Abstract
Necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in premature infants and develops partly from an exaggerated intestinal epithelial immune response to indigenous microbes. There has been interest in administering probiotic bacteria to reduce NEC severity, yet concerns exist regarding infection risk. Mechanisms of probiotic activity in NEC are unknown although activation of the microbial DNA receptor Toll-like receptor-9 (TLR9) has been postulated. We now hypothesize that the Gram-positive bacterium Lactobacillus rhamnosus HN001 can attenuate NEC in small and large animal models, that its microbial DNA is sufficient for its protective effects, and that protection requires activation of the Toll-like receptor 9 (TLR9). We now show that oral administration of live or UV-inactivated Lactobacillus rhamnosus HN001 attenuates NEC severity in newborn mice and premature piglets, as manifest by reduced histology score, attenuation of mucosal cytokine response, and improved gross morphology. TLR9 was required for Lactobacillus rhamnosus-mediated protection against NEC in mice, as the selective decrease of TLR9 from the intestinal epithelium reversed its protective effects. Strikingly, DNA of Lactobacillus rhamnosus HN001 reduced the extent of proinflammatory signaling in cultured enterocytes and in samples of resected human ileum ex vivo, suggesting the therapeutic potential of this probiotic in clinical NEC. Taken together, these findings illustrate that Lactobacillus rhamnosus HN001 is an effective probiotic for NEC via activation of the innate immune receptor TLR9 and that Lactobacillus rhamnosus DNA is sufficient for its protective effects, potentially reducing concerns regarding the infectious risk of this novel therapeutic approach.
Keywords: necrotizing enterocolitis, probiotics, Toll-like receptor 4, Toll-like receptor 9, enterocyte, NF-κB, intestinal inflammation, prematurity, sepsis
necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in premature infants (46). The characteristic features of NEC include the sudden development of abdominal distention, feeding intolerance, and bloody stools in a premature infant who has received oral feeds, which progresses to intestinal necrosis requiring surgical resection in nearly half of cases (22, 24, 26, 41). The overall survival in patients who develop NEC remains ∼50% and has changed little since the disease was first described, stressing the importance and urgency of identifying novel preventative or therapeutic approaches for it. Although a complete understanding of the factors that lead to NEC is lacking, over 90% of cases of NEC develop in infants who receive a nutritional formulation other than breast milk (39). This important association between formula administration and the development of NEC raises the possibility that strategies to modify individual infant formulations may serve to attenuate the severity of NEC and/or prevent the development of the disease in the first place.
In seeking to identify formulations that protect against the development of NEC, much attention has been placed on the potential benefit of supplementing existing nutritive formulas with bacteria that exert positive health benefits. Such “probiotic” bacteria are defined as “live microorganisms that when administered in adequate amounts confer a health benefit on the host” (18, 27), and several randomized trials and two large meta-analyses have suggested the potential benefit of various different probiotics either alone or in combination in reducing the incidence of NEC in premature human infants (3, 6, 8, 14, 35, 38, 42, 58). However, the enthusiasm for administration of probiotics has been dampened by concerns regarding infectious risk in the premature infant (37). In addition, the precise mechanisms of action of probiotics in protecting against NEC remain unknown. A greater understanding of the mechanisms by which probiotics may act and a strategy that avoids the infectious risk may be of great interest to the field.
In this regard, recent studies have identified that microbial DNA from probiotic bacteria may play a critical role in the protective effects of these agents (23, 48–50) and that this protection requires activation of the microbial DNA receptor Toll-like receptor 9 (TLR9). In further studies, TLR9 activation by microbial DNA in the intestinal epithelium protected against NEC development through inhibition of the receptor for lipopolysaccharide, namely TLR4, which we and others have shown to play a critical role in NEC pathogenesis through increasing mucosal injury and delaying mucosal repair (23, 30), although the precise role of DNA from a probiotic species was not examined. These findings raise the exciting possibility that supplementation of an infant nutritive formula with DNA from a probiotic bacteria may offer the potential benefits of the probiotic, without conferring the added risk associated with the delivery of live bacteria. In this regard, Lactobacillus rhamnosus HN001 is a recently identified Gram-positive bacterium, which has demonstrated probiotic activity in inflammatory disorders (19, 56, 60), yet a potential therapeutic role for this probiotic strain or its DNA in NEC remains unexplored. We now hypothesize that Lactobacillus rhamnosus HN001 can attenuate NEC in mouse and piglet models of NEC, that the DNA of Lactobacillus rhamnosus (Lr-DNA) is sufficient to exert its protective effects, and that the mechanism of protective action requires activation of the microbial DNA receptor TLR9.
MATERIALS AND METHODS
Statement of ethics.
The animal experiments described in this study were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocols were approved by the University of Pittsburgh Animal Care and Use Committee (Protocol Numbers: 12040382 and 12030258). All efforts were made to minimize suffering, and any animal that showed evidence of pain or distress, tachypnea, hypotension, or sepsis was immediately euthanized.
All human tissue was obtained via a waiver of consent from the Institutional Review Board of the University of Pittsburgh (Protocol Number 0606072) and was collected after complete review by the attending pediatric pathologist in a deidentified manner without recording of patient demographic or clinical information.
Cells, materials, and reagents.
The small intestinal cell line intestinal epithelial cell (IEC)-6 and RAW 264.7 macrophages were obtained from American Type Culture Collection (ATCC, Manassas, VA) and were maintained in culture as described (47). To generate IEC-6 cells that were stably deficient in TLR9, IEC-6 cells were transduced with lentiviral particles containing TLR9 shRNA (Open Biosystems, Huntsville, AL) using the four-plasmid lentiviral packaging system (Invitrogen, Carlsbad, CA) in permissive human endothelial kidney 293 cells. In parallel, IEC-6 cells were treated with lentiviruses expressing scrambled shRNA, and this stable cell line served as a control. Stable integration of lentivirus was obtained by selection of cells in medium containing puromycin (5 μg/ml), and the extent of knockdown of TLR9 was verified by RT-PCR.
LPS (Escherichia coli 0111:B4 purified by gel filtration chromatography, >99% pure) was obtained from Sigma-Aldrich (St. Louis, MO). Antibodies were obtained as follows: p65 subunit of NF-κB (Cell Signaling, Beverly, MA), F4/80 (Abcam, Cambridge, MA), and sucrase-isomaltase (A-17; Santa Cruz Biotechnology, Santa Cruz, CA).
Lactobacillus rhamnosus HN001 was maintained as a lyophilized seed stock at the Fonterra Research Centre (Palmerston North, New Zealand) and was supplied as a freeze-dried culture by Danisco USA (Madison, WI). Bacterial DNA was isolated from the probiotic Lactobacillus rhamnosus HN001 using a modified approach based on the “Bust n' Grab” technique described by Harju et al. (25) and extraction methods described in Ref. 61. In brief, lyophilized material was thoroughly washed with nuclease-free water, followed by nuclease-free buffer [10 mM Tris, 1 mM EDTA (pH 8.0)] (TE). Cells were then lysed with 10 mg/ml lysozyme and incubated at 37°C for 1 h, followed by three rapid freeze-thaw cycles (from −60°C up to 90°C) in a TE lysis buffer (2% Triton X-100, 1% SDS, 100 mM NaCl, 10 mM Tris·HCl, 1 mM EDTA). DNA was extracted with equal volumes of phenol:chloroform:isoamyl alcohol (25:24:1), followed by extraction with an equal volume of chloroform:isoamyl alcohol (24:1), and precipitation in two volumes of cold 100% ethanol and 0.3 volumes of 3 M sodium acetate. After precipitation, DNA was washed with chilled ethanol, air-dried at ambient temperature, resuspended in 1× TE buffer (10 mM Tris·HCl; 1 mM EDTA, pH 7.8–8.2), and treated with RNAse A (100 μg/ml) and Proteinase K (200 μg/ml). The DNA was then reextracted and precipitated as described above, followed by resuspension in 1× TE buffer. The concentration and purity of DNA preparations were confirmed by measuring the OD260 absorbance and OD260/280 ratio as well as by agarose gel electrophoresis. Endotoxin-free phosphorothioated CpG-DNA, oligodeoxynucleotide (ODN) 1826 (TCCATGACGTTCCTGACGTT) was chemically synthesized at the Integrated DNA Technologies (Coralville, IA) and utilized according to the methodology as described (23).
Cells were treated with Lr-DNA (75 μg/ml media) 1 h before LPS administration (LPS dose 10–25 μg/ml in IEC-6 cells, 10 ng/ml in RAW 264.7 macrophages), and the extent of LPS signaling was determined by the translocation of the p65 subunit of NF-κB from the cytoplasm to the nucleus 1 h after LPS administration (21) and the induction of the TLR4-mediated proinflammatory cytokines by qRT-PCR 6 h after administration of LPS. Immunohistochemistry, immunofluorescence, and qRT-PCR were performed, as in Ref. 1, and evaluated on a Zeiss LSM 710 confocal microscope (Carl Zeiss, Jena, Germany).
Assessment of NF-κB activation in enterocytes.
In serial micrographs of cells that were costained for the p65 subunit of NF-κB and the nuclear stain DAPI, a threshold limit was set based on the emission signal for DAPI, and a corresponding cytoplasmic region was defined by stenciling a circular region 12 pixels beyond the nucleus of each cell. The average integrated pixel intensity pertaining to the corresponding NF-κB staining within the cytoplasmic and nuclear regions was determined for more than 200 cells per treatment group in at least four experiments per group, using MetaMorph software version 6.1 (Molecular Devices, Sunnyvale, CA) (21, 45).
Induction of NEC in neonatal mice.
All animal experiments were approved by the University of Pittsburgh Animal Care and Use Committee. Mice were obtained from Jackson Laboratory. Experimental NEC was induced in 7–10-day-old mice as we have previously described (21, 55) using formula [Similac Advance infant formula (Abbott Nutrition, Columbus, OH): Esbilac (PetAg) canine milk replacer, 2:1], which was supplemented with enteric bacteria made from a stock created from a specimen obtained from an infant with surgical NEC (12.5 μl original stool slurry in 1 ml formula) via gavage five times per day. Additionally, the mice received hypoxia (5% O2-95% N2) for 10 min in a hypoxic chamber (Billups-Rothenberg, Del Mar, CA) twice daily for 4 days. Where indicated, mice were pretreated by oral gavage once daily for 4 days before the induction of NEC with Lr-DNA (1 mg/kg per day), live or UV-irradiated probiotic (57) (3 × 1011 colony-forming units/kg per day, dose equivalent to 1 mg Lr-DNA/kg per day), or oral CpG-DNA (1 mg/kg per day). Control (i.e., non-NEC) animals remained with their mothers and received breast milk.
Bacterial 16s profiling was performed on a stool sample obtained from a human infant with NEC, which was used to induce NEC in mice and piglets. Genomic DNA was isolated from 1 ml of stool slurry using PowerSoil DNA Isolation Kit (MoBiol Laboratories). The V4 region of the 16S rRNA gene was then amplified with forward primer 515F containing the Illumina Flowcell adapter sequences and reverse primer 806R containing the Illumina Flowcell adapter sequences followed by barcode identifiers (11). Amplifications were carried out in triplicate 25-μl reactions using 0.2 μM forward and reverse primers, 25 ng template genomic DNA, and 1× DreamTaq PCR Master Mix (Thermo Fisher Scientific, Rockford, IL). Replicate reactions were pooled and then purified using QIAquick PCR purification kit (Qiagen, Valencia, CA), and purified amplicons were further gel purified and recovered using QIAquick gel extraction kit (Qiagen). Samples were then sequenced on a Illumina MiSeq sequencing platform as previously described (11). Sequence data was analyzed within the MacQIIME (http://www.wernerlab.org/software/macqiime) implementation of QIIME 1.7.0 (10). Sequences were parsed based on sample-specific barcodes and trimmed to a minimum quality score of 20. Operational taxonomic units at 97% were then picked against the Greengenes 13.5 (15) database using UCLUST (17) for taxonomic assignment. Beta diversity analyses were conducted by UNIFRAC (40). This analysis revealed that the human stool largely consisted of Proteobacteria and Enterobacteriacae, typical of human stool (20), with no enrichment for any predominant pathogenic organism.
In experiments designed to assess whether there was any proinflammatory toxicity associated with the administration of Lr-DNA, wild-type mice were orally gavaged with varying doses of Lr-DNA (1–20 mg/kg) once daily for 5 days, and the degree of proinflammatory cytokine induction was assessed by qRT-PCR.
Induction of experimental NEC in premature piglets.
To determine the effects of probiotic DNA in an experimental model of NEC that more closely resembled the human situation, we turned to a piglet model of this disease. We note that the piglet is the approximate size of a human premature infant (1,000–1,200 g), and the intestine of the piglet shares physiological and structural properties with the premature human, making this a useful model for disease interrogation (54). Moreover, previous authors have shown that the piglet intestine expresses both TLR4 and TLR9 (5, 36). Timed pregnant Yorkshire sows were obtained from Animal Biotech Industries (Danboro, PA), and piglets were delivered prematurely via cesarean section at 105–108 days gestation (term, 115 days) without induction of parturition (7, 9, 13, 29, 53). Sows were sedated with a combination of Xylazine (500 mg)/Telazol (500 mg) intramuscularly, and anesthesia was induced with isoflurane gas (3% in 4 liters O2). Once sedated, the sow was intubated, and anesthesia was maintained with isoflurane (2–4%). A lower abdominal midline laparotomy incision was made, and each piglet was removed through separate hysterotomy incisions. Immediately following delivery, the isoflurane was increased to 5%, and the sow was euthanized with pentobarbital-phenytoin sodium (Beuthanasia, 1 ml/4.5 kg intravenously; Schering-Plough, Kenilworth, NJ). Piglets received standard neonatal resuscitation, and each was fitted with an orogastric feeding tube (7F catheter; Vygon, Montgomeryville, PA). The piglets were then housed in a heated humidified incubator (ICS-1; Plas-Laboratories, Lansing, MI) and monitored continuously.
Experimental NEC was induced in piglets by gavage formula feeds at 15 ml/kg every 3 h (120 ml/kg per day) for 4 days (n = 7). The NEC formula consisted of the following per liter of final formula: Pepdite Junior (93.9 g; Nutricia, Allerod, Denmark), MCT Oil (38.3 g, USP grade; Now Foods, Bloomingdale, IL), Whey Protein Isolate (56 g, Now Foods), and 837 g water, which was supplemented with enteric bacteria made from the same stock created from a specimen obtained from an infant with surgical NEC as above (12.5 μl original stool slurry per 1 ml formula, which was enriched in Enterobacteriaceae as determined above). Piglets that were not induced to develop NEC were euthanized at birth (n = 4). Where indicated, piglets were orally gavaged once daily with Lr-DNA (1 mg/kg per day) (n = 3) or live (n = 4) or UV-irradiated probiotic (n = 3) (57) (3 × 1011 colony-forming units/kg per day, dose equivalent to 1 mg Lr-DNA/kg per day). At the conclusion of the study (day 5), animals were euthanized after being placed under anesthesia with isoflurane with pentobarbital-phenytoin sodium (Beuthanasia, 0.5 ml intravenously, Schering-Plough), and intestinal samples were collected from the small intestine for histology according to the techniques of Sangild (13, 29, 53), Burrin (7), and Buddington (9) and mucosal expression of the proinflammatory cytokines (63).
NEC severity assessment.
Histological sections of the terminal ilea of mice and piglets were assessed for the degree of mucosal injury by a pediatric pathologist who was blinded to the study condition according to our previously published scoring system from 0 (normal) to 3 (severe injury) (21) and by expression of the proinflammatory cytokines by qRT-PCR.
Preparation of human intestinal tissue from infants with healed NEC.
All human intestinal samples were obtained and processed as discarded tissue via waiver of consent with approval from the University of Pittsburgh Institutional Review Board and in accordance with the University of Pittsburgh anatomical tissue procurement guidelines. Intestinal samples were obtained from human neonates who previously had intestinal resection for NEC at the time of stoma closure (“Human Healed NEC”, n = 3). After initial review by a pediatric pathologist to ensure that adequate diagnostic information was obtained from the gross tissue specimen, the intestinal resection was divided into several pieces, placed in cell culture media (43), and where indicated pretreated with Lr-DNA (75 μg/ml) or CpG (5 μM), in the presence or absence of LPS (50 μg/ml). After 3 h, tissue was processed for qRT-PCR and assessed for the expression of proinflammatory cytokines (43).
qRT-PCR.
qRT-PCR was performed as previously described using the Bio-Rad CFX96 Real-Time System (Bio-Rad, Hercules, CA) (34) using the primers listed in Table 1 relative to the housekeeping gene RPLO.
Table 1.
Primers utilized for qRT-PCR analysis of the indicated gene
| Gene | Species | Forward Sequence | Reverse Sequence | Amplicon Size, bp |
|---|---|---|---|---|
| iNOS | Mouse/Rat | CTGCTGGTGGTGACAAGCACATTT | ATGTCATGAGCAAAGGCGCAGAAC | 167 |
| Human | AATGAGTCCCCGCAGCCCCT | AGTCATCCCGCTGCCCCAGT | 143 | |
| Pig | TCCAGAAGCAGAACGTGACC | GGGGGTGATGCTTCCTGAAA | 146 | |
| RPLO | Mouse/Rat/Human | GGCGACCTGGAAGTCCAACT | CCATCAGCACCACAGCCTTC | 143 |
| IL-6 | Mouse/Rat | GGCTAAGGACCAAGACCATCCAA | TCTGACCACAGTGAGGAATGTCCA | 138 |
| Human | TCTCCACAAGCGCCTTCG | CTCAGGGCTGAGATGCCG | 193 | |
| IL-1β | Mouse | AGTGTGGATCCCAAGCAATACCCA | TGTCCTGACCACTGTTGTTTCCCA | 175 |
| TNF-α | Mouse/Rat | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC | 175 |
| PCNA | Mouse/Rat | AAAGATGCCGTCGGGTGAATTTGC | AATGTTCCCATTGCCAAGCTCTCC | 130 |
| TLR9−k/d | Mouse/Rat | AAGCTCGACCTGTCCTTCAA | TTGAGCAAGCGGAAGAAGAT | 128 |
iNOS, inducible nitric oxide synthase; TLR, Toll-like receptor.
Statistical analysis.
All experiments were repeated at least in triplicate, with more than 100 cells per high-power field and 200 cells per treatment group. For mouse experiments of NEC, over 10 mouse pups per group were included, and litter-matched controls were included in all cases. For piglet NEC experiments, at least three animals per group were assessed. Animals were each assigned to groups randomly and were limited only by the size of the litter without any bias. Statistical analysis was performed using SPSS 13.0 software. ANOVA, χ2, or two-tailed Student's t-test were used for comparisons as appropriate. In all cases, statistical significance was accepted at P < 0.05 between groups.
RESULTS
Lactobacillus rhamnosus HN001 attenuates the severity of experimental NEC in mice.
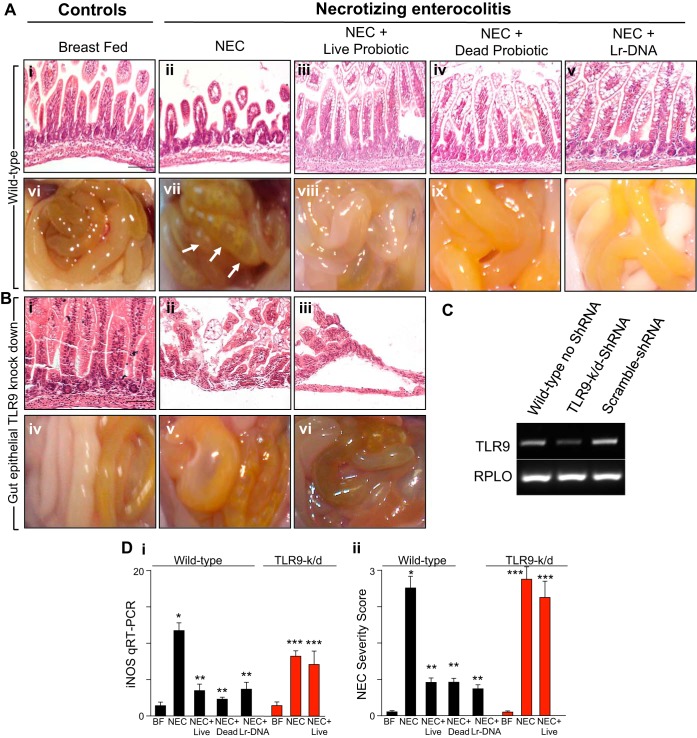
We first sought to determine whether Lactobacillus rhamnosus HN001 in its live probiotic form could attenuate the severity of experimental NEC in mice and, if so, sought to determine the possible mechanisms involved. NEC was induced using a well-validated and highly reproducible model in mouse pups, as we and others have published previously (1, 21, 45), using a combination of formula gavage and intermittent hypoxia. As shown in Fig. 1, newborn mice that were exposed to these conditions exhibited a marked disruption of the intestinal mucosal architecture as measured by histology (Fig. 1A, i and ii) and blind scoring of the histological appearance of the terminal ileum (Fig. 1D, ii). The severity of NEC was confirmed by gross examination of the intestine, which revealed the presence of gas within the intestinal wall, a characteristic feature of NEC that is termed pneumatosis intestinalis (Fig. 1A, vii, see arrows), and by a molecular analysis of the intestinal mucosa, which revealed an increase in the expression of the proinflammatory molecule inducible nitric oxide synthase (iNOS) (Fig. 1D, i) compared with control mice that remained with their mother and were breast fed on demand (Fig. 1A, i vs. ii, vi vs. vii; Fig. 1D, i and ii). Importantly, the daily administration of mice with formula that had been supplemented with the live probiotic Lactobacillus rhamnosus HN001 significantly attenuated the severity of experimental NEC as manifested by preservation of the mucosal architecture (Fig. 1A, iii vs. ii), absence of pneumatosis intestinalis (Fig. 1A, viii vs. vii), decreased expression of intestinal iNOS (Fig. 1D, i), and decreased NEC severity score (Fig. 1D, ii) compared with the wild-type NEC group. Furthermore, with a dose range from 1 mg/kg to 20 mg/kg, Lr-DNA was found to be well tolerated, and the 1 mg/kg dose of Lr-DNA used therapeutically did not induce any proinflammatory cytokines. The expression of the proinflammatory molecule iNOS (Fig. 2A, i) was induced at 10 mg/kg, whereas IL-1β was only elevated at the highest dose tested (Fig. 2A, ii). TNF-α was not upregulated within the intestinal mucosa at doses up to 20 mg/kg (Fig. 2A, iii), nor did any doses tested alter the extent of enterocyte proliferation as determined by evaluation of expression within the intestinal mucosa of proliferating cell nuclear antigen (Fig. 2A, iv). These studies were performed to establish a safety profile for the Lr-DNA, at doses well beyond that which we had determined to be effective against NEC. Taken together, these findings identify a protective effect of Lactobacillus rhamnosus HN001 on the development of NEC in mice. We next sought to evaluate the potential mechanisms involved.
Fig. 1.
Lactobacillus rhamnosus HN001 attenuates the severity of experimental necrotizing enterocolitis (NEC) in mice and requires Toll-like receptor 9 (TLR9) in the intestinal epithelium. A: representative photomicrographs of the terminal ileum (i–v) and gross images of the intestine (vi–x) from neonatal wild-type mice that were either breast fed (BF) (i, vi) or induced to develop NEC in the absence (ii, vii) or presence of either live Lactobacillus rhamnosus (“probiotic”, iii, viii), UV-irradiated probiotic (iv, ix), or DNA that was purified from Lactobacillus rhamnosus HN001 (Lr-DNA) as described in materials and methods (v, x). Arrows indicate areas of pneumatosis. B: representative photomicrographs of the terminal ileum (i–iii) and gross images of the intestine (iv–vi) of neonatal mice in which TLR9 was selectively deleted from the intestinal epithelium as achieved by lentiviral administration of shRNA via oral gavage as described in materials and methods. Mice were either BF (i), induced to develop NEC (ii), or induced to develop NEC in the presence of live Lactobacillus rhamnosus (iii). C: RT-PCR showing TLR9 expression in mucosal scrapings of neonatal mice, which were infected 72 h before the initiation of the experimental NEC model with lentivirus expressing TLR9 or scrambled (control) shRNA. D: NEC severity as assessed by inducible nitric oxide synthase (iNOS) expression by qRT-PCR in the terminal ileum (i); NEC severity score as determined by a pathologist blinded to the groups (ii). In all graphs, *P < 0.05 wild-type NEC vs. BF control; **P < 0.05 wild-type NEC vs. NEC in mice treated with the live, UV-irradiated (dead) probiotic Lactobacillus rhamnosus or Lr-DNA; ***P < 0.05 NEC or NEC + live probiotic Lactobacillus rhamnosus HN001 in TLR9k/d vs. TLR9k/d control. Data are means ± SE. Size bar = 50 μm.
Fig. 2.
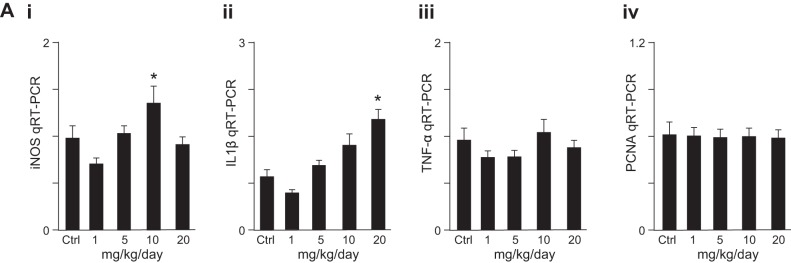
Dose-dependent effects of Lr-DNA on mucosal cytokine expression and enterocyte proliferation in vivo. Expression of iNOS (i), IL-1β (ii), TNF-α (iii), and PCNA (iv) by qRT-PCR in the ilea of neonatal mice treated with Lr-DNA at escalating doses from 0 to 20 mg/kg as indicated once daily for 5 days. Representative of 4 mice per group. In all graphs, *P < 0.05 vs. control.
The reduction in NEC severity by Lactobacillus rhamnosus requires TLR9.
In prior studies of intestinal inflammation, protection against mucosal inflammation may be mediated in part by the innate immune receptor TLR9, which is a receptor in mammalian cells for microbial DNA (12, 48, 50). Moreover, we have previously reported that TLR9 activation with bacterial DNA can attenuate the severity of NEC, in part through a decrease in the extent of proinflammatory signaling that occurs within the intestinal epithelium via the bacterial receptor TLR4 (23). We therefore next sought to test the hypothesis that TLR9 plays a role in the protective effects of Lactobacillus rhamnosus HN001 in experimental NEC in mice. We first examined whether killed Lactobacillus rhamnosus HN001 can protect against NEC, which would indicate that a molecular constituent of the bacterium and not the live bacteria itself may be sufficient. To do so, we administered to mice Lactobacillus rhamnosus HN001 that had been killed by ultraviolet irradiation according to the techniques of van Hoffen et al (57) and induced NEC via formula gavage and hypoxia. As shown in Fig. 1, we still observed a marked protection in NEC severity compared with mice with NEC that had not received Lactobacillus rhamnosus HN001, as measured by histological appearance of the mucosa (Fig. 1A, iv vs. ii), gross appearance of the intestine (Fig. 1A, ix vs. vii), NEC severity score (Fig. 1D, ii), and mucosal expression of iNOS (Fig. 1D, i). These findings suggested that the protective effects of Lactobacillus rhamnosus HN001 could be achieved through a molecular constituent that was independent of cell viability. Given that microbial DNA could serve such a role, we next administered microbial DNA that had been purified from Lactobacillus rhamnosus HN001 (Lr-DNA) as described in materials and methods to mice with experimental NEC. As shown in Fig. 1, we determined that administration of DNA from Lactobacillus rhamnosus HN001 resulted in a profound reduction in NEC severity (Fig. 1D, ii), consistent with the possibility that the protective effects of Lactobacillus rhamnosus HN001 are mediated through its constituent DNA.
To evaluate this possibility directly, we next selectively deleted the receptor for microbial DNA, namely TLR9, from the intestinal epithelium, which we achieved through the oral gavage of lentiviruses that had been packaged with TLR9 shRNA. As shown in Fig. 1, the expression of TLR9 was significantly reduced within the intestinal mucosa 72 h after oral viral administration (Fig. 1C), whereas the administration of lentiviruses expressing scrambled shRNA had no effect (Fig. 1C). Importantly, the oral administration of Lactobacillus rhamnosus HN001 to mice lacking TLR9 within the intestinal epithelium did not protect from the development of NEC (Fig. 1B and Fig. 1D, i and ii), which occurred with equal severity to that in wild-type mice (Fig. 1A, Fig. 1D, i and ii). Taken together, these findings demonstrate that formula supplementation of the probiotic Lactobacillus rhamnosus HN001 protects against the development of NEC in mice and that this occurs at least in part via TLR9.
The oral administration of ODN-1826 CpG-DNA significantly attenuates NEC severity in mice.
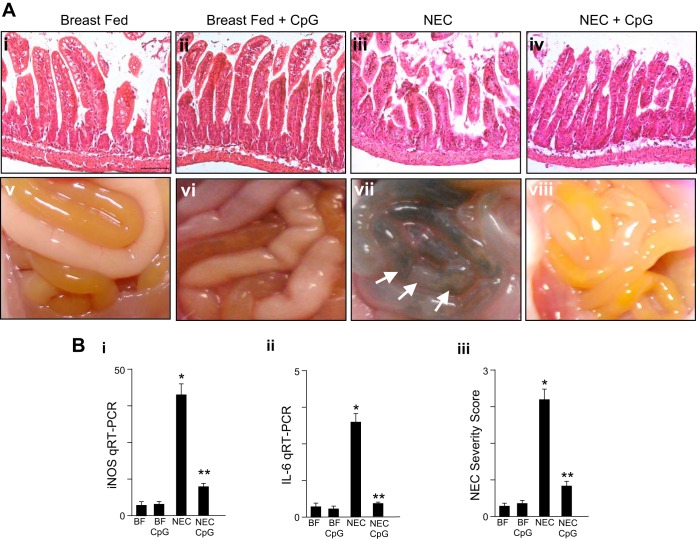
The above findings raise the possibility that the oral activation of TLR9 using a specific agonist added to enteric feeds could exert similar effects as Lactobacillus rhamnosus HN001 and attenuate NEC severity. In support of this possibility, we have previously shown that the activation of TLR9 via intraperitoneal injection of CpG-DNA attenuated the severity of experimental NEC in mice (23) although the oral administration of microbial DNA has not been evaluated in experimental NEC. As shown in Fig. 3, newborn mice were subjected to experimental NEC in the presence or absence of CpG-DNA that was administered once daily via oral gavage for 4 days before the start of the model and continued through the model. As shown in Fig. 3, the oral administration of CpG-DNA significantly attenuated the severity of NEC, as reflected in reduced histological evidence of disruption in the mucosal architecture (Fig. 3A, iv vs. iii), reduced proinflammatory cytokine induction (Fig. 3B, i and ii), improved gross appearance of the intestine (Fig. 3A, viii vs. vii), and lower NEC severity score (Fig. 3B, iii). These findings, together with the results shown in Fig. 1, identify that the activation of TLR9 via the oral route, either with Lactobacillus rhamnosus HN001 or via CpG-DNA, can attenuate the severity of NEC in mice.
Fig. 3.
The oral administration of oral CpG-DNA ODN-1826 attenuates NEC severity in mice. A: representative photomicrographs of the terminal ileum (i–iv) and gross images of the intestines (v–viii) from neonatal wild-type mice that were either BF controls or induced to develop NEC in the presence or absence of oral CpG-DNA (ODN-1826 1 mg/kg) once daily. B: NEC severity as assessed by the terminal ileal expression of iNOS by qRT-PCR (i), IL-6 mRNA expression by qRT-PCR (ii), or NEC severity score as determined by a pathologist blinded to the identity of the groups (iii). In all graphs, *P < 0.05 NEC vs. BF control; **P < 0.05 NEC vs. NEC in mice treated with oral CpG-DNA. Data are means ± SE. Size bar = 50 μm.
Oral gavage with lactobacillus rhamnosus HN001 attenuates the severity of experimental NEC in piglets.
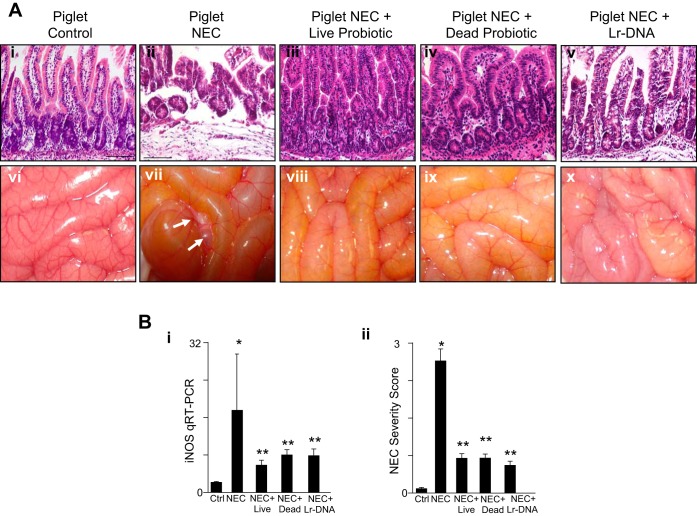
To examine in greater detail the efficacy of Lactobacillus rhamnosus HN001 in protecting against the development of clinical NEC, we next evaluated its use in a large animal model of this disease. Previous authors have shown that NEC may be induced in premature piglets through the oral administration of formulas rich in maltodextrins (29, 52). NEC was therefore induced in premature piglets as described in materials and methods, in the presence of NEC formula or formula containing live Lactobacillus rhamnosus HN001. As shown in Fig. 4, this protocol induced NEC, as demonstrated by a NEC severity score that was assigned by a pathologist blinded to the groups of greater than two, in 57% of piglets, as manifest by a significant disruption of the intestinal architecture (Fig. 4A, ii vs. i), the development of pneumatosis intestinalis (Fig. 4A, vii vs. vi, arrows), and an increase in the proinflammatory molecule iNOS (Fig. 4B, i). Importantly, when the formula was supplemented with live Lactobacillus rhamnosus HN001, the severity of NEC was significantly reduced, such that 0% of piglets developed NEC, as manifest by preservation of the mucosal architecture (Fig. 4A, iii vs. ii), an improvement in the gross appearance of the bowel (Fig. 4A, viii vs. vii), a decrease in the induction of iNOS (Fig. 4B, i), and reduction in NEC severity score (Fig. 4B, ii). In support of a role for DNA from Lactobacillus rhamnosus HN001 in mediating its protective effects against NEC, two additional experiments were performed. First, piglets were administered UV-irradiated Lactobacillus rhamnosus HN001, which also conferred significant protection from NEC, as manifest by reduced mucosal injury (Fig. 4A, iv vs. ii), cytokine expression (Fig. 4B, i), and NEC severity score (Fig. 4B, ii). Second, piglets were administered Lr-DNA, which also was found to protect against experimental NEC (Fig. 4A, v and x, and Fig. 4B, i and ii). Taken together, these findings indicate that live and/or inactivated Lactobacillus rhamnosus HN001 or its purified Lr-DNA can prevent experimental NEC in piglets.
Fig. 4.
Lactobacillus rhamnosus attenuates the severity of experimental NEC in premature piglets. A: representative photomicrographs of the terminal ileum (i–v) and gross images of the intestines (vi–x) of premature piglets that were either untreated and euthanized at birth (controls) or induced to develop experimental NEC in the absence or presence of live or UV-irradiated (dead) probiotic Lactobacillus rhamnosus HN001 or Lr-DNA. Arrows show areas of pneumatosis intestinalis. B: NEC severity in the premature piglets as assessed by iNOS expression by qRT-PCR in the terminal ileum (i) or NEC severity score as determined by a pathologist blinded to the groups (ii). In all graphs, *P < 0.05 NEC vs. control; **P < 0.05 NEC vs. NEC + treatments with the live, UV-irradiated (dead) or the Lr-DNA. Data are means ± SE. Size bar = 50 μm. Combined data from 3 separate experiments in which piglets had been randomly assigned to each group: Ctrl, n = 4; NEC, n = 7; NEC + live probiotic, n = 4; NEC + dead probiotic, n = 3; NEC + DNA, n = 3.
Lr-DNA inhibits TLR4 signaling in intestinal tissue from human infants ex vivo.
Having shown the efficacy of Lactobacillus rhamnosus HN001 in mice and piglets, we next sought to evaluate whether Lr-DNA could inhibit TLR4 signaling in human intestinal tissue that had been removed from patients that were previously treated for NEC. To do so, we obtained freshly resected intestine (ileum) from infants who had undergone surgical resection for NEC at the time of their stoma closure. The resected tissue was sectioned into multiple pieces as in our prior study (43) and treated with LPS in the presence or absence of either Lr-DNA or CpG-DNA, as well as Lr-DNA or CpG-DNA alone. As shown in Fig. 5, LPS significantly increased the expression of the proinflammatory cytokine IL-6, whereas pretreatment with Lr-DNA before LPS administration significantly attenuated the expression of IL-6 in the human tissue. As a control, CpG-DNA pretreatment before LPS administration also inhibited the expression of IL-6. Taken together, these findings indicate that Lr-DNA or the TLR9 ligand CpG-DNA decreases the extent of TLR4-mediated proinflammatory signaling in resected human tissue. These findings raise the exciting possibility that Lr-DNA could represent a novel therapeutic strategy in diseases associated with increased TLR4 signaling, such as NEC.
Fig. 5.
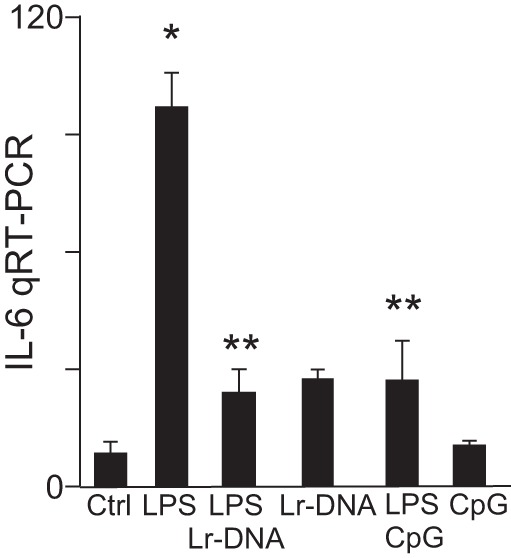
Lr-DNA inhibits TLR4 signaling ex vivo in ileal sections resected from human infants. qPCR showing IL-6 mRNA expression in sections of ileum that had been resected from human infants (n = 3), which was either untreated or treated with LPS (50 μg/ml) for 3 h after 1-h pretreatment with Lr-DNA (75 μg/ml) or CpG-DNA (5 μM) as indicated. *P < 0.05 LPS vs. control. **P < 0.05 LPS + treatments with Lr-DNA or CpG-DNA vs. LPS. Data are means ± SE.
Lr-DNA inhibits TLR4 signaling in cultured enterocytes via TLR9.
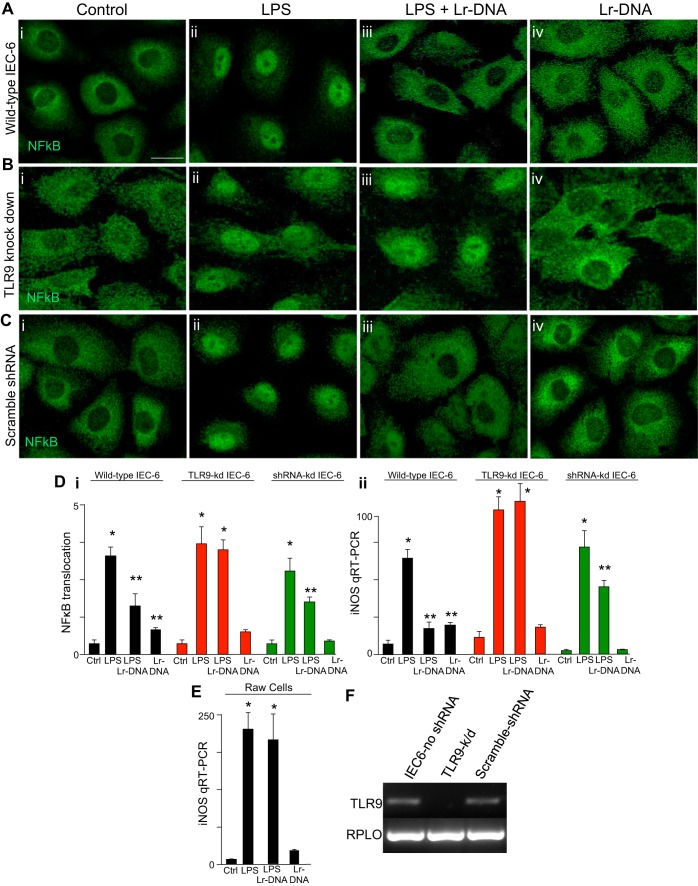
In the final series of studies, we sought to gain insights into the mechanisms by which Lactobacillus rhamnosus HN001 could act to attenuate NEC severity and focused on whether Lr-DNA could inhibit TLR4 signaling in cultured enterocytes. To do so, we studied the cell line IEC-6, which we and others have previously shown to express TLR4 (34, 44, 51). As shown in Fig. 6, treatment of IEC-6 cells with LPS for 1 h significantly increased TLR4 signaling, as measured by an increase in the extent of the translocation of the p65 subunit of NF-κB from the cytoplasm to the nucleus (Fig. 6A, i and ii, and Fig. 6D, i) and the induction of the proinflammatory molecule iNOS (Fig. 6D, ii). Importantly, LPS-induced translocation of NF-κB and induction of iNOS expression were both reduced when IEC-6 cells were first pretreated with Lr-DNA (Fig. 6A, iii, and Fig.6D, i and ii), whereas the treatment of IEC-6 cells with Lr-DNA alone had no effect on either NF-κB translocation or iNOS expression (Fig. 6A, iv, and Fig. 6D, i and ii). Importantly, Lr-DNA did not prevent LPS signaling in RAW macrophages, consistent with the known proinflammatory role of bacterial DNA in myeloid cells (Fig. 6E) (31, 59). To determine whether TLR9 in IEC-6 cells was required for the protective effects of Lr-DNA on TLR4 signaling, we selectively knocked down TLR9 from IEC-6 cells using lentiviral transduction of TLR9 shRNA to generate IECTLR9−k/d cells in which TLR9 expression was significantly reduced without affecting the expression of the housekeeping gene RPLO (Fig. 6F). Importantly, treatment with Lr-DNA did not inhibit TLR4-mediated NF-κB translocation from the cytoplasm to the nucleus nor alter the LPS-induction of iNOS expression (Fig. 6B, iii vs. ii, and Fig. 6D, i and ii). We acknowledge that TLR4 levels were not assessed directly although TLR4 expression levels do not correlate with the extent of signaling that occurs in this pathway. Taken together, these findings reveal that Lr-DNA inhibits TLR4 signaling in IEC-6 enterocytes via TLR9 and provide insights into its protective role in diseases of mucosal inflammation that are mediated by TLR4 activation, including NEC.
Fig. 6.
Lr-DNA inhibits LPS-mediated NF-κB translocation in cultured enterocytes via TLR9. A–C: representative confocal micrographs of wild-type IEC-6 cells (A, i–iv), IEC-6 cells in which TLR9 had been selectively knocked down after transduction with TLR9 shRNA (B, i–iv), or control IEC-6 cells that had been transduced with scrambled shRNA (C, i–iv) and stained for NF-κB p65 subunit. As indicated, cells were treated with LPS (10 μg/ml, 1 h) or were pretreated with DNA that had been purified from with Lactobacillus rhamnosus HN001 along with LPS or with Lr-DNA alone. Size bar = 10 μm. D: extent of NF-κB translocation (i) and iNOS expression by qRT-PCR in the cell types and conditions indicated (LPS 25 μg/ml, 6 h; Lr-DNA 75 μg/ml 1-h pretreatment) (ii). E: iNOS expression by qRT-PCR in RAW 264.7 macrophages treated with LPS (10 ng, 6 h) or 1-h pretreatment of Lr-DNA. F: RT-PCR showing expression of TLR9 or RPLO (loading control) in wild-type IEC-6 cells or IEC-6 cells that were infected 48 h prior with lentivirus-expressing target or scrambled (control) shRNA. In all graphs, *P < 0.05 LPS or LPS + Lr-DNA vs. control. **P < 0.05 LPS + Lr-DNA vs. LPS. Data are means ± SE.
DISCUSSION
In the current study, we describe the process in which supplementation of infant formula with live or ultraviolet light-inactivated Lactobacillus rhamnosus HN001 can decrease the severity of experimental NEC in mice and piglets. We further determined that the protective effects of Lactobacillus rhamnosus HN001 were not seen in mice in which the bacterial DNA receptor TLR9 had been knocked down, suggesting a pivotal role for microbial DNA signaling in the protective response of Lactobacillus rhamnosus HN001. In support of this possibility, the administration of DNA from Lactobacillus rhamnosus HN001 exerted a similar effect, in both mice and piglets. Strikingly, and perhaps most importantly, the potential protective effects of DNA from Lactobacillus rhamnosus HN001 on inflammatory signaling in the pediatric intestine were suggested by the observation that treatment of intestinal specimens obtained from infants who underwent resection and subsequent stoma closure in the treatment of NEC reduced the extent of LPS-induced proinflammatory mediator induction. Taken together, these findings raise the exciting possibility that Lactobacillus rhamnosus HN001, or its DNA, could serve as a novel agent for the prevention of NEC in humans, acting in part through the microbial DNA receptor TLR9.
The current findings expand the growing literature on the role of probiotics in the prevention or treatment of NEC. In particular, because the first randomized controlled trials showed that probiotic administration could reduce the expected incidence of NEC (14, 38), there has been growing interest in the potential use of probiotics as protective agents for this disease. Several recent meta-analyses of over 20 randomized controlled trials have indicated that probiotics can reduce the incidence of NEC (3, 6, 8, 14, 35, 38, 42, 58) and raise the possibility that routine use of probiotics may be beneficial. However, upon closer inspection, it is clear that several areas of concern exist regarding the generalizability of trial results to the population most at risk for NEC development. First, the precise probiotic agent(s) to use remains uncertain, a problem that is compounded by the fact that many trials use different probiotics in varying combinations, making the precise determination of what the effective agent actually is nearly impossible (4, 6, 16). Second, there remain significant concerns regarding the potential development of sepsis as a result of the administration of live bacteria to premature infants, and at least one widely reported case has added greatly to this concern (28, 32, 33). Third, and perhaps most importantly, is the fact that the precise mechanism of action of protective effect of probiotics remains largely unknown, increasing the anxiety among clinicians regarding either potential off-target effects and potential for toxicity. Although the present study was not designed to address the first four issues, it is hoped that, by identifying a critical role for TLR9 signaling in the protective effects of probiotics in NEC, a more informative approach to the mechanistic actions of probiotics in NEC may be undertaken.
The present findings highlight the potential role of modulation of the innate immune system by probiotics as an important mechanism to explain their protective actions. In this regard, we have previously shown that parenteral administration of microbial DNA can activate the innate immune receptor TLR9 in enterocytes (23), which leads to a reduction in the extent of signaling via the lipopolysaccharide receptor TLR4, a receptor that we and others have determined to play a critical role in NEC development (23, 30). The current study extends those prior findings and provides important mechanistic information into the protective role of Lactobacillus rhamnosus HN001 by revealing that TLR9 signaling is required, and further, by demonstrating that Lr-DNA can limit the extent of TLR4 signaling in intestinal epithelial cells. It is important to note that the administration of Lr-DNA alone did not increase the extent of proinflammatory signaling when administered alone to mice or piglets or in cells (Figs. 1, 3, 4, and 6), providing additional reassurance of the potential safety of this agent. Although these findings suggest that TLR9 activation is critical for the protective effects of Lactobacillus rhamnosus HN001, they do not exclude the possibility that other potential biological mechanisms may contribute to the beneficial effects of probiotics. In this regard, previous authors have suggested that probiotics can favorably alter the intestinal microflora, improve intestinal epithelial integrity, reduce apoptosis, and modulate proinflammatory gene expression within the intestinal epithelium (2, 12, 62). Although no prior study has explored the role of Lactobacillus rhamnosus HN001 or its DNA in models of experimental NEC, it is possible that each of these pathways may be involved to varying degrees. Additional studies with this bacterium and/or its DNA along these mechanistic lines may serve to provide a deeper understanding regarding how probiotic therapies may work in general, with the goal of understanding in greater detail the degree to which agents such as Lactobacillus rhamnosus HN001 or its DNA may be brought to clinical use. We fully acknowledge that, although there exist advantages of using bacterial DNA, such as the avoidance of potential infection that may follow the use of whole live bacteria, there are also several limitations, as bacterial DNA can deleteriously activate the immune system of the host. The fact that the gastrointestinal administration of bacterial DNA did not result in any significant proinflammatory activation at levels in which NEC was protected (i.e., 1 mg/kg) is reassuring (Fig. 2), but further studies need to be performed to establish a full safety and toxicity profile.
In summary, the current findings identify that Lactobacillus rhamnosus HN001 or its DNA can protect against the development of NEC in small and large animal models, in a process that requires activation of the DNA receptor TLR9, with no evidence of toxicity. Importantly, Lr-DNA reduced the extent of TLR4 signaling in human tissue that was treated ex vivo, raising the exciting possibility that this bacterium may serve a therapeutic role in patients at risk for NEC development. Only through further, careful study of the safety and efficacy of promising agents such as Lactobacillus rhamnosus HN001 or its DNA can the field hope to offer preventive strategies for infants with this devastating disease.
GRANTS
This research was funded in part via 5R01GM078238 and 5R01DK083752 from the National Institutes of Health to D. Hackam.
DISCLOSURES
The research was funded in part by Abbott Nutrition. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.G., C.P.S., J.A.O., R.H.B., K.C.G., D.L.T., A.V., K.B., M.J.M., B.F., P.L., and D.J.H. conception and design of research; M.G., C.P.S., K.C.G., A.V., K.B., M.J.M., B.F., P.L., and D.J.H. performed experiments; M.G., C.P.S., J.A.O., R.H.B., K.C.G., A.V., K.B., M.J.M., B.F., P.L., and D.J.H. analyzed data; M.G., C.P.S., J.A.O., R.H.B., K.C.G., A.V., K.B., M.J.M., B.F., and D.J.H. interpreted results of experiments; M.G., C.P.S, R.H.B., K.C.G., and D.J.H. prepared figures; M.G., R.H.B., K.C.G., D.L.T., K.B., and D.J.H. drafted manuscript; M.G., R.H.B., K.C.G., D.L.T., and D.J.H. edited and revised manuscript; M.G., C.P.S., J.A.O., R.H.B., K.C.G., D.L.T., A.V., K.B., M.J.M., B.F., P.L., and D.J.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the expertise of Maria Branca, Thomas Prindle, Congrong Ma, and Samantha Weyandt for technical assistance and expertise with the animal experiments and processing of the histology. The authors thank Steven Davis for editorial review. The authors acknowledge the advice and support of Dr. Per Sangild (University of Copenhagen), Dr. Randy Buddington (University of Memphis), and Dr. Douglas Burrin (Baylor College of Medicine) in the performance of the NEC model in piglets.
REFERENCES
- 1.Afrazi A, Sodhi CP, Good M, Jia H, Siggers R, Yazji I, Ma C, Neal MD, Prindle T, Grant ZS, Branca MF, Ozolek J, Chang EB, Hackam DJ. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J Immunol 188: 4543–4557, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal R, Sharma N, Chaudhry R, Deorari A, Paul VK, Gewolb IH, Panigrahi P. Effects of oral Lactobacillus GG on enteric microflora in low-birth-weight neonates. J Pediatr Gastroenterol Nutr 36: 397–402, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev CD005496: CD005496, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Alfaleh K, Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev CD005496: CD005496, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bering SB, Bai S, Zhang K, Sangild PT. Prematurity does not markedly affect intestinal sensitivity to endotoxins and feeding in pigs. Br J Nutr 108: 672–681, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 147: 192–196, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bjornvad CR, Thymann T, Deutz NE, Burrin DG, Jensen SK, Jensen BB, Molbak L, Boye M, Larsson LI, Schmidt M, Michaelsen KF, Sangild PT. Enteral feeding induces diet-dependent mucosal dysfunction, bacterial proliferation, and necrotizing enterocolitis in preterm pigs on parenteral nutrition. Am J Physiol Gastrointest Liver Physiol 295: G1092–G1103, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Braga TD, da Silva GA, de Lira PI, de Carvalho Lima M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 93: 81–86, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Buddington RK, Bering SB, Thymann T, Sangild PT. Aldohexose malabsorption in preterm pigs is directly related to the severity of necrotizing enterocolitis. Pediatr Res 63: 382–387, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6: 1621–1624, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo NA, Perdigon G, de Moreno de Leblanc A. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol 11: 177, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cilieborg MS, Boye M, Thymann T, Jensen BB, Sangild PT. Diet-dependent effects of minimal enteral nutrition on intestinal function and necrotizing enterocolitis in preterm pigs. JPEN J Parenter Enteral Nutr 35: 32–42, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 82: 103–108, 2002 [DOI] [PubMed] [Google Scholar]
- 15.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72: 5069–5072, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet 369: 1614–1620, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461, 2010 [DOI] [PubMed] [Google Scholar]
- 18.FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food Guidelines for the Evaluation of Probiotics in Food. London, Ontario, Canada: FAO/WHO, 2002 [Google Scholar]
- 19.Gill HS, Rutherfurd KJ, Prasad J, Gopal PK. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br J Nutr 83: 167–176, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science 312: 1355–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good M, Siggers RH, Sodhi CP, Afrazi A, Alkhudari F, Egan CE, Neal MD, Yazji I, Jia H, Lin J, Branca MF, Ma C, Prindle T, Grant Z, Shah S, Slagle D, Paredes J, 2nd, Ozolek J, Gittes GK, Hackam DJ. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci USA 109: 11330–11335, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, Higgins R, Raju TN. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res 62: 510–514, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, Jakub A, Shi XH, Shah S, Ozolek JA, Hackam DJ. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol 182: 636–646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol 23: 278–285, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Harju S, Fedosyuk H, Peterson KR. Rapid isolation of yeast genomic DNA: Bust n' Grab. BMC Biotechnol 4: 8, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry MC, Moss RL. Necrotizing enterocolitis. Annu Rev Med 60: 111–124, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Indian Council of Medical Research Task F, Coordinating Unit I, Coordinating Unit DBT. ICMR-DBT guidelines for evaluation of probiotics in food. Indian J Med Res 134: 22–25, 2011 [PMC free article] [PubMed] [Google Scholar]
- 28.Jenke A, Ruf EM, Hoppe T, Heldmann M, Wirth S. Bifidobacterium septicaemia in an extremely low-birthweight infant under probiotic therapy. Arch Dis Child 97: F217–F218, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Jensen ML, Sangild PT, Lykke M, Schmidt M, Boye M, Jensen BB, Thymann T. Similar efficacy of human banked milk and bovine colostrum to decrease incidence of necrotizing enterocolitis in preterm piglets. Am J Physiol Regul Integr Comp Physiol 305: R4–R12, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 177: 3273–3282, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20: 709–760, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Kunz AN, Noel JM, Fairchok MP. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J Pediatr Gastroenterol Nutr 38: 457–458, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 115: 178–181, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 179: 4808–4820, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Li D, Rosito G, Slagle T. Probiotics for the prevention of necrotizing enterocolitis in neonates: an 8-year retrospective cohort study. J Clin Pharm Ther 38: 445–449, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Li XQ, Zhu YH, Zhang HF, Yue Y, Cai ZX, Lu QP, Zhang L, Weng XG, Zhang FJ, Zhou D, Yang JC, Wang JF. Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet diarrhoea: intestinal microbiota and immune imbalances. PLoS One 7: e40666, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, Tsao LY, Chen CH, Su BH. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 122: 693–700, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, Oh W. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 115: 1–4, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 368: 1271–1283, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71: 8228–8235, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luig M, Lui K. Epidemiology of necrotizing enterocolitis. I. Changing regional trends in extremely preterm infants over 14 years. J Paediatr Child Health 41: 169–173, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Manzoni P, Mostert M, Leonessa ML, Priolo C, Farina D, Monetti C, Latino MA, Gomirato G. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin Infect Dis 42: 1735–1742, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Neal MD, Jia H, Eyer B, Good M, Guerriero CJ, Sodhi CP, Afrazi A, Prindle T, Jr, Ma C, Branca M, Ozolek J, Brodsky JL, Wipf P, Hackam DJ. Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PloS One 8: e65779, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol 176: 3070–3079, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Neal MD, Sodhi CP, Dyer M, Craig BT, Good M, Jia H, Yazji I, Afrazi A, Richardson WM, Beer-Stolz D, Ma C, Prindle T, Grant Z, Branca MF, Ozolek J, Hackam DJ. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J Immunol 190: 3541–3551, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 364: 255–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quaroni A, Isselbacher KJ, Ruoslahti E. Fibronectin synthesis by epithelial crypt cells of rat small intestine. Proc Natl Acad Sci USA 75: 5548–5552, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rachmilewitz D, Karmeli F, Shteingart S, Lee J, Takabayashi K, Raz E. Immunostimulatory oligonucleotides inhibit colonic proinflammatory cytokine production in ulcerative colitis. Inflamm Bowel Dis 12: 339–345, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Rachmilewitz D, Karmeli F, Takabayashi K, Hayashi T, Leider-Trejo L, Lee J, Leoni LM, Raz E. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology 122: 1428–1441, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126: 520–528, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Ruemmele FM, Beaulieu JF, Dionne S, Levy E, Seidman EG, Cerf-Bensussan N, Lentze MJ. Lipopolysaccharide modulation of normal enterocyte turnover by toll-like receptors is mediated by endogenously produced tumour necrosis factor alpha. Gut 51: 842–848, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sangild P. Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med (Maywood) 231: 1695–1711, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Sangild PT, Siggers RH, Schmidt M, Elnif J, Bjornvad CR, Thymann T, Grondahl ML, Hansen AK, Jensen SK, Boye M. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130: 1776–1792, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Sangild PT, Thymann T, Schmidt M, Stoll B, Burrin DG, Buddington RK. Invited Review: The preterm pig as a model in pediatric gastroenterology. J Anim Sci 91: 4713–4729, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T, Jr, Russo AM, Afrazi A, Good M, Brower-Sinning R, Firek B, Morowitz MJ, Ozolek JA, Gittes GK, Billiar TR, Hackam DJ. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143: 708–718; e701–705, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas DJ, Husmann RJ, Villamar M, Winship TR, Buck RH, Zuckermann FA. Lactobacillus rhamnosus HN001 attenuates allergy development in a pig model. PloS One 6: e16577, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Hoffen E, Korthagen NM, de Kivit S, Schouten B, Bardoel B, Duivelshof A, Knol J, Garssen J, Willemsen LE. Exposure of intestinal epithelial cells to UV-killed Lactobacillus GG but not Bifidobacterium breve enhances the effector immune response in vitro. Int Arch Allergy Immunol 152: 159–168, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg 47: 241–248, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Watson JL, McKay DM. The immunophysiological impact of bacterial CpG DNA on the gut. Clin Chim Acta 364: 1–11, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Wickens K, Black P, Stanley TV, Mitchell E, Barthow C, Fitzharris P, Purdie G, Crane J. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin Exp Allergy 42: 1071–1079, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Wilson K. Preparation of genomic DNA from bacteria. In: Current Protocols in Molecular Biology, edited by Ausubel FM. Hoboken, NJ: Wiley, 2001, pp. 2.–4.1–. [DOI] [PubMed] [Google Scholar]
- 62.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 277: 50959–50965, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, Neal MD, Jia H, Lin J, Ma C, Branca MF, Prindle T, Richardson WM, Ozolek J, Billiar TR, Binion DG, Gladwin MT, Hackam DJ. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci USA 110: 9451–9456, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]