Abstract
Nephron number (Nglom) and size (Vglom) are correlated with risk for chronic cardiovascular and kidney disease and may be predictive of renal allograft viability. Unfortunately, there are no techniques to assess Nglom and Vglom in intact kidneys. This work demonstrates the use of cationized ferritin (CF) as a magnetic resonance imaging (MRI) contrast agent to measure Nglom and Vglom in viable human kidneys donated to science. The kidneys were obtained from patients with varying levels of cardiovascular and renal disease. CF was intravenously injected into three viable human kidneys. A fourth control kidney was perfused with saline. After fixation, immunofluorescence and electron microscopy confirmed binding of CF to the glomerulus. The intact kidneys were imaged with three-dimensional MRI and CF-labeled glomeruli appeared as punctate spots. Custom software identified, counted, and measured the apparent volumes of CF-labeled glomeruli, with an ∼6% false positive rate. These measurements were comparable to stereological estimates. The MRI-based technique yielded a novel whole kidney distribution of glomerular volumes. Histopathology demonstrated that the distribution of CF-labeled glomeruli may be predictive of glomerular and vascular disease. Variations in CF distribution were quantified using image texture analyses, which be a useful marker of glomerular sclerosis. This is the first report of direct measurement of glomerular number and volume in intact human kidneys.
Keywords: chronic kidney disease, magnetic resonance imaging, cationized ferritin
chronic kidney disease (CKD) affects 1 in 10 American adults (10) and its prevalence is rapidly increasing. In the United States alone, ∼90,000 patients will die from end-stage renal disease (ESRD) each year, and over $40 billion is spent on treatment of CKD and ESRD (23). While most of the burden of CKD is focused on ESRD, studies have shown that even the mild stages of CKD are not benign and result in both higher risks of cardiovascular disease (17) and increased health care costs (28). It is therefore critical to develop diagnostic tools to detect and monitor kidney disease at early stages.
It is now well-known that lower nephron number is associated with higher susceptibility to kidney disease and hypertension. It is likely that a low number or a loss of nephrons leads to glomerular hypertrophy and hyperfiltration. The theory behind this is that a kidney with a low number of nephrons must filter the same amount of fluid per unit time as a kidney with a high nephron number. Therefore, to maintain an acceptable total filtration surface area and a constant glomerular filtration rate (GFR), each glomerulus in a poorly endowed kidney must increase in surface area and filter more fluid than its healthy counterpart. While this likely compensatory mechanism may maintain homeostasis in the early stages, it complicates the use of GFR measurements for early detection of kidney diseases involving changes in nephron number and glomerular volume (29). Ideally, clinical measurements of glomerular number and size could be used to detect and monitor loss of nephrons and glomerular hypertrophy in patients at risk of CKD, such as those with diabetes and hypertension.
Currently, measurements of total nephron number (Nglom) and mean glomerular volume (Vglom) require histological sectioning, quantitation of a fraction of a kidney and extrapolation to a total glomerular number and volume (8, 15). Studies employing these methods have provided significant insights into renal physiology and the role of Nglom and Vglom in both kidney-specific and systemic diseases (7, 9, 19, 22, 25). Unfortunately, these methods require resection and destruction of the kidney. At this time, there are no methods available for direct measurements of Nglom, Vglom, or protein leakage of individual glomeruli in vivo. Such methods would provide a window for early intervention and may also prove vital in assessing renal allograft viability before transplant.
One potential strategy for noninvasive measurements of glomerular morphology is the development of a magnetic resonance imaging (MRI) contrast agent that specifically targets the glomerular basement membrane (GBM). We and others recently showed that the cationized ferritin (CF) nanoparticle can be used as an intravenous MRI contrast agent to detect functional structures in fenestrated organs, including kidney glomeruli (1–6, 11, 18, 26). The application of CF in the kidney is based on electrostatic binding of CF to anionic proteoglycans of the GBM, allowing each perfused glomerulus in the kidney to be located, counted, and measured using MRI. This method has also been used to detect glomerular permeability to macromolecules in a rat model of focal and segmental glomerulosclerosis (5). Three-dimensional (3D) MRI, after CF injection, also enables novel measurements of glomerular size distributions and pathologies in the kidney (3, 18). Because ferritin is naturally occurring in mammalian tissue, CF may be relatively nontoxic (1).
Here, we demonstrate that CF can be used as an MRI contrast agent to visualize, count, and measure the size of glomeruli in whole excised human kidneys. We further demonstrate MRI-detectable changes in glomerular and vascular morphology with renal vascular disease and hypertension.
MATERIALS AND METHODS
Sample Preparation
Cationized horse spleen ferritin (CF; molecular weight = 475 kDa) was synthesized according to Danon et al. (16). Four human kidneys were obtained at autopsy through a donor network (The International Institute for the Advancement of Medicine, Edison, NJ) after Institutional Review Board approval and informed consent. The kidneys were deemed unsuitable for transplant by the Organ Procurement Officer. A request and informed consent for research were obtained only after the kidneys were deemed unsuitable for transplant. The kidneys were infused at autopsy with heparinized saline and stored in University of Wisconsin preservation solution and, within 24 h, the renal artery was catheterized and the kidneys were perfused with 120 ml of PBS. Three kidneys (hereafter referred to as kidneys CF1, CF2, and CF3) were perfused with 300 mg of CF in PBS per kg by kidney weight. The kidneys were then perfused with 120 ml of PBS to remove any unbound CF and then perfused with 10% neutral buffered formalin. One kidney received no CF (as a control), but received the same number of PBS and formalin perfusions. A minimum of three biopsies (∼1 mm3) were taken from random locations in the cortex from each kidney and prepared for immunofluorescence (IF) and transmission electron microscopy (detailed below). Targeted biopsies were also taken from the cortex of the CF2 kidney. The reasons for this are discussed below. All kidneys were stored in 10% neutral buffered formalin at 4°C.
Before imaging, the CF-labeled kidneys and the unlabeled control kidney were removed from formalin and washed three times in 500 ml PBS (for a total of 1.5 l of PBS) over 24 h. The kidneys were first imaged using a Bruker 7T/35 MRI scanner and a 72-mm quadrature transmit/receive radio frequency coil (Bruker, Billerica, MA). The whole kidneys were imaged in PBS in a sealed plastic container to keep the tissue hydrated. A T2*-weighted (TE/TR = 20/39 ms) 3D gradient echo fast low angle shot (FLASH) sequence was used to image the entire kidney. MR images were acquired with 3D acquisition using a 117 × 117 × 117 μm3 resolution (field of view = 6 × 6 × 10.5 cm3, matrix size = 512 × 512 × 896, 5 averages, total scan time = 10 h 39 min/kidney).
After 7T MRI, IF, and electron microscopy, kidneys were imaged using a Siemens 3T Skyra MRI scanner and a 32-channel transmit/receive radio frequency head coil (Siemens, Erlangen, Germany). Whole kidneys were imaged in PBS in a sealed plastic container. A T2*-weighted (TE/TR = 20/32 ms) 3D gradient recalled echo (GRE) sequence was used to image the kidney. MR images were acquired with a resolution of 270 × 270 × 540 μm3 (field of view = 12 × 4.9 × 7.8 cm, matrix size = 448 × 182 × 144, scan time = 4.2 h in the CF-labeled kidney; FOV = 12 × 5.4 × 6.9 cm, matrix size = 448 × 200 × 128, scan time = 4 h in the unlabeled control kidney). A T2*-weighted (TE/TR = 83/2,000 ms, FA = 150, matrix size = 320 × 120 × 51, spatial resolution = 400 × 400 × 130 μm3, 24 averages, total scan time = 41.6 min) half fourier acquisition single shot turbo spin echo (HASTE) pulse sequence was also used to acquire an image of the entire control kidney to distinguish cortex and medulla. The CF-labeled kidneys did not require HASTE imaging to distinguish cortex from medulla. One slice was selected from the 3D reconstructed image data and corrected for inhomogeneities by subtracting a low-pass filtered version of the image (50 × 50 Gaussian kernel with SD = 20 voxels). The slice image intensities were normalized to a range of 0–1 and the cortex and medulla were manually segmented (directly from the T2*-weighted GRE for the CF-labeled kidney and from the coregistered HASTE T2-weighted image for the naive control). The T2-weighted HASTE image was linearly registered to the gradient echo image using FSL FLIRT (21).
Transmission Electron Microscopy
Approximately 1 mm3 pieces of tissue were collected from the cortex of each kidney after perfusion of formalin and immediately placed in 2% glutaraldehyde/0.1 M cacodylate solution for overnight fixation. Samples were dehydrated in graded ethanol solutions ranging from 70 to 100% and then infiltrated with and embedded in epoxy resin. The resulting blocks were cut into 70-nm sections and stained with 0.2% osmium tetroxide. Osmium tetroxide precipitates were digested with 1% periodic acid for 12 min. A Philips CM12 transmission electron microscope was used to collect images at ×53 k magnification with an accelerating voltage of 80 kV.
IF
Frozen tissue.
We performed IF microscopy to confirm labeling of the GBM with CF. Because CF is a protein, it is readily detected with IF. Several ∼1-mm3 tissue samples were taken from each kidney after perfusion. The biopsies were placed in 10% neutral buffered formalin for 4 h and stored in PBS overnight. The samples were cryoprotected in 15% sucrose followed by 30% sucrose and then rapidly frozen to −80°C and cut into 35-μm sections. The sections were washed in PBS, permeabilized with 0.5% Triton X-100 (Sigma, St. Louis, MO), incubated in rabbit anti-horse spleen ferritin (Sigma), immunostained with an Alexa 594 goat anti-rabbit secondary antibody and 4′,6-diamidino-2-phenylindole (Invitrogen, Carlsbad, CA), and imaged on a Zeiss 710 laser-scanning confocal microscope.
Formalin-fixed tissue.
A second round of IF was performed in formalin-fixed tissue after MRI and stereological analysis. Two approaches were used for tissue sampling and paraffin embedding: 1) targeted sampling and 2) random sampling. Targeted sampling, based on MRI, was used for analysis of the CF2 kidney to extract 2-mm3 samples from areas of cortex with good ferritin labeling and areas of cortex with poor ferritin labeling. For CF1 and CF3, similar size blocks were randomly cut from the cortex of the formalin-fixed tissue. All formalin-fixed tissue samples were embedded in paraffin. Three serial sections (4-μm-thick each) were cut from each block. The first section was used for IF while the second section was used for periodic acid Schiff (PAS) staining. For IF, sections were rehydrated in 100% ethanol (5 min), 70% ethanol (5 min), and then PBS (5 min). Sections were then subjected to an antigen retrieval step, which involved immersion in Target Retrieval Solution (DAKO, S1699) for 20 min at a controlled temperature of 90°C in a DAKO PT Link PT10126 system. After being cooled, slides were washed in buffer (DAKO, K8007) and then in 1% filtered BSA in PBS for 1 h. Sections were then immunostained using an antibody against Wilms' Tumor-1 (WT1) antigen (monoclonal mouse anti-human WT1-DAKO, M356101, clone 6F-H2), a well-known podocyte marker that allowed us to confirm glomerular localization and the same rabbit anti-horse spleen ferritin (Sigma), as previously described. After 1-h incubation at room temperature, sections were labeled with goat anti-mouse Alexa 488 (1:2,000; Invitrogen A-11008) and goat anti-rabbit Alexa 555 (1:1,000; Invitrogen A-11001) for another hour at room temperature under light protection. Finally, Prolong Gold with DAPI (Invitrogen P-36931; anti-fade mounting medium) was used for permanent coverslipping and left for 24 h. Confocal images were taken on a Leica SP5 laser confocal microscope (Leica MicroSystems, Manheim, Germany). Images were obtained using a ×40 objective lens (1.25 NA), using sequential imaging for 488 nm, 555 nm and UV light.
Stereology and Histopathology
The MRI-based measurements of Nglom and Vglom were validated (after MRI) using the physical disector/fractionator design-based stereological method described by Cullen-McEwen et al. (12–14). In brief, kidneys were weighed and a series of sampling and subsampling steps were applied to select a systematic uniform random sample of 10–15 tissue blocks from the cortex. These blocks were embedded in glycolmethacrylate (Kulzer GmbH), serially sectioned at 20 μm, and every 10th and 11th section pair was collected and stained with PAS. The section pairs were viewed with a pair of light microscopes modified for projection. Glomeruli present in one section (the reference section) but not in the paired section (look-up section) were counted according to the disector principle. At the same time glomeruli were counted with the disector principle, stereological grid points overlying glomeruli were counted and used to estimate Vglom.
PAS-stained glycolmethacrylate sections were assessed by a specialist renal pathologist (JD). Sections from ∼10 blocks per kidney were examined. Seventy two, 61, and 66 glomeruli were assessed in kidneys CF1, CF2, and CF3, respectively.
Image Processing
Nglom and the individual volumes of all glomeruli were calculated from the MR images using an in-house algorithm written for MATLAB (The Mathworks, Natick, MA). First, a Hessian for each voxel of the raw MRI volume (in 3D) was used to flag candidate glomerular regions and discern glomeruli in close proximity to each other. This step populated candidate regions and, as a result, dramatically reduced the data size. Five features, including average intensity, divergence, region volumes, shape index, and the Laplacian of Gaussian, were extracted to remove false positive glomeruli. With those features, a Gaussian mixture model clustering algorithm was used to group candidate regions (black dots) into several clusters throughout the volume. Next, all clusters were overlain individually onto the original MRI volume, and clusters that did not identify populations of glomeruli were identified manually and eliminated from further analyses. The remaining black dots were counted as glomeruli and their sizes were measured based on the number of voxels comprising each dot. Clusters of black dots in the control kidney of similar locations and appearance to those of CF-labeled kidneys were counted as false glomeruli to quantify the negative contribution of blood artifact.
To validate the algorithm, we counted, by eye, the number of labeled glomeruli in six 35-mm2 sections of the original MRI volume. The same 35-mm2 sections were then compared with the glomeruli in the same images identified by the algorithm. These glomerular counts were compared using a paired Student's two-tailed t-test and were not statistically different (P > 0.05) and were well correlated (R2 = 0.88).
Image Texture Analysis
We performed image texture analysis to assess the pattern of glomerular labeling in the MR images of CF-labeled kidneys. We manually drew 16 lines (each 64 voxels in length) through the cortex of each kidney in 7T MRI volumes (all of which have the same FOV and matrix size) in ImageJ and plotted the signal line profile. The location of each line profile was randomly chosen and each profile was oriented through glomeruli that appeared to originate from the same interlobular artery. The spatial power spectrum from each line profile was calculated based on the Fast Fourier Transform in MATLAB. Spatial power spectra of CF-perfused and control kidneys were compared at each spatial frequency using Student's two-tailed t-tests (α = 0.05).
Statistics
Statistical analyses were calculated in MATLAB by either two-sample or paired two-tailed Student's t-tests to test the hypothesis that the mean difference between groups is zero (α = 0.05).
RESULTS
To investigate the use of CF as a glomerulus-specific MRI contrast agent in humans, CF was injected into the renal artery of three viable (but untransplantable) human donor kidneys within 24 h of resection. Saline was injected into one kidney instead of CF as a control. Donor data were investigated to establish possible reasons for any variability in nephron number and CF accumulation in the kidneys measured by MRI (see Pathology section). These data are shown in Table 1. Notably, the donor of kidney CF2 suffered from severe, untreated hypertension, and the donor of kidney CF1 suffered from mild, treated hypertension.
Table 1.
Clinical data, stereological estimates, and MRI-based data
|
Stereology |
MRI |
% Difference Between Methods |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, yr | Gender | Race | Cause of Death | Initial Creatinine, mg/dl | Initial GFR, ml/min* | Peak Creatinine, mg/dl | Last Creatinine, mg/dl | Nglom (×106) | Vglom (×10−3 mm3) | Kidney Wt, g | aNglom (×106) | Median aVglom (×10−3 mm3) | aVglom Skewness | Nglom % | Vglom % | |
| CF1 | 68 | M | C | Cardiac arrest | 1.6 | 43 | 2.9 | 2.5 | 1.13 | 5.01 | 167 | 1.27 | 4.8 | 1.5 | 12 | 4 |
| CF2 | 45 | F | AA | Hypertensive stroke | 1.1 | 65 | 2.7 | 2.7 | 0.74 | 4.68 | 110 | 0.92 | 3.2 | 2.2 | 25 | 32 |
| CF3 | 37 | F | C | Cardiac arrest | 1.9 | 30 | 6.05 | 6.05 | 1.46 | 2.82 | 186 | 1.52 | 3.2 | 1.7 | 4 | 13 |
| Ctrl | 55 | F | C | Stroke | 1.6 | 45 | 1.8 | 1.2 | — | — | — | 0.057 | 1.6 | 3.2 | — | — |
Stereological estimates and magnetic resonance imaging (MRI)-based measurements for total nephron number (Nglom) and mean glomerular volume (Vglom) follow a similar trend, although differences between the 2 techniques are apparent. It is important to note that, due to the difference in the number of glomeruli sampled using each method and the heterogeneous nature of human kidneys, we do not expect these 2 measurements to be in perfect agreement.
MDRD Study Equation was used to calculate glomerular filtration rate (GFR).
MRI
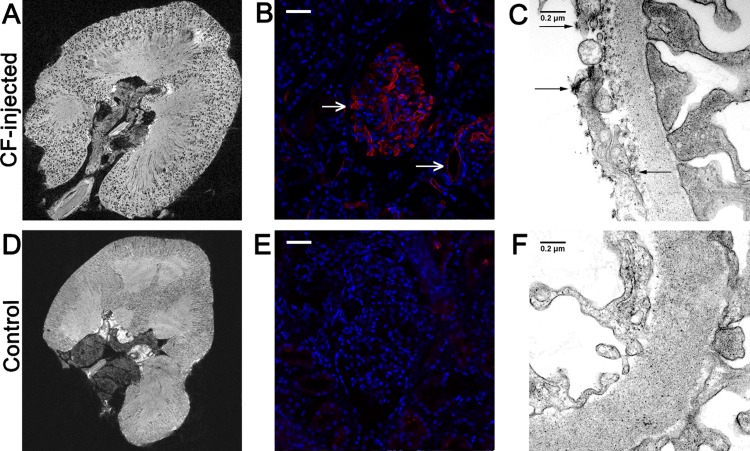
We imaged the intact, fixed donor kidneys on a 7T MRI scanner using a 3D GRE pulse sequence. As shown in Fig. 1A, the MR images exhibited dark spots throughout the renal cortex of the CF-labeled kidneys. Each dark spot in the cortex is ∼50–80% darker than the surrounding cortex. These dark spots were not present in the unlabeled control kidney (Fig. 1D). The punctate darkening of each glomerulus was caused by the accumulation of the superparamagnetic CF in the GBM. The labeled glomeruli defined the boundary between the cortex and medulla and revealed the individual lobes and papillas of the kidney. The specific binding of CF to the glomerulus was confirmed with IF microscopy (Fig. 1, B, E). Transmission electron microscopy showed CF bound to the GBM as well as to the glycocalyx of glomerular endothelial cells (Fig. 1C). Transmission electron microscopy revealed no CF in the control kidney (Fig. 1F).
Fig. 1.
Intravenously injected cationized ferritin (CF) specifically labels glomeruli in perfused human donor kidneys, making them visible with 7T magnetic resonance imaging (MRI). A 2D slice of a 3D MR image of a CF-labeled human kidney contains punctate, hypointense spots throughout the cortex (A). Images of an unlabeled control kidney contained no hypointense spots but did show minimal image darkening caused by residual blood (D). Immunofluorescence confirmed the accumulation of CF (red) in glomeruli (B, top arrow) and leakage of CF into tubules of CF-perfused kidneys (B, bottom arrow). Unlabeled glomeruli of the control kidney were clear of CF (E). Cell nuclei are shown in blue (DAPI). Transmission electron microscopy (TEM) confirmed the accumulation of CF in the glomerular basement membrane (GBM) and endothelial glycocalyx (arrows, C). The glomerular capillary walls of the unlabeled control kidney were clear of CF (F). White scale bars = 50 μm. Black scale bars = 0.2 μm.
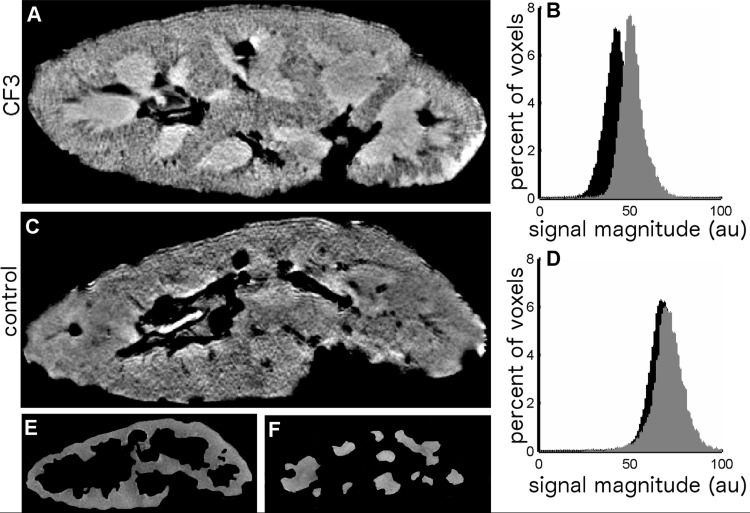
To assess the possibility of detecting glomeruli in typical clinical MRI systems, CF-labeled kidneys were also imaged at lower resolution on a clinical 3T MRI scanner (Fig. 2). While individual glomeruli were not visible at the lower resolution of 3T MRI, the average image magnitude in the cortex of CF-labeled kidneys was ∼20% lower than in the medulla. Minimal difference between image magnitude in the cortex and medulla (<2%) was seen in the unlabeled control kidney. Thus, CF labeling can be detected with typical clinical MRI systems by measuring the ratio of cortical to medullary image intensity.
Fig. 2.
CF3 (A) and unlabeled control (C) kidneys were imaged at 3T using T2*-weighted and T2-weighted MRI. While individual glomeruli are not visible with these imaging parameters, the labeling of glomeruli in the CF3 kidney is evident in histograms of T2*-weighted signal magnitude in the cortex compared with those of the medulla. A broad (∼20%) downward shift in the cortical T2*-weighted signal magnitude with respect to the medulla was observed in the CF3 kidney (B), while minimal (∼2%) differences in cortical and medullary signals were seen in the naive control kidney (D). Regions of interest (ROIs) for the T2*-weighted control images were defined using the half fourier acquisition single shot turbo spin echo (HASTE; T2-weighted) MR images, which provided better contrast between the cortex and the medulla. Shown are examples of the cortical (E) and medullary (F) ROIs defined from the 3D HASTE MR images of the unlabeled control kidney. In addition to separating the cortex from the medulla, these ROIs eliminate large blood vessels, calyxes, and the pelvis from the analysis.
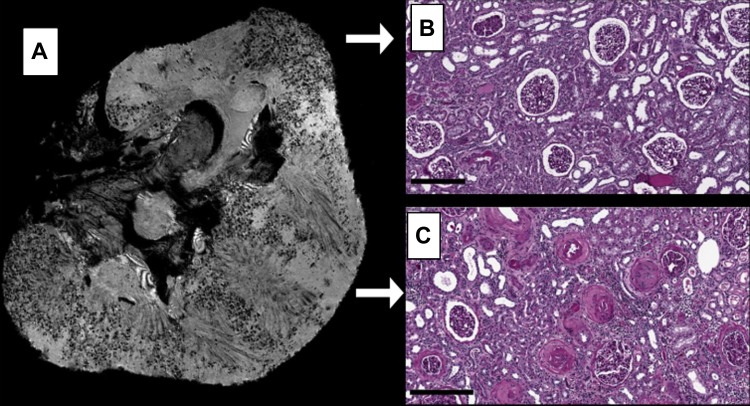
Leakage of CF through the GBM into the proximal tubule was visible in 7T MRI and IF images of kidneys CF1 and CF2 (Fig. 3A, Fig. 4G, Fig. 5, A and D, and magnified MRI panels of Fig. 6). The leakage of CF past the glomerular capillary wall appeared as diffuse darkening of the MR image, similar to leakage previously observed in a rat model of focal and segmental glomerulosclerosis (5). CF was visible by IF in the tubules (Fig. 1B, lower arrow) and Bowman's capsules (Fig. 4N) of kidneys CF1 and CF2, consistent with the MRI-visible CF leakage in kidneys CF1 and CF2. Based on MRI (Fig. 3A) and IF (Fig. 4, E–H), large regions of the cortex of kidney CF2 lacked CF-labeled glomeruli. Histopathological examination of this kidney suggested that this was due to severe glomerular and arteriolar sclerosis that prevented perfusion of those areas of the cortex (Fig. 3). In comparison, the MR image darkening in glomeruli of kidney CF3 (histopathologically deemed the healthiest of the group) was punctate and appeared throughout the entire cortex of the kidney (see Fig. 5G), suggesting minimal protein leakage and vascular damage. This was supported by IF (Fig. 4, A–D).
Fig. 3.
7T MR image of the CF2 kidney revealed large regions of cortex lacking CF-labeled glomeruli (A). Histopathology performed on the cortical regions that lacked CF-related signal darkening revealed severe sclerosis of glomeruli and arterioles (C), which likely prevented perfusion of glomeruli in these regions. The regions of the CF2 cortex that did have CF-labeled glomeruli showed mild sclerosis. These glomeruli and arterioles appeared substantially healthier than those located in regions that lacked CF-related contrast (B). Scale bars = 400 μm.
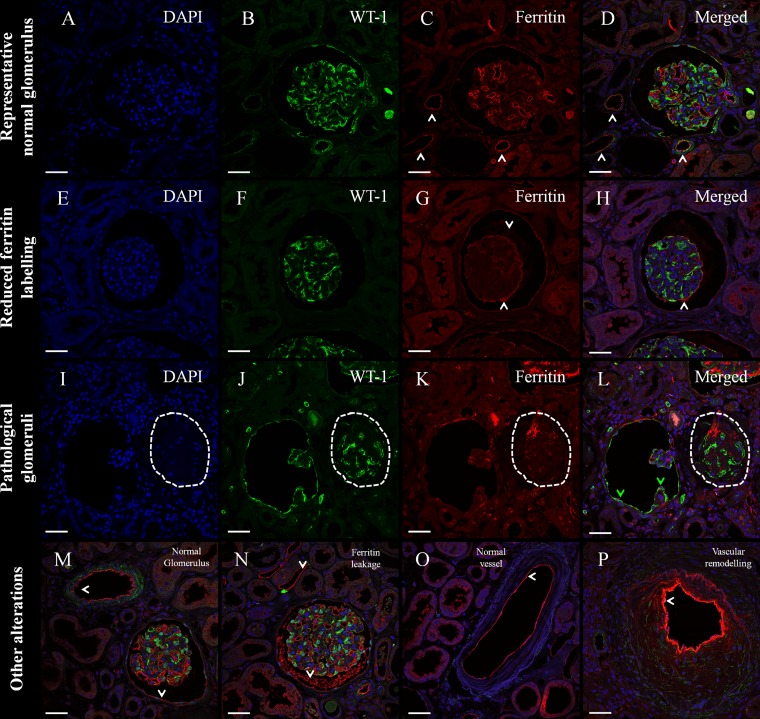
Fig. 4.
Immunofluorescence of intravenous CF labeling and histopathology in human donor kidneys. Triple immunofluorescence with an antibody against Wilms Tumor 1 antigen (WT-1) as a marker of healthy mature podocytes (green), ferritin as a marker of injected CF (red), and DAPI as a nuclear marker (blue). A–D: normal glomerulus from patient CF3 with strong podocyte labeling and strong ferritin labeling on the GBM and fine ferritin labeling in peritubular capillaries and Bowman's capsule. E–H: glomerulus with reduced ferritin labeling in the tuft, and ferritin signal on Bowman's space (very frequent in CF2, regular in CF1, and very rare in CF3). I–L: 2 pathological glomeruli. On the left, a glomerulus with expanded Bowman's space, collapsed tuft, and WT-1+ cells on Bowman's capsule: all 3 features suggest an atubular glomerulus or obstructed collecting ducts. On the right (dashed ellipsis), a collapsed glomerular tuft without open capillaries and indirect signs of glomerulosclerosis. M: normal glomerulus (present in all subjects; very frequent in CF3). N: glomerulus with ferritin “leakage” into the Bowman's space (frequent finding in CF1 and CF2). O: normal vessel (frequent finding in CF3). P: vessel with vascular remodeling (very common in CF2, and sporadic finding in CF1). White arrowheads represent ferritin labeling outside the glomerular tuft; green arrowheads show WT-1 expression in cells on Bowman's capsule. Scale bars = 50 μm.
Fig. 5.
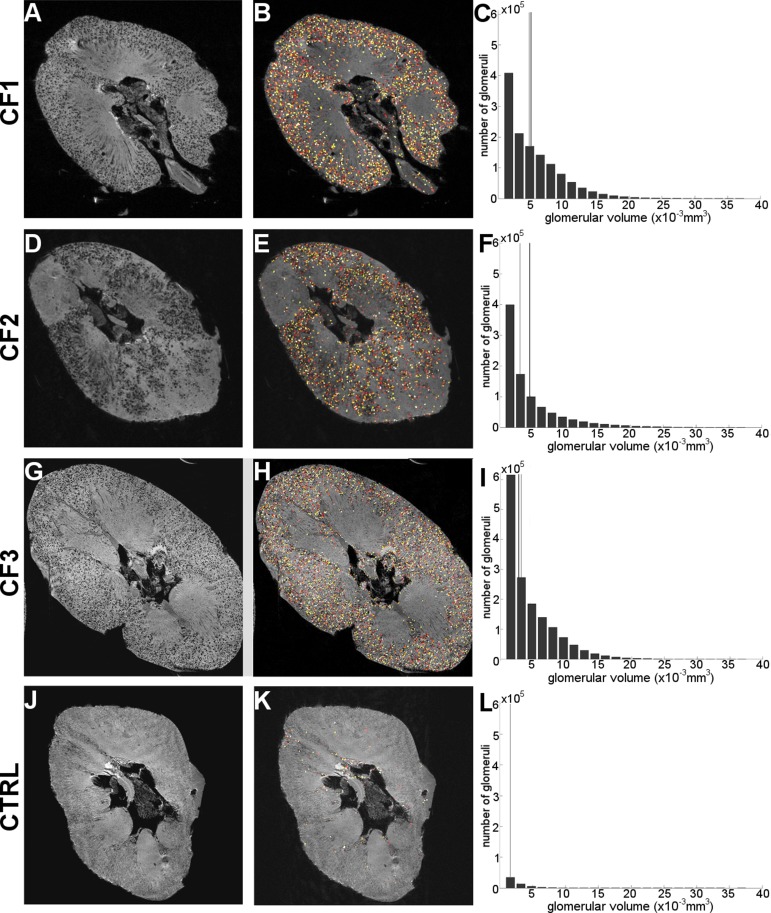
Glomeruli are made visible in all 3 CF-labeled kidneys (A, D, G) and the glomerular segmentation algorithm was able to identify, count, and measure the size of labeled glomeruli (B, E, H). Identified glomeruli are assigned an arbitrary color for visualization purposes in these panels. The majority of regions defined as glomeruli by the algorithm exist in the cortical and juxtamedullary regions of the representative slice. The control kidney (J, K) shows very few regions defined as “glomeruli”—most of which are likely attributed to residual blood. The MRI-measured apparent glomerular volume (aVglom) distribution for each kidney is shown, along with a gray line showing the median MRI-measured aVglom and a black line showing the Vglom estimate obtained using stereology (C, F, I, and L). Note that no stereological measurement is available for the control kidney.
Fig. 6.
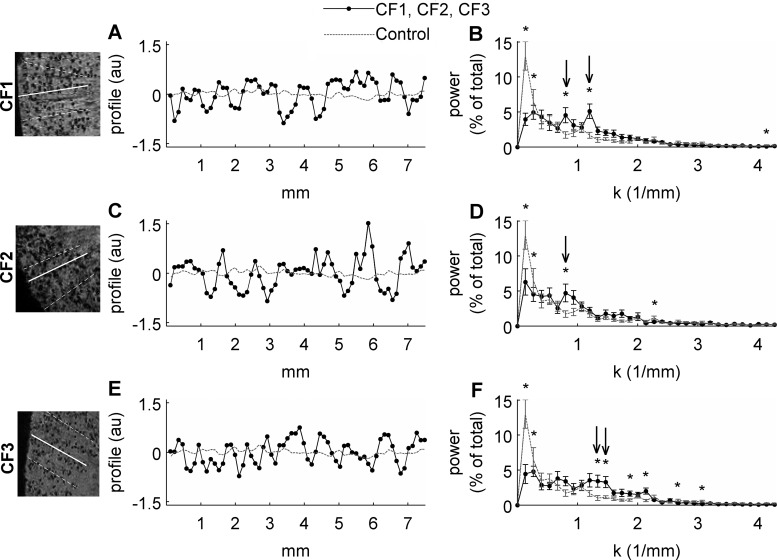
Line profiles (16 per kidney) were drawn through the cortex of each CF-labeled kidney in 7T MRI volumes (all of which have the same FOV and matrix size). Here, the line signal profiles of the solid white profiles are plotted (A, C, and E) and the mean power spectrum for the 16 line profiles is shown (B, D, and F). Black traces are data from CF-labeled kidneys and gray traces are data from the naive control. Arrows point to spatial spectral peaks of particular interest. Qualitatively, the line profiles for the CF-labeled kidneys appear different from one another, with the CF3 line profiles being mostly composed of relatively high-frequency oscillations, the CF2 oscillations being mostly composed of relatively low-frequency oscillations, and the CF1 kidney being composed of a mixture of high- and low-frequency components. The line profile signal changes associated with the appearance and disappearance of sites of CF accumulation in the CF1 kidney correspond to 2 spectral peaks; one at k = 0.8 mm−1 (4.5% of total signal) and one at k = 1.2 mm−1 (5% of total signal; B). The line profile signal changes associated with CF accumulation in the CF2 kidney correspond to only a low-frequency spatial signal oscillation at k = 0.8 mm−1 and account for 5% of the total signal along the line profiles (D). The line profile signal changes associated with the accumulation of CF in the CF3 kidney correspond only to high-frequency spatial oscillations between k = 1.2 and 1.5 mm−1, which account for 10% of the total signal along the line profiles (F). Histopathology showed that CF2 had substantial nephrosclerosis, CF1 had mild nephrosclerosis, and CF3 had minimal nephrosclerosis; therefore, the prominence of low-frequency spectral peaks may suggest the advancement of nephrosclerosis. Stars represent a statistically significant difference between the CF-labeled kidney and the naive control (P < 0.04) and arrows indicate peaks of particular interest. Error bars represent means ± 1 SD between power spectra of 16 randomly chosen line profiles.
IF
IF analysis for ferritin and WT-1 revealed that the CF1 kidney contained a large number of apparently healthy glomeruli, with moderate leakage of CF into Bowman's capsule. The CF2 kidney contained a large number of poorly labeled glomeruli (Fig. 4, E–H). CF2 also contained a large number of glomeruli with an expanded Bowman's space, collapsed glomerular tufts, and WT-1-positive cells—all features consistent with atubular glomeruli or obstructed collecting ducts (Fig. 4, I–L). There was heavy leakage of CF into Bowman's space and signs of vascular remodeling or thickening of the vessel wall (Fig. 4N). Signs of vascular remodeling (Fig. 4P) were particularly apparent in the regions of the CF2 kidney that lacked CF-related MRI signal changes. Interestingly, remodeling vessels appeared to accumulate a large amount of CF in this kidney, suggesting highly charged endothelial surfaces. The CF3 kidney was dominated by healthy glomeruli, with a large accumulation of CF in the GBM and light accumulation of CF in the peritubular capillaries and Bowman's capsules (Fig. 4, A–D).
Histopathology
Histopathology of kidney CF1 revealed four sclerotic glomeruli of the 72 examined. There was widespread patchy fibrosis and tubular dilation and atrophy with mild diffuse lymphohistiocytic leukocytic infiltration within the interstitium. The arteries were sclerotic with variable hyalinosis. Overall, there was minor nephrosclerosis and acute tubular injury.
In CF2, five totally sclerosed glomeruli of the 61 were examined and one with perihilar segmental sclerosis. In the regions of CF-labeled tissue (Fig. 3B), the interstitium had widespread mild, patchy fibrosis, tubules were mildly dilated and atrophic, and a moderate lymphohistiocytic infiltrate was present that included eosinophils. The arterioles were tortuous and showed marked hyalinosis with intimal sclerosis. Within the unlabeled regions of CF2, the degree and number of severely sclerotic glomeruli were striking (Fig. 3C). Vascular involvement was evident, with both the arteries and arterioles severely thickened. In some unlabeled areas, it was impossible to distinguish sclerotic glomeruli from obstructed arterioles. The tubules in the unlabeled areas were unaffected overall, but did occasionally contain cast material. We conclude that the observed lack of CF-labeled glomeruli in some regions of kidney CF2 was correlated with focal sclerosis and vascular damage at those locations.
Kidney CF3 had one sclerotic glomerulus of the 66 glomeruli examined, with no mesangial proliferation or segmental sclerosis within the glomeruli. The interstitium showed slight fibrosis and tubular atrophy with minimal lymphohistiocytic interstitial inflammatory infiltrate. The arteries were either normal or had mild sclerosis of the intima. The tubules were mildly dilated with scattered uromodulin casts. There were only very mild changes of acute tubular injury and very mild background nephrosclerosis.
Quantitative Morphology
We developed and applied custom software to measure glomerular number and individual glomerular volume from the MR images. The custom 3D image processing software identified (Fig. 5, B, E, H, K) and measured the size of labeled glomeruli (Fig. 5, C, F, I, L) in the MR images of CF-labeled kidneys.
The number of glomeruli identified in the MR images by the software yielded the total apparent number of glomeruli per kidney (aNglom). These data were compared with stereological estimates of Nglom (Table 1). Both MRI- and stereology-based measurements were consistent with the range of Nglom reported in the literature (24). The algorithm counted 0.057 × 106 false glomeruli in the one unlabeled control kidney, yielding an estimated false positive rate of the algorithm of ∼6%.
Using the same software, we estimated the median apparent glomerular volumes (aVglom) using the MR images and compared them with stereological estimates (Table 1). These median volumes are consistent with those reported in the literature (24). The MRI-based measurements were used to generate the glomerular size distribution for each CF-labeled kidney, which cannot be obtained with other techniques (Fig. 5, C, F, I, L). We observed a large number of glomeruli in these distributions with volumes of 2.4 × 10−3 mm3 or less. This was unexpected, because prior stereological estimates of glomerular volumes in human kidneys suggest that only ∼10% of the total number of glomeruli in a kidney should have volumes this small (data not shown). In MRI, these glomeruli represented ∼30% of the total number of glomeruli.
Image Texture Analysis
To detect morphological differences between MR images of CF-labeled donor kidneys, we performed image texture analysis (Fig. 6). This analysis consisted of spatial power spectra associated with line profiles randomly drawn in the cortex in the MR images. The line signal profiles in the CF1 kidney, which showed only mild nephrosclerosis, were composed of a mix of high- and low-frequency oscillations with CF-related spatial spectral peaks at k = 0.8 mm−1 (4.5% of total signal power) and k = 1.2 mm−1 (5% of total signal power). The line signal profiles in kidney CF2, histopathologically assessed as the least healthy kidney of the group, were composed of low-frequency CF-related oscillations corresponding to a CF-related spatial spectral peak at k = 0.8 mm−1, which accounted for 5% of the total signal power along the line profiles. The line signal profiles in CF3, defined by histopathology as the healthiest kidney of the group, demonstrated high-spatial frequency oscillations between k = 1.2 and 1.5 mm−1 and account for 10% of the total signal power along the line profiles.
DISCUSSION
This work demonstrates that individual glomeruli in human kidneys can be detected using intravenous injection of CF, followed by MRI. With this approach, the apparent number (aNglom) and volume (aVglom) of glomeruli can be measured in the whole kidney. We refer to these as “apparent” measurements of glomerular number and volume because they are based on detection of glomeruli by an exogenous agent and on a computer algorithm to measure the sites of agent accumulation. Nevertheless, this technique expands the number of glomeruli that can be practically sampled by many orders of magnitude; from hundreds of glomeruli using stereology to all functioning glomeruli in the kidney (of the order of 105-106 glomeruli). The MRI technique has potential for direct translation to clinical practice to aid in the evaluation of transplant allografts, the diagnosis of kidney disease, and the quantitation of nephron endowment in children born early or with low birth weight.
MRI-based measurements of aNglom and aVglom for CF1 and CF3 agreed well with the estimates obtained using stereology. Due to the difference in the number of glomeruli sampled using each method and the heterogenous nature of human kidneys, we did not expect these two measurements to be in perfect agreement. Furthermore, we counted 57,000 glomerulus-like dark spots in the unlabeled control kidney, most of which were likely due to small regions of residual blood. This ∼6% false positive rate of this counting technique is of the order of previous reports in rat kidneys (3).
For CF2, the kidney with marked glomerular, interstitial, and vascular pathology, the MRI and stereological estimates of glomerular number and volume were more disparate. Surprisingly, the MRI estimate of glomerular number was higher than the stereological estimate for this kidney, despite the fact that ∼20% of the cortex lacked CF-labeled glomeruli. This patient had significant and uncontrolled hypertension resulting in vascular and glomerular pathology. Histopathology revealed that glomeruli and arterioles were severely sclerosed in the regions of the CF2 cortex that lacked CF-labeled glomeruli. The glomeruli in these regions were likely underperfused or abnormal. We observed glomeruli with poor ferritin labeling, sclerotic glomeruli with no open capillaries, and possibly atubular glomeruli (27) or obstruction of collecting ducts; all of them with little, if any, ferritin signal and therefore representing a population of nonfunctioning glomeruli. To the best of our knowledge, this is the first time large regions of nonfunctioning glomeruli have been detected in an intact kidney. We also found evidence of vascular remodeling and of CF accumulation at sites of vascular remodeling in this kidney. The over counting of glomeruli in the CF2 kidney can likely be attributed to CF accumulation to vasculature undergoing remodeling, which would appear glomerulus-like to the image processing algorithm. Future work will focus on separating CF-related MRI signal changes in glomeruli from those that occur due to vascular changes and blood- and CF-artifact (discussed below). These structures will likely be distinguishable based on their morphology.
The 3D MRI-based technique also enabled calculation of the glomerular volume distribution in CF-labeled kidneys. This is the first report of the glomerular size distribution in human kidneys. The glomerular size distributions show a large number of small “glomeruli.” From previous stereological estimates of individual glomerular volumes (IGV) in human kidneys, we predict that ∼10% of the glomeruli should have volumes of 2.4 × 10−3 mm3 or less (20), yet from MRI we find ∼30% of apparent glomeruli have volumes in this range. It is thus likely that ∼1/3rd of the glomeruli detected by MRI with volumes less than 2.4 × 10−3 mm3 are true glomeruli that make up ∼10% of all glomeruli in the kidney. We speculate that a remaining 1/6th of the small apparent glomeruli are due to the same systematic artifacts observed in the unlabeled control kidney. There are then two possible sources of the remaining 1/2 of the small of apparent glomeruli: spurious labeling of CF in nonglomerular structures or partial CF labeling in some glomeruli, or both. Future work will focus on improving image analysis and MRI acquisition to reduce the number of falsely identified glomeruli. It may be possible to exclude these erroneous glomeruli by setting a strict lower image processing threshold on the IGV measurements. Our data illustrate that caution is required in applying this threshold to ensure that IGV is not simply adjusted to give a “correct” result. Nonetheless, the intrarenal distribution of glomerular volumes may emerge as a powerful new parameter for assessing glomerular hypertrophy and shrinkage in health and disease.
We quantified the spatial distribution of CF accumulation in the kidney by image texture analysis. The CF-related image darkening in the CF3 kidney—the kidney with the least reported nephrosclerosis—was punctuate and was associated with spatial power spectral peaks between k = 1.2 and 1.5 mm−1. With profound arteriolosclerosis and nephrosclerosis, as in kidney CF2, the CF-related signal darkening appeared diffuse and was associated with a spatial power spectral peak at k = 0.8 mm−1. Previous work in rats showed that this diffuse labeling, quantified using the spatial power spectrum, can result from leakage of protein past the glomerular capillary wall into Bowman's space and the proximal tubule (5). This analysis is supported by the histopathological and spectral analyses of kidney CF1, which exhibited only mild nephrosclerosis and spectral peaks at both k = 0.8 and 1.2 mm−1, suggesting populations of healthy (k = 1.2 mm−1) and sclerotic (k = 0.8 mm−1) glomeruli. Image texture analysis may prove useful to quantify morphological changes with disease progression.
CF creates contrast in T2*-weighted MRI by dephasing the spins of water protons surrounding the site of CF accumulation. The volume over which this dephasing is seen in T2*-weighted MRI images depends on the amount of accumulated CF and the image acquisition parameters. It is thus important to consider imaging parameters and to minimize CF dosage when measuring aVglom with T2*-weighted MRI. While this work used a large CF dose of 300 mg/kg kidney wt, intravenous doses of just 0.6–1 mg/kg body wt of CF should be sufficient to visualize rat glomeruli with T2*-weighted MRI and have minimal effects on the kidney, liver, and immune function biomarkers (1–3). Substantial work must be done in the future to optimize dosing of donor kidney with CF. Recent advances in MRI contrast agent design have also opened the door to labeling glomeruli with a highly sensitive T1-shortening (bright) MRI “paraCF” contrast agents (11). ParaCF allows improved in vivo detection of glomeruli with T1-weighted MRI and elimination of the dephasing artifacts found in T2*-weighted MRI that might affect volume measurements, and a further 100-fold reduction in required dose. Such an agent would greatly improve glomerular detection in vivo against the dark blood background and allow for doses that may have a minimal effect on renal function.
The ability to clinically measure glomerular morphology and local protein leakage has the potential to directly improve patient care and clinical outcomes. Measurements of glomerular morphology could be used to assess the viability of kidneys from both living and deceased donors, ensuring that a donor kidney has sufficient filtration surface area. The relationship between glomerular morphology and kidney viability, measured by in vivo GFR and survival rates, will be the subject of future work in preclinical models of kidney transplants. Noninvasive glomerular morphological measurements would also allow younger recipients to receive kidneys possessing a nephron number sufficient to match their future life span. Individuals at risk for CKD could receive an individualized risk assessment using this technique, enabling early detection and regular monitoring of kidney disease. Still, the use of such a technique in the clinic requires substantial work to address scan time and contrast agent toxicity. Many of these studies are already underway. Development of highly sensitive, T1-shortening (bright) contrast agents (11) and advancements in radio frequency hardware for high-resolution in vivo MRI of the kidney (26) have made it possible to visualize glomeruli in vivo in a matter of minutes, although specialized contrast agents and semi-invasive hardware are currently required to do so. Initial studies of the toxicity and biodistribution of CF (1) suggest MRI-detectable doses of CF are minimally toxic. Production of recombinant human ferritin may further reduce toxicity (30). It will also be increasingly important to determine the minimum dose of CF needed to detect glomeruli in human studies with MRI. Based on our previous in vivo studies, and accounting for allometric differences between rats and humans, we approximate the in vivo detectable limit of intravenous CF in humans to be 0.56 mg/kg.
In conclusion, glomerular number and volume in viable human kidneys can be measured with MRI. To the best of our knowledge, this is the first technique to measure the volume of every glomerulus in the human kidney and to identify large regions of arteriolar and glomerular sclerosis. This technique is nondestructive and therefore has the potential for translation to the clinic. This study is thus a first step toward characterizing human kidney glomeruli in vivo.
GRANTS
G. F. Egan is a National Health and Medical Research Council Principal Research Fellow. This work was funded by a grant to K. M. Bennett from the National Institutes of Health (NIH) Diabetic Complications Consortium and by NIH Grant DK-091722. V. G. Puelles received a Monash Research Graduate School Scholarship and a Faculty of Medicine International Postgraduate Scholarship to support his PhD candidature.
DISCLOSURES
K.M. Bennett owns Nanodiagnostics, LLC.
AUTHOR CONTRIBUTIONS
Author contributions: S.C.B., V.G.P., M.Z., T.W., J.A.A., J.F.B., and K.M.B. conception and design of research; S.C.B., L.A.C.-M., V.G.P., E.J.B., J.D., A.N., and K.M.B. performed experiments; S.C.B., L.A.C.-M., V.G.P., M.Z., E.J.B., J.D., J.R.C., M.S.F., and A.N. analyzed data; S.C.B., L.A.C.-M., V.G.P., E.J.B., J.D., J.R.C., M.S.F., A.N., Q.-z.W., J.A.A., G.F.E., J.F.B., and K.M.B. interpreted results of experiments; S.C.B., V.G.P., E.J.B., J.R.C., and A.N. prepared figures; S.C.B., V.G.P., M.Z., J.R.C., A.N., J.F.B., and K.M.B. drafted manuscript; S.C.B., L.A.C.-M., V.G.P., T.W., E.J.B., J.R.C., J.A.A., G.F.E., J.F.B., and K.M.B. edited and revised manuscript; S.C.B., L.A.C.-M., V.G.P., J.F.B., and K.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the joint Barrow Neurological Institute–Arizona State University Center for Preclinical Imaging and the support from the Monash Biomedical Imaging Research Platform. We also acknowledge support from David Lowry at the ASU Electron Microscopy Facility, Joseph Georges at the Translational Genomics Research Institute (confocal microscopy), and Monash Micro Imaging (confocal microscopy). Histological sectioning and staining for pathological analysis were performed by staff at the Monash Histology Platform. We acknowledge the outstanding staff of the International Institute for the Advancement of Medicine of the Musculoskeletal Tissue Foundation for tissue procurement and support.
REFERENCES
- 1.Beeman SC, Georges JF, Bennett KM. Toxicity, biodistribution, and ex vivo MRI detection of intravenously injected cationized ferritin. Magn Reson Med 69: 853–861, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Beeman SC, Mandarino LJ, Georges JF, Bennett KM. Cationized ferritin as a magnetic resonance imaging probe to detect microstructural changes in a rat model of non-alcoholic steatohepatitis. Magn Reson Med 70: 1728–1738, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beeman SC, Zhang M, Gubhaju L, Wu T, Bertram JF, Frakes DH, Cherry BR, Bennett KM. Measuring glomerular number and size in perfused kidneys using MRI. Am J Physiol Renal Physiol 300: F1454–F1457, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Bennett KM, Bertram JF, Beeman SC, Gretz N. The emerging role of MRI in quantitative renal glomerular morphology. Am J Physiol Renal Physiol 304: F1252–F1257, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett KM, Zhou H, Sumner JP, Dodd SJ, Bouraoud N, Doi K, Star RA, Koretsky AP. MRI of the basement membrane using charged nanoparticles as contrast agents. Magn Reson Med 60: 564–574, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertram JF, Cullen-McEwen LA, Egan GF, Gretz N, Baldelomar E, Beeman SC, Bennett KM. Why and how we determine nephron number. Pediatr Nephrol 29: 575–580, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol 26: 1529–1533, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Bertram JF, Soosaipillai MC, Ricardo SD, Ryan GB. Total numbers of glomeruli and individual glomerular cell types in the normal rat kidney. Cell Tissue Res 270: 37–45, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Brenner B, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more of the other? Am J Hypertens 1: 335–347, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010 [Google Scholar]
- 11.Clavijo-Jordan V, Beeman SC, Baldelomar EJ, Bennett KM. Disruptive chemical doping in a ferritin-based iron oxide nanoparticle to decrease r2 and enhance detection with T1-weighted MRI. Contrast Media Mol Imaging DOI: 10.1002/cmmi.1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cullen-McEwen LA, Armitage JA, Nyengaard JR, Bertram JF. Estimating nephron number in the developing kidney using the physical disector/fractionator combination. Methods Mol Biol 886: 109–119, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Cullen-McEwen LA, Douglas-Denton RN, Bertram JF. Estimating total nephron number in the adult kidney using the physical disector/fractionator combination. Methods Mol Biol 886: 333–350, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Cullen-McEwen LA, Drago J, Bertram JF. Nephron endowment in glial cell line-derived neurotrophic factor (GDNF) heterozygous mice. Kidney Int 60: 31–36, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Damadian R, Shwayri E, Bricker N. On the existence of non-urine forming nephrons in the diseased kidney of the dog. J Lab Clin Med 65: 26–39, 1965 [PubMed] [Google Scholar]
- 16.Danon D, Goldstein L, Marikovsky Y, Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res 38: 500–510, 1972 [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Heilmann M, Neudecker S, Wolf I, Gubhaju L, Sticht C, Schock-Kusch D, Kriz W, Bertram JF, Schad LR, Gretz N. Quantification of glomerular number and size distribution in normal rat kidneys using magnetic resonance imaging. Nephrol Dial Transplant 27: 100–107, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Hoy WE, Bertram JF, Denton RD, Zimanyi M, Samuel T, Hughson MD. Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens 17: 258–265, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Hoy WE, Hughson MD, Diouf B, Zimanyi M, Samuel T, McNamara BJ, Douglas-Denton RN, Holden L, Mott SA, Bertram JF. Distribution of volumes of individual glomeruli in kidneys at autopsy: association with physical and clinical characteristics and with ethnic group. Am J Nephrol 33, Suppl 1: 15–20, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Menini S, Ricci C, Iacobini C, Bianchi G, Pugliese G, Pesce C. Glomerular number and size in Milan hypertensive and normotensive rats: their relationship to susceptibility and resistance to hypertension and renal disease. J Hypertens 22: 2185–2192, 2004 [DOI] [PubMed] [Google Scholar]
- 23.National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. [Google Scholar]
- 24.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Puelles VG, Zimanyi MA, Samuel T, Hughson MD, Douglas-Denton RN, Bertram JF, Armitage JA. Estimating individual glomerular volume in the human kidney: clinical perspectives. Nephrol Dial Transplant 27: 1880–1888, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian C, Yu X, Chen DY, Dodd S, Bouraoud N, Pothayee N, Chen Y, Beeman S, Bennett K, Murphy-Boesch J, Koretsky A. Wireless amplified nuclear MR detector (WAND) for high-spatial-resolution MR imaging of internal organs: preclinical demonstration in a rodent model. Radiology 268: 228–236, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulte K, Berger K, Boor P, Jirak P. Origin of parietal podocytes in atubular glomeruli mapped by lineage tracing. Clin J Am Soc Nephrol 25: 129–141, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith DH, Gullion CM, Nichols G, Keith DS, Brown JB. Cost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO population. J Am Soc Nephrol 15: 1300–1306, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Stevens L, Greene T, Levey A. Surrogate end points for clinical trials of kidney disease progression. Clin J Am Soc Nephrol 1: 874–884, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Uchida M, Terashima M, Cunningham CH, Suzuki Y, Willits DA, Willis AF, Yang PC, Tsao PS, McConnell MV, Young MJ, Douglas T. A human ferritin iron oxide nano-composite magnetic resonance contrast agent. Magn Reson Med 60: 1073–1081, 2008 [DOI] [PubMed] [Google Scholar]