Abstract
Local weather factors are widely considered to influence the transmission of infectious gastroenteritis. Few studies, however, have examined the non-stationary relationships between global climatic factors and transmission of infectious gastroenteritis. We analyzed monthly data for cases of infectious gastroenteritis in Fukuoka, Japan from 2000 to 2012 using cross-wavelet coherency analysis to assess the pattern of associations between indices for the Indian Ocean Dipole (IOD) and El Niño Southern Oscillation (ENSO). Infectious gastroenteritis cases were non-stationary and significantly associated with the IOD and ENSO (Multivariate ENSO Index [MEI], Niño 1 + 2, Niño 3, Niño 4, and Niño 3.4) for a period of approximately 1 to 2 years. This association was non-stationary and appeared to have a major influence on the synchrony of infectious gastroenteritis transmission. Our results suggest that non-stationary patterns of association between global climate factors and incidence of infectious gastroenteritis should be considered when developing early warning systems for epidemics of infectious gastroenteritis.
Infectious gastroenteritis contributes significantly to the 1 billion episodes of diarrhea and 3 million deaths in children under 5 years of age per year, and is the fifth-leading cause of death worldwide1,2,3,4. The transmission of infectious gastroenteritis is complex and multifactorial, involving both host and environmental factors.
Local weather factors such as temperature, relative humidity, and rainfall have been suggested as important factors in the spread and seasonality of infectious gastroenteritis5,6,7,8,9,10,11,12. In addition to local weather factors, several studies reported that the El Niño Southern Oscillation (ENSO) and Indian Ocean Dipole (IOD) play important roles in the transmission of infectious diseases, including dengue13,14,15,16, malaria17,18,19,20,21 and cholera22,23,24,25. The ENSO is the most prominent source of interannual global climate variability which affects weather conditions, such as temperature, rainfall, wind speed and direction, and storm tracking throughout the world20,26. These effects, however, fluctuate and vary from region to region20,26. The IOD is another global climate phenomenon that arises from ocean-atmosphere interactions which affect climate patterns in the tropical Indian Ocean17,20,22,24. Moreover, the World Health Organization (WHO) quantified the impact of global warming on diarrhea, and reported that warming by 1°C was associated with a 5% increase in diarrhea27. Although regional differences and contrasting effects of temperature on different kinds of diarrhea are evident28, few studies have examined the non-stationary associations of infectious gastroenteritis and global climate variability.
Wavelet analysis is useful in the investigation of non-stationary associations using time series data29 as it can measure associations (coherency) between two time-series at any frequency (period) band and time-window period. This analysis has been used to determine whether the presence of a particular periodic cycle at a given time in disease incidence corresponds to the presence of the same periodical cycle at the same time in an exposure covariate29. Wavelet analyses have been used to analyze the transmission of infectious diseases13,14,17,22,30,31. Therefore a better understanding of the sensitivity of these analyses to climate variability might help develop a reliable climate-based prediction system for epidemics of gastroenteritis.
Here, we explored the time-varying relationship between climate variation and monthly incidence of infectious gastroenteritis between 2000 and 2012 in Fukuoka, Japan. To our knowledge, this is the first report to quantify the time-varying impact of climatic factors on the number of infectious gastroenteritis cases using cross-wavelet analysis.
Results
A total of 654,254 cases of infectious gastroenteritis from 2000 to 2012 were included in our analyses, of which 392,514 (60.0%) were children aged 0 to 4 years, 171,750 (26.3%) aged 5 to 9 years, 47,541 (7.3%) aged 10 to 14 years, and 42,449 (6.5%) aged 15 years or older.
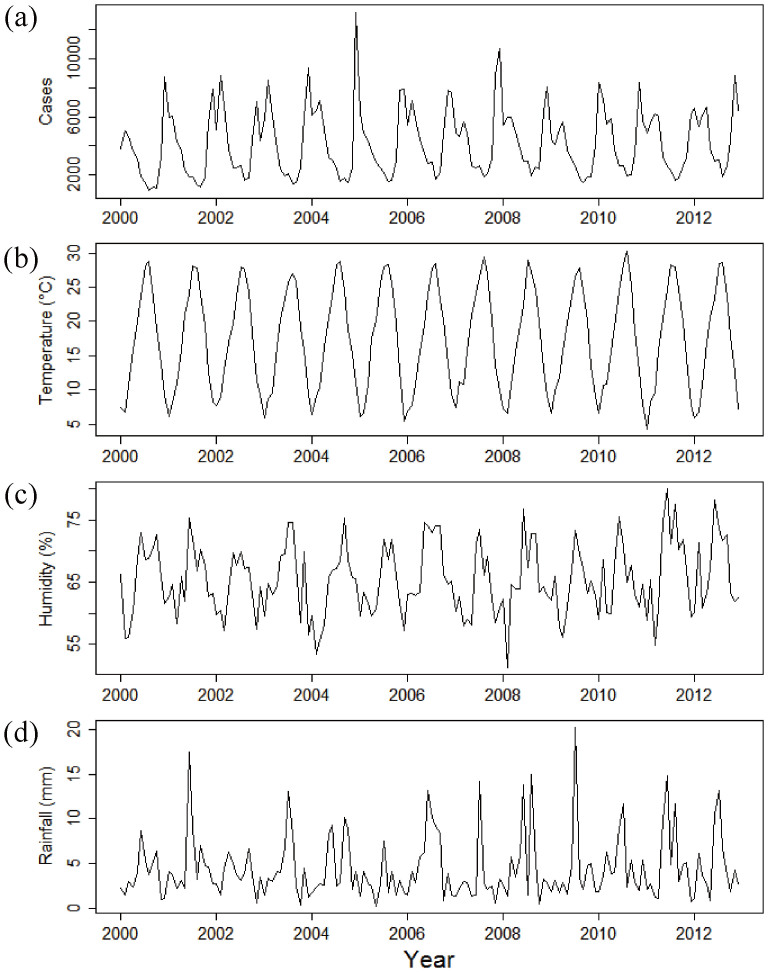
The time series for the number of infectious gastroenteritis cases per month, ambient temperature, relative humidity and rainfall during the study period are shown in Figure 1. As noted above, the incidence of infectious gastroenteritis displays a seasonal pattern in temperate areas, with marked peaks in winter (Fig. 1).
Figure 1. Monthly time series data in Fukuoka, Japan (2000–2012).
(a) Infectious gastroenteritis cases; (b) Temperature; (c) Relative humidity; (d) Rainfall.
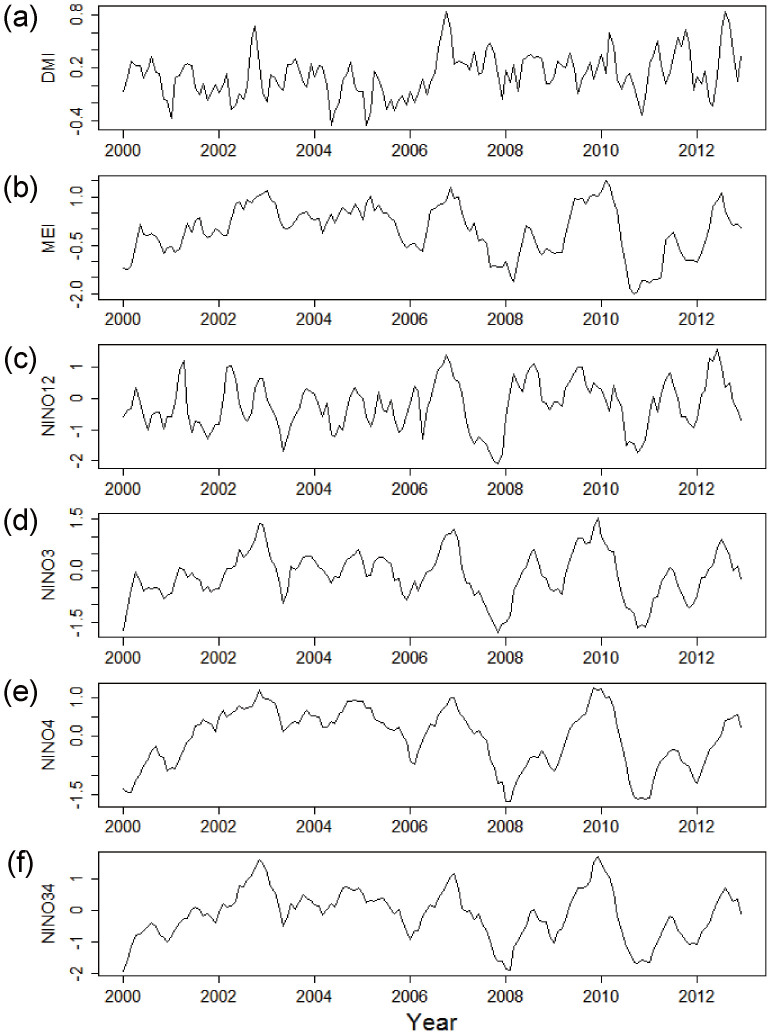
The time series for DMI and ENSO indices (MEI, Niño 1 + 2, Niño 3, Niño 4, and Niño 3.4) during the same period are shown in Figure 2. Strong positive IOD events (indicated by large DMI values) occurred in 2006, with a peak DMI in October, and in 2012, with a peak DMI in August. Strong ENSO events (indicated by large MEI values) were observed in 2006 and again in 2009 to 2010 (Fig. 2).
Figure 2. Monthly time series data for global climatic indices (2000–2012).
(a) DMI (dipole mode index); (b) MEI (multivariate ENSO index); (c) Niño 1 + 2 (ENSO index); (d) Niño 3 (ENSO index); (e) Niño 4 (ENSO index); (f) Niño 3.4 (ENSO index).
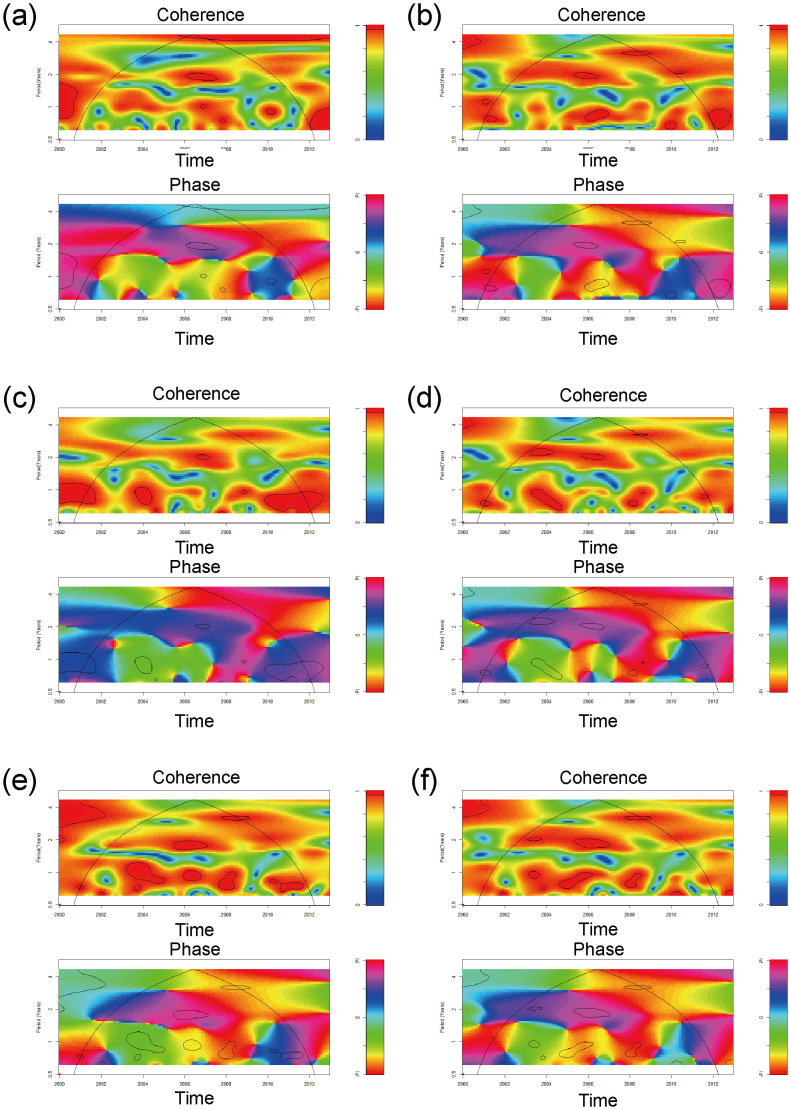
Cross-wavelet coherence and cross-wavelet phase analysis of the global climatic time series (DMI and ENSO indices) with infectious gastroenteritis cases by month are shown in Figure 3. The wavelet coherence provides information about whether two non-stationary signals are linearly correlated at a particular time and frequency14,30. The wavelet coherence is equal to 1 when there is a perfect linear relationship at a particular time and frequency between the two signals14,30. In fact, cross-wavelet coherency analysis revealed that infectious gastroenteritis cases were significantly (p < 0.05) coherent with DMI for 2 years (2006–2007) and 1 year (2010). With regard to ENSO indices, MEI was significantly coherent at periods of approximately 1 to 2 years (2005–2006). Niño 1 + 2 was significantly coherent at a period of approximately 1 year (2003–2004) and approximately 2 years (2006). Niño 3, Niño 4, and Niño 3.4 were significantly coherent at a period of approximately 1 to 2 years between 2003 to 2008. DMI and ENSO indices were predictive of disease data, as shown by the cross-wavelet phases (Fig. 3). All local weather factors had non-stationary relationships with both DMI and ENSO indices (Supplementary Fig. S1).
Figure 3. Cross-wavelet coherency and phase of the infectious gastroenteritis time series with global climatic indices.
(a) DMI (dipole mode index); (b) MEI (multivariate ENSO index); (c) Niño 1 + 2 (ENSO index); (d) Niño 3 (ENSO index); (e) Niño 4 (ENSO index); (f) Niño 3.4 (ENSO index). Red regions in the upper part of the plots indicate frequencies and times for which the two series share variability. Cone of influence (within which results are not influenced by the edges of the data) and significant coherent time-frequency regions (p < 0.05) are indicated by solid lines. In cross-wavelet phase plots, colors correspond to different lags between the variability in the two series for a given time and frequency, measured in angles from −PI to PI. A value of PI corresponds to a lag of 16 months.
Discussion
In this study, we observed that the monthly incidence of infectious gastroenteritis cases is significantly associated with changes in the IOD and ENSO (MEI, Niño 1 + 2, Niño 3, Niño 4, and Niño 3.4) for the 1- to 2-year periodic mode. This association is non-stationary and appears to have a major influence on the synchrony of infectious gastroenteritis transmission. These findings indicate that early warning systems for epidemics of infectious gastroenteritis should consider non-stationary, and possibly non-linear patterns of association between climatic factors and infectious gastroenteritis cases.
Predicting the potential effects of changes in climate on the incidence and distribution of infectious gastroenteritis requires an understanding of the relationship between non-stationary climate and transmission of disease. Our results demonstrate that the incidence of infectious gastroenteritis is strongly associated with non-stationary climate variables, including DMI and ENSO indices, with coherent cycles of approximately 1 to 2 years. A study in Peru reported that during El Niño there was a marked increase in the number of cases of diarrhea and dehydration in infants and young children32. Further, another study in Peru indicated that the number of hospital admissions for infectious gastroenteritis increased during the El Niño event, and that this increase was greater than that explained by seasonal temperature changes5. Thus, our results are consistent with previous studies and demonstrate the importance of climate change in the spread of infectious gastroenteritis.
Extreme IOD and El Niño are two dominant drivers of year-to-year climate variability33. In recent observations, IOD and El Niño repeatedly co-occurred, indicating the interactive nature of the two major climate modes34. A recent study indicated that the warming of the Indian Ocean relative to that of the Pacific might play an important role in the climate changes of the latter35. Another study suggested that the Indian Ocean might significantly enhance El Niño and its forecast of onset, which in turn enhances the IOD and its long-range predictability33. Thus, our combined IOD and ENSO results demonstrate the importance of global climate factors on the transmission of infectious gastroenteritis.
Our study also found that the incidence of infectious gastroenteritis is strongly associated with local weather factors, such as temperature, relative humidity, and rainfall, with coherent cycles throughout the year. The positive relationship between infectious gastroenteritis cases and temperature, humidity, and rainfall in our present study is broadly consistent with previous studies in Japan, China, Peru, Fiji, and Bangladesh5,8,9,11,12,36. Further, temperature increases were suggested to be positively associated with Salmonella, Campylobacter, and Escherichia coli28,37,38,39,40. Relative humidity and rainfall also affect water and sanitation infrastructure and the number of pathogens, and might impact the replication rate of certain bacterial and viral pathogens28. Our results suggest that previous findings based on this assumption can be improved by assuming a non-stationary association and more accurately evaluating the possible non-linear association between climatic factors and infectious gastroenteritis transmission.
Although data on the cause of infectious gastroenteritis were not available for analysis, a striking increase was suggested for both the number of cases of bacterial diarrhea during summer and of rotavirus during winter41. Hot weather might cause certain behavioral patterns, such as higher water consumption and less conscientious hygiene practices, which promote diarrhea transmission6. In addition, this study found long-term lag effects of global climate variability on infectious gastroenteritis transmission, which might be related to the fact that hot weather affects contamination in food production and distribution systems37,39. This suggests that temperature control during food production, processing, transport, preparation or storage might interact with weather patterns and thus have an impact on the risk of disease. For childhood infectious diseases, another study also suggested that periodic diseases become chaotic with introduction of low-level vaccination42. The Ministry of Health, Labour and Welfare, Japan has not adopted routine rotavirus vaccination, and the results of this study might be affected by the effects of rotavirus vaccination. This characteristic may also indicate the need for more precise modeling to elucidate the complex relationships of global climate variability and infectious gastroenteritis transmission, and further investigation would be a critical topic for future study. Although other behavioral factors unrelated to changes in climate might also affect the seasonality of diarrhea, such as changes in patterns of food availability, we have no evidence for such factors.
Our findings with the IOD and ENSO indices support the recent proposal that large-scale climate indices might be more useful for forecasting ecological effects than local weather variables43. Climate can affect the transmission of infectious diseases through several linear and non-linear pathways in a host population. Climate can also affect several biological traits of the organisms involved in the life cycle of parasites, from individual life histories to population dynamics44. The WHO and the Intergovernmental Panel on Climate Change (IPCC) have identified changes in the incidence of diarrhea as one of the most important future health effects of climate change45. While local weather most likely only affects the biological components of disease transmission, large-scale climate patterns might also influence contextual components of disease transmission, such as population susceptibility.
Our results for infectious gastroenteritis in this paper do not consider etiology despite the fact that various pathogens have different seasonal patterns. As the response of bacterial and viral pathogens to the effects of climate variability might differ, our results might only reflect the responses of the most common cause of infectious gastroenteritis. Our results should therefore be interpreted with caution, and further studies on the effects of climate variability on specific pathogen-induced infectious gastroenteritis are warranted.
The present study has several limitations. First, not all cases in the community are represented in the surveillance data. This under-reporting can occur anywhere in the reporting chain, from the initial decision of a patient not to seek health care to failure to record cases in the disease registry. However, we consider this did not result in substantial bias because the degree of under-reporting is unlikely to have varied over time. Second, the participating sentinel medical institutions were recruited on a voluntary basis. However, this did not pose a threat to the validity of the comparisons over time, which is the subject of this study. Third, we analyzed monthly data, spanning from 2000 to 2012. Modeling accuracy of these associations would be improved by a longer study period or more detailed data.
The results of this study have practical implications for public health officials. Elucidation of the relationship between climate variability and infectious gastroenteritis transmission is important for disease control and prevention. The present findings may help public health officials to predict epidemics and prepare for the effects of climatic change on infectious gastroenteritis through the implementation of preventive public health measures, such as warning health workers, active community-based campaigns promoting oral rehydration, increasing staff and the number of beds in oral rehydration units in hospitals, early warning weather forecasts, and improving infectious gastroenteritis control programs.
In conclusion, this study found quantitative evidence that non-stationary associations have a major influence on synchrony between changes in both the IOD and ENSO and the monthly incidence of infectious gastroenteritis cases for the 1- to 2-year periodic mode. Our results suggest that non-stationary patterns of association between global climate factors and infectious gastroenteritis cases should be considered when developing early warning systems for epidemics of infectious gastroenteritis.
Methods
Data sources
Under the Infectious Disease Control Law, the number of infectious gastroenteritis patients is reported on a weekly basis from 120 sentinel medical institutions in Fukuoka Prefecture in southwest Japan11,12. A case of infectious gastroenteritis is defined by clinical factors of sudden stomach ache, vomiting, and diarrhea. However, under this law it is not necessary for sentinel medical institutions to report information from laboratory tests. We therefore obtained clinical data that were recorded and reported by sentinel volunteers to the Fukuoka Institute of Health and Environmental Sciences, which is the municipal public health institute of the Fukuoka Prefectural Government. Monthly cases of infectious gastroenteritis were calculated from daily records based on the day of diagnosis. Large outbreaks were removed from the data set.
The strength of the IOD was measured by the Dipole Mode Index (DMI), defined as the difference in sea surface temperature (SST) anomalies between the western (10°S–10°N, 50°–70°E) and eastern (10°S–0°, 90°–110°E) tropical Indian Oceans46. DMI data were obtained from the Japan Agency for Marine-Earth Science and Technology (JAMSTEC) (http://www.jamstec.go.jp/frcgc/research/d1/iod/). The ENSO indices (i.e. Multivariate ENSO Index [MEI], Niño 1 + 2, Niño 3, Niño 4 and Niño 3.4) were also extracted from the National Oceanic and Atmospheric Administration (NOAA) Climate Prediction Center data (http://www.cpc.ncep.noaa.gov).
We also obtained data on daily average temperature, relative humidity and rainfall in Fukuoka Prefecture from the Japan Meteorological Agency. Monthly means for average temperatures, relative humidity and rainfall were calculated from daily records.
This study was approved by the ethics committee of the Fukuoka Prefectural Government. The requirement for written informed consent was waived. Patient records and other patient information remained anonymous and de-identified prior to analysis. All methods were carried out in accordance with approved guidelines and regulations.
Statistical analysis
We examined the frequency of reported infectious gastroenteritis cases using cross-wavelet coherency analysis17,22,29,30. This analysis enables the investigation and quantification of whether the presence of infectious gastroenteritis at a particular frequency at a given time corresponds to that of a climate covariate at the same frequency and time. Specifically, cross wavelet coherency analysis was used to determine whether a particular frequency of reported cases at a particular time during an infectious gastroenteritis epidemic corresponded to the same frequency of a climate covariate at the same time. Coherence analysis is similar to correlation analysis as it is normalized between 0 and 1, where 0 corresponds to the total absence of cycles with the same period in the analyzed time series, and 1 corresponds to the presence of cycles with exactly the same periods in the analyzed time series17,22,29,30,47. Briefly, for the two time series, the cross wavelet spectrum (CWS) is estimated over time for a series of frequencies (or period, i.e., 1/frequency). The CWS is normalized by the power spectra of two variables in the same manner that covariance is normalized by the product of the standard deviation of two variables when their correlation is estimated. Therefore, CWS can be considered equivalent to covariance. When the magnitude of the CWS is similar to the normalized product of the time series spectra the ratio is equal to 1, which indicates that the two studied time series have cycles with exactly the same periodicity17,22,29,30,47.
We also estimated the cone of influence, where inferences from the wavelet analysis outside the cone are not valid because of the manipulations performed to generate the wavelet spectrum in the absence of data for more frequent periods17,22,29,30. Cross wavelet coherence significance was estimated using the method described by Maraun and Kurths48 for a minimum of 6 months (i.e., the minimum period of interest in cycles studied with cross wavelet analysis was 6 months), with a total smoothing window of 31 (i.e., 15 months) and dimensionless parameter of 6 for the Morlet Wavelet. Further details concerning cross wavelets and software are described by Maraun and Kurths48 and Chaves and Pascual30. All statistical analyses were conducted using R version 3.0.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Author Contributions
DO made substantial contributions to conception and design, analyzed data and wrote the manuscript.
Supplementary Material
Figure s1
Acknowledgments
The authors thank the Fukuoka Prefectural Government, Department of Public Health and Medical Affairs, Division of Public Health for conducting infectious disease surveillance. This study was supported by a grant from the Ministry of Health, Labour and Welfare, Japan and research budget of the Department of Public Health and Medical Affairs, Fukuoka Prefectural Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Guerrant R. L., Hughes J. M., Lima N. L. & Crane J. Diarrhea in developed and developing countries: magnitude, special settings, and etiologies. Rev Infect Dis 12 Suppl1, S41–S50 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern C., Martines J., de Zoysa I. & Glass R. I. The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull World Health Organ 70, 705–714 (1992). [PMC free article] [PubMed] [Google Scholar]
- Kosek M., Bern C. & Guerrant R. L. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ 81, 197–204 (2003). [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). The Global Burden of Disease: 2004 Update (WHO, Geneva, 2008). [Google Scholar]
- Checkley W. et al. Effect of El Niño and ambient temperature on hospital admissions for diarrhoeal diseases in Peruvian children. Lancet 355, 442–450 (2000). [DOI] [PubMed] [Google Scholar]
- Black R. E. & Lanata C. F. [Epidemiology of diarrhoeal diseases in developing countries]. Infections of the gastrointestinal tract [Blaster, M. J., Smith, P. D., Ravdin, J. I., Greenberg, H. B., Guerrant, R. I. (ed.)] [13–16] (Raven Press, New York, 1995) [Google Scholar]
- Konno T. et al. Influence of temperature and relative humidity on human rotavirus infection in Japan. J Infect Dis 147, 125–128 (1983). [DOI] [PubMed] [Google Scholar]
- Singh R. B. et al. The influence of climate variation and change on diarrheal disease in the Pacific Islands. Environ Health Perspect 109, 155–159 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume M. et al. Association between climate variability and hospital visits for non-cholera diarrhoea in Bangladesh: effects and vulnerable groups. Int J Epidemiol 36, 1030–1037 (2007). [DOI] [PubMed] [Google Scholar]
- Hashizume M. et al. Rotavirus infections and climate variability in Dhaka, Bangladesh: a time-series analysis. Epidemiol Infect 136, 1281–1289 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onozuka D., Hashizume M. & Hagihara A. Effects of weather variability on infectious gastroenteritis. Epidemiol Infect 138, 236–243 (2010). [DOI] [PubMed] [Google Scholar]
- Onozuka D. & Hashizume M. Weather variability and paediatric infectious gastroenteritis. Epidemiol Infect 139, 1369–1378 (2011). [DOI] [PubMed] [Google Scholar]
- Thai K. T. et al. Dengue dynamics in Binh Thuan province, southern Vietnam: periodicity, synchronicity and climate variability. PLoS Negl Trop Dis 4, e747 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazelles B., Chavez M., McMichael A. J. & Hales S. Nonstationary influence of El Niño on the synchronous dengue epidemics in Thailand. PLoS Med 2, e106 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest A., Tan S. B. & Wilder-Smith A. Meteorological factors and El Niño Southern Oscillation are independently associated with dengue infections. Epidemiol Infect 140, 1244–1251 (2012). [DOI] [PubMed] [Google Scholar]
- Johansson M. A., Cummings D. A. & Glass G. E. Multiyear climate variability and dengue-El Niño southern oscillation, weather, and dengue incidence in Puerto Rico, Mexico, and Thailand: a longitudinal data analysis. PLoS Med 6, e1000168 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume M., Chaves L. F. & Minakawa N. Indian Ocean Dipole drives malaria resurgence in East African highlands. Sci Rep 2, 269 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Minakawa N., Githeko A. K. & Yan G. Association between climate variability and malaria epidemics in the East African highlands. Proc Natl Acad Sci U S A 101, 2375–2380 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson M. C., Mason S. J., Phindela T. & Connor S. J. Use of rainfall and sea surface temperature monitoring for malaria early warning in Botswana. Am J Trop Med Hyg 73, 214–221 (2005). [PubMed] [Google Scholar]
- Hashizume M., Terao T. & Minakawa N. The Indian Ocean Dipole and malaria risk in the highlands of western Kenya. Proc Natl Acad Sci U S A 106, 1857–1862 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats R. S., Bouma M. J., Hajat S., Worrall E. & Haines A. El Niño and health. Lancet 362, 1481–1489 (2003). [DOI] [PubMed] [Google Scholar]
- Hashizume M. et al. A differential effect of Indian ocean dipole and El Niño on cholera dynamics in Bangladesh. PLoS One 8, e60001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner R. C. Jr, et al. Highly localized sensitivity to climate forcing drives endemic cholera in a megacity. Proc Natl Acad Sci U S A 109, 2033–2036 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume M. et al. The Indian Ocean dipole and cholera incidence in Bangladesh: a time-series analysis. Environ Health Perspect 119, 239–244 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle K., Rodo X., Pascual M., Yunus M. & Mostafa G. Refractory periods and climate forcing in cholera dynamics. Nature 436, 696–700 (2005). [DOI] [PubMed] [Google Scholar]
- Shaman J. & Lipsitch M. The El Niño-Southern Oscillation (ENSO)-pandemic influenza connection: coincident or causal? Proc Natl Acad Sci U S A 110 Suppl 1, 3689–3691 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). Climate change and human health: risks and responses (WHO, Geneva, 2004). [Google Scholar]
- Kolstad E. W. & Johansson K. A. Uncertainties associated with quantifying climate change impacts on human health: a case study for diarrhea. Environ Health Perspect 119, 299–305 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrence C. & Compo G. P. A practical guide to wavelet analysis. Bulletin of the American Meteorological Society 79, 61–78 (1998). [Google Scholar]
- Chaves L. F. & Pascual M. Climate cycles and forecasts of cutaneous leishmaniasis, a nonstationary vector-borne disease. PLoS Med 3, e295 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin de Magny G., Guegan J. F., Petit M. & Cazelles B. Regional-scale climate-variability synchrony of cholera epidemics in West Africa. BMC Infect Dis 7, 20 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Lindo E., Pinell-Salles P., Maruy A. & Chea-Woo E. El Niño and diarrhoea and dehydration in Lima, Peru. Lancet 350, 1597–1598 (1997). [DOI] [PubMed] [Google Scholar]
- Luo J. J., Zhang R., Behera S. K. & Masumoto Y. Interaction between El Niño and Extreme Indian Ocean Dipole. J Climate 23, 726–742 (2010). [Google Scholar]
- Annamalai H., Xie S. P. & McCreary J. P. Impact of Indian Ocean sea surface temperature on developing El Niño. J Climate 18, 302–319 (2005). [Google Scholar]
- Luo J. J., Sasaki W. & Masumoto Y. Indian Ocean warming modulates Pacific climate change. Proc Natl Acad Sci U S A 109, 18701–18706 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Bi P., Hiller J. E., Sun Y. & Ryan P. Climate variations and bacillary dysentery in northern and southern cities of China. J Infect 55, 194–200 (2007). [DOI] [PubMed] [Google Scholar]
- D'Souza R. M., Becker N. G., Hall G. & Moodie K. B. Does ambient temperature affect foodborne disease? Epidemiology 15, 86–92 (2004). [DOI] [PubMed] [Google Scholar]
- Kovats R. S. et al. The effect of temperature on food poisoning: a time-series analysis of salmonellosis in ten European countries. Epidemiol Infect 132, 443–453 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury M., Charron D. F., Holt J. D., Allen O. B. & Maarouf A. R. A time series analysis of the relationship of ambient temperature and common bacterial enteric infections in two Canadian provinces. Int J Biometeorol 50, 385–391 (2006). [DOI] [PubMed] [Google Scholar]
- Naumova E. N. et al. Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiol Infect 135, 281–292 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cama R. I. et al. Enteropathogens and other factors associated with severe disease in children with acute watery diarrhea in Lima, Peru. J Infect Dis 179, 1139–1144 (1999). [DOI] [PubMed] [Google Scholar]
- Earn D. J., Rohani P., Bolker B. M. & Grenfell B. T. A simple model for complex dynamical transitions in epidemics. Science 287, 667–670 (2000). [DOI] [PubMed] [Google Scholar]
- Stenseth N. C. et al. Review article. Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Niño Southern Oscillation and beyond. Proc Biol Sci 270, 2087–2096 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett T. B. et al. Why large-scale climate indices seem to predict ecological processes better than local weather. Nature 430, 71–75 (2004). [DOI] [PubMed] [Google Scholar]
- Walker C. L. et al. Global burden of childhood pneumonia and diarrhoea. Lancet 381, 1405–1416 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji N. H., Goswami B. N., Vinayachandran P. N. & Yamagata T. A dipole mode in the tropical Indian Ocean. Nature 401, 360–363 (1999). [DOI] [PubMed] [Google Scholar]
- Shumway R. H. & Stoffer D. S. Time series analysis and its applications (Springer, New York, 2000). [Google Scholar]
- Maraun D. & Kurths J. Cross wavelet analysis: significance testing and pitfalls. Nonlinear Processes in Geophysics 11, 505–514 (2004). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure s1