Abstract
Acute kidney injury (AKI) is associated with mitochondrial fragmentation, which contributes to mitochondrial damage and tubular cell apoptosis. Mitochondrial fragmentation involves the cleavage of both mitochondrial outer and inner membranes. Cleavage of the outer membrane results from Drp-1-mediated fission activation and Bak-promoted fusion arrest, but the molecular mechanism of inner membrane cleavage remains elusive. OMA1-mediated proteolysis of OPA1, a key inner membrane fusion protein, was recently suggested to account for inner membrane cleavage during cell stress. In this study, we determined the role of OMA1 in OPA1 proteolysis and mitochondrial fragmentation in experimental models of ischemic AKI. In ATP-depletion injury, knockdown of OMA1 suppressed OPA1 proteolysis, mitochondrial fragmentation, cytochrome c release, and consequent apoptosis in renal proximal tubular cells. In mice, OMA1 deficiency prevented ischemic AKI as indicated by better renal function, less tubular damage, and lower apoptosis. OPA1 proteolysis and mitochondrial injury during ischemic AKI were ameliorated in OMA1-deficient mice. Thus, OMA1-mediated OPA1 proteolysis plays an important role in the disruption of mitochondrial dynamics in ischemic AKI.
Keywords: acute kidney injury, ischemia-reperfusion, mitochondria, apoptosis
acute kidney injury (AKI) is a major kidney disease that is associated with high mortality and an increased incidence of chronic kidney disease (9–11, 32). One of the main causes of AKI in clinical settings is renal ischemia-reperfusion, which occurs during kidney transplantation, vascular occlusion in major surgeries, renal vascular obstruction, and hypoperfusion of kidneys due to dehydration, hypotention, and decreased cardiac output (2, 28). Due to decreased blood flow in the kidneys, ischemia leads to the deprivation of oxygen and nutrients in tissues, resulting in ATP depletion and related injury in kidney cells. Restoration of blood flow following ischemia further induces reperfusion injury.
Despite decades of study, the cellular and molecular mechanism of AKI induced by renal ischemia-reperfusion remains elusive (2, 28). The research in recent years has demonstrated that the cell death pathway centered on mitochondrial damage contributes remarkably to renal tubular cell injury and death in AKI (18, 26). Especially, it is now known that mitochondria are a class of dynamic organelles that constantly undergo fission and fusion under physiological conditions. During ATP depletion in vitro and ischemia AKI in vivo, mitochondrial dynamics are shifted to fission, resulting in mitochondrial fragmentation, which, along with prodeath molecules such as Bax and Bak, induces mitochondrial membrane permeabilization and the release of apoptotic factors (e.g., cytochrome c) to initiate the cascade of apoptosis (5, 37).
As double-membrane organelles, mitochondria maintain their dynamics at both inner and outer membranes (8, 24, 36). At the outer membrane, fission is mainly controlled by Drp1, while fusion depends on the functional interaction between mitofusin proteins (13, 29, 36). Relatively little is known about the regulation of inner membrane dynamics. Nonetheless, OPA1 is known to be the fusion protein for mitochondrial inner membrane in mammalian cells and the regulation of OPA1 depends on proteolytic processing (1, 30). In 2009, two groups reported separately that OMA1, a zinc metalloprotease located at mitochondrial inner membrane, is responsible for OPA1 proteolysis and inactivation during cell stress (14, 19). The proteolysis of OPA1 by OMA1 leads to the loss of the long isoforms of OPA1 (L-OPA1), resulting in the inactivation of OPA1 and cessation of inner membrane fusion (14, 19, 30). More recently, OMA1 has been implicated in OPA1 proteolysis in vivo in metabolic stress (27). However, it remains unclear whether OMA1 mediates OPA1 proteolysis and inactivation and alterations of mitochondrial dynamics in vivo under other pathological conditions such as ischemia-reperfusion. In addition, the regulation of OPA1 and OMA1 has not been examined in renal cells or tissues. Therefore, the current study was designed to investigate the role of OMA1 in OPA1 proteolysis, mitochondrial fragmentation, and tubular cell apoptosis in experimental models of ischemic AKI.
MATERIALS AND METHODS
Cells and OMA1 knockdown.
The immortalized rat kidney proximal tubular cell line (RPTC) used in this study was originally from Dr. Ulrich Hopfer's lab (Case Western Reserve University, Cleveland, OH). OMA1-shRNA was purchased from Origene Technologies (Rockville, MD). To generate OMA1 knockdown stable RPTC cells, OMA1-shRNA was transfected into RPTCs at the confluence of 60% using Lipofectamine LTX (Life Technologies, Grand Island, NY). Twenty-four hours after transfection, cells were subjected to 20 μM puromycin (Clontech Laboratories, Mountain View, CA) selection for 2 wk. The knockdown effect was confirmed by immunoblot analysis with anti-OMA1 antibody (Abcam, Cambridge, MA). RPTCs stably transfected with scramble-shRNA were used as control cells.
ATP depletion of RPTC.
RPTCs were treated with 10 mM sodium azide (Sigma, St. Louis, MO) for 2.5 h in a glucose-free Krebs-Ringer bicarbonate buffer followed by reperfusion with fresh culture medium for 3 h. Cells were then fixed with 4% paraformaldehyde and stained with Hoechst 33342. Cells were examined by phase contrast and fluorescence microscopy to evaluate the percentage of apoptotic cells, which showed typical morphology of cellular condensation, formation of apoptotic bodies, and nuclear condensation and fragmentation (6).
Animals and renal ischemia-reperfusion.
The mice used in this study were housed and treated following the protocols approved by the Institutional Animal Care and Use Committees of Charlie Norwood VA Medical Center and Medical College of Georgia at Georgia Regent University. C57BL/6 mice were originally purchased from Jackson Laboratory (Bar Harbor, ME). The OMA1 germ line knockout (OMA1-KO) mouse model was established as described (27). OMA1-KO mice were mated with C57BL/6 mice to generate heterozygotes as breeders for the production of wild-type (WT) and KO littermates for experiment. The genotypes of the mice were determined by PCR with the primers: 1) forward: gagtgctgtttctctgggtgt; 2) reverse for WT: tgccctaaactgaaggtgtg; 3) reverse for KO: tagaccgcggctagaggta. The WT allele product was 379 bp and the KO allele product was 252 bp.
For experiment, male mice of 8 wk old (WT and OMA1-KO) were subjected to 25 min of bilateral renal ischemia as detailed in previous studies (33, 35). Briefly, mice were given 60 mg/kg pentobarbital sodium by intraperitoneal injection for anesthesia. Renal pedicles were clamped for 25 min followed by the clips for reperfusion being released. Mice were killed at different reperfusion times as indicated to collect blood samples and kidney tissues. Sham-operated mice had the similar procedures but without renal pedicle clamping. The body temperature of the mice was maintained at 36.5°C using a homeothermic blanket system (Harvard Apparatus, Holliston, MA).
Renal function measurement.
Blood samples were collected from tail clip or at the time of death. After clotting at room temperature, serum was collected after centrifugation at 12,000 g for 5 min. Blood urea nitrogen (BUN) and serum creatinine were measured with analytical kits from Stanbio Laboratory (Boerne, TX).
Histology.
Kidneys were collected freshly and fixed with 4% paraformaldehyde at 4°C overnight, followed by dehydration and paraffin embedding. The paraffin-embedded tissues were cut into 5-μm sections for the hematoxylin and eosin staining. Tubular damage was indicated by loss of brush border, tubular dilation, cast formation, and cell lysis. Tubular damage was scored as follows: 1: 0–25% of damage, 2: 26–50% of damage, 3: 51–75% of damage, and 4: >75% of damage. The slides were checked in a blind manner and the representative images were taken with a light microscope.
TUNEL staining.
Paraffin-embedded kidney tissue sections were rehydrated and permeabilized with 0.1 M sodium citrate, pH 6.0 for 60 min at 60°C. The slides were then incubated with a TdT-mediated dUTP nick end labeling (TUNEL) reaction enzyme mixture from in situ Cell Death Detection kit (Roche Applied Science, Indianapolis, IN) for 40 min at 37°C. The slides were mounted with Prolong Gold Anti-fade Reagent (Life Technologies). For quantification, 10–20 fields were randomly selected from each tissue section and the amount of TUNEL-positive cells per 1 mm2 was evaluated as before (5, 20, 33).
Analysis of mitochondrial fragmentation.
To evaluate mitochondrial fragmentation in cultured cells, the pAcGFP1-Mito–MitoGreen (Clontech Laboratories, Mountain View, CA) was transiently transfected into RPTC. Following treatment, the cells were fixed with 4% paraformaldehyde and mounted with Prolong Gold Anti-fade Reagent (Life Technologies). Mitochondrial fragmentation was evaluated as described in our previous studies (6, 12). Briefly, the morphology of mitochondria in individual cells was examined. The fragmented mitochondria displayed shortened and punctated morphologies while the filamentous mitochondria had thread-like or tubular structures. Totally 100–200 cells were examined to determine the percentage of cells with fragmented mitochondria in each group and five separated experiments were conducted for statistical analysis. To analyze mitochondrial fragmentation in vivo, mice were perfused with heparin (10 ml of 10 U/ml for each mouse) and 50 ml fixative (100 mM sodium cacodylate, 2 mM CaCl2, 4 mM MgSO4, 4% paraformaldehyde, and 2.5% glutaraldehyde) followed by overnight postfixation at 4°C. Tissue blocks of ∼1 mm3 containing cortex and outer medulla were cut from each kidney, which were then processed in the electron microscopy core of Georgia Regent University. The length of mitochondria in the cells was measured using ImageJ software (http://imagej.nih.gov/ij). Mitochondria with more than 2 μm of length were considered filamentous. The cells with <1% of filamentous mitochondria were counted cells with mitochondrial fragmentation (6, 33).
Analysis of cytochrome c release.
Cytochrome c release was detected by immunoblot analysis for its expression in mitochondria and cytosol, respectively (6). To examine cytochrome c release in RPTCs, the cells were fractionated using an isotonic sucrose buffer containing 0.05% digitonin (wt/vol) for 5 min. The cytosol and mitochondrial fractions were separated by centrifugation. The digitonin-soluble portion was the cytosolic fraction and the pellet was mitochondria-enriched membrane fraction. To analyze cytochrome c release in mouse kidney tissues, fresh mouse kidney cortical tissues were collected and homogenized with lysis buffer containing 0.27 M sucrose, 1 mM EGTA, and 5 mM Tris·HCl (pH 7.4). After 600 g of centrifugation for 10 min at 4°C, the supernatant was collected for further centrifugation in 4°C with a speed of 100,000 g for 1 h to separate the cytosol and mitochondrial fractions. The soluble portion was the cytosolic fraction and the pellet was mitochondrial fraction (33).
Immunoblot analysis.
Protein samples were separated on the denatured SDS-PAGE gels and then transferred to PVDF membrane for immunoblot analysis. After being blocked in 5% milk, the blots were incubated in primary antibodies and secondary antibodies subsequently. The specific signals were detected by chemiluminescence.
Statistics.
Microsoft Excel (14.1.2) was used for all the data analysis. Student's t-test and ANOVA test with P < 0.05 were considered as statistically significant difference.
RESULTS
Knockdown of OMA1 suppresses ATP depletion-induced apoptosis in RPTC.
We initially examined the role of OMA1 in apoptosis following ATP depletion in RPTCs. RPTCs were stably transfected with shRNAs to knockdown OMA1. As shown in Fig. 1, A and B, OMA1-shRNA transfection induced ∼50% decrease of OMA1 expression comparing with the scramble shRNA transfection. Both OMA1 knockdown cells and scrambled sequence transfected cells were treated with sodium azide (a mitochondrial respiration inhibitor) in glucose-free medium to induce ATP depletion, followed by recovery in full culture medium (5). The treatment induced 35% apoptosis in scrambled sequence transfected cells, but only 15% in OMA1 knockdown cells (Fig. 1, C and D). Consistently, scrambled sequence transfected cells had a significantly higher level of cleaved/active caspase-3 than the OMA1 knockdown cells (Fig. 1E: lane 5 vs. 6; Fig. 1F). These results indicate that the loss of OMA1 alleviates the apoptosis induced by ATP depletion in RPTCs, supporting a role of OMA1 in tubular cell apoptosis in this model.
Fig. 1.
OMA1 knockdown suppresses ATP depletion-induced apoptosis in rat kidney proximal tubular cell line cells (RPTCs). RPTCs were transfected with OMA1-shRNA or scrambled sequence shRNA plasmids to generate stable cell lines. The cells were then left untreated (control) or treated with 10 mM sodium azide in glucose-free buffer for 2.5 h to induce ATP depletion (A), followed by 3 h of recovery in fresh culture medium (A/R). A: representative immunoblots showing OMA1 knockdown in OMA1-shRNA transfected cells; β-actin was probed as internal protein control. B: densitometry quantification of immunoblots confirming OMA1 knockdown in OMA1-shRNA transfected cells (n = 3). *P < 0.05. C: representative images of cell morphology (top) and nuclear morphology after Hochest staining (bottom). D: percentage of apoptosis evaluated by counting the cells with typical apoptotic morphology (n = 3). *P < 0.05. **P < 0.01. E: immunoblot of cleaved, active caspase 3. F: quantitative analysis of cleaved, active caspase 3 by densitometry. *P < 0.05. **P < 0.01.
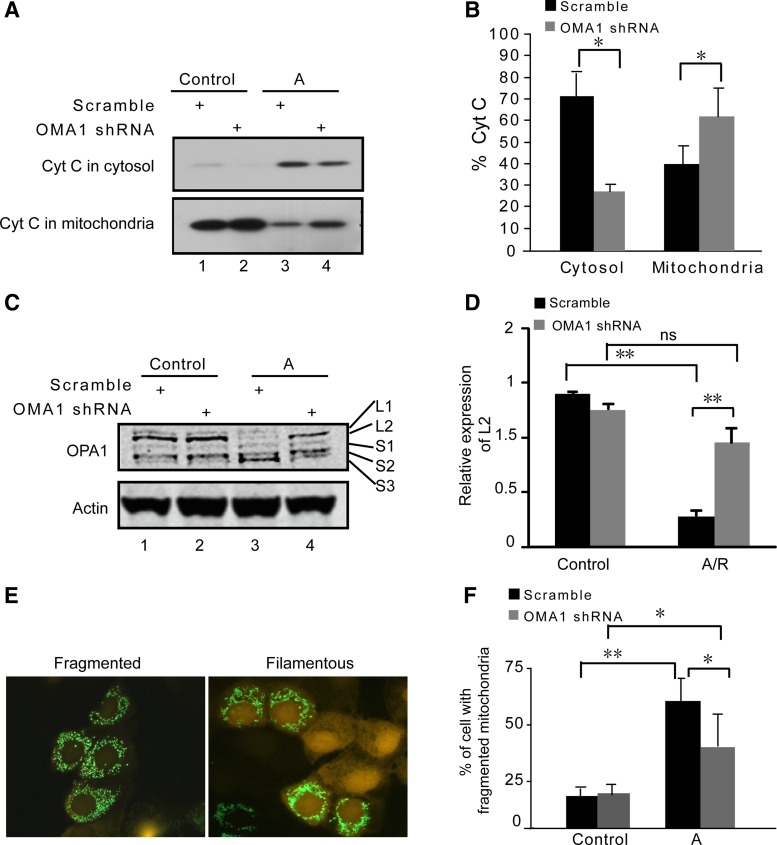
Knockdown of OMA1 inhibits OPA1 proteolysis and mitochondrial fragmentation during ATP depletion.
Cytochrome c release from mitochondria to cytosol is a hallmark of the intrinsic pathway of apoptosis (7). As shown in Fig. 2, A and B, cytochrome c was released during azide treatment of RPTCs (lane 3) and this release was inhibited in OMA1 knockdown cells (lane 4). As OMA1 has been implicated in OPA1 cleavage during mitochondrial injury and stress (14, 19, 27), we further examined the spectrum of OPA1 isoforms in those cells. Two long isoforms (L1 and L2) and three short isoforms (S1, S2, and S3) of OPA1 were detected by immunoblot analysis in control cells (Fig. 2C: lanes 1, 2). Following azide treatment, the long isoforms of OPA1 (especially OPA1-L2) disappeared and, concomitantly, there was a marked accumulation of OPA1-S3 short form (Fig. 2C: lane 3), indicative of OPA1 proteolysis. Notably, the OPA1 proteolysis was suppressed in OMA1 knockdown cells (Fig. 2C: lane 4). This conclusion is further supported by quantification of OPA1- L2 (Fig. 2D).
Fig. 2.
OMA1 knockdown inhibits ATP depletion-induced OPA1 proteolysis, mitochondrial fragmentation, and cytochrome c (Cyt c) release. OMA1 shRNA or scrambled sequence transfected RPTCs were left untreated (control) or treated with 10 mM sodium azide to induce ATP depletion. A: immunoblots of Cyt c. Cells were fractionated into mitochondria-enriched membrane fraction and cytosolic fraction for immunoblot analysis. B: quantification of percentage of Cyt c in cytosol and mitochondria. The Cyt c bands were analyzed by densitometry. The signals were used to calculate the percentages. *P < 0.05. C: immunoblot of OPA1 in whole cell lysate. The long (L1, L2) and short (S1, S2, S3) OPA1 isoforms are indicated. β-Actin was used as a loading control. D: OPA-L2 bands were analyzed by densitometry to calculate its degradation upon treatment (n = 3). **P < 0.01. ns, No significance. E: representative images of RPTCs with fragmented or filamentous mitochondria. F: quantification of percentage of cells with fragmented mitochondria (n = 3). *P < 0.05. **P < 0.01.
OPA1 is the key fusion protein for mitochondrial inner membrane. Excessive proteolysis leads to the inactivation of OPA1 followed by the arrest of inner membrane fusion, contributing to inner membrane cleavage (1). We reasoned that the preservation of OPA1 in OMA1-knockdown cells (Fig. 2, C and D) would prevent mitochondrial fragmentation as a consequence of lack of inner membrane cleavage. To monitor mitochondrial morphology, the cells were transfected with Mito-Green (pAcGFP1-Mito) to label mitochondria and then subjected to azide treatment. Representative images of cells with filamentous (right) and fragmented (left) mitochondria are shown in Fig. 2E. By cell counting, we detected some (<25%) cells with fragmented mitochondria under control conditions, which were not affected by OMA1 knockdown. After azide treatment, 52% cells showed fragmented mitochondria, which was suppressed to 37% when OMA1 was knocked down (Fig. 2F).
Together, these in vitro experimental results suggest that under ATP depletion conditions, OMA1 may regulate apoptosis by mediating OPA1 proteolysis and inducing mitochondrial fragmentation and proapoptotic factors leakage in kidney tubular cells.
OPA1 proteolysis occurs during renal ischemia-reperfusion in mice.
To assess whether OMA1-dependent OPA1 proteolysis plays a pathological role in vivo, we examined a mouse model of renal ischemia-reperfusion. C57BL/6 mice were subjected to 25 min of bilateral renal ischemia followed by 48 h of reperfusion. Figure 3, A and B, shows that after ischemia-reperfusion the BUN and serum creatinine values significantly increased compared with control. Meanwhile, the kidney injury molecule-1 (Kim-1), a biomarker of kidney proximal tubular injury (31), was markedly induced at 48 h of reperfusion (Fig. 3, C and D). The loss of renal function was accompanied with OPA1 proteolysis. As shown in Fig. 3, E and F, after ischemia-reperfusion, the long isoforms of OPA1 (L1 and L2) showed obvious decreases, while the shortest OPA1 accumulated.
Fig. 3.
OPA1 proteolysis occurs during renal ischemia-reperfusion in mice. C57BL/6 mice were subjected to 25 min of bilateral renal ischemia followed by 48 h of reperfusion (I25R48). A: blood urea nitrogen (BUN) values for mice before (control) and I25R24 and I25R48 (n = 4). **P < 0.01. B: serum creatinine values at 48 h of reperfusion (n = 4). *P < 0.05. C: immunoblot of kidney injury molecule (Kim 1) at conditions of sham and I25R48. D: quantification of Kim 1 (n = 4). **P < 0.01. E: immunoblot of OPA1 showing OPA1 proteolysis during renal ischemia-reperfusion. F: densitometry analysis of OPA1-L2 degradation during renal ischemia-reperfusion (n = 4). **P < 0.01.
OMA1 knockout protects renal function and reduces OPA1 proteolysis in ischemic AKI.
Since OPA1 proteolysis occurs in ischemic AKI in mouse, we determined whether the proteolysis is OMA1 dependent. To this end, we used a recently described OMA1 knockout mouse model (27). The deficiency of OMA1 in this model was verified by genotyping (Fig. 4A). OMA1 knockout did not induce noticeable defects in renal function and histology at the age of 8 wk of our experiment (Figs. 4, B and C, and 5A). Male littermates of OMA1-KO and WT were subjected to 25 min of bilateral renal ischemia. At 24 h of reperfusion, BUN in WT mice increased to 159 mg/dl, whereas 93 mg/dl in KO mice (Fig. 4B). The BUN in WT mice further increased to over 200 mg/dl at 48 h of reperfusion, while that of KO started to decrease. Consistently, serum creatinine in KO mice (0.52 mg/dl) was significantly lower than that of WT mice (1.35 mg/dl; Fig. 4C). Immunoblot analysis showed a dramatic induction of Kim-1 in the kidney tissues of WT mice after ischemia-reperfusion, which was markedly lower in KO tissues (Fig. 4, D and E). Notably, OPA1-L2 isoform disappeared during renal ischemia-reperfusion in WT tissues but was preserved in KO tissues (Fig. 4, F and G), suggesting a role of OMA1 in mediating OPA1 proteolysis and ischemic AKI.
Fig. 4.
OMA1 knockout (KO) protects renal function and reduces OPA1 proteolysis in ischemic acute kidney injury (AKI). Male littermates of wild-type (WT) and OMA1-KO mice were subjected to 25 min of bilateral renal ischemia and 48 h of reperfusion. A: PCR-based genotyping results to confirm OMA1 deletion in KO mice. WT allele, 379 bp; OMA1-KO allele, 252 bp. B: BUN values for WT and KO mice before surgery (control) and during renal ischemia-reperfusion (I25R24 and I25R48; n = 5 pairs). #P < 0.05 vs. KO mice at I25R48. *P < 0.05, **P < 0.01 vs. control. C: serum creatinine for WT and KO mice before surgery (control) and at 48 h of reperfusion (I25R48; n = 5 pairs). **P < 0.01. D: immunoblots of Kim-1 for WT and KO mice with sham and I25R48 operations. E: quantification of relative expression of Kim 1 (n = 3 pairs). *P < 0.05, **P < 0.01. F: immunoblot of OPA1 showing the changes of various OPA1 isoforms during renal ischemia-reperfusion in the WT and KO mice. β-Actin was probed as loading control. G: densitometry analysis of OPA1-L2 (n = 3 pairs). **P < 0.01.
Fig. 5.
Tubular damage and apoptosis are attenuated in OMA1-KO mice during renal ischemia-reperfusion. Kidney tissues with or without 25 min of ischemia and 48 h of reperfusion were fixed and processed for paraffin embedding, followed by hematoxylin and eosin (H & E) or TdT-mediated dUTP nick end labeling (TUNEL) staining. A: representative images of H & E staining. B: kidney tubular damage score from H & E staining for WT and KO mice with ischemic AKI (I25R48). *P < 0.05. C: representative images of TUNEL staining. D: quantification of apoptotic cells from TUNEL images (bottom; n = 5). *P < 0.05. E: immunoblot analysis of cleaved caspase 3 during renal ischemia-reperfusion in WT and KO mice. F: densitometry quantification of cleaved caspase 3 (n = 3 pairs). **P < 0.01.
Tubular damage and apoptosis are attenuated in OMA1-KO mice during renal ischemia-reperfusion.
In histology, renal ischemia-reperfusion induced significant tubular damage in WT mice, as indicated by lysis of tubules and massive cast formation (Fig. 5A: left bottom). Tubular damage was also detected in OMA1-KO tissues (Fig. 5A: right bottom). Our results showed that WT tissues had a tubular damage score of 2 and score of 1.25 was for KO tissues (Fig. 5B). We further evaluated renal apoptosis by TUNEL assay. After renal ischemia-reperfusion, ∼50 TUNEL-positive cells were detected in each mm2 of WT kidney tissues, whereas 35 in OMA1-KO tissues (Fig. 5, C and D). Cleaved, active caspase 3 was detected in both WT and OMA1-KO mice after ischemia-reperfusion (Fig. 5H: lanes 2–4 and lanes 6–8). However, the level of active caspase 3 in WT kidney tissue was markedly higher than that in OMA1-KO tissue (Fig. 5, E and F). Together, these results indicate that OMA1 contributes to apoptosis, tubular damage, and ischemic kidney injury.
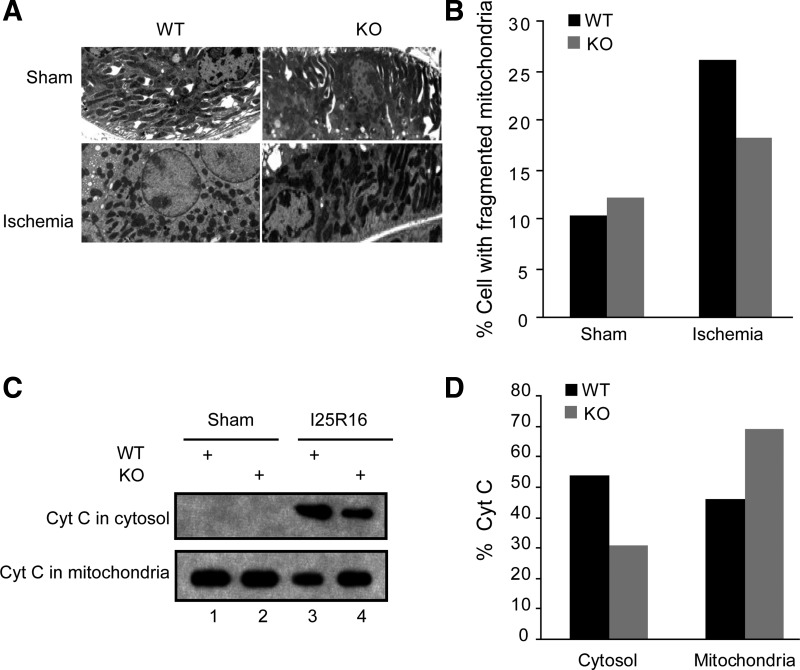
Resistance of OMA1-KO tissues to mitochondrial fragmentation and cytochrome c release in ischemic AKI.
Our results shown above support a role of OMA1 in tubular cell injury and death in ischemic AKI. Mechanistically, OMA1 mediates OPA1 proteolysis under the disease condition. OPA1 is a mitochondria inner membrane fusion protein, which plays an important role in cytochrome c retention in mitochondria (15). Thus, to further understand the involvement of OMA1 in ischemia AKI, we examined mitochondrial morphology and cytochrome c release in the WT and KO kidneys (Fig. 6, A and B). By electron microscopy, we observed proximal tubular cells in kidneys with filamentous or fragmented mitochondria (Fig. 6A). The basal level of tubular cells with fragmented mitochondria was ∼10% in both WT and OMA1-KO mice. Renal ischemia induced mitochondrial fragmentation in 26.1% of tubular cells in WT mice, but only 18.2% in KO mice (Fig. 6B), supporting a role of OMA1 in mitochondrial fragmentation. We further examined cytochrome c release in WT and OMA1-KO mouse kidneys (Fig. 6, C and D). No cytochrome c release from mitochondria was detected in sham-operated kidneys (Fig. 6C: lanes 1 and 2). After 25 min of ischemia and 16 h of reperfusion, there was significant release of cytochrome c in WT mouse kidneys (lane 3), which was partially suppressed in OMA1-KO mouse kidneys (lane 4).
Fig. 6.
Resistance of OMA1-KO tissues to mitochondrial fragmentation and cytochrome c release in ischemic AKI. A, B: male littermates of WT and OMA1-KO mice were subjected to 25 min of bilateral renal ischemia and a brief period (∼15 min) of reperfusion. Kidney tissues were fixed and processed for electron microscopy to record representative images (A) and quantify the cells with fragmented mitochondria in collected images (B). C: mice were subjected to 25 min of bilateral renal ischemia and 16 h of reperfusion. Kidney tissues were collected to separate mitochondrial and cytosol fractions for immunoblot analysis of cytochrome c. D: densitometry analysis of cytochrome c in cytosol and mitochondria.
DISCUSSION
Tubular cell apoptosis contributes significantly to the pathogenesis of AKI (18, 26). Especially, the intrinsic pathway of apoptosis plays an important role in tubular apoptosis under this disease condition (18, 37). The intrinsic pathway is characterized by Bax/Bak-mediated mitochondrial membrane permeabilization and the release of apoptogenic factors, such as cytochrome c. Our recent work demonstrated that Bax or Bak-deficient mice are protected from AKI following renal ischemia-reperfusion (33). Interestingly, before mitochondrial membrane permeabilization, mitochondria become fragmented during tubular cell apoptosis and inhibition of mitochondrial fragmentation prevents mitochondrial damage and reduces apoptosis and kidney injury, supporting a pathogenic role of mitochondrial fragmentation (5). We and others further showed that fragmented mitochondria are sensitized to Bax “attack” of mitochondria (3, 16), providing an explanation as to how mitochondrial fragmentation participates in apoptosis. Of note, all these studies focused on the molecular events at mitochondrial outer membrane. In contrast, relatively little is known about inner membrane cleavage.
In the present study, we investigated the regulation of mitochondrial inner membrane during cell stress following ATP depletion in vitro and renal ischemia-reperfusion in vivo. Specifically, two inner membrane proteins were studied: OPA1 and OMA1. OPA1 is the key fusion protein for mitochondrial inner membrane. Due to alternative splicing and proteolytic processing, OPA1 exists in cells in multiple forms (1, 30). Consistently, our immunoblot analysis detected two long and three short OPA1 isoforms in RPTCs and kidney tissues (Figs. 2 and 4). Interestingly, the fusion activity of OPA1 depends on the presence of both long and short OPA1 isoforms. During cell stress, OPA1 is excessively proteolyzed, leading to the loss of the long isoforms resulting in the inactivation of OPA1, which contributes to the cleavage of mitochondrial inner membrane (21). Importantly, it has been suggested that OMA1, a zinc metalloprotease located in mitochondrial inner membrane, may be the key protease responsible for the excessive OPA1 proteolysis during cell stress or apoptosis (14, 19). To examine this possibility in renal cells and tissues, we first determined the effects of OMA1 knockdown. OMA1-knockdown suppressed OPA1 proteolysis, inhibited mitochondrial fragmentation and leakage, and attenuated apoptosis following ATP depletion injury in RPTCs (Figs. 1 and 2). To demonstrate the role of OMA1 in vivo, we examined a newly established OMA1-KO mouse model. Compared with WT mice, OMA1-KO mice exhibited better kidney function, less OPA1 proteolysis, tubular damage, and apoptosis in response to ischemic challenge (Figs. 4–6). Collectively, these results support a role of OMA1 in OPA1 proteolysis and the associated mitochondrial damage and apoptosis.
As highly dynamic organelles, mitochondria frequently undergo fission and fusion in normal physiological conditions and the dynamics are important to the homeostasis, function, and viability of mitochondria and the cell (8, 24, 36). Mitochondrial fission depends on the activation of fission proteins such as Drp1, while fusion is controlled by mitofusin proteins at the outer membrane and OPA1 at the inner membrane. In response to cell stress, mitochondrial fission is activated and fusion is arrested, resulting in fragmentation of the organelles. Inhibition of mitochondrial fragmentation by blocking Drp1 or expressing mitofusins prevents mitochondrial damage and apoptosis, supporting a critical role of mitochondrial fragmentation in cell injury and death under pathological conditions. Notably, blockade of mitochondrial fragmentation leads to the prevention of apoptosis and tissue damage in disease models (5, 17, 23, 25), further supporting a pathogenic role of mitochondrial fragmentation. Mitochondrial fragmentation plays a critical role in ischemic and cisplatin nephrotoxic kidney injury (5). Mitochondrial fragmentation involves the cleavage of both outer and inner membranes. However, most previous studies were focused on the cleavage of the outer membrane. Our present results have verified the role of OMA1 in OPA1 proteolysis and inactivation for inner membrane cleavage. Together with previous work (4–6, 12), it is suggested that upon cell stress, Drp1 moves to mitochondria to accelerate fission and Bak is activated to block outer membrane fusion, leading to outer membrane cleavage. Meanwhile, OMA1 is activated to induce excessive proteolysis and inactivation of OPA1, resulting in inner membrane cleavage. Together, these molecular events coordinate mitochondrial fragmentation during cell stress and apoptosis.
Despite the recognition of the role of OMA1 in OPA1 proteolysis, it remains elusive as to how OMA1 is activated for OPA1 proteolysis during cell stress. In this regard, OMA1 has been proposed to be normally sequestered by prohibitin complexes and, upon cell stress, OMA1 is released (22). This scenario, while being attractive, remains to be investigated. For example, it is unclear whether OMA1 is indeed sequestered in prohibitin complexes and whether the complexes are disrupted to release OMA1 during cell stress. In addition, the factors governing the integrity of prohibitin complexes are completely unknown. Addressing these questions would gain significant new insights into the molecular regulation of mitochondrial inner membrane dynamics under pathophysiological conditions.
GRANTS
The study was supported in part by grants from National Natural Science Foundation of China (81370791), National Basic Research Program of China 973 Program No. 2012CB517600, and the National Institutes of Health and Department of Veterans Administration of USA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.X. and Z.D. conception and design of research; X.X., P.m.Q., and C.L.-O. performed experiments; X.X. and Z.D. analyzed data; X.X., Y.H., Q.W., and Z.D. interpreted results of experiments; X.X. prepared figures; X.X. drafted manuscript; Y.H., Q.W., C.L.-O., and Z.D. edited and revised manuscript; Y.H. and Z.D. approved final version of manuscript.
REFERENCES
- 1.Belenguer P, Pellegrini L. The dynamin GTPase OPA1: more than mitochondria? Biochim Biophys Acta 1833: 176–183, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks C, Cho SG, Wang CY, Yang T, Dong Z. Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol 300: C447–C455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks C, Dong Z. Regulation of mitochondrial morphological dynamics during apoptosis by Bcl-2 family proteins: a key in Bak? Cell Cycle 6: 3043–3047, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Brooks C, Wei Q, Cho S, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci USA 104: 11649–11654, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 15: 269–290, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet 46: 265–287, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 82: 516–524, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 104: 343–348, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Cho SG, Du Q, Huang S, Dong Z. Drp1 dephosphorylation in ATP depletion-induced mitochondrial injury and tubular cell apoptosis. Am J Physiol Renal Physiol 299: F199–F206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detmer SA, Chan DC. Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J Cell Biol 176: 405–414, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol 187: 1023–1036, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126: 177–189, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Gall JM, Wang Z, Liesa M, Molina A, Havasi A, Schwartz JH, Shirihai O, Borkan SC, Bonegio RG. Role of mitofusin 2 in the renal stress response. PLos One 7: e31074, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X, Disatnik MH, Monbureau M, Shamloo M, Mochly-Rosen D, Qi X. Inhibition of mitochondrial fragmentation diminishes Huntington's disease-associated neurodegeneration. J Clin Invest 123: 5371–5388, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int 80: 29–40, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol 187: 959–966, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int 82: 1271–1283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landes T, Leroy I, Bertholet A, Diot A, Khosrobakhsh F, Daloyau M, Davezac N, Miquel MC, Courilleau D, Guillou E, Olichon A, Lenaers G, Arnaune-Pelloquin L, Emorine LJ, Belenguer P. OPA1 (dys)functions. Semin Cell Dev Biol 21: 593–598, 2010 [DOI] [PubMed] [Google Scholar]
- 22.McBride H, Soubannier V. Mitochondrial function: OMA1 and OPA1, the grandmasters of mitochondrial health. Curr Biol 20: R274–R276, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, Walzer G, Twig G, Katz S, Corkey BE, Shirihai OS. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes 58: 2303–2315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell 148: 1145–1159, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Quiros PM, Ramsay AJ, Sala D, Fernandez-Vizarra E, Rodriguez F, Peinado JR, Fernandez-Garcia MS, Vega JA, Enriquez JA, Zorzano A, Lopez-Otin C. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J 31: 2117–2133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol 178: 749–755, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol 48: 463–493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int 84: 138–148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: technical notes and tricks. Am J Physiol Renal Physiol 303: F1487–F1494, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, stress. Science 337: 1062–1065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhan M, Brooks C, Liu F, Sun L, Dong Z. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int 83: 568–581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]