Abstract
We used an unbiased approach of gene expression profiling to determine differential gene expression of all the macromolecular modulators (MMs) considered to be involved in stone formation, in hyperoxaluric rats, with and without treatment with the NADPH oxidase inhibitor apocynin. Male rats were fed rat chow or chow supplemented with 5% wt/wt hydroxy-l-proline (HLP) with or without apocynin-supplemented water. After 28 days, rats were euthanized and their kidneys explanted. Total RNA was isolated and microarray analysis was conducted using the Illumina bead array reader. Gene ontology analysis and the pathway analyses of the genes were done using Database for Annotation, Visualization of Integrated Discovery enrichment analysis tool. Quantitative RT-PCR of selected genes was carried out to verify the microarray results. Expression of selected gene products was confirmed using immunohistochemistry. Administration of HLP led to crystal deposition. Genes encoding for fibronectin, CD 44, fetuin B, osteopontin, and matrix-gla protein were upregulated while those encoding for heavy chains of inter-alpha-inhibitor 1, 3, and 4, calgranulin B, prothrombin, and Tamm-Horsfall protein were downregulated. HLP-fed rats receiving apocynin had a significant reversal in gene expression profiles: those that were upregulated came down while those that were downregulated stepped up. Apocynin treatment resulted in near complete absence of crystals. Clearly, there are two types of MMs; one is downregulated while the other is upregulated during hyperoxaluria and crystal deposition. Apparently gene and protein expressions of known macromolecular modulators of CaOx crystallization are likely regulated by ROS produced in part through the activation of NADPH oxidase.
Keywords: reactive oxygen species, fetuin, matrix Gla protein, osteopontin, kidney stone
formation of kidney stones is the culmination of a series of events starting with crystal nucleation followed by growth, aggregation, and their retention within the kidneys (10, 47). All of these processes are modulated by macromolecules produced by the kidneys and/or liver. Numerous studies have been performed to identify crystallization modulators and determine their mode of action (6, 43, 67, 72, 83, 84). However, regulation of the production of modulating macromolecules during stone formation is poorly understood.
Studies using both animal models and tissue culture have shown that renal epithelial cells produce reactive oxygen species (ROS) when exposed to a variety of crystals, including calcium oxalate (CaOx), calcium phosphate (CaP), and uric acid (36). In addition, production of crystallization modulators, such as osteopontin (Opn) (41, 76, 78), Tamm-Horsfall protein (Umod, uromodulin) (13, 58), urinary prothrombin fragment-1 (Uptf-1) (17, 18), bikunin (Bk) (32), and inter-α-trypsin inhibitors (Itih) (61), is altered when cells are exposed to CaOx crystals. In support of this, we have shown that inhibition of NADPH oxidase reduces the production of Opn by renal epithelial cells exposed to CaOx crystals, while CaOx monohydrate crystal exposure of renal epithelial NRK52E cells, pretreated with the NADPH oxidase inhibitor diphenyleneiodium (DPI), reduced the production of ROS compared with cells not treated with DPI (78). The relative expression of Opn mRNA and production of Opn, which increases on exposure to crystals, was also decreased with DPI treatment. More importantly, treatment of hyperoxaluric rats with the angiotensin receptor blocker (ARB) candesartan reduced both the production of Opn and deposition of CaOx crystals in the kidneys (76).
We have suggested that ROS play a significant role in the production of macromolecules known to affect crystallization in the kidneys (37, 39), hypothesizing that, during crystallization of CaOx, kidneys produce ROS via upstream involvement of both the renin-angiotensin-aldosterone system (RAAS) and NADPH oxidases. This set of macromolecules includes α-1-microglobulin/bikunin precursor (Ambp), Opn, Fetuin (Fetub), matrix Gla protein (Mgp), fibronectin (Fn1), Umod, thrombin/coagulation factor 2 (F2), calgranulin B (S100a9), hyaluronan synthase (Has1), CD44, heparanase (Hpse), and several heavy chains of inter-alpha-trypsin inhibitor (Itih1, Itih3, Itih4).
To test our hypothesis, first we determined the changes in gene expression and production of these macromolecules identified as being involved in biomineralization, stone formation, and retention in hyperoxaluric rats, and second, the subsequent effects of treatment with apocynin, an antioxidant and inhibitor of NADPH oxidases. This report shows the temporal changes that occur in the expressions of these various molecular modulators in renal tissues in response to sustained hyperoxaluria, crystal formation, and the intervention treatment with apocynin. We discuss how these changes point to a number of molecular and bioprocesses that help decipher the response of renal tissue to CaOx crystals.
MATERIALS AND METHODS
Animal procedures.
The experiments described herein were performed in Sprague-Dawley rats purchased from Harlan Labs. The studies were approved by the University of Florida's IACUC and were conducted in accord with the recommendations of the NIH Guide for the Care and Use of Laboratory Animals. All procedures are detailed in our earlier publications (34, 40, 86). In brief, three groups of six rats each, average weight of 150 g, were placed in metabolic cages with free access to food and water. Rats in group 1 were fed a normal rat chow diet and given sterile water. Rats in both group 2 and group 3 were fed the same chow as group 1 rats, but supplemented with 5% (wt/wt) hydroxy-l-proline (HLP); however, rats in group 3 were placed on water supplemented with 4 mM apocynin. Apocynin dosage was based upon previous publications (50). Urine samples were collected weekly. At the end of day 28, all rats were euthanized and their kidneys removed. From each rat, one kidney was used for RNA isolation, while the second was placed in 10% phosphate buffered formalin for histological analyses.
RNA extraction and differential expression of genes by microarray analysis.
Each rat kidney excised for RNA isolation was surgically separated into medulla and cortex, then snap frozen in liquid nitrogen and stored at −800C. Total RNA was isolated concurrently from each of the 36 tissue specimens using the RNeasy Mini-Kit (QIAGEN, Valencia, CA) as per the manufacturer's instructions, and as described previously (42). This resulted in RNA from both cortex and medulla for each rat within all three treatment groups. Microarray hybridizations were carried out with each of the 36 RNA specimens using the Illumina RatRef-12 Expression Bead Chip containing >22,000 genes expressed in the rat genome. Expressed values were determined using the Illumina bead array reader. All microarray data have been deposited with the Gene Expression Omnibus (GSE36446).
Gene expression data analysis was performed using the Genome Studio Gene Expression Module V1.0. Before the analysis, the individual signal intensity values retrieved from the microarray probes were log transformed (using 2 as a base) and normalization was done for all the individual samples within each study group. After normalizing the signal intensity values for each of the 36 arrays, the Student's t-test was used to do a probe-by-probe comparison between two groups concurrently. For each comparison, the fold change (FC) and P value was calculated for each gene based on the n = 6 replicate samples within each experimental group, and volcano plots were drawn for each comparison. Differential gene expressions were compared between the cortex and medulla tissues from control vs. HLP-treated rats and between control and HLP-apocynin-treated rats using Database for Annotation, Visualization of Integrated Discovery (DAVID) enrichment analysis tool (Bioinformatics Resources, National Institute of Allergy and Infectious Diseases) for GO: TERM and KEGG pathway analysis (26, 27). Cluster analysis of genes was also done for the identification of biological processes, cellular component, and molecular function ontology.
Histological examinations.
Formalin-fixed tissues were embedded in paraffin and sectioned to a thickness of 5 μm. Deparaffinization of paraffin-embedded slides was performed by xylene immersion followed by dehydration in ethanol. Kidney sections were processed for immunohistochemistry using specific antibodies against Fn1, Mgp, Umod, Opn, A2m, S100a9, fetuin, and prothrombin. Staining was developed by the addition of diaminobenzidine (DAB) substrate (Vector Labs, Burlingame, CA) and counterstained with hematoxylin. Images were taken using the Zeiss Axiovert 200M microscope (Carl Zeiss Microimaging, Thornwood, NY).
Real-time PCR.
cDNA was generated using Invitrogen's SuperScript III First-Strand Synthesis System (Carlsbad, CA; cat. no. 18080-051), as described previously (42). Quantitative real-time PCR was carried out to determine the mRNA expression of Fn1, Mgp, Opn, A2m, S100a9, and Umod. The mRNA of these genes was PCR amplified and detected using the Roche's FastStart High Fidelity PCR System (Indianapolis, IN; cat. no. 03553426001) (42). The forward and reverse primers used are as follows: Fn1, forward 5′-GTGGCTGCCTTCAACTTCTC-3′ and reverse 5′-GTGGGTTGCAAACCTTCAAT-3′; Mgp, forward 5′-CTACTTCTCGGCGCTGCCTGAAG-3′ and reverse 5′-GCCTGCCCAGGAGATCAAC-3′; Opn, forward 5′-CCGATGAGGCTATCAAGGTC-3′ and reverse 5′-ACTGCTCCAGGCTGTGTGTT -3′; S100a9, forward 5′-GCTCCTTAGCTTTGAGCAAGA-3′ and reverse 5′-TTTCTTTGAATTCCGCCTTG-3′. Umod primers (QT00187012) were purchased from Qiagen.
Statistical analysis.
The statistical analysis was performed using GraphPad Prism version 5.0 for Windows (GraphPad Software, La Jolla, CA). P values were calculated by one-way ANOVA for nonparametric data. P < 0.05 is considered statistically significant.
RESULTS
Histology.
As we previously published (39, 40), HLP administration to male rats for 28 days produces hyperoxaluria and CaOx crystal deposition in the rat kidneys, while nontreated rats remain normo-oxaluric and devoid of any crystals. Urinary excretion of oxalate by HLP-treated rats increases many-fold, which in humans can only be achieved in conditions of primary hyperoxaluria. The extent of crystal deposition in HLP-treated rats ranges from large extensive deposits (Fig. 1A) to a few scattered small deposits. Even though all segments of the kidneys, including cortex, medulla, and papilla, contained crystals, the majority of the crystals were seen in the tubular lumens of the distal tubules and collecting ducts of the cortex and outer medulla. The tubules that contained crystals were dilated and showed destruction of the lining epithelium, while neighboring tubules appeared normal with no overt signs of injury. Although HLP-treated rats receiving apocynin remained hyperoxaluric, their kidneys contained only a few scattered small crystal deposits, mostly at the corticomedullary junctions (Fig. 1B).
Fig. 1.
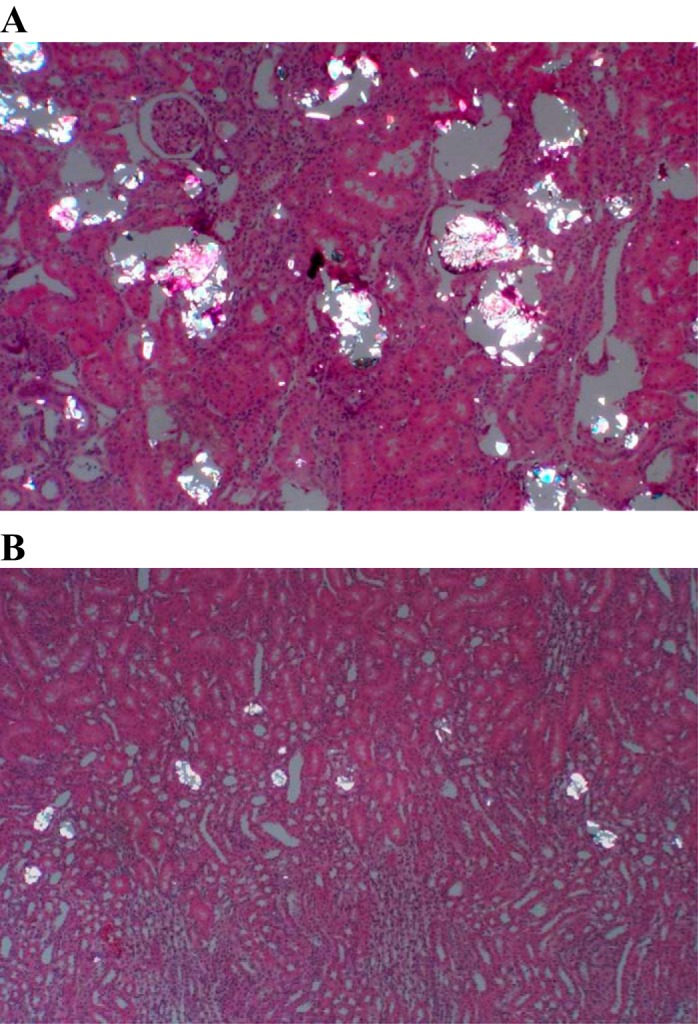
Hematoxylin- and eosin-stained sections of paraffin-embedded kidneys examined under polarized lights. Crystals of CaOx are birefringent. A: kidneys of rats fed hydroxy-l-proline (HLP) for 28 days. B: kidneys of rats fed HLP and treated with apocynin. Original magnification, ×10.
Microarray analyses.
Microarray profiles of renal cortex and medulla tissue for control vs. HLP-fed rats identified 3,302 and 2,894 genes, respectively, that were significantly differentially expressed (>2-fold change). On the other hand, a similar comparison of cortex and medulla tissues for control vs. HLP + apocynin-treated rats revealed 3,000 and 2,505 differentially expressed genes, respectively. Although global microarray transcriptome data permit extensive simultaneous analyses of multiple molecular and biological processes defined by differentially expressed genes, in this case the broad activity of apocynin in lowering renal crystallization, we have focused here specifically on the set of macromolecules known to modulate CaOx crystal formation and depositions.
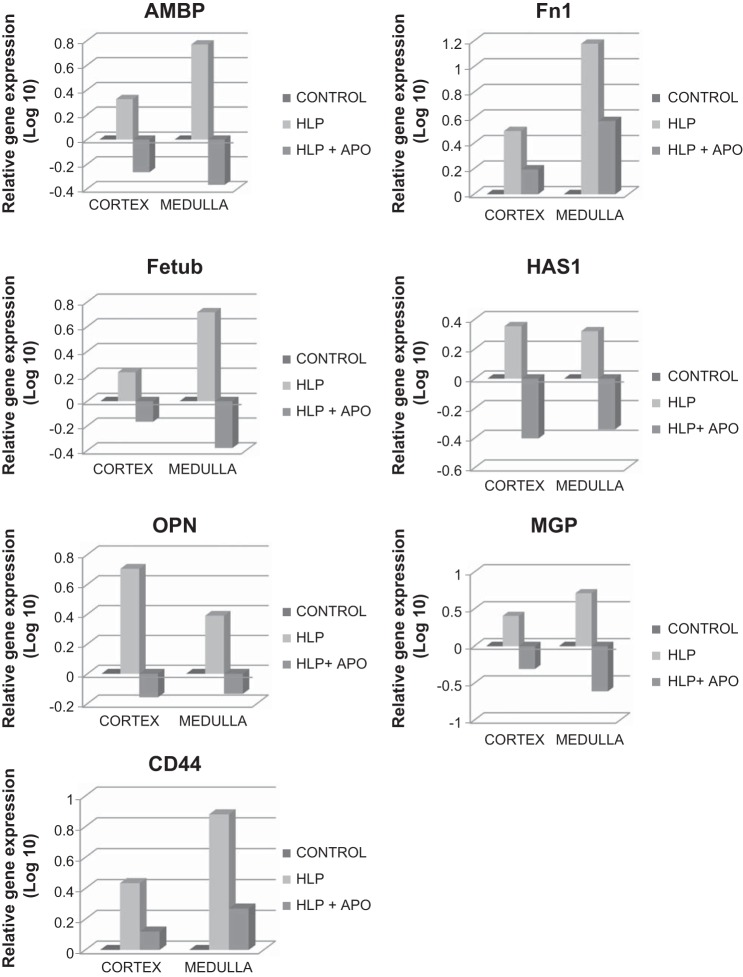
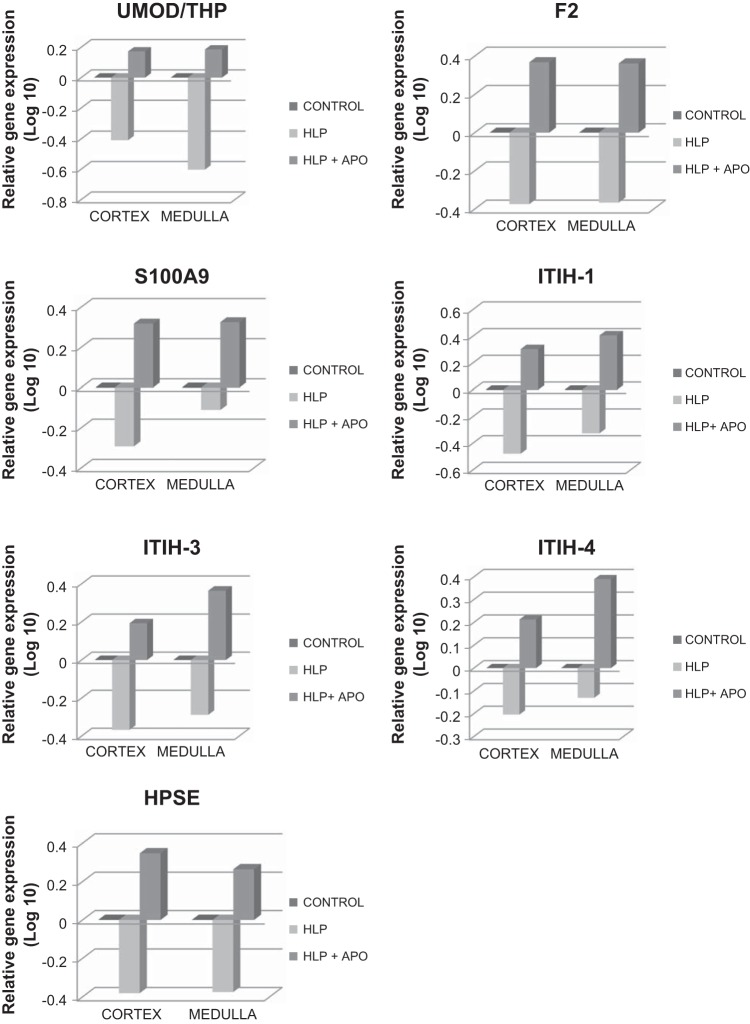
Analyses of renal tissues for genes encoding these macromolecular modulators revealed that several were upregulated while others were downregulated in HLP-treated rats compared with nontreated control rats (Table 1). Expressions of genes encoding for α-1-microglobulin/bikunin precursor (Ambp), fibronectin (Fn-1), fetuin (Fetub), hyaluronan synthase (Has1), osteopontin (Opn), matrix gla protein (Mgp), and CD44 (CD44) were highly increased in both the cortical as well as medullary sections of the kidneys (Fig. 2). On the other hand, expression of genes encoding for prothrombin/coagulation factor 2 (F2), Tamm-Horsfall Protein/Uromodulin (Umod), calgranulin-B (S100a9), and inter-α-trypsin inhibitor heavy chains-1,3,4 (Itih1, Itih3, Itih4), as well as heparanase (Hpse), were significantly decreased (Fig. 3). When HLP-fed rats were simultaneously treated with apocynin, an antioxidant and inhibitor of NADPH oxidase, the effects of HLP on expressions of these molecular modulator genes were reversed, i.e., the upregulated gene expressions of Ambp, Fetub, Has1, Opn, and Mgp were now downregulated, the upregulated expressions of Fn1 and CD44 were decreased (Fig. 2), while the downregulated expressions of Umod, F2, S100a9, Itih1, Itih3, Itih4, and Hpse were reversed, exhibiting upregulated expressions (Fig. 3).
Table 1.
Alterations in gene expression of macromolecules involved in the nucleation, growth, aggregation, and retention within the kidneys of hyperoxaluric rats
| Macromolecule/Gene | Gene Expression | Crystallization (Nucleation/Growth) | Crystal Retention (Aggregation and Adhesion) |
|---|---|---|---|
| Fibronectin/Fn1 | + | Inhibitor of aggregation and adhesion | |
| CD44/CD44 | + | Promoter of adhesion | |
| Hyaluronan synthase/Has1 | + | Hyaluronic acid is promoter of adhesion | |
| Bikunin/Ambp | + | Inhibitor of nucleation, growth | Inhibitor of aggregation and adhesion |
| Fetuin/Fetub | + | Inhibitor of crystallization | |
| MGP/Mgp | + | Inhibitor of crystallization | |
| Osteopontin/Opn | + | Free OPN inhibits nucleation, growth | Free OPN inhibits aggregation and attachment; immobilized OPN promotes crystal attachment |
| THP/Umod | − | Inhibitor of aggregation | |
| Prothrombin fragment-1/F2 | − | Inhibitor of growth | Inhibitor of aggregation |
| Calgranulin-B/S100A9 | − | Inhibitor of growth | Inhibitor of aggregation |
| ITIH 1, 3, 4/Itih1, 3, 4 | − | Inhibitor of nucleation, growth | Inhibitor of aggregation |
| Heparanase/Hpse | − | Heparan sulfate is inhibitor of aggregation and adhesion |
+, Upregulation; −, downregulation. Has1 is responsible for synthesis of hyaluronic acid and Hpse for heparan sulfate.
Fig. 2.
Relative gene expression in cortex and medullas of kidneys of control, HLP-fed, and HLP-fed rats treated with apocynin (Apo). Genes are highly upregulated in HLP-treated rats, and gene expression is decreased on treatment with apocynin. AMBP, α-1-microglobulin/bikunin precursor; Fn1, fibronectin; Fetub, fetuinB; HAS1, hyaluronan synthase; OPN, osteopontin; MGP, matrix-gla-protein; CD44.
Fig. 3.
Relative gene expression in cortex and medullas of kidneys of control, HLP-fed, and HLP-fed rats treated with apocynin (Apo). Genes are highly downregulated in HLP-treated rats, and gene expression is increased on treatment with apocynin. UMOD/THP, uromodulin/Tamm Horsfall protein; F2, prothrombin/coagulation factor 2; S100A9, calgranulin B; ITIH-1, ITIH-3, and ITIH-4 are heavy chain 1, 3 and 4 of inter α inhibitor; HPSE, heparanase.
Quantitative real-time PCR.
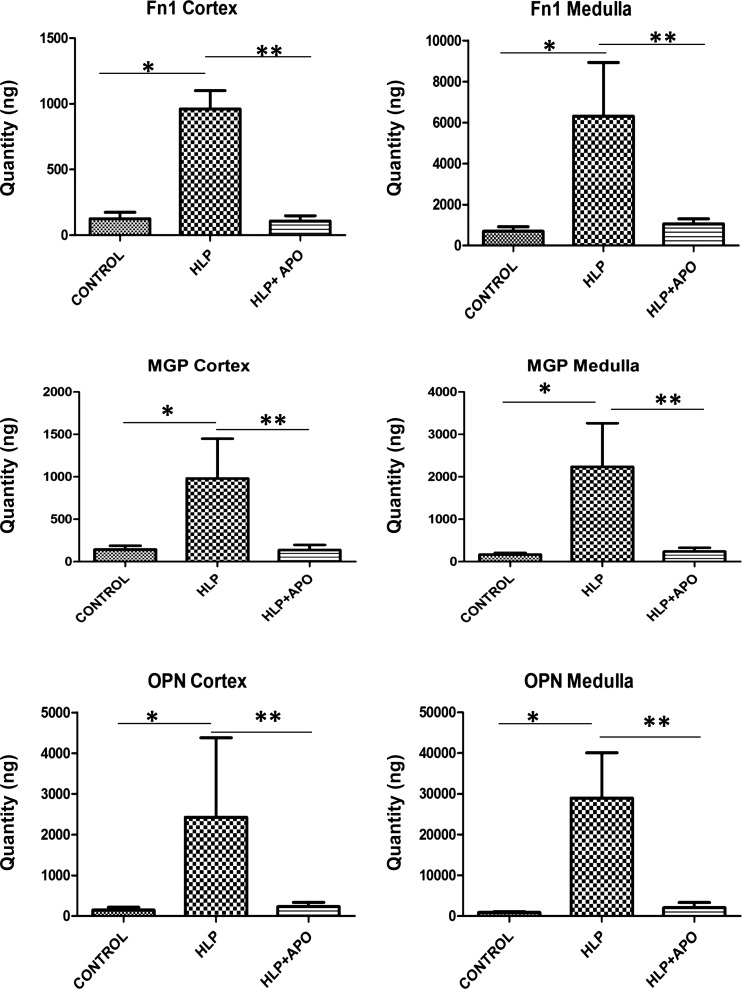
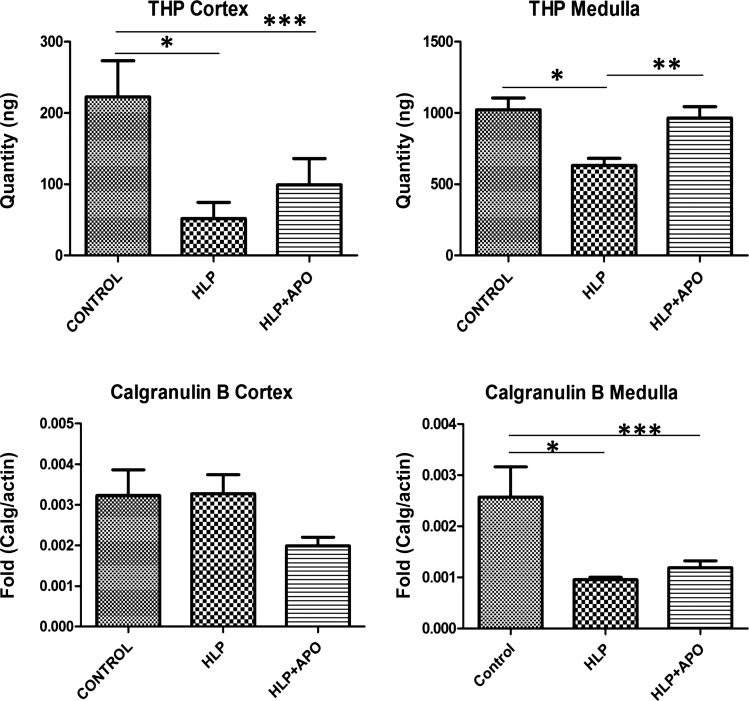
Quantitative Real-Time PCR of selected modulator genes, i.e., Fn1, Mgp, and Opn, that were upregulated in response to HLP-treatment, and Umod and S100a9, that were downregulated in HLP-fed rats, was carried out to verify the microarray results. In line with the microarray results, there were significant differences between the gene expressions seen by qPCR in controls vs. HLP-treated and HLP-fed vs. HLP-fed plus apocynin-treated rats. Expressions of Fn1, Mgp, and Opn were highly increased in both renal cortex and medulla of HLP-treated rats (Fig. 4). Treatment with apocynin reduced expressions of these genes to control levels. On the other hand, Umod expression was reduced in renal tissues of HLP-fed rats (Fig. 5), while apocynin treatment appeared to have little effect on Umod. There was no significant change in S100a9 expression in the kidneys of HLP-treated rats with little or no significant effect through apocynin treatment of the hyperoxaluric rats.
Fig. 4.
Results of real-time PCR showing for fibronectin (Fn1), matrix-gla-protein (MGP), osteopontin (OPN) in the cortex and medullas of the control, HLP-fed, and HLP-fed rats treated with apocynin (Apo). HLP treatment increased and apocynin treatment reduced the gene expressions. *Control vs. HLP group, P < 0.05. **HLP vs. HLP+APO group, P < 0.05.
Fig. 5.
Results of real-time PCR showing for Tamm-Horsfall protein/Uromodulin (THP), and calgranulin B in the cortex and medullas of the control, HLP-fed, and HLP-fed rats treated with apocynin (Apo). HLP treatment significantly decreased THP in cortex of HLP-fed rats. There were no significant differences in the others. *Control vs. HLP group, P < 0.05. **HLP vs. HLP+APO group, P < 0.05. ***Control vs. HLP+APO group, P < 0.05.
Immunohistochemical staining.
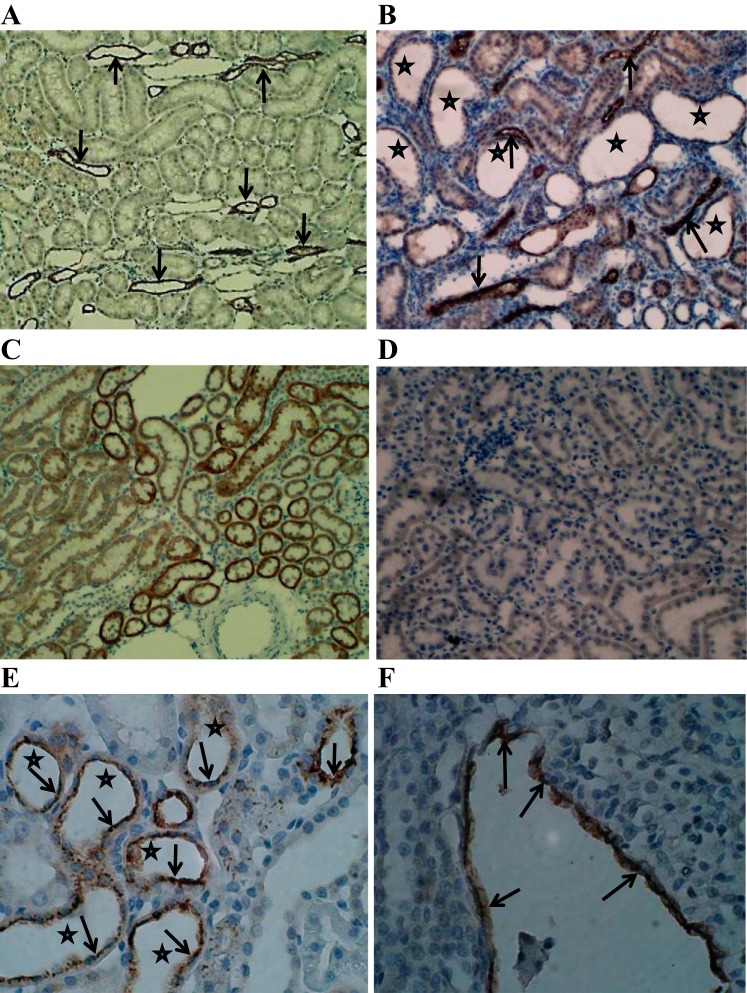
Immunohistochemical staining of kidney sections from untreated control, HLP-fed, and HLP-fed plus apocynin-treated rats provided further supportive data confirming the differential expressions of and effects of apocynin treatment on known macromolecular modulators of CaOx crystallization and retention. As expected, there was no or only patchy staining for Opn, Mgp, Fn1, F2, CD44, Fetuin B, and Tamm-Horsfall protein in the normal control kidneys. Crystal depositions in kidneys were associated with increased expressions of the molecular modulators examined with the exception of calgranulin B. Tamm-Horsfall protein expression was generally restricted to the loops of Henle in both controls and HLP-fed rats (Fig. 6, A and B). The expression of calgranulin B was more intense in tubular epithelial cells of the control rats (Fig. 6C) compared with the HLP-treated rats (Fig. 6D). Expression of Opn was mostly seen associated with crystals or the epithelium lining tubules containing crystals (Fig. 6E). In addition, the epithelium lining the papillary surface of inner medulla in the renal calyces was also heavily stained for Opn (Fig. 6F). In general, differences in the IHC staining between HLP-fed and HLP-fed but apocynin-treated rats were not noticeably distinct.
Fig. 6.
Immunohistochemical staining for selected macromolecules in kidneys. A: Tamm Horsfall (Uromodulin) protein expression in the kidney of a control rat is limited to limbs of the loop of Henle (arrows) in the outer medulla. Magnification, ×10. B: kidney of the HLP-treated rat shows intense THP expression is still limited (arrows). Crystals have dropped out during processing leaving behind large distended tubules (stars). Magnification, ×10. C: the expression of calgranulin B is seen in most epithelial cells lining the renal cortical tubules of the control rats. Staining is very intense. Magnification, ×10. D: calgranulin B expression is reduced in renal tubular epithelial cells of the HLP-treated rats. Magnification, ×10. E: OPN expression in HLP-treated rats is mostly limited to the epithelial cells lining the tubules that contain crystals. OPN is expressed in the cytoplasm as well as the luminal membrane (arrows). Stars mark the sites of crystal deposits. Magnification, ×40. F: kidneys of the HLP-treated rats also showed heavy staining of the papillary surface epithelium (arrows). Magnification, ×40.
DISCUSSION
A number of animal models and tissue culture studies have been used to investigate the effect of renal cell exposure to oxalate and CaOx or CaP crystals on the production of crystallization modulators. In most studies, the production and secretion/excretion of individual modulators were determined; however, determining the biological importance of these is complicated since different cell lines and animal models were often used. More recently, a few studies have examined changes in the mRNA and gene expression profiles, and these studies have begun to reveal the coordinated increases in the macromolecular expression (43, 67). In the present study, we investigated the gene expression profiles of the various known modulators of stone formation in a single animal model under controlled conditions, then verifying the transcriptional results using QT-PCR and immunohistochemical analyses of a selected set of genes and macromolecules.
During induction of renal crystallization by HLP, genes encoding for osteopontin, fibronectin 1, alpha-1-microglobulin/bikunin precursor, matrix gla protein, fetuin-B, CD44, and hyaluronan/hyaluronic acid synthase were all upregulated. Opn is a well-recognized modulator of biomineralization and crystallization of both CaOx and CaP (28, 29, 43, 46, 82). A percentage of Opn deficient mice spontaneously produce interstitial calcifications (60) and produce intratubular CaOx deposits when challenged with the hyperoxaluria-inducing agent ethylene glycol (82). Results have shown that production of Opn by renal epithelial cells is increased when they are exposed to high oxalate and/or CaOx or CaP crystals (9, 14, 38, 40, 41, 44, 46, 49, 57, 59, 63, 76–78). Opn expression is mostly seen in association with the crystal deposits in the renal tubules (14, 40, 41, 64), both as part of the crystal matrix as well as tubular and cellular contents. Fibronectin, considered an inhibitor of CaOx nephrolithiasis (73), impedes adhesion of CaOx crystals to renal tubular cells in culture (74). Renal tubular expression of fibronectin is increased in hyperoxaluric mice and is associated with tubules which contain crystals (64). Microarray analyses of the genes in renal epithelial cells exposed to CaOx crystals have also shown upregulation of Opn and fibronectin genes (59, 64, 65).
Hyaluronan synthase (HAS) catalyzes the production of hyaluronic acid (HA),a negatively charged high molecular weight polysaccharide produced during wound healing and inflammation. CD44 is a transmembrane protein and the main cell surface receptor for HA as well as Opn. Both CD44 and HA are upregulated during injury, inflammation, and wound healing and are involved in the formation of a cell coat or pericellular matrix on surfaces of proliferating and migrating cells. HA is restricted to the inner medullary interstitium of the normal kidneys but is also synthesized and secreted from the apical membrane of tubular epithelial cells. Distal collecting duct cells express both CD44 and HA on apical cell surfaces of the proliferating cells (80, 81). Proliferating cells are receptive to adhesion of CaOx crystals, a property lost when cells become confluent. In addition, removal of the pericellular matrix by hyaluronidase treatment also results in loss of crystal adhesion property of the proliferating cells (80). Expression of CD44 is also increased in kidneys of the hyperoxaluric rats (40). Results of our transcriptional study show increased expressions of CD44 and HAS1 genes. Thus CD44, HA, and Opn are likely to be involved in crystal retention within the kidneys of the hyperoxaluric rats (1, 41, 46, 80).
Bikunin and α1-microglobulin are encoded by a single gene, Ambp. Both products have been individually shown to be inhibitors of CaOx crystallization (3, 4, 71). Expression of Ambp, as well as its two proteins, is increased in the renal tubular cells of the hyperoxaluric rats (16, 32). Oxalate exposure results in a time- and concentration-dependent induction of α1-microglobulin in LLC-PK1 cells (16). Expression of bikunin mRNA is also increased on exposure of renal epithelial cells to oxalate and CaOx crystals (31).
Matrix gla protein is a vitamin K-dependent protein functioning primarily as an inhibitor of vascular calcification (69). Results of studies show increased expression of Mgp in renal tubules of hyperoxaluric rats (35, 85), as well as in renal tubular epithelial cell lines NRK-52E and MDCK after exposure to oxalate or CaOx monohydrate crystals (11, 35). Hyperoxaluria also induces Mgp expression in the peritubular vessels of the renal medulla of hyperoxaluric rats (35).
Fetuin-A and -B belong to the cystatin superfamily and are expressed in humans, rats, and mice at both the mRNA and protein levels (7). Both actively inhibit precipitation of basic calcium phosphate in vitro. Fetuin A is considered an important inhibitor of pathological calcification in humans (23). Fetuin A deficient mice develop soft tissue calcification, including nephrocalcinosis (68). The role of fetuin in kidney stone formation has not been analytically examined; however, it has been reported that kidney stone patients have lower urinary Fetuin A levels than normal controls (70). We found IHC staining for Fetuin B in proximal tubules of the kidneys of the hyperoxaluric rats and significant increase in the expression of Fetuinb gene in their cortex and medullary tissues.
Umod, S100a9, F2, Hpse, Itih1, Itih3, and Itih4 were coordinately downregulated in renal tissue of HLP-fed rats. In vitro studies have provided evidence that Umod inhibits CaOx crystal aggregation (33). Umod-deficient mice spontaneously produce interstitial calcification in their renal papillae (51) and produce intratubular CaOx crystal deposits when ethylene glycol is administered (60). Umod expression is also increased in kidneys of hyperoxaluric rats and seen associated with CaOx crystals (15). However, Umod production, as determined by Western and Northern blot analyses (54), appears to be decreased. Urinary prothrombin fragment-1 is an inhibitor of CaOx crystal growth and aggregation (20) that can be found in matrices of stones as well as CaOx crystals produced in the urine (2). Whereas F2 mRNA is expressed in human and rat kidneys (19), there is a reduction in prothrombin mRNA in kidneys of hyperoxaluric rats with CaOx crystals (19). S100a9 is also an inhibitor of growth and aggregation of CaOx crystals (66), excreted in urine, expressed in mammalian kidneys (66), and a constituent of kidney stone matrix (62). To our knowledge, this is the first study showing involvement of S100a9 in CaOx nephrolithiasis using an animal model.
The members of the Itih family of plasma serine protease inhibitors are normally involved in extracellular matrix stabilization and are expressed in the kidneys at both the gene and protein level (43, 61). Individual heavy chains have not shown crystallization inhibitory properties in vitro (45), but urinary excretion of Itih proteins has been linked to stone formation (5, 22, 55). Urinary GAGs, such as chondroitin sulfate, heparin sulfate, and hyaluronic acid, are considered to play a significant role in stone formation (43). Heparin sulfate has been shown to inhibit CaOx crystal aggregation (43) possibly by its adhesive properties to renal epithelial cells (30).
In general, it appears that most of the upregulated genes encode macromolecules that have been shown in vitro to be crystallization inhibitors. Bikunin, osteopontin, fetuin, fibronectin, and MGP have all been shown to inhibit crystallization of CaP and/or CaOx. Animal model studies show that these proteins are normally present at low levels in the kidneys and urine and their production is increased during nephrolithiasis. Interestingly, osteopontin is normally expressed in the thick ascending limbs of the loops of the Henle, but cell culture studies show that even epithelial cells of the proximal and collecting ducts can be induced to produce OPN by exposure to CaOx crystals. The gene encoding for hyaluronan synthase (Has1), a protein involved in the synthesis of hyaluronic acid, is also upregulated; thus OPN, CD44, and hyaluronic acid are each involved in crystal adhesion, a step toward their endocytosis, movement into the lysosomes and/or exocytosis into the interstitium, where intracellular or interstitial crystals are destroyed. Upregulation of these genes is most likely a protective response of the cells that come in contact with high oxalate and/or CaOx/CaP crystals.
In contrast, genes encoding Tamm-Horsfall protein, calgranulin B, coagulation factor 2, and heavy chains of inter-α-inhibitor were downregulated in HLP-fed rats using microarray analyses, although qPCR showed no significant change in either Umod or S100a9 gene expressions. Earlier studies have shown that hyperoxaluria and CaOx nephrolithiasis do not significantly alter production of Tamm-Horsfall protein in rat models. Of molecular interest, Hpse, the gene encoding heparanase, was also downregulated, a fact that could be considered a protective response since heparin sulfate is an inhibitor of CaOx crystallization.
We have hypothesized that ROS produced in response to hyperoxaluria and nephrolithiasis are also involved in the production of macromolecules that modulate stone formation (36, 37, 39). To validate our hypothesis we determined the effect of specific and nonspecific antioxidants on the production of selected macromolecules by renal epithelial cells exposed to oxalate and various types of stone crystals in culture or animal models. Diphenyleneiodium (DPI), an inhibitor of NADPH oxidase, significantly reduced the production of hydrogen peroxide by NRK52E cells exposed to oxalate, CaOx monohydrate, or brushite crystals (75). DPI also reduced the expression of Opn mRNA and production of Opn induced by CaOx monohydrate crystals (78).
Our transcriptome study revealed that genes encoding for both the cytosolic and membrane-associated units of the NADPH oxidase complex are upregulated in both renal cortex and medullary tissue of HLP-fed rats (34). Activation appears to occur via the renin-angiotensin system. Concomitant treatment with apocynin, an antioxidant and inhibitor of NADPH oxidase, significantly reduced the production and urinary excretion of hydrogen peroxide and Opn (86), while simultaneously downregulating genes encoding ROS scavenging enzymes. We have also shown that treatment of hyperoxaluric rats with angiotensin II receptor blocker significantly reduces renal synthesis and excretion of Opn (76). NADPH oxidase is a major source of ROS in the kidneys (12, 48), particularly in the presence of angiotensin II (21).
Treating hyperoxaluric rats with apocynin significantly affected the genes for macromolecular modulators important in stone formation, uniformly reversing the effects of oxalate and CaOx crystals on gene expression. Of the various macromolecules investigated here, regulation of Opn gene expression and production of Opn has been investigated in greatest detail because of its involvement in a variety of physiological and pathological processes (8, 56). It is now generally agreed that Opn expression is regulated by ROS sensitive transcription factors. Expression of Opn mRNA and protein in vascular muscle cells is upregulated by hydrogen peroxide in a dose-dependent manner (25), mediated through both transcriptional and translational regulations (53). Hydrogen peroxide has been shown to regulate Opn expression in a murine model of postischemic neovascularization (52). In contrast, resveratol suppresses mRNA expression of NADPH oxidase subunits p22phox and p47phox, as well as the in vitro expression of Opn, monocyte chemoattractant-1 (Mcp1), and synthesis of hyaluronan in cultures of human renal epithelial cells (24). It also reduces the in vivo expression of Opn and hyaluronan in the kidneys of hyperoxaluric rats.
In conclusion, results of our transcriptional study demonstrate that the expressions of genes encoding macromolecular modulators considered significantly involved in kidney stone formation are altered in hyperoxaluria and CaOx nephrolithiasis. Most genes encoding for inhibitors of crystal aggregation are downregulated while those encoding for promoters of crystal adhesion are upregulated, thereby promoting conditions conducive to crystal retention within the tubules (Table 1). These gene expressions are reversed when animals are treated with apocynin, an antioxidant and inhibitor of NADPH oxidase assembly. Strengths of the study include investigation of 14 known urinary crystallization modulators in one single model using microarray, immunohistochemistry, and QT-PCR; demonstration that there are two types of modulators, some of which are upregulated while the others are downregulated; and providing evidence that inhibition of NADPH oxidase abolishes crystal deposition. A limitation of the study includes the difficulty of separating whether changes in macromolecular expression are a direct response to hyperoxaluria, crystallization, or to renal/epithelial injury/inflammation or ROS.
GRANTS
This study was supported in part by National Institute of Health (NIH) Grant 5R01-DK 078602.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.R.K. and A.B.P. conception and design of research; S.R.K., S.J., and A.B.P. analyzed data; S.R.K., S.J., and A.B.P. interpreted results of experiments; S.R.K., S.J., and W.W. prepared figures; S.R.K. drafted manuscript; S.R.K. and A.B.P. edited and revised manuscript; S.R.K., S.J., and A.B.P. approved final version of manuscript; S.J. and W.W. performed experiments.
ACKNOWLEDGMENTS
We thank G. Clark, Dr. J. Yao, and Dr. Y. Sun from the Univ. of Florida's Interdisciplinary Center for Biotechnology Research (ICBR) for running the microarrays and providing expert assistance in data analyses.
REFERENCES
- 1.Asselman M, Verhulst A, Van Ballegooijen ES, Bangma CH, Verkoelen CF, De Broe ME. Hyaluronan is apically secreted and expressed by proliferating or regenerating renal tubular cells. Kidney Int 68: 71–83, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Atmani F, Khan SR. Quantification of proteins extracted from calcium oxalate and calcium phosphate crystals induced in vitro in the urine of healthy controls and stone-forming patients. Urol Int 68: 54–59, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Atmani F, Khan SR. Role of urinary bikunin in the inhibition of calcium oxalate crystallization. J Am Soc Nephrol 10, Suppl 14: S385–S388, 1999 [PubMed] [Google Scholar]
- 4.Atmani F, Mizon J, Khan SR. Identification of uronic-acid-rich protein as urinary bikunin, the light chain of inter-alpha-inhibitor. Eur J Biochem 236: 984–990, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Bergsland KJ, Kelly JK, Coe BJ, Coe FL. Urine protein markers distinguish stone-forming from non-stone-forming relatives of calcium stone formers. Am J Physiol Renal Physiol 291: F530–F536, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Beshensky AM, Wesson JA, Worcester EM, Sorokina EJ, Snyder CJ, Kleinman JG. Effects of urinary macromolecules on hydroxyapatite crystal formation. J Am Soc Nephrol 12: 2108–2116, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Denecke B, Graber S, Schafer C, Heiss A, Woltje M, Jahnen-Dechent W. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem J 376: 135–145, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denhardt DT, Guo X. Osteopontin: a protein with diverse functions. FASEB J 7: 1475–1482, 1993 [PubMed] [Google Scholar]
- 9.Evan AP, Bledsoe SB, Smith SB, Bushinsky DA. Calcium oxalate crystal localization and osteopontin immunostaining in genetic hypercalciuric stone-forming rats. Kidney Int 65: 154–161, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Finlayson B, Khan SR, Hackett RL. Theoretical chemical models of urinary stone. In: Renal Tract Stone: Metabolic Basis and Clinical Practice, edited by Wickham JE, Buck AC. New York: Churchill Livingston, 1990, p. 133–147 [Google Scholar]
- 11.Gao B, Yasui T, Lu X, Zhou H, Liu J, Liu P, Okada A, Xiao C, Kohri K. Matrix Gla protein expression in NRK-52E cells exposed to oxalate and calcium oxalate monohydrate crystals. Urol Int 85: 237–241, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gokhale JA, Glenton PA, Khan SR. Characterization of Tamm-Horsfall protein in a rat nephrolithiasis model. J Urol 166: 1492–1497, 2001 [PubMed] [Google Scholar]
- 14.Gokhale JA, Glenton PA, Khan SR. Localization of tamm-horsfall protein and osteopontin in a rat nephrolithiasis model. Nephron 73: 456–461, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Gokhale JA, McKee MD, Khan SR. Immunocytochemical localization of Tamm-Horsfall protein in the kidneys of normal and nephrolithic rats. Urol Res 24: 201–209, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Grewal JS, Tsai JY, Khan SR. Oxalate-inducible AMBP gene and its regulatory mechanism in renal tubular epithelial cells. Biochem J 387: 609–616, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grover PK, Dogra SC, Davidson BP, Stapleton AM, Ryall RL. The prothrombin gene is expressed in the rat kidney: implications for urolithiasis research. Eur J Biochem 267: 61–67, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Grover PK, Miyazawa K, Coleman M, Stahl J, Ryall RL. Renal prothrombin mRNA is significantly decreased in a hyperoxaluric rat model of nephrolithiasis. J Pathol 210: 273–281, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Grover PK, Miyazawa K, Ryall RL. Renal prothrombin: quantification of mRNA using reverse transcription-polymerase chain reaction in a rat model. Electrophoresis 25: 797–803, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Grover PK, Ryall RL. Inhibition of calcium oxalate crystal growth and aggregation by prothrombin and its fragments in vitro: relationship between protein structure and inhibitory activity. Eur J Biochem 263: 50–56, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal 4: 899–914, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Hedgepeth RC, Yang L, Resnick MI, Marengo SR. Expression of proteins that inhibit calcium oxalate crystallization in vitro in the urine of normal and stone-forming individuals. Am J Kidney Dis 37: 104–112, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Herrmann M, Kinkeldey A, Jahnen-Dechent W. Fetuin-A function in systemic mineral metabolism. Trends Cardiovasc Med 22: 197–201, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Hong SH, Lee HJ, Sohn EJ, Ko HS, Shim BS, Ahn KS, Kim SH. Anti-nephrolithic potential of resveratrol via inhibition of ROS, MCP-1, hyaluronan and osteopontin in vitro and in vivo. Pharmacol Rep 65: 970–979, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Hu T, Luan R, Zhang H, Lau WB, Wang Q, Zhang Y, Wang HC, Tao L. Hydrogen peroxide enhances osteopontin expression and matrix metalloproteinase activity in aortic vascular smooth muscle cells. Clin Exp Pharmacol Physiol 36: 626–630, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Prot 4: 44–57, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Hunter GK. Role of osteopontin in modulation of hydroxyapatite formation. Calcif Tissue Int 93: 348–354, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Hunter GK, Grohe B, Jeffrey S, O'Young J, Sorensen ES, Goldberg HA. Role of phosphate groups in inhibition of calcium oxalate crystal growth by osteopontin. Cells Tissues Organs 189: 44–50, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Iida S, Ishimatsu M, Chikama S, Inoue M, Matsuoka K, Akasu T, Noda S, Khan SR. Protective role of heparin/heparan sulfate on oxalate-induced changes in cell morphology and intracellular Ca2+. Urol Res 31: 198–206, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Iida S, Peck AB, Byer KJ, Khan SR. Expression of bikunin mRNA in renal epithelial cells after oxalate exposure. J Urol 162: 1480–1486, 1999 [PubMed] [Google Scholar]
- 32.Iida S, Peck AB, Johnson-Tardieu J, Moriyama M, Glenton PA, Byer KJ, Khan SR. Temporal changes in mRNA expression for bikunin in the kidneys of rats during calcium oxalate nephrolithiasis. J Am Soc Nephrol 10: 986–996, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Jaggi M, Nakagawa Y, Zipperle L, Hess B. Tamm-Horsfall protein in recurrent calcium kidney stone formers with positive family history: abnormalities in urinary excretion, molecular structure and function. Urol Res 35: 55–62, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Joshi S, Saylor BT, Wang W, Peck AB, Khan SR. Apocynin-treatment reverses hyperoxaluria induced changes in NADPH oxidase system expression in rat kidneys: a transcriptional study. PLos One 7: e47738, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan A, Wang W, Khan SR. Calcium oxalate nephrolithiasis and expression of matrix GLA protein in the kidneys. World J Urol 32: 123–130, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol 8: 75–88, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 33: 349–357, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Khan SR. Nephrocalcinosis in animal models with and without stones. Urol Res 38: 429–438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan SR. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 189: 803–811, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan SR, Glenton PA, Byer KJ. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-l-proline. Kidney Int 70: 914–923, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Khan SR, Johnson JM, Peck AB, Cornelius JG, Glenton PA. Expression of osteopontin in rat kidneys: induction during ethylene glycol induced calcium oxalate nephrolithiasis. J Urol 168: 1173–1181, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Khan SR, Khan A, Byer KJ. Temporal changes in the expression of mRNA of NADPH oxidase subunits in renal epithelial cells exposed to oxalate or calcium oxalate crystals. Nephrol Dial Transplant 26: 1778–1785, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan SR, Kok DJ. Modulators of urinary stone formation. Front Biosci 9: 1450–1482, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Kleinman JG, Beshensky A, Worcester EM, Brown D. Expression of osteopontin, a urinary inhibitor of stone mineral crystal growth, in rat kidney. Kidney Int 47: 1585–1596, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi H, Shibata K, Fujie M, Sugino D, Terao T. Identification of structural domains in inter-alpha-trypsin involved in calcium oxalate crystallization. Kidney Int 53: 1727–1735, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Kohri K, Yasui T, Okada A, Hirose M, Hamamoto S, Fujii Y, Niimi K, Taguchi K. Biomolecular mechanism of urinary stone formation involving osteopontin. Urol Res 40: 623–637, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Kok DJ. Clinical implications of physicochemistry of stone formation. Endocrinol Metab Clin North Am 31: 855–867, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol 282: F1111–F1119, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Lieske JC, Hammes MS, Hoyer JR, Toback FG. Renal cell osteopontin production is stimulated by calcium oxalate monohydrate crystals. Kidney Int 51: 679–686, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Liu F, Wei CC, Wu SJ, Chenier I, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Apocynin attenuates tubular apoptosis and tubulointerstitial fibrosis in transgenic mice independent of hypertension. Kidney Int 75: 156–166, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Mo L, Goldfarb DS, Evan AP, Liang F, Khan SR, Lieske JC, Wu XR. Progressive renal papillary calcification and ureteral stone formation in mice deficient for Tamm-Horsfall protein. Am J Physiol Renal Physiol 299: F469–F478, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyle AN, Joseph G, Fan AE, Weiss D, Landazuri N, Taylor WR. Reactive oxygen species regulate osteopontin expression in a murine model of postischemic neovascularization. Arterioscler Thromb Vasc Biol 32: 1383–1391, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyle AN, Remus EW, Fan AE, Lassegue B, Walter GA, Kiyosue A, Griendling KK, Taylor WR. Hydrogen peroxide regulates osteopontin expression through activation of transcriptional and translational pathways. J Biol Chem 289: 275–285, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marengo SR, Chen DH, Kaung HL, Resnick MI, Yang L. Decreased renal expression of the putative calcium oxalate inhibitor Tamm-Horsfall protein in the ethylene glycol rat model of calcium oxalate urolithiasis. J Urol 167: 2192–2197, 2002 [PubMed] [Google Scholar]
- 55.Marengo SR, Resnick MI, Yang L, Chung JY. Differential expression of urinary inter-alpha-trypsin inhibitor trimers and dimers in normal compared to active calcium oxalate stone forming men. J Urol 159: 1444–1450, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J. Osteopontin–a molecule for all seasons. QJM 95: 3–13, 2002 [DOI] [PubMed] [Google Scholar]
- 57.McKee MD, Nanci A, Khan SR. Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi. J Bone Miner Res 10: 1913–1929, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Miyake O, Yoshioka T, Yoshimura K, Honda M, Yamaguchi S, Koide T, Okuyama A. Expression of Tamm-Horsfall protein in stone-forming rat models. Br J Urol 81: 14–19, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Miyazawa K, Aihara K, Ikeda R, Moriyama MT, Suzuki K. cDNA macroarray analysis of genes in renal epithelial cells exposed to calcium oxalate crystals. Urol Res 37: 27–33, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Mo L, Liaw L, Evan AP, Sommer AJ, Lieske JC, Wu XR. Renal calcinosis and stone formation in mice lacking osteopontin, Tamm-Horsfall protein, or both. Am J Physiol Renal Physiol 293: F1935–F1943, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Moriyama MT, Glenton PA, Khan SR. Expression of inter-alpha inhibitor related proteins in kidneys and urine of hyperoxaluric rats. J Urol 165: 1687–1692, 2001 [PubMed] [Google Scholar]
- 62.Mushtaq S, Siddiqui AA, Naqvi ZA, Rattani A, Talati J, Palmberg C, Shafqat J. Identification of myeloperoxidase, alpha-defensin and calgranulin in calcium oxalate renal stones. Clin Chim Acta 384: 41–47, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Okada A, Nomura S, Higashibata Y, Hirose M, Gao B, Yoshimura M, Itoh Y, Yasui T, Tozawa K, Kohri K. Successful formation of calcium oxalate crystal deposition in mouse kidney by intraabdominal glyoxylate injection. Urol Res 35: 89–99, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Okada A, Yasui T, Fujii Y, Niimi K, Hamamoto S, Hirose M, Kojima Y, Itoh Y, Tozawa K, Hayashi Y, Kohri K. Renal macrophage migration and crystal phagocytosis via inflammatory-related gene expression during kidney stone formation and elimination in mice: Detection by association analysis of stone-related gene expression and microstructural observation. J Bone Miner Res 25: 2701–2711, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Okada A, Yasui T, Hamamoto S, Hirose M, Kubota Y, Itoh Y, Tozawa K, Hayashi Y, Kohri K. Genome-wide analysis of genes related to kidney stone formation and elimination in the calcium oxalate nephrolithiasis model mouse: detection of stone-preventive factors and involvement of macrophage activity. J Bone Miner Res 24: 908–924, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Pillay SN, Asplin JR, Coe FL. Evidence that calgranulin is produced by kidney cells and is an inhibitor of calcium oxalate crystallization. Am J Physiol Renal Physiol 275: F255–F261, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Ryall RL. Macromolecules and urolithiasis: parallels and paradoxes. Nephron Physiol 98: p37–p42, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 112: 357–366, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schurgers LJ, Cranenburg EC, Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost 100: 593–603, 2008 [PubMed] [Google Scholar]
- 70.Stejskal D, Karpisek M, Vrtal R, Student V, Solichova P, Fiala R, Stejskal P. Urine fetuin-A values in relation to the presence of urolithiasis. BJU Int 101: 1151–1154, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Tardivel S, Medetognon J, Randoux C, Kebede M, Drueke T, Daudon M, Hennequin C, Lacour B. Alpha-1-microglobulin: inhibitory effect on calcium oxalate crystallization in vitro and decreased urinary concentration in calcium oxalate stone formers. Urol Res 27: 243–249, 1999 [DOI] [PubMed] [Google Scholar]
- 72.Thongboonkerd V. Proteomics and kidney stone disease. Contrib Nephrol 160: 142–158, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Tsujihata M, Miyake O, Yoshimura K, Kakimoto KI, Takahara S, Okuyama A. Fibronectin as a potent inhibitor of calcium oxalate urolithiasis. J Urol 164: 1718–1723, 2000 [PubMed] [Google Scholar]
- 74.Tsujikawa K, Tsujihata M, Tei N, Yoshimura K, Nonomura N, Okuyama A. Elucidation of the mechanism of crystal-cell interaction using fibronectin-overexpressing Madin-Darby canine kidney cells. Urol Int 79: 157–163, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Umekawa T, Byer K, Uemura H, Khan SR. Diphenyleneiodium (DPI) reduces oxalate ion- and calcium oxalate monohydrate and brushite crystal-induced upregulation of MCP-1 in NRK 52E cells. Nephrol Dial Transplant 20: 870–878, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Umekawa T, Hatanaka Y, Kurita T, Khan SR. Effect of angiotensin II receptor blockage on osteopontin expression and calcium oxalate crystal deposition in rat kidneys. J Am Soc Nephrol 15: 635–644, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Umekawa T, Iguchi M, Uemura H, Khan SR. Oxalate ions and calcium oxalate crystal-induced up-regulation of osteopontin and monocyte chemoattractant protein-1 in renal fibroblasts. BJU Int 98: 656–660, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Umekawa T, Tsuji H, Uemura H, Khan SR. Superoxide from NADPH oxidase as second messenger for the expression of osteopontin and monocyte chemoattractant protein-1 in renal epithelial cells exposed to calcium oxalate crystals. BJU Int 104: 115–120, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verhulst A, Asselman M, Persy VP, Schepers MS, Helbert MF, Verkoelen CF, De Broe ME. Crystal retention capacity of cells in the human nephron: involvement of CD44 and its ligands hyaluronic acid and osteopontin in the transition of a crystal binding- into a nonadherent epithelium. J Am Soc Nephrol 14: 107–115, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Verkoelen CF, Van Der Boom BG, Romijn JC. Identification of hyaluronan as a crystal-binding molecule at the surface of migrating and proliferating MDCK cells. Kidney Int 58: 1045–1054, 2000 [DOI] [PubMed] [Google Scholar]
- 82.Wesson JA, Johnson RJ, Mazzali M, Beshensky AM, Stietz S, Giachelli C, Liaw L, Alpers CE, Couser WG, Kleinman JG, Hughes J. Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol 14: 139–147, 2003 [DOI] [PubMed] [Google Scholar]
- 83.Wesson JA, Worcester EM, Kleinman JG. Role of anionic proteins in kidney stone formation: interaction between model anionic polypeptides and calcium oxalate crystals. J Urol 163: 1343–1348, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Wesson JA, Worcester EM, Wiessner JH, Mandel NS, Kleinman JG. Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules. Kidney Int 53: 952–957, 1998 [DOI] [PubMed] [Google Scholar]
- 85.Yasui T, Fujita K, Sasaki S, Sato M, Sugimoto M, Hirota S, Kitamura Y, Nomura S, Kohri K. Expression of bone matrix proteins in urolithiasis model rats. Urol Res 27: 255–261, 1999 [DOI] [PubMed] [Google Scholar]
- 86.Zuo J, Khan A, Glenton PA, Khan SR. Effect of NADPH oxidase inhibition on the expression of kidney injury molecule and calcium oxalate crystal deposition in hydroxy-l-proline-induced hyperoxaluria in the male Sprague-Dawley rats. Nephrol Dial Transplant 26: 1785–1796, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]