Abstract
The intestinal epithelium forms a selective barrier maintained by tight junctions (TJs) and separating the luminal environment from the submucosal tissues. N-acylhomoserine lactone (AHL) quorum-sensing molecules produced by gram-negative bacteria in the gut can influence homeostasis of the host intestinal epithelium. In the present study, we evaluated the regulatory mechanisms affecting the impact of two representative long- and short-chain AHLs, N-3-(oxododecanoyl)-homoserine lactone (C12-HSL) and N-butyryl homoserine lactone (C4-HSL), on barrier function of human intestinal epithelial Caco-2 cells. Treatment with C12-HSL, but not with C4-HSL, perturbed Caco-2 barrier function; the effect was associated with decreased levels of the TJ proteins occludin and tricellulin and their delocalization from the TJs. C12-HSL also induced matrix metalloprotease (MMP)-2 and MMP-3 activation via lipid raft- and protease-activated receptor (PAR)-dependent signaling. Pretreatment with lipid raft disruptors, PAR antagonists, or MMP inhibitors restored the C12-HSL-induced loss of the TJ proteins and increased permeability of Caco-2 cell monolayers. These results indicate that PAR/lipid raft-dependent MMP-2 and -3 activation followed by degradation of occludin and tricellulin are involved in C12-HSL-induced alterations of epithelial paracellular barrier functions.
Keywords: N-acylhomoserine lactone, matrix metalloprotease, occludin, tricellulin, intestinal epithelium
the intestinal epithelium functions as a physical and metabolic barrier for selective uptake of nutrients and water, while protecting against permeation of luminal noxious contents, such as bacteria, antigens, toxins, and pathogens, into the mucosal tissues and circulatory system (38, 56). A defective intestinal epithelial barrier, characterized by an increase in intestinal permeability, allows the penetration of bacteria and bacterial products and results in the activation of mucosal immune system, leading to inflammation and tissue damage (16). This notion is supported by emerging clinical studies that have shown increased intestinal permeability in close relatives of patients with inflammatory intestinal diseases such as inflammatory bowel disease, celiac disease, and food allergies (9, 23, 27, 49, 60, 69). These findings suggest that perturbation of intestinal epithelial barrier function precedes and predicts a potential development of intestinal and systemic diseases (11).
The intestinal barrier is achieved by the formation of tight junctions (TJs) between adjacent cells, which encircle the apical ends of the lateral membranes of epithelial cells (2, 56). TJs are multiprotein complexes composed of integral transmembrane proteins and cytoplasmic scaffolding proteins in association with a variety of regulatory proteins (56, 58, 59). TJs regulate the selective paracellular permeability of epithelial cell layers by functioning both as a barrier against noxious molecules and providing a pore for ions, solutes, and water (1, 58, 61). Of the TJ components, four families of integral transmembrane proteins, occludin, tricellulin, claudins, and junctional adhesion molecules (JAMs), directly form intercellular homophilic and heterophilic interactions to seal the space between cells (1, 19, 24, 67). TJs of intestinal epithelium are highly dynamic structures. Their integrity and permeability can be altered by a variety of exogenous stimuli, including cytokines, toxins, immune cells, and pathogenic bacteria (7, 11). For example, the disruption of TJs during pathogenic bacterial infection results in gastrointestinal barrier failure, which subsequently facilitates the translocation of bacteria and luminal noxious molecules across the damaged epithelial layers and further promotes barrier disruption and disease development (31, 47, 54, 68).
Quorum sensing (QS) is a cell-density-dependent mechanism that bacteria use to communicate with each other using small diffusible signal molecules, termed QS molecules. They modulate bacterial cooperative activities and pathophysiological processes, including virulence factor production, host colonization, and biofilm formation (3, 13). Among QS molecules, N-acyl homoserine lactones (AHLs) are commonly produced by a wide variety of gram-negative bacteria and have been reported for their potential effects on host cell homeostasis (12, 13). For example, N-(3-oxododecanoyl)-l-homoserine lactone (C12-HSL) and N-butyryl-l-homoserine lactone (C4-HSL) were shown to induce proinflammatory cytokine expression, apoptosis, cell migration, and barrier disruption in several types of cells (29, 42, 52, 55, 63, 64, 72). Although an increasing body of evidence has indicated that some of AHLs can modulate epithelial homeostasis (63, 64), much less is known about underlying mechanisms by which QS molecules induce the disruption of intestinal epithelial TJs.
In the present study, we defined the molecular mechanisms of C12-HSL-induced paracellular permeability across intestinal epithelial Caco-2 cells that were associated with the diminished expression and dislocation of the TJ proteins occludin and tricellulin. We demonstrated that the C12-HSL-stimulated loss of TJ proteins was mediated by matrix metalloprotease (MMP)-2 and MMP-3 activation via a lipid raft- and protease-activated receptor (PAR)-dependent mechanism. Thus our data indicate that MMP-2 and MMP-3 activation plays the critical role in C12-HSL-induced perturbation of TJ integrity and barrier function in human intestinal epithelial cells.
MATERIALS AND METHODS
Materials.
C12-HSL and C4-HSL were purchased from Sigma-Aldrich (St. Louis, MO) and their stock solutions (100 mM) were prepared in dimethyl sulfoxide (DMSO). ARP100 (MMP-2 inhibitor III), N-isobutyl-N-(4-methoxyphenylsulfonyl)-glycylhydroxamic acid (NNGH, MMP-3 inhibitor II) and GM1489 were obtained from Calbiochem (La Jolla, CA). Methyl-β-cyclodextrin and filipin III were purchased from Sigma-Aldrich. The PAR antagonists FR 171113, RWJ 56110, and FSLLRY-NH2 were purchased from Tocris Bioscience (Bristol, UK). Rat tail collagen type I was obtained from BD Biosciences (Bedford, MA). Antibodies against occludin and tricellulin were purchased from Life Technologies (Grand Island, NY). Anti-GAPDH antibody, anti-actin antibody, and horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture and treatment factors.
Human intestinal adenocarcinoma Caco-2 cells (American Type Culture Collection) were cultured in DMEM medium (Life Technologies) supplemented with GlutaMAX, 10% fetal bovine serum, 100 units/ml penicillin G, 100 μg/ml of streptomycin, and 1% nonessential amino acids (all from Life Technologies) at 37°C in 5% CO2. Culture medium was changed three times per week and cells were subcultured weekly upon reaching 80% confluence. For experiments, confluent cells were grown for 2–3 wk and then incubated with phenol red and serum-free DMEM medium for 12 h prior to treatment.
C12-HSL concentrations can reach up to 300–600 μM in the bacterial biofilm produced by Pseudomonas aeruginosa culture in flow cells (10). Therefore, cells were treated with 10–400 μM concentration range of C12-HSL or C4-HSL. In selective experiments, Caco-2 cells were pretreated for 0.5–1 h with pharmacological inhibitors, followed by exposure to C12-HSL in the presence of the inhibitors. Treatment with C12-HSL or pharmacological inhibitors did not show cytotoxic effects over the time course of the experiments as determined by the MTT conversion assay (data not shown).
Permeability assay.
Cells were seeded on collagen type I-coated Transwell polyester filters (12-mm diameter, 0.4 μm pore size, Corning Costar) and cultured for 2–3 wk to obtain intestinal epithelial properties (34, 48). Treatment factors were added both to the lower and the upper compartments of the Transwell system. Then 0.5 ml of FITC-dextran 20 (FD-20, 1 mg/ml in KRG solution) was loaded into the upper chamber for 1 h. Fluorescence of FD-20 in 0.1-ml aliquots from the lower chamber was determined with a microplate spectrofluorometer (SPECTRAMax Gemini EM, Molecular Devices) using 483 nm as excitation and 517 nm as emission wavelengths. Relative permeability was expressed by the ratio of FD-20 transported into the lower chamber compared with untreated or vehicle control group. All assays were performed at least in quadruplicate.
Immunoblotting.
Treated cells were washed with cold PBS twice and lysed with RIPA lysis buffer [1.0% Nonidet P-40, 0.5% deoxycholic acid, 0.2% SDS, 40 mM Tris·HCl (pH 7.6), 1 mM EDTA, 1 mM EGTA, 10 mM MgCl2, 150 mM NaCl, 1 mM Na3VO4, 1 mM NaF, 1 × EDTA-free protease inhibitor cocktail (Roche Applied Science), and 1 mM phenylmethylsulfonyl fluoride] for the total cell extract. Membrane proteins were isolated by using BioVision plasma membrane protein extraction kit according to the manufacturer's manual. The extracted proteins where then dissolved with RIPA lysis buffer containing 0.5% SDS. The lysates were sonicated on ice by three 5-s bursts with 10-s intervals and centrifuged at 15,000 g for 0.5 h at 4°C. Protein concentration was determined with a BCA protein assay kit (Thermo Scientific, Rockford, IL). Then 10 μg protein of cell lysates were electrophoresed on SDS-polyacrylamide gels, transferred to a polyvinylidene fluoride membrane, blocked with 3% BSA in PBS-0.1% Tween-20 solution, and incubated with the primary antibodies overnight at 4°C. After incubation with the secondary antibody for 2 h, immunoblots were visualized by using the ECL detection system (Amersham Biosciences). GAPDH or actin was determined as the loading control. The band density was measured with Image J software (NIH).
MMP activity assay.
Following treatment, the conditioned media were collected and stored at −70°C. Changes in total (active and pro-) levels of MMP-2 and MMP-3 activities were determined by using the AnaSpec SensoLyte 520 MMP-2 Assay Kit and the AnaSpec SensoLyte 520 MMP-3 Assay Kit (AnaSpec, San Jose, CA), according to the manufacturer's instructions. Pro-MMP-2 and -3 were activated with 1 mM p-aminophenylmercuric acetate. Both kits use a 5-FAM/QXL520 fluorescence resonance energy transfer (FRET) peptide as an MMP substrate. In the intact FRET peptide, the fluorescence of 5-FAM is quenched by QXL520. Upon cleavage into separate fragments by the respective MMPs, fluorescence intensity was measured at 490/520 nm with a microplate spectrofluorometer (SPECTRAMax Gemini EM).
MMP activation was also determined by zymographic analysis. Briefly, aliquots of 400 μl of the conditioned media were concentrated by use of a Nanosep centrifugal device (10-kDa cutoff, Pall Life Sciences, Ann Arbor, MI) and mixed with 2 × nonreducing zymogram sample buffer (Bio-Rad Laboratories). Zymographic analysis was performed by electrophoresis on 10% SDS-polyacrylamide gel containing gelatin for MMP-2 or 12% SDS-polyacrylamide gel containing casein for MMP-3. After electrophoresis, SDS was removed by incubating the gel twice with 1 × zymogram renaturation buffer and MMP activity was revealed by 3-day incubation at 37°C with zymogram development buffer (both from Bio-Rad), followed by staining with 0.1% Coomassie Brilliant Blue solution.
Immunofluorescence.
Cells grown on collagen type I-coated culture slides (Biocoat, BD Biosciences, Bedford, MA) were fixed with 4% paraformaldehyde for 20 min and permeabilized with 0.2% Triton X-100 in PBS for 5 min. Nonspecific binding was prevented by incubation with 1% BSA in PBS for 1 h. The cells were incubated overnight with anti-occludin or anti-tricellulin antibodies diluted at 1:100 in blocking solution (1% BSA in PBS) in a wet chamber and then incubated with Alexa 647-conjugated secondary antibodies (1:200 dilution in blocking solution) for 1 h. The slides were mounted with ProLong Gold Antifade reagent (Life Technologies). The images were captured by a confocal microscope (Olympus Fluoview V5, Olympus America, Melville, NY) using constant image-acquisition settings.
Statistical analysis.
Data were statistically analyzed by one-way ANOVA, followed by Student's t-test. Statistical probability of P < 0.05 was considered significant. Results are expressed as means ± SD.
RESULTS
C12-HSL, but not C4-HSL, increases epithelial paracellular permeability.
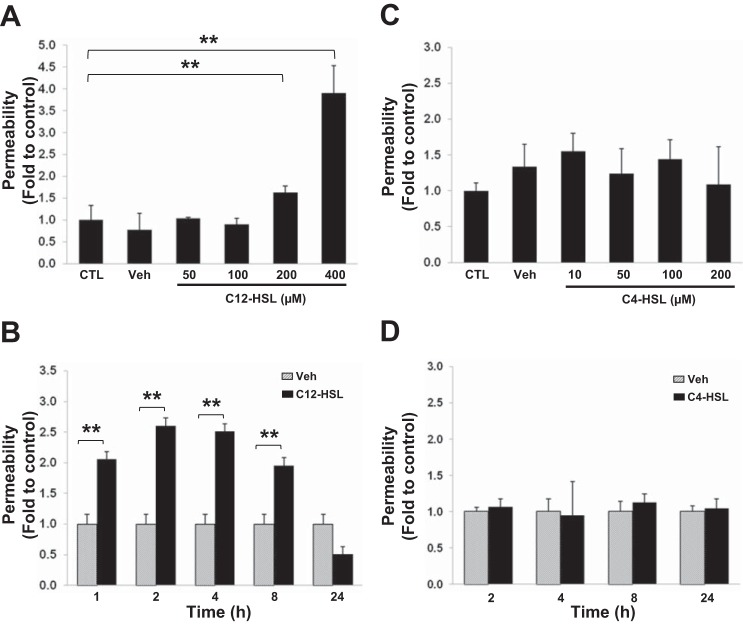
Gram-negative bacteria produce AHLs containing different lengths of carbon chains, which have distinct biological functions (12). To examine the impact of AHLs on barrier function of intestinal epithelial cells, human Caco-2 cells were treated with a long carbon chain AHL, C12-HSL, or a short carbon chain AHL, C4-HSL. Epithelial barrier permeability was measured by the paracellular flux of fluorescent FITC-dextran 20. As shown in Fig. 1, A and B, exposure to C12-HSL at the concentrations of 200 and 400 μM increased passage of FITC-dextran across Caco-2 monolayers. The effects were significant already after a 1 h treatment, reached the maximum 2–4 h following stimulation with C12-HSL, and returned to the control levels after 24 h. In contrast, C4-HSL did not induce any significant changes in permeability. The dose-dependent effects were evaluated 4 h posttreatment with C4-HSL levels of up to 200 μM (Fig. 1C). In addition, time-dependent impact was evaluated with 200 μM C4-HSL for up to 24 h (Fig. 1D).
Fig. 1.
N-3-(oxododecanoyl)-homoserine lactone (C12-HSL), but not N-butyryl homoserine lactone (C4-HSL), increases paracellular permeability across Caco-2 monolayers. Caco-2 cells grown on collagen-coated Transwell filters (12-mm diameter, 0.4-μm pore size) were incubated with the indicated concentrations of C12-HSL (A) or C4-HSL (C) for 4 h. In separate experiments, cells were incubated with 200 μM C12-HSL (B) or 200 μM C4-HSL (D) for the indicated times. Controls were either untreated (CTL) or incubated with vehicle (0.4% DMSO, Veh). Epithelial permeability was determined by measuring paracellular passage of FITC-dextran 20 from the apical side to the basolateral side across Caco-2 monolayers. Values (means ± SD) are expressed as fold change compared with CTL or Veh; n ≥ 4. **P < 0.01 vs. control.
These results indicate that exposure to a long carbon acyl chain homoserine lactone, C12-HSL, but not a short carbon acyl chain homoserine lactone, C4-HSL, affects barrier integrity of intestinal epithelium in a dose- and time-dependent manner. Based on these data, we selected treatment with 200 μM C12-HSL for 4 h as the experimental conditions to evaluate potential mechanisms involved C12-HSL-induced epithelial barrier dysfunction.
C12-HSL affects the expression and distribution of transmembrane TJ proteins.
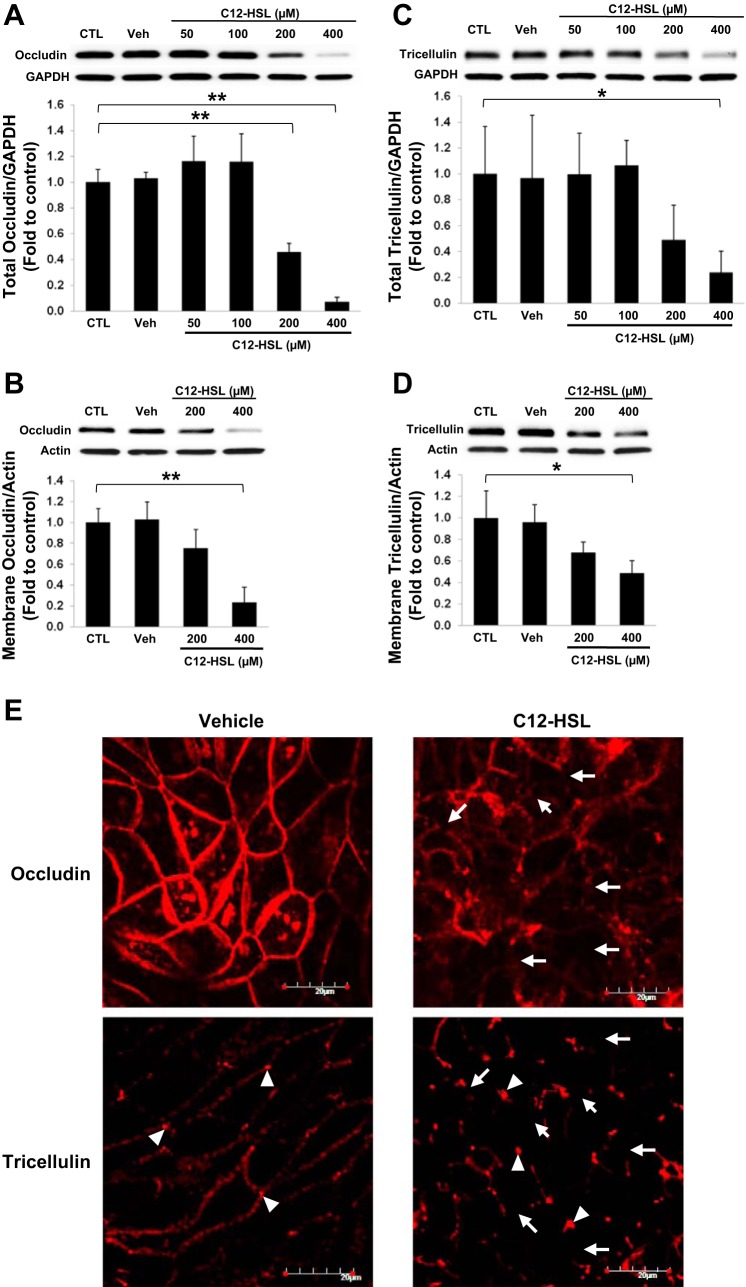
The intestinal epithelial barrier is maintained and modulated by TJ complexes, which connect adjacent cells at the apical end of the lateral membrane ensuring structural integrity and low permeability of the intestinal epithelial barrier. Therefore, we examined the expression level and distribution of occludin and tricellulin in total cell lysates and cell membrane fractions. These two TJ transmembrane proteins are directly responsible for the formation of TJs between adjacent epithelial cells. Caco-2 cells were exposed to 50–400 μM C12-HSL for 4 h and the levels of TJ proteins were analyzed by immunoblotting, followed by densitometric quantitation of the specific bands (Fig. 2). Treatment with C12-HSL at 200 μM and 400 μM caused statistically significant and dose-dependent decrease in occludin and tricellulin in total cell lysates (Fig. 2, A and C, respectively) and in membrane fractions (Fig. 2, B and D, respectively), compared with those of untreated cells (control) or 0.4% DMSO-treated cells (vehicle).
Fig. 2.
C12-HSL decreases the expression and distribution of occludin and tricellulin. Caco-2 cells grown on collagen-coated wells of cell culture plates were treated with the indicated concentrations of C12-HSL for 4 h. Controls were either untreated (CTL) or incubated with vehicle (0.4% DMSO, Veh). The levels of occludin (A and B) and tricellulin (C and D) in total cell lysates (A and C) or in cell membrane fractions (B and D) were assessed by immunoblotting. Blots are representative from at least 3 experiments. Band intensity was determined by densitometric analysis using Image J program. Values are means ± SD. *P < 0.05, **P < 0.01 vs. control. E: distribution of occludin and tricellulin was analyzed by immunofluorescence staining and confocal microscopy after 4 h treatment with 200 μM C12-HSL or 0.2% DMSO (Vehicle). The images are representative data from 1 of 3 experiments. Arrows indicate the diminished expression of occludin (top) or tricellulin (bottom). Arrowheads designate the presence of tricellulin at the tricellular junctions. Scale bar = 20 μm.
We next evaluated the effects of C12-HSL on junctional distribution of occludin and tricellulin by immunofluorescent antibody labeling and confocal microscopy. Consistent with the immunoblotting results, C12-HSL decreased the intensity and displaced occludin from cell-cell junctions. In addition, expression of tricellulin at bicellular junctions was diminished in cells treated with C12-HSL with the preserved expression at tricellular junctions (Fig. 2E). It should be noted that treatment with C12-HSL did not affect mRNA levels of these transmembrane proteins under the employed experimental conditions.
C12-HSL induces expression and activation of MMP-2 and MMP-3.
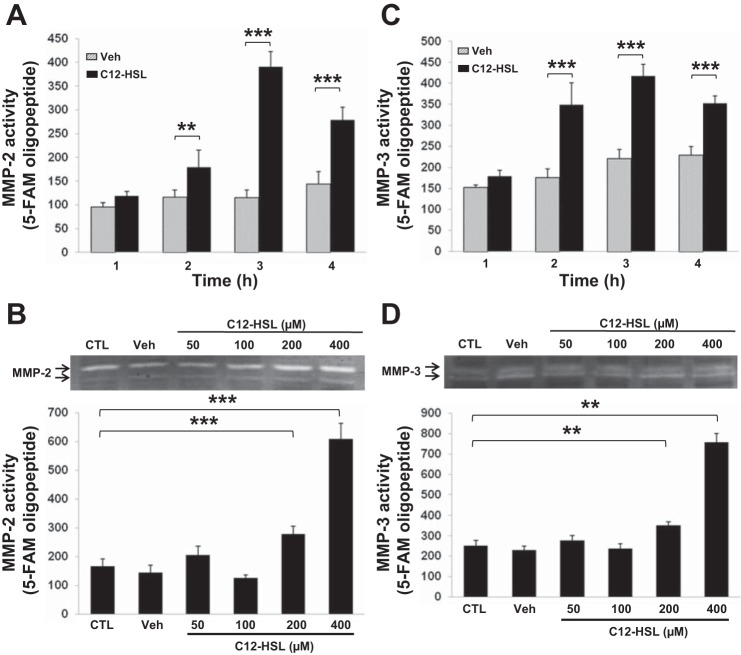
Recent studies have demonstrated that MMP activation induces degradation of TJ proteins and results in disruption of TJ integrity in epithelial cells and endothelial cells (36, 37, 71). Therefore, we examined whether MMPs contribute to C12-HSL-induced changes in permeability of Caco-2 monolayers. As shown in Fig. 3, treatment with C12-HSL increased secretion and total activity of MMP-2 and MMP-3 in a time- and dose-dependent manner. MMP activity was determined by zymograms and MMP activity assay kits using a specific fluorescent peptide substrate for each MMP. A significant increase both MMP-2 and MMP-3 activity was observed in cells exposed for 2–4 h to 200 μM C12-HSL (Fig. 3, A and C). In dose-dependent experiments, treatment with both 200 and 400 μM C12-HSL for 4 h induced expression of these MMPs (Fig. 3, B and D).
Fig. 3.
C12-HSL increases activity of matrix metalloprotease (MMP)-2 and MMP-3. Cells were incubated with 200 μM C12-HSL for the indicated time points (A and C) or with the indicated concentrations of C12-HSL for 4 h (B and D). Controls were either untreated (CTL) or incubated with 0.4% DMSO (B and D) or 0.2% DMSO (A and C) as vehicle (Veh). MMP-2 (A and B) and MMP-3 (C and D) activity was determined by using activity assay kits and zymography (blots in B and D). Values are means ± SD; n ≥ 4. **P < 0.01, ***P < 0.001 vs. CTL (B and D) or Veh (A and C) at the indicated time points.
MMP-2 and MMP-3 mediate C12-HSL-induced alterations of barrier permeability and TJ protein expression.
To evaluate whether upregulation of MMP-2 and MMP-3 participate in C12-HSL-induced barrier dysfunction in Caco-2 cells, we first evaluated the dose-dependent correlation plots between permeability and MMP activities. A significant correlation between a permeability increase and MMP-2 activity (r = 0.9941, P < 0.001) or MMP-3 activity (r = 0.9986, P < 0.001) was confirmed in C12-HSL-treated cultures by analysis of the Pearson coefficients (Fig. 4, A and B, respectively).
Fig. 4.
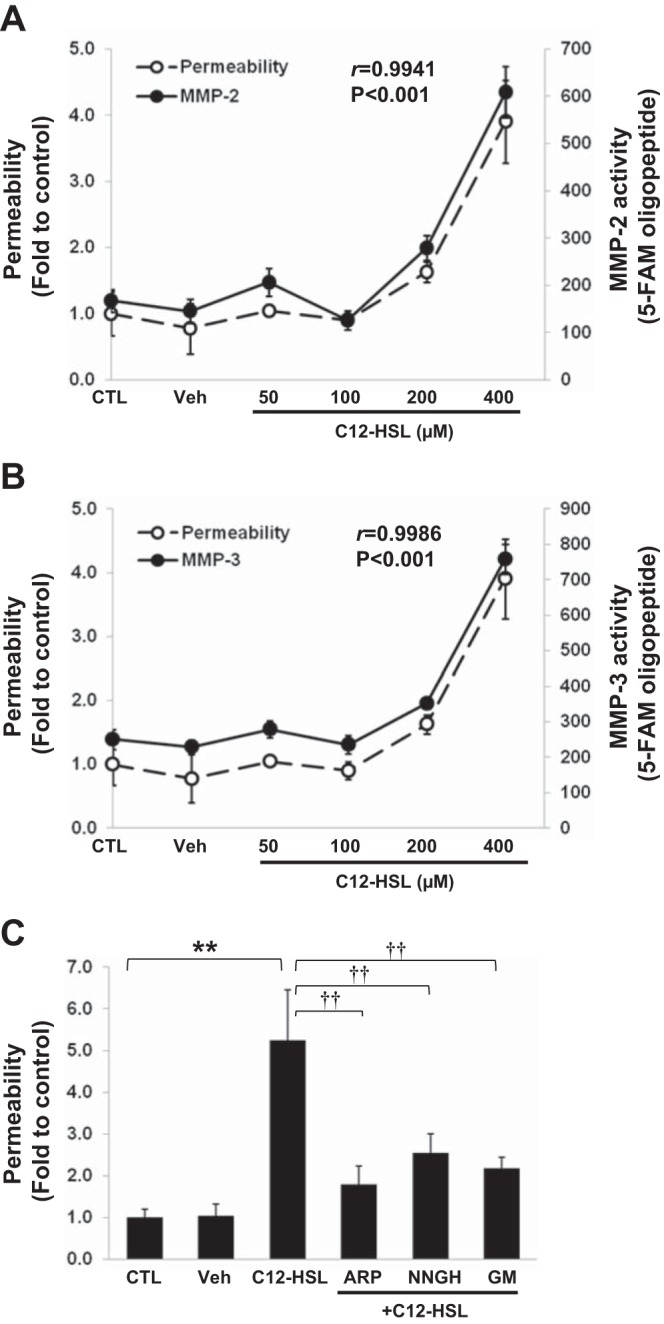
MMP-2 and MMP-3 modulate C12-HSL-induced paracellular permeability. C12-HSL-induced changes in paracellular permeability were plotted against the alterations of MMP-2 (A) and MMP-3 (B) activity. The significant correlations between permeability and MMP-2 activity (r = 0.9941, P < 0.001) or MMP-3 activity (r = 0.9986, P < 0.001) were detected by analysis of the Pearson coefficients. C: cells grown on the Transwell filters were pretreated with 25 μM ARP100 (ARP, MMP-2 inhibitor), 25 μM NNGH (MMP-3 inhibitor), or 25 μM GM1489 (GM, general MMP inhibitor) for 1 h prior to incubation with 200 μM C12-HSL for 4 h. Controls were either untreated (CTL) or incubated with vehicle (0.4% DMSO, Veh). Permeability was determined as in Fig. 1. Values are means ± SD; n ≥ 4. **P < 0.01 vs. CTL, ††P < 0.01 vs. C12-HSL-treated cells.
To confirm the contribution of MMPs to C12-HSL-induced disruption of barrier integrity, cells were next pretreated for 1 h with specific pharmacological inhibitors against MMPs, namely, ARP100 (MMP-2 inhibitor), NNGH (MMP-3 inhibitor), or GM1489 (general MMP inhibitor), followed by stimulation with 200 μM C12-HSL for 4 h. All pharmacological inhibitors significantly diminished C12-HSL-induced increase in permeability (Fig. 4C). Inhibition of MMP-3 activity by NNGH was also effective in protecting against C12-HSL-induced reduction of occludin (Fig. 5A) and tricellulin (Fig. 5B) levels.
Fig. 5.
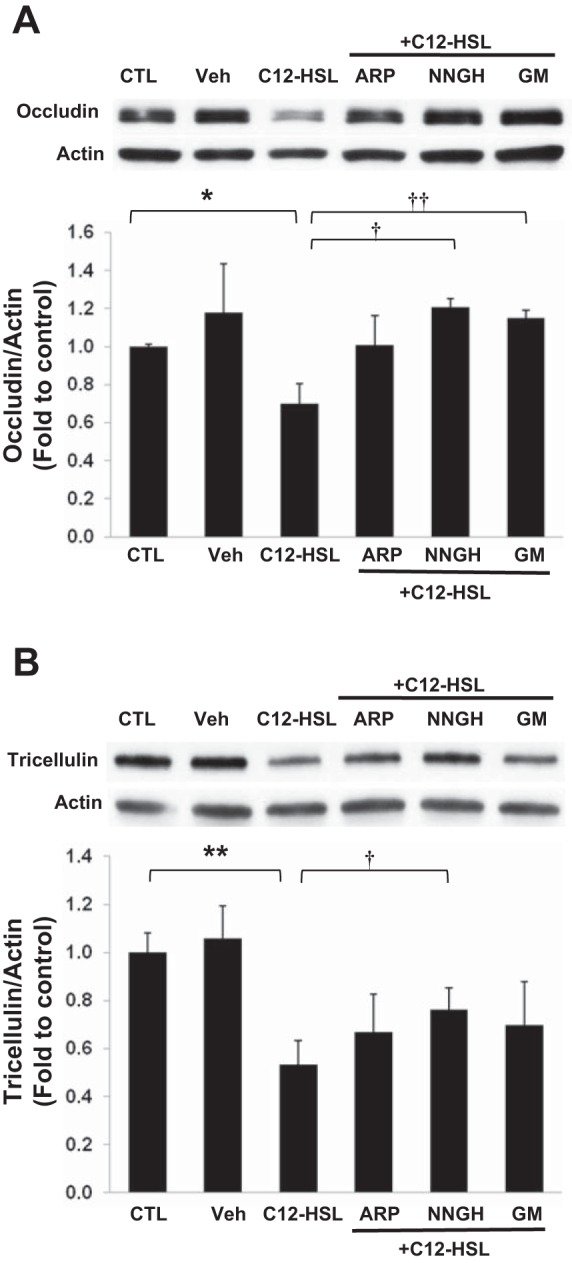
Inhibition of MMP activation attenuates C12-HSL-induced decrease in occludin and tricellulin expression. Cells were pretreated with 25 μM ARP100 (ARP), 25 μM NNGH, or 25 μM GM1489 (GM) for 1 h prior to incubation with 200 μM C12-HSL for 4 h. Controls were either untreated (CTL) or incubated with vehicle (0.4% DMSO, Veh). Expression levels of occludin (A) and tricellulin (B) were assessed by immunoblotting. Representative blots of 3 independent experiments. Densitometric analysis of the specific bands was performed with the Image J program. Values are means ± SD. *P < 0.05, **P < 0.01 vs. CTL, †P < 0.05, ††P < 0.01 vs. C12-HSL-treated cells.
Lipid rafts and PARs are required for C12-HSL-induced activation of MMPs and loss of TJ proteins.
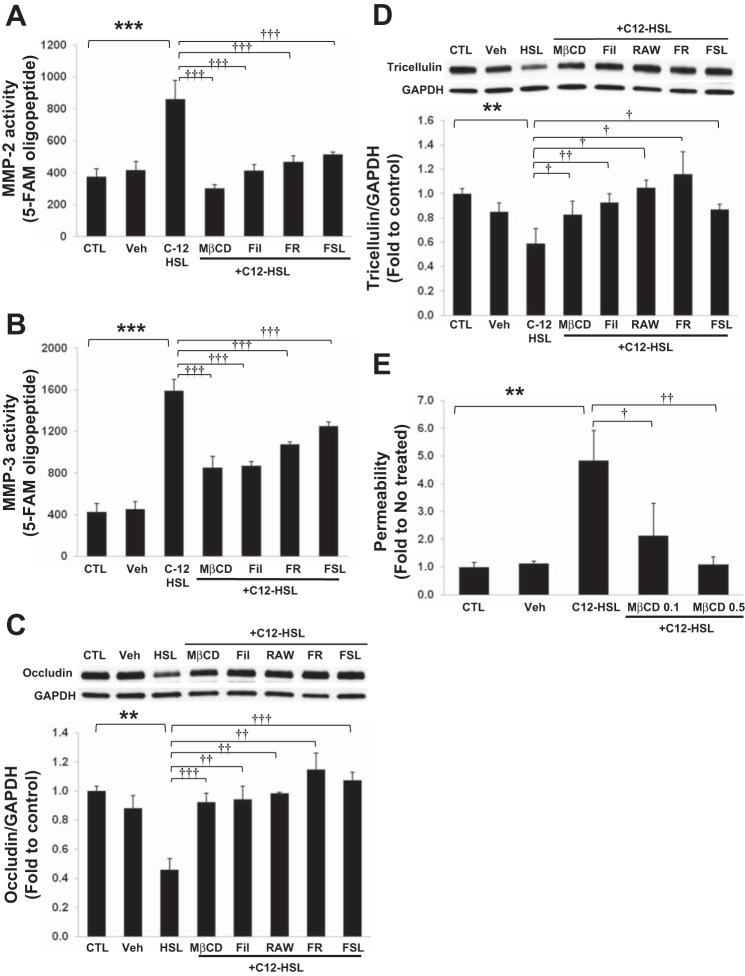
MMP/PAR systems play important roles in the regulation of barrier function of epithelial and endothelial cells (20, 28, 57). In addition, emerging studies indicated localization of MMPs, PARs, and TJ proteins in lipid rafts (5, 21, 33, 44, 50). Therefore, we examined the involvement of cholesterol-enriched lipid rafts and PARs in C12-HSL-induced MMP activation and alterations of TJ protein expression.
Lipid raft disruption by cholesterol depleting agents, methyl-β-cyclodextran (MβCD) and filipin III, completely abolished C12-HSL-induced activation of MMP-2 (Fig. 6A) and attenuated MMP-3 activation (Fig. 6B). Similar protective effects were also observed in cultures pretreated with the selective PAR-1 antagonists FR 171113 and RWJ 56110 or with the PAR-2 peptide antagonist FSLLRY-NH2 (Fig. 6, A and B). Consistent with these results, the loss of occludin and tricellulin was also prevented by cholesterol-depleting agents and PAR antagonists (Fig. 6, C and D). In addition, cholesterol depletion by MβCD dose dependently inhibited C12-HSL-induced an increase in permeability across Caco-2 monolayers (Fig. 6E). Together, these data indicate that cholesterol-rich lipid raft membrane domains and PAR/MMP-mediated signaling are required for C12-HSL-induced MMP activation and the loss of TJ proteins.
Fig. 6.
Lipid rafts and protease-activated receptors (PARs) are required for C12-HSL-induced MMP activation and degradation of occludin and tricellulin. Cells were pretreated with 0.5 mM methyl-β-cyclodextrin (MβCD), 1 mg/ml filipin III (Fil), 5 μM FR 171113 (FR), 10 μM RAW 56110 (RAW), or 12.5 μM FSLLRY-NHs (FSL), followed by treatment with 200 μM C12-HSL for 4 h in the continuous presence of the inhibitors. Controls were either untreated (CTL) or incubated with vehicle (0.4% DMSO, Veh). MMP-2 (A) and MMP-3 (B) activity in the conditioned medium was determined as in Fig. 3, A and C, respectively. Levels of occludin (C) and tricellulin (D) were analyzed by immunoblotting and quantified by densitometric analysis using Image J. Blots show representative images of occludin and tricellulin from 1 of 3 independent experiments. E: cells grown on the Transwell filters were pretreated with MβCD at 0.1 mg/ml (MβCD 0.1) or 0.5 mg/ml (MβCD 0.5) for 1 h prior to incubation with 200 μM C12-HSL for 4 h. Controls were either untreated (CTL) or incubated with vehicle (0.2% DMSO, Veh). Permeability was determined as in Fig. 1. Values are means ± SD. **P < 0.01, ***P < 0.001 vs. CTL, †P < 0.05, ††P < 0.01, †††P < 0.001 vs. C12-HSL treated cells.
DISCUSSION
The gastrointestinal epithelium provides a selective permeability barrier, which separates the lumen environment and the submucosal tissues (56). QS molecules produced by bacteria in the gut have been recognized to be associated with perturbation of host epithelial cell homeostasis and development of intestinal diseases (3). Recently, a number of studies have reported the detrimental effects of AHLs produced by gram-negative bacteria on intestinal epithelial barrier function, inflammation, and cell migration (29, 55, 64). Studies on AHLs produced by P. aeruginosa, an opportunistic, pathogenic, gram-negative bacteria (13), revealed that the QS system consists of two separate but subordinated systems, las and rhl. The LasI and RhlI synthases produce C12-HSL and C4-HSL, respectively. Emerging data suggest that C12-HSL can induce epithelial barrier disruption, characterized by reduced transepithelial electric resistance, increased paracellular permeability, and the alterations of TJ and adherence junction proteins (62–64). Because the mechanisms of these effects are still poorly understood, we employed an in vitro cell culture system using human intestinal epithelial Caco-2 cells to delineate the molecular mechanisms underlying AHL-mediated disruption of intestinal epithelial barrier integrity.
In response to the report that the concentrations of C12-HSL can reach 300–600 μM in P. aeruginosa biofilms (10), we examined the effects of AHLs in the range of 0–400 μM on permeability of Caco-2 cell monolayers. Disruption of the epithelial barrier function was dose and time dependently increased in C12-HSL-treated cells, but not in C4-HSL-treated cells. This reversible response to C12-HSL was similar to the observation of previous reports (62, 64). Consistent with diminished barrier integrity, the levels of occludin and tricellulin were significantly and dose dependently reduced by C12-HSL in total cell lysates and in cell membrane fractions. These data indicate that C12-HSL induces an increase in permeability of Caco-2 monolayers, at least in part, through the loss of occludin and tricellulin.
Both occludin and tricellulin belong to the MARVEL family and are involved in modulation of paracellular permeability of epithelial and endothelial cells (40). Occludin, the first identified TJ protein, forms a barrier against macromolecules, but not against small ions (1). Previous studies reported that occludin level in intestinal tissues was markedly decreased in patients with intestinal diseases, including Crohn's disease, ulcerative colitis, and celiac disease, and in animal models of inflammatory bowel disease (15, 17). Recent study demonstrated that occludin knockdown in Caco-2 cells and mouse intestines induced an increase in paracellular permeability of macromolecules (1). These results support the critical role of occludin in intestinal epithelial barrier function. Although TJ formation or differentiation into polarized epithelial cells were not affected in occludin-deficient mice, the animals exhibited histological and functional abnormalities such as chronic inflammation and hyperplasia of the gastric epithelium, testicular atrophy, and loss of cytoplasmic granules in the salivary gland (46). Tricellulin is a newly defined tetraspanin membrane TJ protein with 51% homology of occludin (24). Two extracellular loops of this protein contribute to homophilic and heterophilic interaction. The COOH- and NH2-terminal cytoplasmic domains of tricellulin are known to determine its translocation and localization into cell junctional sites (24). Although tricellulin is preferentially localized at tricellular junctions, forming a barrier against passage of macromolecules, tricellulin is also observed at bicellular junctions in cells with high levels of this protein, which exhibit decreased permeability of both macromolecules and small ions (32, 40). Interestingly, occludin and tricellulin are known to affect each other's cellular localization. In MDCK cells, occludin knockdown induces the localization of tricellulin to bicellular junctions rather than tricellular junctions, and the reintroduction of occludin expression abolishes bicellular tricellulin (25). Furthermore, in mammary epithelial Eph4 cells, the knockdown of tricellulin resulted in discontinuous localization of occludin at bicellular junctions and accumulation of occludin at tricellular junctions with disruption of barrier properties (24). Consistent with these observations, our data strongly suggest that simultaneous loss and delocalization of both occludin and tricellulin are responsible for C12-HSL-mediated increase in paracellular permeability across Caco-2 cell monolayers. Considering the cooperative interactions between occludin and tricellulin in TJs, concurrent loss of both TJ proteins as observed in the present study may aggravate the alterations in intestinal epithelial permeability.
Although we focused on the involvement of transmembranal occludin and tricellulin, exposure to C12-HSL has been reported to result in the alterations of TJ-associated proteins, including zonula occludens (ZO)-1 and ZO-3, and adherens junctions, which can affect the barrier integrity (62–64). Therefore, we cannot exclude the possibility that changes in expression levels or distribution of other junctional proteins by C12-HSL contributed to increased paracellular permeability of Caco-2 cells observed in the present study.
Novel results from the present study suggest that C12-HSL-induced alterations of epithelial permeability are mediated via MMP-2- and MMP-3-induced degradation of occludin and tricellulin. MMPs are the family of zinc-dependent proteases classically described in the contest of extracellular matrix (ECM) remodeling under physiological and pathophysiological conditions (43). MMPs have been shown to be expressed by multiple cell populations within the gastrointestinal tract, including human colonic primary epithelial cells under normal healthy conditions (65). Elevated mRNA levels of MMP-2 and MMP-3 were reported in colon tissues of patients with inflammatory bowel disease (43, 45). Recent observations also indicated that MMPs can mediate degradation of TJ proteins including occludin, ZO-1, and claudins, causing alterations of barrier function in epithelial and endothelial cells (20, 30, 70). For example, MMP-2 was reported to degrade occludin and claudin-5 in the blood-brain barrier during ischemia and reperfusion injury (36, 37). In human nonpigmented ciliary epithelial cells, TNF-α upregulated MMP-3 expression and degraded TJ proteins, such as occludin and claudin-1, leading to increased permeability through the cellular barrier (70). In the present study, C12-HSL-mediated loss of occludin and tricellulin was attenuated by inhibitors against MMP-2 or MMP-3, suggesting that both TJ proteins constitute substrates of these MMPs.
MMP activation is regulated at multiple levels, including transcription, proteolytic degradation of precursor zymogens by proteases and other MMPs, interaction with specific ECM components, and inhibition by tissue inhibitors of matrix metalloproteinases (TIMPs) (22). Our results indicate that PAR-1 and -2 modulate C12-HSL-induced upregulation of MMP-2 and MMP-3. These results are consistent with literature data indicating the contribution of PARs to MMP expression and activation in epithelial and endothelial cells (20, 57). PARs, discovered as thrombin receptors, are members of the G protein-coupled receptor (GPCR) superfamily and are known to be the key players in modulation of permeability and inflammation in epithelial and endothelial cells (21, 35). Furthermore, PAR activation was shown to modulate the degradation of TJ proteins and the disassembly of TJs, which cause TJ barrier disruption (18, 20). For example, exposure of intestinal epithelial cells to mast cell chymase induced PAR-2-mediated MMP-2 expression and an increase in paracellular permeability through MMP-2-mediated degradation of claudin-5 protein (20). Zonula occludens toxin (Zot), a Vibrio cholera-derived toxin, was also shown to TJ disassembly through PAR-2-mediated phosphorylation of ZO-1 (18). In addition, PAR-1 activation in endothelial cells stimulated cytoskeletal reorganization and changed junctional barrier integrity through lipid raft-dependent signaling (8). The requirement of intact lipid rafts was also demonstrated in the present study as ablation of lipid raft structures by two different lipid raft disruptors, MβCD and filipin III, inhibited C12-HSL-induced MMP activation and the loss of TJ proteins. In addition, pretreatment with MβCD effectively blocked the increase in C12-HSL-induced permeability across Caco-2 monolayers. Lipid raft microdomains are an integral part of the TJ spatial organization and several TJ proteins reside in lipid raft domains (33). PAR-1 and -2 are also known to be localized in lipid raft domains in epithelial and endothelial cells (4, 8). Thus our data indicate that lipid raft domains and the associated signaling pathways are involved in C12-HSL-induced epithelial barrier dysfunction.
Although the present study focused on the role of PARs and MMPs in C12-HSL-induced disruption of barrier function and dissociation of TJ proteins from cell membranes, several other mechanisms were demonstrated to be also involved in these processes under different experimental conditions. For example, TJ disruption may result from internalization of TJ proteins through vesicle trafficking processes including clathrin-mediated endocytosis (26), macropinocytosis (6), and caveolae-mediated endocytosis (51, 53). Postinternalization, TJ protein-containing vesicles can be fused with early endosomes and then recycled to the plasma membrane or directed to lysosomal degradation (14, 41). In addition, proinflammatory cytokines, such as TNF-α, LIGHT, and IFN-γ can cause occludin removal from TJs via the myosin light chain (MLC) kinase-dependent caveolar endocytosis (39, 51, 66). Stimulation of PAR-1 also can induce lipid raft-dependent MLC phosphorylation and cytoskeletal reorganization, a process that may be involved in TJ delocalization from cell membrane and the loss of cellular barrier functions (8).
Taken together, our results demonstrate that C12-HSL induces disruption of epithelial barrier integrity associated with degradation of occludin and tricellulin through MMP-2 and -3 activation. These processes are mediated by PAR signaling and require intact lipid rafts. Because the opening of TJs is indispensable for bacteria invasion, C12-HSL-mediated disruption of TJ integrity is a potential mechanism for the translocation of bacteria through the epithelial barriers (Fig. 7).
Fig. 7.
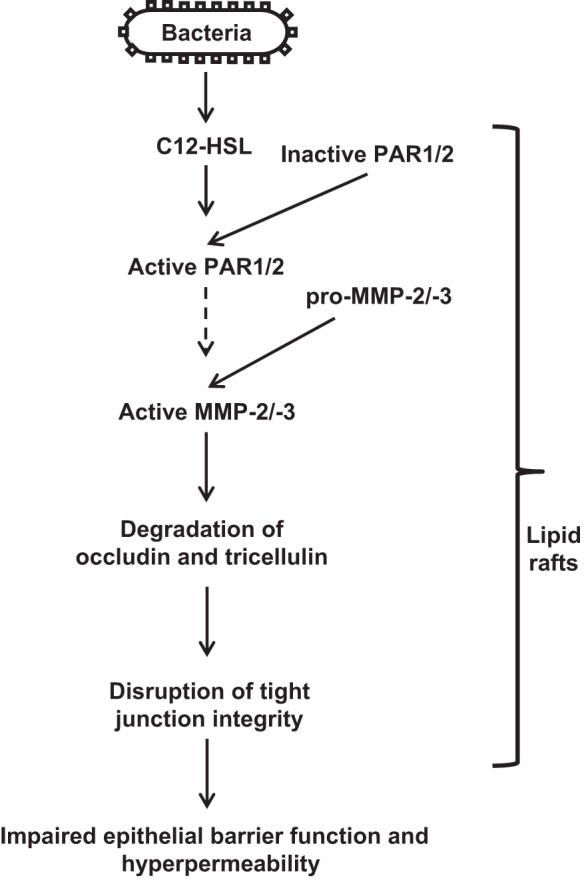
Proposed diagram of C12-HSL-induced loss of occludin and tricellulin leading to impaired intestinal epithelial barrier function.
GRANTS
This study was supported in part by the American Heart Association SDG award 09SDG2300037 (S. Y. Eum) and NIH awards CA133257, DA027569, MH072567, and MH098891 (M. Toborek).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.Y.E. and M.T. conception and design of research; S.Y.E., D.J., L.B., and I.E.A. performed experiments; S.Y.E. and D.J. analyzed data; S.Y.E., L.B., I.E.A., and M.T. interpreted results of experiments; S.Y.E. and D.J. prepared figures; S.Y.E. drafted manuscript; S.Y.E., D.J., L.B., I.E.A., and M.T. edited and revised manuscript; S.Y.E., D.J., L.B., I.E.A., and M.T. approved final version of manuscript.
REFERENCES
- 1.Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M, Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 300: G1054–G1064, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1: a002584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashida H, Ogawa M, Kim M, Mimuro H, Sasakawa C. Bacteria and host interactions in the gut epithelial barrier. Nat Chem Biol 8: 36–45, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Awasthi V, Mandal SK, Papanna V, Rao LV, Pendurthi UR. Modulation of tissue factor-factor VIIa signaling by lipid rafts and caveolae. Arterioscler Thromb Vasc Biol 27: 1447–1455, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae JS, Yang L, Rezaie AR. Lipid raft localization regulates the cleavage specificity of protease activated receptor 1 in endothelial cells. J Thromb Haemost 6: 954–961, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J 19: 923–933, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta 1788: 864–871, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlile-Klusacek M, Rizzo V. Endothelial cytoskeletal reorganization in response to PAR1 stimulation is mediated by membrane rafts but not caveolae. Am J Physiol Heart Circ Physiol 293: H366–H375, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Carratu R, Secondulfo M, de Magistris L, Iafusco D, Urio A, Carbone MG, Pontoni G, Carteni M, Prisco F. Altered intestinal permeability to mannitol in diabetes mellitus type I. J Pediatr Gastroenterol Nutr 28: 264–269, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Charlton TS, de Nys R, Netting A, Kumar N, Hentzer M, Givskov M, Kjelleberg S. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Environ Microbiol 2: 530–541, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest 84: 282–291, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Cooley M, Chhabra SR, Williams P. N-Acylhomoserine lactone-mediated quorum sensing: a twist in the tail and a blow for host immunity. Chem Biol 15: 1141–1147, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Deep A, Chaudhary U, Gupta V. Quorum sensing and bacterial pathogenicity: from molecules to disease. J Lab Physicians 3: 4–11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher SJ, Rappoport JZ. Tight junction regulation through vesicle trafficking: bringing cells together. Biochem Soc Trans 42: 195–200 [DOI] [PubMed] [Google Scholar]
- 15.Fries W, Mazzon E, Squarzoni S, Martin A, Martines D, Micali A, Sturniolo GC, Citi S, Longo G. Experimental colitis increases small intestine permeability in the rat. Lab Invest 79: 49–57, 1999 [PubMed] [Google Scholar]
- 16.Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell 140: 859–870, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol 281: G216–G228, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Goldblum SE, Rai U, Tripathi A, Thakar M, De Leo L, Di Toro N, Not T, Ramachandran R, Puche AC, Hollenberg MD, Fasano A. The active Zot domain (aa 288–293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J 25: 144–158, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol 81: 1–44, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Groschwitz KR, Wu D, Osterfeld H, Ahrens R, Hogan SP. Chymase-mediated intestinal epithelial permeability is regulated by a protease-activating receptor/matrix metalloproteinase-2-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 304: G479–G489, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, Choi EH, Uchida Y, Brown BE, Feingold KR, Elias PM. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol 126: 2074–2086, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. FEBS J 278: 28–45, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med 105: 883–885, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171: 939–945, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikenouchi J, Sasaki H, Tsukita S, Furuse M, Tsukita S. Loss of occludin affects tricellular localization of tricellulin. Mol Biol Cell 19: 4687–4693, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell 15: 176–188, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson PG, Lessof MH, Baker RW, Ferrett J, MacDonald DM. Intestinal permeability in patients with eczema and food allergy. Lancet 1: 1285–1286, 1981 [DOI] [PubMed] [Google Scholar]
- 28.Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis 38: 376–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlsson T, Turkina MV, Yakymenko O, Magnusson KE, Vikstrom E. The Pseudomonas aeruginosa N-acylhomoserine lactone quorum sensing molecules target IQGAP1 and modulate epithelial cell migration. PLoS Pathog 8: e1002953, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Kim CS, Lee YM, Jo K, Shin SD, Kim JS. Methylglyoxal induces hyperpermeability of the blood-retinal barrier via the loss of tight junction proteins and the activation of matrix metalloproteinases. Graefes Arch Clin Exp Ophthalmol 250: 691–697, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Kohler H, Sakaguchi T, Hurley BP, Kase BA, Reinecker HC, McCormick BA. Salmonella enterica serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. Am J Physiol Gastrointest Liver Physiol 293: G178–G187, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Krug SM, Amasheh S, Richter JF, Milatz S, Gunzel D, Westphal JK, Huber O, Schulzke JD, Fromm M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell 20: 3713–3724, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambert D, O'Neill CA, Padfield PJ. Methyl-beta-cyclodextrin increases permeability of Caco-2 cell monolayers by displacing specific claudins from cholesterol rich domains associated with tight junctions. Cell Physiol Biochem 20: 495–506, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Le Ferrec E, Chesne C, Artusson P, Brayden D, Fabre G, Gires P, Guillou F, Rousset M, Rubas W, Scarino ML. In vitro models of the intestinal barrier. The report and recommendations of ECVAM Workshop 46 European Centre for the Validation of Alternative methods. Altern Lab Anim 29: 649–668, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Lee H, Hamilton JR. Physiology, pharmacology, and therapeutic potential of protease-activated receptors in vascular disease. Pharmacol Ther 134: 246–259, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci 32: 3044–3057, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu A, Suofu Y, Guan F, Broderick JP, Wagner KR, Clark JF. Matrix metalloproteinase-2 deletions protect against hemorrhagic transformation after 1 h of cerebral ischemia and 23 h of reperfusion. Neuroscience 253: 361–367, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol 5: 119–144, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, Turner JR. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol 189: 111–126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mariano C, Sasaki H, Brites D, Brito MA. A look at tricellulin and its role in tight junction formation and maintenance. Eur J Cell Biol 90: 787–796, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Mayer ML, Sheridan JA, Blohmke CJ, Turvey SE, Hancock RE. The Pseudomonas aeruginosa autoinducer 3O-C12 homoserine lactone provokes hyperinflammatory responses from cystic fibrosis airway epithelial cells. PLoS One 6: e16246, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naito Y, Yoshikawa T. Role of matrix metalloproteinases in inflammatory bowel disease. Mol Aspects Med 26: 379–390, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. J Cell Sci 113: 1771–1781, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Pedersen G, Saermark T, Kirkegaard T, Brynskov J. Spontaneous and cytokine induced expression and activity of matrix metalloproteinases in human colonic epithelium. Clin Exp Immunol 155: 257–265, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11: 4131–4142, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaguchi T, Kohler H, Gu X, McCormick BA, Reinecker HC. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol 4: 367–381, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Sambuy Y, De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol 21: 1–26, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Carteni M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 55: 1443–1449, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Schwab W, Harada H, Goetz W, Nowicki M, Witt M, Kasper M, Barth K. Immunocytochemical and biochemical detection of EMMPRIN in the rat tooth germ: differentiation-dependent co-expression with MMPs and co-localization with caveolin-1 in membrane rafts of dental epithelial cells. Histochem Cell Biol 128: 195–203, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, Fu YX, Turner JR. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology 132: 2383–2394, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwarzer C, Fu Z, Patanwala M, Hum L, Lopez-Guzman M, Illek B, Kong W, Lynch SV, Machen TE. Pseudomonas aeruginosa biofilm-associated homoserine lactone C12 rapidly activates apoptosis in airway epithelia. Cell Microbiol 14: 698–709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell 16: 3919–3936, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simovitch M, Sason H, Cohen S, Zahavi EE, Melamed-Book N, Weiss A, Aroeti B, Rosenshine I. EspM inhibits pedestal formation by enterohaemorrhagic Escherichia coli and enteropathogenic E. coli and disrupts the architecture of a polarized epithelial monolayer. Cell Microbiol 12: 489–505, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Smith RS, Fedyk ER, Springer TA, Mukaida N, Iglewski BH, Phipps RP. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-kappa B and activator protein-2. J Immunol 167: 366–374, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 70: 631–659, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tressel SL, Kaneider NC, Kasuda S, Foley C, Koukos G, Austin K, Agarwal A, Covic L, Opal SM, Kuliopulos A. A matrix metalloprotease-PAR1 system regulates vascular integrity, systemic inflammation and death in sepsis. EMBO Mol Med 3: 370–384, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2: 285–293, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol Cell Physiol 273: C1378–C1385, 1997 [DOI] [PubMed] [Google Scholar]
- 60.van Elburg RM, Uil JJ, Mulder CJ, Heymans HS. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut 34: 354–357, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 68: 403–429, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Vikstrom E, Bui L, Konradsson P, Magnusson KE. The junctional integrity of epithelial cells is modulated by Pseudomonas aeruginosa quorum sensing molecule through phosphorylation-dependent mechanisms. Exp Cell Res 315: 313–326, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Vikstrom E, Bui L, Konradsson P, Magnusson KE. Role of calcium signalling and phosphorylations in disruption of the epithelial junctions by Pseudomonas aeruginosa quorum sensing molecule. Eur J Cell Biol 89: 584–597, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Vikstrom E, Tafazoli F, Magnusson KE. Pseudomonas aeruginosa quorum sensing molecule N-(3 oxododecanoyl)-l-homoserine lactone disrupts epithelial barrier integrity of Caco-2 cells. FEBS Lett 580: 6921–6928, 2006 [DOI] [PubMed] [Google Scholar]
- 65.von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut 47: 63–73, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol 166: 409–419, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Westphal JK, Dorfel MJ, Krug SM, Cording JD, Piontek J, Blasig IE, Tauber R, Fromm M, Huber O. Tricellulin forms homomeric and heteromeric tight junctional complexes. Cell Mol Life Sci 67: 2057–2068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wroblewski LE, Shen L, Ogden S, Romero-Gallo J, Lapierre LA, Israel DA, Turner JR, Peek RM., Jr Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology 136: 236–246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 341: 1437–1439, 1993 [DOI] [PubMed] [Google Scholar]
- 70.Yamada H, Yoneda M, Inaguma S, Watanabe D, Banno S, Yoshikawa K, Mizutani K, Iwaki M, Zako M. Infliximab counteracts tumor necrosis factor-alpha-enhanced induction of matrix metalloproteinases that degrade claudin and occludin in non-pigmented ciliary epithelium. Biochem Pharmacol 85: 1770–1782, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Zeni P, Doepker E, Schulze-Topphoff U, Huewel S, Tenenbaum T, Galla HJ. MMPs contribute to TNF-α-induced alteration of the blood-cerebrospinal fluid barrier in vitro. Am J Physiol Cell Physiol 293: C855–C864, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Zhu H, Conibear TC, Thuruthyil SJ, Willcox MD. Pseudomonas aeruginosa quorum-sensing signal molecules induce IL-8 production by human corneal epithelial cells. Eye Contact Lens 34: 179–181, 2008 [DOI] [PubMed] [Google Scholar]