Abstract
Hepatosteatosis, the ectopic accumulation of lipid in the liver, is one of the earliest clinical signs of alcoholic liver disease (ALD). Alcohol-dependent deregulation of liver ceramide levels as well as inhibition of AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor α (PPAR-α) activity are thought to contribute to hepatosteatosis development. Adiponectin can regulate lipid handling in the liver and has been shown to reduce ceramide levels and activate AMPK and PPAR-α. However, the mechanisms by which adiponectin prevents alcoholic hepatosteatosis remain incompletely characterized. To address this question, we assessed ALD progression in wild-type (WT) and adiponectin knockout (KO) mice fed an ethanol-containing liquid diet or isocaloric control diet. Adiponectin KO mice relative to WT had increased alcohol-induced hepatosteatosis and hepatomegaly, similar modest increases in serum alanine aminotransferase, and reduced liver TNF. Restoring circulating adiponectin levels using recombinant adiponectin ameliorated alcohol-induced hepatosteatosis and hepatomegaly in adiponectin KO mice. Alcohol-fed WT and adiponectin KO animals had equivalent reductions in AMPK protein and PPAR-α DNA binding activity compared with control-fed animals. No difference in P-AMPK/AMPK ratio was detected, suggesting that alcohol-dependent deregulation of AMPK and PPAR-α in the absence of adiponectin are not primary causes of the observed increase in hepatosteatosis in these animals. By contrast, alcohol treatment increased liver ceramide levels in adiponectin KO but not WT mice. Importantly, pharmacological inhibition of de novo ceramide synthesis in adiponectin KO mice abrogated alcohol-mediated increases in liver ceramides, steatosis, and hepatomegaly. These data suggest that adiponectin reduces alcohol-induced steatosis and hepatomegaly through regulation of liver ceramides, but its absence does not exacerbate alcohol-induced liver damage.
Keywords: adiponectin, alcohol, ceramide, hepatosteatosis
alcoholic liver disease (ALD) and nonalcoholic liver disease (NALD) are major public health concerns worldwide (11, 72). ALD and NALD have similar spectra of clinical presentation, ranging from hepatosteatosis to steatohepatitis, progressive fibrosis, and cirrhosis (4). Hepatosteatosis, characterized by ectopic liver triglyceride (TG) accumulation (86) and often accompanied by hepatomegaly (9, 42, 65), occurs in 90% of heavy drinkers (31) and is thought to be promoted by insulin resistance due to overnutrition (6). Although long considered a benign condition, recent studies demonstrate that hepatosteatosis is a major susceptibility factor for subsequent liver insult (21, 37, 89).
Among the many identified factors contributing to hepatosteatosis, there has been recent interest in the role of the sphingolipid ceramide. Ceramides are important cell membrane components that also act as second messengers in the cell, influencing diverse functions such as cell growth, apoptosis and insulin signaling (13, 33). Two major pathways for ceramide production are de novo ceramide synthesis, with the rate-limiting step catalyzed by serine palmitate transferase (SPT) and hydrolysis of sphingomyelin by sphingomyelinase (SMase) (13). Elevated ceramides contribute to insulin resistance that is often associated with nonalcoholic hepatosteatosis (14) attributable in part to their ability to inhibit Akt, an important mediator of insulin action (13). Insulin resistance and elevated liver ceramides have also been observed in alcohol-fed animals (19, 52, 71), and pharmacological inhibition of ceramide elevation prevents alcohol-induced insulin resistance and steatosis (52, 57).
Ceramides inhibit insulin signaling in part by activation of the protein phosphatase 2A (PP2A), which limits Akt activation (13). However, PP2A can also influence other intracellular signaling molecules, including AMP-activated protein kinase (AMPK) (53), an important regulator of lipid handling in hepatocytes (36). It remains unclear whether ceramide-dependent inhibition of Akt or AMPK are primary causes of fatty liver. Ceramides can affect many other pathways (78), and elevated ceramides may contribute to hepatosteatosis through yet unknown mechanisms (14).
In addition to elevating ceramides, chronic alcohol intake can also contribute to hepatosteatosis development through stimulation of fatty acid synthesis and inhibition of fatty acid oxidation. Alcohol-mediated inhibition of AMPK and peroxisome proliferator-activated receptor α (PPAR-α) are important contributors to this effect (22). AMPK is a heterotrimeric protein kinase activated by increased cellular energy demand and involves upstream kinases such as liver kinase B 1 and calcium/calmodulin-dependent protein kinase kinase-β (36). Activated AMPK inhibits fatty acid synthesis by phosphorylating and inhibiting acetyl-CoA carboxylase (ACC), which catalyzes the carboxylation of acetyl-CoA to produce malonyl-CoA, the rate-limiting step in fatty acid synthesis (36). ACC inhibition by AMPK indirectly stimulates mitochondrial fatty acid oxidation, as malonyl-CoA inhibits transport of fatty acids into the mitochondria for oxidation (36). AMPK activation may also inhibit fatty acid synthesis by inhibiting transcription of sterol response element-binding protein 1c (SREBP-1c) (76, 84, 103), a transcription factor that drives production of genes involved in de novo lipid synthesis (15). Hepatocytes isolated from alcohol-fed rats exhibit reduced AMPK phosphorylation in response to treatment with the AMPK agonist 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) (32). Chronic alcohol consumption in animals is associated with reductions in AMPK protein and steady-state phosphorylation levels (3, 103), and AICAR treatment reverses alcoholic steatosis (91). PPAR-α is a transcription factor that drives expression of genes important for fatty acid oxidation (62). Alcohol treatment of hepatocytes abrogated PPAR-α DNA-binding activity (30). Alcohol-fed animals have reduced transcript levels of PPAR-α target genes, and alcoholic steatosis can be alleviated by PPAR-α agonist treatment (29, 63). Together, these data suggest that alcohol-dependent inhibition of AMPK and PPAR-α contribute to the development of hepatosteatosis.
Adiponectin, an abundant serum adipokine, has many beneficial effects, including stimulating fatty acid oxidation and enhancing insulin sensitivity (98). Adiponectin generates these effects in part through reduction of ceramide levels and activation of AMPK and PPAR-α (98), leading to suggestions that alcohol-mediated dysregulation of adiponectin may present a common mechanism contributing to hepatosteatosis development (104). Adiponectin is a 30-kDa protein produced primarily by adipose tissue that circulates as trimeric (low-molecular-weight, LMW), hexameric (middle-molecular-weight, MMW), and high-molecular-weight (HMW) oligomers (92). Adiponectin reduces target tissue ceramide levels likely through activation of ceramidase activity in two identified adiponectin cell surface receptors, adiponectin receptor (AdipoR) 1 and AdipoR2 (38). Adiponectin also activates AMPK and PPAR-α through AdipoR1 and AdipoR2, respectively (98). However, the intracellular signals linking adiponectin receptor activation to AMPK and PPAR-α activation in vivo remain poorly understood (98).
Although there has been much interest in the protective role of adiponectin in ALD progression, important questions remain. Some reports from clinical studies and animal models have shown that alcohol consumption increases adiponectin levels (17, 43, 88, 97), whereas others observed the opposite effect (44, 96, 102). Studies in alcohol-fed animals demonstrate that elevation of adiponectin to supraphysiological levels either with recombinant adiponectin or dietary modification was sufficient to reduce hepatosteatosis and liver damage (3, 85, 96). However, it remains unclear how ALD progresses in the absence of adiponectin. Also, increased adiponectin has been associated with increases in AMPK phosphorylation and expression of PPAR-α target genes in the liver of alcohol-fed mice (102), but whether adiponectin is directly or indirectly responsible for this effect is unclear. Finally, no data on adiponectin-dependent regulation of ceramide levels in alcohol-fed animals are currently available.
To begin to address these questions, we compared markers of ALD progression and adiponectin-sensitive pathways in alcohol-fed WT and adiponectin KO mice. Our data show that adiponectin KO mice are more susceptible to alcohol-mediated increases in hepatosteatosis and hepatomegaly. However, in the absence of increased inflammation in adiponectin KO mice, no differences in alcohol-induced increases in serum alanine aminotransferase (ALT), an important marker of liver damage, were found between WT and adiponectin KO mice. Steatosis and hepatomegaly were alleviated in alcohol-fed adiponectin KO mice by restoring circulating adiponectin levels using recombinant protein. An alcohol-induced increase in liver ceramide levels was observed in adiponectin KO but not WT mice. Importantly, treatment with myriocin, a specific SPT inhibitor, inhibited alcohol-dependent increases in liver ceramide levels in adiponectin KO mice and reduced steatosis and hepatomegaly. Together, these data suggest that adiponectin regulates lipid handling in alcohol-fed animals predominantly through regulation of liver ceramide levels.
MATERIALS AND METHODS
Animals studies.
All animal procedures were approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University. Adiponectin KO mice (B6.129-Adipoqtm1Chan) (58), originally made available by Dr. Lawrence Chan (Baylor College of Medicine), or congenic C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) 10–12 wk of age were housed in shoebox cages with α-cob bedding and mouse igloos (Bioserv, Frenchtown, NJ). Animals were pair-fed Reyes chocolate-flavored alcohol diet (Bioserv) (15.1% protein, 35.9% fat, 49% carbohydrate) or isocaloric Reyes chocolate-flavored control diet, substituting ethanol calories for carbohydrate (maltose dextrin). The Reyes diet, comparable in composition to the Lieber-Decarli diet, was employed to overcome the rodents' natural alcohol aversion (69) and enhance alcohol intake (73–75). Consistent with this, we observed that mice fed the Reyes diet formulated with 34% ethanol-derived calories (EDC) consumed on average 25–30 g ethanol/kg body wt per day (Table 2). This intake is elevated relative to reported consumption by mice fed a standard Lieber-DeCarli diet containing similar alcohol concentrations (17–20 g ethanol/kg body wt per day) (34, 96, 105). Unless otherwise noted, mice were fed control diet for 3 days, then diet with 22% EDC for 7 days, followed by 27% EDC for 7 days and 7 days on 34% EDC.
Table 2.
Comparison of alcohol consumed and caloric intake
| WT |
Adn KO |
|||
|---|---|---|---|---|
| Ethanol-derived calories | 27% | 34% | 27% | 34% |
| Daily alcohol consumption, g ethanol/kg body wt | 21.96 ± 3.49 | 29.08 ± 8.85* | 20.36 ± 4.50 | 26.2 ± 4.5* |
| Daily caloric intake, kcal/g body wt | 0.53 ± 0.08 | 0.60 ± 0.18 | 0.53 ± 0.12 | 0.54 ± 0.10 |
Data are mean daily values ± SD. Mice were fed liquid alcohol diet with either 27 or 34% ethanol-derived calories (EDC), and consumption of alcohol and total caloric intake were assessed.
WT, wild-type; Adn, adiponectin; KO, knockout.
(n =16,
P < 0.01 compared with 27% diet)
Blood was collected from the tail of live animals and from the vena cava at death. Collected blood was incubated at room temperature for 30 min and centrifuged, and serum was collected for analysis. For liver analysis, animals were anesthetized and weighed, and blood was collected. Following death, the liver was dissected out and weighed, the left-lateral and medial lobes were freeze-clamped using liquid nitrogen-cooled aluminum clamps for biochemical analysis, and the right lobes were embedded in optimal cutting temperature compound (OCT) for histology. For AMPK Western blots, animals were anesthetized in 5% isoflurane, and the liver was immediately (within 10 s) freeze clamped as described (23) to prevent rapid postmortem changes in metabolite levels and AMPK phosphorylation.
Myriocin (Enzo Life Sciences, Farmingdale, NY) was dissolved in sterile 1× PBS and administered by intraperitoneal (IP) injection (0.5 mg/kg) every 48 h for 8 days before death. For glucose tolerance tests (GTTs), animals were fasted 6 h and weighed, and an initial glucose reading was taken using Precision Xtra glucose meters (Abbott Diabetes Care, Witney, UK). Mice were given glucose (2 g/kg) by IP injection, and serum glucose levels were measured at 15, 30, 60, and 120 min following injection. Area under the curve (AUC) was calculated using GraphPad Prism 5 software (San Diego, CA).
Recombinant adiponectin treatment.
Recombinant adiponectin was purified from HEK-293 cells, generously donated by Drs. Russell Miller and Morris Birnbaum, as described (10). Briefly, HEK-293 cells stably expressing a secreted form of adiponectin were maintained in high glucose Dulbecco's Modified Eagle Medium (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (Life Technologies), 20 mM HEPES (Sigma, St. Louis, MO) and 1× penicillin/streptomycin solution (Life Technologies). For purification, serum-free culture supernatants containing adiponectin were precipitated with ammonium sulfate and resuspended in 10 mM HEPES, 50 mM NaCl, and 1 mM CaCl2. Resuspended adiponectin was bound to a MonoQ HP HiTrap column (GE Healthcare Life Sciences, Pittsburgh, PA) and eluted using a 50–500 mM NaCl gradient. Dialyzed eluent was quantitated using the BCA Protein Assay Reagent (Pierce Biotechnology, Rockford, IL) and adiponectin ELISA (B-Bridge International, Cupertino, CA). The presence of LMW, MMW, and HMW adiponectin oligomers was confirmed by SDS-PAGE under nondenaturing conditions (data not shown) and by HMW adiponectin ELISA (B-Bridge International). Alzet pumps (model 1007D; Alzet, Cupertino, CA) were filled with either 100 μl of saline or recombinant adiponectin (10 μg/μl). Adiponectin- or saline-containing Alzet pumps were implanted in the interscapular region under anesthesia according to the manufacturer's instructions, and mice were treated postsurgically with Buprenex Injectable (0.1 mg/kg, Reckitt Benckiser Healthcare, Hull, UK).
Histological analysis.
OCT-embedded liver samples were sectioned and stained with hematoxylin and eosin by the Kimmel Cancer Center pathology core facility (Thomas Jefferson University). Oil red O staining was done with NovaUltra Oil Red O stain kit (IHC World, Woodstock, MD). Images were captured using an Olympus CKX41 microscope fitted with a Qcolor3 camera (Olympus, Center Valley, PA). Liver sections were scored for hepatosteatosis using the Brunt scoring system (16). Tissues that had <10% of total cell number with notable steatosis were scored 0, those with 10–35% were scored a 1, 35–60% were scored a 2, and samples with >60% steatosis were scored a 3. Five fields/sample were scored.
Biochemical analysis.
For Western blotting, tissue lysates were generated by homogenizing frozen tissue in RIPA buffer (Sigma) supplemented with phosphatase and protease inhibitor cocktails (Sigma), and sample protein levels were determined by BCA protein assay. Protein (20 μg) was loaded onto an SDS-PAGE gel, and Western blotting was performed as described (23). For analysis of serum hormone and cytokine levels, ELISA kits were used for adiponectin (B-Bridge International), HMW adiponectin (B-Bridge International), and TNF (eBioscience, San Diego, CA) according to the manufacturers' instructions. For TG measurements, 20 mg of tissue was lysed with 2:1 (vol/vol) ethanol/30% potassium hydroxide solution and precipitated by adding MgCl2 to a final concentration of 0.5 M. Samples were centrifuged, and supernatants were analyzed for TG content using Stanbio Triglyceride Liquicolor (Stanbio, Boerne, TX) according to the manufacturer's instructions. Serum ALT levels were analyzed using the ALT/glutamic-pyruvic transaminase assay kit (Stanbio) according to the manufacturer's instructions. For ceramide analysis, 30 mg of tissue was homogenized in 100 μl of water followed by addition of 750 μl of methanol. Ceramide species were measured by liquid chromatography/tandem mass spectrometry at the Medical University of South Carolina lipidomics core facility as described (12). Ceramide concentrations were normalized to sample protein content as assessed by BCA analysis.
Transcription factor binding activity was assessed from nuclear extracts prepared from frozen tissue using a nuclear extraction kit (Origene, Rockville, MD). PPAR-α DNA-binding activity in 100 μg of nuclear extract was measured using the PPAR-α transcription factor assay kit (Cayman Chemical, Ann Arbor, MI) according to manufacturer's instructions.
For real-time PCR analysis, UPL probe-compatible primers (Table 1) were designed using Universal Probe Library Assay Design Center (Roche Applied Science, Indianapolis, IN, https://www.roche-applied-science.com/sis/rtpcr/upl/index.jsp). RNA was isolated from liver tissue using the Animal Tissue RNA Purification Kit (Norgen Biotek, Thorold, ON, Canada) and was reverse transcribed using EasyScript cDNA Synthesis Kit (Applied Biological Materials, Richmond, BC, Canada). cDNA (100 ng) was preamplified for 12 cycles using TaqMan PreAmp Master Mix according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). qPCR reactions were performed using 48.48 BioMark Dynamic Arrays (Fluidigm, South San Francisco, CA) with 40 cycles of amplification (15 s at 95°C, 5 s at 70°C, 60 s at 60°C). CT values were calculated by the Real-Time PCR Analysis Software (Fluidigm). Relative gene expression was determined by the 2−ΔΔCT method (56). Mean CT values for TATA-binding protein, Mrpl4, and Erlin2 were used for housekeeping normalization. Relative gene expression is reported as a fold change compared with wild-type control-fed samples.
Table 1.
List of primers and Universal Probe Library (UPL) probes used for high-throughput qPCR
| Gene Symbol | GenBank Accession Number | UPL Probe | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|---|
| IL-6 | NM_031168.1 | 6 | gctaccaaactggatataatcagga | ccaggtagctatggtactccagaa |
| TNF | NM_0136932 | 79 | cgtagcccacgtcgtagc | ggttgtctttgagatccatgc |
| SOCS3 | NM_007707.3 | 13 | cacacaaggagccaaacaca | tagccacctgggtgaatcc |
| MCP-1 | NM_011333.3 | 62 | ggctggagagctacaagagg | ctcttgagcttggtgacaaaaa |
| Sptlc1 | NM_009269.2 | 108 | cctccagtctccaagaacca | accacgatgttgtgggttg |
| Sptlc2 | NM_011479.3 | 15 | gacctatggcatgggatttg | cactcagaatcaggcaacctt |
| CerS1 (Lass1) | NM_138647.3 | 26 | taggggtggtgcctaccat | tagaggcggaaccagaacc |
| CerS2 (Lass2) | NM_029789.1 | 82 | tctgatgtcaagcgaaagga | acgtaattggcaaaccagga |
| CerS4 (Lass4) | NM_026058.4 | 18 | tgaagcagtgtccagaggag | agtctgccgaagcgtgag |
| CerS5 (Lass5) | NM_028015.2 | 89 | gctggcagtgtgcatcttc | tttaatgccaacacggagtg |
| CerS6 (Lass6) | NM_172856.3 | 2 | gctggtttcgacaaagacg | agaggtaaaaggaaaatctccaca |
| Smpd1 | NM_011421.2 | 108 | cccgcctgcaaagtcttat | ccacattgggctccttctt |
| Smpd2 | NM_009213.2 | 26 | ctggcccagttcatccac | agccctgtccactctttcag |
| Smpd3 | NM_021491.3 | 29 | gtgtaccccggacaacctt | cccttcagcagatccttcc |
| Smpd4 | NM_001164609.1 | 79 | agcgtacctccaccactctg | ggctagtgtggaggccatag |
| Asah1 | NM_019734.2 | 55 | tgaagatggtggatcaaaagc | acatctgcaattcccctca |
| Asah2 | NM_018830.1 | 78 | ggtgtgcttagaggcatcg | tgctcaggctgatttctcaa |
| AdipoR1 | NM_028320.3 | 15 | tttgccactcccaagcac | acaccactcaagccaagtcc |
| AdipoR2 | NM_197985.3 | 98 | ggcaacatttggacacatctc | atttgggcgaaacatataaaagat |
| Tbp | NM_013684.3 | 97 | ggggagctgtgatgtgaagt | ccaggaaataattctggctca |
| Mrpl4 | NM_023167.2 | 77 | tctggcagaggaacttcagg | ttctgttgccagggcttg |
| Erlin2 | NM_153592.1 | 7 | cagagaagaagatctcagaaattgaa | atcttcagcgctgtgtagca |
UPL compatible primers were designed using UPL Assay Design Center.
SOCS3, suppressor of cytokine signaling 3; MCP-1, monocyte chemoattractant protein 1; Sptlc1, serine palmitate transferase long chain base subunit; CerS1 ceramide synthase 1; Smpd, sphingomyelinase; Asah, acid ceramidase; AdipoR, adiponectin receptor; Tbp, TATA-binding protein.
Statistical analyses were performed using ANOVA unless otherwise noted. Data are presented are means ± SD or means ± SE.
RESULTS
Effect of alcohol consumption on circulating adiponectin levels.
Initial experiments examined serum adiponectin levels in WT mice fed Reyes chocolate-flavored alcohol diet, a modification of the standard Lieber-DeCarli diet. Using an isocaloric pair-feeding protocol (54), diet alcohol concentration was elevated stepwise from 22% EDC diet to 27% EDC and finally 34% EDC, with animals maintained on each alcohol concentration for 7 days. Maintaining animals on 34% EDC for longer than 7 days was associated with significant weight loss (data not shown). Alternatively, mice were fed a diet containing 22% EDC for 7 days followed by 5 wk on 27% EDC. No weight loss was observed in animals on this feeding protocol (data not shown). Animals on the 34% EDC diet had similar average total daily caloric intake to mice fed the 27% EDC diet (0.53 ± 0.08 vs. 0.60 ± 0.18 kcal/g body wt) (Table 2) and to mice fed a standard chow diet (46) but had significantly higher average daily alcohol intake (22.0 ± 3.5 vs. 29.1 ± 8.9 g ethanol/kg body wt per day, mean ± SD, P < 0.01) (Table 2). The serum adiponectin levels in mice fed the 34% ECD diet were significantly elevated relative to control-fed mice (Table 3), whereas serum adiponectin levels in mice maintained on the 27% EDC protocol were not significantly different from control-fed animals. These data demonstrate that, although total caloric intake is similar, average daily alcohol consumption by mice is increased by feeding the 34% EDC diet relative to 27% EDC. Additionally, 34% EDC feeding is associated with elevated serum adiponectin levels relative to control-fed animals.
Table 3.
Serum adiponectin levels in WT mice
| EDC | 27% |
34% |
||
|---|---|---|---|---|
| Diet | Control | Alcohol | Control | Alcohol |
| Serum Adiponectin (μg/ml) | 13.38 ± 1.74 | 18.81 ± 2.34 | 16.27 ± 0.37 | 26.1 ± 1.13* |
Data are means ± SE. Mice were fed liquid control diet or alcohol-containing diet with either 27 or 34% EDC, and serum adiponectin levels were assessed at harvest. (n = 4,
P < 0.01)
Impact of alcohol feeding on liver damage markers in WT and adiponectin KO mice.
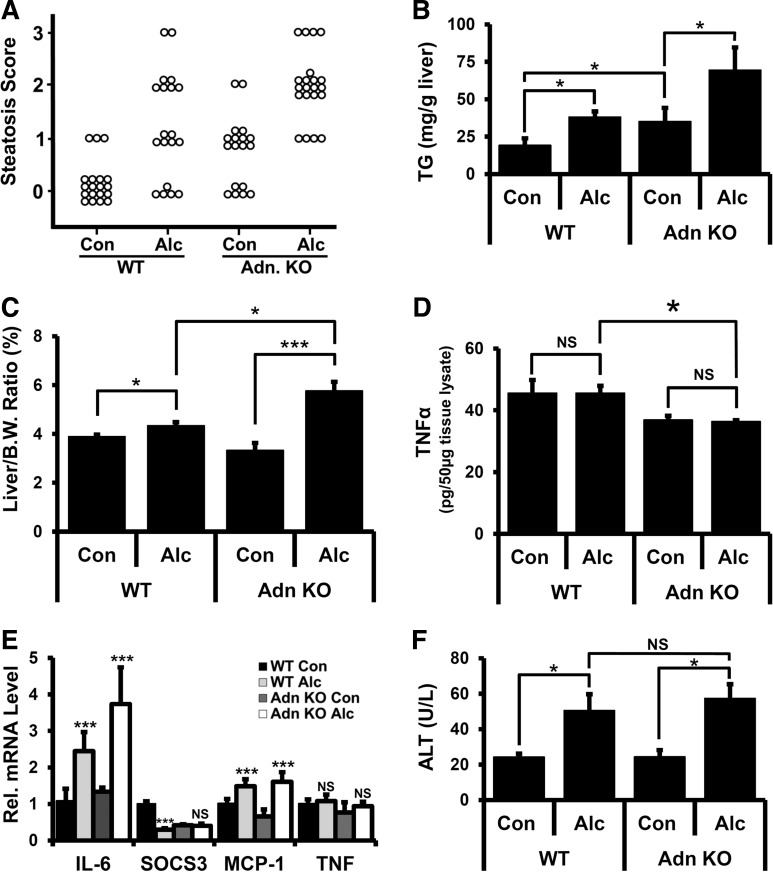
Because 34% EDC diet feeding was associated with elevated alcohol intake and increased serum adiponectin levels in WT mice, we used this protocol to assess the effects of alcohol feeding on liver damage in adiponectin KO mice. No significant differences in alcohol or caloric intake between WT and adiponectin KO mice were detected (Table 2). Liver sections from alcohol- and control-fed WT and adiponectin KO mice were graded for steatosis using the Brunt scoring system. In grades 0–2, lipid accumulation was primarily periportal, whereas grade 3 samples displayed panlobular lipid accumulation (data not shown). No evidence of gross necrotic liver injury was observed under any of these conditions. Each treatment group exhibited variations in steatosis scores. and differences in distribution were assessed by χ2 test. As shown in Fig. 1A, alcohol feeding in WT mice was associated with increased incidence of steatosis compared with control-fed animals [χ2(3) = 34.7, P < 0.01, n = 19]. Whereas only 16% of examined livers from control-fed WT mice had evidence of steatosis (score ≥ 1), 66% of liver sections from control-fed adiponectin KO mice had steatosis [χ2(3) = 19.8, P < 0.01, n = 18]. Alcohol-fed adiponectin KO mice had significantly increased steatosis scores relative to both control-fed adiponectin KO mice [χ2(3) = 54.1, P < 0.01, n = 18] and alcohol-fed WT mice [χ2(3) = 14.5, P < 0.01, n = 18].
Fig. 1.
Liver and serum analysis after alcohol (Alc) feeding in wild-type (WT) and adiponectin (Adn) knockout (KO) mice. A: histological sections were scored for hepatosteatosis, and animals/score were plotted. B: quantitation of triglyceride (TG) from liver extracts. C: liver-to-body weight (B.W.) ratio. D: TNF-α levels were determined by ELISA using tissue lysates. E: mRNA extracted from livers of treated animals were assessed for IL-6, suppressor of cytokine signaling 3 (SOCS3), monocyte chemoattractant protein (MCP)-1 and TNF levels. F: alanine aminotransferase (ALT) measurements were determined in serum. Data presented as means ± SE. NS, not significant, *P < 0.05, ***P < 0.01.
Consistent with the histological scoring data, liver TG levels were significantly increased in alcohol-fed WT mice relative to control-fed WT mice (Fig. 1B). Elevated TG levels were observed in control-fed adiponectin KO mice relative to control-fed WT mice, and alcohol-fed adiponectin KO mice had significantly more TG than adiponectin KO mice or alcohol-fed WT mice (Fig. 1B). Together, these data show that alcohol-fed adiponectin KO mice have more liver TG and hepatosteatosis relative to WT mice, suggesting that they are more sensitive to alcohol-mediated dysregulation of lipid handling.
Liver-to-body weight ratios were assessed as an indicator of hepatomegaly, which is found in both clinical (61, 80) and experimental (40, 42, 65) ALD. WT alcohol-fed mice had a modest increase in liver-to-body weight ratio compared with control-fed animals (Fig. 1C, 3.7% vs. 4.36%), in agreement with previous literature reports (102). Alcohol-fed adiponectin KO mice had a significantly larger increase in liver-to-body weight ratio relative to control-fed animals (Fig. 1C, 3.32% vs. 5.76%), and this ratio was also significantly elevated over alcohol-fed WT mice, suggesting that adiponectin KO mice are more susceptible to alcohol-induced hepatomegaly.
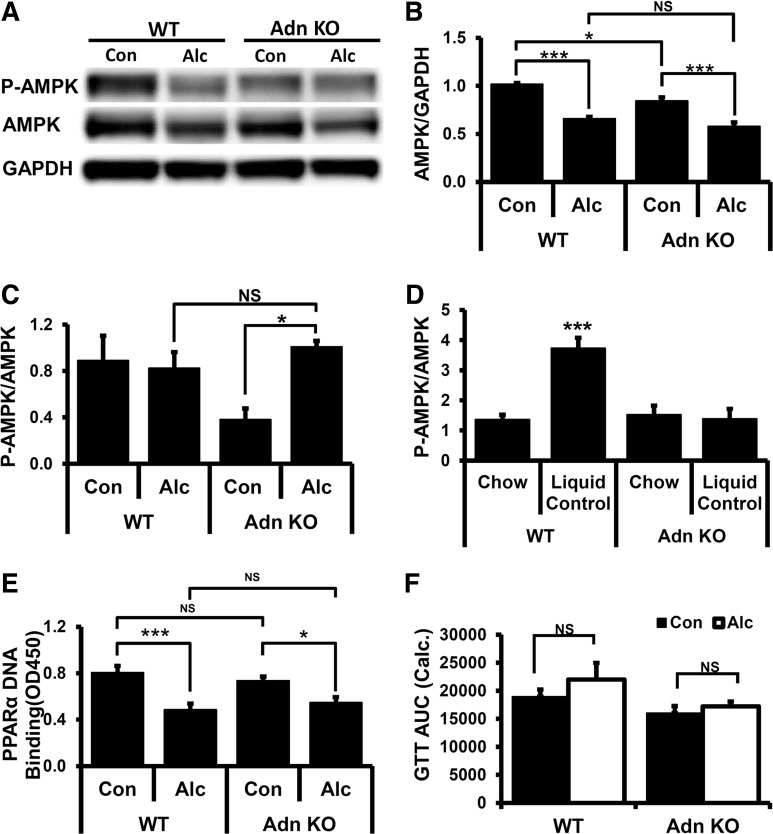
Fig. 3.
AMP-activated protein kinase (AMPK) phosphorylation, peroxisome proliferator-activated receptor α (PPAR-α) activation, and glucose tolerance test (GTT) after alcohol feeding. A: Western blot of representative samples probed with antibodies specific for AMPK-α subunit phosphorylated on Thr172 (P-AMPK), total AMPK-α protein (AMPK), and GAPDH. B: quantitation of AMPK protein levels from Western blots (n = 4/group). C: quantitation of P-AMPK relative to AMPK protein from Western blots in control and alcohol-fed animals. D: comparison of P-AMPK/AMPK ratio in chow-fed and liquid control diet-fed animals (n = 4/group). E: liver nuclear extracts assayed for PPAR-α-binding to PPAR DNA-binding element. F: GTT administered following alcohol feeding. AUC, calculated area under the curve. Data presented as mean ± SE. *P < 0.05, ***P < 0.01.
Inflammation, and in particular TNF, is an important driver of both steatosis and steatohepatitis (1, 2, 5, 101). Under conditions used in these studies, TNF levels in serum from alcohol-fed animals were below the level of detection (data not shown). Therefore, TNF levels were assessed in liver homogenates. In control-fed animals, TNF levels were lower in adiponectin KO than in WT mice (Fig. 1D), whereas no alcohol-dependent changes in liver TNF protein levels were evident in either WT or adiponectin KO mice. Similar results were obtained when liver TNF mRNA transcript levels were assessed by RT-PCR (Fig. 1E). By contrast, IL-6 and monocyte chemotactic protein-1 (MCP-1) transcript levels were elevated in both WT and adiponectin KO alcohol-fed mice relative to control-fed animals (Fig. 1E), but no difference was detected between alcohol-fed WT and adiponectin KO mice. Alcohol feeding significantly reduced suppressor of cytokine signaling 3 (SOCS3) transcripts in WT mice but not adiponectin KO mice (Fig. 1E). This observation shows that adiponectin KO mice have reduced liver TNF protein levels and comparable alcohol-dependent changes in IL-6 and MCP-1 transcripts to WT animals, suggesting similar susceptibility to alcohol-dependent changes in inflammation in WT and adiponectin KO mice. When liver sections were assessed for inflammatory foci, 12 out of 16 WT mice that were fed the control liquid diet had at least one inflammatory focus per five fields examined, whereas 11 out of 19 alcohol-fed WT animals had inflammatory foci. By contrast, only 3 out of 19 liver sections from control-fed adiponectin KO mice showed evidence of inflammatory foci, whereas 6 out of 20 liver sections from alcohol-fed adiponectin KO mice had inflammatory infiltrate. We noted that none of the tissues examined had more than one focus per field, indicating that lymphocyte recruitment to the liver was not strongly induced using this feeding protocol. This is consistent with our TNF analysis and further suggests that increased inflammation in adiponectin KO mice does not contribute to alterations in lipid handling and hepatomegaly observed after alcohol feeding in these animals.
ALT, a clinically important measure of liver damage, was elevated in alcohol-fed compared with control-fed WT and adiponectin KO mice. However, no differences in serum ALT levels were observed between alcohol-fed WT and adiponectin KO mice (Fig. 1F). Taken together, our data show that, with equivalent alcohol intake, adiponectin KO mice display higher levels of alcohol-mediated steatosis and hepatomegaly than WT mice but had lower liver TNF protein levels, with similar increases in inflammatory markers IL-6 and MCP-1 and comparable degrees of liver damage.
Adiponectin treatment reduces steatosis and TG accumulation in alcohol-fed adiponectin KO mice.
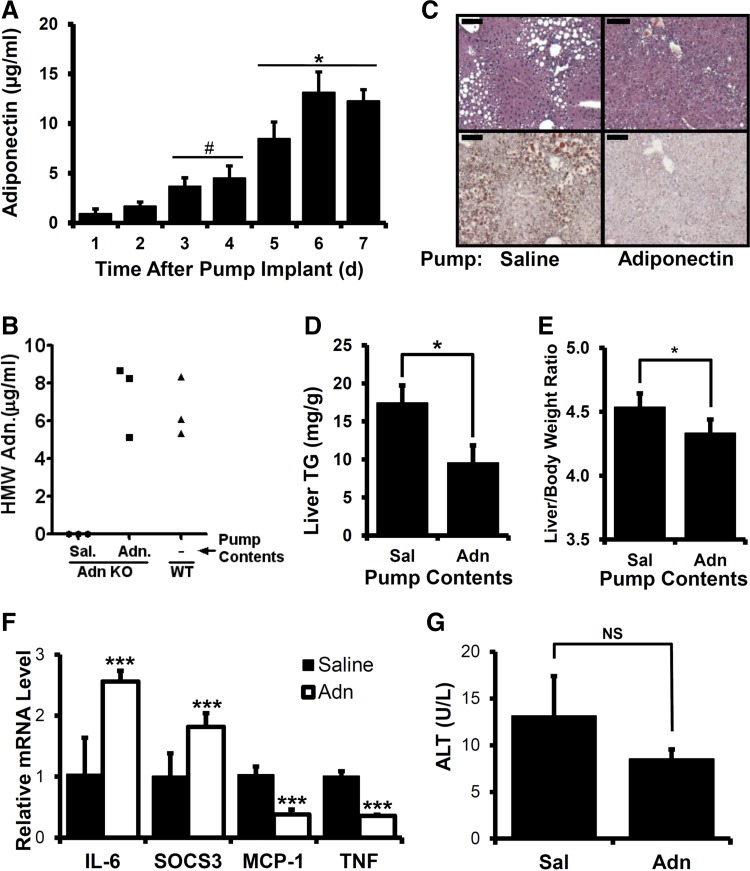
To assess whether the increased alcohol-induced steatosis and hepatomegaly observed in adiponectin KO mice was directly or indirectly attributable to the absence of adiponectin, serum adiponectin was reconstituted in adiponectin KO mice using recombinant protein. Adiponectin purified from HEK-293 cells, which contains LMW, MMW, and HMW oligomers, was administered using Alzet osmotic pumps capable of delivering adiponectin or saline control solution for 7 days. Because pump implantation on day 1 of 34% EDC diet feeding was associated with a significant decrease in daily caloric and alcohol intake (data not shown), pumps were implanted on day 5 of 27% EDC feeding, and livers were harvested on day 5 of 34% EDC diet feeding. No serum adiponectin was detected in animals implanted with saline-delivering pumps (data not shown). By contrast, daily analysis of total serum adiponectin levels in animals with adiponectin-delivering pumps showed stepwise increases on days 1–4 after pump implantation (Fig. 2A) and levels comparable to control-fed WT animals on days 5–7 (Table 3, Fig. 2A). The HMW adiponectin level in implanted adiponectin KO mice was also similar to that observed in alcohol-fed WT mice at the time of harvest (Fig. 2B).
Fig. 2.
Effect of recombinant adiponectin treatment in alcohol-fed adiponectin KO mice. A: daily serum samples from alcohol-fed adiponectin KO mice implanted with adiponectin-delivering Alzet pumps were measured for adiponectin by ELISA. #P < 0.05 compared with day 1. *P < 0.05 compared with day 3. B: serum high-molecular-weight (HMW) adiponectin assessed by ELISA at harvest. Sal, saline. C: representative liver histological sections from alcohol-fed adiponectin KO mice implanted with either saline- or adiponectin-delivering pumps stained with hematoxylin and eosin (top) or oil red O (bottom), scale bar = 100 μm. D: quantitation of triglyceride levels in liver extracts. E: liver-to-body weight ratio. F: mRNA extracted from livers of treated animals was analyzed for IL-6, SOCS3, MCP-1, and TNF levels by qRT-PCR. G: ALT activities in serum. Data presented as means ± SE. *P < 0.05, ***P < 0.01.
When steatosis was examined in adiponectin-infused adiponectin KO mice, a notable decrease in oil red O-positive areas was observed relative to saline-infused animals after alcohol feeding, indicative of reduced liver TGs (Fig. 2C). Consistent with this, a significantly reduced liver TG level was observed in adiponectin-reconstituted animals relative to saline-infused controls (Fig. 2D). We noted lower total liver TG levels in pump-implanted mice (Fig. 2D) relative to unimplanted animals (Fig. 1B), which may be attributable to reduced duration of the 34% EDC diet feeding period (5 days for implanted animals vs. 7 days for unimplanted). Restoration of serum adiponectin also alleviated alcohol-dependent changes in liver-to-body weight ratio (Fig. 2E). No differences in liver TNF levels were found between adiponectin- and saline-treated mice (data not shown). Interestingly, when transcripts for inflammatory markers were examined, we found that mice given recombinant adiponectin had reduced MCP-1 and TNF transcripts but elevated IL-6 and SOCS3 transcripts (Fig. 2F). Also, no significant differences in serum ALT were observed in these animals (Fig. 2G). These results show that restoration of serum adiponectin in adiponectin KO mice is associated with reduced steatosis and hepatomegaly after alcohol feeding, suggesting that adiponectin functions to directly counter alcohol-induced hepatosteatosis and hepatomegaly.
AMPK and PPAR-α activation in alcohol-fed mice.
Because alcohol treatment adversely affects activation of liver AMPK and PPAR-α (103, 104) and AMPK and PPAR-α are reported to be responsive to adiponectin action (98), we investigated how alcohol treatment affects AMPK or PPAR-α in the absence of adiponectin. Liver AMPK protein level was lower in control-fed adiponectin KO mice relative to WT (Fig. 3, A and B). Both WT and adiponectin KO mice had reduced AMPK protein levels in alcohol-fed animals relative to control. However, no difference in AMPK protein was found between alcohol-fed WT and adiponectin KO mice (Fig. 3, A and B). Absolute levels of phosphorylated AMPK (P-AMPK), a marker of AMPK activation, were reduced in both WT and adiponectin KO mice relative to control-fed animals (Fig. 3A). However, because AMPK protein levels were also reduced, no change in the P-AMPK/AMPK ratio was observed (Fig. 3, A and C). Only control-fed adiponectin KO mice showed a significant reduction in the P-AMPK/AMPK ratio (Fig. 3, A and C). One possible explanation for this difference is that adiponectin is a determinant of the P-AMPK/AMPK ratio in control-fed but not alcohol-fed mice. We noted that pair-fed control animals routinely consume all the provided diet, which may create a nutrient-restricted state. If this is correct, we predict that no difference in P-AMPK/AMPK would be detected in ad libitum chow-fed WT or adiponectin KO mice, whereas enhanced P-AMPK/AMPK levels would be found in control-fed WT but not control-fed adiponectin KO mice. Consistent with these idea, we found that liquid control-fed WT but not adiponectin KO mice had an elevated P-AMPK/AMPK ratio relative to chow-fed animals (Fig. 3D). This suggests that adiponectin is required for AMPK activation in control-fed animals. Taken together, these data show that alcohol-fed WT and adiponectin KO mice have similar reductions in AMPK protein and similar P-AMPK/AMPK ratios, suggesting that alcohol-dependent changes in AMPK are independent of adiponectin action.
To assess PPAR-α activation, we measured PPAR-α DNA-binding activity in liver nuclear extracts from WT and adiponectin KO mice. Significant reductions in PPAR-α-binding activity were observed in alcohol-fed WT and adiponectin KO mice relative to control-fed animals (Fig. 3E). However, no difference in PPAR-α DNA-binding activity was detected between WT and adiponectin KO mice (Fig. 3E), supporting the interpretation that alcohol-dependent deregulation of PPAR-α is adiponectin independent in the liver. Taken together, these data indicate that adiponectin is not the causal factor in the deregulation of AMPK and PPAR-α in alcohol-fed animals. Importantly, this also implies that the increased steatosis and hepatomegaly seen in alcohol-fed adiponectin KO mice are not driven primarily by increased deregulation of PPAR-α or AMPK in the absence of adiponectin.
GTTs in alcohol-fed mice.
Recent studies suggest that insulin resistance contributes to alcohol-mediated hepatosteatosis (19, 52, 71). As adiponectin has been reported to have insulin-sensitizing properties (98), we tested whether alterations in insulin sensitivity attributable to alcohol feeding are enhanced in adiponectin KO mice using a GTT. With the use of our standard alcohol-feeding protocol, GTTs were administered on day 7 of the 34% EDC feeding, and integrated AUC was calculated from changes in blood glucose levels as a function of time. As depicted in Fig. 3F, no significant alterations were detected in GTT AUC in alcohol-fed WT or adiponectin KO mice relative to control-fed animals. Because ethanol had no effect on glucose handling, it is unlikely that changes in glucose handling contribute to increased steatosis and hepatomegaly observed in alcohol-fed adiponectin KO mice. We also observed no differences in Akt phosphorylation levels in alcohol-fed WT and adiponectin KO mice (data not shown).
Myriocin treatment reduces alcohol-induced liver phenotypes in adiponectin KO mice.
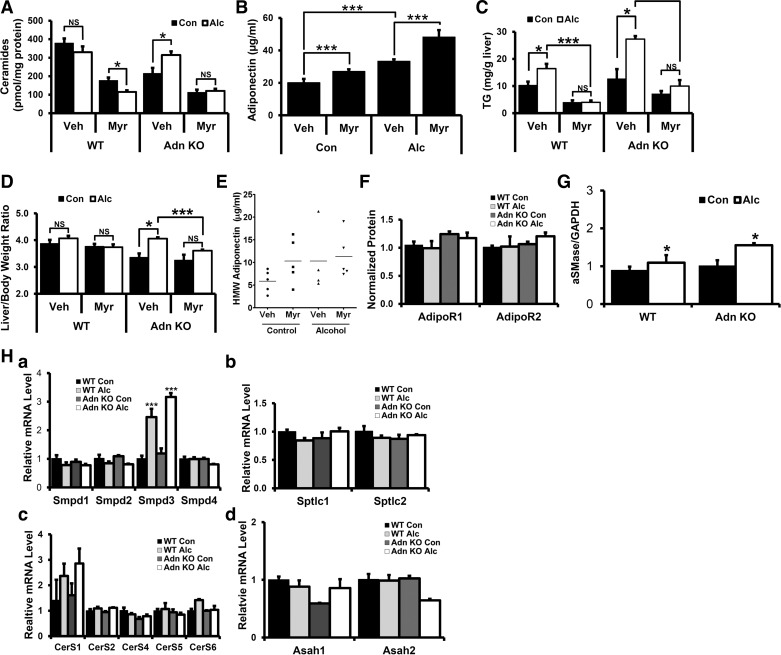
Alcohol feeding can alter liver ceramides (19, 52, 71), and adiponectin-dependent regulation of ceramidase activity is important for many of its beneficial effects (38). However, the role of adiponectin in the regulation of liver ceramide levels in alcohol-fed animals is unknown. Therefore, we investigated alcohol-dependent changes in liver ceramide levels between alcohol-fed WT and adiponectin KO mice by measuring both total ceramide levels and individual ceramide species in liver lipid extracts. Total ceramide levels were lower in control-fed adiponectin KO relative to WT mice (Fig. 4A). Individual ceramide analysis showed that, although the average value of all individual ceramide species examined were lower in control-fed adiponectin KO relative to WT mice (Table 4), ceramides C14:0, C16:0, C18:0, C20:0, C20:1, C24:0, and C24:1 were significantly lower in adiponectin KO animals (Table 4). Interestingly, alcohol-dependent increases in total liver ceramides were observed in adiponectin KO but not in WT mice (Fig. 4A). When alcohol-dependent changes in individual ceramide species were examined, increases in ceramides C24:0 and C26:0 were observed in both WT and adiponectin KO mice. By contrast, whereas no changes in ceramides C14:0, C16:0, C18:0, C18:1, C22:0, and C26:1 were observed in WT mice, adiponectin KO mice had significant increases in these species. Alcohol-dependent decreases in ceramides C20:0, C20:1, and C22:1 were observed in WT mice, whereas no change in these ceramide species was observed in adiponectin KO mice (Table 4).
Fig. 4.
Effects of alcohol feeding and myriocin (Myr) treatment on alcohol-fed WT and adiponectin KO mice. A: liver lipid extracts were analyzed for ceramide levels by liquid chromatography/tandem mass spectrometry and normalized to protein content. B: serum adiponectin levels at harvest analyzed by ELISA. C: liver triglycerides at harvest. D: liver-to-body weight ratio at harvest. E: serum HMW adiponectin levels at harvest in WT animals analyzed by ELISA. F and G: quantitation of Western blots of liver lysates probed with specific antibodies for adiponectin receptor 1 (AdipoR1) and AdipoR2 (F) or acid sphingomyelinases (aSMase) (G) and normalized to GAPDH. H: mRNA extracted from livers of treated animals were assessed for sphingomyelinase (Smpd) isoforms (a), serine palmitate transferase (SPT) subunits (b), ceramide synthase (CerS) isoforms (c), and acid ceramidase (Asah) isoforms (d). Data presented as mean ± SE. (n ≥ 4) *P < 0.05, ***P < 0.01.
Table 4.
Liver ceramides in control- and alcohol-fed WT and adiponectin KO mice treated with myriocin
| WT |
Adiponectin KO |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control |
Alcohol |
Control |
Alcohol |
|||||
| Vehicle | Myriocin | Vehicle | Myriocin | Vehicle | Myriocin | Vehicle | Myriocin | |
| C14:0 | 1.94 ± 0.29 | 1.78 ± 0.17 | 2.28 ± 0.11 | 1.54 ± 0.16† | 0.96 ± 0.10‡ | 1.01 ± 0.07 | 2.26 ± 0.64* | 2.25 ± 0.28 |
| C16:0 | 36.51 ± 6.59 | 36.08 ± 4.04 | 33.03 ± 1.22 | 18.30 ± 1.81† | 14.21 ± 0.82‡ | 17.56 ± 2.40 | 27.90 ± 3.69* | 18.54 ± 2.12 |
| C18:0 | 12.23 ± 2.26 | 8.04 ± 1.47 | 15.42 ± 1.85 | 6.78 ± 0.83† | 4.05 ± 0.34‡ | 3.90 ± 0.47 | 8.48 ± 1.31* | 6.39 ± 0.66 |
| C18:1 | 1.14 ± 0.42 | 0.91 ± 0.18 | 1.40 ± 0.24 | 1.12 ± 0.10 | 0.23 ± 0.05 | 0.34 ± 0.02 | 0.57 ± 0.06* | 0.80 ± 0.05† |
| C20:0 | 64.56 ± 5.25 | 24.78 ± 4.40† | 39.34 ± 5.29* | 15.15 ± 2.70† | 37.35 ± 7.89‡ | 17.88 ± 1.32 | 38.29 ± 1.90 | 19.31 ± 0.58† |
| C20:1 | 4.21 ± 0.30 | 1.82 ± 0.33† | 1.37 ± 0.34* | 0.78 ± 0.12 | 1.65 ± 0.26‡ | 1.25 ± 0.15 | 2.43 ± 0.73 | 0.78 ± 0.21 |
| C22:0 | 65.11 ± 12.02 | 15.96 ± 0.73† | 40.32 ± 5.99 | 12.09 ± 0.73† | 46.10 ± 7.97 | 16.27 ± 4.85† | 48.96 ± 7.91 | 13.60 ± 1.41† |
| C22:1 | 20.64 ± 2.85 | 6.15 ± 0.58† | 9.61 ± 1.21* | 3.45 ± 0.40† | 14.82 ± 2.26 | 6.20 ± 1.74† | 12.50 ± 2.02 | 4.68 ± 0.24† |
| C24:0 | 55.46 ± 4.93 | 20.95 ± 1.24† | 91.45 ± 12.78* | 18.79 ± 1.77† | 34.59 ± 6.48‡ | 14.25 ± 2.03† | 87.66 ± 9.89* | 18.43 ± 2.55† |
| C24:1 | 115.67 ± 12.44 | 58.56 ± 5.35† | 93.06 ± 7.46 | 36.12 ± 2.07† | 59.49 ± 7.47‡ | 33.18 ± 4.13† | 83.40 ± 10.08 | 34.85 ± 4.63† |
| C26:0 | 0.60 ± 0.06 | 1.25 ± 0.67 | 1.66 ± 0.23* | 0.44 ± 0.09† | 0.57 ± 0.06 | 0.50 ± 0.08 | 1.47 ± 0.26* | 0.74 ± 0.32 |
| C26:1 | 0.45 ± 0.12 | 0.37 ± 0.10 | 0.79 ± 0.15 | 0.20 ± 0.07† | 0.29 ± 0.06 | 0.18 ± 0.03 | 0.59 ± 0.10* | 0.24 ± 0.12 |
| dhC16:0 | 0.86 ± 0.28 | 1.04 ± 0.09 | 1.45 ± 0.16 | 1.09 ± 0.11 | 0.47 ± 0.03 | 0.55 ± 0.06 | 1.05 ± 0.20* | 0.99 ± 0.14 |
| dhSph | 8.10 ± 1.10 | 4.83 ± 1.13 | 8.97 ± 1.84 | 2.55 ± 0.38† | 5.92 ± 1.07 | 3.52 ± 0.76 | 5.12 ± 1.82 | 2.19 ± 0.43 |
| dhSph-1P | 0.28 ± 0.06 | 0.09 ± 0.05† | 0.41 ± 0.09 | 0.05 ± 0.02† | 0.28 ± 0.05 | 0.08 ± 0.02† | 0.31 ± 0.12 | 0.02 ± 0.01 |
| Sph | 30.37 ± 3.01 | 23.89 ± 2.05 | 32.64 ± 3.39 | 15.86 ± 0.95† | 18.83 ± 1.19 | 16.07 ± 1.91 | 18.14 ± 2.75 | 14.00 ± 0.97 |
| Sph-1P | 2.86 ± 0.80 | 2.54 ± 0.59 | 4.91 ± 0.69 | 2.05 ± 0.51† | 2.25 ± 0.26 | 1.39 ± 0.13 | 3.73 ± 1.33 | 1.43 ± 0.25 |
Data are means ± SE. (n ≥ 4) Liver lipid extracts from vehicle- or myriocin-treated animals fed control or alcohol diet analyzed at harvest for liver ceramide levels by liquid chromatography/tandem mass spectrometry and normalized to protein content.
Alcohol-fed significantly different from control in vehicle-treated WT and adiponectin KO mice.
Myriocin-treated mice significantly different from vehicle-treated mice.
Control-fed adiponectin KO significantly different from control-fed WT mice.
Sph, sphingomyelin.
To investigate the functional impact of alcohol-dependent changes in liver ceramide levels on steatosis and hepatomegaly in adiponectin KO mice, animals were treated with the SPT inhibitor myriocin, which specifically inhibits de novo ceramide synthesis (38, 93, 95, 99). With the use of our standard feeding protocol, control- and alcohol-fed animals were treated with myriocin or vehicle control every other day during the 7 days of 34% EDC feeding. Surprisingly, we found that myriocin treatment was associated with elevated serum adiponectin levels in WT mice relative to vehicle-treated controls in both alcohol- and control-fed animals (Fig. 4B), whereas no significant differences were observed in the HMW adiponectin oligomer (Fig. 4E). Liver ceramide analysis showed reduced liver ceramide levels in myriocin-treated relative to vehicle-treated control in both WT and adiponectin KO mice regardless of diet (Fig. 4A). Interestingly, myriocin treatment inhibited the alcohol-mediated increase in total liver ceramides observed in adiponectin KO mice (Fig. 4A). This inhibition is driven primarily by suppression of alcohol-mediated increases in ceramide C24:0 and C24:1 and significant reductions in ceramides C20:0, C22:0, and C22:1 (Table 4).
Myriocin treatment also suppressed alcohol-dependent elevations in liver TGs in both WT and adiponectin KO mice relative to vehicle-treated controls (Fig. 4C). Additionally, myriocin treatment significantly reduced the alcohol-induced increase in hepatomegaly in adiponectin KO mice (Fig. 4D). Taken together, these data show that adiponectin KO mice are more susceptible to alcohol-mediated increases in liver ceramides. Furthermore, inhibiting the alcohol-mediated increase in liver ceramides with myriocin prevents increases in liver TG accumulation in adiponectin KO mice, suggesting that adiponectin suppresses alcohol-induced steatosis and hepatomegaly by inhibiting alcohol-mediated deregulation of liver ceramide levels.
Because adiponectin KO mice had alcohol-dependent increases in liver ceramide levels, we examined changes in the expression of enzymes known to regulate liver ceramide levels. AdipoR1/2 are thought to have intrinsic ceramidase activity (38) and are expressed at similar levels in WT and adiponectin KO mice (Fig. 4F). We found that alcohol treatment increased acid SMase (aSMase) protein levels similarly in WT and adiponectin KO mice (Fig. 4G). Interestingly, we found no change in aSMase transcript levels (Smpd1, Fig. 4H, a.) Significant elevations in one of neutral SMase2 transcripts (Smpd3) were the only observed alcohol-dependent change at the transcript level (Fig. 4H, a). Neutral SMase2 is thought to be the major neutral SMase that is involved in the response to cytokines and oxidative stress (20). However, these effects of alcohol treatment were observed in both WT and KO mice. No alcohol-dependent changes in SPT subunits (SPTLC1,2; Fig. 4H, b), ceramide synthases (CerS; Fig. 4H, c), or acid ceramidases (Asah 1/2; Fig. 4H, d) were observed. We also measured transcript levels of SPT, CerS, and SMase in alcohol-fed adiponectin KO mice implanted with pumps delivering either saline or adiponectin and found no difference in any transcripts (data not shown), suggesting that adiponectin does not regulate ceramide levels through transcriptional control of the expression of these proteins. Taken together, these data show no evidence of alcohol-dependent differences between WT and adiponectin KO mice in the expression of adipoRs, SMases, SPT, CerSs, or acid ceramidase levels, suggesting that alcohol-dependent changes in liver ceramide levels in the adiponectin KO mice may be attributable to posttranslational effects on protein activity.
DISCUSSION
The beneficial metabolic effects of adiponectin are well documented (98), and it has also been argued that it protects the liver from alcohol-mediated damage (5, 76). In particular, several authors found that supraphysiological adiponectin levels protect against alcohol-induced liver damage (83, 96). However, it remains unclear how ALD progresses in the absence of adiponectin and how adiponectin exerts its positive effects in the liver. To directly address these questions, we compared markers of ALD progression and interrogated adiponectin-dependent intracellular pathways in WT and adiponectin KO alcohol-fed mice. We show that adiponectin KO relative to WT mice had alcohol-dependent increases in hepatosteatosis, liver-to-body weight ratio, and liver TG accumulation. However, transcripts of inflammatory markers and serum ALT levels were similar, and tissue levels of TNF were reduced. This suggests that adiponectin KO mice are more susceptible to alcohol-mediated dysregulation in lipid handling, but, in the absence of enhanced alcohol-induced inflammation, this did not lead to increased liver damage. We show that restoring circulating total and HMW adiponectin with recombinant protein in adiponectin KO mice lowers liver TG and hepatomegaly in alcohol-fed mice, indicating that adiponectin is directly responsible for these effects. Additionally, our study shows that adiponectin KO mice but not WT mice have alcohol-mediated increases in liver ceramide levels and that pharmacologically reducing ceramide levels is associated with decreased liver TG and hepatomegaly. Together, these findings suggest that, under conditions tested, adiponectin does not directly protect against liver damage, but is a critical regulator of liver ceramide levels that contribute to alleviating alcohol-mediated steatosis and hepatomegaly.
For these experiments, mice were fed Reyes chocolate-flavored liquid diet to enhance alcohol intake. Although animals consumed high levels of alcohol, alcohol-dependent increases in MCP-1 and IL-6 were similar even in the absence of adiponectin, which is surprising given the literature suggesting that adiponectin is a suppressor of alcohol-induced inflammation (5, 60, 96). Although adiponectin does have reported proinflammatory effects under some conditions (26, 66, 77), the literature on the balance between pro- and anti-inflammatory effects of adiponectin is inconsistent (79). For instance, different independently derived strains of adiponectin KO mice have varying levels of TNF (49, 59) and susceptibility to inflammatory stimuli. Adiponectin KO mice generated by Matsuzawa and coworkers (59) were shown to be more susceptible to developing colitis than WT mice (64). By contrast, adiponectin KO mice derived by Chan and coworkers (58), which were used in this study, were protected from colitis (27). Furthermore, the Chan adiponectin KO mice tended to have lower serum TNF levels in response to LPS injection compared with WT animals (68). The underlying reasons for these different responses are poorly understood (100) but are consistent with results presented in this study. The complexity of the regulation of inflammation by adiponectin is further illustrated by our results showing that adiponectin treatment of alcohol-fed adiponectin KO mice reduced TNF and MCP-1 expression but enhanced IL-6 and SOCS3 transcript levels relative to controls. This result is consistent with literature showing that adiponectin treatment induces IL-6 in mice (8) and that adiponectin can suppress alcohol-stimulated macrophage TNF production (90).
The observed alcohol-dependent increase in serum adiponectin in WT mice after 1 wk of 34% EDC feeding adds to a growing literature demonstrating a complex relationship between alcohol intake and serum adiponectin levels. Interestingly, although an alcohol-dependent increase in total adiponectin levels was observed, no differences were detected between control- and alcohol-fed WT mice in HMW adiponectin, considered by some the most metabolically active form (35, 81, 94). The increase in total adiponectin in 34% EDC-fed mice may be due in part to the lack of an alcohol-dependent increase in serum TNF in our animals, as TNF can antagonize adiponectin production by adipocytes (55). In contrast to the increase in serum adiponectin after 1 wk of 34% EDC feeding, we did not observe significant changes in serum adiponectin after 5 wk of 27% EDC feeding. A possible explanation for this differential effect is that alcohol intake dynamically regulates adiponectin levels. Consistent with this idea, some authors have observed a transient increase in serum adiponectin in alcohol-fed mice (88), whereas others have demonstrated no change for the first 3 wk of 34% EDC diet feeding, after which adiponectin levels decrease (96). Another potential explanation is that increased alcohol intake observed in mice fed the 34% EDC diet contributed to increased adiponectin, as no change in adiponectin was observed at lower alcohol intake on the 27% EDC diet. This effect may be indirect, as increased alcohol intake was associated with weight loss in 34% EDC but not 27% EDC animals despite similar caloric intake. Adiponectin is upregulated during weight loss but is reduced under conditions of weight gain (7, 24). This is consistent with the proposal by Kim et al. (47) that adiponectin production serves as a “starvation signal” for adipocytes, with adiponectin production diminishing as adipocyte hypertrophy progresses.
Steatosis and liver damage.
Whereas alcohol-fed adiponectin KO mice had more steatosis, hepatomegaly, and TG accumulation relative to WT animals, serum ALT levels were similar, suggesting that steatosis and liver damage are separable phenomena. Although these phenotypes are often coincident, steatosis without increased liver damage has also been observed in alcohol-fed C5 KO mice and in mice harboring a hepatocyte-specific deletion of STAT3 (hsSTAT3 KO) (39, 70). C5 is a member of the complement membrane attack complex, the terminal effector of the complement cascade initiated by C1q, which in turn sequentially activates C3 and C5. Adiponectin has considerable homology to complement cascade initiator C1q (41) and can also activate C1q (67). Reduced activation of the complement cascade in the absence of adiponectin may contribute to the alcohol-dependent phenotypes in adiponectin KO mice. However, it is currently unclear mechanistically how complement activation contributes to hepatosteatosis development. Complicating the assessment of the role of the complement in the development of alcoholic liver disease is the observation that, whereas C5 KO mice have increased hepatosteatosis without increased liver damage, mice deficient in complement C3 are resistant to alcohol-mediated increases in liver TGs but are more susceptible to liver damage (70). Interestingly, an alcohol-dependent increase in serum adiponectin was observed in C3 KO but not WT mice (18), which could contribute to the reduced hepatosteatosis in these animals.
Alcohol-fed hsSTAT3 KO mice also show increased steatosis without increases in ALT relative to WT. STAT3 is a transcription factor driving production of many target genes including SOCS3, activated downstream of serum factors such as IL-6, IL-10, and leptin. Adiponectin has been shown to acutely activate hepatocyte STAT3 through an indirect mechanism involving stimulation of macrophage IL-6 production (8). It is interesting to note in this context that control-fed adiponectin KO relative to WT mice have reduced SOCS3 transcripts (Fig. 1E) and that treatment with adiponectin induces both IL-6 and SOCS3 (Fig. 2F). However, we did not observe any change in SREBP-1 DNA-binding activity in alcohol-fed WT or adiponectin KO mice (data not shown). This transcription factor was reported to mediate the increased steatosis in hsSTAT3 KO mice (39).
It is also interesting to note that reduced inflammation relative to WT mice was consistently observed in C5 and hs-STAT3, as in our adiponectin KO mice, in response to alcohol feeding (39, 70). Given the important contribution that inflammation plays in ALD progression, this raises the possibility that the similarity in phenotype is not attributable to adiponectin-dependent interactions with either the complement or STAT3 pathways but rather is attributable to abrogated inflammation.
Previous studies have demonstrated that elevating adiponectin above normal levels using either pharmacological or recombinant-protein treatments prevents both hepatosteatosis and liver damage (83, 96). Our study shows that alcohol-mediated liver damage is not enhanced in the absence of adiponectin and further that restoring adiponectin to physiological levels does not have an effect on liver damage. This suggests that elevating adiponectin above normal levels may have additional effects not observed under normal physiological conditions.
Adiponectin effectors: the role of ceramide.
Our data are consistent with studies demonstrating reductions in AMPK protein and PPAR-α DNA-binding activity in alcohol-fed animals (30, 103). However, similar reductions were observed in WT and adiponectin KO mice, suggesting that this effect is adiponectin-independent. This conclusion is further supported by the finding that a decline in AMPK protein level and PPAR-α DNA-binding activity was observed in WT mice, where an alcohol-dependent increase in circulating adiponectin was observed. AMPK phosphorylation was also similar between alcohol-fed WT and adiponectin KO mice, suggesting that AMPK phosphorylation is also adiponectin independent in our animal model. These observations suggest that, in our system, enhanced alcohol-dependent hepatosteatosis and hepatomegaly in adiponectin KO mice are independent of AMPK. By contrast, our data suggest that adiponectin is required for AMPK phosphorylation in pair-fed control-fed animals. It is possible that alcohol treatment adds additional energetic stress to the livers of alcohol-fed adiponectin KO mice, activating AMPK in the absence of adiponectin. Importantly, our data suggest that neither AMPK nor PPAR-α dysregulation contributes to the increased steatosis observed in alcohol-fed adiponectin KO mice.
Because AMPK and PPAR-α likely did not contribute to alcohol-mediated increases in steatosis in adiponectin KO mice, alcohol-dependent changes in ceramide levels were investigated as mediators of adiponectin action. Consistent with a role for adiponectin-dependent stimulation of ceramidase activity (38), we found that adiponectin KO mice were more susceptible to an alcohol-mediated increase in liver ceramides. Reductions in TG and hepatomegaly in adiponectin KO mice following pharmacological reduction of ceramide levels suggest that an elevation in ceramides contributes to hepatosteatosis development in these animals. In contrast to other studies, we observed no change in total ceramide levels in alcohol-fed WT mice relative to control (52, 82). This difference may be related to the observed alcohol-mediated increase in serum adiponectin in WT mice, which can counter ceramide increases through activation of ceramidase activity.
Our data showing that treatment with the SPT inhibitor myriocin suppressed the alcohol-dependent increase in total liver ceramides in adiponectin KO mice suggest that alcohol increases liver ceramides at least in part by stimulating de novo ceramide synthesis. The observation that alcohol-dependent increases in liver ceramide levels were not observed in WT animals further suggests that this increased flux through the de novo synthesis pathway is countered by adiponectin. Our data show no difference between WT and adiponectin KO mice in levels of proteins involved in ceramide synthesis and degradation pathways, consistent with the interpretation that this effect is posttranslationally mediated. This opens the possibility that adiponectin-dependent ceramidase activity is responsible for this effect. Supakul and Liangpunsakul have suggested that acid SMase plays a role in ceramide elevation in alcohol-fed animals (87). Our data also demonstrate that alcohol increases acid SMase protein levels. However, treatment of alcohol-fed animals with the acid SMase inhibitor imipramine only partially inhibited the alcohol-mediated increase in liver ceramide levels (52), suggesting that alcohol contributes to the elevation of ceramide levels through additional mechanisms. Myriocin treatment of control-fed WT mice also led to significant reductions in liver ceramides, which suggests that de novo ceramide synthesis is an important determinant of steady-state ceramide levels. Together, our data are consistent with the conclusion that de novo synthesis is the predominant contributor to alcohol-dependent accumulation of ceramides in the liver and that this effect is countered by adiponectin-dependent activation of ceramidase activity.
Contrary to expectations, we noted that total ceramide levels were reduced in control-fed adiponectin KO mice compared with WT. Reduced liver ceramidase activity in the absence of adiponectin would be expected to contribute to higher liver ceramide levels in adiponectin KO mice. This unexpected finding may be related to reduced TNF levels observed in adiponectin KO mice, as TNF increases liver ceramide production through stimulation of aSMase (28), as well as through an inverse agonist effect of TNF on adipoR1-dependent ceramidase activity (50). Consistent with a role for TNF in controlling steady-state ceramide levels, ob/ob mice, a genetic model of insulin resistance, have elevated ceramide levels relative to WT, whereas ob/ob mice lacking TNF receptors have normal ceramide levels, suggesting that TNF is an important determinant of ceramide levels (99). On the basis of this evidence, we consider it likely that the observed differences in ceramide levels between control-fed WT and adiponectin KO mice are homeostatic and related to reduced TNF in the adiponectin KO mice. In further support for the hypothesis that the difference is homeostatic, adiponectin KO, but not WT mice, are susceptible to alcohol-mediated increases in liver ceramides, which suggests that ceramidase activity is in fact reduced in the absence of adiponectin.
When we examined individual ceramide species, we found that alcohol-fed WT mice had increases in only C24:0 and C26:0 ceramides, which were also seen in adiponectin KO mice. Increased C24:0 was also observed by Liangpunsakul et al. after alcohol feeding (52). Ceramide synthesis is thought to be controlled in part by substrate availability (13), which opens the possibility that hepatocytes in alcohol-fed animals accumulate C24:0 fatty acids. Accumulation of very-long-chain fatty acids may be indicative of peroxisome dysfunction (45), as peroxisomes are critical for oxidation of these lipids. This would also be consistent with the observation from our study and others (29) showing decreased PPAR-α activation, as PPAR-α is an important driver of peroxisome proliferation (25). Additionally, there may be alcohol-dependent increases in ceramide synthase CerS2, which is highly expressed in the liver and principally involved in the synthesis of C24:0 and C26:0 (51), both of which were significantly elevated in alcohol-fed animals in our study. However, no difference in CerS2 mRNA was observed in our analyses (Fig. 4H, c).
In control-fed WT mice, myriocin treatment only reduced ceramides of chain length from C20:0-C24:1. By contrast, alcohol-fed WT mice had significant myriocin-dependent reductions in all ceramide species examined although changes in C18:1 and C20:1 did not reach significance. This suggests that alcohol-fed WT animals are more sensitive to the effects of myriocin. This may be due to increased adiponectin levels in alcohol-fed, myriocin-treated animals, which are elevated almost 2.5-fold over vehicle-treated control-fed animals. In support of the idea that increased adiponectin is required for enhanced myriocin effects in alcohol-fed WT mice, no difference in myriocin sensitivity was observed between control- and alcohol-fed adiponectin KO animals.
Our study of individual ceramide species also demonstrates that, when alcohol-dependent ceramide elevations were observed in WT mice, there was a larger increase in adiponectin KO mice; however, when no alcohol-dependent change was found in WT mice, there was an increase in adiponectin KO mice; when an alcohol-dependent decrease was observed in WT mice, no change was observed in adiponectin KO mice. This suggests that adiponectin KO mice had uniform increases in all ceramide species examined relative to WT, irrespective of saturation or fatty acid chain length. This observation is consistent with the idea that adiponectin-dependent ceramidase activity is not specific for fatty acid chain length or saturation. This is also supported by our observation that increased adiponectin observed in myriocin-treated alcohol-fed WT mice was associated with reductions in nearly all ceramide species examined.
Systemic myriocin administration had profound effects on liver ceramides but may also affect other organ systems in ways that impact hepatosteatosis. In clinical studies, elevated adipose tissue ceramide levels were positively correlated with hepatosteatosis severity, suggesting that liver-adipose crosstalk is an important determinant of hepatosteatosis (48). Our data show profound myriocin-dependent effects on adiponectin levels, suggesting that adiponectin production by adipose tissue was directly affected by myriocin treatment and may contribute to the observed decrease in hepatosteatosis. Interestingly, our observation that myriocin treatment is associated with increased serum adiponectin is, to our knowledge, the first suggestion that adipose tissue ceramide levels can influence adiponectin production/secretion.
Summary.
Taken together, our data show that adiponectin is an important regulator of lipid homeostasis in alcohol-fed mice but does not protect against liver damage in the absence of alcohol-induced inflammation. Our data also indicate that alcohol promotes hepatosteatosis through stimulation of de novo ceramide synthesis and that adiponectin opposes this effect, possibly through activation of ceramidase activity. Our study leaves open the question of whether increased hepatosteatosis in alcohol-fed adiponectin KO mice renders them more susceptible to further insult, and future studies utilizing alcohol-feeding protocols known to induce inflammation will be employed to address this question.
GRANTS
The study was supported by NIH grants R01 AA018873, R01 AA008714, and K05 AA017261 to J. Hoek. J. Correnti and E. Juskeviciute were supported by institutional training grant T32 AA007463. Research was supported in part by the Lipidomics Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313), and the Lipidomics Core in the SC Lipidomics and Pathobiology COBRE, Department Biochemistry, MUSC (P20 RR017677).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.C., E.J., and A.S. performed experiments; J.M.C., E.J., and A.S. analyzed data; J.M.C. and J.B.H. interpreted results of experiments; J.M.C. prepared figures; J.M.C. drafted manuscript; J.M.C. and J.B.H. edited and revised manuscript; J.M.C. and J.B.H. approved final version of manuscript; J.B.H. was responsible for conception and design of research.
ACKNOWLEDGMENTS
The authors acknowledge Amanda Riccio, Rhonda Walters, and Zhijiu Zhong for experimental support and Drs. Sara Crumm, Rotonya Carr, Linda Greenbaum, Bruce Fenderson, and Ashlie Burkart for expert technical advice. We thank Dr. Russell A. Miller and Dr. Morris J. Birnbaum of the University of Pennsylvania for the adiponectin-producing cells and Dr. Lawrence C. B. Chan of Baylor College of Medicine for the adiponectin KO mice.
REFERENCES
- 1.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology 20: 453–460, 1994 [PubMed] [Google Scholar]
- 2.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 108: 218–224, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 295: G833–G842, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol 8: 491–501, 2011 [DOI] [PubMed] [Google Scholar]
- 5.An L, Wang X, Cederbaum AI. Cytokines in alcoholic liver disease. Arch Toxicol 86: 1337–1348, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol 17, Suppl: S186–S190, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. 1999. Biochem Biophys Res Commun 425: 560–564, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, Kaneko K, Kobayashi M, Iwane A, Sasako T, Okazaki Y, Ohsugi M, Takamoto I, Yamashita S, Asahara H, Akira S, Kasuga M, Kadowaki T. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab 13: 401–412, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Baraona E, Leo MA, Borowsky SA, Lieber CS. Alcoholic hepatomegaly: accumulation of protein in the liver. Science 190: 794–795, 1975 [DOI] [PubMed] [Google Scholar]
- 10.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, Adams LA, Charatcharoenwitthaya P, Topping JH, Bugianesi E, Day CP, George J. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 54: 1208–1216, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A. Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Adv Exp Med Biol 688: 46–59, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest 121: 4222–4230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bikman BT, Summers SA. Sphingolipids and hepatic steatosis. Adv Exp Med Biol 721: 87–97, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 114: 147–152, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis 21: 3–16, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Buechler C, Schaffler A, Johann M, Neumeier M, Kohl P, Weiss T, Wodarz N, Kiefer P, Hellerbrand C. Elevated adiponectin serum levels in patients with chronic alcohol abuse rapidly decline during alcohol withdrawal. J Gastroenterol Hepatol 24: 558–563, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Bykov I, Jauhiainen M, Olkkonen VM, Saarikoski ST, Ehnholm C, Junnikkala S, Vakeva A, Lindros KO, Meri S. Hepatic gene expression and lipid parameters in complement C3(−/−) mice that do not develop ethanol-induced steatosis. J Hepatol 46: 907–914, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Carr RM, Dhir R, Yin X, Agarwal B, Ahima RS. Temporal effects of ethanol consumption on energy homeostasis, hepatic steatosis, and insulin sensitivity in mice. Alcohol Clin Exp Res 37: 1091–1099, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry 45: 11247–11256, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Clouston AD, Powell EE. Interaction of non-alcoholic fatty liver disease with other liver diseases. Best Pract Res Clin Gastroenterol 16: 767–781, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Crabb DW, Galli A, Fischer M, You M. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha. Alcohol 34: 35–38, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Crumm S, Cofan M, Juskeviciute E, Hoek JB. Adenine nucleotide changes in the remnant liver: An early signal for regeneration after partial hepatectomy. Hepatology 48: 898–908, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delporte ML, Brichard SM, Hermans MP, Beguin C, Lambert M. Hyperadiponectinaemia in anorexia nervosa. Clin Endocrinol (Oxf) 58: 22–29, 2003 [DOI] [PubMed] [Google Scholar]
- 25.DeLuca JG, Doebber TW, Kelly LJ, Kemp RK, Molon-Noblot S, Sahoo SP, Ventre J, Wu MS, Peters JM, Gonzalez FJ, Moller DE. Evidence for peroxisome proliferator-activated receptor (PPAR)alpha-independent peroxisome proliferation: effects of PPARgamma/delta-specific agonists in PPARalpha-null mice. Mol Pharmacol 58: 470–476, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Ehling A, Schaffler A, Herfarth H, Tarner IH, Anders S, Distler O, Paul G, Distler J, Gay S, Scholmerich J, Neumann E, Muller-Ladner U. The potential of adiponectin in driving arthritis. J Immunol 176: 4468–4478, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Fayad R, Pini M, Sennello JA, Cabay RJ, Chan L, Xu A, Fantuzzi G. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology 132: 601–614, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Checa JC, Colell A, Mari M, Garcia-Ruiz C. Ceramide, tumor necrosis factor and alcohol-induced liver disease. Alcohol Clin Exp Res 29, Suppl 11: 151S–157S, 2005 [PubMed] [Google Scholar]
- 29.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem 278: 27997–28004, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Galli A, Pinaire J, Fischer M, Dorris R, Crabb DW. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem 276: 68–75, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572–1585, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Villafranca J, Guillen A, Castro J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie 90: 460–466, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Grosch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res 51: 50–62, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Hackney JF, Engelman RW, Good RA. Ethanol calories do not enhance breast cancer in isocalorically fed C3H/Ou mice. Nutr Cancer 18: 245–253, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Hada Y, Yamauchi T, Waki H, Tsuchida A, Hara K, Yago H, Miyazaki O, Ebinuma H, Kadowaki T. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem Biophys Res Commun 356: 487–493, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison SA, Diehl AM. Fat and the liver–a molecular overview. Semin Gastrointest Dis 13: 3–16, 2002 [PubMed] [Google Scholar]
- 38.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17: 55–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, Osei-Hyiaman D, Moh A, Fu XY, Pacher P, Kunos G, Gao B. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology 134: 1148–1158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howarth DL, Passeri M, Sadler KC. Drinks like a fish: using zebrafish to understand alcoholic liver disease. Alcohol Clin Exp Res 35: 826–829, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271: 10697–10703, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Israel Y, Orrego H, Colman JC, Britton RS. Alcohol-induced hepatomegaly: pathogenesis and role in the production of portal hypertension. Fed Proc 41: 2472–2477, 1982 [PubMed] [Google Scholar]
- 43.Joosten MM, van Erk MJ, Pellis L, Witkamp RF, Hendriks HF. Moderate alcohol consumption alters both leucocyte gene expression profiles and circulating proteins related to immune response and lipid metabolism in men. Br J Nutr 108: 620–627, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Kawamoto R, Tabara Y, Kohara K, Miki T, Ohtsuka N, Kusunoki T, Abe M. Alcohol drinking status is associated with serum high molecular weight adiponectin in community-dwelling Japanese men. J Atheroscler Thromb 17: 953–962, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Kemp S, Berger J, Aubourg P. X-linked adrenoleukodystrophy: clinical, metabolic, genetic and pathophysiological aspects. Biochim Biophys Acta 1822: 1465–1474, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Kentish SJ, O'Donnell TA, Frisby CL, Li H, Wittert GA, Page AJ. Altered gastric vagal mechanosensitivity in diet-induced obesity persists on return to normal chow and is accompanied by increased food intake. Int J Obes (Lond). In press [DOI] [PubMed] [Google Scholar]
- 47.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J, Rissanen A, Hakkinen AM, Lindell M, Bergholm R, Hamsten A, Eriksson P, Fisher RM, Oresic M, Yki-Jarvinen H. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 56: 1960–1968, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277: 25863–25866, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Kupchak BR, Garitaonandia I, Villa NY, Smith JL, Lyons TJ. Antagonism of human adiponectin receptors and their membrane progesterone receptor paralogs by TNFalpha and a ceramidase inhibitor. Biochemistry 48: 5504–5506, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laviad EL, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH, Jr, Futerman AH. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem 283: 5677–5684, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Liangpunsakul S, Rahmini Y, Ross RA, Zhao Z, Xu Y, Crabb DW. Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice. Am J Physiol Gastrointest Liver Physiol 302: G515–G523, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liangpunsakul S, Sozio MS, Shin E, Zhao Z, Xu Y, Ross RA, Zeng Y, Crabb DW. Inhibitory effect of ethanol on AMPK phosphorylation is mediated in part through elevated ceramide levels. Am J Physiol Gastrointest Liver Physiol 298: G1004–G1012, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24: 197–211, 1989 [PubMed] [Google Scholar]
- 55.Lim JY, Kim WH, Park SI. GO6976 prevents TNF-alpha-induced suppression of adiponectin expression in 3T3–L1 adipocytes: putative involvement of protein kinase C. FEBS Lett 582: 3473–3478, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Lizarazo D, Zabala V, Tong M, Longato L, de la Monte SM. Ceramide inhibitor myriocin restores insulin/insulin growth factor signaling for liver remodeling in experimental alcohol-related steatohepatitis. J Gastroenterol Hepatol 28: 1660–1668, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, Paul A, Chan L. Increased beta -oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem 277: 34658–34661, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8: 731–737, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Mandal P, Roychowdhury S, Park PH, Pratt BT, Roger T, Nagy LE. Adiponectin and heme oxygenase-1 suppress TLR4/MyD88-independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. J Immunol 185: 4928–4937, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morgan MY. The treatment of alcoholic hepatitis. Alcohol Alcohol 31: 117–134, 1996 [DOI] [PubMed] [Google Scholar]
- 62.Moriya T, Naito H, Ito Y, Nakajima T. “Hypothesis of seven balances”: molecular mechanisms behind alcoholic liver diseases and association with PPARalpha. J Occup Health 51: 391–403, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Nanji AA, Dannenberg AJ, Jokelainen K, Bass NM. Alcoholic liver injury in the rat is associated with reduced expression of peroxisome proliferator-alpha (PPARalpha)-regulated genes and is ameliorated by PPARalpha activation. J Pharmacol Exp Ther 310: 417–424, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Nishihara T, Matsuda M, Araki H, Oshima K, Kihara S, Funahashi T, Shimomura I. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology 131: 853–861, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Orrego H, Blendis LM, Crossley IR, Medline A, Macdonald A, Ritchie S, Israel Y. Correlation of intrahepatic pressure with collagen in the Disse space and hepatomegaly in humans and in the rat. Gastroenterology 80: 546–556, 1981 [PubMed] [Google Scholar]
- 66.Park PH, McMullen MR, Huang H, Thakur V, Nagy LE. Short-term treatment of RAW264.7 macrophages with adiponectin increases tumor necrosis factor-alpha (TNF-alpha) expression via ERK1/2 activation and Egr-1 expression: role of TNF-alpha in adiponectin-stimulated interleukin-10 production. J Biol Chem 282: 21695–21703, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peake PW, Shen Y, Walther A, Charlesworth JA. Adiponectin binds C1q and activates the classical pathway of complement. Biochem Biophys Res Commun 367: 560–565, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Pini M, Sennello JA, Chan L, Fantuzzi G. Adiponectin deficiency does not affect the inflammatory response to endotoxin or concanavalin a in mice. Endocrinology 147: 5019–5022, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Pohorecky LA. Animal analog of alcohol dependence. Fed Proc 40: 2056–2064, 1981 [PubMed] [Google Scholar]
- 70.Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, Medof ME, Nagy LE. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology 132: 1117–1126, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramirez T, Longato L, Dostalek M, Tong M, Wands JR, de la Monte SM. Insulin resistance, ceramide accumulation and endoplasmic reticulum stress in experimental chronic alcohol-induced steatohepatitis. Alcohol Alcohol 48: 39–52, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 59: 160–168, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Reyes E, Garcia KD, Jones BC. Effects of the maternal consumption of alcohol on alcohol selection in rats. Alcohol 2: 323–326, 1985 [DOI] [PubMed] [Google Scholar]
- 74.Reyes E, Murray RW. Gamma glutamyl transpeptidase as a sensitive marker of fetal alcohol exposure. Neurobehav Toxicol Teratol 7: 177–180, 1985 [PubMed] [Google Scholar]
- 75.Reyes E, Wolfe J, Savage DD. The effects of prenatal alcohol exposure on radial arm maze performance in adult rats. Physiol Behav 46: 45–48, 1989 [DOI] [PubMed] [Google Scholar]
- 76.Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life 60: 790–797, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Rovin BH, Song H. Chemokine induction by the adipocyte-derived cytokine adiponectin. Clin Immunol 120: 99–105, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Ruvolo PP. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res 47: 383–392, 2003 [DOI] [PubMed] [Google Scholar]
- 79.Saxena A, Fletcher E, Larsen B, Baliga MS, Durstine JL, Fayad R. Effect of exercise on chemically-induced colitis in adiponectin deficient mice. J Inflamm 9: 30, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwartz JM, Reinus JF. Prevalence and natural history of alcoholic liver disease. Clin Liver Dis 16: 659–666, 2012 [DOI] [PubMed] [Google Scholar]
- 81.Seino Y, Hirose H, Saito I, Itoh H. High molecular weight multimer form of adiponectin as a useful marker to evaluate insulin resistance and metabolic syndrome in Japanese men. Metabolism 56: 1493–1499, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Setshedi M, Longato L, Petersen DR, Ronis M, Chen WC, Wands JR, de la Monte SM. Limited therapeutic effect of N-acetylcysteine on hepatic insulin resistance in an experimental model of alcohol-induced steatohepatitis. Alcohol Clin Exp Res 35: 2139–2151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 298: G364–G374, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek EW, Scarpace PJ, Zolotukhin S. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci USA 100: 14217–14222, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song Z, Zhou Z, Deaciuc I, Chen T, McClain CJ. Inhibition of adiponectin production by homocysteine: a potential mechanism for alcoholic liver disease. Hepatology 47: 867–879, 2008 [DOI] [PubMed] [Google Scholar]
- 86.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 45: 42–72, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Supakul R, Liangpunsakul S. Alcoholic-induced hepatic steatosis—role of ceramide and protein phosphatase 2A. Transl Res 158: 77–81, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tan X, Sun X, Li Q, Zhao Y, Zhong W, Sun X, Jia W, McClain CJ, Zhou Z. Leptin deficiency contributes to the pathogenesis of alcoholic fatty liver disease in mice. Am J Pathol 181: 1279–1286, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 346: 987–990, 1995 [DOI] [PubMed] [Google Scholar]
- 90.Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-α production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol 290: G998–G1007, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tomita K, Tamiya G, Ando S, Kitamura N, Koizumi H, Kato S, Horie Y, Kaneko T, Azuma T, Nagata H, Ishii H, Hibi T. AICAR, an AMPK activator, has protective effects on alcohol-induced fatty liver in rats. Alcohol Clin Exp Res 29, Suppl 12: 240S–245S, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia 55: 2319–2326, 2012 [DOI] [PubMed] [Google Scholar]
- 93.Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM, Lopaschuk GD. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59: 2453–2464, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, Xu A, Knight C, Xu LY, Cooper GJ. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem 277: 19521–19529, 2002 [DOI] [PubMed] [Google Scholar]
- 95.Watt MJ, Barnett AC, Bruce CR, Schenk S, Horowitz JF, Hoy AJ. Regulation of plasma ceramide levels with fatty acid oversupply: evidence that the liver detects and secretes de novo synthesised ceramide. Diabetologia 55: 2741–2746, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112: 91–100, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu J, Lai KK, Verlinsky A, Lugea A, French SW, Cooper MP, Ji C, Tsukamoto H. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J Hepatol 55: 673–682, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab 17: 185–196, 2013 [DOI] [PubMed] [Google Scholar]
- 99.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab 297: E211–E224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab 2: 133–141, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology 117: 942–952, 1999 [DOI] [PubMed] [Google Scholar]
- 102.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 42: 568–577, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 127: 1798–1808, 2004 [DOI] [PubMed] [Google Scholar]
- 104.You M, Rogers CQ. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood) 234: 850–859, 2009 [DOI] [PubMed] [Google Scholar]
- 105.Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol 166: 1681–1690, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]