatment with neuronostatin (1,000 nM) enhanced low-glucose-induced glucagon release compared with islets treated with control medium alone (Fig. 1A). This effect appeared to be a direct one on the α-cell, since exposure to neuronostatin significantly increased low-glucose-induced glucagon release from αTC1-9 cells as well (Fig. 1B).
Fig. 1.
Neuronostatin (NST) enhances low-glucose-induced glucagon secretion and enhances glucagon expression. A: isolated rat islets (15 islets/200 μl KRB) were incubated for 30 min in KRB containing 20 mM glucose in the presence or absence of NST. Low-glucose-induced glucagon secretion was initiated by changing the incubation medium to KRB containing 20, 10, or 3 mM glucose ± NST. NST significantly enhanced low glucose-stimulated glucagon release. B: immortalized α-cell line, αTC1-9, was incubated in KRB containing 25 mM glucose ± NST. Following 1-h equilibration, glucagon secretion was initiated by switching the medium to KRB containing 25 or 2 mM glucose ± indicated peptides, and cells were incubated for 2 h. As with isolated whole islets, NST significantly enhanced glucagon secretion. To assess the effect of NST on glucagon mRNA expression, islets (C) or αTC1-9 cells (D) were treated with 100 nM NST for indicated times. Real-time PCR was performed and glucagon message normalized to the internal control GAPDH (C) or actin (D) and presented as fold increase over untreated controls. Statistical significance: **P < 0.01, ***P < 0.001 vs. no peptide control. No significant within-group differences were observed by 2-way ANOVA with Bonferroni post hoc analysis.
Unlike insulin in β-cells, α-cells do not contain an overabundance of glucagon-containing vesicles, and glucagon secretion is regulated tightly at the transcriptional level (3). Therefore it is possible that neuronostatin increased glucagon secretion via an effect on transcription. Indeed, rat islets treated with 100 nM neuronostatin for either 1 or 3 h exhibited a significant increase in glucagon mRNA expression compared with vehicle-treated controls, as determined by RT-PCR (Fig. 1C). To examine this effect without the influence of the other islet cell types, we treated αTC1-9 cells with neuronostatin for 0.5, 1, 3, and 6 h and determined glucagon mRNA levels using RT-PCR. Treatment with neuronostatin for 1 h led to a significant increase in the accumulation of glucagon mRNA compared with vehicle-treated control cells (Fig. 1D). Following prolonged exposures of 3–6 h with neuronostatin glucagon mRNA accumulation returned to basal levels.
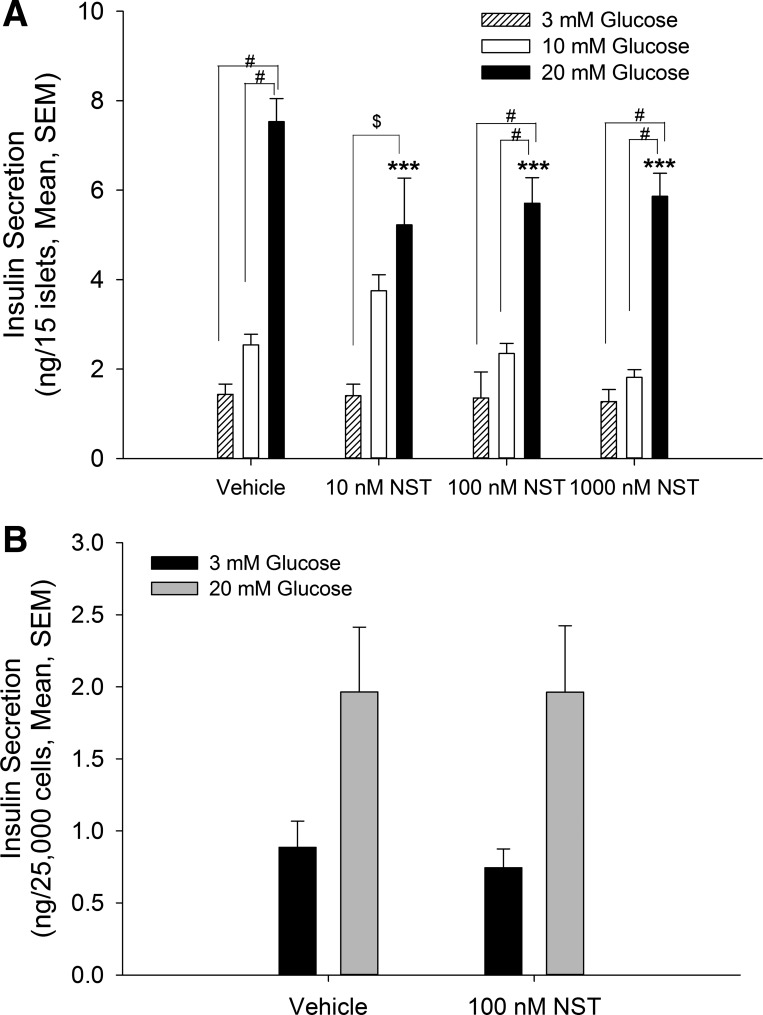
It has been demonstrated that a full secretory response from β-cells in the presence of physiological levels of glucose depends on intraislet communication with the resident α-cells (3, 5, 8, 13, 15). We therefore tested whether neuronostatin could alter insulin secretion in isolated islets. Indeed, exposure of islets to neuronstatin resulted in an attenuation of glucose-stimulated insulin secretion (20 mM; Fig. 2A). This effect did not appear to be concentration dependent, since neuronostatin concentrations of 10, 100, and 1,000 nM all yielded similar levels of inhibition. Importantly, this effect seemed to require islet cell types in addition to β-cells since, although exposure to high glucose (20 mM) induced insulin release (F = 10.94), neuronstatin failed to alter insulin release from the immortalized β-cell line INS 832/13 (Fig. 2B). Furthermore, neuronostatin did not alter proinsulin mRNA production in isolated rat islets [relative proinsulin expression: control (n = 8), 1.0 ± 0.32; neuronostatin (n = 8): 0.95 ± 0.30].
Fig. 2.
NST attenuates glucose-stimulated insulin secretion in rat islets but not in INS 832/13 cells. A: isolated rat islets (15 islets/200 μl KRB) were incubated for 30 min in KRB containing 3 mM glucose in the presence or absence of indicated peptides. Glucose-induced insulin secretion was initiated by changing the incubation medium to KRB containing 3, 10, or 20 mM glucose ± NST. Incubation with 10, 100, or 1,000 nM NST significantly inhibited glucose-stimulated insulin secretion. B: INS 832/13 cells were incubated with KRB containing 3 mM glucose ± NST. Following 1-h equilibration, glucose-induced insulin secretion was initiated by removing the incubation medium and replacing it with KRB containing 3 or 20 mM glucose ± NST. NST failed to alter glucose-stimulated insulin release in INS 832/13 cells. ***P < 0.001 vs. no peptide control; $P < 0.01; #P < 0.001 vs. indicated treatment groups as determined by 2 -way ANOVA with Bonferroni post hoc analysis.
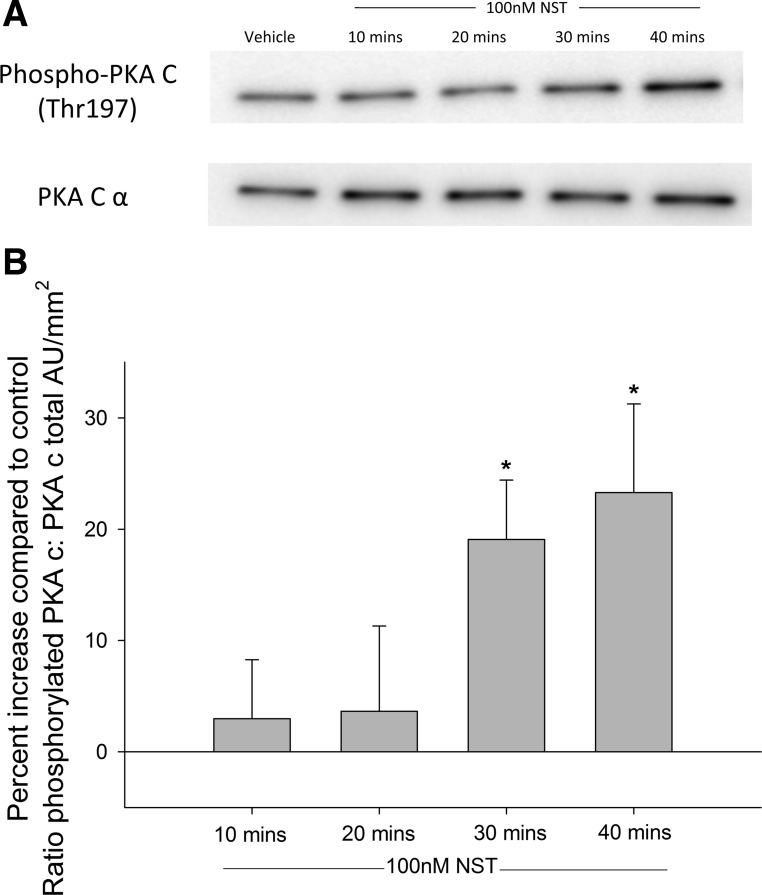
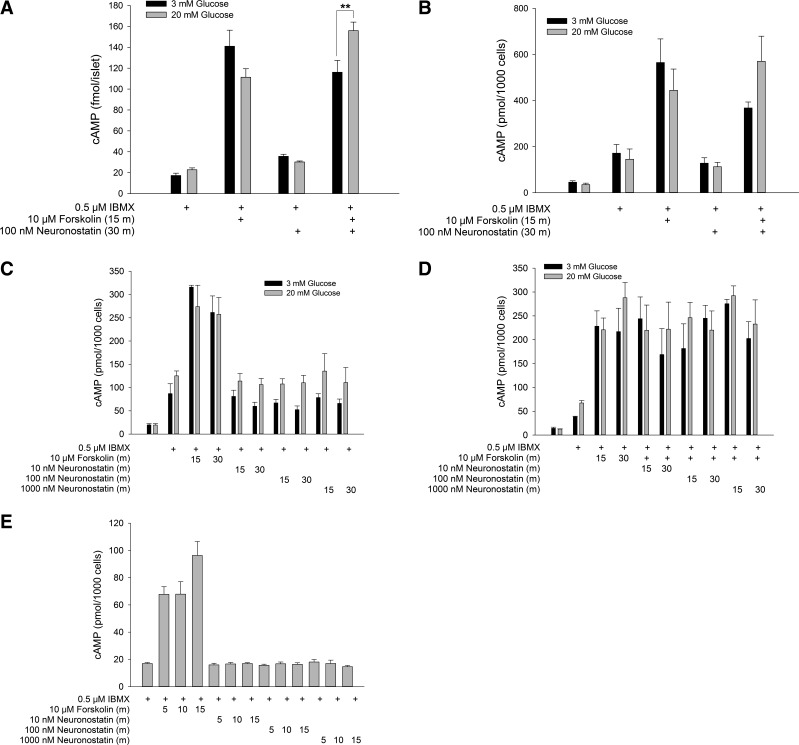
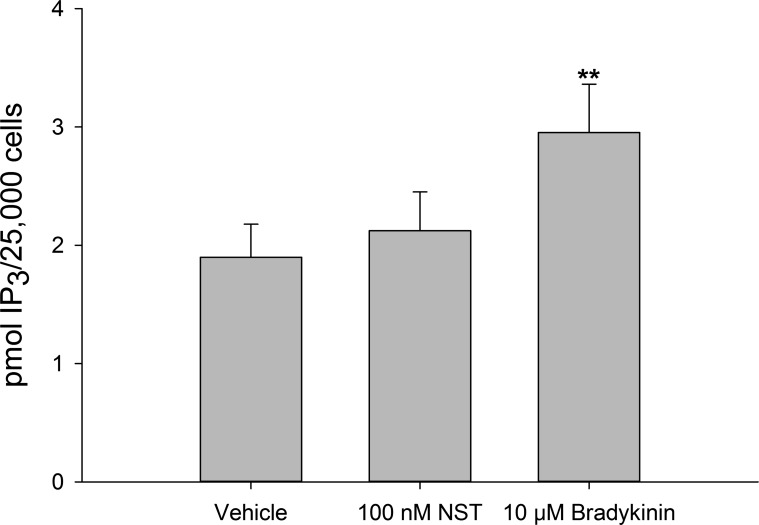
Neuronostatin has been shown to activate PKA-dependent signaling pathways in murine cardiomyocytes (6). In αTC1-9 α-cells, treatment with 100 nM neuronostatin led to an increase in phosphorylated PKA at 30 and 40 min, as determined by Western blot (Fig. 3, A and B). Because activation of PKA usually involves an increase in cytoplasmic cAMP levels (16), we tested the ability of neuronostatin to stimulate cAMP formation. In isolated rat islets, treatment with the diterpene forskolin (10 μM, 15 min), led to an increase in cAMP during both low (filled bars) and high (gray bars) glucose conditions (Fig. 4A). However, neuronostatin failed to alter cAMP levels during either glycemic condition. Neuronostatin also failed to influence either basal or forskolin-stimulated cAMP production in INS 832/13 cells (Fig. 4B), Min6 cells (Fig. 4, C and D), or αTC1-9 cells (Fig. 4E). Because the activation of PKA may also be associated with upstream PI 3-kinase activity and IP3 formation (10), we tested the effect of neuronostatin on IP3 levels in αTC1-9 cells. Incubation with bradykinin (10 μM for 1 min) led to a significant rise in IP3 levels (Fig. 5). However, treatment with neuronostatin (100 nM for 1 min) did not affect the formation of IP3.
Fig. 3.
NST activates PKA in αTC1-9 cells. αTC1-9 cells were serum starved overnight in 1 mM glucose and treated with 100 nM NST for indicated times. Cells were lysed and analyzed by Western blot using phosphospecific or total antibodies. A: treatment with NST for 30 or 40 min increased phosphorylated PKA, whereas total PKA levels were unaffected. B: quantification of band density. *P < 0.05 vs. no peptide control. Results are representative of the average ± SE of 3 independent experiments.
Fig. 4.
NST fails to modulate cAMP levels. Rat islets (A), INS 832/13 (B), or Min6 (C and D) cells were preincubated in KRB containing 3 mM glucose for 30 min in the presence or absence of IBMX, as indicated. Preincubation buffer was removed and replaced with KRB containing 3 mM glucose (open bars) or 20 mM glucose (filled bars) and treated as indicated. In conditions where cells were treated with both forskolin and NST, NST was included in the preincubation and stimulatory buffers. Alternatively, αTC1-9 cells were plated in complete medium and treated as indicated (E). Following treatment, all cells were lysed in 100% ethanol and samples dried by rotary evaporator. cAMP levels were determined by RIA. Data are represented as means ± SE of ≥3 experiments performed in duplicate (B–E) or of one experiment performed in quadruplicate (A). **P < 0.01 vs. indicated treatment groups as determined by 2-way ANOVA with Bonferroni post hoc analysis.
Fig. 5.
NST fails to modulate IP3 levels. αTC1-9 cells were preincubated for 30 min in KRB. Cells were treated with NST or bradykinin and lysed in ice cold 100% EtOH. IP3 was measured by RIA. NST failed to alter IP3 levels after 1 min of exposure. **P < 0.01 vs. untreated control.
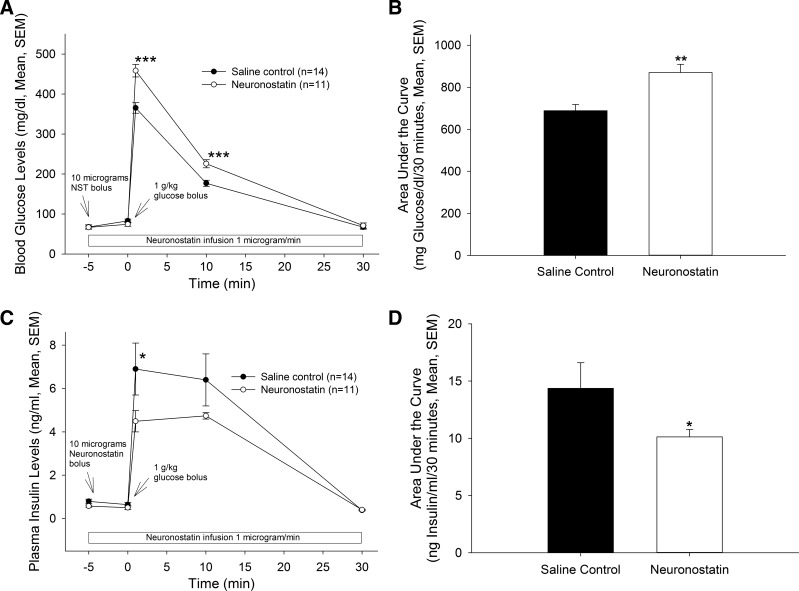
To determine whether the in vitro results translated to an effect on glucose homeostasis in vivo, male rats were infused with either saline vehicle or neuronostatin (10 μg bolus followed by an infusion of 1 μg/min) via a carotid catheter. These rats were then challenged with glucose, and insulin production and glucose clearance were evaluated. Infusion with neuronostatin delayed glucose clearance in this model, such that blood glucose levels in neuronstatin-treated animals were significantly higher at 1 and 10 min following intra-arterial injection of a glucose bolus (Fig. 6, A and B). This effect of neuronstatin resolved 30 min after glucose administration. The treatment of isolated islets with neuronostatin resulted in an inhibition of glucose-stimulated insulin secretion, and, consistent with these findings, neuronostatin also significantly reduced plasma insulin levels in rats subjected to the glucose challenge (Fig. 6, C and D) at 1 min following injection of the glucose bolus. Although there was a trend toward reduced insulin levels at 10 min after glucose administration, significance was not attained. By 30 min, both saline- and neuronostatin-infused animals exhibited similar plasma insulin levels.
Fig. 6.
Intra-arterial infusion of NST delays glucose disposal and attenuates insulin release during a glucose challenge in conscious, unrestrained rats. Rats bearing carotid and jugular cannulae received a bolus of saline vehicle or 10 μg of NST via carotid catheter followed by infusion of saline or NST (1 μg/min). At 1, 10, and 30 min following a bolus of glucose (1 g/kg body wt ip), blood was drawn via jugular catheter for measurement of blood glucose and insulin levels. NST significantly delayed the clearance of glucose (A and B) and inhibited insulin release (C and D). *P < 0.05, **P < 0.01 vs. saline-treated control animals.
DISCUSSION
Neuronostatin is produced in δ-cells of the endocrine pancreas, and intraperitoneal injection of the peptide led to an induction of c-Jun in the islets of Langerhans in a pattern consistent with the activation of pancreatic α-cells (17). Here, we report that neuronostatin stimulates glucagon mRNA accumulation and release while inhibiting glucose-stimulated insulin secretion in primary rat islets. The effect of neuronostatin to inhibit high-glucose-stimulated-insulin release is likely secondary to a direct effect on the α-cell (or another cellular component of the islet), since neuronostatin failed to alter insulin release in the immortalized β-cell line INS 832/13, yet it significantly increased glucagon release from both αTC1-9 cells and isolated rat islets. Multiple reports have described the unique interactions between β- and α-cells in islets and that insulin and glucagon, respectively, and somatostatin from δ-cells, can act in an autocrine and paracrine fashion to regulate their own secretion and the secretion of the other hormones (3, 5, 8, 13, 15). For example, insulin is known to be a potent inhibitor of glucagon release (3, 7, 14). It has also been observed that FACS-purified β-cells secrete significantly less insulin than whole islets (∼1/30th) when exposed to high glucose alone, yet they regain full secretion potential when reconstituted with purified α-cells (15, 22). Thus a full secretory response from β-cells in the presence of physiological levels of glucose depends on intraislet communication with the resident α-cells. On the basis of these findings, it is tempting to speculate that neuronostatin may regulate insulin release as a result of its direct action on α cells.
Neuronostatin has been shown to signal via a PKA-dependent mechanism in murine cardiomyocytes (6, 21), and our data indicate that neuronostatin initiates a similar signaling pathway in αTC1-9 cells. On the basis of this signaling cascade, we hypothesized that the neuronostatin receptor was a G protein-coupled receptor (GPCR), in particular the orphan GPCR GPR107 (26), and we have confirmed GPR107 expression by αTC1-9 cells (26). Because neuronostatin treatment led to an increase in phosphorylated PKA, we reasoned that the peptide, through an interaction with GPR107, could activate Gs, thus leading to the formation of cAMP. However, neuronstatin failed to alter cAMP levels in either isolated islets or immortalized α- or β-cell lines, indicating that neuronostatin led to the activation of PKA via an alternative pathway. Potential alternative pathways include RhoA (18), the NF-κB pathway (27), or the heterotrimeric protein Gα13 (12). Future studies will investigate the possible importance of those alternative pathways in the action of neuronostatin to increase PKA phosphorylation in α-cells.
In these studies, relatively high concentrations (100 or 1,000 nM) of neuronostatin were required to elicit significant changes in vitro. Although it is impossible to know the exact number of molecules of neuronostatin required to initiate intracellular changes in a single cell, the concentrations of peptide used in these studies likely are physiologically relevant. We have previously reported that male pancreas tissue (endocrine and exocrine pancreas combined) contains ∼35.6 ng/g neuronostatin (17). The concentration of neuronostatin in the pancreas is therefore ∼2.5 μmol when adjusted for islet weight (1–2% of total pancreas weight), since neuronostatin is produced exclusively in the pancreatic δ-cells. Thus, the concentrations used in these studies are far less than the concentration of neuronostatin that is normally present in healthy rat endocrine pancreas tissue. Furthermore, it has been observed that similar concentrations of somatostatin are required to elicit significant effects on glucagon secretion in vitro (5).
Perspectives
Are the actions of neuronostatin in the islet physiologically relevant? Any attempt to compromise the production of neuronostatin would affect somatostatin production as well; thus, any investigation into the physiological relevance of neuronostatin must utilize passive neutralization or, preferably, compromise of the neuronostatin receptor. We currently are developing a strategy to compromise GPR107 production uniquely in α-cells of the islet. Once confirmed, this approached will be utilized in future studies to determine the importance of neuronostatin in the physiological mechanisms controlling glucose homeostasis. However, our results suggest that neuronostatin may play a physiologically relevant role in glucose homeostasis, since our in vivo experiments revealed an effect of the peptide on glucose-stimulated insulin secretion and glucose clearance. These effects appear to be the result of a direct action of neuronostatin on α-cells. The exact mechanism by which the α-cell communicates with the β-cell has been speculated upon by others but remains undetermined. The results presented here suggest that, in addition to effects in central nervous system and the heart, neuronostatin may also contribute to the physiological control of glucose homeostasis through intraislet actions of the endocrine cells in a paracrine manner attenuating insulin secretion from β-cells.
GRANTS
This work was supported by NIH Grants HL-06623 to W. K. Samson and DK-052194 to J. A. Corbett.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S.S., M.M.E., W.K.S., J.A.C., and G.L.C.Y. conception and design of research; A.S.S., M.M.E., W.K.S., and G.L.C.Y. performed experiments; A.S.S., M.M.E., W.K.S., and G.L.C.Y. analyzed data; A.S.S., M.M.E., W.K.S., J.A.C., and G.L.C.Y. interpreted results of experiments; A.S.S., M.M.E., and G.L.C.Y. prepared figures; A.S.S. and G.L.C.Y. drafted manuscript; A.S.S., M.M.E., W.K.S., J.A.C., and G.L.C.Y. approved final version of manuscript; M.M.E., W.K.S., J.A.C., and G.L.C.Y. edited and revised manuscript.
REFERENCES
- 1.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179: 77–79, 1973 [DOI] [PubMed] [Google Scholar]
- 2.Chambers KT, Unverferth JA, Weber SM, Wek RC, Urano F, Corbett JA. The role of nitric oxide and the unfolded protein response in cytokine-induced beta-cell death. Diabetes 57: 124–132, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28: 84–116, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Harms PG, Ojeda SR. A rapid and simple procedure for chronic cannulation of the rat jugular vein. J Appl Physiol 36: 391–392, 1974 [DOI] [PubMed] [Google Scholar]
- 5.Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson IC, Low MJ, et al. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 58: 403–411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua Y, Ma H, Samson WK, Ren J. Neuronostatin inhibits cardiac contractile function via a protein kinase A- and JNK-dependent mechanism in murine hearts. Am J Physiol Regul Integr Comp Physiol 297: R682–R689, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol 5: 330–335, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Jo J, Choi MY, Koh DS. Beneficial effects of intercellular interactions between pancreatic islet cells in blood glucose regulation. J Theoret Biol 257: 312–319, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Kelly CB, Blair LA, Corbett JA, Scarim AL. Isolation of islets of Langerhans from rodent pancreas. Methods Mol Med 83: 3–14, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Ji G, Neugebauer V. Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J Neurosci 31: 1114–1127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinnon CM, Ravier MA, Rutter GA. Foxo1 is required for the regulation of preproglucagon gene expression by insulin in pancreatic αTC1-9 cells. J Biol Chem 281: 39358–39369, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Niu J, Vaiskunaite R, Suzuki N, Kozasa T, Carr DW, Dulin N, Voyno-Yasenetskaya TA. Interaction of heterotrimeric G13 protein with an A-kinase-anchoring protein 110 (AKAP110) mediates cAMP-independent PKA activation. Curr Biol 11: 1686–1690, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Orci L, Baetens D, Rufener C, Amherdt M, Ravazzola M, Studer P, Malaisse-Lagae F, Unger RH. Hypertrophy and hyperplasia of somatostatin-containing D-cells in diabetes. Proc Natl Acad Sci USA 73: 1338–1342, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oskarsson PR, Lins PE, Ahre B, Adamson UC. Circulating insulin inhibits glucagon secretion induced by arginine in type 1 diabetes. Eur J Endocrinol 142: 30–34, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Pipeleers D, in't Veld PI, Maes E, Van De Winkel M. Glucose-induced insulin release depends on functional cooperation between islet cells. Proc Natl Acad Sci USA 79: 7322–7325, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salazar NC, Chen J, Rockman HA. Cardiac GPCRs: GPCR signaling in healthy and failing hearts. Biochim Biophys Acta 1768: 1006–1018, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samson WK, Zhang JV, Avsian-Kretchmer O, Cui K, Yosten GLC, Klein C, Lyu RM, Wang YX, Chen XQ, Yang J, Price CJ, Hoyda TD, Ferguson AV, Yuan XB, Chang JK, Hsueh AJ. Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J Biol Chem 283: 31949–31959, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sriwai W, Zhou H, Murthy KS. Gq-dependent signaling by the lysophosphatidic acid receptor LPA3 in gastric smooth muscle: reciprocal regulation of MYPT1 phosphorylation by Rho kinase and cAMP-independent PKA. Biochem J 411: 543–551, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unger RH, Dobbs RE, Orci L. Insulin, glucagon, and somatostatin secretion in the regulation of metabolism. Annu Rev Physiol 40: 307–343, 1978 [DOI] [PubMed] [Google Scholar]
- 20.Unger RH, Orci L. Possible roles of the pancreatic D-cell in the normal and diabetic states. Diabetes 26: 241–244, 1977 [DOI] [PubMed] [Google Scholar]
- 21.Vainio L, Perjes A, Ryti N, Magga J, Alakoski T, Serpi R, Kaikkonen L, Piuhola J, Szokoki I, Ruskoaho H, Kerkela R. Neuronostatin, a novel peptide encoded by somatostatin gene, regulates cardiac contractile function and cardiomyocyte survival. J Biol Chem 287: 4572–4580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JL, Corbett JA, Marshall CA, McDaniel ML. Glucose-induced insulin secretion from purified beta-cells. A role for modulation of Ca2+ influx by cAMP- and protein kinase C-dependent signal transduction pathways. J Biol Chem 268: 7785–7791, 1993 [PubMed] [Google Scholar]
- 23.Weber SM, Scarim AL, Corbett JA. Inhibition of IFN-γ-induced STAT1 activation by 15-deoxy-Δ12,14-prostaglandin J2. Am J Physiol Endocrinol Metab 284: E883–E891, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Yosten GLC, Pate AT, Samson WK. Neuronostatin acts in brain to biphasically increase mean arterial pressure through sympatho-activation followed by vasopressin secretion: the role of melanocortin receptor. Am J Physiol Regul Integr Comp Physiol 300: R1194–R1199, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yosten GLC, Samson WK. The melanocortins, not oxytocin, mediate the anorexigenic and antidipsogenic effects of neuronostatin. Peptides 31: 1711–1714, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yosten GLC, Redlinger LJ, Samson WK. Evidence for an interaction of neuronostatin with the orphan G protein-coupled receptor GPR107. Am J Physiol Regul Integr Comp Physiol 303: R941–R949, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89: 413–424, 1997 [DOI] [PubMed] [Google Scholar]