Abstract
One approach to enhance the disinfection of root canals in endodontic treatment is ultrasonic irrigation with sodium hypochlorite. Reactive oxygen species, such as hydroxyl radical, are generated by biological defense systems to kill invading bacteria. Ultrasonic irrigation with hydrogen peroxide may be a promising option to increase hydroxyl radical generation. We examined the bactericidal effects of hydroxyl radical generated from low concentration hydrogen peroxide with ultrasound in vitro. An ultrasonic tip was submerged in 0.5 or 1.0 M hydrogen peroxide in a microfuge tube. hydrogen peroxide was irradiated with the ultrasound, the tip of which was maintained centered in the tube to mimic ultrasonic irrigation. Hydroxyl radical generation was assessed by electron spin resonance spectroscopy. Subsequently, Enterococcus faecalis suspension in hydrogen peroxide was prepared and irradiated as described above. Bactericidal effects were assessed by viable counting. Electron spin resonance measurements showed that hydroxyl radical generation increased significantly in a time- and dose-dependent manner (two-way analysis of variance and Tukey’s test, p<0.05). Moreover, the bactericidal effects of hydrogen peroxide against Enterococcus faecalis were enhanced by ultrasonic irradiation in a time- and dose-dependent manner. These results suggest that ultrasonic irrigation in the presence of low concentration hydrogen peroxide can serve as a disinfection strategy in endodontic treatment.
Keywords: hydroxyl radical, ultrasound, hydrogen peroxide, bactericidal effect, electron spin resonance
Introduction
Bacteria and their products are considered to be major etiological agents of endodontic infections.(1) Thus, the primary objective of root canal treatment is the elimination of these bacteria from the root canal system with subsequent repair of the periodontal tissue.(2) However, the complexity of the root canal renders complete shaping and cleaning using various instrumentation techniques difficult.(3)
Studies have shown that the methods presently available for chemomechanical debridement of the root canal result in a considerable number of cases with detectable remaining bacteria.(4,5) Because residual bacteria may place the treatment outcome at risk, supplementary approaches have been developed to improve root canal disinfection. One approach to enhance disinfection is ultrasonic irrigation, which involves ultrasonic activation of an endodontic irrigant, such as sodium hypochlorite. Such ultrasonic irrigation has been reported to enhance disinfection in the root canal, possibly because of ultrasonic cavitation and acoustic streaming.(6,7) However, the findings of previous antibacterial studies have been inconclusive.(8,9) As a result, additional research is needed to determine the effects of ultrasonic irrigation in root canal treatment.
Bactericidal effects of hydrogen peroxide (H2O2) in biological systems have been reported, with growth inhibition and/or inactivation of pathogenic bacteria when H2O2 is used at an appropriate disinfecting concentration and under suitable operating conditions.(10) Concentrations in the range of 3–5% H2O2 have been used as endodontic irrigants.(11) However, the antimicrobial efficacy and tissue-dissolving capacity of H2O2 used in root canal treatment are lower than those of the commonly used endodontic irrigant, sodium hypochlorite.(11)
H2O2 is generally considered to be a reactive oxygen species (ROS). ROS are chemically reactive molecules containing oxygen that are generated in biological defense systems as part of the immunological response to invading bacteria.(12) Additionally, H2O2 can be converted into hydroxyl radical (HO•) by the Fenton(13) and Haber-Weiss(14) reactions. HO• is also a ROS and has one unpaired electron in its structure, so it is apt to remove an electron from other substances, thereby oxidizing them.(15) This makes HO• reactive and toxic to bacteria because it oxidizes sulfhydryl groups and double bonds in proteins, lipids, and membrane surfaces.(16) Moreover, HO• is formed due to the energy involved during cavitation bubble collapse when water is treated with ultrasound.(17)
Thus, the use of ultrasound in the presence of H2O2 seems a promising option to increase the generation of HO•, which can be used for root canal disinfection in endodontic treatment. The purpose of this study was to qualitatively assess HO• generation from H2O2 activated by an ultrasonic unit in vitro and the bactericidal effect of such HO• generation against Enterococcus faecalis (E. faecalis), which has been implicated in persistent root canal infections.
Materials and Methods
Reagents and ultrasonic unit
5-(2,2-dimethyl-1,3-propoxycyclophosphoryl)-5-methyl-1-pyrroline-N-oxide (CYPMPO) was purchased from Radical Research (Tokyo, Japan). Dimethyl sulfoxide (DMSO) and H2O2 were purchased from Wako Pure Chem. Ind., Ltd. (Osaka, Japan). The Handy Sonic UR-20P (Tomy Seiko Co., Ltd., Tokyo, Japan) with an active ultrasonic tip (φ 2.5-mm) was used as the ultrasonic unit, operated at a fixed driving frequency of 28 kHz with an output power of 10 or 20 W.
Experimental design
Experimental solutions (360 µl) consisted of 0.5 or 1.0 M H2O2 diluted with 0.025 M Tris-HCl buffer (pH 7.0); 0.025 M Tris-HCl buffer alone was used as a control. The ultrasonic tip was inserted into the experimental solution in a 600-µl microfuge tube. The soaking length was fixed at 20 mm of the ultrasonic tip. Then, the experimental solution was activated with ultrasonic irradiation (UI) for 1, 2, or 3 min on ice to avoid temperature change, during which the ultrasonic tip was maintained centered in the microfuge tube to mimic endodontic ultrasonic irrigation. Four experimental conditions were tested: (i) Tris-HCl without UI, (ii) H2O2 without UI, (iii) Tris-HCl with UI, and (iv) H2O2 with UI.
HO• generation from H2O2 with UI
HO• generation under the four experimental conditions was analyzed quantitatively using an electron spin resonance (ESR) spin-trapping technique. This analysis was conducted using a ROS-generating system containing CYPMPO. Briefly, CYPMPO (40 µl) was added to each solution, to yield a final H2O2 concentration of 0.45 or 0.90 M (1.5 or 3.0 w/v%). HO• generation was assessed using the experimental conditions described above. The ESR observations were performed with a JES-RE1X (JEOL, Tokyo, Japan) connected to a WIN-RAD ESR data analyzer (Radical Research, Tokyo, Japan) with the following instrument settings: microwave power, 8.00 mW; magnetic field, 335.6 ± 7.5 mT; field modulation width, 0.079 mT; sweep time, 1 min; and time constant, 0.03 s. The results are expressed as the signal intensity (peak height).
Viable counting for bactericidal activity
E. faecalis JCM5803 stock culture was obtained from the Japan Collection of Microorganisms (RIKEN BioResource Center, Tsukuba, Japan). Bacteria were cultured aerobically in brain–heart infusion (BHI) broth (Becton Dickinson Labware, Franklin Lakes, NJ) at 37°C and, after harvesting by centrifugation, were washed once in 0.025 M Tris-HCl buffer (pH 7.0) and re-suspended in the same buffer. The cell density of suspensions was adjusted to ~2.0 × 107 cells/ml. In a 600-µl microfuge tube, 200 µl of the suspension was mixed with 200 µl of 0.9 M H2O2 diluted with Tris-HCl buffer to yield a final concentration of 1.0 × 107 cells/ml and 0.45 or 0.90 M (1.5 or 3.0 w/v%) H2O2, as for the ESR measurement. Immediately after mixing, the suspension was exposed to ultrasound as described above. A 100-fold serial dilution of the mixture was then prepared using Tris-HCl buffer and 50 µl was spread on BHI agar (Becton Dickinson Labware). Plates were cultured at 37°C for 18 h under the conditions described above, and then numbers of colony-forming unit (CFU)/ml were determined.
Statistical analysis
All tests were performed in six sets (n = 6). To assess the statistical significance of differences among groups, two-way analysis of variance and Tukey’s test were used (p<0.05).
Results
HO• generation from H2O2 with UI
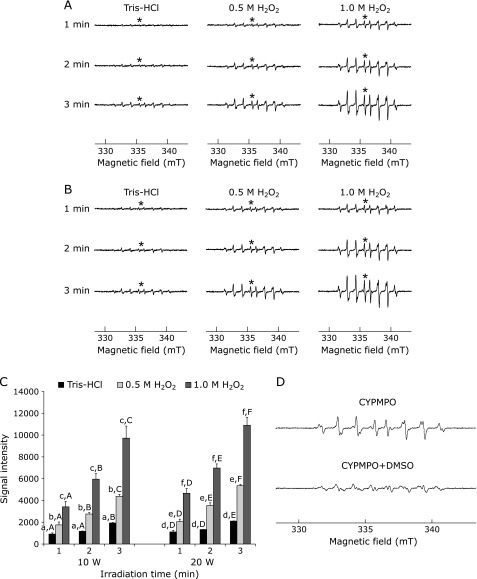
HO• generation from H2O2 with UI was investigated using the ESR spin-trapping technique with CYPMPO. We observed that HO• generated with 10 or 20 W irradiation in the presence of CYPMPO led to the formation of a characteristic CYPMPO-OH spin adduct spectrum (hyperfine coupling constants: AN = 1.37 mT, AH = 1.37 mT, and AP = 4.88 mT), with hyperfine splitting, giving rise to 14 resolved peaks (Fig. 1A and B). CYPMPO-OH spin adduct formation increased significantly in a time- and dose-dependent manner (p<0.05; Fig. 1C). Moreover, the presence of HO• was confirmed because the intensity of CYPMPO-OH spin adduct was decreased by the addition of 10.0 M DMSO, a scavenger of HO• (Fig. 1D). In the experimental solutions without UI, little CYPMPO-OH signal intensity was observed, regardless of the experimental duration (data not shown).
Fig. 1.
HO• generation from H2O2 by ultrasound irradiation. (A) and (B) ESR spin trapping measurement of HO• generated from H2O2 by 10 W (A) and 20 W (B) ultrasound with CYPMPO as the spin trap. The asterisk (*) indicates the signal intensity used for the analysis of HO• generation. (C) Signal intensities of the ESR spectrum of CYPMPO-OH by 10 and 20 W ultrasound. Results are expressed as the mean ± SD. Within experimental solutions, means sharing the same upper-case letter are not significantly different (p>0.05). Between experimental solutions at the same irradiation time, means sharing the same lower-case letter are not significantly different (p>0.05). (D) Influence of DMSO on ESR spectra of HO• generated from H2O2 by 20 W ultrasound with CYPMPO as the spin trap.
Viable counting for bactericidal activity
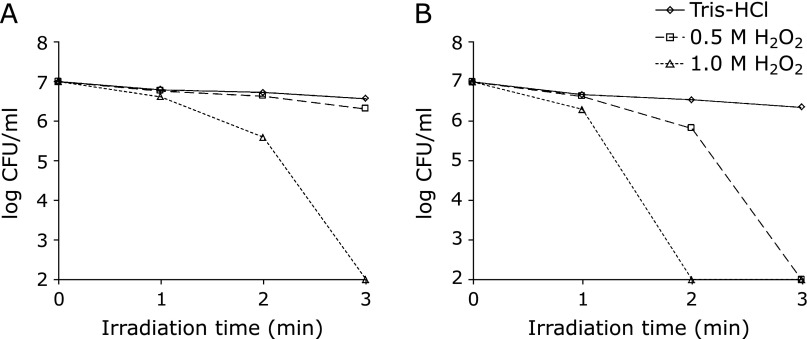
The bactericidal effect against E. faecalis in H2O2 with UI was examined by viable counting. E. faecalis were effectively killed, with a 4-log reduction under the conditions of 1.0 M H2O2 with 10 W irradiation in 3 min (Fig. 2A). On the other hand, no bactericidal effects were observed in both 0.5 M H2O2 and Tris-HCl with UI regardless experimental duration. Fig. 2B shows the bactericidal effect in H2O2 with 20 W irradiation. The number of CFU/ml dramatically decreased in 1.0 M H2O2, and an approximately 4-log reduction was obtained within 2 min. Moreover, the bacteria were killed effectively in 0.5 M H2O2 with UI in 3 min (~4-log reduction). The condition with Tris-HCl alone killed almost no bacteria, even after 3 min UI.
Fig. 2.
Viable counting bactericidal activity in the suspension after ultrasound irradiation. The data points indicate the mean values (n = 6). (A) Number of CFU/ml of 10 W irradiation in each experimental solution. (B) Number of CFU/ml of 20 W irradiation in each experimental condition.
Discussion
Ultrasonic irrigation for root canal treatment relies on the transmission of acoustic energy from an oscillating ultrasonic instrument to an irrigant in the root canal. The energy is transmitted by means of ultrasonic waves and can induce acoustic streaming and cavitation of the irrigant.(6,11) H2O2 has been reported to act as a source of HO• in the dissociation process(13) and HO• is formed due to the energy available generated during cavitation bubble collapse.(17) We confirmed HO• generation from H2O2 with UI using our system because of the identical hyperfine coupling constants to previously reported constants of HO• by Kamibayashi et al.(18) and spectrum elimination with DMSO in a same way by Mukohda et al.(19) Thus, the mechanism of HO• generation may be explained by both acceleration of H2O2 dissociation by UI and ultrasonic cavitation activity. On the other hand, the small amount of CYPMPO-OH spin adduct spectrum was observed during UI of Tris-HCl (control solution). This phenomenon may be caused of HO• formation due to ultrasonic cavitation activity in aqueous solutions.
The ESR spin-trapping technique is used for the quantitative assessment of ROS, such as HO•. This technique involves compounds that readily react with free radicals to produce a relatively long-lived free radical product (spin adduct), which can then be identified by its ESR spectrum.(20,21) We developed an ESR-based technique to detect free radical reactions induced by ROS in biological systems in vitro and in vivo.(20,21) Among in vitro ESR applications, the spin-trapping technique is well known for its abilities to detect ROS and quantify spin adduct concentration in experimental systems.(20,21) Although the half-life of HO• is extremely short,(22) total HO• generation throughout the experimental period can be directly and specifically assessed using the ESR technique. In the present study, the amounts of HO• generated from H2O2 with UI at 10 and 20 W increased significantly in a time- and dose-dependent manner. These results would suggest that UI continuously formed HO• throughout the ultrasonic exposure period. Moreover, it appears that the amount of HO• generation increased in proportion to output power in this system. Therefore, further researches are needed to assess HO• generation in several output power strengths such as more than 30 W.
The bactericidal effect of H2O2 with UI on E. faecalis was also examined. E. faecalis, a Gram-positive anaerobic facultative coccus, has been recovered from several oral sites.(23) This bacterium was used because it exhibits a high level of resistance to a wide range of medications,(24) and is commonly found in cases of root canal treatment failure associated with persistent apical periodontitis.(25,26) Our results indicated that the bactericidal effects were time- and dose-dependently enhanced by UI. The number of CFU/ml after 10 and 20 W irradiation in 1.0 M H2O2 markedly decreased in 3 and 2 min irradiation time, respectively. In addition, E. faecalis was effectively killed with the conditions of 20 W irradiation for 3 min in 0.5 M H2O2. These findings indicated that even lower concentration of H2O2 could be effective against E. faecalis in our system. This may depend upon the amount of HO• from H2O2 because the ESR measurements showed more HO• with longer irradiation times and higher H2O2 concentration. In biological system, HO• causes radical chain reactions and leads to generation of many types of ROS including alkoxyl- and alkylperoxyl-radicals. These reactions in lipids of cell membrane refer to lipid peroxidation, which might be toxic to bacteria or cells.(27) Therefore, bactericidal effect of this system may be due to not only HO• but also other types of ROS. Thus, our results suggest that appropriate H2O2 concentration and irradiation time are important factors in the achievement of an optimal bactericidal effect in this system.
The conventional H2O2 concentration for endodontic application is approximately 3–5 w/v%,(11) whereas 1.5 and 3.0 w/v% H2O2 were used in this study. Chemical disinfectants, such as the commonly used endodontic irrigant, sodium hypochlorite, can cause problems, such as tissue damage or accidental injury caused by leakage.(28,29) Additionally, a subcommittee of the US Food and Drug Administration (FDA, 2003) concluded that H2O2 was safe at concentrations of up to 3 w/v%. Accordingly, a low-concentration endodontic irrigant with bactericidal effects may be clinically desirable from patient-safety viewpoint.
ROS cause oxidative damage to tissues or cells if not controlled.(30) Although no detrimental effect on the oral mucosa or healing of wounded skin in HO• generation from H2O2 by laser irradiation was reported,(31) it is difficult to compare UI with laser irradiation. Further studies are required to assess the safety for clinical use of present method.
According to our system, ultrasonic irrigation in the presence of H2O2 could provide the capacity for disinfection by not only ultrasonic cavitation and acoustic streaming, but also HO• generation. In conclusions, the ESR spin-trapping technique and a bactericidal assay in the present study showed that HO• was generated from even low concentration of H2O2 activated by ultrasound, and that it exerted a bactericidal effect against E. faecalis. Thus, this method may be usefully applied in root canal disinfection.
Acknowledgments
This study was supported by Research Grants from the Sato Fund for 2012 and Grant from Dental Research Center for 2012 and 2013, Nihon University School of Dentistry.
Abbreviations
- BHI
brain-heart infusion
- CFU
colony forming unit
- CYPMPO
5-(2,2-dimethyl-1,3-propoxycyclophosphoryl)-5-methyl-1-pyrroline-N-oxide
- DMSO
dimethyl sulfoxide
- ESR
electron spin resonance
- H2O2
hydrogen peroxide
- HO•
hydroxyl radical
- ROS
reactive oxygen species
- UI
ultrasonic irradiation
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–349. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 2.Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15:348–381. doi: 10.1177/154411130401500604. [DOI] [PubMed] [Google Scholar]
- 3.Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009;35:791–804. doi: 10.1016/j.joen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Shuping GB, Orstavik D, Sigurdsson A, Trope M. Reduction of intracanal bacteria using nickel-titanium rotary instrumentation and various medications. J Endod. 2000;26:751–755. doi: 10.1097/00004770-200012000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Rôças IN, Siqueira JF., Jr Comparison of the in vivo antimicrobial effectiveness of sodium hypochlorite and chlorhexidine used as root canal irrigants: a molecular microbiology study. J Endod. 2011;37:143–150. doi: 10.1016/j.joen.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 6.van der, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40:415–426. doi: 10.1111/j.1365-2591.2007.01243.x. [DOI] [PubMed] [Google Scholar]
- 7.Huque J, Kota K, Yamaga M, Iwaku M, Hoshino E. Bacterial eradication from root dentine by ultrasonic irrigation with sodium hypochlorite. Int Endod J. 1998;31:242–250. doi: 10.1046/j.1365-2591.1998.00156.x. [DOI] [PubMed] [Google Scholar]
- 8.Paiva SS, Siqueira JF, Jr, Rôças IN, et al. Supplementing the antimicrobial effects of chemomechanical debridement with either passive ultrasonic irrigation or a final rinse with chlorhexidine: a clinical study. J Endod. 2012;38:1202–1206. doi: 10.1016/j.joen.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Tardivo D, Pommel L, La Scola, About I, Camps J. Antibacterial efficiency of passive ultrasonic versus sonic irrigation. Ultrasonic root canal irrigation. Odontostomatol Trop. 2010;33:29–35. [PubMed] [Google Scholar]
- 10.Labas MD, Zalazar CS, Brandi RJ, Cassano AE. Reaction kinetics of bacteria disinfection employing hydrogen peroxide. Biochemical Engineering Journal. 2008;38:78–87. [Google Scholar]
- 11.Metzger Z, Basrani B, Goodis HE. Instruments, materials, and devices. In: Hargreaves KM, Cohen S, Berman LH, editors. Pathways of the Pulp (10th ed) St. Louis: Mosby Elsevia (USA); 2011. pp. 223–282. [Google Scholar]
- 12.DeLeo FR, Allen LA, Apicella M, Nauseef WM. NADPH oxidase activation and assembly during phagocytosis. J Immunol. 1999;163:6732–6740. [PubMed] [Google Scholar]
- 13.Neyens E, Baeyens J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater. 2003;98:33–50. doi: 10.1016/s0304-3894(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 14.Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986;247:1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- 15.Ikai H, Nakamura K, Shirato M, et al. Photolysis of hydrogen peroxide, an effective disinfection system via hydroxyl radical formation. Antimicrob Agents Chemother. 2010;54:5086–5091. doi: 10.1128/AAC.00751-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heling I, Chandler NP. Antimicrobial effect of irrigant combinations within dentinal tubules. Int Endod J. 1998;31:8–14. [PubMed] [Google Scholar]
- 17.Hu Y, Zhang Z, Yang C. Measurement of hydroxyl radical production in ultrasonic aqueous solutions by a novel chemiluminescence method. Ultrason Sonochem. 2008;15:665–672. doi: 10.1016/j.ultsonch.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Kamibayashi M, Oowada S, Kameda H, et al. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) Free Radic Res. 2006;40:1166–1172. doi: 10.1080/10715760600883254. [DOI] [PubMed] [Google Scholar]
- 19.Mukohda M, Ueno S, Kamibayashi M, Okada M, Yamawaki H, Hara Y. Influences of organic solvents on CYPMPO-electron spin resonance spectra in vitro radical generating systems. J Vet Med Sci. 2010;72:1547–1550. doi: 10.1292/jvms.10-0232. [DOI] [PubMed] [Google Scholar]
- 20.Janzen EG. Spin trapping. Methods Enzymol. 1984;105:188–198. doi: 10.1016/s0076-6879(84)05025-4. [DOI] [PubMed] [Google Scholar]
- 21.Lee MC. Assessment of oxidative stress and antioxidant property using electron spin resonance (ESR) spectroscopy. J Clin Biochem Nutr. 2013;52:1–8. doi: 10.3164/jcbn.12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci. 1992;669:7–20. doi: 10.1111/j.1749-6632.1992.tb17085.x. [DOI] [PubMed] [Google Scholar]
- 23.Rams TE, Feik D, Young V, Hammond BF, Slots J. Enterococci in human periodontitis. Oral Microbiol Immunol. 1992;7:249–252. doi: 10.1111/j.1399-302x.1992.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 24.Siqueira JF, Jr, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999;32:361–369. doi: 10.1046/j.1365-2591.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 25.Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J. 2003;36:1–11. doi: 10.1046/j.1365-2591.2003.00603.x. [DOI] [PubMed] [Google Scholar]
- 26.Hancock HH, 3rd, Sigurdsson A, Trope M, Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:579–586. doi: 10.1067/moe.2001.113587. [DOI] [PubMed] [Google Scholar]
- 27.Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57:715–725. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 28.Marshall MV, Cancro LP, Fischman SL. Hydrogen peroxide: a review of its use in dentistry. J Periodontol. 1995;66:786–796. doi: 10.1902/jop.1995.66.9.786. [DOI] [PubMed] [Google Scholar]
- 29.Neaverth EJ, Swindle R. A serious complication following the inadvertent injection of sodium hypochlorite outside the root canal system. Compendium. 1990;11:474–481. [PubMed] [Google Scholar]
- 30.Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222:1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada Y, Mokudai T, Nakamura K, et al. Topical treatment of oral cavity and wounded skin with a new disinfection system utilizing photolysis of hydrogen peroxide in rats. J Toxicol Sci. 2012;37:329–335. doi: 10.2131/jts.37.329. [DOI] [PubMed] [Google Scholar]