Abstract
Besides the glomerulus, the tubulointerstitium is often concomitantly affected in certain diseases, e.g., diabetic nephropathy, and activation of the renin-angiotensin system, to a certain extent, worsens its outcome because of perturbations in hemodynamics and possibly tubuloglomerular feedback. Certain studies suggest that pathobiology of the tubulointerstitium is influenced by small GTPases, e.g., Rap1. We investigated the effect of ANG II on inflammatory cytokines, while at the same time focusing on upstream effector of Rap1, i.e., Epac1, and some of the downstream tubular transport molecules, i.e., Na/H exchanger 3 (NHE3). ANG II treatment of LLC-PK1 cells decreased Rap1a GTPase activity in a time- and dose-dependent manner. ANG II treatment led to an increased membrane translocation of NHE3, which was reduced with Epac1 and PKA activators. ANG II-induced NHE3 translocation was notably reduced with the transfection of Rap1a dominant positive mutants, i.e., Rap1a-G12V or Rap1a-T35A. Transfection of cells with dominant negative Rap1a mutants, i.e., Rap1a-S17A, or Epac1 mutant, i.e., EPAC-ΔcAMP, normalized ANG II-induced translocation of NHE3. In addition, ANG II treatment led to an increased expression of inflammatory cytokines, i.e., IL-1β, IL-6, IL-8, and TNF-α, which was reduced with Rap1a-G12V or Rap1a-T35A transfection, while it reverted to previous comparable levels following transfection of Rap1a-S17A or EPAC-ΔcAMP. ANG II-induced expression of cytokines was reduced with the treatment with NHE3 inhibitor S3226 or with Epac1 and PKA activators. These data suggest that this novel Epac1-Rap1a-NHE3 pathway conceivably modulates ANG II-induced expression of inflammatory cytokines, and this information may yield the impetus for developing strategies to reduce tubulointertstitial inflammation in various renal diseases.
Keywords: AKI, cytokines, angiotensin II, Epac1-Rap1a, NHE3, inflammation, tubulointerstitium, diabetic nephropathy
compared with the glomerular compartment, the tubulointerstitium represents the bulk (∼90%) of the renal parenchymal mass and is thus amenable to a wide variety of injuries, the nature of which may be ischemic, toxic, immunological, or inflammatory. At times, tubulointerstitial injury may be part and parcel of a renal glomerular disease process (36). Interestingly, in such a scenario, it is likely that the tubulointerstitial injury would be mainly reflected in the functional derangements of the kidney, i.e., glomerular filtration rate (GFR), or at least it could worsen the outcome over what would be expected when only the glomerulus is involved (44, 45). It is well known that the biology of the glomerular compartment is heavily influenced by the renin-angiotensin system (RAS) that regulates local intrarenal hemodynamics, which apparently worsens the pathological outcome of a given glomerular disease. Prime examples where the activation of the RAS or ANG II contributes to or amplifies the injury are diabetic nephropathy and cardiovascular smooth cell dysfunctions, as reported in several studies, reviews, and perspectives (13, 24, 35, 49, 62, 70). In any event, it is conceivable that the activation of the RAS besides causing perturbation in the glomerular compartment may also modulate the tubulointerstitial injury since some of the RAS components are believed to be expressed within this compartment (33, 37). For instance, mRNAs of angiotensinogen, angiotensin-converting enzyme, and ANG II receptors have been reported to be expressed in proximal tubules (33). Being expressed in this compartment, the RAS would be able to regulate various biophysiological processes, including GFR, tubuloglomerular feedback, and sodium transport, the latter process being modulated by various exchangers, including Na+/H+ exchanger 3 (NHE3) (5, 7, 33, 57). ANG II, besides regulating intrarenal or -glomerular hemodynamics and induction of oxidant stress and MAP kinase signaling cascades, also regulates cell growth, proliferation, gene expression of hormones and growth factors, extracellular matrix genes, and inflammatory cytokines directly or by utilizing its receptors (19, 21, 26, 28, 34, 56). Intriguingly, ANG II has been detected in renal interstitial fluid (RIF), and its levels rise in states where the tubulointerstitial compartment is adversely affected, suggesting it plays a role in tubulointerstitial inflammation and cellular processes associated with it, besides vasoconstriction and modulation of cAMP-sensitive and ATP-dependent mechanism, i.e., regulation of sodium transport by NHE3 (9, 33, 37, 46). Overall, it is conceivable that ANG II may influence the pathobiology of proximal tubules, the compartment enriched with mitochondria, which modulate various ATP-dependent mechanisms.
Perturbations in tubular homeostasis are known to be associated with dysfunctions of mitochondria, as seen in two of the common renal disorders, i.e., diabetic nephropathy and acute kidney injury (15, 65, 68). In acute tubular injury, these dysfunctions lead to a multitude of pathobiological consequences, including oxidant stress, apoptosis, DNA fragmentation, and induction of various profibrogenic as well as inflammatory cytokines, such as transforming growth factor (TGF)-β, IL-10, TNF-α, and monocyte chemoattractant protein-1 (MCP-1) (72, 73). Interestingly, amelioration of some of these dysfunctions and aberrant tubular homeostasis has been reported with the overexpression of Rap1 (65). Rap1 belongs to the Ras family of small GTPases that cycle between an active GTP-bound form and inactive GDP-bound form (17, 66). Their activity is modulated by a guanine exchange factor Epac (exchange protein activated by cAMP) via its disheveled/Egl-10/Pleckstrin domain (14, 60). Epac was identified when cAMP-induced activation of the Ras-like small GTPase Rap1 was found to be insensitive to inhibition of PKA (14, 23). Besides modulating GTPase activity, Epac is known to modulate several cAMP-regulated processes as well as diverse signaling cascades or pathways related to Akt, phospholipase Cε, and ERK via activation of the Rap1/B-Raf pathway (23, 39, 42, 60, 64).
The above brief review of the literature suggests that a multitude of molecules or processes channeling into different pathways modulate the complex pathophysiology of the tubular compartment which if overexpressed eventually may lead to the manifestation of a tubulointerstitial disease. In view of this contention, we were curious to explore the yet to be defined downstream events initiated by the treatment of proximal tubular cells, i.e., LLC-PK1, with ANG II that ultimately may lead to induction of inflammatory cytokines, while taking into consideration both the physiological as well as biological processes that maintain homeostasis of the tubular compartment. In other words, the question addressed was, is there a signaling pathway that is at the crossroad of molecules that modulate the physiology vs. biology of the renal tubules, in particular those downstream of ANG II, since the latter is regarded by some of the investigators akin to a morphogenetic cytokine (56)? Importantly, we focused on the molecules that are expressed within the kidney and could adversely affect the pathobiology of the tubules in the event they are inappropriately expressed following local or systemic stimuli utilizing in vitro systems.
MATERIALS AND METHODS
Reagents.
The LLC-PK1 cell line (renal proximal tubular cell line) was purchased from the American Type Culture Collection (ATCC). Lipofectamine 2000, G418, Zeocin, TRIzol, and the pcDNA3.1 plasmid vector were obtained from Invitrogen. Other reagents were purchased from the following vendors: Sigma-Aldrich: M199 media, ANG II, quabain octahydrate, forskolin, and Epac activator, a cell-permeable cAMP analog, 8-CPT-2-O-Me-cAMP (8-cAMP) that selectively binds to Epac1 and triggers Epac1 signaling, and PKA activator (6-MB-cAMP); Life Technologies: Fast SYBRR Green Master Mix; and Abcam: ELISA kits for IL-1β, IL-6, IL-8, and TNF-α. The NHE3-specific inhibitor S3226 was a generous gift from Sanofi-Aventis (Frankfurt, Germany).
Animals.
Two-month-old Sprague-Dawley male rats were purchased from Harlan (Indianapolis, IN) and housed at Northwestern University animal facilities. The animals were kept for 1 wk in rooms with a 12:12-h light-dark cycle with maintenance of a temperature of 22°C with 50% humidity. The rats were anesthetized with an intraperitoneal injection of 2% avertin solution (240 mg/kg body wt). This protocol was approved by the Animal Care and Use Committee of Northwestern University.
Cell culture studies.
LLC-PK1 cells were grown in low-glucose M199 medium supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml of streptomycin. The cells were maintained in a humidified atmosphere of 5% CO2-95% air at 37°C. Approximately 5 × 105 cells were seeded in 25-cm2 culture flasks and maintained to achieve 80% confluence. The cells were then transfected with various Rap1a plasmid constructs following the manufacturer's instructions (FuGene). Rap1a (Ras-proximate small GTPase) plasmids included Rap1a-G12V and Rap1a-T35A (both constitutively active mutants) and Rap1a S17A (dominant negative mutant). Similarly, Epac1 plasmid and its dominant negative mutant (EPAC-ΔcAMP) were transfected into LLC-PK1 cells. Both the Epac1 plasmids were originally a gift from Dr. Johannes Bos (University Medical Center, Utrecht, The Netherlands) and have been propagated and used by our laboratory, as described in our previous publications (64, 65). The LLC-PK1 cells and transfectants were treated with various concentrations of ANG II (10−8-10−11 mol/l) in the presence or absence of various inhibitors of NHE3 (quabain: 100 nM and S3226: 0.5–10 μM), Epac activator (10 μM), PKA activator (10 μM), and forskolin (10 μM). The latter is known to raise cAMP levels, which in turn modulate cAMP-sensitive signaling pathways modulated by PKA and Epac1. ANG II was used at a concentration of 10−9 mol/l, with treatment extending to 30 min for Rap1a activity and NHE3 membrane translocation experiments, while treatment was extended to 12 h for experiments with gene and protein expression of proinflammatory cytokines.
Ex vivo detection of NHE3 in kidney slices.
Kidneys of anesthetized rats were perfused with 100 ml of warm 140 mM sucrose-phosphate buffer, pH 7.4, via the abdominal aortic route. The kidneys were then harvested under aseptic conditions. Their capsule and fibroconnective tissue was dissected out. Multiple 1-mm-thick kidney slices from each group of experiments were prepared and immediately placed in 5 ml of prewarmed and -gassed (5% CO2-95% O2) Hanks' balanced salt buffer, pH 7.4, for 10 min. The buffer was supplemented with 4 mM sodium acetate, 1 mM sodium citrate, and 6 mM l-alanine (30). Then, kidney slices for each group of experiments were incubated for 30 min with the activator of EPAC (8-pCPT-2-O-Me-cAMP) and PKA or with forskolin in the absence or presence of 10−9 mol/l ANG II. The slices were then processed for NHE3 immunofluorescence microscopy. The kidney slices were fixed with a freshly prepared periodate-lysine-paraformaldehyde (PLP) solution (0.01 M NaIO4, 0.075 M lysine HCl, 0.25% paraformaldehyde in 0.1 M phosphate buffer, pH 7.2) for 3 h (67). The slices were then cut into 1-mm3 pieces, and fixation in PLP solution continued for 12 h at 4°C. Fixed renal tissues were embedded in an OCT compound to prepare 5-μm-thick cryostat sections. The sections were then incubated with polyclonal antibodies against NHE3 followed by reincubation with goat anti-rabbit IgG conjugated with FITC (Abcam). For double staining, the sections were also stained for F-actin with rhodamine-phalloidin (Molecular Probes).
Assay of NHE3 protein in cellular membrane.
The LLC-PK1 cells were grown in M199 media with a low concentration of d-glucose (5 mM), supplemented with FBS and antibiotics, as indicated above. After achieving 80% confluence, the cells were incubated for 30 min with PKA activator (6-MB-cAMP), EPAC activator (8-pCPT-2-O-Me-cAMP), or forskolin in the absence or presence of 10−9 mol/l ANG II. The cells were then washed with PBS three times. The surface-expressed proteins were then biotinylated with EZ-Link sulfo-LC-NHS-Biotin (Pierce) for 30 min at 25°C, per instructions of the vendor. After a rerinsing with PBS, the cells were incubated for 5 min with 100 mM glycine to quench and remove excess biotin reagent and reaction by-products. The cells were then lysed with RIPA buffer (50 mm Tris·HCl, pH 7.5, 150 mm NaCl, 0.1% SDS, 0.5% deoxycholate, 1% Nonidet P-40, 1 mm Na3V04, 1 mm PMSF, 10 μg/ml leupeptin, and 1 μg/ml aprotinin). Cellular debris was removed from the lysates by centrifuging at 2,500 g at 4°C for 30 min. The supernatants were collected and incubated with 50 μl of prewashed streptavidin-agarose beads (Pierce) at 4°C for 12 h in a microfuge Eppendorf tube. After a brief centrifugation, the pellets were washed sequentially with buffer A (50 mM Tris·HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA), buffer B (50 mM Tris·HCl, pH 7.4, 500 mM NaCl), and buffer C (50 mM Tris·HCl, pH 7.4). Proteins were eluted with 30 μl of 2× SDS-PAGE loading buffer and boiled for 5 min. The eluted proteins were fractionated by 10% of SDS-PAGE. The fractionated proteins were electroblotted onto nitrocellulose membranes in a wet transfer chamber under constant voltage. The membrane blots were probed with various NHE3 antibodies, and autoradiograms were developed using a chemiluminescence kit (Thermo Scientific).
Generation of eukaryotic expression constructs and stable transfectants.
Rap1a cDNA was generated by RT-PCR with Rap1a-specific primers, and it was cloned into pcDNA3.1 using the following primers: sense, 5′-CGGAGGGCTAGCACATCATGCGTGAGTACAAG-3′ with an Nhe1 site and antisense, 5′-CGGAGGGAATTCCTAGAGCAGCAGACA-3′ with an EcoRI site. The restriction sites in the primers are underscored. The sense orientation of the pcDNA3.1-Rap1a vector was identified by nucleotide sequencing. A mutant plasmid was generated by PCR mutagenesis of pcDNA3.1-Rap1a using the following primers: sense, 5′-TGGTCCTTGGTTCAGTAGGCGTTGGGAAGTC-3′ and antisense, 5′-GACTTCCCAACGCCTACTGAACCAAGGACCA-3′. This mutant was designated as Rap1a-G12V, in which there was a substitution of Gly → Val amino acid residue. Another mutant plasmid was generated by PCR mutagenesis of pcDNA3.1-Rap1a using the following primers: sense, 5′-GGAGGCGTTGGGAAGGCTGCTCTGACAGTTC-3′ and antisense, 5′-GAACTGTCAGAGCAGCCTTCCCAACGCCTCC-3′. This mutant was designated as Rap1a-S17A, in which there was a substitution of Ser → Ala amino acid residue. A third mutant plasmid was generated by PCR mutagenesis of pcDNA3.1-Rap1a using the following primers: sense, 5′-AGGGAATTTTTGTTGAAAAATATGACCCAGCGATAGAAATTCCTACA-3′ and antisense, 5′-TGTAGGAATCTTCTATCGCTGGGTCATATTTTTCAACAAAATTCCCT-3′. This mutant was designated as Rap1a-T35A, in which there was a substitution of Thr → Ala amino acid residue.
The plasmid constructs were then transfected into LLC-PK1 cells, and stable transfectants were selected by growing cells in the presence of G418 (800 μg/ml). The selected transfectants were then propagated in the presence of a relatively low concentration of G418 (200 μg/ml) and used for various studies.
Assay of Rap1a activity.
Rap1a activity (GTP form) was determined by a Rap1 GTP pull-down assay, as described in our previous publications (40, 64, 65). Briefly, the treated cells were harvested and disrupted with RIPA lysis buffer (50 mm Tris·HCl, pH 7.5, 150 mm NaCl, 0.1% SDS, 0.5% deoxycholate, 1% Nonidet P-40, 1 mm Na3V04, 1 mm PMSF, 10 μg/ml leupeptin, and 1 μg/ml aprotinin), and the lysate was centrifuged for 10 min at 10,000 g. The resulting supernatants were incubated with freshly prepared glutathione-Sepharose 4B beads coupled to a glutathione S-transferase (GST) fusion protein containing a Rap binding domain (RBD) of RalGDS proteins for 1 h to allow the association of activated Rap1a with the effector-GST fusion protein. The beads were washed three times with the RIPA buffer, suspended in sample buffer, and subjected to 12.5% SDS-PAGE. The fractionated gel proteins were transferred onto nitrocellulose membranes by electroblotting and processed for Western blot analysis, using anti-Rap1a antibodies and then the ECL Chemiluminescence system.
Total protein assay.
Proteins from cellular extracts from various experiments were prepared using RIPA buffer as described above. Protein concentration in the extracts was measured by a Bio-Rad assay, it was adjusted to 1 mg/ml, and equal amounts of protein were loaded in each lane of the gels subjected SDS-PAGE for Western blot analyses.
Gene expression of proinflammatory cytokines.
To quantify the gene expression of various inflammatory cytokines (IL-1β, IL-6, IL-8, and TNF-α), real-time-RT-PCR was employed. The culture media was saved for ELISAs to measure the levels of proinflammatory cytokines, while the cells were used for their gene expression studies. Total RNA was extracted from cells subjected to various treatments and transfected with Rap1a and Epac1 mutants, using TRIzol reagent (Life Technologies). Briefly, cells were cultured to achieve 80% confluence as indicated above. Culture medium replaced with M199 devoid of FBS. ANG II was added at a concentration of 10−9 mol/l to cells treated with NHE3 inhibitors or transfected with Rap1a mutants or Epac mutants. After 12 h, the media was aspirated and TRIzol was added to the culture petri dish for extraction of RNA. The genomic DNA was removed with RNAase-free DNAase treatment, and the RNA was reprecipitated. The concentration of RNA was determined by spectrometric analyses. It was then subjected to 1% agarose gel electrophoresis to assess the integrity of 28S and 18S RNA. First-strand cDNA was synthesized using a GoScript Reverse Transcriptase RT-PCR kit by following the vendor's instructions (Promega). Real-time RT-PCR was performed using the Step One Plus system (Life Technologies) following the manufacturer's instructions. Porcine primers for various cytokines were designed using Primer3 Software and synthesized by Integrated DNA Technologies. The respective sense and antisense sequences of primers of various cytokines were as follows: IL-1β: 5′-CAAAGGCCGCCAAGATATAA-3′ and 5′-GAAATTCAGGCAGCAACAT-3′; IL-6: 5′-AAGGTGATGCCACCTCAGAC-3′ and 5′-TCTGCCAGTACCTCCTTGCT-3′; IL-8: 5′-TGGCAGTTTTCCTGCTTTCT-3′ and 5′-CAGTGGGGTCCACTCTCAAT-3′; TNF-α: 5′-ATGGATGGGTGGATGAGAAA-3′ and 5′-TGGAAACTGTTGGGGAGAAG-3′; and β-actin: 5′-GGATGCAGAAGGAGATCACG-3′ and 5′-ATCTGCTGGAAGGTGGACAG-3′.
The real-time PCR mixture of template cDNA, sense and antisense primers, and Fast SYBRR Green Master Mix was placed in each well of a MicroAmp Optical 96-well plate in quadriplicate and covered with an optical adhesive cover. The PCR conditions were as follows: denaturation 30 s at 95°C, annealing 3 s at 95°C, and extension 30 s at 60°C. The PCR for various cytokines was performed at the same time under identical conditions in quadriplicate. Gene expression values were normalized against endogenous control β-actin utilizing the standard curve method for quantification. The results were expressed as the degree of change relative to the respective control under basal conditions.
Protein expression of proinflammatory cytokines.
To measure the amount of cytokines (IL-1β, IL-6, IL-8, and TNF-α), sandwich ELISAs were employed. The LLC-PK1 cells were plated onto six-well plates and maintained to achieve 80% confluence and transfected with Rap1a or Epac1 mutants. Culture medium was replaced with M199 devoid of FBS, and cells were treated with NHE3 inhibitors (quabain: 100 nm or S3226: 0.5–10 μm) in the presence or absence of 10−9 mol/l ANG II. After 12 h of treatment, the supernatants were saved and frozen at −80°C for assaying of various cytokines. Commercial kits (Abcam, Cambridge, MA) were used to determine their amounts in the media per instructions of the vendor. The reaction products in each well of the ELISA plates were subjected to spectroscopy at 450 nm on a Multiskan RC plate reader. The concentrations of cytokines were determined from a reference curve generated by using standards of recombinant porcine IL-1β, IL-6, IL-8, and TNF-α provided by the vendor in various kits. The minimum limits of detection of cytokines were as follows: IL-1β: 6 pg/ml; IL-6: 45 pg/ml; IL-8: 10 pg/ml; and TNF-α: 20 pg/ml.
Statistical analysis.
All results are depicted as means ± SD. Data were analyzed by one-way analysis of variance, and a P value of <0.05 was considered statistically significant.
RESULTS
Rap1, a GTPase, and Epac1, a cAMP-sensitive guanine exchange factor (GEF), have been shown to modulate pathobiology of proximal renal tubular epithelium (64, 65). In this regard, ANG II has also been reported to influence profoundly the intracellular levels of cAMP to regulate various transport mechanisms in the proximal epithelium (41) while also affecting the inflammatory cytokines in several other cell types (1, 20, 27, 43, 54, 55, 56, 58). These observations led us to delineate the interrelationship among ANG II, Epac1, Rap1a, NHE3, and inflammatory cytokines, which may ultimately dictate the pathogenetic outcome of the tubulointerstitial compartment.
Effect of ANG II on signaling molecules downstream of Epac1 (Rap1a) in LLC-PK1 cells.
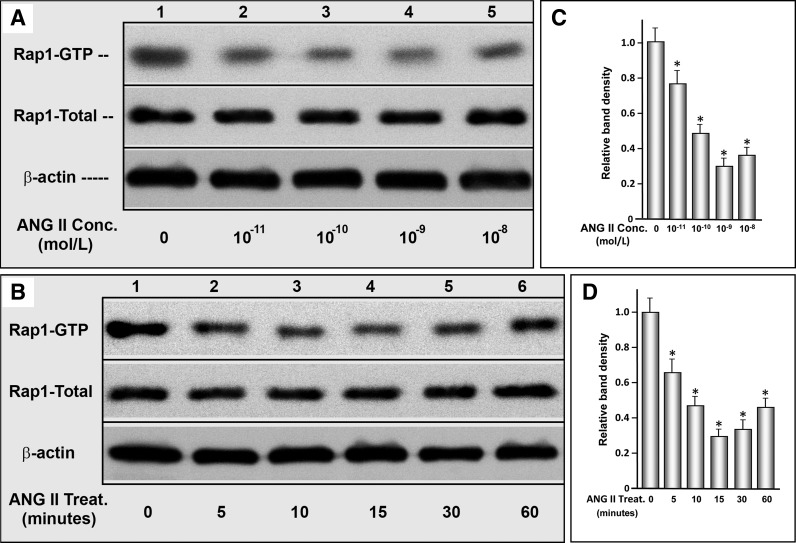
Rap1a is one of the downstream molecules that is directly affected by Epac1. Exposure of cells to various concentrations of ANG II (10−11-10−8 M) yielded a dose-dependent decrease in the activity of Rap1-GTP, as assessed by a pull-down assay in conjunction with Western blot analyses (Fig. 1, A and C, lanes 1–5, columns 1–5). In this assay, only the activated form Rap1, i.e., GTP form, can bind to the Rap1a binding protein. A maximal decrease in Rap1 activity was observed at 10−9 M ANG II; however, a further significant decrease in Rap1 activity was not seen with higher concentrations of ANG II. The expression of total Rap1, which conceivably included the bulk of the GDP form, was unchanged (Fig. 1A). Using this concentration of ANG II (10−9 M), the time course activity of Rap1 was investigated. A maximal decrease was observed between 15- and 30-min time points (Fig. 1, B and D). A further decrease extending up to 12 h was minimal (data not shown). The expression of total Rap1 was unchanged for up to several hours. In view of the time- and dose-dependent results, ANG II exposure was restricted to 30 min for Rap1 activity and NHE3 localization experiments, while its exposure was extended to 12 h for the de novo synthesized proinflammatory cytokine experiments.
Fig. 1.
Effect of ANG II on signaling molecules downstream of guanine exchange factor exchange protein activated by cAMP (Epac1; small GTPase Rap1a) in LLC-PK1 cells. A dose-dependent decrease in the activity of Rap1-GTP was observed in cells treated with various concentrations of ANG II (A and C, lanes 1–5, columns 1–5). A maximal decrease in Rap1 activity was observed at 10−9 M ANG II. Similarly, ANG II (10−9 M) treatment led to a time-dependent decrease in Rap1 activity. A maximal decrease was observed between the 15- and 30-min time points (B and D). Total Rap1a activity was unchanged. *P < 0.01 compared with control cells at 0 concentration of ANG II or at 0 time point.
Localization of NHE3 following treatment with ANG II, Epac, and PKA activators and forskolin.
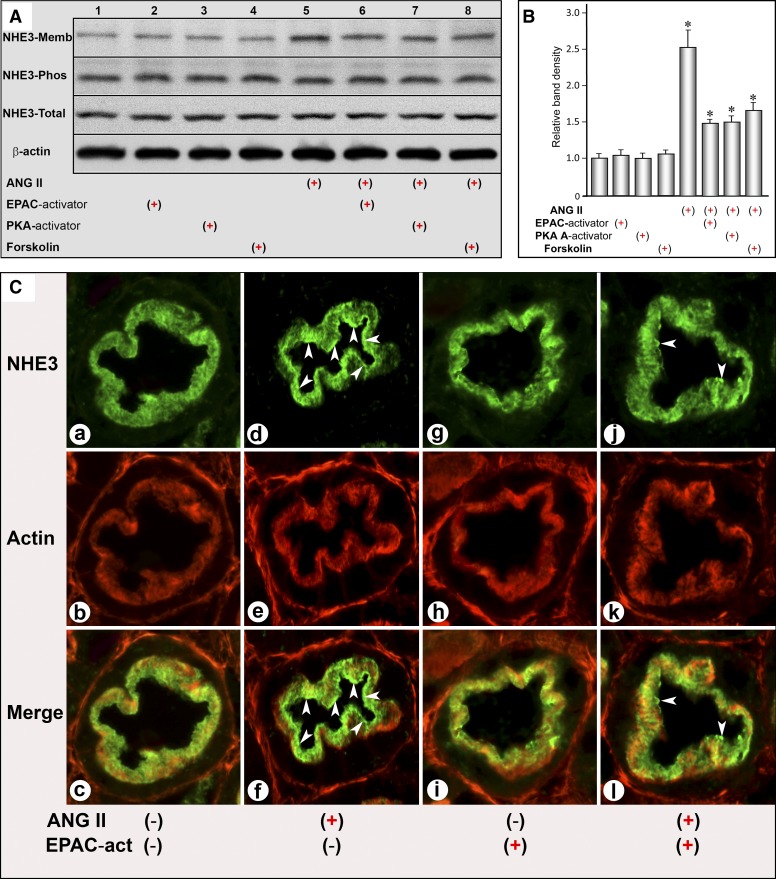
Morphological (ex vivo kidney slices) as well as biochemical (Western blotting) studies were performed to assess the localization of NHE3. For biochemical studies, the LLC-PK1 cells were treated with 10−9 M ANG II for 30 min. Then, cell fractionation was carried out. Treatment with ANG II led to an increased translocation or expression of NHE3 in the plasma membrane fraction, while total NHE3 and its phosphorylated form remained unchanged compared with the control, as assessed by immunoblot analyses (Figs. 2, A and B, lane 5, and column 5). Similarly, ex vivo kidney slices revealed an increased translocation of NHE3 from the basal toward the apical domain of the proximal tubular epithelia following ANG II exposure (Fig. 2C, D and F, arrowheads), suggesting NHE3 redistribution in the plasmalemma comparable to the observations made in LLC-PK1 cells by Western blot analyses. Concomitant treatment with ANG II and the Epac1 activator (8-pCPT-2′-O-Me-cAMP) significantly reduced the expression of NHE3 in the membrane fraction of LLC-PK1 cells (Fig. 2, A and B, lane 6, column 6), and it also decreased the localization in the apical domain of the proximal tubular epithelia in ex vivo kidney slices (Fig. 2C, J and L, arrowheads). Treatment of kidney slices or LLC-PK1 cells with the Epac1 activator alone did not induce any alterations in the NHE3 expression or its localization (Fig. 2, A and B, lane 2, column 2 and C, g and i). The Epac1 activator of PKA (6-MB-cAMP) or forskolin, which modulates the activities of Epac1 and PKA, significantly reduced the ANG II-induced increased expression of NHE3 in the cellular membrane fraction (Fig. 2, A and B, lanes 7 and 8, columns 7 and 8).
Fig. 2.
Localization of Na/H exchanger type 3 (NHE3) following treatment with ANG II, Epac and PKA activators, and forskolin. Western blot analyses showed that ANG II treatment caused an increased translocation of NHE3 in the plasma membrane fraction, while total NHE3 and its phosphorylated form were not significantly altered (Fig. 2, A and B, lane 5, column 5). Treatment of ex vivo kidney slices with ANG II also led to an increased translocation of NHE3 from base toward the apical domain of the proximal tubular epithelia (C, d and f, arrowheads). Concomitant treatment with ANG II and Epac1 activator (8-pCPT-2′-O-Me-cAMP) significantly reduced the expression of NHE3 in the membrane fraction of LLC-PK1 cells (A and B, lane 6, column 6) as well as in the proximal tubular epithelia of ex vivo kidney slices (C, j and l, arrowheads). Treatment of kidney slices or LLC-PK1 cells with Epac1 activator alone did not induce any alterations in NHE3 expression or its localization (A and B, lane 2, column 2; C, g and i). Treatment with an activator of PKA (6-MB-cAMP) or forskolin also reduced the ANG II-induced increased expression of NHE3 in the membrane fraction (A and B, lanes 7 and 8, columns 7 and 8). *P < 0.01 compared with control cells with no treatment with ANG II.
Authentication of Rap1a activity in Rap1a mutants and effect of various mutants on NHE3 localization/expression following exposure to ANG II or Epac activator in LLC-PK1 cells.
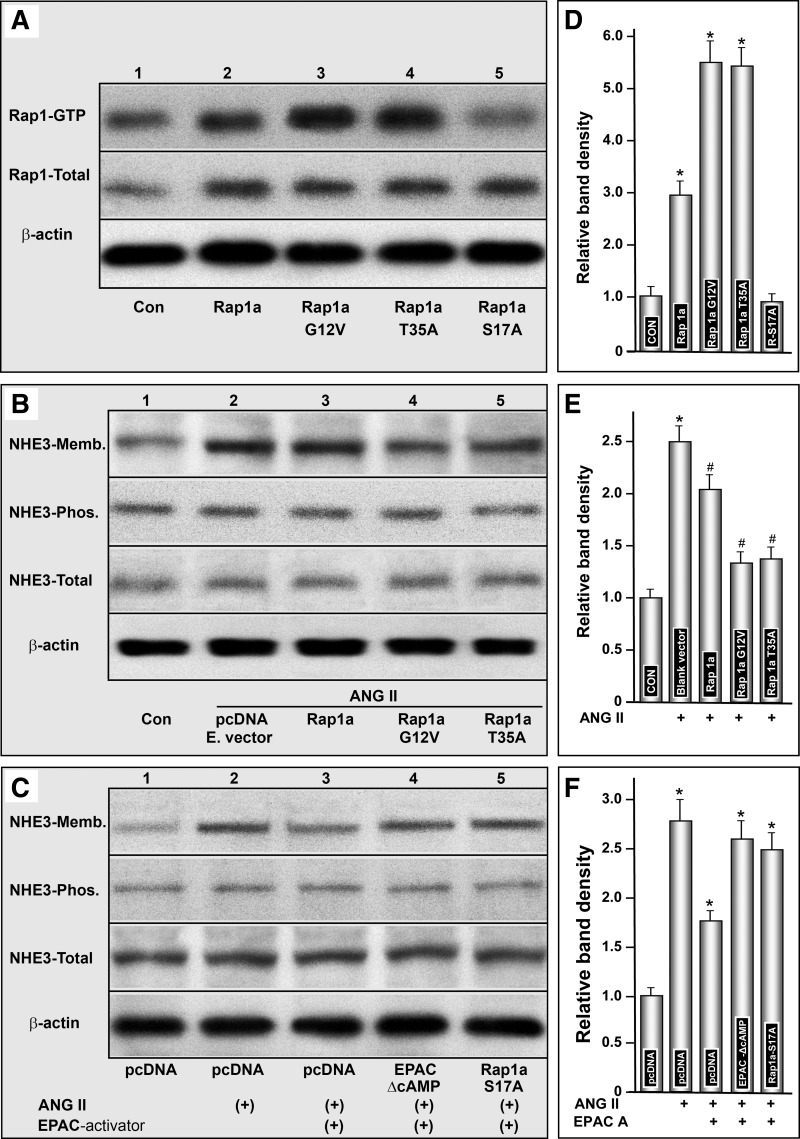
To assess whether the effects of ANG II are mediated via Epac1 and its downstream signaling GTPase Rap1a, which in turn modulates the translocation of NHE3, a detailed characterization of Rap1a was carried out. First, various putative dominant negative and positive mutants were generated and their activities were tested. Transfection of Rap1a pcDNA in LLC-PK1 cells increased the total as well as GTP form activity. However, the increase in the activated GTP form was highly significant (Fig. 3, A and D, lane 2, and column 2). No significant further increase in total Rap1 activity was observed with the transfection of Rap1a mutants (Fig. 3A, middle). Interestingly, transfection of Rap1a-G12V and Rap1a-T35A mutants markedly increased the activated form of Rap1a-GTP activity, compared with nontransfected or cells transfected with Ra1a pcDNA alone (Fig. 3, A and D, lanes 3 and 4, columns 3 and 4). Since these mutants increased the activity dramatically, they were designated as dominant-positive mutants. Transfection of Rap1a-S17A decreased the Rap1a-GTP activity lower than even in nontransfected cells (Fig. 3, A and D, lane 5, and column 5). This mutant was designated as dominant-negative.
Fig. 3.
Authentication of activity in various Rap1a mutants and their effect on NHE3 localization/expression following treatment with ANG II and Epac activator in LLC-PK1 cells. Transfection of Rap1a pcDNA in LLC-PK1 cells increased the total as well as GTP form of activity (A and D, lane 2, column 2). Transfection of Rap1a-G12V and Rap1a-T35A mutants (dominant positive) markedly increased the activated form of Rap1a-GTP activity, while the total activity was similar to that seen with the transfection of Rap1a pcDNA alone (A and D, lanes 3 and 4, columns 3 and 4). Transfection of Rap1a-S17A (dominant negative) decreased the Rap1a-GTP activity more than even in nontransfected cells (A and D, lane 5, column 5). Treatment with ANG II induced a remarkable increase in the translocation of NHE3 to the membrane fraction of LLC-PK1 cells transfected with the empty vector (B and E, lane 2, column 2). ANG II-induced translocation of NHE3 was slightly reduced with the transfection of Rap1a pcDNA (B and E, lane 3, column 3), while dramatically reduced with the transfection of dominant-positive mutants (Rap1a-G12V and Rap1a-T35A; B and E, lanes 4 and 5, columns 4 and 5). In another series of experiments, the ANG II-induced expression of NH3 in the membrane fraction was found to be decreased with concomitant treatment with the Epac1 activator in cells transfected with empty pcDNA (C and F, lane 3 vs. 2, column 3 vs. 2). NHE3 translocation was largely restored with the transfection of cells with either Epac1 mutant EPAC-ΔcAMP or Rap1a S17A dominant-negative mutant, and it was comparable to ANG II-induced redistribution of NHE3 in cells transfected with empty pcDNA even in the presence of the Epac1 activator (C and F, lanes 4 and 5 vs. 2, columns 4 and 5 vs. 2). These observations suggested an interrelationship among the effects exerted by ANG II, Epac1, GTPase, Rap1a, and NHE3, which conceivably may mean that this cascade of events would modulate diverse biological processes, including various inflammatory processes. *P < 0.01 compared with control cells with no treatment with ANG II. #P < 0.01 compared with cells transfected with empty pcDNA and treated with ANG II.
These mutants were utilized to assess the translocation of NHE3 in the presence or absence of ANG II (10−9 M) or the Epac1 dominant-negative mutant (EPAC-ΔcAMP) or activator (8-pCPT-2′-O-Me-cAMP). In cells transfected with empty vector, ANG II induced a remarkable increase in the translocation of NHE3 to the membrane fraction of LLC-PK1 cells (Fig. 3, B and E, lane 2, and column 2). Transfection of Rap1a pcDNA reduced to a certain extent the ANG II-induced translocation of NHE3 (Fig. 3, B and E, lane 3, and column 3). Remarkably, transfection of the dominant-positive mutant (Rap1a-G12V and Rap1a-T35A) notably reduced the translocation of NHE3 (Fig. 3, B and E, lanes 4 and 5, columns 4 and 5). Interestingly, the expression of total NHE3 and its phosphorylated form remained unchanged compared with the control, as assessed by Western blot analyses (Fig. 3, B and E).
In an another series of experiments, the expression of NHE3 in the membrane fraction was noted to be increased in cells transfected with empty pcDNA vector and exposed to ANG II (Fig. 3, C and F, lane 2, and column 2). While addition of the Epac1 activator attenuated the membrane translocation that was induced with the ANG II exposure (Fig. 3, C and F, lane 3, and column 3). The membrane translocation was largely restored with the transfection of cells with either Epac1 mutant EPAC-ΔcAMP or dominant-negative mutant Rap1a S17A, and it was comparable to ANG II-induced redistribution of NHE3 in cells transfected with empty pcDNA even in the presence of the Epac1 activator (Fig. 3, C and F, lanes 4 and 5 vs. 2, columns 4 and 5 vs. 2). These observations suggested that Rap1a directly or upon activation of upstream effector Epac1 reduces the ANG II-induced translocation of NHE3. Since ANG II is intimately involved in diverse biological processes, including inflammation, we explored the possibility of whether ANG II induction of proinflammatory cytokines is mediated via Epac1-modulated Rap1a GTPase and NHE3.
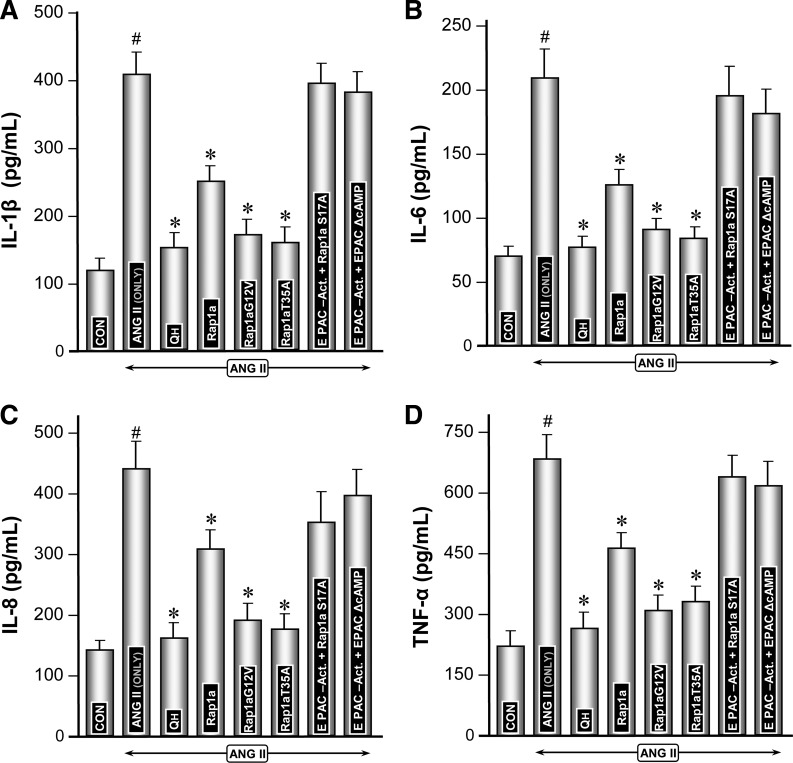
Modulation of expression of proflammatory cytokines by Rap1a GTPase and Epac1 and Rap1a mutants under the influence of ANG II.
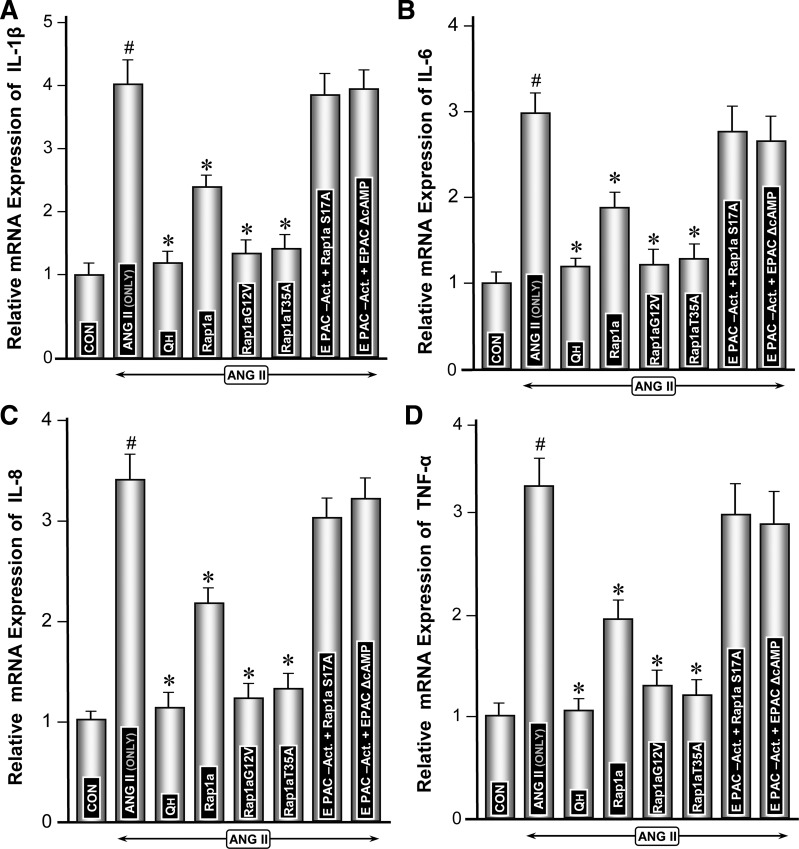
The expression of major proinflammatory cytokines, i.e., IL-1β, IL-6, IL-8, and TNF-α, was assessed. The gene expression of cytokines in LLC-PK1 cells was quantified by real-time PCR while ELISAs were performed to determine their levels in the culture media. The relative mRNA expression was considered as one in the control untreated cells, while cytokine levels were expressed as picograms per milliliter compared with the curve generated from standards (0–1,000 pg/ml) provided in each of the ELISA kits for various cytokines. Overall, the change in expression of various cytokines was more or less similar following a given treatment in LLC-PK1 cells (Figs. 4 and 5). ANG II treatment led to a remarkable induction (3- to 4-fold) of all the proinflammatory cytokines compared with the control (Figs. 4, A–D, and 5, A–D, column 2 vs. 1). Transfection of Rap1a-pcDNA reduced ANG II induction of cytokines by ∼30% (Figs. 4, A–D, and 5, A–D, column 4). Transfection of dominant-positive mutants (Rap1a-G12V and Rap1a-T35A) significantly reduced the expression of cytokines (Figs. 4, A–D, and 5, A–D, columns 5 and 6). Interestingly, the Rap1a dominant-negative mutant (Ra1a-S17A) normalized the ANG II induction of cytokines even in presence of the Epac1 activator (Fig. 4, A–D, and 5, A–D, column 7). Similarly, Epac1 mutant EPAC-ΔcAMP did not reduce the mRNA as well as protein expression induced by ANG II despite the concomitant treatment of cells with the Epac1 activator (Figs. 4, A–D, and 5, A–D, column 8). Intriguingly, treatment of cells with NHE3 inhibitor quabain markedly reduced the ANG II-induced expression of all the cytokines (Figs. 4, A–D, and 5, A–D, column 3), and reduction could be achieved comparable to basal levels. These observations suggested that Epac1, Rap1a, and NHE3 together play a role in the modulation of expression of proinflammatory cytokines at various steps of the signaling cascade.
Fig. 4.
Modulation of gene expression of proinflammatory cytokines (IL-1β, IL-6, IL-8, and TNF-α) by Rap1a GTPase and Epac1 and Rap1a mutants under the influence of ANG II. Real-time PCR analyses revealed that the change in mRNA expression of various cytokines was similar following a given treatment of LLC-PK1 cells, and the relative mRNA level under basal control conditions was designated as 1 on the ordinate scale (A–D). ANG II treatment caused a marked increase in the proinflammatory cytokines (A–D, column 2 vs. 1). Transfection of Rap1a-pcDNA slightly reduced the ANG II-induced expression (A–D, column 4). Transfection of dominant-positive mutants (Rap1a-G12V and Rap1a-T35A) markedly reduced their expression (A–D, columns 5 and 6). Transfection of Rap1a dominant-negative mutant (Ra1a-S17A) or Epac1 mutant (EPAC-ΔcAMP) normalized the ANG II induction of cytokines even in the presence of the Epac1 activator (A–D, columns 7 and 8). Treatment of cells with quabain (QH) markedly reduced the ANG II-induced expression of all the 4 cytokines (A–D, column 3). #P < 0.01 compared with control cells with no treatment with ANG II. *P < 0.01 compared with cells treated with ANG II.
Fig. 5.
Protein expression of proinflammatory cytokines following ANG II treatment of LLC-PK1 cells transfected with Rap1a GTPase and Epac1 and Rap1a mutants. The basal protein expression levels for each of the cytokines (IL-1β, IL-6, IL-8, and TNF-α) in culture media were different, and they are expressed as pg/ml. However, the expression of all the cytokines increased by 3- to 4-fold following ANG II treatment (A–D, column 2 vs. 1). Like mRNA expression, the transfection of Rap1a-pcDNA or its mutants, Rap1a-G12V and Rap1a-T35A, attenuated the expression of cytokines, and the reduction was much more notable in cells transfected with dominant-positive mutants (A–D, columns 4–6). Transfection of the dominant-negative Rap1a mutant (Rap1a-S17A) or Epac1 mutant (EPAC-ΔcAMP) restored the levels of cytokines in cells comparable to those treated with ANG II alone (A–D, columns 7 and 8). Reduction of ANG II-induced increased cytokine de novo synthesis was highly attenuated following quabain treatment (A–D, column 3). #P < 0.01 compared with control cells with no treatment with ANG II. *P < 0.01 compared with cells treated with ANG II.
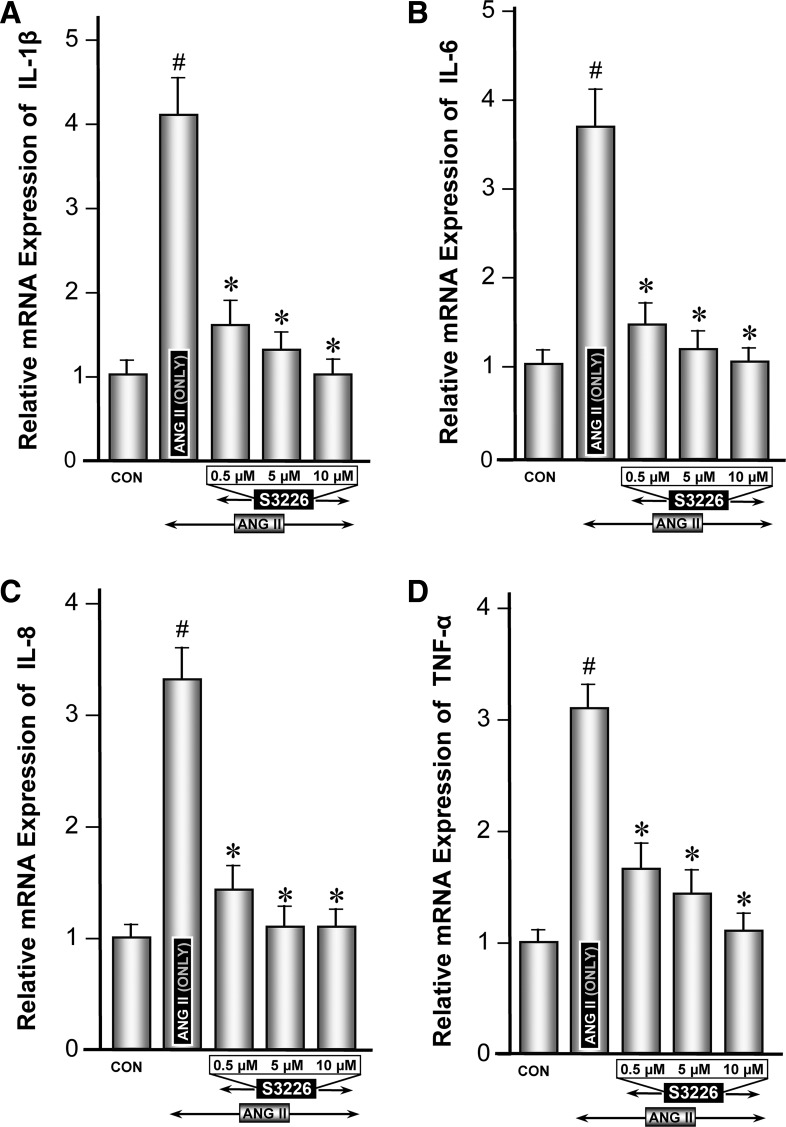
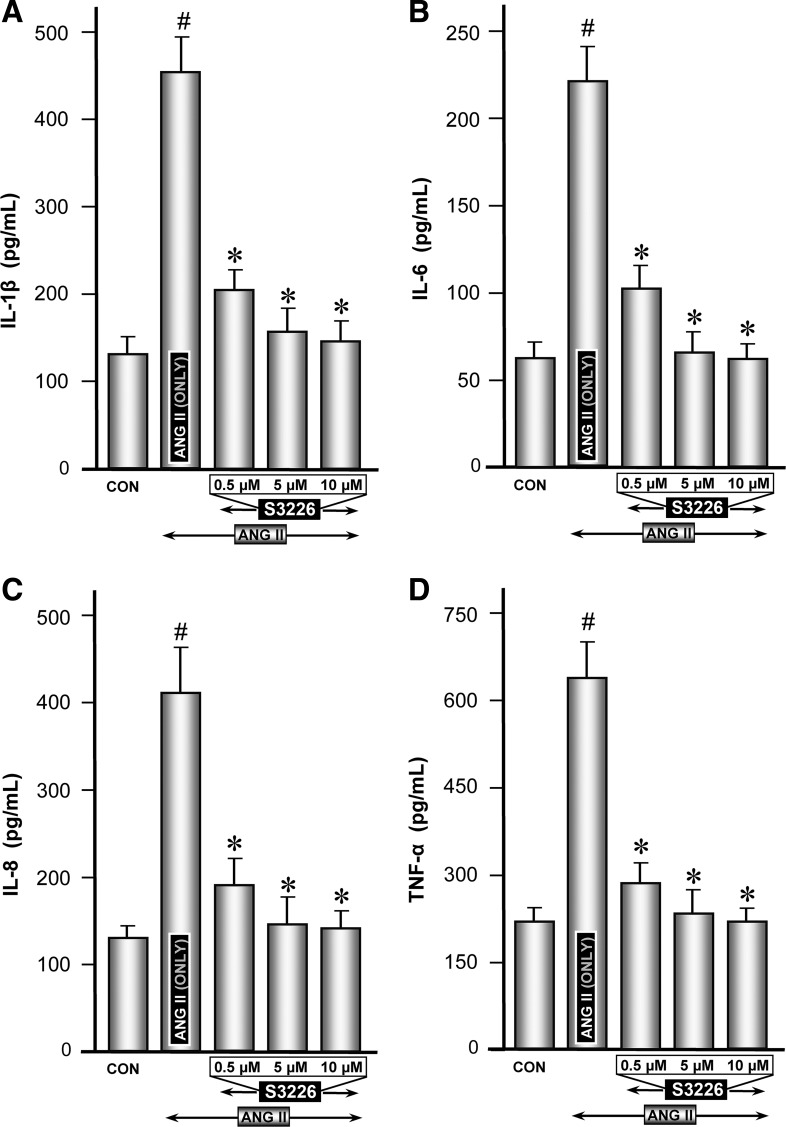
Modulation of ANG II-induced expression of proinflammatory cytokines by NHE3-specific inhibitor S3226.
Inhibition of NHE3 by quabain may not be considered highly specific; thus experiments pertaining to proinflammatory cytokines were repeated with one of the specific inhibitor of NHE3, i.e., S3226. Like the experiments described above, ANG II induced a remarkable induction in the mRNA and protein expression of all the cytokines, compared with the control (Figs. 6, A–D, and 7, A–D, column 2 vs. 1). In general, S3226 inhibition of ANG II-induced mRNA or protein expression of proinflammatory cytokines was universally seen for all the cytokines, but to a varying degree (Figs. 6, A–D, and 7, A–D, columns 3–5). Above all, the inhibition of S3226 (Mr ∼415) was dose dependent in the range 0.5–10 μM. No discernible cellular toxicity was observed up to a concentration of 20 μM in the culture medium, as monitored by trypan blue exclusion and MTT assay methods. The cell cultures exhibiting >95% viability were considered suitable for various experiments. The degree of inhibition by S3226 was more reflected in mRNA levels than protein expression. These findings suggested that S3226 may be a specific inhibitor of NHE3, a molecule downstream of Rap1a, and it modulates the expression of ANG II-induced expression of inflammatory cytokines.
Fig. 6.
Modulation of ANG II-induced gene expression of proinflammatory cytokines by NHE3-specific inhibitor S3226. Real-time PCR analyses revealed that ANG II induced a remarkable induction in the mRNA expression of cytokines, compared with control (A–D, column 2 vs. 1). Concomitant treatment with S3226 (0.5–10 μM) caused a dose-dependent inhibition of ANG II-induction of proinflammatory cytokines (A–D, columns 3–5). #P < 0.01 compared with control cells with no treatment with ANG II. *P < 0.01 compared with cells treated with ANG II.
Fig. 7.
Status of protein expression of proinflammatory cytokines following treatment with ANG II and NHE3-specific inhibitor S3226. Protein levels were measured in the culture media of cells, which were utilized for mRNA expression. ANG II treatment induced a marked increase in the secretion of de novo synthesized cytokines in the culture media (A–D, column 2 vs. 1). Concomitant treatment with S3226 caused a significant attenuation of ANG II-induced de novo synthesized proinflammatory cytokines in a dose-dependent manner (A–D, columns 3–5). #P < 0.01 compared with control cells with no treatment with ANG II. *P < 0.01 compared with cells treated with ANG II.
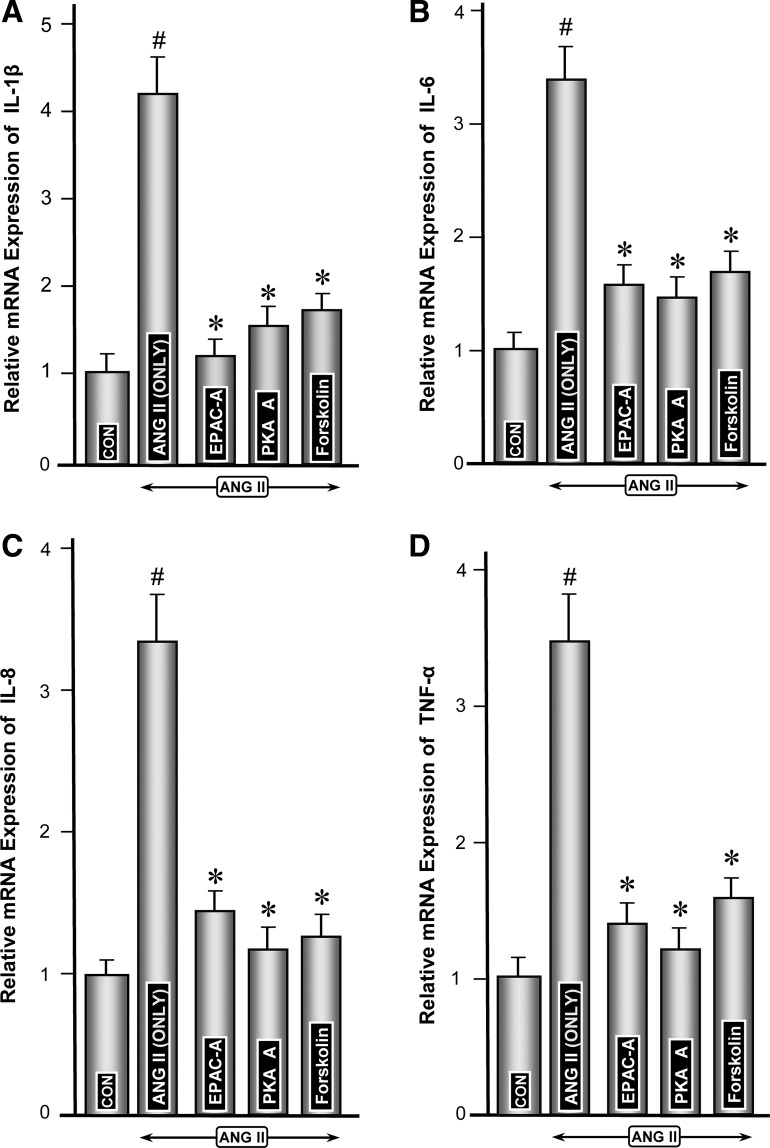
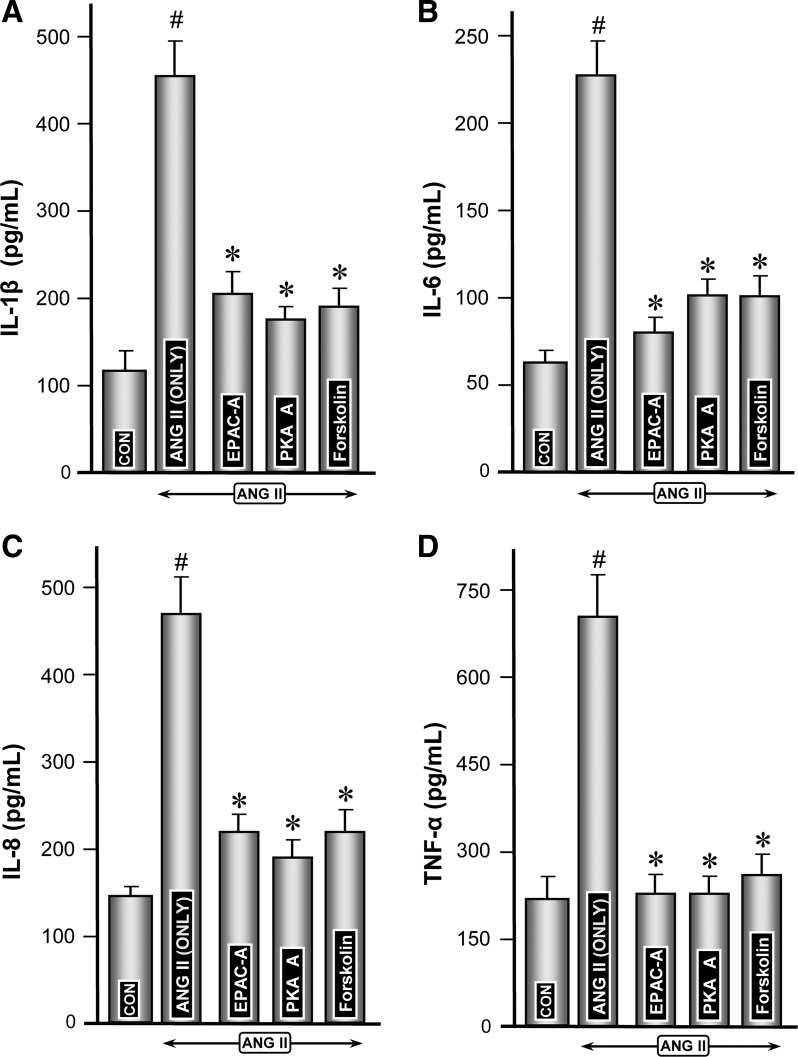
Modulation of ANG II-induced expression of proinflammatory cytokines by activators of Epac (8-pCPT-2′-O-Me-cAMP) and PKA (6-MB-cAMP) and forskolin.
To assess whether the cAMP-sensitive signaling pathways and molecules modulate the ANG II-induced expression of proinflammatory cytokines, experiments with these two activators were performed. Markedly increased ANG II-induced mRNA expression of various cytokines was highly attenuated in cells concomitantly treated with activators of Epac1 or PKA (Fig. 8, A–D, columns 3 and 4 vs. 2). Both exhibited comparable reduction in cytokine expression. Forskolin, which modulates cAMP-sensitive Epac1 and PKA, also significantly reduced the mRNA expression of various cytokines (Fig. 8, A–D, column 5 vs. 2). Attenuation of protein expression of various cytokines following treatment with Epac1 or PKA activators or forskolin paralleled their gene expression (Fig. 9, A–D, columns 3, 4, and 5 vs. 2), suggesting Epac1, a cAMP-sensitive GEF, is intimately involved in the modulation of ANG II-induced changes in the major proinflammatory cytokines.
Fig. 8.
Modulation of ANG II-induced mRNA expression of proinflammatory cytokines by activators of Epac (8-pCPT-2′-O-Me-cAMP) and PKA (6-MB-cAMP) and forskolin. Highly induced ANG II-induced mRNA expression of various cytokines was notably reduced in cells treated with activators of Epac1 and PKA (A–D, columns 3 and 4 vs. 2). Forskolin also reduced the mRNA expression (A–D, column 5 vs. 2). The degree of reduction in mRNA caused by activators of Epac1 and PKA and forskolin were comparable among various cytokines. #P < 0.01 compared with control cells with no treatment with ANG II. *P < 0.01 compared with cells treated with ANG II.
Fig. 9.
Protein expression of cytokines following treatment with ANG II and molecules pertinent to cAMP-sensitive signaling pathway molecules, i.e., Epac (8-pCPT-2′-O-Me-cAMP) and PKA (6-MB-cAMP) and forskolin. Both the Epac1 and PKA activators significantly attenuated the ANG II-induced expression of de novo synthesized cytokines in the culture media (A–D, columns 3 and 4 vs. 2). Similarly, a comparable reduction in ANG II-induced protein expression was observed following forskolin treatment (A–D, column 5 vs. 2), suggesting cAMP-sensitive molecules are intimately involved in the modulation of ANG II-induced major proinflammatory cytokines. #P < 0.01 compared with control cells with no treatment with ANG II. *P < 0.01 compared with cells treated with ANG II.
DISCUSSION
The results of this investigation suggest that ANG II could modulate the biology of inflammatory cytokines in tubular cells by a series of events while having the small GTPases, like Rap1a, as a central nodal point with certain upstream and downstream molecules essentially participating in this pathway.
Our previous work suggests that small monomeric G proteins, i.e., the Ras family of proteins, modulate the biology of glomerular as well as tubular cells, especially in diabetic states where the RAS is actively operative (36, 40, 64, 65). However, it seems that the information in regard to the ANG II modulation of specific small GTPase is rather scantly reported in the literature. The role of heterodimeric G large proteins in the biology of tubules, including ANG II-induced hypertrophy, activation of Na+-K+-ATPase in normal and diabetic states, and regulation of NHE3 activity, has been reported (3, 4, 52, 61, 69). Interestingly, the effects of ANG II on various signaling pathways are biphasic, meaning thereby that at low concentrations it increases the Na/H exchanger activity while inhibition is observed at higher concentrations (31). Since the effects of ANG II on the Ras family of proteins, i.e., Rap1, are unknown and the fact they seem to be biphasic, it was obligatory to first assess the dose at which one would have notable effect on the activity of Rap1. A dose-dependent decrease in Rap1 activity was observed with the treatment with ANG II, the maximal being at a concentration of 10−9 mol/l (Fig. 1, A and C). Using this concentration, the effect was remarkably discernible between 15 and 30 min (Fig. 1, B and D). This time point and dose were then used to assess the status of upstream, i.e., Epac1, and downstream, i.e., NHE3, signaling molecules following ANG II treatment of tubular cells. Of note, the total activity of Rap1 was not significantly altered, suggesting that these effects on the GTP form of Rap1a are authentic.
The effects of ANG II upstream and downstream of small GTPase Rap1a were investigated simultaneously using various cAMP-sensitive activators of Epac1 (8-cAMP) and of PKA (6-MB-cAMP) since NHE3 expression, activity, and trafficking or translocation are to a variable extent modulated by cAMP levels as well as by (de)phosphorylation-dependent and -independent processes (10, 11, 16, 38, 41, 74). In this regard, there are studies which indicate that ANG II via its receptors increases NHE3 activity and translocation with the decrease in the cAMP concentration in the cellular compartment or tubular fluid (11, 30, 41). In line with these observations, we noted that following ANG II treatment of LLC-PK1 cells an increase in the expression or translocation of NHE3 in the membrane fraction was observed (Fig. 2, A and B). Similarly, the experiments with ex vivo slices indicated a strong apical expression of NHE3 (Fig. 2C, d and f), suggesting that ANG II heavily influences the translocation of the transporter besides expression or activity, as reported previously (30). The ANG II-induced apical expression was markedly reduced with the concomitant treatment with the Epac1 activator 8-cAMP (Fig. 2C, j and i). Similarly, NHE3 expression was reduced significantly in the membrane fraction following concomitant treatment with 8-cAMP without any change in the total or phosphorylated form of NHE3 (Fig. 2, A and B), suggesting cAMP-regulated pathways initiated by ANG II treatment are involved. In addition to the Epac1 activator, we also investigated other cAMP-sensitive molecules, such as PKA and forskolin, since Rap1a is believed to be the major substrate of PKA that can be activated by this kinase (13, 50). Treatment with both the PKA activator and forskolin significantly reduced the ANG II-induced expression or translocation of NHE3 in the membrane fraction (Fig. 2, A and B), thus confirming that the cAMP-dependent processes are involved in functionality of this exchanger, as has been also observed in other systems (11, 30, 41).
To strengthen the notion of an anticipated interrelationship among Epac1, Rap1a, and NHE3, mutants of Epac1 and Rap1a were employed. Epac1 includes a GEF and a cAMP domain, and the latter upon binding to Rap1a modulates its activity (13, 23). A mutant of Epac1 was obtained in which cAMP was modified with the loss of its activity such that it behaves as a dominant-negative construct, as reported in our previous publications, and it was designated EPAC-ΔcAMP (64). Another three constructs of Rap1a were generated as well like the ones we had constructed for its isoform Rap1b previously (40). Both the isoforms exhibit 95% homology, although certain of their functions may differ (66). Nevertheless, the sites of mutation introduced had identical amino acid residues. The constructs with mutation at positions 12 and 35 behaved as dominant-positive while at position 17 exerted a dominant-negative effect. Their effects were authenticated by activity assays, where an increased Rap1a activity was observed following transfection of Rap1a-G12V and Rap1a-T35A pcDNA into LLC-PK1 cells, while a significant decrease was seen upon transfection of Rap1a-S17A pcDNA (Fig. 3, A and D). These mutant Epac1 and Rap1a constructs were instrumental to assess their influence on NHE3 translocation following ANG II treatment. Transfection of Rap1a-pcDNA alone caused a mild decrease in the ANG II-induced translocation/expression of NHE3 in the membrane fraction, and this decrease was accentuated with the transfection of dominant-positive Rap1a-pcDNA constructs (Fig. 3, B and E). On the other hand, transfection of the dominant-negative Rap1a-pcDNA construct or EPAC-ΔcAMP normalized the ANG II effects on NHE3 membrane expression in the presence of Epac1 activators (Fig. 3, C and F), suggesting indeed there is cross talk among these molecules, i.e., Epac1, Rap1a, and NHE3, following ANG II treatment. Certainly, regulation of NHE3 by Epac1 is known (30), but this investigation identifies a small GTPase, i.e., Rap1a, as an intermediary molecule between Epac1 and NHE3 in a scenario of the events downstream of ANG II. Besides Rap1a, modulation of expression or translocation or activity of NHE3 by heterodimeric G or the Rho family of proteins has been reported (3, 4); however, their direct interaction, if any, remains to be defined as is the case of dipeptidyl peptidase IV that forms a multimeric complex with NHE3 and conceivably thus regulates its activity/expression/translocation (22).
Another major aspect of this investigation was to delineate whether this pathway leads to the modulation of inflammatory cytokines since ANG II does exert inflammatory effects relevant to tubular pathobiology. Overall, the information gathered from many clinical literature reports supplemented with in vivo experiments suggests that the biology of the RAS or ANG II, oxidant stress, and inflammatory cytokines is intertwined such that the pathological effects manifest in all the compartments of the kidney, like in chronic kidney disease, such as diabetic nephropathy and tubulointerstitial fibrosis (47, 53, 75). Interestingly, the oxidant stress whether originating because of inflammation or ANG II stimulation can upregulate ANG II type 1 receptor (AT1R) with a multitude of responses, including activation of signaling pathways and transcription factors (6, 18). One of the important transcription factors that is induced by ANG II or following AT1R or AT2R upregulation or activation of small GTPase (Rho-kinase) seems to be NF-κB. The latter apparently regulates a wide variety of cellular processes as well as being involved in the modulation of inflammatory cytokines like TNF-α, IL-6, and IL-8 and chemokines such asMCP-1 (54, 55, 58). The induction of cytokines and activation of transcription factor NF-κB following ANG II treatment have been described in a number cell culture systems, including mesangial, vascular smooth muscle (VSM), neuronal cells, and hepatocytes (1, 8, 20, 43). However, the mechanism(s) of activation of NF-κB by ANG II may be different depending upon the cell type (8). For instance, in VSM cells the ANG II-induced activation and translocation of NF-κB may occur following proteolytic degradation of its inhibitor, IkB, that under basal conditions partners with the transcription factor and remains in a heterdimeric state. In hepatocytes, ANG II induces larger precursor forms, e.g., NF-κB1 (p50/p105), that undergo ubiquitin/proteasomsal processing of the C-terminal region of ankyrin repeats to generate mature NF-κB, and this process is independent of IkB turnover (8). These cell culture studies would indicate that the in vitro system used in this investigation may be appropriate to investigate the yet to be defined effects of ANG II on the induction of inflammatory cytokines via the small GTPase Rap1a in LLC-PK1 cells, a proximal tubular cell line. The fact that the induction of a vast number of inflammatory cytokines has been also reported in ischemic and/or toxic form of tubular injury make it seem that LLC-PK1, a well-characterized tubular epithelial cell line, would be certainly suitable for our current ANG II-related studies (2, 51).
Following ANG II treatment of LLC-PK1 cells, a three- to fourfold increase in mRNA expression, as determined by real-time PCR, of IL-1β, IL-6, IL-8, and TNF-α was observed (Fig. 4, A–D). Similarly, an increase in the protein expression, as measured by ELISA, was observed (Fig. 5, A–D). The selection of these inflammatory biomarkers was based upon the Chronic Renal Insufficiency Cohorts study that highlights their value in the deterioration of renal functions in chronic kidney disease patients (25). These results were also reassuring since they were in line with those reported for in vivo ANG II infusion studies (54, 55, 58). Interestingly, ANG II induction of inflammatory cytokines was slightly reduced in cells transfected with Rap1a-pcDNA, and the effect was distinctly notable in those transfected with dominant-positive Rap1a plasmid constructs (Figs. 4, A–D, and 5, A–D). The cells transfected with the Epac mutant EPAC-ΔcAMP or the dominant-negative Rap1a mutant construct did not exhibit any significant change in ANG II-induced expression of cytokine in the presence of the Epac activator 8-cAMP. In general, mRNA and protein expression profiles were similar for all four cytokines investigated following various treatments. These results established that ANG II modulates cytokine expression by involving Epac1 and Rap1a signaling molecules, and the small GTPases are intricately linked to exert the ANG II downstream effects.
The next question of whether or not NHE3, a molecule downstream of Rap1a, modulates the ANG II-induced effect on inflammatory cytokines was addressed. As indicated earlier that ANG II is known to modulate the activity of the Na+/H+ exchanger by utilizing various receptors in different pathways and consequential anticipated further downstream effects, we therefore elected to use inhibitors of NHE3 activity to assess the effect on the expression of various inflammatory cytokines (12, 29). Initially, quabain, a cardiac glycoside that is known to attenuate NHE3 activity and expression, was used (48). Treatment of cells with quabain significantly attenuated the mRNA as well as protein expression of various cytokines (Figs. 4, A–D, and 5, A–D). Since the effects of quabain may not be specific, thus S3226, a specific inhibitor of NHE3 (32, 63), was employed to assess the ANG II-induced expression on various cytokines. A dose-dependent decrease in the expression of inflammatory cytokines was observed following S3226 treatment (Figs. 6, A–D, and 7, A–D). The effect of NHE3 inhibitor S3226 most likely is specific since the decrease in both mRNA and protein expression was similar in all the cytokines studied. These observations may go well together in situations like acute tubular injury, where there is an induced inflammatory cytokine expression, and improvement in renal functions are observed following the administration of NHE3 inhibitor S3226 (2, 32, 51). Intriguingly, severe inflammation induced by LPS injection or administration of inflammatory cytokines TNF-α or IL-1β leads to a downregulation of the Na+/H+ exchanger and associated acute renal failure (59). Similarly, in patients with ulcerative colitis, inflammatory cytokines cause NHE3 dysfunctions, although these may be related to the downregulation of NHE3 interacting PDZ-domain protein (PDZK1) rather than NHE3 itself (71). These deviations in various results in different studies would mean that molecular mechanisms regulating the functionality or expression of NHE3 may be complex, and there could be multiple levels of its modulation which above all may be organ and disease specific. Thus more investigative work is needed to understand NHE3 functionality, trafficking, expression, and phosphorylation or dephosphorylation status with respect to the pathobiology of inflammatory cytokines in various target organ systems and disease processes. Finally, since we had established that NHE3 trafficking or apical translocation of NHE3 was cAMP sensitive (Fig. 2, A–C), the status of inflammatory cytokines was assessed following the treatment of LLC-PK1 cells with activators of Epac1, PKA, and forskolin. These activators significantly reduced the ANG II-induced mRNA as well as protein expression of all the inflammatory cytokines investigated (Figs. 8, A–D, and 9, A–D), suggesting an intricate relationship between ANG II and cAMP-sensitive guanine exchange factor Epac1 and subsequent downstream signaling events.
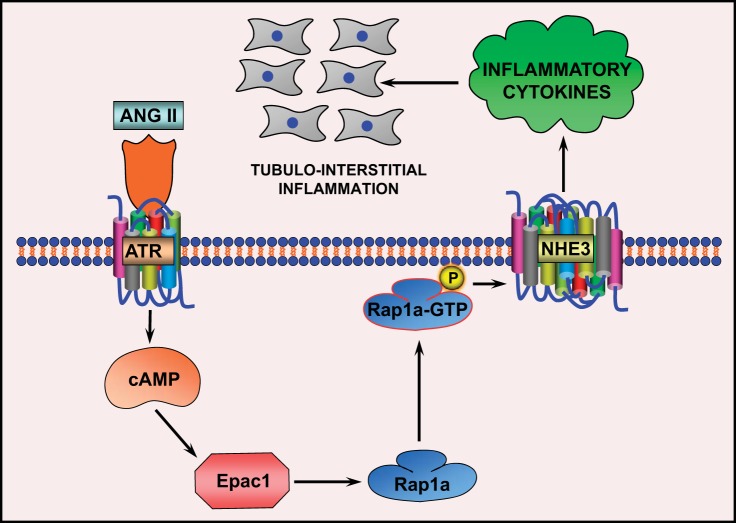
In summary, the current study delineates the sequence of events initiated by ANG II that leads to upregulation of inflammatory cytokines with intervening steps regulated by a guanine exchange factor, Epac1, a small GTPase, Rap1a, and Na+/H+ exchanger, NHE3, in LLC-PK1 cells, a tubular cell line (Fig. 10). The delineation of this Epac1-Rap1a-NHE3 pathway to highlight the ANG II induction of inflammatory cytokines may have some bearing on tubulointerstitial pathobiology relevant to various renal diseases. The question as to how individual steps of this pathway modulate transcription, translation, and posttranslation of downstream molecules would be the subject of future investigations.
Fig. 10.
Schematic drawing depicting the flow of conceivable events that follow exposure of LLC-PK1 cells to ANG II, leading to perturbation in cAMP levels and modulation of Epac1. This would lead to alterations in Rap1a activity, and that in turn would affect the trafficking of NHE3 and finally the expression of critical cytokines that are involved in the pathogenesis of tubulointerstitial inflammation.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK60635.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.X. and D.J. performed experiments; P.X. prepared figures; D.J. drafted manuscript; P.K.D. and Y.S.K. provided conception and design of research; L.S. analyzed data; Y.S.K. edited and revised manuscript; Y.S.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Elisabeth I. Wallner for proofreading the manuscript.
Since the subject matter of this study is somewhat diverse, the authors apologize if relevant work of any investigator is not cited in the text.
REFERENCES
- 1.Agarwal D, Dange RB, Raizada MK, Francis J. Angiotensin II causes imbalance between pro- and anti-inflammatory cytokines by modulating GSK-3b in neuronal culture. Br J Pharmacol 169: 860–874, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm 2009: 10.1155/2009/137072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht FE, Xu J, Moe OW, Hopfer U, Simonds WF, Orlowski J, Jose PA. Regulation of NHE3 activity by G protein subunits in renal brush-border membranes. Am J Physiol Regul Integr Comp Physiol 278: R1064–R1073, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Alexander RT, Furuya W, Szaszi K, Orlowski J, Grinstein S. Rho GTPases dictate the mobility of the Na-H exchanger NHE3 in epithelia: role in apical retention and targeting. Proc Natl Acad Sci USA 102: 12253–1258, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banday AA, Lokhandwala MF. Angiotensin II-mediated biphasic regulation of proximal tubular Na+-H+ exchanger 3 is impaired during oxidant stress. Am J Physiol Renal Physiol 301: F364–F370, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Banday AA, Lokhanwala MF. Oxidative stress causes renal angiotensin II type 1 receptor upregulation, Na+/H+ exchanger 3 overstimulation, and hypertension. Hypertension 57: 452–459, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reilly R, Igarashi P, Aronson PS. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am J Physiol Renal Fluid Electrolyte Physiol 265: F736–F742, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol Cell Biochem 212: 155–169, 2000 [PubMed] [Google Scholar]
- 9.Cabado AG, Yu FH, Kapu A, Lukacs G, Grinstein S, Orlowski J. Distinct structural domains confer cAMP sensitivity and ATP dependence to the Na+/H+ exchanger NHE3 isoform. J Biol Chem 271: 3590–3599, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Chalumeau C, Cheyron D, Defontaine Kellerman O, Paillard M, Poggioli AJ. NHE3 activity and trafficking depend on the state of actin organization in proximal tubule. Am J Physiol Renal Physiol 280: F283–F290, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Clark JD, Cragoe EJ, Jr, Limbird LE. α2-Adrenergic receptors regulate Na+-H+ exchange via a cAMP-dependent mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 259: F977–F985, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Costa-Pessoa JM, Figeiredo CF, Thieme K, Oliveira-Souza M. The regulation of NH1 and NHE3 activity by angiotensin II is mediated by the activation of angiotensin II type 1 receptor/phospholipase C/calcium/calmodulin pathway in distal nephron cells. Eur J Pharmacol 721: 322–331, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Decieves AE, Sharma K. New pharmacological treatments for improving renal outcomes in diabetes. Nat Rev Nephrol 6: 371–380, 2010 [DOI] [PubMed] [Google Scholar]
- 14.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Dong Z, Saikumar P, Patel Y, Weinberg JM, Venkatachalam MA. Serine protease inhibitors suppress cytochrome c-mediated caspases-9 activation and apoptosis during hypoxia-reoxygenation. Biochem J 347: 669–677, 2000 [PMC free article] [PubMed] [Google Scholar]
- 16.Dynia DW, Steinmetz AG, Kocinsky HS. NHE3 function and phosphorylation are regulated by a calyculin A-sensitive phosphatase. Am J Physiol Renal Physiol 298: F745–F753, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans T, Hart MJ, Cerione RA. Ras superfamilies: regulatory proteins and post-translational modifications. Curr Opin Cell Biol 2: 185–191, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Fanelli C, Zatz R. Linking oxidative stress, the renin-angiotensin system, and hypertension. Hypertension 57: 373–374, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Ferrario CM. Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. J Renin Angiotensin Aldosterone Syst 7: 3–14, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Funakoshi Y, Ichiki T, Ito K, Takeshita A. Induction of interleukin-6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension 34: 118–125, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol 302: 148–158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girardi AC, Knauf F, Dermuth HU, Aronson PS. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am J Physiol Cell Physiol 287: C1238–C1245, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP. Annu Rev Pharmacol 50: 355–375, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Griffin KA, Bidani AK. Progression of renal disease: renoprotective specificity of renin-angiotensin system blockade. Clin J Am Soc Nephrol 1: 1054–1065, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, Periera BG, Feldman HJ, Kusek JW, Joffe MM, Raj DS. Association between albuminuria, kidney function and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 7: 1938–1946, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris RC, Martinez-Maldonado M. Angiotensin II-mediated renal injury. Miner Electrolyte Metab 21: 328–335, 1995 [PubMed] [Google Scholar]
- 27.Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Front Physiol 3: 1–8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison-Bernard LM. The renal renin-angiotensin system. Adv Physiol Educ 33: 270–274, 2009 [DOI] [PubMed] [Google Scholar]
- 29.He P, Klein J, Yun CC. Activation of Na+/H+ exchanger NHE3 by angiotensin II is mediated by IP3 with IRBIT and Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 285: 27869–27878, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honegger KJ, Capuano P, Winter C, Bacic D, Stange G, Wagner CA, Biber J, Murer H, Hernando N. Regulation of sodium-proton exchanger isoform 3 (NHE3) by PKA and exchange protein directly activated by cAMP (EPAC). Proc Natl Acad Sci USA 103: 803–808, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houillier P, Chanbrey R, Achard JM, Frossart M, Poggioli J, Pailard M. Signalling pathways in the biphasic effect of angiotensin on apical Na/H antiporter activity in proximal tubule. Kidney Int 50: 1496–1506, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Hropot M, Juretschke HP, Langer KH, Schwark JR. S3226, a novel NHE3 inhibitor, attenuates ischemia-induced acute renal failure. Kidney Int 60: 2283–2289, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Ichihara A, Kobori H, Nishiyama A, Navar LG, Suzuki H, Saruta T. Renal renin-angiotensin system. Contrib Nephrol 143: 117–130, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia L, Li Y, Xiao C, Du J. Angiotensin II induces inflammation leading to cardiac remodeling. Front Biosci 17: 221–231, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Jin W, Reddy MA, Chen Z, Putta S, Lanting L, Kato M, Park JT, Chandra M, Wang C, Tangirala RK, Natarajan R. Small RNA sequencing reveals microRNAs that modulate angiotensin II effects in vascular smooth muscle cells. J Biol Chem 287: 15672–15683, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 6: 395–423, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev 52: 11–34, 2000 [PubMed] [Google Scholar]
- 38.Kurashima K, Yu FH, Cabado AG, Szabo EZ, Grinstein S, Orlowski J. Identification of sites required for downregulation of Na1/H1 exchanger NHE3 activity by cAMP-dependent protein kinase: phosphorylation-dependent and -independent mechanisms. J Biol Chem 272: 28672–28679, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Laroche-Joubert N, Marsy S, Michelet S, Imbert-Teboul M, Doucet A. Protein kinase A-independent activation of ERK and H, K-ATPase by cAMP in native kidney cells: role of Epac1. J Biol Chem 277: 18598–18604, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Lin S, Sahai A, Chugh SS, Pan X, Wallner EI, Danesh FR, Lomasney JW, Kanwar YS. High glucose stimulates synthesis of fibronectin via a novel protein kinase C, Rap1b, and B-Raf signaling pathway. J Biol Chem 277: 41725–41735, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Liu FY, Cogan MG. Angiotensin II stimulates early proximal biocarbonate absorption in the rat by decreasing cAMP. J Clin Invest 84: 83–91, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cAMP opposing effects of exchange protein directly activated by cAMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 277: 11497–11504, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Moriyama T, Fujibayashi M, Fujiwara Y, Kaneko T, Xia C, Imai E, Kamada T, Ando A, Ueda N. Angiotensin II stimulates interleukin-6 release from cultured mouse mesangial cells. J Am Soc Nephrol 6: 95–101, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Nath KA. The tubulo-interstitium in progressive renal disease. Kidney Int 54: 992–994, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–7, 1992 [DOI] [PubMed] [Google Scholar]
- 46.Nishiyama A, Seth DM, Navar LG. AT1 receptor-mediated augmentation of renal interstitial fluid (RIF) angiotensin II in angiotensin II-induced hypertension. J Hypertens 21: 1897–1903, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 65: 1009–1016, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Oweis S, Wu L, Kiela PR, Zhao H, Malhotra D, Ghishan FK, Xie Z, Shapiro JI, Liu J. Cardiac glycoside downregulates NHE3 activity and expression in LLC-PK1 cells. Am J Physiol Renal Physiol 290: F997–F1009, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Peti-Peterdi J, Kang JJ, Toma I. Activation of renal renin-angiotensin system in diabetes—new concepts. Nephrol Dial Transplant 23: 3047–3049, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quilliam LA, Mueller H, Bohl BP, Prossnitz V, Sklar LA, Der CJ, Bokoch GM. Rap1a is a substrate for cAMP-dependent protein kinase in human neutrophils. J Immunol 147: 1628–1635, 1991 [PubMed] [Google Scholar]
- 51.Ramesh G, Reeves WB. Inflammatory cytokines in acute renal failure. Kidney Int Suppl 91: S56–S61, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Rangel LB, Caruso-Neves C, Lara LS, Brasil FL, Lopes AG. Angiotensin II activates the quabain-insensitive Na+-ATPase from proximal tubules through a G-protein. Biochim Biophys Acta 1416: 309–319, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Ritz E, Haxsen V. Angiotensin II and oxidative stress. J Am Soc Nephrol 14: 2985–2987, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 82: S12–S22, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Ruperez M, Sanchez-Lopez E, Blanco-Colio LM, Esteban V, Rodriguez-Vita J, Plaza JJ, Egido J, Ruiz-Ortega M. The Rho-kinase pathway regulates angiotensin II-induced renal damage. Kidney Int Suppl 99: S39–S45, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Ruster C, Wolf G. Angiotensin II as a morphogenetic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol 22: 1189–1199, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Saccomani G, Mitchell KD, Navar LG. Angiotensin II stimulation of Na+-H+ exchange in proximal tubule cells. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1188–F1195, 1990 [DOI] [PubMed] [Google Scholar]
- 58.Sadoshima J. Cytokine actions of angiotensin II. Circ Res 86: 1187–1189, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Schmidt C, Hocherl K, Schweda F, Kurtz A, Bucher M. Regulation of renal sodium transporters during severe inflammation. J Am Soc Nephrol 18: 1072–1083, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C-calcium signaling mediated by cAMP and Rap GTPase. Nat Cell Biol 3: 1020–1024, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Shah S, Hussain T. Enhanced angiotensin II-induced activation of N+, K+-ATPase in the proximal tubules of obese Zucker rats. Clin Exp Hypertens 28: 29–40, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Sharma K, Ziyadeh FN. Biochemical events and cytokine interactions linking glucose metabolism to development of diabetic nephropathy. Semin Nephrol 17: 80–82, 1997 [PubMed] [Google Scholar]
- 63.Singh V, Raheja G, Borthakur A, Kumar A, Gill RK, Alakkam A, Malakooti J, Dudeja PK. Lactobacillus acidophilus upregulates intestinal NHE3 expression and function. Am J Physiol Gastrointest Liver Physiol 303: G1393–G1401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun L, Kondeti VK, Xie P, Raparia K, Kanwar YS. Epac1-mediated high glucose-induced renal proximal tubular cells hypertrophy via Akt/P21 pathway. Am J Pathol 179: 1706–1718, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun L, Xie P, Wada J, Kashihara N, Liu FY, Zhao Y, Kumar D, Chugh SS, Danesh FR, Kanwar YS. Rap1b GTPase ameliorates glucose-induced mitochondrial dysfunction. J Am Soc Nephrol 19: 2293–2301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev 81: 153- 208, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Tramonti G, Xie P, Wallner EI, Danesh FR, Kanwar YS. Expression and functional characteristics of tubular transporters: P-glycoprotein, PEPT1, and PEPT2 in renal mass reduction and diabetes. Am J Physiol Renal Physiol 291: F972–F980, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Wang W, Wang Y, Long J, Hasudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR. Mitochondria fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab 15: 186–200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolf G, Neilson EG. Angiotensin II induces cellular hypertrophy in cultured proximal tubular cells. Am J Physiol Renal Fluid Electrolyte Physiol 259: F768–F777, 1990 [DOI] [PubMed] [Google Scholar]
- 70.Wolf G, Ziyadeh FN. Renal tubular hypertrophy induced by angiotensin II. Semin Nephrol 17: 448–454, 1997 [PubMed] [Google Scholar]
- 71.Yeruva S, Chodisetti G, Min L, Goldstein J, Singh A, Bleich A, Gereke M, Bruder D, Yun CC, Seidler U. Inflammatory cytokines down-regulate the Na+/H+ exchanger (NHE3) interacting PDZ-domain protein PDZK1 in ulcerative colitis patients, colitis mice and in Caco-2bbe cells: link to inflammation-associated NHE3 dysfunction. Proc Physiol Soc Lond 27: C43, 2012 [Google Scholar]
- 72.Zager RA, Johnson ACM, Becker K. Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and “end-stage” kidney disease. Am J Physiol Renal Physiol 301: F1334–F1345, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zager RA, Johnson ACM, Lund S. Uremia impacts renal inflammatory cytokine gene expression in the setting of experimental acute kidney injury. Am J Physiol Renal Physiol 297: F961–F970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao H, Wiederkehr MR, Fan L, Collazo RL, Crowder LA, Moe OW. Acute inhibition of Na/H exchanger NHE-3 by cAMP. Role of protein kinase A and NHE-3 phosphoserines 552 and 605. J Biol Chem 274: 3978–3987, 1999 [DOI] [PubMed] [Google Scholar]
- 75.Zhong JC, Guo D, Chen CB, Wang W, Schuster M, Loibner H, Penninger JM, Scholey JW, Kassiri Z, Oudit GY. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension 57: 314–322, 2011 [DOI] [PubMed] [Google Scholar]