Abstract
Pancreatic cancer is a devastating disease with a survival rate of <5%. Moreover, pancreatic cancer aggressiveness is closely related to high levels of prosurvival mediators, which can ultimately lead to rapid disease progression. One of the mechanisms that enables tumor cells to evade cellular stress and promote unhindered proliferation is the endoplasmic reticulum (ER) stress response. Disturbances in the normal functions of the ER lead to an evolutionarily conserved cell stress response, the unfolded protein response (UPR). The UPR initially compensates for damage, but it eventually triggers cell death if ER dysfunction is severe or prolonged. Triptolide, a diterpene triepoxide, has been shown to be an effective compound against pancreatic cancer. Our results show that triptolide induces the UPR by activating the PKR-like ER kinase-eukaryotic initiation factor 2α axis and the inositol-requiring enzyme 1α-X-box-binding protein 1 axis of the UPR and leads to chronic ER stress in pancreatic cancer. Our results further show that glucose-regulated protein 78 (GRP78), one of the major regulators of ER stress, is downregulated by triptolide, leading to cell death by apoptosis in MIA PaCa-2 cells and autophagy in S2-VP10 cells.
Keywords: endoplasmic reticulum stress, apoptosis, autophagy, pancreatic cancer, triptolide, glucose-regulated protein 78
pancreatic adenocarcinoma is the fourth-leading cause of cancer-related death in the United States, with a 5-yr survival rate of <5% (17). Thus, understanding the pathobiology of pancreatic cancer is of utmost importance in developing innovative and effective therapies against it.
Proper folding of secreted and transmembrane proteins occurs in the endoplasmic reticulum (ER). Many different physiological processes, highly secretory cells such as pancreatic β-cells, plasma B lymphocytes, and salivary glands, and pathological conditions such as hypoxia, ER Ca2+ depletion, oxidative stress, viral infections, and cancer can cause an imbalance between ER protein folding load and capacity, leading to accumulation of unfolded proteins in the ER lumen, a condition known as “ER stress” (15, 31). Adaptation to ER stress is mediated by induction of the unfolded protein response (UPR), a signal transduction pathway that transmits information about protein folding status in the ER lumen to the nucleus to increase folding capacity, aiding in cell survival. The UPR involves three distinct components: 1) transcriptional induction of genes encoding ER-resident chaperones to facilitate protein folding, 2) translational attenuation to decrease the demands on the organelle, and 3) ER-associated degradation to degrade the accumulated unfolded proteins via the ubiquitin-proteasome pathway (18).
In mammalian cells, the UPR is controlled by three transmembrane ER sensors, namely, PKR-like ER kinase (PERK), inositol-requiring enzyme 1α (Ire1α), and activating transcription factor (ATF) 6, which are kept in an inactive state by binding to the ER chaperone glucose-regulated protein 78 (GRP78), preventing their oligomerization-induced activation (14). When ER homeostasis is perturbed, the accumulated misfolded proteins bind to GRP78 and titrate it away from the ER stress sensors, thereby activating the UPR. Activation of Ire1α promotes splicing of a 26-nucleotide intron from the X-box-binding protein 1 (XBP1) mRNA to give rise to its spliced variant XBP1s. Activation of PERK leads to phosphorylation of the translational initiation factor eukaryotic initiation factor 2α (eIF2α), which inhibits global translation of mRNAs and reduces influx of new proteins into the ER. In contrast, translation of a subset of mRNAs, such as ATF4 is upregulated, which controls the expression of ER chaperones downstream. A known downstream target of ATF4 is CCAAT-enhancer-binding protein homologous protein (CHOP), a transcription factor implicated in control of translation and apoptosis. Activation of the transcription factors XBP1, ATF4, and ATF6 activates the UPR target genes, including ER chaperones and ER-associated protein degradation genes. Studies have shown that if the function of the ER cannot be reestablished by the UPR, excessive or sustained ER stress will induce cell death. The cell death mechanisms triggered by ER stress include caspase-dependent apoptosis and caspase-independent necrosis, as well as autophagy (38).
GRP78, a main target of UPR signaling that promotes cell survival, is required for proper protein folding, targeting of misfolded proteins for degradation, ER Ca2+ binding, and control of the activation of ER stress sensors. Although GRP78 expression is maintained at low basal levels in major adult organs, it has often been found to be upregulated in cancer cells (2, 16, 26, 34, 36). Studies have shown that although the majority of GRP78 resides in the ER lumen, a fraction of it exists as an ER transmembrane protein, with its NH2 portion in the cytosol. This can directly bind and inhibit the activity of proapoptotic effectors, such as caspase-7 and the BH3-only proapoptotic protein BIK and its downstream target BAX (13, 22). It is intriguing that increased expression of GRP78, which is a mechanism that tolerates a low level of chronic ER stress to thrive under suboptimal conditions, not only causes prosurvival robustness but also confers increased chemoresistance (2, 26). Hence, to decrease the ability of tumors to survive and proliferate under suboptimal conditions, it would be highly desirable to block GRP78 expression.
Triptolide, a diterpene triepoxide extract from the Chinese herb Tripterygium wilfordii, has been shown to inhibit pancreatic cancer cell viability in vitro (1, 10, 28) and to block growth and metastatic spread in vivo (5, 6). We and others have shown that triptolide inhibits the growth of a number of cancers in nude mice (3, 6, 8, 19). Our previous study showed that triptolide activates differential cell death pathways in different pancreatic cancer cell lines: apoptotic cell death in the primary cell lines MIA PaCa-2 and Capan-1 and autophagy-associated cell death in the metastatic cell lines S2-013, S2-VP10, and Hs766T (27). In the current study, we show for the first time that triptolide kills pancreatic cancer cell lines by inducing the UPR, resulting in ER stress by inhibiting expression of the survival protein GRP78, a negative regulator of the ER stress sensors. Furthermore, we show that a specific knockdown of GRP78 using siRNA also kills pancreatic cancer cells by activating apoptosis in MIA PaCa-2 cells and autophagy in S2-VP10 cells, which is in accordance with our earlier study with triptolide. Furthermore, we also show that triptolide-induced ER stress is important in cell death, since inhibition of ER stress by knockdown of XBP1 shows a significant rescue of triptolide-mediated cell death.
EXPERIMENTAL PROCEDURES
Reagents.
Triptolide was purchased from Calbiochem (San Diego, CA); GRP78 siRNA pool, BECLIN1 siRNA pool, and nonsilencing small interfering RNA (siRNA) from Dharmacon (Lafayette, CO); and Opti-MEM I, DMEM, and RPMI 1640 tissue culture medium from Invitrogen (Carlsbad, CA). The WST-8 viability assay was purchased from Dojindo Molecular Technologies (Gaithersburg, MD), the Caspase-Glo 3/7 assay kit from Promega (San Luis Obispo, CA), and the bicinchoninic acid protein assay kit from Pierce (Rockford, IL). All other reagents were obtained from Sigma Aldrich (St. Louis, MO).
Cell culture.
The pancreatic cancer cell line MIA PaCa-2 [American Type Culture Collection (ATCC)] was grown and propagated in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin; S2-013 and S2-VP10 cell lines (kind gift from Prof. D. Buschbaum, University of Alabama) were cultured in RPMI 1640 medium supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin, while AsPC1 cells (ATCC) were cultured in RPMI 1640 medium supplemented with 20% FBS. The human pancreatic ductal epithelial cells (ATCC) were cultured in keratinocyte medium supplemented with bovine pituitary hormone and EGF. All cells were maintained at 37°C in a humidified air atmosphere with 5% CO2.
ON-TARGETplus SMARTpool human GRP78 siRNA, human BECLIN1 siRNA, and heat shock protein 70 (HSP70) siRNA (Dharmacon) were used to silence expression of the respective genes in the pancreatic cancer cell lines MIA PaCa-2 and S2-VP10. Transfections were done using HiPerFect (Qiagen) according to the manufacturer's instructions. A pool of four siRNAs was used for all the above-mentioned genes.
Triptolide was used at 25–200 nM to study viability. Cells were treated with triptolide for 1–24 h after treatment with the compound at 100 nM.
Cell viability assay.
Three different pancreatic cancer cell lines were seeded into 96-well plates (5,000 cells/well) and allowed to adhere for 24 h. Cells were treated with increasing concentrations of triptolide or with siRNA, and cell viability was determined by the WST-8 viability assay according to the manufacturer's protocol. After the cells were treated for different time periods, 10 μl of the CCK-8 reagent were added to the wells and the cells were incubated for 1 h in darkness at 37°C in a humidified air atmosphere with 5% CO2. Absorbance at 450 nm was measured using a BioTek plate reader.
Measurement of annexin V-positive cells.
Pancreatic cancer cell lines were seeded in six-well plates (2 × 105 cells/well) and allowed to adhere for 24 h. Cells were treated with increasing concentrations of triptolide or with siRNA, and externalization of phosphatidylserine was analyzed using the Guava Nexin kit and Guava PCA flow cytometer according to the manufacturer's protocol, as previously described (28).
Caspase-3 assay.
Three different pancreatic cancer cell lines were seeded into 96-well plates (5,000 cells/well) and allowed to adhere for 24 h. Cells were treated with increasing concentrations of triptolide or with siRNA, and caspase-3 activity was measured by the Caspase-Glo 3/7 assay according to the manufacturer's protocol, as previously described (28).
Quantitative real-time PCR.
Quantitative RT-PCR for GRP78 and CHOP was carried out using primers procured from Qiagen (Valencia, CA). RNA was isolated from the different cell lines and from the tumor samples according to the manufacturer's instruction using TRIzol (Life Technologies, Carlsbad, CA). Total RNA (1 μg) was used to perform real-time PCR (Applied Biosystems 7300 real-time PCR system) using the QuantiTect SYBR Green PCR kit (Qiagen) according to the manufacturer's instructions. All data were normalized to the housekeeping gene 18S (18S QuantiTect primer assay, Qiagen).
Western blotting.
Cell lysates for Western blotting were prepared as described previously (28). Equal amounts of protein samples were resolved by SDS-PAGE using precast 10% or 4–15% Tris·HCl gels (Bio-Rad), transferred onto nitrocellulose membranes (Bio-Rad), processed for immunoblotting with specific antibodies, and detected using the enhanced chemiluminescence system. Anti-LC3B, anti-Grp78, anti-phosphorylated (Ser51) eIF2α, anti-total eIF2α, and Ire1α antibodies were purchased from Cell Signaling Technology. Anti-β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunofluorescence.
Pancreatic cancer cells were plated in chamber slides and incubated for 24 h at 37°C. The slides were treated with triptolide for 24 h, fixed with 3.7% paraformaldehyde, and permeabilized with 0.1% Triton X-100. The slides were incubated with a 1:200 dilution of rabbit polyclonal anti-LC3B antibody (Cell signaling Technologies) and a 1:800 dilution of Alexa 488-conjugated donkey anti-mouse IgG (Molecular Probes) for LC3 staining. The slides were mounted using Prolong Gold antifade with 4′,6-diaminido-2-phenylindole (Molecular Probes). Immunofluorescence images were obtained on a confocal microscope (Nikon Eclipse Ti) with a ×60 oil-immersion objective. EZ-C software version 3.80 was used to obtain z-stack images. The LC3 dots were quantified using the ImageJ software command “Analyze Particles,” which counts and measures objects in binary or thresholded images.
Immunohistochemistry.
For immunohistochemistry, paraffin tissue sections were received mounted on charged slides. The slides were deparaffinized in xylene and hydrated through graded ethanols. Slides were steamed with a Reveal Decloaker (Biocare Medical, Concord, CA) to minimize background staining. Sniper universal blocking agent (Biocare Medical) was used throughout the protocol. The slides were stained using an antibody against Grp78 (rabbit polyclonal, Thermo Scientific, Rockford, IL). A diaminobenzidine peroxidase substrate kit (Vector Laboratories) was used to reveal staining for Grp78. The tissue sections were counterstained with Gill's hematoxylin (Vector Laboratories). The antibody was omitted for the negative-omission controls.
Statistical analysis.
Values are means ± SE. All experiments with cells were repeated at least three times. The significance of the difference between the control and each experimental test condition was analyzed by unpaired Student's t test; P < 0.05 was considered statistically significant.
RESULTS
Triptolide induces ER stress in pancreatic cancer cells via activation of the PERK-eIF2α and Ire1α-XBP1 arms of the UPR cascade.
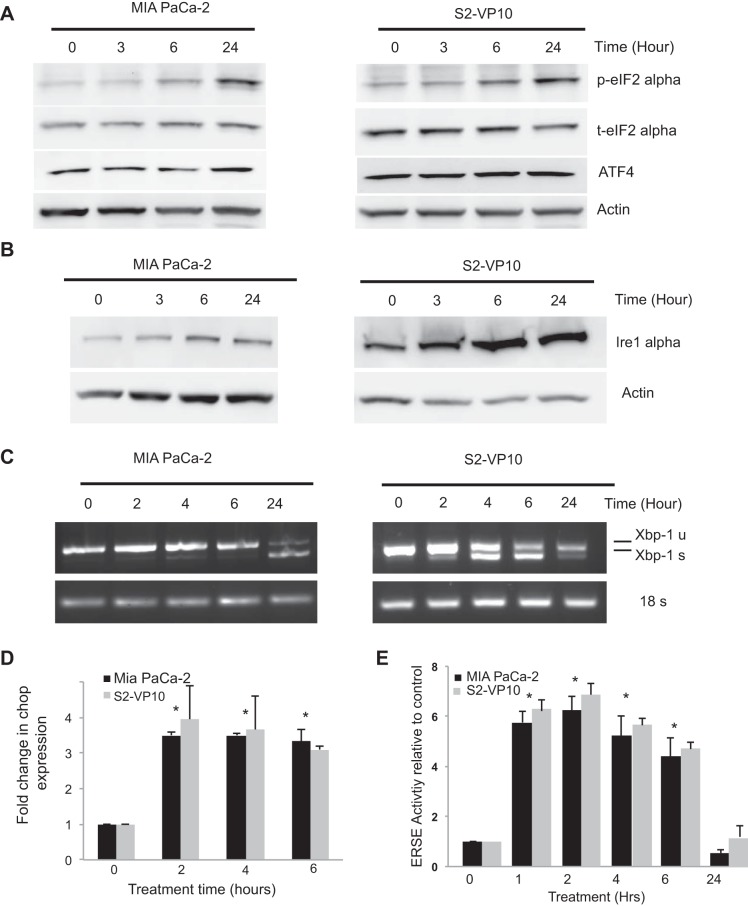
Previous results from our laboratory showed that triptolide downregulated HSP70, one of the major chaperones in a cancer cell (28). To study if this downregulation of HSP70 results in induction of the UPR by triptolide, we first evaluated this effect on the PERK-eIF2α arm of the UPR. Treatment of MIA PaCa-2 and S2-VP10 cells with 100 nM triptolide showed a sustained increase in the levels of phosphorylated eIF2α, which is downstream to the PERK activation (Fig. 1A). There was no change in the levels of total eIF2α. Consistent with this finding, there was an increase in the expression of ATF4 at 24 h following triptolide treatment. We also assessed the effect of triptolide on the Ire1α-XBP1 arm of ER stress. As shown in Fig. 1B, treatment of MIA PaCa-2 and S2-VP10 cells with 100 nM triptolide showed a sustained increase in the levels of Ire1α. This further corroborated an increased splicing of XBP1, a downstream effector of Ire1α, in response to triptolide (Fig. 1C). Splicing of XBP1 differed between the two cell lines: MIA PaCa-2 showed a delayed splicing of XBP1 by 24 h, whereas S2-VP10 cells showed a peak in XBP1 splicing at 4 h and a subsequent decline. There was no change in the levels of ATF6 in response to triptolide in both cell lines, indicating that this arm of ER stress was not activated by triptolide (data not shown). Also, the levels of CHOP mRNA increased in response to triptolide in both cell lines as assessed by quantitative RT-PCR (Fig. 1D). The effect of triptolide on the ER stress pathway was further validated by using the ER stress response element (ERSE) luciferase reporter assay, where the luciferase gene is under the control of the ERSE. Treatment of MIA PaCa-2 and S2-VP10 cells with 100 nM triptolide showed a significant increase in the luciferase activity until 6 h of treatment (Fig. 1E). Similar results were obtained in the metastatic cell line S2-013 (data not shown). These results indicate that treatment with triptolide induces a UPR in pancreatic cancer cells.
Fig. 1.
Effect of triptolide treatment on markers of the endoplasmic reticulum (ER) stress response in pancreatic cancer cells. A and B: MIA PaCa-2 and S2-VP10 cells were exposed to 100 nM triptolide for 0–24 h, and protein was extracted and assayed for expression of phosphorylated eukaryotic initiation factor 2α (p-eIF2α), total eIF2α (t-eIF2α), activating transcription factor 4 (ATF4), and inositol-requiring enzyme 1α (Ire1α). C and D: MIA PaCa-2 and S2-VP10 cells were exposed to 100 nM triptolide, and RNA was extracted and assayed for X-box-binding protein 1 (XBP1) splicing and expression of C/EBP homology protein (CHOP). Actin and 18S were used as respective loading controls. u, Unspliced; s, spliced. E: MIA PaCa-2 and S2-VP10 cells were transfected with ER stress element (ERSE) luciferase construct and treated with 100 nM triptolide for 0–24 h, assayed for luciferase activity, and compared with untreated cells. Values are means ± SE; n = 3. *P < 0.01.
Triptolide downregulates GRP78 expression in pancreatic cancer cells.
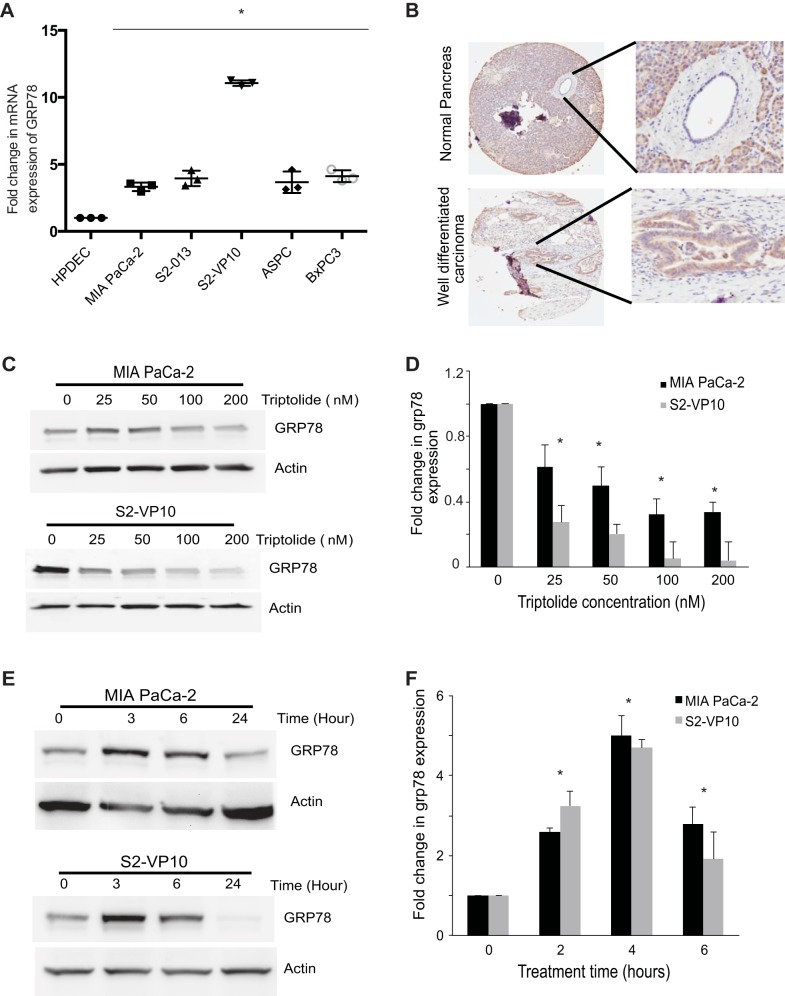
GRP78 is overexpressed in colon cancer (13, 39) and prostate cancer (9). Our studies showed overexpression of GRP78 in pancreatic cancer cell lines and in tumor compared with normal ductal cells (Fig. 2, A and B).
Fig. 2.
Glucose-regulated protein 78 (GRP78) is upregulated in tumor cells and downregulated by triptolide treatment. A: fold change in GRP78 gene expression in pancreatic cancer cell lines and normal pancreatic ductal cells. B: immunohistochemistry of pancreatic tumor tissue shows increased expression of GRP78. C–F: MIA PaCa-2 and S2-VP10 cells were exposed to 0–200 nM triptolide for 24 h or 100 nM triptolide for 0–6 h, and RNA and protein were extracted and assayed for GRP78 expression by Western blotting or quantitative RT-PCR. C and E: representative Western blots. D and F: fold change in GRP78 expression. Values are means ± SE; n = 3. *P < 0.01.
Treatment of the pancreatic cancer cell lines MIA PaCa-2 (a primary cell line) and S2-VP10 (a metastatic cell line) with increasing doses of triptolide showed decreased expression of GRP78 at mRNA and protein levels (Fig. 2, C and D). This finding indicates that triptolide inhibits GRP78 expression in a dose-dependent manner.
However, treatment of MIA PaCa-2 and S2-VP10 cells with 100 nM triptolide showed a transient increase in GRP78 expression (3 h at the protein level and 4 h at the mRNA level) followed by a decrease in its expression at 24 h (Fig. 2, E and F). This increase in GRP78 expression at 3–4 h was consistent with the increase in the ERSE reporter activity assay (Fig. 1E). Our results demonstrate that short-term treatment with triptolide induces the UPR in pancreatic cancer cells, causing a transient increase in GRP78, while an extended treatment results in a decrease in GRP78 (Fig. 2, C and D).
Downregulation of GRP78 in pancreatic cancer results in cell death.
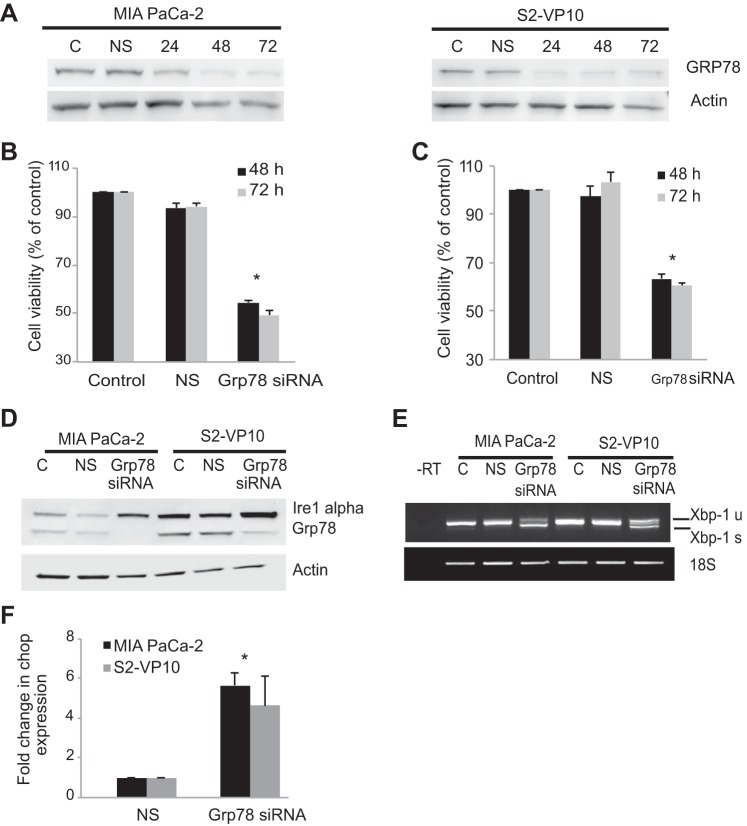
To test whether triptolide-induced downregulation of GRP78 results in cell death, we used a specific siRNA pool to inhibit the expression of GRP78 in MIA PaCa-2 and S2-VP10 cells and evaluated its effect on cell viability. Figure 3A, a representative Western blot, shows a decrease in GRP78 expression after siRNA treatment at different time points in MIA PaCa-2 and S2-VP10 cells. Cells treated with the transfection reagent alone (control) or with nonsilencing siRNA do not show a change in GRP78 expression. We next tested the effect of knockdown of GRP78 expression on cell viability in MIA PaCa-2 and S2-VP10 cells. As shown in Fig. 3, B and C, a specific knockdown of GRP78 in MIA PaCa-2 and S2-VP10 cells showed a significant decrease in cell viability (∼50%) compared with cells treated with the transfection reagent alone (control) or with the nonsilencing siRNA. Since GRP78 functions as a negative regulator of the ER stress sensors, we next evaluated the effect of inhibition of GRP78 expression on the ER stress pathway. Inhibition of GRP78 induced the expression of the ER stress sensor Ire1α, increased the splicing of XBP1, and induced the expression of CHOP mRNA in MIA PaCa2 and S2-VP10 cells (Fig. 3, D–F) compared with cells treated with the transfection reagent alone (control) or with nonsilencing siRNA.
Fig. 3.
Effect of inhibition of GRP78 on cell viability and the ER stress response in MIA PaCa-2 and S2-VP10 cells. A: representative blot showing knockdown of GRP78 by small interfering RNA (siRNA) pool at 24, 48, and 72 h in MIA PaCa-2 and S2-VP10 cells. B and C: knockdown of GRP78 by the siRNA pool results in a significant decrease in cell viability in MIA PaCa-2 and S2-VP10 cells compared with cells treated with transfection reagent alone [control (C)] or with nonsilencing siRNA (NS). D–F: effect of knockdown of GRP78 by the siRNA pool in MIA PaCa-2 and S2-VP10 cells on Ire1α expression, XBP1 splicing, and CHOP expression compared with cells treated with transfection reagent alone (control) or with nonsilencing siRNA. Values are means ± SE; n = 3. *P < 0.01.
Inhibition of GRP78 induces apoptotic cell death in MIA PaCa-2 cells, whereas it induces autophagy-associated cell death in S2-VP10 cells.
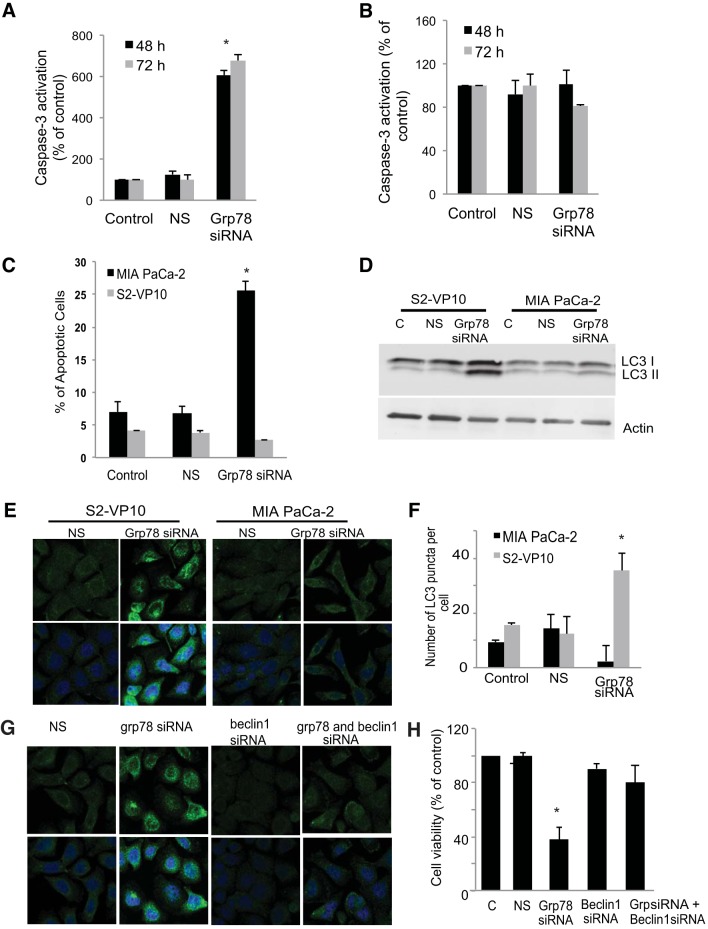
To further characterize the mechanism by which inhibition of GRP78 induces cell death in MIA PaCa-2 and S2-VP10 cells, two different markers of apoptosis were monitored: caspase-3 activation and annexin V staining. As shown in Fig. 4A, knockdown of GRP78 expression showed a significant increase in caspase-3 activation after 48 and 72 h compared with cells treated with the transfection reagent alone (control) or with nonsilencing siRNA. However, despite a significant decrease in cell viability after GRP78 knockdown in S2-VP10 cells, no caspase-3 activation was seen (Fig. 4B). In accordance with this observation, MIA PaCa-2, but not S2-VP10, cells showed a significant increase in annexin V staining after inhibition of GRP78 expression compared with cells treated with the transfection reagent alone or with nonsilencing siRNA. The loss of cell viability in the absence of apoptotic markers in S2-VP10 cells after knockdown of GRP78 suggests the utilization of an alternate pathway of cell death (Fig. 4C).
Fig. 4.
Effect of inhibition of GRP78 on cell death pathways in MIA PaCa-2 and S2-VP10 cells. A–C: MIA PaCa-2 and S2-VP10 cells were treated with GRP78 siRNA pool and assayed for caspase-3 activation at 48 and 72 h posttransfection (A and B) and annexin V staining at 48 h posttransfection (C) and compared with cells treated with transfection reagent alone (control) or with nonsilencing siRNA. D–F: autophagy was assayed by monitoring LC3B levels by Western blotting (D) or by immunofluorescence (E) and quantitated (F). Data are representative of 3 independent experiments. G and H: autophagy was inhibited by knockdown of BECLIN1 and/or GRP78, and the effect on autophagy was monitored by assaying for LC3B by immunofluorescence (G) and shown as cell viability (H) compared with cells treated with nonsilencing siRNA or the GRP78 siRNA pool alone. Results are representative of 4 independent experiments. Values are means ± SE; n = 3. *P < 0.01.
Since our previous study showed that triptolide induces autophagy-associated cell death in S2-VP10 cells (27), we examined the effect of inhibition of GRP78 expression on the markers of autophagy. We determined the induction of autophagy by monitoring formation of the autophagososme-specific protein LC3B by Western blotting and immunofluorescence. As shown in Fig. 4D, inhibition of GRP78 resulted in a significant increase in an autophagy-specific form of LC3B in S2-VP10 cells compared with control cells treated with the transfection reagent alone or cells treated with nonsilencing siRNA. No such increase in this LC3B form was seen in MIA PaCa-2 cells after knockdown of GRP78 expression (Fig. 4D). In accordance with the Western blot data, knockdown of GRP78 in S2-VP10 cells induced autophagy, as evidenced by a significant increase in the punctate staining pattern for LC3B, indicating a membrane localization (Fig. 4, E and F). Cells treated with the transfection reagent alone (control) or with nonsilencing siRNA showed a diffuse staining pattern for LC3B, indicating cytosolic localization and the absence of induction of autophagy. Inhibition of GRP78 in MIA PaCa-2 cells showed a diffuse staining pattern for LC3B, indicating the absence of autophagy and corroborating our Western blot data (Fig. 4, E and F).
Next, we evaluated the role of autophagy induced by inhibition of GRP78 in S2-VP10 cells. Autophagy was inhibited using the specific siRNA pool against BECLIN1, a gene essential in activating the process of autophagy (12), and GRP78 was inhibited using specific siRNA, and the effect on autophagy and cell viability was monitored. As shown in Fig. 4G, unlike GRP78 knockdown, a dual knockdown of BECLIN1 and GRP78 in S2-VP10 cells shows a diffuse staining pattern for LC3, indicating a cytosolic localization comparable to that in cells treated with nonsilencing siRNA or BECLIN1 siRNA, indicating the absence of autophagy. Furthermore, a dual knockdown of BECLIN1 and GRP78 in S2-VP10 cells showed a significant rescue of viability of S2-VP10 cells compared with cells treated with GRP78 siRNA alone (Fig. 4H). A knockdown of BECLIN1 alone had no effect on cell viability.
Triptolide-induced UPR leads to pancreatic cancer cell death.
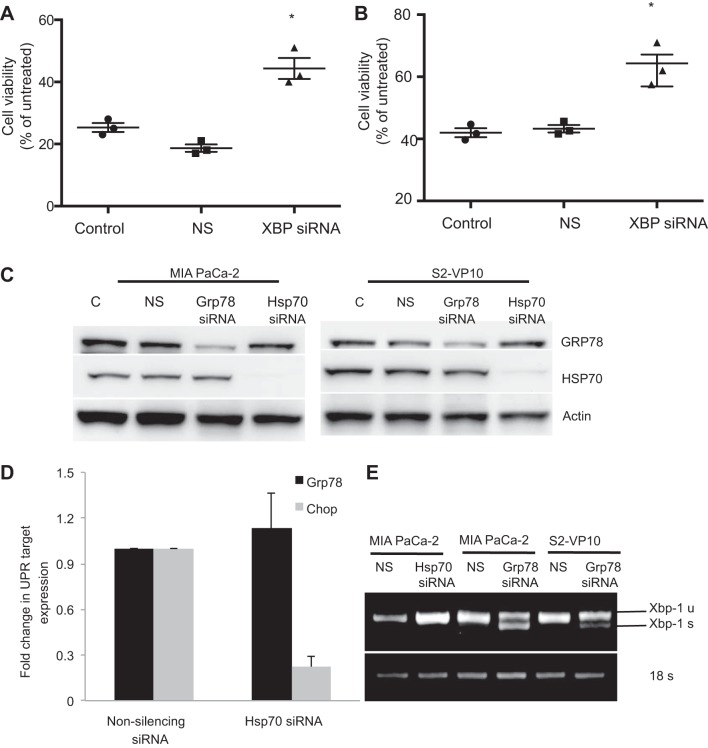
To study if triptolide-induced UPR results in cell death, MIA PaCa-2 and S2-VP10 cells were transfected with a specific siRNA pool against XBP1 to inhibit the UPR and treated with 100 nM triptolide, and cell viability was assessed. As shown in Fig. 5, A and B, inhibition of XBP1 expression in MIA PaCa-2 and S2-VP10 cells showed a rescue of triptolide-mediated cell death compared with cells treated with the transfection reagent alone (control) or with nonsilencing siRNA. The rescue was much greater in S2-VP10 than MIA PaCa-2 cells. This observation indicates that triptolide-induced UPR indeed mediates cell death in pancreatic cancer cells and that inhibition of ER stress rescues cells from this cell death.
Fig. 5.
Effect of knockdown of HSP70 on GRP78 expression and ER stress response markers. A and B: MIA PaCa-2 and S2-VP10 cells were transfected with the pool of XBP1 siRNA and treated with 100 nM triptolide for 48 h, and cell viability was assessed. C: MIA PaCa-2 and S2-VP10 cells were transfected with the pool of GRP78 siRNA or HSP70 siRNA, and the effect on HSP70 and GRP78 expression was monitored by Western blotting. D and E: effect of knockdown of HSP70 expression on the ER stress makers does not affect GRP78 or CHOP expression (D) or XBP1 splicing (E) compared with cells treated with nonsilencing siRNA or with GRP78 siRNA. Values are means ± SE; n = 3. *P < 0.01.
Previous data from our laboratory show that triptolide-induced cell death in pancreatic cancer cells is mediated by downregulation of HSP70 expression. Since in the current study we showed that GRP78, a member of the heat shock family of proteins, is also inhibited by triptolide, which triggers cell death by induction of the ER stress pathway, we monitored HSP70 expression in MIA PaCa-2 and S2-VP10 cells after inhibition of HSP70 and vice versa. Inhibition of GRP78 by siRNA had no effect on HSP70 expression (Fig. 5C). Also, inhibition of HSP70 had no effect on GRP78 expression. Furthermore, inhibition of HSP70 expression did not induce expression of the key ER stress players GRP78 and CHOP (Fig. 5D). Consistent with these observations, downregulation of HSP70 expression did not induce XBP1 splicing, which is seen by the downregulation of GRP78 (Fig. 5E).
DISCUSSION
Cancer cells exhibit increased cellular stresses and are more dependent on stress support pathways for survival. A key player in this process is the survival protein GRP78. GRP78 is overexpressed in many human cancers, where it mediates tumor growth by enhancing proliferation and protecting against apoptosis (16, 21, 41). Our findings of an increased expression of GRP78 not only in the human pancreatic cancer cell lines, but also in the human pancreatic cancer tissue samples (Fig. 2, A and B), support these observations. An increased expression of GRP78 is a mechanism whereby a low level of chronic ER stress is allowed to thrive under suboptimal conditions. In support of this, we observed a transient increase in GRP78 expression at mRNA (4 h) and protein (3 h) levels at an early time point following triptolide treatment (Fig. 2, E and F). This could reflect an initial survival response of cells to triptolide. Although activation of the ER stress response leads to adaptations that may aid in cell survival, it is well known that, under severe and prolonged ER stress conditions where the cells fail to restore ER homeostasis, the ER stress response activates pathways that lead to apoptotic cell death (32, 33, 37, 41). In accordance with this finding, our results show that prolonged (24 h) treatment with triptolide inhibits expression of GRP78, a known inhibitor of ER stress sensors, and as a result activates the ER stress pathway, which results in cell death (Fig. 1).
Our study shows that triptolide induces two different arms of ER stress: 1) the PERK-eIF2α axis and 2) the Ire1α-XBP1 axis (Fig. 1A). Although some reports suggest that compounds that induce sustained eIF2α phosphorylation provide cytoprotection in situations of ER stress (11), other reports have shown a prolonged suppression of protein synthesis, which is incompatible with cell survival and leads to autophagy (20). Moreover, involvement of the PERK-eIF2α axis of ER stress in apoptotic cell death has also been well documented (40). This is consistent with our current observations and our earlier finding that triptolide treatment causes a sustained increase in the levels of phosphorylated eIF2α and causes autophagy-associated cell death in S2-VP10 cells (Figs. 1A and 4, D–F) and apoptotic cell death in MIA PaCa-2 cells (Figs. 1A and 4C). Similarly, studies have implicated Ire1α in modulation of ER stress-induced cell death. It has been shown that, in ER-stressed cells, XBP1 splicing and XBP1 protein expression decline with time (24, 25, 35). This decline correlates with cell death, and reconstitution of Ire1α activity improves cell survival (15, 18). This is consistent with our current finding that although treatment with triptolide resulted in a sustained increase in Ire1α expression, splicing of XBP1 declines with time and correlates with cell death (Fig. 1, B and C). Moreover, it has been reported that CHOP and GRP78 contain an ERSE region that is recognized by the XBP1 protein, and, hence, shows a correlation between XBP1 splicing and expression of the ER stress markers GRP78 and CHOP (35). This is also in agreement with our current findings of correlations between the expression patterns of XBP1, GRP78, and CHOP, as shown by ERSE reporter assay (Fig. 1, C–E).
Furthermore, the present study confirms that triptolide-induced ER stress kills pancreatic cancer cells, since inhibition of GRP78, a negative regulator of ER stress, causes cell death in pancreatic cancer cells (Fig. 3, B and C). Moreover, an inhibition of XBP1 confers partial protection from triptolide-mediated cell death (Fig. 5, A and B). Interestingly, we have found that inhibition of GRP78 activates two different cell death pathways: 1) it triggers apoptosis in MIA PaCa-2 cells, whereas 2) it triggers autophagy in S2-VP10 cells. We further prove that the autophagy induced in S2-VP10 cells by inhibition of GRP78 expression is responsible for cell death, since inhibition of autophagy using BECLIN1 siRNA, along with inhibition of GRP78, rescues cells from death associated with GRP78 inhibition alone (Fig. 4H). Our previous work showed that triptolide is able to induce autophagy-mediated cell death in some pancreatic cancer cell lines and apoptotic cell death in others (27). Although, many reports suggest that the ER stress response pathway induces autophagy, it has always been in the context of cell survival (29, 30). This study not only demonstrates that triptolide-mediated ER stress induces autophagy, but it provides convincing evidence that the autophagy aids in cell death, instead of cell survival.
We previously showed that one of the mechanisms by which triptolide induces cell death in pancreatic cancer cells is inhibition of HSP70 expression (10, 23). Our more recent study explored this further and showed that triptolide inhibits the transcription factor Sp1, which is upstream of a number of signaling pathways (4). This study further shows that HSF1, the transcription factor for HSP70, and NF-κB, the transcription factor for a number of ER stress genes including GRP78, are regulated by Sp1. This also explains our findings in the present study that triptolide-mediated downregulation of GRP78 is independent of HSP70 (Fig. 5C). Furthermore, although GRP78, like HSP70, belongs to the heat shock family of proteins, the mechanism by which inhibition of these two proteins kill pancreatic cancer cells is very different: not only is inhibition of HSP70 expression independent of GRP78 expression (Fig. 5C), but it is also not associated with induction of ER stress (Fig. 5, D and E).
Our current findings also show that inhibition of GRP78 expression induces two different cell death pathways in MIA PaCa-2 and S2-VP10 cells. This is consistent with our previous finding that triptolide causes cell death in pancreatic cancer cell lines by activating two different cell death pathways: 1) apoptosis in MIA PaCa-2, Capan-1, BxPC-3 cells and 2) autophagy in S2-013, S2-VP10, and Hs766T cells (27). Thus this study further elucidates the mode of action of triptolide, a promising therapeutic drug against pancreatic cancer.
In conclusion, our study shows that although increased expression of GRP78 confers a survival advantage to the tumor cells, prolonged exposure to triptolide induces chronic ER stress, which eventually leads to cell death.
A water-soluble prodrug of triptolide, Minnelide, has been recently evaluated very exhaustively in a preclinical study on multiple mouse models (6). This study showed that the compound is well tolerated in animals, with no damage to normal cells. Minnelide is also undergoing clinical trials at the University of Minnesota. In this context, inhibition of GRP78 by activation of the ER stress pathway by triptolide offers a novel mechanism for inhibiting the growth and survival of pancreatic cancer cells. To the best of our knowledge, this is the first report (6) that triptolide causes cell death in pancreatic cancer cells by inhibiting GRP78 expression and inducing ER stress.
GRANTS
This study was supported by University of Alabama Birmingham/University of Minnesota National Cancer Institute (NCI) Specialized Program of Research Excellence in Pancreatic Cancer Grant P50 CA-101955 (to S. M. Vickers) and NCI Grants R01 CA-170946 and CA-124723 (to A. K. Saluja). R. Chugh was supported by NCI Training Grant T32 CA-132715.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.M., S.B., V.D., and A.K.S. are responsible for conception and design of the research; N.M., Z.C., V.S., and R.C. performed the experiments; N.M., S.B., Z.C., V.S., R.C., V.D., M.Y., and S.M.V. analyzed the data; N.M., S.B., V.S., V.D., M.Y., and S.M.V. interpreted the results of the experiments; N.M., S.B., and R.C. prepared the figures; S.B. and S.M.V. drafted the manuscript; S.B., R.C., V.D., M.Y., and S.M.V. edited and revised the manuscript; M.Y., S.M.V., and A.K.S. approved the final version of the manuscript.
REFERENCES
- 1.Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, Sharif R, Dawra R, Lerch MM, Saluja A. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res 67: 616–625, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Al-Rawashdeh FY, Scriven P, Cameron IC, Vergani PV, Wyld L. Unfolded protein response activation contributes to chemoresistance in hepatocellular carcinoma. Eur J Gastroenterol Hepatol 22: 1099–1105, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Antonoff MB, Chugh R, Borja-Cacho D, Dudeja V, Clawson KA, Skube SJ, Sorenson BS, Saltzman DA, Vickers SM, Saluja AK. Triptolide therapy for neuroblastoma decreases cell viability in vitro and inhibits tumor growth in vivo. Surgery 146: 282–290, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S, Sangwan V, McGinn O, Chugh R, Dudeja V, Vickers SM, Saluja AK. Triptolide-induced cell death in pancreatic cancer is mediated by O-GlcNAc modification of transcription factor Sp1. J Biol Chem 288: 33927–33938, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee S, Thayanithy V, Sangwan V, Mackenzie TN, Saluja AK, Subramanian S. Minnelide reduces tumor burden in preclinical models of osteosarcoma. Cancer Lett 335: 412–420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers SM, Saluja AK. A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med 4: 156ra139, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clawson KA, Borja-Cacho D, Antonoff MB, Saluja AK, Vickers SM. Triptolide and TRAIL combination enhances apoptosis in cholangiocarcinoma. J Surg Res 163: 244–249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D, Cai J, Groshen S, Lieskovsky G, Skinner DG, Lee AS, Pinski J. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol 38: 1547–1552, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Dudeja V, Mujumdar N, Phillips P, Chugh R, Borja-Cacho D, Dawra RK, Vickers SM, Saluja AK. Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology 136: 1772–1782, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fullwood MJ, Zhou W, Shenolikar S. Targeting phosphorylation of eukaryotic initiation factor-2α to treat human disease. Prog Mol Biol Transl Sci 106: 75–106, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Graf MR, Jia W, Johnson RS, Dent P, Mitchell C, Loria RM. Autophagy and the functional roles of Atg5 and beclin-1 in the anti-tumor effects of 3β-androstene 17α-diol neuro-steroid on malignant glioma cells. J Steroid Biochem Mol Biol 115: 137–145, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Hardy B, Raiter A, Yakimov M, Vilkin A, Niv Y. Colon cancer cells expressing cell surface GRP78 as a marker for reduced tumorigenicity. Cell Oncol (Dordr) 35: 345–354, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Hetz C. The UPR as a survival factor of cancer cells: more than folding proteins? Leuk Res 33: 880–882, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hetz C, Glimcher LH. Fine-tuning of the unfolded protein response: assembling the IRE1α interactome. Mol Cell 35: 551–561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang LW, Lin CY, Lee CC, Liu TZ, Jeng CJ. Overexpression of GRP78 is associated with malignant transformation in epithelial ovarian tumors. Appl Immunohistochem Mol Morphol 20: 381–385, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 19: 1893–1907, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Kaufman RJ. Molecular chaperones and the heat shock response. Biochim Biophys Acta 1423: R13–R27, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Krosch TC, Sangwan V, Banerjee S, Mujumdar N, Dudeja V, Saluja AK, Vickers SM. Triptolide-mediated cell death in neuroblastoma occurs by both apoptosis and autophagy pathways and results in inhibition of nuclear factor-κB activity. Am J Surg 205: 387–396, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai WL, Wong NS. The PERK/eIF2α signaling pathway of unfolded protein response is essential for N-(4-hydroxyphenyl)retinamide (4HPR)-induced cytotoxicity in cancer cells. Exp Cell Res 314: 1667–1682, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Li H, Song H, Luo J, Liang J, Zhao S, Su R. Knockdown of glucose-regulated protein 78 decreases the invasion, metalloproteinase expression and ECM degradation in hepatocellular carcinoma cells. J Exp Clin Cancer Res 31: 39, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, Glavic A, Kress C, Lin JH, Walter P, Reed JC, Glimcher LH, Hetz C. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1α. Mol Cell 33: 679–691, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackenzie TN, Mujumdar N, Banerjee S, Sangwan V, Sarver A, Vickers S, Subramanian S, Saluja AK. Triptolide induces the expression of mir-142–3p: a negative regulator of heat shock protein 70 and pancreatic cancer cell proliferation. Mol Cancer Ther 12: 1266–1275, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matus S, Nassif M, Glimcher LH, Hetz C. XBP-1 deficiency in the nervous system reveals a homeostatic switch to activate autophagy. Autophagy 5: 1226–1228, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Misiewicz M, Dery MA, Foveau B, Jodoin J, Ruths D, Leblanc AC. Identification of a novel endoplasmic reticulum stress response element regulated by XBP1. J Biol Chem 288: 20378–20391, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mozos A, Roue G, Lopez-Guillermo A, Jares P, Campo E, Colomer D, Martinez A. The expression of the endoplasmic reticulum stress sensor BiP/GRP78 predicts response to chemotherapy and determines the efficacy of proteasome inhibitors in diffuse large B-cell lymphoma. Am J Pathol 179: 2601–2610, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mujumdar N, Mackenzie TN, Dudeja V, Chugh R, Antonoff MB, Borja-Cacho D, Sangwan V, Dawra R, Vickers SM, Saluja AK. Triptolide induces cell death in pancreatic cancer cells by apoptotic and autophagic pathways. Gastroenterology 139: 598–608, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res 67: 9407–9416, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem 277: 21836–21842, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, Goldsmith PC, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett 514: 122–128, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Scull CM, Tabas I. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol 31: 2792–2797, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13: 184–190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi H, Wang JP, Zheng HC, Masuda S, Takano Y. Overexpression of GRP78 and GRP94 is involved in colorectal carcinogenesis. Histol Histopathol 26: 663–671, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Taylor RC, Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153: 1435–1447, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uematsu K, Ogata S, Nakanishi K, Hiroi S, Tominaga S, Aida S, Kawai T. Glucose-regulated protein 78 expression in urothelial carcinoma of the upper urinary tract. BJU Int 106: 873–878, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Verfaillie T, van Vliet A, Garg AD, Dewaele M, Rubio N, Gupta S, de Witte P, Samali A, Agostinis P. Pro-apoptotic signaling induced by photo-oxidative ER stress is amplified by Noxa, not Bim. Biochem Biophys Res Commun 438: 500–506, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Wang G, Yang ZQ, Zhang K. Endoplasmic reticulum stress response in cancer: molecular mechanism and therapeutic potential. Am J Transl Res 2: 65–74, 2010 [PMC free article] [PubMed] [Google Scholar]
- 39.Xing X, Lai M, Wang Y, Xu E, Huang Q. Overexpression of glucose-regulated protein 78 in colon cancer. Clin Chim Acta 364: 308–315, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Zhao C, Yin S, Dong Y, Guo X, Fan L, Ye M, Hu H. Autophagy-dependent EIF2AK3 activation compromises ursolic acid-induced apoptosis through upregulation of MCL1 in MCF-7 human breast cancer cells. Autophagy 9: 196–207, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou H, Zhang Y, Fu Y, Chan L, Lee AS. Novel mechanism of anti-apoptotic function of 78-kDa glucose-regulated protein (GRP78): endocrine resistance factor in breast cancer, through release of B-cell lymphoma 2 (BCL-2) from BCL-2-interacting killer (BIK). J Biol Chem 286: 25687–25696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]