Abstract
A wide network of interconnected areas was previously found in neuroimaging studies involving normal as well as pathological subjects; however literature seems to suffer from a lack of investigation in glucose metabolism behaviour under olfactory condition. Thus, the present work describe for the first time a pure olfactory related brain response of metabolism by using 18F-fluorodeoxyglucose-Positron Emission Tomography/Computer Tomography in eleven resting subjects undergoing a neutral and a pure olfactory condition. By contrasting these experimental phases, it was possible to depict a re-organization pattern of default mode network structures in a relatively ecological environment. Moreover, by correlating such pattern with a battery of validated olfactory and neuropsychological tests, our work allowed in showing peculiar correlation data that could cluster the subjects sample in a certain range of normality. We believe the present study could integrate the current knowledge in olfactory research and could be a start-up for future contributions.
The existing literature on brain activation changes during olfactory tasks is mainly based on neuroimaging techniques that unify the stimulation and the acquisition phases in the camera gantry often perceived as an unfriendly, noisy and not odorless environment (i.e. functional magnetic resonance imaging - fMRI - shows strong unintended auditory and olfactory stimulation through the period of experimental stimulation and data acquisition), constituting a common bias to the interpretation of the majority of existing data1,2. Furthermore MRI examinations are often difficult if not impossible to stand for many patients bearers of neurodegenerative, psychiatric and emotional disorders. Conversely, 18F-fluorodeoxyglucose-Positron Emission Tomography/Computer Tomography (FDG-PET/CT) is a functional neuroimaging methodology that allows to investigate the biochemical changes coupled to the cerebral glucose metabolism in relatively ecological environments1,3 - i.e. in a comfortable quiet, light and airy room - avoiding possible biases resulting from physical and psychological discomfort for the patient1,2. For these reasons it seems important to report the metabolic changes associated to an olfactory stimulation under these conditions. Furthermore the wide availability of this facility and the relatively standardized image acquisition protocols favour the reproducibility of FDG-PET/CT studies. To the best of our knowledge there is a noteworthy lack of investigation of olfaction neural correlates in resting-state by using FDG-PET/CT. In fact, in the majority of imaging studies the neural correlates of olfactory stimulation have been indirectly related to cerebral blood flow using techniques as fMRI and H215O-PET4,5,6,7,8, being in the latter compulsory the presence of an in-house cyclotron along with a complicated and sensitive methodology.
The literature on olfaction neural correlates highlights two other important issues which may benefit by the use of FDG-PET/CT. The first one concerns the perception and the sensation of smell: in fact these two phenomena are largely dependent on sniffing behaviors which may be modulated by attentional mechanisms in order to increase the probability of detecting odors. Indeed the subsystems controlling sniffing and smelling are separate in the human olfactory cortex and the different airflows they produce could result in asymmetries in cerebral activations9. This imposes a limitation to fMRI studies where these two behaviours strongly affect the on-line metabolic activity while this is not the case for FDG-PET/CT studies where the metabolic activity is delayed with respect to the moment of inhalation and any attentional mechanism and specific sniffing behaviour.
The second important issue concerns the fact that the chemosensory inputs can automatically induce an unwanted hedonic response, implying an involvement of the limbic system. In fact, in studies where a pure odorant compound such as vanilline (VAN) was used, H215O-PET scanning showed metabolic changes not only in the olfactory bulb but also in the limbic system, e.g., in the piriform, orbito-frontal, and anterior cingular cortices and the agranular insular region5.
In the present study we aimed at assessing the cortical and subcortical metabolic involvement related to a pure olfactory stimulation by using FDG-PET/CT in normal resting subjects. Moreover, in order to integrate data resulting from the functional imaging literature on olfactory neurobiology we correlated neuroimaging data resulting from neural glucose consumption with a battery of validated questionnaires (VQ).
Results
FDG-PET/CT
A significantly higher glucose metabolism in Neutral Condition (NC) was found in left superior, inferior, middle, medial frontal and orbital gyrus (orbitofrontal cortex, OFC) (Cluster A) as well as in anterior cingulate cortex (ACC) (Table 1, Figure 1) as compared to Olfactory Condition (OC). In the opposite comparison a relative higher glucose metabolism was found in cerebellum declive (DoC), cuneus (Cu), lingual gyrus (LG) and parahippocampal gyrus (PHG), mainly in the left hemisphere (Table 2, Figure 2).
Table 1. Numerical results of SPM comparisons between 18F-FDG uptake in NC and OC (N = 11).
| 1. Cluster level | 2. Voxel level | ||||||
|---|---|---|---|---|---|---|---|
| Comparison | Cluster extent | Corrected p value | Cortical Region | Z score of maximum | MNI coordinates | Cortical region | BA |
| NC - OC | 2060 | 0.041 | L Frontal Lobe | 4.15 | −30,48, −4 | Middle Frontal Gyrus | 10 |
| L Frontal Lobe | 3.62 | −24,40, −6 | Middle Frontal Gyrus | 11 | |||
| L Frontal Lobe | 3.43 | −28,30, −22 | Inferior Frontal Gyrus | 11 | |||
| L Frontal Lobe | 3.25 | −12,52,14 | Medial Frontal Gyrus | 10 | |||
| L Frontal Lobe | 2.96 | −18,28, −26 | Orbital Gyrus | 47 | |||
| L Limbic Lobe | 2.93 | −20,44,6 | Anterior Cingulate | 32 | |||
| L Frontal Lobe | 2.80 | −22,66,8 | Superior Frontal Gyrus | 10 | |||
| L Frontal Lobe | 2.55 | −36,38, −2 | Middle Frontal Gyrus | 47 | |||
| L Frontal Lobe | 2.54 | −14,46, −12 | Superior Frontal Gyrus | 11 | |||
Table 1. In the ‘cluster level' section on left, the number of voxels, the corrected P value of significance and the cortical region where the voxel is found, are all reported for each significant cluster. In the ‘voxel level' section, all of the coordinates of the correlation sites (with the Z-score of the maximum correlation point), the corresponding cortical region and BA are reported for each significant cluster. L, left; R, right; BA, Brodmann's area. In the case that the maximum correlation is achieved outside the grey matter, the nearest grey matter (within a range of 5 mm) is indicated with the corresponding BA.
Figure 1. 3D rendering showing the cluster of voxels in the left orbitofrontal and anterior cingulate cortex in which FDG uptake was significantly higher at NC (n = 11) as compared to OC (n = 11).
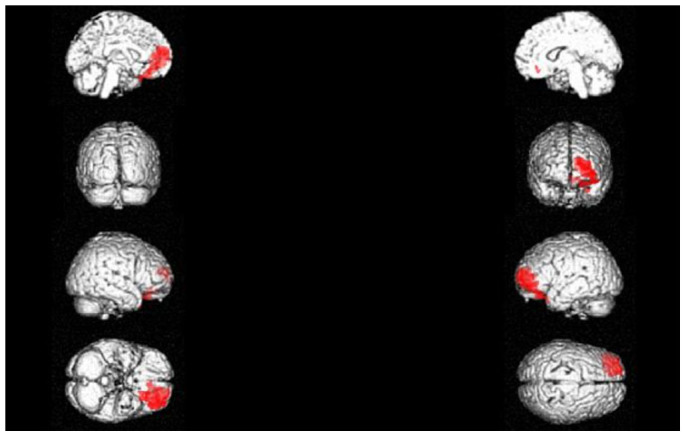
The first row represents the medial aspect of left (on the left) and right (on the right) hemispheres; the second row represents the anterior (on the left) and posterior (on the right) aspect on the brain; the third row represents the lateral aspect of the right (on the left) and of the left (on the right) hemispheres; the fourth row represents the inferior (on the left) and the superior (on the right) aspects of the brain. Coordinates and regional details are presented in Table 1.
Table 2. Numerical results of SPM comparisons between 18F-FDG uptake in OC and NC (N = 11).
| 3. Cluster level | 4. Voxel level | ||||||
|---|---|---|---|---|---|---|---|
| Comparison | Cluster extent | Corrected p value | Brain Region | Z score of maximum | MNI coordinates | Cortical region | BA |
| OC - NC | 4008 | 0.000 | L Cerebellum | 3.86 | −16, −74, −24 | Declive of Cerebellum | |
| L Occipital Lobe | 3.66 | −2, −102,0 | Cuneus | 18 | |||
| L Occipital Lobe | 3.16 | −2, −98,18 | Lingual Gyrus | 18 | |||
| R Occipital Lobe | 3.09 | 12, −76, −4 | Lingual Gyrus | 18 | |||
| L Limbic Lobe | 2.50 | −22, −54, −8 | Parahippocampal Gyrus | 19 | |||
Table 2. In the ‘cluster level' section on left, the number of voxels, the corrected P value of significance and the cortical region where the voxel is found, are all reported for each significant cluster. In the ‘voxel level' section, all of the coordinates of the correlation sites (with the Z-score of the maximum correlation point), the corresponding cortical region and BA are reported for each significant cluster. L, left; R, right; BA, Brodmann's area. In the case that the maximum correlation is achieved outside the grey matter, the nearest grey matter (within a range of 5 mm) is indicated with the corresponding BA.
Figure 2. 3D rendering showing the cluster of voxels in the cuneus, lingual gyrus, parahippocampal gyrus and declive of cerebellum mainly in the left hemisphere, in which FDG uptake was significantly higher at OC (n = 11) as compared to NC (n = 11).
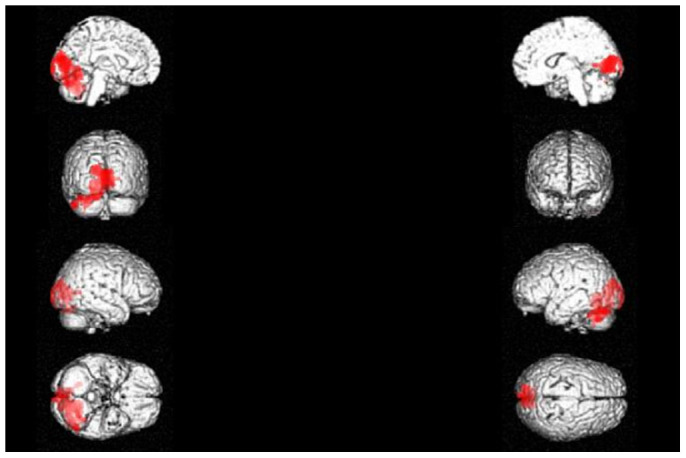
The first row represents the medial aspect of left (on the left) and right (on the right) hemispheres; the second row represents the anterior (on the left) and posterior (on the right) aspect on the brain; the third row represents the lateral aspect of the right (on the left) and of the left (on the right) hemispheres; the fourth row represents the inferior (on the left) and the superior (on the right) aspects of the brain. Coordinates and regional details are presented in Table 2.
VQ and Correlation Analysis
Olfactory and VQ results are shown as means ± SD in Table 3 and figure 3.
Table 3. Mean and Standard Deviations (SD) of olfactory and Validated Questionnaires scores.
| TESTS | MEAN | SD |
|---|---|---|
| QOD NS | 0.18182 | 0.404520 |
| QOD PS | 5.36364 | 0.674200 |
| QOD SD | 16.27273 | 1.272078 |
| Anxiety | 21.54545 | 3.856518 |
| OT | 9.27273 | 1.131672 |
| OD | 13.81818 | 0.873863 |
| OI | 13.09091 | 1.136182 |
| TDI | 36.18182 | 1.877801 |
| IVAS NC | 0.09091 | 0.301511 |
| IVAS OC | 6.36364 | 0.839372 |
| PVAS OC | 8.77273 | 0.719848 |
Table 3. QOD NS: questionnaire of olfactory disorders - negative statements; QOD PS: questionnaire of olfactory disorders – postitive statements; QOD SD: questionnaire of olfactory disorders – socially desired statement; Anxiety: self-rating anxiety scale; OT: odor threshold; OD: odor discrimination; OI: odor identification; TDI: sum of OT, OD and OI; IVAS NC: intensity visuo-analogue scale after neutral condition; IVAS OC: intensity visuo-analogue scale after olfactory condition; PVAS OC: pleasantness visuo-analogue scale after olfactory condition.
Figure 3. Subjects' scores of olfactory tests and validated questionnaires.
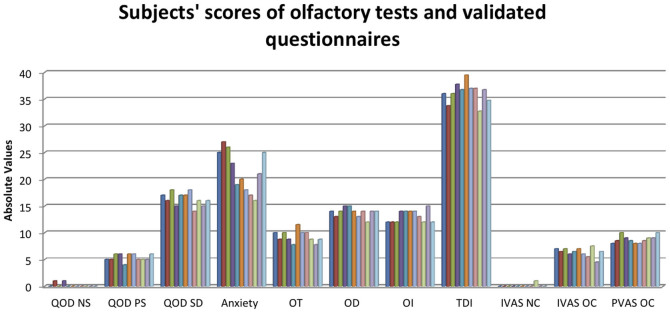
QOD NS: questionnaire of olfactory disorders - negative statements; QOD PS: questionnaire of olfactory disorders – postitive statements; QOD SD: questionnaire of olfactory disorders – socially desired statement; Anxiety: self-rating anxiety scale; OT: odor threshold; OD: odor discrimination; OI: odor identification; TDI: sum of OT, OD and OI; IVAS NC: intensity visuo-analogue scale after neutral condition; IVAS OC: intensity visuo-analogue scale after olfactory condition; PVAS OC: pleasantness visuo-analogue scale after olfactory condition.
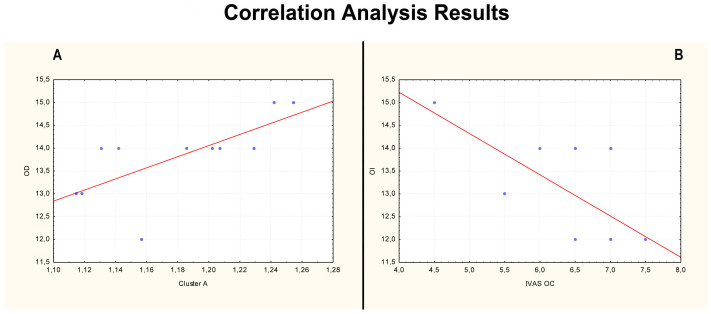
The correlation analysis resulted in significant (p < 0.05) positive correlation (r = 0.78) between Cluster A and the scores of Odor Discrimination (OD) (Figure 4A). Moreover, a significant (p < 0.05) negative correlation (r = −0.65) was found between Odor Identification (OI) and Intensity Visuo-Analogue Scale scores after the OC (IVAS OC) (Figure 4B).
Figure 4. A) Positive correlation (r = 0.78) between odor discrimination (OD) and Cluster A and B) negative correlation (r = −0.65) between odor identification (OI) and intensity visuo-analogue scale after olfactory condition (IVAS OC).
Discussion
A review of the literature since the original work of Zatorre in 199210 to now, shows that many functional and structural imaging techniques were applied to study the olfactory system using a number of different olfactory tasks. Thus, due to the heterogeneity of methods used across the studies the interpretation and comparison of results from these experiments is often difficult4,5,6,7,8,9,10,11,12,13,14,15,16,17. Furthermore, many results support the idea that the neural correlates of odor processing are strongly task dependent, involving a distributed network of structures - even outside olfactory core regions - determined by the nature of the context and of the olfactory and sensory task17.
Although as compared to fMRI and H215O-PET/CT the temporal resolution of brain activation as recorded by FDG-PET is inferior, in our study we could replicate for the first time - due to the robust within subjects experimental model allowing fairly good statistics - the results previously found by these two techniques.
In particular, completing data from previous neuroimaging studies, the first interesting finding is that OC might increase the activity in the entorhinal cortex and hippocampus, as well as in PHG8, suggesting that the visual cortex may be involved in visual-olfactory interaction12,18,19,20 and addressing the decreased glucose uptake found in OFC to the ‘on-off switch' behavior16. This region was similarly activated in response to all odor mixtures and deactivated in response to single odorants. Interestingly, by means of the found positive correlation (r = 0.78; Figure 4A) between the OD scores and the relative regional cerebral glucose metabolism (rCGM) increase in OFC during the NC (Cluster A), the present data add further novelty in understanding the OFC-related role in olfactory discrimination. In fact, this aspect suggest that a relative OFC rCGM increase, during resting condition, is always present when the cortical olfactory system is trying to discriminate odors – even if they are not present as in NC - and that its relative rCGM decrease, achieved by means of a single and pure odor condition (as is OC) could be accompanied by a decrease in OD. Moreover the found OFC activation/deactivation pattern and the relative correlation with OD confirm and further expand data previously shown by Boyle and colleagues16.
In addition, for the first time, an rCGM increase in DoC was found when comparing OC to NC. According to the sensorimotor-cognitive dichotomy21,22 and to comparative anatomy of cerebellum for which the Larsell' DoC23 is actually thought to be overlapped with Lobule VI, it appears feasible that a OC-related rCGM increase, found for the first time in the present within-subject pure and passive olfactory condition devoid of attentional tasks, could be not only related to attentional processes and maintenance of the sniffing magnitude24 but also to the olfactory condition ‘per se'. This intriguing data could not only confirm the role of DoC in estimating the valence of salient, emotional cues and selecting appropriate behavioral responses25, but add further contributions in unraveling many roles postulated regarding the olfactory-related cerebellar involvement24.
Noticeably, the present study found a global leftward asymmetry both when comparing OC to NC and viceversa. Previous olfactory neuroimaging studies have demonstrated a bilateral activation in primary olfactory cortex and greater activation in the right than in the left orbitofrontal cortex17,26,27. Possible explanation for this inconsistency could be related to the experimental model setting in which any retrieval/recognition task of the incoming stimulus was generated. Regarding these aspects, a switching of lateralization from right to the left hemisphere associated to a switch from recognition to semantic processing of the on-line information, for both verbal and non-verbal materials, has been widely assessed by the hemispheric encoding/retrieval asymmetry model28.
Furthermore, the improved sensitivity of the state-of-the-art PET cameras allows to detect reliable signal changes also under sub-optimal conditions. The possibility to create a defined ecologic baseline condition to the exploration of olfactory neural underpinnings helped in avoiding possible cortical activation related to unwanted attentional processes and odour sensitivity enhancement29 due to the examination environment.
This increases the dynamic range of brain metabolism as an informative correlate of neural activity. Thus, this notion of baseline implies that during a particular task not only activation is observed but also deactivation in certain areas is found3 and depicts an organized default mode network (DMN)30 in which some regions are most active during resting state30,31,32. These regions have been thought to be involved in monitoring the internal and external milieu30,31,32,33,34 and an emerging point is that the DMN is composed of a group of relatively large and interconnected areas, including the ACC and ventromedial prefrontal cortex (BA 10)30. Hence, when introducing a pure odor compound in the present experimental model a cortical reorganization pattern from anterior (OFC and ACC in NC) to posterior (Cu, LG and PHG in OC) and subcortical (DoC) regions was in line with Magistretti's observations. This specific neural behavior could suggest, for the first time, a possible rearrangement of cortical activity when experimenting a pure and passive olfactory stimulation condition.
To this end, Visual, Somatosensory and Cerebellum hub networks, including PHG, Cu, LG and DoC have been previously found to be negatively correlated with DMN activity, suggesting that an anticorrelated activity between this network and the former regions could be engaged by sensory processing tasks35.
Finally, by using a panel of the above-mentioned VQ we had the opportunity to cluster the subjects sample in a normal range and, thus, to correlate them each other. Interestingly a significant negative correlation (r = −0.65; Figure 4B) between a quantitative aspect of a pure odorant (IVAS OC) with a qualitative one (OI) was found in the present study by using validated tests36. In particular this trend of correlation could depict a daily life aspect in which an increase in subjective intensity of a pure odorant target is commonly associated to reduction in general odor identification. On the other side, it could partially offer an explanatory model, at normal subjects level, of the peculiar pathological behavior for which a higher level of intensity score with a less percentage of identification was found in some mood affected patients37. However, literature is still lacking of a neurobiological correlate of these aspects in homogeneous experimental conditions. Nevertheless, this pattern of correlation could suggest a connected sway of these tests and could advantage future investigations regarding psychophysical dimensions of olfaction, by starting out a defined cluster of psychological and neurobiological results.
Methods
Subjects
Eleven right-handed individuals (six women and five men; mean age 45.7 ± 11 years) without otorhinolaryngologic or neurological diseases were enrolled in the study. All of them were assessed as normosmic when evaluated with the multiple-forced choice Sniffin' Sticks Screeinng test38 in four main domains: Odor Threshold (OT), Odor Discrimination (OD), Odor Identification (OI) and their sum (TDI).
A detailed case history was collected for all subjects who underwent Ear-Nose-Throat examination with fiberoptic check of the upper airways. Neurological diseases were excluded with the Mini-Mental State Examination and MRI. All those conditions that could potentially develop an olfactory dysfunction were considered as exclusion criteria. Thus, patients with sino-nasal disorders or surgery history, head trauma, neuro-psychiatric disorders (Parkinson's disease, Alzheimer's disease, schizophrenia, multiple sclerosis, depression and Multiple Chemical Sensitivity/Idiopatic Environmental Intolerance), lower airways and/or lung diseases, active hepatitis, cirrhosis, chronic renal failure, Vitamin B12 deficiency, alcohol, tobacco or drug abuse, cerebral vascular accidents, insulin dependent diabetes mellitus, hypothyroidism and Cushing Syndrome, were not included in the study.
Moreover, we excluded all patients in treatment with drugs that could interfere with 18F -FDG uptake and distribution in the brain39. No patients were pregnant or breastfeeding.
The Ethics Committee of the “Tor Vergata” University School of Medicine approved the protocol research. The study adhered to the principles of the Declaration of Helsinki and all of the participants provided written informed consent after receiving a detailed explanation of the study.
Experimental Procedure
All subjects underwent FDG-PET/CT after a neutral olfactory stimulation by using a common aerosol facial mask containing in its ampoule only 5 ml of saline sodium chloride (NaCl) 0.9% (Neutral Condition – NC). After one month, the same sample of individuals underwent a second FDG-PET/CT scan after a pure olfactory stimulation by using the same aerosol facial mask containing in its ampoule a solution of 1.5 ml of VAN 100% (Dacor Ltd | Sarandrea Ltd) and 5 ml of saline sodium chloride (NaCl) 0.9% (Olfactory Condition – OC). In both conditions oxygen flow rate was conveyed at 3.5 liters per minute and the stimulation consisted of one continuous 9 minutes block without any sniffing generated instructions. At the end of the third minute, each subject was injected with 3 MBq/Kg (210–350 MBq) of FDG i.v. and the olfactory stimulation continued for six more minutes. After both NC and OC all subjects laid down in a semi-darkened, noiseless and odorless room, without any artificial stimulation, with their eyes closed for 20 minutes.
PET/CT scanning
The PET/CT system Discovery ST16 (GE Medical Systems, TN, USA) was used for the whole population in exam. This system combines a high-speed ultra 16-detector-row (912 detectors per row) CT unit and a PET scanner with 10080 bismuth germanate (BGO) crystals in 24 rings with a 128 × 128 matrix. Crystal size 6,2 × 6,2 × 30 mm, coincidence window 11,7 nsec, system sensitivity 9,3 cps/kBq in 3D mode, dispersion fraction 44%, maximum count rate in cps at 50% dead time 63 kcps @ 12 kBq/mL (3-D), axial FWHM 1 cm radius 5,2 mm in 3D mode, axial FOV 157 mm.
Before and after the FDG injection, hydratation (500 ml of i.v. NaCl) 0.9%) was performed in order to reduce the pooling of the radiotracer in the kidneys. All subjects had normal serum glucose level and fasted for at least 5 h before the 18F -FDG injection.
Validated questionnaires (VQ)
Before undergoing to olfactory stimulation all subjects completed the following VQ:
Questionnaire of Olfactory Disorders (QOD) - completed only before NC phase - to assess daily life problems due to olfactory impairment, we used a short modified version of an olfactory-specific QOD developed by Frasnelli and Hummel40 which has recently been used by many researchers41. The modified short version of the QOD consists of 25 statements that are divided into three general domains: 17 negative statements (QOD-NS), two positive statements (QOD-PS), and six socially desired statements (QOD-SD);
Zung Self-Rating Anxiety Scale42 (Anxiety) - completed only before NC phase – is a 20-item scale with some of the items keyed positively and some negatively on a four-point scale ranging from 1 = none or a little of the time to 4 = most or all of the time. The final score ranges from 20–80, a score between 20 and 44 is considered in the normality range, 45–59 is mild to moderate anxiety, 60–74 is severe, and 75–80 is very severe;
Intensity Visuo-Analogue Scale (IVAS), in which each subject may score from 0 to 10 the subjective intensity of olfactory perception after both NC and OC37;
Pleasantness Visuo-Analogue Scale (PVAS), in which each subject may score from 0 to 10 the subjective pleasantness of olfactory perception after the OC37.
Statistical analysis
FGD-PET/CT
Differences in brain FDG uptake were analyzed using statistical parametric mapping (SPM2, Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab 6.5 (Mathworks, Natick, Massachusset, USA). PET data were subjected to affine and non-linear spatial normalization into the MNI space. The spatially normalized set of images were then smoothed with a 8 mm isotropic Gaussian filter to blur individual variations in gyral anatomy and to increase the signal-to-noise ratio. Images were globally normalized using proportional scaling to remove confounding effects to global CBF changes, with a threshold masking of 0.8. The resulting statistical parametric maps, SPM{t}, were transformed into normal distribution (SPM{z}) unit. Correction of SPM coordinates to match the Talairach coordinates was achieved by the subroutine implemented by Matthew Brett (http://www.mrc-cbu.cam.ac.uk/Imaging). Brodmann areas (BAs) were then identified at a range of 0 to 3 mm from the corrected Talairach coordinates of the SPM output isocentres, after importing them by Talairach client (http://www.talairach.org/index.html). A statistical height thresholds equal or lower than p < 0.05 at both cluster and voxel level was accepted as significant. A statistical height thresholds equal or lower than p < 0.05 at voxel level and p < 0.05 corrected for multiple comparison at cluster level was accepted as significant.
This more liberal choice was adopted to avoid type II errors attributable to overconservative thresholds43. In fact, given the exploratory nature of this analysis and considering the relatively low sensitivity of PET without repeated measures, higher thresholds could lead to false-negative results in PET studies. Only those clusters containing more than 125 (5 × 5 × 5 voxels, i.e. 11 × 11 × 11 mm) contiguous voxels were accepted as significant, based on the calculation of the partial volume effect resulting from the spatial resolution of the PET camera (about the double of FWHM).
The OC vs NC comparisons were performed by means of the ‘compare populations: 1 scan/subject (Ancova)' option, using age and sex as covariates.
VQ and Correlation Analysis
Data description included calculation of means and standard deviations (SD) for all VQ. In addition, Spearman's rank correlation was performed between QOD (and relative subscales), Anxiety, OT, OD, OI, IVAS NC, IVAS OC and PVAS OC. (STATISTICA 7 package for Windows).
Moreover, in order to evaluate the correlations between brain metabolism and subjective variables, the clusters of voxels found to be significantly different from zero in the NC – OC and OC – NC comparisons were segmented and the semi-quantitative raw data values in each of them were individually normalized to the relative FDG uptake in the cerebellum. All values were then transformed into z-scores and submitted to statistical analyses seeking for Spearman's rank correlations with VQ total scores. The correlation analyses have been performed considering the values at NC and OC (as well as the relative VQ scores) as a continuum.
Author Contributions
Conceived and designed the experiments: M.A., A.M., A.C., O.S. Performed the experiments: M.A., A.M., A.C., B.D.P., E.B. Analyzed the data: A.M., M.P., M.A., M.C. Contributed reagents/materials/analysis tools: A.C., M.P., O.S., B.D.P., E.B. Wrote the paper: A.M., M.A., M.C., M.P.
References
- Mazard A. et al. Impact of fMRI acoustic noise on the functional anatomy of visual mental imagery. J. Cogn. Neurosci. 14, 172–186 (2002). [DOI] [PubMed] [Google Scholar]
- Benjamin C. et al. The influence of rest period instructions on the default mode network. Front. Hum. Neurosci. 4, 218 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti P. J. Neuron-glia metabolic coupling and plasticity. J. Exp. Biol. 209, 2304–2311 (2006). [DOI] [PubMed] [Google Scholar]
- Savic I. Brain imaging studies of the functional organization of human olfaction. Chem. Senses 30, i222–i223 (2005). [DOI] [PubMed] [Google Scholar]
- Savic-Berglund I. Imaging of Olfaction and Gustation. Nutr. Rev. 62, 205–207 (2004). [DOI] [PubMed] [Google Scholar]
- Hillert L., Musabasic V., Berglund H., Ciumas C. & Savic I. Odor processing in multiple chemical sensitivity. Hum. Brain Mapp. 28, 172–182 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp R., Sommer M., Müller J. & Hajak G. Olfactometry in fMRI studies: odor presentation using nasal continuous positive airway pressure. Acta Neurobiol. Exp. 64, 171–176 (2004). [DOI] [PubMed] [Google Scholar]
- Kjelvik G., Evensmoen H. R., Brezova V. & Haberg A. K. The human brain representation of odor identification. J. Neurophysiol. 108, 645–657 (2012). [DOI] [PubMed] [Google Scholar]
- Sobel N. et al. Sniffing and smelling:separate subsystems in the human olfactory cortex. Nature 392, 282–286 (1998). [DOI] [PubMed] [Google Scholar]
- Zatorre R. J., Jones-Gotman M., Evans A. C. & Meyer E. Functional localization and lateralization of human olfactory cortex. Nature 360, 339–340 (1992). [DOI] [PubMed] [Google Scholar]
- Bengtsson S., Berglund H., Gulyas B., Cohen E. & Savic I. Brain activation during odor perception in males and females. Neuroreport 12, 2027–33 (2001). [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B. & Murphy C. Neural substrates of cross-modal olfactory recognition memory: An fMRI study. Neuroimage 31, 386–396 (2006). [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B. & Murphy C. Age-related differences in the neural substrates of cross-modal olfactory recognition memory: an fMRI investigation. Brain Res. 1285, 88–98 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet J. P. et al. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J. Neurosci. 20, 7752–7759 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken D. A. et al. Olfactory system activation from sniffing: effects in piriform and orbitofrontal cortex. Neuroimage 22, 456–465 (2004). [DOI] [PubMed] [Google Scholar]
- Boyle J. A., Djordjevic J., Olsson M. J., Lundstrom J. M. & Jones-Gotman M. The Human Brain Distinguishes between Single Odorants and Binary Mixtures. Cereb. Cortex 19, 66–71 (2009). [DOI] [PubMed] [Google Scholar]
- Savic I. & Gulyas B. PET shows that odors are processed both ipsilaterally and contralaterally to the stimulated nostril. Neuroreport 11, 2861–2866 (2000). [DOI] [PubMed] [Google Scholar]
- Royet J. P. et al. Functional anatomy of perceptual and semantic processing for odors. J. Cogn. Neurosci. 11, 94–109 (1999). [DOI] [PubMed] [Google Scholar]
- Qureshy A. et al. Functional Mapping of Human Brain in Olfactory Processing: A PET Study. J. Neurophysiol. 84, 1656–1666 (2000). [DOI] [PubMed] [Google Scholar]
- Kroupi E., Yazdani A., Vesin J. M. & Ebrahimi T. Multivariate spectral analysis for identifying the brain activations during olfactory perception. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 6172–6175 (2012). [DOI] [PubMed] [Google Scholar]
- Manni E. & Petrosini L. A century of cerebellar somatotopy: a debated representation. Nat. Rev. Neurosci. 5, 241–9 (2004). [DOI] [PubMed] [Google Scholar]
- Stoodley C. J. & Schmahmann J. D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44, 489–501 (2009). [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D. et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage 10, 233–60 (1999). [DOI] [PubMed] [Google Scholar]
- Moscovich M. et al. Olfactory impairment in familial ataxias. J. Neurol. Neurosurg. Psychiatry 83, 970–4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C. et al. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 29, 8586–94 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre R. J. & Jones-Gotman M. [Functional imaging of the chemical senses]. Brain Mapping: The Applications [Toga, A. W., Mazziotta, J. C. (ed.)] [403–424] (Academic Press, San Diego, 2000). [Google Scholar]
- Olofsson J. K. et al. Laterality of the olfactory event-related potential response. Chem. Senses 31, 699–704 (2006). [DOI] [PubMed] [Google Scholar]
- Habib R., Nyberg L. & Tulving E. Hemispheric asymmetries of memory: the HERA model revisited. Trends Cogn. Sci. 7, 241–245 (2003). [DOI] [PubMed] [Google Scholar]
- Åhs F., Miller S. S., Gordon A. R. & Lundström J. N. Aversive learning increases sensory detection sensitivity. Biol. Psychol. 92, 135–41 (2013). [DOI] [PubMed] [Google Scholar]
- Sheline Y. I. et al. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. 106, 1942–1947 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E. & Snyder A. Z. A default mode of brain function: a brief history of an evolving idea. Neuroimage 37, 1083–1090 (2007). [DOI] [PubMed] [Google Scholar]
- Buckner R., Andrews-Hanna J. & Schacter D. The brain's default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38 (2008). [DOI] [PubMed] [Google Scholar]
- Pagani M. et al. Brief Report: alterations in cerebral blood flow as assessed by PET/CT in adults with autism spectrum disorder with normal IQ. J. Autism. Dev. Disord. 42, 313–8 (2012). [DOI] [PubMed] [Google Scholar]
- Micarelli A., Jacobsson H., Larsson S. A., Jonsson C. & Pagani M. Neurobiological insight into hyperbaric hyperoxia. Acta Physiol. (Oxf) 209, 69–76 (2013). [DOI] [PubMed] [Google Scholar]
- Tomasi D. & Volkow N. D. Association between Functional Connectivity Hubs and Brain Networks. Cereb. Cortex 21, 2003–2013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burón E. & Bulbena A. Olfaction in affective and anxiety disorders: a review of the literature. Psychopathology 46, 63–74 (2013). [DOI] [PubMed] [Google Scholar]
- Lombion-Pouthier S., Vandel P., Nezelof S., Haffen E. & Millot J. L. Odor perception in patients with mood disorders. J. Affect. Disord. 90, 187–91 (2006). [DOI] [PubMed] [Google Scholar]
- Kobal G., Hummel T., Sekinger B., Barz S., Roscher S. & Wolf S. “Sniffin' sticks”: screening of olfactory performance. Rhinology 34, 222–226 (1996). [PubMed] [Google Scholar]
- Varrone A. et al. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur. J. Nucl. Med. Mol. Imaging 36, 2103–2110 (2009). [DOI] [PubMed] [Google Scholar]
- Simopoulos E. et al. Olfaction-Associated Quality of Life in Chronic Rhinosinusitis: Adaptation and Validation of an Olfaction-Specific Questionnaire. Laryngoscope 122, 1450–1454 (2012). [DOI] [PubMed] [Google Scholar]
- Alessandrini M. et al. Intranasal administration of hyaluronan as a further resource in olfactory performance in multiple chemical sensitivity syndrome. Int. J. Immunopathol. Pharmacol. 26, 1019–25 (2013). [DOI] [PubMed] [Google Scholar]
- Zung W. W. & Gianturco J. A. Personality dimension and the Self-Rating Depression Scale. J. Clin. Psychol. 27, 247–8 (1971). [DOI] [PubMed] [Google Scholar]
- Oishi N. et al. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology 65, 1708–1715 (2005). [DOI] [PubMed] [Google Scholar]