Abstract
Extracellular acidification has been observed in allergic inflammatory diseases. Recently, we demonstrated that the proton-sensing receptor G protein-coupled receptor 65 (GPR65) regulates eosinophil survival in an acidic environment in vitro and eosinophil accumulation in an allergic lung inflammation model. For mast cells, another inflammatory cell type critical for allergic responses, it remains unknown whether GPR65 is expressed and/or regulates mast cell viability. Thus, in the present study, we employed in vitro experiments and an intestinal anaphylaxis model in which both mastocytosis and eosinophilia can be observed, particularly in the gastrointestinal tract, to enable us to directly compare the effect of GPR65 expression on these two cell types. We identified GPR65 expression on mast cells; however, unlike eosinophil viability, mast cell viability in vitro is not affected by acidification or GPR65 expression. Mechanistically, we determined that mast cells do not respond to extracellular acidification with increased cAMP levels. Furthermore, in the intestinal anaphylaxis model, we observed a significant reduction of eosinophils (59.1 ± 9.2% decrease) in the jejunum of allergen-challenged GPR65-deficient mice compared with allergen-challenged wild-type mice, despite the degree of antigen sensitization and the expression levels of Th2 cytokines (Il4, Il13) and eosinophil chemokines (Ccl11, Ccl24) in the jejunum being comparable. In contrast, the accumulation of mast cells in allergen-challenged mice was not affected by GPR65 deficiency. In conclusion, our study demonstrates differential regulation of eosinophils and mast cells in inflammatory tissue, with mast cell viability and accumulation being independent of GPR65.
Keywords: allergic inflammation, extracellular acidification, G protein-coupled receptor 65
extracellular acidification has been observed in many inflammatory disorders, including allergic diseases (7, 13, 26), as well as in solid tumors (31). Multiple mechanisms have been proposed to contribute to extracellular acidification, including 1) anaerobic glycolysis and enhanced production of lactic acid due to hypoxia and intense cellular metabolism (27) and 2) proton efflux to maintain NADPH oxidase activity and physiological intracellular pH in the inflammatory cells (3, 18). However, the consequences of acidification are incompletely understood.
A few studies have examined the responses of specific immune cells to acidosis, with neutrophils being the most studied cell type. These studies have shown that neutrophil functions, such as chemokinesis and survival, are enhanced in acidic pH (5, 11, 24, 28, 32). Additionally, lymphocyte motility is activated by acidic pH (23). Dendritic cell activation, as well as antigen uptake, is also increased with mild acidosis (29). Recently, we demonstrated that eosinophil viability is enhanced in acidic conditions (10). However, the mechanism of how immune cells sense the acidification is incompletely understood.
Acidity can affect cells in many ways, including through activation of proton-sensing receptors. G protein-coupled receptor 65 (GPR65, also known as T cell death-associated gene 8) belongs to a group of acid-sensing receptors in the G2 accumulation receptor (G2A) family. This family includes three additional structurally related members, G2A, ovarian cancer G protein-coupled receptor 1 (OGR1), and G protein-coupled receptor 4 (GPR4). Members of this family, including GPR65, have been shown to sense extracellular acidity by proton transfer to the histidines in the first loop of GPR65, presumably causing a conformational change in GPR65 that activates Gsα (13). This activity has been further demonstrated to induce cyclic adenosine 5′-monophosphate (cAMP) accumulation associated with adenylyl cyclase activation (9, 10, 22, 30).
Increasing evidence demonstrates that extracellular acidification may affect cell functions through GPR65. For instance, GPR65 was suggested to be involved in extracellular acidification-induced inhibition of superoxide anion production in human neutrophils (20) and proinflammatory cytokine [tumor necrosis factor-α and interleukin (IL)-6] production in mouse macrophages (16). Moreover, GPR65 was shown to facilitate tumor development by promoting adaptation to the acidic environment and thereby enhancing cell survival and proliferation (8). More recently, we reported that eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner (10). Furthermore, in an allergic asthma model, we found that GPR65 is required for optimal eosinophil accumulation in the pulmonary acidic environment and acts by affecting eosinophil survival (10). Collectively, these in vitro and in vivo studies suggest that GPR65 has different roles in different cell types.
Mast cells are critical for allergic responses, especially in the gastrointestinal tract. In this study, we aimed to examine 1) whether mast cells express GPR65; 2) whether GPR65 has an important role in regulating mast cell viability in vitro like it does for eosinophil viability; and 3) whether GPR65 is required for optimal mast cell and/or eosinophil accumulation by using an intestinal anaphylaxis model in which pronounced mastocytosis and eosinophilia develop in the jejunum. First, we identified GPR65 expression on mast cells. However, unlike for eosinophil survival, GPR65 was not required for mast cell survival in the acidic environment in vitro. Second, in the allergic gastrointestinal inflammation model, we found that GPR65 deficiency did not affect mast cell accumulation; however, it downregulated eosinophil accumulation in the inflammatory tissue, which is consistent with the phenotype in our prior asthma model (10). In conclusion, our present study demonstrated that mast cell viability and accumulation in inflammatory tissue are independent of proton-sensing receptor GPR65.
MATERIALS AND METHODS
Mice.
Male and female 6- to 8-wk-old Gpr65+/+ and Gpr65−/− mice (BALB/c background) were housed under specific pathogen-free conditions. Gpr65−/− mice contain a disrupted Gpr65 locus with an enhanced green fluorescent protein (EGFP) reporter knocked into the exon 2 to allow the analysis of GPR65 expression in living cells. All studies were reviewed and approved by the Cincinnati Children's Hospital Medical Center Institutional Animal Care and Use Committee.
Culture of mast cells and eosinophils.
To obtain mast cells for in vitro study, bone marrow (BM) cells were cultured in RPMI 1640 (Invitrogen) with 10% fetal bovine serum (FBS), 100 IU/ml penicillin and 10 μg/ml streptomycin, 2 mM glutamine, 50 μM 2-mercaptoethanol (2-ME), 1 mM pyruvate (Invitrogen), 1× nonessential amino acids (Invitrogen), and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES; Invitrogen). In addition, during the first 3 wk, the medium contained 10 ng/ml stem cell factor (SCF; PeproTech) and 20 ng/ml recombinant mouse IL-3 (PeproTech). During the 4th and 5th wk, the medium contained 20 ng/ml IL-3 only. The cell phenotype was subsequently identified by flow cytometry following FcϵRIα-PE (eBioscience) and c-Kit-APC (R&D System) staining and morphological examination after toluidine blue staining on the cytospun slides. Approximately 90–95% of harvested cells were consistently FcϵRIα and c-Kit double-positive mast cells (data not shown).
As a source of eosinophils, we primarily used BM-derived eosinophils, which were generated as described previously (4) with minor modifications. Briefly, BM cells were cultured in IMDM (Invitrogen) supplemented with 10% FBS (Cambrex), 100 IU/ml penicillin and 10 μg/ml streptomycin (Cellgro), 2 mM glutamine (Invitrogen), and 50 μM 2-ME (Sigma-Aldrich). From day 0 to 4, the medium contained 100 ng/ml SCF and 100 ng/ml FLT3 ligand (PeproTech). On day 5, the medium was replaced with fresh medium containing 10 ng/ml recombinant mouse IL-5 (PeproTech). On day 14, the cell phenotype was identified by flow cytometry following CCR3-FITC (R&D Systems) and Siglec-F-PE (BD Bioscience) staining and morphological examination following a modified Giemsa staining (Diff Quik) on the cytospun slides. Approximately 90–95% of harvested cells were consistently CCR3 and Siglec-F double-positive eosinophils (data not shown). Because our original studies (10) were performed with eosinophils from the spleen of IL-5 transgenic mice, some experiments were confirmed using splenic eosinophils (e.g., cAMP levels in Fig. 1F).
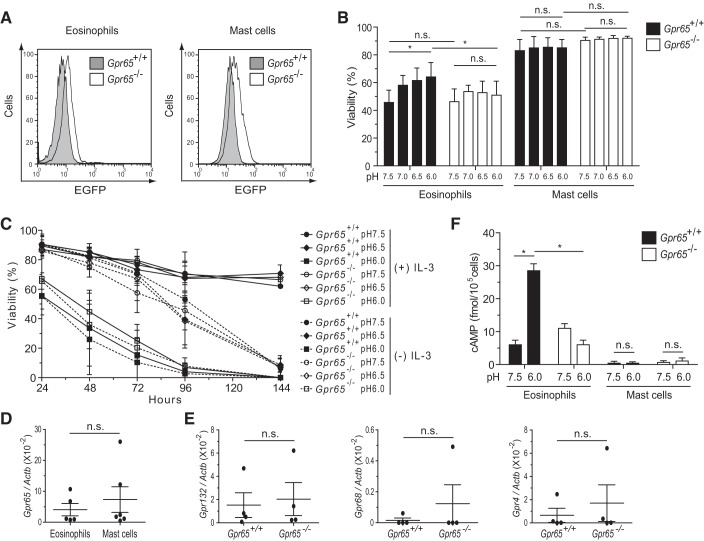
Fig. 1.
In vitro viability and intracellular cAMP assessment. A: flow cytometric identification of enhanced green fluorescent protein (EGFP) on mast cells and eosinophils from G protein-coupled receptor (Gpr) 65-positive (+/+) and Gpr65−/− (EGFP knock-in) mice. Representative out of 6 experiments is shown. B: flow cytometric assessment of eosinophil and mast cell viability after a 7-h incubation in media at the indicated pH (n = 5 experiments). The viable cells were defined as 7-AAD and Annexin V double negative. C: viable mast cell counting by trypan blue exclusion assay after incubation for the indicated time in media at pH 7.5–6.0 in the presence (solid lines) or absence (dashed lines) of interleukin (IL)-3 (10 ng/ml) (n = 3 experiments). D: comparison of Gpr65 transcript levels between wild-type (WT) mast cells and WT eosinophils by real-time RT-PCR following normalization by the housekeeping gene Actb. E: comparison of Gpr132 [encoding G2 accumulation receptor (G2A)], Gpr68 [encoding ovarian cancer G protein-coupled receptor 1 (OGR1)], and Gpr4 [encoding G protein-coupled receptor 4 (GPR4)] transcript levels between WT and GPR65-deficient mast cells by real-time RT-PCR following normalization by Actb. F: eosinophils and mast cells were incubated at the indicated pH for 30 min in the presence of 3-isobutyl-1-methylxanthine (IBMX), and accumulated intracellular cAMP was measured via a competitive enzyme-linked immunosorbent assay (ELISA) from cell lysates (n = 3 experiments). Data are expressed as means ± SD, and paired t-test was used for statistical analysis (B, C, and F). *P < 0.05; ns, no significant difference.
Assessment of cell viability.
For acidic exposure, the complete RPMI 1640 (for mast cells) and IMDM (for eosinophils) media were prepared by using 30 mM biological buffer [either HEPES or 2-(N-morpholino)ethanesulfonic acid for pH ranges 7.0–7.5 and 5.5–6.5, respectively]. After the media were adjusted to the appropriate pH, NaCl was added to maintain consistent osmolarity in all pH media. In each experiment, cells (1 × 106/ml) were incubated in the pH-adjusted media (7.0–7.5 or 5.5–6.5) at 37°C with 5% CO2 in the presence or absence of IL-3 (20 ng/ml) for mast cells or in the presence of IL-5 (10 ng/ml) for eosinophils. The cells were then washed and stained with 7-AAD and APC-conjugated Annexin V (BD Biosciences) in 1× Annexin V binding buffer (BD Biosciences). These samples were incubated at room temperature for 15 min and analyzed immediately on FACS Canto II (BD Bioscience). The viable cells were defined as 7-AAD negative and Annexin V negative. Alternatively, viable cells were manually counted by trypan blue exclusion assay.
Intracellular cAMP measurement.
Mast cells (and eosinophils as a positive control) were incubated in the buffered media containing 0.5 mM 3-isobutyl-1-methylxanthine (a phosphodiesterase inhibitor) to promote cAMP accumulation in cells. After a 30-min incubation at 37°C with 5% CO2, cells were lysed immediately for cAMP quantification using a competitive enzyme-linked immunosorbent assay (ELISA; Cyclic AMP XP Chemiluminescent Assay Kit; Cell Signaling).
Experimental oral antigen-induced intestinal anaphylaxis model.
As previously described (2), mice were sensitized two times, 2 wk apart, with 50 μg of ovalbulmin (OVA; grade V; Sigma-Aldrich) and 1 mg of alum (Thermo Scientific) in sterile saline by the means of intraperitoneal injection. Two weeks later, mice were orally gavaged with 50 mg of OVA (in 250 μl of saline), with controls receiving 250 μl of saline, every other day for a total of six times. Before each intragastric challenge, mice were deprived of food for 3–4 h. Challenges were performed with intragastric feeding needles (01-290-2B; Fisher Scientific). Rectal temperatures were measured before and 30 min after OVA challenge. Diarrhea was assessed by visually monitoring mice for up to 60 min after intragastric challenge. Mice demonstrating profuse liquid stool were recorded as diarrhea-positive animals.
Preparation of single cell suspensions of the jejunum.
For examination of EGFP expression in jejunal mast cells, fragments of the jejunum from OVA-challenged mice were dissected out and cut open longitudinally. Next, the tissue was first incubated in HBSS with EDTA (5 mM) on ice. After removal of EDTA, the tissue was minced into small pieces and incubated in serum-free RPMI 1640 containing collagenase A (2.4 mg/ml; Roche) and DNase I (0.1 mg/ml; Roche) with gentle shaking for 30 min at 37°C. Finally, the tissue was homogenized by pushing it through a 19.5-gauge needle and resuspended in PBS with 0.2% BSA for flow cytometry. FcϵRIα+/c-Kit+/7-AAD− cells were considered as live mast cells for examining EGFP expression under the control of GPR65 promoter activity.
ELISA.
Total IgE in the serum was measured with the OptEIA ELISA Kit (BD Bioscience) according to the manufacturer's protocol. In brief, diluted serum samples were applied to anti-mouse IgE monoclonal antibody (mAb)-coated 96-well ELISA plates (Costar, Corning) after blocking with 10% FBS. After a 2-h incubation at room temperature, the plates were washed and added with the premixed Working Detector [biotinylated anti-mouse IgE mAb and streptavidin-horseradish peroxidase (HRP) conjugate] into each well. After a 1-h incubation, the colorimetric reaction was developed following the addition of tetramethylbenzidine substrate solution (BD Bioscience) and then stopped with 1 M H2SO4. Optical density at 450 nm was quantified with an ELISA plate reader (Synergy 2; BioTek). OVA-specific IgG1 in the serum was measured in a very similar way; OVA (100 μg/ml) was used to coat the ELISA plates, and diluted (1:1,000) HRP-conjugated anti-mouse IgG1 (X56; 0.5 mg/ml; BD Biosciences) was used to detect IgG1 in the serum. All diluted serum samples were performed in duplicate.
Total RNA extraction and real-time RT-PCR.
Total RNA was extracted using TRIzol Reagent (Invitrogen) as per the manufacturer's instructions. RNA (1 μg) was treated by DNase I (Qiagen) for 15 min at room temperature before reverse transcription using the iScript cDNA Synthesis Kit (Bio-Rad). cDNA (2 μl) were subjected to real-time RT-PCR set up with iQ SYBR Green Supermix (Bio-Rad) and the following individual primer sets: Gpr65 (encoding GPR65), forward 5′-CAGATTTGCCAGCCTCCTCAGTC, reverse 5′-GCCTCTTGCTTGCCCTTTTGAA; Gpr132 (encoding G2A), forward 5′-TCACAAGGGGGTCCACAGAACTC, reverse 5′-ACGGCACTGTACACCACCACCA; Gpr68 (encoding OGR1), forward 5′-ACTGCCTTCCTTTGCCCTACCA, reverse 5′-GAGCCAATCCCTCTCTTGCCAT; Gpr4 (encoding GPR4), forward 5′-CTGTGCAGAGTCGGGACCAAGT, reverse 5′-AAGGGGGTTCCAGGAGACTCAG; Il4 (encoding IL-4), forward 5′-CTGTAGGGCTTCCAAGGTGCTTCG, reverse 5′-CCATTTGCATGATGCTCTTTAGGC; Il13 (encoding IL-13), forward 5′-CATGGCGCTCTGGGTGACTG, reverse 5′-CGGCCAGGTCCACACTCCATAC; Ccl11 (encoding eotaxin-1), forward 5′-GGCTCACCCAGGCTCCATCC, reverse 5′-TTTTGGTCCAGGTGCTTTGTGG; Ccl24 (encoding eotaxin-2), forward 5′-CTCCTTCTCCTGGTAGCCTGC, reverse 5′-GTGATGAAGATGACCCCTGCCTT; Mcpt1, Mcpt2, and Mcpt4 (encoding mast cell protease 1, 2, and 4, respectively), forward 5′-GCTGGAGCTGAGGAGATTATTG, Mcpt1 reverse 5′-CTCCCATGTATGCTGTTTTTAACT, Mcpt2 reverse 5′-CCTCTCCTTCGAACCGTTCTTA, Mcpt4 reverse 5′-TGCCAATAGTTTTTACAGGCCTC; and the housekeeping gene Actb, forward 5′-CGATGCCCTGAGGCTCTTTTCC, reverse: 5′-CATCCTGTCAGCAATGCCTGGG. The relative gene expression levels were normalized to Actb.
Immunohistochemistry for detection and quantification of intestinal eosinophils and mast cells.
The jejunum segment was fixed with 4% paraformaldehyde, processed with standard histological techniques, and immunostained with antiserum against murine major basic protein (MBP) as previously described (15). Briefly, 5-μm sections were mounted on glass slides, quenched with H2O2, blocked with normal goat serum, and stained with rabbit anti-murine eosinophil MBP antiserum (a gift of J. and N. Lee, Mayo Clinic, Scottsdale, AZ). The slides were washed and incubated with biotinylated goat anti-rabbit antibody and avidin-peroxidase complex (Vectastain ABC Peroxidase Elite kit; Vector Laboratories, Burlingame, CA). The slides were then developed by nickel diaminobenzidine, enhanced with cobalt chloride to form a black precipitate, and counterstained with nuclear fast red. The sections were taken from the same position in the jejunum (3–5 cm distal to the stomach), and at least two random sections per mouse were analyzed. Mast cells were identified by staining for chloroacetate esterase (CAE) activity as previously described (6, 12). The eosinophils and mast cells within the jejunal lamina propria and crypt areas were counted and normalized from 15 to 25 fields of view (magnification ×100) individually by an observer blinded to treatment and genotype. Values were normalized by quantifying the total number of eosinophils or mast cells per square millimeter of tissue.
Statistical analysis.
Student's t-test (for experiments with 2 groups) and ANOVA (for experiments with >2 groups) were used to assess statistical significance. A P value of <0.05 was considered significant. All analyses were performed with Graphpad Prism 5.0 software.
RESULTS
GPR65 deficiency has no effect on mast cell viability in vitro.
We and other groups have reported that a variety of leukocytes, including eosinophils, neutrophils, and T and B lymphocytes, express GPR65 (10, 20, 21). Another critical cell type that accumulates in allergic inflammation is the mast cell. First, we examined BM-derived mast cells to determine whether they express GPR65 by analyzing EGFP reporter that is knocked into the Gpr65 locus and is thus under the control of the endogenous Gpr65 promoter (21). We identified by flow cytometry that murine mast cells also express Gpr65 to a level that is comparable to eosinophils (P = 0.35, n = 6 experiments; Fig. 1A). This was further confirmed by comparing the Gpr65 transcript levels between wild-type (WT) mast cells and WT eosinophils as shown in Fig. 1D. No significant effect of EGFP expression was observed on the development of BM mast cells, in that the number of cells and expression levels of FcϵRIα/c-Kit were comparable after 35 days in culture (data not shown). Next, to investigate whether GPR65 regulates mast cell viability in the acidic environment, we incubated mast cells in media buffered to pH 6.0–7.5 in parallel with eosinophils. Consistent with our prior study in which eosinophils isolated from CD2-IL-5 transgenic mouse spleen were studied (10), WT eosinophils derived from BM also showed an increasing trend of viability from pH 7.5 to 6.0 after a 7-h incubation. Specifically, the viability at pH 6.0 was significantly higher than it was at pH 7.5 (Fig. 1B). However, the viability of GPR65-deficent eosinophils at pH 6.0 was significantly lower than that of WT eosinophils (Fig. 1B), showing that eosinophil survival in an acidic environment is dependent on GPR65. In contrast, WT mast cells did not show significant difference in viability between pH 7.5 and 6.0 after a 7-h incubation. Moreover, no significant difference was observed between WT and GPR65-deficient mast cells in their viability at pH 7.5 or 6.0 (Fig. 1B). We further investigated whether mast cell viability would be affected under acidic conditions with different incubation time (3–5 h or longer than overnight), with cytokine IL-3 withdrawal, or of even greater acidity (e.g., pH 5.5). Under no condition did we observe pH-dependent viability of WT mast cells or a significant difference in the viability between WT and GPR65-deficient mast cells (Fig. 1C and data not shown).
To explore the mechanism accounting for the lack of change in mast cell viability in response to acidity, we investigated several possibilities. First, we examined whether other proton-sensing receptors, including G2A, OGR1, and GPR4, are overexpressed in GPR65-deficient mast cells. No significant difference was observed in the expression of other proton-sensing receptors between WT and GPR65-deficient mast cells (Fig. 1E). Second, we tested whether mast cells signal in response to acidity. Because proton binding to GPR65 has been shown to induce intracellular cAMP accumulation and eosinophil viability under acidic conditions is dependent on cAMP (9, 10, 22, 30), we hypothesized that mast cells do not accumulate cAMP in response to acidic pH. Consistent with our prior report (10), WT eosinophils exhibited a significant, GPR65-dependent increase in cAMP at pH 6.0 compared with pH 7.5. In contrast, WT mast cells did not show cAMP induction at pH 6.0 compared with pH 7.5 (Fig. 1F). Collectively, these results demonstrate that GPR65 is expressed on mast cells but, unlike eosinophils, does not lead to cAMP induction or enhanced cell survival in response to an acidic environment.
Comparable sensitization and local inflammatory responses between Gpr65+/+ and Gpr65−/− mice.
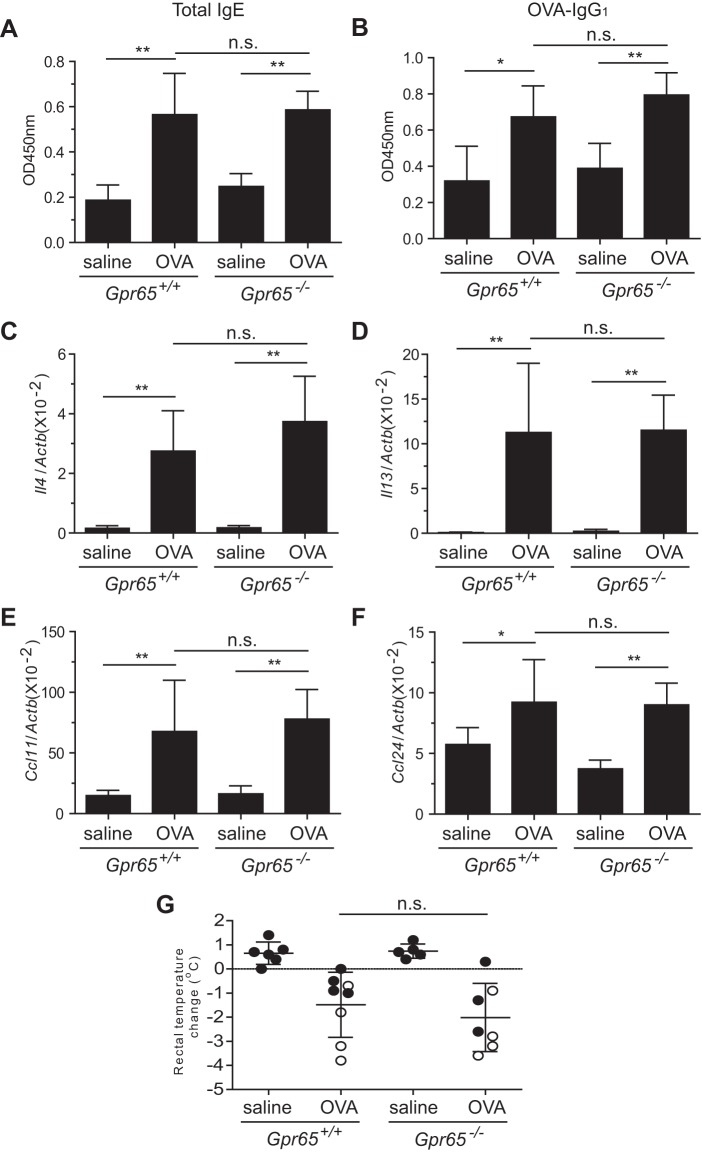
Our prior asthma study model together with in vitro data has suggested that GPR65 regulates eosinophil accumulation by affecting eosinophil survival (10). Because our present in vitro results suggested that GPR65 deficiency has no effect on mast cell survival, we speculated that GPR65 deficiency would not affect mast cell accumulation in vivo. To test this hypothesis and to directly compare the effect of GPR65 on eosinophils and mast cells within the same tissue, we used an oral antigen-induced intestinal anaphylaxis model that develops pronounced eosinophilia and mastocytosis in the jejunum. We first tested whether GPR65 deficiency would affect antigen sensitization, which in turn could affect the accumulation of mast cells and eosinophils in the GPR65-deficient mice following OVA challenges, by measuring the levels of total IgE and OVA-specific IgG1 in the serum of WT and GPR65-deficient mice by ELISA. We found that both of these Th2-type antibodies were significantly increased in OVA-challenged WT and GPR65-deficient mice compared with in saline-challenged controls (Fig. 2, A and B). However, there was no significant difference between WT and GPR65-deficient mice following OVA challenges (Fig. 2, A and B), suggesting that the sensitization to OVA antigen in WT and GPR65-deficient mice was comparable. Second, we examined the transcript levels of the major Th2 cytokines IL-4 and IL-13, as well as the eosinophil-selective chemokines eotaxin-1 and eotaxin-2, in the jejunum by real-time RT-PCR. The transcripts of all of these mediators were significantly increased in WT and GPR65-deficient mice following OVA challenges compared with in the saline-challenged control mice, but none of them was significantly different between OVA-challenged WT and GPR65-deficient mice (Fig. 2, C–F). Third, when performing OVA challenge, we recorded rectal temperature decrease and diarrhea occurrence, the two characteristic features in the intestinal anaphylaxis model. Neither the rectal temperature change nor the diarrhea occurrence showed a significant difference between WT and GPR65-deficient mice following OVA challenge (Fig. 2G). Collectively, these findings suggest that GPR65 deficiency does not have an effect on the antigen-induced systemic and local inflammatory responses.
Fig. 2.
Major immune responses and anaphylaxis outcomes in WT and GPR65-deficient mice. A and B: total IgE (1:20 dilution; A) and ovalbulmin (OVA)-specific IgG1 (1:125,000 dilution; B) levels in the serum samples from saline- and OVA-challenged WT and GPR65-deficient mice were determined by ELISA. C–F: real-time RT-PCR analysis of the Th2 cytokines Il4 (C) and Il13 (D) and the eosinophil chemokines Ccl11 (E) and Ccl24 (F) in the jejunum of WT and GPR65-deficient mice after normalization by the housekeeping gene Actb. G: anaphylaxis was assessed by recording rectal temperature change and diarrhea occurrence following six challenges of either intragastric saline or OVA in WT and GPR65-deficient mice. The mice that evidenced diarrheal symptoms are in open symbols. Data shown are a representative of 4 experiments and expressed as means ± SD (n = 4–6 mice/saline group, n = 7–9 mice/OVA group). *P < 0.05; **P < 0.01; ns, no significant difference.
Downregulated eosinophilia in the jejunum of OVA-challenged Gpr65−/− mice.
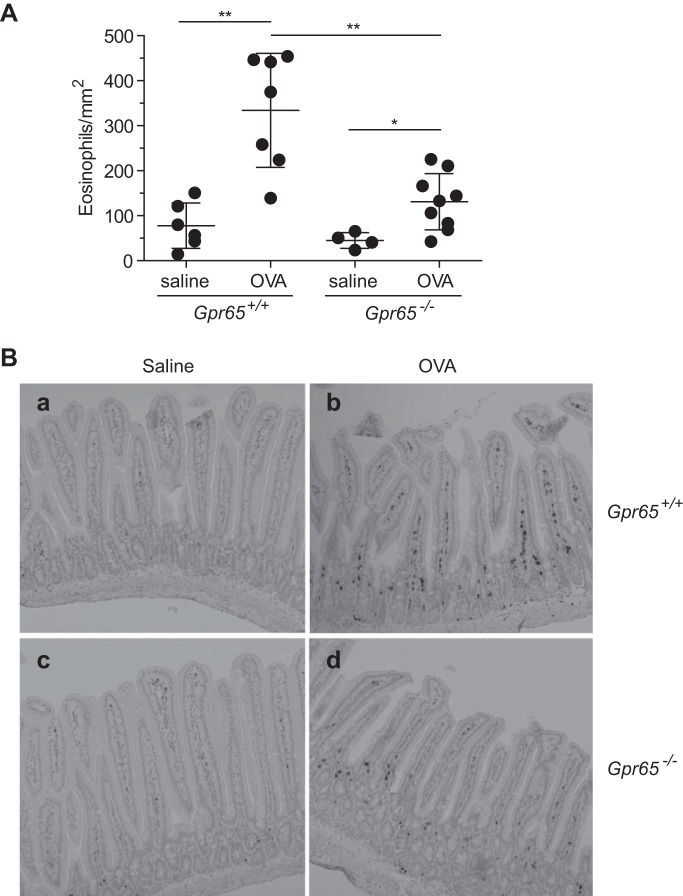
We have observed that Gpr65−/− mice had attenuated airway eosinophilia in an allergic asthma model (10), suggesting that GPR65 was required for optimal eosinophil accumulation in allergic lung inflammation. Thus, given that extracellular acidification is commonly observed in inflammatory disorders, including allergic diseases (7, 13, 26), we hypothesized that GPR65 would also regulate eosinophil accumulation in the intestinal anaphylaxis model. Both WT and GPR65-deficent mice had significantly increased eosinophilia in the jejunum following multiple intragastric OVA challenges compared with the saline-challenged controls (Fig. 3). However, OVA-challenged GPR65-deficient mice had significantly reduced eosinophilia in their jejunum compared with the OVA-challenged WT mice (59.1 ± 9.2% decrease, n = 4 experiments). These data suggest that GPR65 regulates eosinophil accumulation in the jejunum during allergic gastrointestinal inflammation.
Fig. 3.
Quantification of eosinophils in the jejunum of WT and GPR65-deficient mice. A: the eosinophils within the jejunual lamina propria and crypt areas were identified as major basic protein (MBP)-positive (dark brown) cells by immunohistochemistry staining and quantified by an observer blinded to treatment and genotype. Data shown are a representative of 4 experiments and expressed as means ± SD. *P < 0.05; **P < 0.01. B: representative images of anti-MBP staining on the jejunum from saline-challenged WT mice (a), OVA-challenged WT mice (b), saline-challenged GPR65-deficient mice (c), and OVA-challenged GPR65-deficient mice (d).
Mastocytosis in the jejunum of OVA-challenged Gpr65−/− mice.
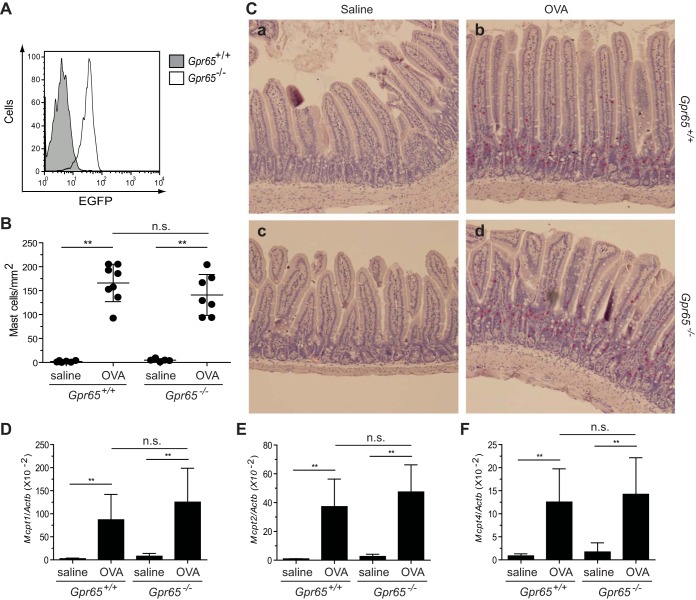
We further investigated whether GPR65 deficiency affects mast cell accumulation during the same allergic gastrointestinal inflammation. Following the confirmation of Gpr65 expression on the jejunal mast cells (Fig. 4A), we evaluated the mastocytosis in WT and GPR65-deficient mice following OVA challenges. Both WT and GPR65-deficient mice developed significant mastocytosis in the jejunum following OVA challenges, and no significant difference was observed between these mice in terms of the overall mast cell numbers or their distribution within lamina propria and crypt areas (Fig. 4, B and C). Moreover, we compared the transcript levels of marker genes (reviewed in Ref. 14) for mucosal mast cells [mast cell protease (MCP)-1 and MCP-2] and connective mast cells (MCP-4) in the jejunum. The transcription of all of these genes was significantly increased in OVA-challenged WT and GPR65-deficient mice compared with in saline-challenged control mice (Fig. 4, D–F). Notably, none of these markers had significantly different expression between WT and GPR65-deficient mice following OVA challenges, suggesting that GPR65 deficiency has no effect on these two mast cell subpopulations. Collectively, these findings suggest that GPR65 deficiency does not affect mast cell accumulation in allergic gastrointestinal inflammation.
Fig. 4.
Quantification of mast cells and mast cell proteases in the jejunum of WT and GPR65-deficient mice. A: flow cytometric examination of EGFP in the jejunal mast cells from Gpr65+/+ and Gpr65−/− (EGFP knock-in) mice following OVA challenges. B: the mast cells within jejunal lamina propria and crypt areas were identified as chloroacetate esterase (CAE) activity-positive (red) cells and quantified by an observer blinded to treatment and genotype. C: representative images of CAE staining on the jejunum from saline-challenged WT mice (a), OVA-challenged WT mice (b), saline-challenged GPR65-deficient mice (c), and OVA-challenged GPR65-deficient mice (d). D–F: the transcript levels of murine Mcpt1 (D), Mcpt2 (E), and Mcpt4 (F) relative to the housekeeping gene Actb in the jejunum of WT and GPR65-deficient mice were determined by real-time RT-PCR. All data are a representative of 4 experiments and expressed as means ± SD (n = 4–6 mice/saline group, n = 7–9 mice/OVA group). **P < 0.01; ns, no significant difference.
DISCUSSION
In the present study, we demonstrated downregulated eosinophilia but unaffected mastocytosis in GPR65-deficient mice during allergic gastrointestinal inflammation. GPR65 deficiency did not affect systemic sensitization or local inflammatory responses, suggesting that a cell-intrinsic mechanism leads to decreased eosinophil accumulation. Mechanistic in vitro studies demonstrated that the viability of mast cells, in contrast to eosinophils, was not affected by acidification or GPR65 deficiency. This difference may explain our finding that GPR65 selectively affected eosinophil accumulation in allergic gastrointestinal inflammation.
Our previous study has shown that eosinophil development in the BM and eosinophil numbers in the blood and tissue at baseline are not affected by GPR65 deficiency (10). In the present study, we found no effect of GPR65 deficiency on allergic sensitization or local expression of major Th2 cytokines and eosinophil chemokines in the inflamed gastrointestinal tissue, all of which could affect eosinophil accumulation. These results are consistent with previous studies showing that GPR65-deficient mice have normal major immune responses, including cytokine and antibody production in response to antigen (10, 21). On the basis of these data and our previous study (10) showing that GPR65 regulates eosinophil viability in response to acidic pH in vitro (also shown as control for mast cells in Fig. 1B), we postulate that the observed decreased intestinal eosinophilia in OVA-challenged GPR65-deficient mice is due to reduced eosinophil viability in the local microenvironment.
In contrast to the reduced eosinophilia in the intestine, comparable mastocytosis was observed in OVA-challenged WT and GPR65-deficient mice. Consistent with previous findings that disease outcomes, such as rectal temperature decrease (hypothermia) and diarrhea occurrence, are dependent on IgE and mast cells but not eosinophils in the allergic gastrointestinal inflammation model (2), we did not observe a significant difference in disease outcomes between allergen-challenged WT and GPR65-deficient mice (Fig. 2G). We were interested in the mechanism underlying the differential regulation of mast cells and eosinophils by GPR65. While mast cells express GPR65 in vitro and in vivo (Fig. 1A and data not shown), GPR65 deficiency did not have an effect on the viability of mast cells in physiological or acidic environment in vitro. There are several potential explanations for these findings. First, GPR65 function may be redundant on mast cells, since the other three structurally related G2A subfamily members (G2A, OGR1, and GPR4) were all shown to bind extracellular protons as their primary ligands (13, 19, 30). Moreover, these receptors (at least G2A and GPR65) may have differential proton sensitivity in different cell types (22). Second, following extracellular proton-induced GPR65 activation, adenylyl cyclase activation leads to cAMP accumulation (9, 22, 30). However, cAMP may have different functions in different cell types, possibly stemming from cross talk with other pathways or differential levels of cAMP at baseline in individual cell types (1, 25). For instance, the level of cAMP in regulatory T cells is >10-fold higher than in CD4 effector T cells (1). Indeed, our study also demonstrated that eosinophils have a relatively higher baseline level of cAMP compared with mast cells (Fig. 1F). Third, cAMP accumulation may not necessarily be the only intracellular signaling pathway to be induced through GPR65. As a matter of fact, we observed no remarkable cAMP induction in WT mast cells in the acidic environment (pH 6.0 vs. 7.5) nor a significant cAMP reduction in GPR65-deficient mast cells (Fig. 1F). Thus, it remains possible that other signaling pathways, such as the inositol triphosphate signaling pathway associated with phospholipase C activation (13, 17) or the Rho signaling pathway (19), could be induced. However, it is unclear whether these possible early signaling events would have the potential to affect cell viability. Further studies are needed to address the cell-specific functions of GPR65 in inflammatory tissue and allergic disease.
In summary, our results have established these conclusions: 1) mast cells express GPR65; 2) GPR65 is not required for mast cell survival in an acidic environment; and 3) GPR65 regulates eosinophil but not mast cell accumulation in allergic gastrointestinal inflammation.
GRANTS
This work was supported by National Institute of Allergy and Infectious Diseases Grant R21-AI-088559 (N. Zimmermann).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: X.Z. and E.M. performed experiments; X.Z. and E.M. analyzed data; X.Z. prepared figures; X.Z. drafted manuscript; X.Z., E.M., S.P.H., and N.Z. edited and revised manuscript; X.Z., E.M., S.P.H., and N.Z. approved final version of manuscript; S.P.H. and N.Z. conception and design of research; S.P.H. and N.Z. interpreted results of experiments.
ACKNOWLEDGMENTS
We thank Shawna Hottinger for editorial assistance and Dr. Owen N. Witte for providing the Gpr65−/− mice.
REFERENCES
- 1.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, Stoll S, Schild H, Staege MS, Stassen M, Jonuleit H, Schmitt E. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med 204: 1303–1310, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest 112: 1666–1677, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature 422: 531–534, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol 181: 4004–4009, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dziezanowski MA, DeStefano MJ, Rabinovitch M. Effect of antitubulins on spontaneous and chemotactic migration of neutrophils under agarose. J Cell Sci 42: 379–388, 1980 [DOI] [PubMed] [Google Scholar]
- 6.Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol 135: 279–290, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med 161: 694–699, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Ihara Y, Kihara Y, Hamano F, Yanagida K, Morishita Y, Kunita A, Yamori T, Fukayama M, Aburatani H, Shimizu T, Ishii S. The G protein-coupled receptor T-cell death-associated gene 8 (TDAG8) facilitates tumor development by serving as an extracellular pH sensor. Proc Natl Acad Sci USA 107: 17309–17314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii S, Kihara Y, Shimizu T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem 280: 9083–9087, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Kottyan LC, Collier AR, Cao KH, Niese KA, Hedgebeth M, Radu CG, Witte ON, Khurana Hershey GK, Rothenberg ME, Zimmermann N. Eosinophil viability is increased by acidic pH in a cAMP- and GPR65-dependent manner. Blood 114: 2774–2782, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol 69: 522–530, 2001 [PubMed] [Google Scholar]
- 12.Leder LD. The chloroacetate esterase reaction. A useful means of histological diagnosis of hematological disorders from paraffin sections of skin. Am J Dermatopathol 1: 39–42, 1979 [PubMed] [Google Scholar]
- 13.Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature 425: 93–98, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Miller HR, Pemberton AD. Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology 105: 375–390, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest 103: 1719–1727, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mogi C, Tobo M, Tomura H, Murata N, He XD, Sato K, Kimura T, Ishizuka T, Sasaki T, Sato T, Kihara Y, Ishii S, Harada A, Okajima F. Involvement of proton-sensing TDAG8 in extracellular acidification-induced inhibition of proinflammatory cytokine production in peritoneal macrophages. J Immunol 182: 3243–3251, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Mogi C, Tomura H, Tobo M, Wang JQ, Damirin A, Kon J, Komachi M, Hashimoto K, Sato K, Okajima F. Sphingosylphosphorylcholine antagonizes proton-sensing ovarian cancer G-protein-coupled receptor 1 (OGR1)-mediated inositol phosphate production and cAMP accumulation. J Pharmacol Sci 99: 160–167, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Morgan D, Cherny VV, Murphy R, Katz BZ, DeCoursey TE. The pH dependence of NADPH oxidase in human eosinophils. J Physiol 569: 419–431, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami N, Yokomizo T, Okuno T, Shimizu T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J Biol Chem 279: 42484–42491, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Murata N, Mogi C, Tobo M, Nakakura T, Sato K, Tomura H, Okajima F. Inhibition of superoxide anion production by extracellular acidification in neutrophils. Cell Immunol 259: 21–26, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Radu CG, Cheng D, Nijagal A, Riedinger M, McLaughlin J, Yang LV, Johnson J, Witte ON. Normal immune development and glucocorticoid-induced thymocyte apoptosis in mice deficient for the T-cell death-associated gene 8 receptor. Mol Cell Biol 26: 668–677, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radu CG, Nijagal A, McLaughlin J, Wang L, Witte ON. Differential proton sensitivity of related G protein-coupled receptors T cell death-associated gene 8 and G2A expressed in immune cells. Proc Natl Acad Sci USA 102: 1632–1637, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratner S. Motility of IL-2-stimulated lymphocytes in neutral and acidified extracellular matrix. Cell Immunol 139: 399–410, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol 113: 610–619, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Robison GA, Butcher RW, Sutherland EW. Cyclic AMP. Annu Rev Biochem 37: 149–174, 1968 [DOI] [PubMed] [Google Scholar]
- 26.Simmen HP, Blaser J. Analysis of pH and pO2 in abscesses, peritoneal fluid, and drainage fluid in the presence or absence of bacterial infection during and after abdominal surgery. Am J Surg 166: 24–27, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res 49: 4373–4384, 1989 [PubMed] [Google Scholar]
- 28.Trevani AS, Andonegui G, Giordano M, Lopez DH, Gamberale R, Minucci F, Geffner JR. Extracellular acidification induces human neutrophil activation. J Immunol 162: 4849–4857, 1999 [PubMed] [Google Scholar]
- 29.Vermeulen M, Giordano M, Trevani AS, Sedlik C, Gamberale R, Fernandez-Calotti P, Salamone G, Raiden S, Sanjurjo J, Geffner JR. Acidosis improves uptake of antigens and MHC class I-restricted presentation by dendritic cells. J Immunol 172: 3196–3204, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Wang JQ, Kon J, Mogi C, Tobo M, Damirin A, Sato K, Komachi M, Malchinkhuu E, Murata N, Kimura T, Kuwabara A, Wakamatsu K, Koizumi H, Uede T, Tsujimoto G, Kurose H, Sato T, Harada A, Misawa N, Tomura H, Okajima F. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J Biol Chem 279: 45626–45633, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol 2: 343–366, 1984 [DOI] [PubMed] [Google Scholar]
- 32.Zigmond SH, Hargrove RL. Orientation of PMN in a pH gradient: acid-induced release of a chemotactic factor. J Immunol 126: 478–481, 1981 [PubMed] [Google Scholar]