Abstract
Epidemiological and experimental studies suggest that the consumption of flavonoid-rich diets decreases the risk of various chronic diseases such as cardiovascular diseases. Although studies on the bioavailability of flavonoids have been well-characterized, the tissue and cellular localizations underlying their biological mechanisms are largely unknown. The development and application of novel monoclonal antibodies revealed that macrophages could be the major target of dietary flavonoids in vivo. Using macrophage-like cell lines in vitro, we examined the molecular basis of the interaction between the macrophages and flavonoids, especially the glucuronide metabolites. We have found that extracellular β-glucuronidase secreted from macrophages is essential for the bioactivation of the glucuronide conjugates into the aglycone, and that the enzymatic activity, which requires an acidic pH, is promoted by the increased secretion of lactate in response to the mitochondrial dysfunction. This review describes our recent findings indicating the molecular mechanisms responsible for the anti-inflammatory activity of dietary flavonoids within the inflammation sites. We propose that the extracellular activity of β-glucuronidase associated with the status of the mitochondrial function in the target cells might be important biomarkers for the specific sites where the glucuronides of dietary flavonoids can act as anti-atherosclerotic and anti-inflammatory agents in vivo.
Keywords: flavonoid, glucuronide, macrophage, β-glucuronidase, mitochondria
Flavonoids in Our Diet and Health
Polyphenols are a large family of natural compounds widely distributed in plant foods and beverages, and therefore are regularly ingested by humans. Polyphenols are defined according to the nature of their backbone structures; i.e., phenolic acids, flavonoids and the less common stilbenes and lignans. Among them, flavonoids are the most abundant polyphenols in our diets. Flavonoids are further divided into several classes; i.e., flavones, flavonols, isoflavones, flavanols, anthocyanins, proanthocyanidins, flavanones etc. In 1936, Rusznyak and Szent-Gyoygi(1,2) reported that citrus flavonoids reduced capillary fragility and permeability in blood vessels. Thereafter, they have been called “vitamin P” (“P” for permeability), and numerous studies on the biological activities of flavonoids, leading to the good health, have been described. Quercetin (3,3',4',5,7-pentahydroxyflavone) is the major flavonoid in our diet, and most of the quercetin in plants is in the glycoside form. In our daily diet, the quercetin glycosides are particularly abundant in onion (0.3 g/g fresh weight),(3) tea (10–25 mg/L),(4) and buckwheat. Epidemiological studies suggested the tight links between diets rich in quercetin and other flavonoids and the decreased incidence of cardiovascular, neoplastic, and neurodegenerative diseases.(5–12) Another example of the major flavonoids in our diet is flavanols (catechins). They are abundant in green tea (~150 mg/100 ml)(13–15) and to a lesser extent in black tea (13.9 mg/100 ml),(16) in which the parent catechins are oligomerized during fermentation. Red wine (270 mg/L)(17) and chocolate (black chocolate, 53.5 mg/100 g; milk chocolate, 15.9 mg/100 g)(16) are also dietary sources of catechins. Four major tea catechins are well-characterized; i.e., (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epigallocatechin gallate (EGCg), and (−)-epicatechin gallate (ECg) at 6.4%, 19%, 59%, and 13.6% of the total catechins in green tea, respectively.(15) Much attention has focused on the cancer-preventive effects of EGCg, most abundant catechin in green tea. The anti-atherosclerotic effects of EC, as well as its oligomeric procyanidins, have also been reported using atherosclerotic animal models.(18–20)
Bioavailability of Flavonoids
The bioavailability of quercetin, catechins and other polyphenols in human and rodents has been investigated in vivo. After oral intake, the glycosides of the flavonoids are rapidly hydrolyzed during passage across the small intestine (by glycosidases) or the colon (by bacterial activity) to transiently generate the aglycones, which are further metabolized into glucuronides and/or sulfates by phase II reactions, and some (if containing the catecholic moiety) are further methylated by catechol-O-methyltransferase (COMT). As the results of the efficient and multiple metabolic modifications, non-conjugated aglycones are scarcely detected in the human plasma. Previous reports have clearly shown that quercetin-3-O-glucuronide (Q3GA) and quercetin-3'-O-sulfate are the major quercetin conjugates in rat and human plasma, while the aglycone could not be detected.(21–23) The concentrations of the total quercetin metabolites in human plasma after the intake of quercetin (150 mg from onion) are around 1 µM at maximum.(24) We also measured the concentration of Q3GA in human plasma to be 264 nM.(23) In contrast to the observation that no quercetin aglycone was detected in human plasma, tea catechins, especially ECg and EGCg, can be detected as the aglycones (20 and 60 ng/ml, respectively, at maximum concentrations 1–2 h after ingestion) in human plasma after intake of green tea.(25)
Development and Application of Novel Monoclonal Antibodies
Analysis of the specific localization of tissue and/or cellular flavonoids has been difficult, because the analytical methods are limited to chromatography. To overcome the difficulties, we planned to develop the specific antibodies to flavonoids to immunohistochemically characterize their localizations in vivo. To prepare the antibodies to flavonoids, we first utilized chemically synthesized Q3GA(22) as the immunogen, because (i) Q3GA is a major metabolite in human and rat plasma(21,22) and, (ii) the glucuronide metabolites have one carboxyl group, which is available for coupling with the carrier proteins. For catechins, because we did not have authentic catechin glucuronides, a succinylated derivative of EC, which contains a backbone structure common to four major tea catechins, was chemically synthesized. The obtained 3-succinylated EC was then coupled with the carrier proteins and injected into mice.(26) Finally, we developed two monoclonal antibodies, termed 14A2 and 5A3. The 14A2 significantly recognized Q3GA, but not the quercetin aglycone, quercetin-3-O-sulfate, and 3'-methylated metabolite isorhamnetin.(23) The 5A3 antibody specifically recognized ECg, but not the other catechins including EC used as the starting material for the immunogen preparation. The unexpected specificity of 5A3 to require the 3-galloyl moiety for recognition may be explained by the structural analogy between 3-succinyl-EC and ECg. In other words, the 5A3 is the anti-“3-esterified EC” antibody.(26)
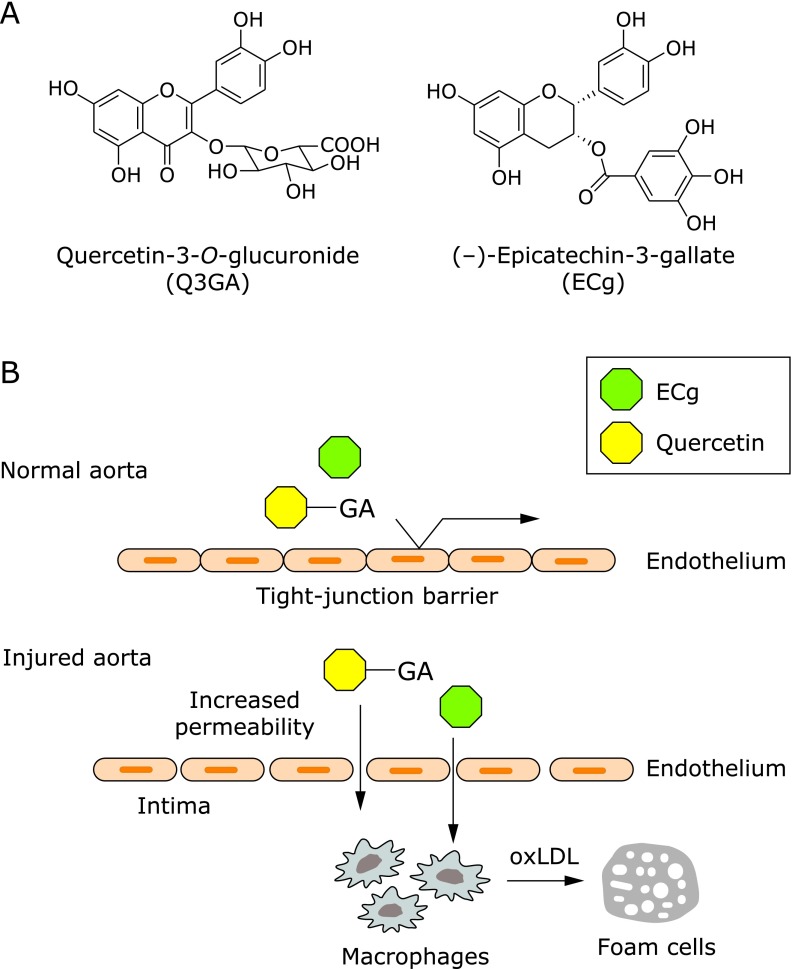
To examine the localization of Q3GA and ECg (see the structures in Fig. 1A), both the 14A2 and 5A3 antibodies were applied to the immunohistochemical staining of human aortic tissues. Although no significant immunoreactivity was obtained in normal-appearing regions, the immunoreaction products of both antibodies were specifically detected in atherosclerotic lesions and were mainly co-localized in the macrophage-derived foam cells.(23,26) In contrast, the endothelial cells, smooth muscle cells, and extracellular matrix in the subendothelial layer, the so-called intima, were scarcely stained with these antibodies. Our data clearly showed that, at least two different types of flavonoids, Q3GA and ECg, specifically accumulated in the macrophage-derived foam cells in a human atherosclerotic aorta. The previous observations that inflammatory or other stimuli result in the increased tight-junction permeability of endothelial cells(27) might explain the accumulation of these flavonoids in the atherosclerotic aorta. Increased permeability of the endothelial cells allows certain plasma molecules to permeate and interact with the intimal cells, such as macrophages (Fig. 1B). Mochizuki et al.(28) demonstrated the increased permeation of Q3GA through the interleukin-1α-stimulated human aortic endothelial cells, raising the possibility that plasma circulating Q3GA and other molecules could permeate injured endothelial cells at the sites of inflammation and atherosclerosis. As compared with the lower plasma concentrations of flavonoids in the human studies, most of the in vitro studies previously reported using cultured cells that required relatively higher concentrations (over µM) of the flavonoids to exert their activities. Therefore, the specific localization of the flavonoids in the injured aorta might be important for their anti-atherosclerotic and anti-inflammatory effects in the inflammatory sites.
Fig. 1.
Specific localization of flavonoids in macrophage cells in aorta. (A) Chemical structures of quercetin-3-O-glucuronide (Q3GA) and (–)-epicatechin-3-gallate (ECg), which were shown to accumulate in the atherosclerotic macrophage foam cells.(23,26) (B) Proposed mechanism for the specific accumulation of Q3GA and ECg in macrophage cells in the atherosclerotic lesions. GA, glucuronides.
Accumulation of Flavonoids in Macrophage Cells
We next examined the biological consequences of the accumulation of flavonoids in macrophages in vitro. Consistent with the results obtained from the immunohistostaining, the significant accumulation of Q3GA and ECg was also reproduced in the macrophage-like cell lines (RAW264 and/or THP-1) in vitro.(23,26) Using a quick trypsinization experiment, we demonstrated that Q3GA could bind to the cell surface proteins via anionic binding on the RAW264 cells, but not intracellularly accumulate. In contrast, it was found that the aglycones are incorporated into cells, presumably via simple diffusion.(29) Surprisingly, the quercetin aglycone and the methylated quercetins (3'- or 4'-methylated quercetins), as well as Q3GA itself, were significantly detected in the Q3GA-treated RAW264 cells.(23) The generation of quercetin and methylquercetins from Q3GA reflects the presence of the enzymatic activities of β-glucuronidase and COMT in the macrophages. These non-conjugated derivatives were also detected in other lines of macrophage cells (J774-1, differentiated THP-1 and peritoneal primary macrophages) upon treatment with Q3GA, but not detected in the vascular endothelial cells (HUVEC and BAEC) and many other cell lines from different tissues.(23,29) Given that COMT is generally localized inside the cells(30) and that Q3GA cannot enter into the cells, the β-glucuronidase-catalyzed deconjugation of Q3GA into quercetin could be the first step in the extracellular fluid or cell surface, followed by simple diffusion inside the cells, followed by the COMT-catalyzed methylation. The possibility whether ECg glucuronides could also be deconjugated into the aglycone remains unproven. In contrast, the enzymatic activity of sulfatase, another important deconjugation enzyme, was scarcely observed in the cultured medium of the RAW264 cells. These results suggest that β-glucuronidase is a key enzyme for the bioactivation of inactive flavonoid metabolites in vivo.
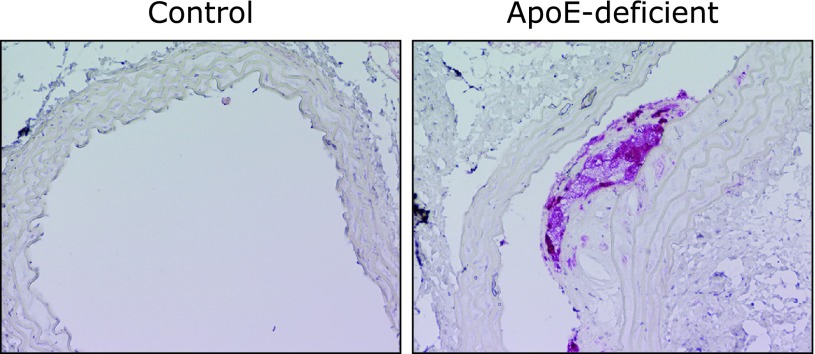
The in vivo expression of β-glucuronidase is also demonstrated immunohistochemically in the foamy cells in atherosclerotic aorta of the apoE-deficient mice (Fig. 2), showing the tight link between inflammation and the deconjugation in vivo. We also examined the tissue distribution of β-glucuronidase by immunoblot analysis of various tissues from ICR mice, and found that a significant expression of β-glucuronidase, as well as in the peritoneal macrophages, was observed in the thymus, liver and spleen, which may contain inflammatory cells such as lymphocytes, kupffer cells (in liver), and/or macrophages.
Fig. 2.
β-Glucuronidase localization in the foamy macrophage cells in the atherosclerotic lesions of apoE-deficient mice. Aortic sections (left, wild-type control mice; right, apoE-deficient mice) were immunostained with anti-β-glucuronidase antibody.
β-Glucuronidase Activity is Linked with Mitochondrial Dysfunction
It has been reported that lysosomal enzymes including β-glucuronidase are released from inflammatory cells, such as macrophages and neutrophils, under inflammatory conditions.(31–34) Using zymography with a chromogenic substrate and immunoblot analysis of the cultured medium, we demonstrated that β-glucuronidase is indeed the major deglucuronidation enzyme secreted from the RAW264 cells.(29) Although the β-glucuronidase-catalyzed deconjugation of flavonoid glucuronides has been suggested at the site of inflammation,(35–37) the molecular basis has not fully been understood. We also found that the lipopolysaccharide (LPS) stimulation of RAW264 cells resulted in the significant accumulation of Q3GA and the aglycone,(23) however, in spite of the significant deconjugation, the basal levels of both expression and secretion of β-glucuronidase was not enhanced upon LPS treatment.(29) It is speculated that the lysosomal enzyme β-glucuronidase requires the acidic conditions for its catalytic activity. Indeed, the in vitro deconjugation of Q3GA in the cultured medium was significantly enhanced by adjustment of the pH to 5. Furthermore, the LPS stimulation of the RAW264 cells resulted in the acidification of the phenol-red containing medium, turning it yellow,(29) showing that the activation of the macrophages induced the acidic conditions around the cells. Lactate is a major acidic product secreted from the cells as the result of glycolysis, and its increased levels in the medium are generally used as an indicator of mitochondrial dysfunction. We then measured the lactate levels in the cultured medium by a liquid chromatography-tandem mass spectrometry and found increased levels of lactate upon treatment with LPS, as well as a mitochondrial inhibitor antimycin-A. Furthermore, the involvement of increased lactate in the deconjugation of Q3GA was supported by the observation that the direct addition of lactate to the fresh medium enhanced the deconjugation of Q3GA during the RAW264 culturing. These results strongly suggested that the acidification around the macrophage cells through lactate secretion associated with the mitochondrial dysfunction might assist the deconjugation of the glucuronide metabolites.
Much attention has been paid to the mitochondrial dysfunction as a result of autophagy/mitophagy impairment. Autophagy deficiency characterized in Atg5 or Atg7-deifient mice is implicated in various age-related diseases including neurodegeneration,(38,39) diabetes,(40,41) and hepatocarcinoma.(42,43) Recent studies further demonstrated that mitochondrial dysfunction derived from autophagy/mitophagy impairment in the inflammatory cells could be implicated in the induction of chronic inflammation.(44–46) We have demonstrated that the autophagy impairment by Atg7 knockdown induced the β-glucuronidase activity in the medium, and conversely that autophagy inducers reduced the β-glucuronidase activity.(29) These observations suggested that autophagy impairment could be a trigger for mitochondrial dysfunction that induces the β-glucuronidase activity.
Deconjugation and the Biological Activities of Flavonoids in Macrophage Cells
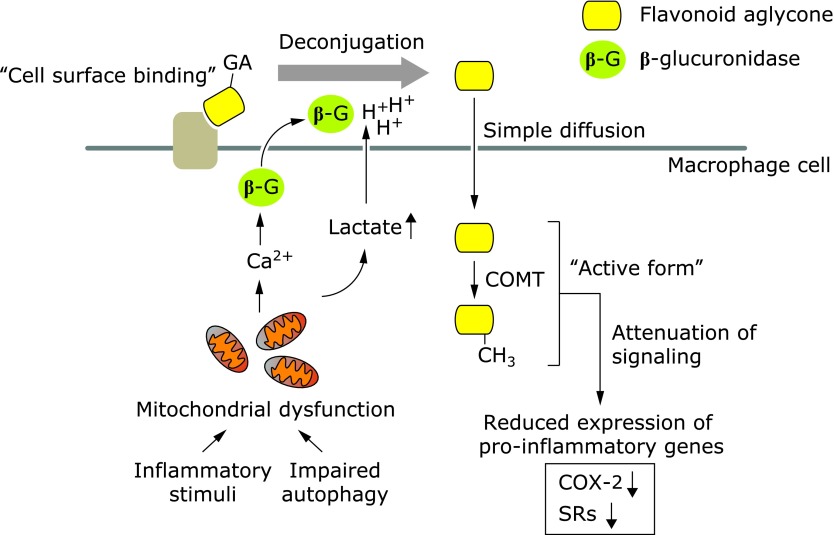
To examine the biological consequences of the localization of Q3GA and ECg in the macrophages in the aorta, the effects of these flavonoids on the expression of scavenger receptors were determined in RAW264 cells. Treatment of the cells with Q3GA and ECg significantly suppressed the expression of a class A scavenger receptor (SR-A) and CD36, respectively, in the RAW264 cells.(23,26) Many papers have suggested the anti-inflammatory and anti-atherosclerotic activities of the conjugated metabolites of the flavonoids. However, in most in vitro studies,(47) the biological activities of the conjugates were examined after longer incubation periods, which might lead to the deconjugation in some cell types, such as macrophages. We confirmed that Q3GA itself, in the absence of the deconjugation activity, failed to inhibit the expressions of the pro-inflammatory genes, such as scavenger receptors and cyclooxygenase-2.(29) In contrast, quercetin aglycone and/or the methylated forms significantly inhibited the pro-inflammatory signaling pathways, in particular, the JNK pathway. These results strongly suggested that the deconjugation of the conjugated metabolites is essential for the anti-inflammatory and anti-atherosclerotic activities inside the cells. Therefore, the primary accumulation of the glucuronide on the cell surface of the macrophages might also be important for the activities, because the glucuronides are expected to be efficiently deconjugated into the aglycone on the cell surface, where the β-glucuronidase activity is relatively concentrated. In other words, the cell surface proteins might play a role as a scaffold of the flavonoid glucuronides, leading to the efficient deconjugation into the bioactive aglycones. Recent our findings on the accumulation and deconjugation of flavonoid conjugates are summarized and illustrated in Fig. 3.
Fig. 3.
Proposed scheme for the deconjugation of flavonoid glucuronides by macrophages. Flavonoid glucuronides bound to the cell surface proteins of macrophages were readily doconjugated into the aglycone by the β-glucuronidase (β-G) activity under acidic conditions derived from increased lactate secretion associated with mitochondria dysfunction. The β-glucuronidase secretion was promoted by increased intracellular calcium ions (Ca2+). The deconjugated aglycones (or the methylated form) accumulated in the cells could act as the active form for the anti-inflammatory and anti-atherosclerotic activities. The suppression of the gene expressions of cyclooxygenase-2 (COX-2) and scavenger receptors (SR-A and CD36) has been previously demonstrated.(23,26) The scheme is modified from our recent paper (Ishisaka et al.).(29)
Are Flavonoids Bioactive In Vivo?
We have recently demonstrated that the injection of LPS into mice resulted in the increased accumulation of quercetin sulfates in the spleen and plasma in vivo.(29) This result may reflect the deglucuronidation of the sulfoglucuronides at the inflammatory sites. It has also been reported that the sulfated metabolites of quercetin, such as quercetin-3'-O-sulfate and quercetin-sulfoglucuronides, are also the major metabolites in the plasma and urine of humans.(21,48) In contrast to the significant β-glucuronidase activity, the sulfatase activity was scarcely observed in macrophage cells. Thus, the quercetin sulfates could also be the major products after the macrophage-mediated deconjugation at inflammatory sites. Although the biological activity of quercetin sulfates has not yet been examined in this study, they could affect the intracellular events by accumulating inside the cells, because the sulfates are generally more hydrophobic than the glucuronides.
Although the potent mutagenicity and cytotoxicity of quercetin have been demonstrated in vitro, they have not yet been confirmed in vivo after the oral intake of quercetin.(49) The safety of orally administered quercetin and other flavonoids in vivo may be explained by the fact that most flavonoids are metabolized during absorption and circulation.(21) The phase II detoxification converts hydrophobic chemicals into hydrophilic metabolites, and then helps to limit the entry of the chemicals into cells. Indeed, the entry of Q3GA into the macrophage cells was limited only to the cell surface.(29) The conjugation (glucuronidation and/or sulfation) of hydroxyl group(s) on polyphenols attenuates the anti-oxidative and anti-inflammatory activities(22,47) and promotes their rapid excretion. It has long been controversial whether the conjugated metabolites of the flavonoids can act as health-beneficial agents in vivo. As already described, we have reported that Q3GA itself failed to inhibit the anti-inflammatory gene expressions in macrophages and that the β-glucuronidase-mediated conversion of Q3GA into the aglycone is essential for the inhibitory effects.(23,29) Galindo et al.(50) also reported the deconjugation-mediated activity of the glucuronidated quercetin, resulting in the lowering of blood pressure in spontaneously hypertensive rats. The quercetin aglycone was quite unstable under the biological conditions and the anti-inflammatory activity was almost completely attenuated after the degradation of quercetin (our unpublished results). In contrast, quercetin conjugates including Q3GA are quite stable under the biological conditions.(51) Hence, the macrophage-mediated deconjugation plays an important role in providing bioactive/unstable aglycones in the biological fluids, in particular, at the sites of inflammation. The selective deconjugation within the sites of inflammation might also ensure the safety of flavonoids in normal tissues.
Acknowledgments
I thank all of my co-workers, especially Professor Junji Terao (The University of Tokushima), Dr. Akari Ishisaka (University of Hyogo), Professor Noriyuki Shibata (Tokyo Women’s Medical University) and Professor Michitaka Naito (Sugiyama Jogakuen University). This study was supported in part by a Grant-in-Aid for Young Scientists (B) (2005–2006), by a Grant-in-Aid for Young Scientists (A) (2007–2010) from the Ministry of Education, Culture, Sports, Science, and Technology, by the Ministry of Agriculture, Forestry and Fishery Food Project, Japan, and by the Center of Excellence Program in the 21st Century in Japan.
Abbreviations
- COMT
catechol-O-methyltransferase
- EC
(−)-epicatechin
- ECg
(−)-epicatechin gallate
- EGC
(−)-epigallocatechin
- EGCg
(−)-epigallocatechin gallate
- LPS
lipopolysaccharide
- Q3GA
quercetin-3-O-glucuronide
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Rusznyák ST, Szent-Györgyi A. Vitamin P: flavonols as vitamins. Nature. 1936;138:27. [Google Scholar]
- 2.Bentsáth A, Rusznyák ST, Szent-Györgyi A. Vitamin nature of flavones. Nature. 1936;138:798. [Google Scholar]
- 3.Hertog MG, Hollman PC, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J Agr Food Chem. 1992;40:2379–2383. [Google Scholar]
- 4.Hertog MG, Hollman PC, van de Putte B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J Agr Food Chem. 1993;41:1242–1246. [Google Scholar]
- 5.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 6.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary flavonoids and cancer risk in the Zutphen Elderly Study. Nutr Cancer. 1994;22:175–184. doi: 10.1080/01635589409514342. [DOI] [PubMed] [Google Scholar]
- 7.Hertog MG, Kromhout D, Aravanis C, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995;155:381–386. [PubMed] [Google Scholar]
- 8.Hertog MG, Hollman PC. Potential health effects of the dietary flavonol quercetin. Eur J Clin Nutr. 1996;50:63–71. [PubMed] [Google Scholar]
- 9.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156:637–642. [PubMed] [Google Scholar]
- 10.Le Marchand L, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. J Natl Cancer Inst. 2000;92:154–160. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- 11.Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris) 1997;153:185–192. [PubMed] [Google Scholar]
- 12.Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 13.Lee MJ, Wang ZY, Li H, et al. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol Biomarkers Prev. 1995;4:393–399. [PubMed] [Google Scholar]
- 14.Hasegawa R, Chujo T, Sai-Kato K, Umemura T, Tanimura A, Kurokawa Y. Preventive effects of green tea against liver oxidative DNA damage and hepatotoxicity in rats treated with 2-nitropropane. Food Chem Toxicol. 1995;33:961–970. doi: 10.1016/0278-6915(95)00064-9. [DOI] [PubMed] [Google Scholar]
- 15.Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea--a review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 16.Arts IC, Hollman PC, Kromhout D. Chocolate as a source of tea flavonoids. Lancet. 1999;354:488. doi: 10.1016/S0140-6736(99)02267-9. [DOI] [PubMed] [Google Scholar]
- 17.Frankel EN, Waterhouse AL, Teissedre PL. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J Agric Food Chem. 1995;43:890–894. [Google Scholar]
- 18.Osakabe N, Natsume M, Adachi T, et al. Effects of cacao liquor polyphenols on the susceptibility of low-density lipoprotein to oxidation in hypercholesterolemic rabbits. J Atheroscler Thromb. 2000;7:164–168. doi: 10.5551/jat1994.7.164. [DOI] [PubMed] [Google Scholar]
- 19.Kurosawa T, Itoh F, Nozaki A, et al. Suppressive effect of cocoa powder on atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. J Atheroscler Thromb. 2005;12:20–28. doi: 10.5551/jat.12.20. [DOI] [PubMed] [Google Scholar]
- 20.Kurosawa T, Itoh F, Nozaki A, et al. Suppressive effects of cacao liquor polyphenols (CLP) on LDL oxidation and the development of atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. Atherosclerosis. 2005;179:237–246. doi: 10.1016/j.atherosclerosis.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Day AJ, Mellon F, Barron D, Sarrazin G, Morgan MR, Williamson G. Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radic Res. 2001;35:941–952. doi: 10.1080/10715760100301441. [DOI] [PubMed] [Google Scholar]
- 22.Moon JH, Tsushida T, Nakahara K, Terao J. Identification of quercetin 3-O-β-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic Biol Med. 2001;30:1274–1285. doi: 10.1016/s0891-5849(01)00522-6. [DOI] [PubMed] [Google Scholar]
- 23.Kawai Y, Nishikawa T, Shiba Y, et al. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem. 2008;283:9424–9434. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- 24.Murota K, Hotta A, Ido H, et al. Antioxidant capacity of albumin-bound quercetin metabolites after onion consumption in humans. J Med Invest. 2007;54:370–374. doi: 10.2152/jmi.54.370. [DOI] [PubMed] [Google Scholar]
- 25.Masukawa Y, Matsui Y, Shimizu N, et al. Determination of green tea catechins in human plasma using liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;834:26–34. doi: 10.1016/j.jchromb.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Kawai Y, Tanaka H, Murota K, Naito M, Terao J. (−)-Epicatechin gallate accumulates in foamy macrophages in human atherosclerotic aorta: implication in the anti-atherosclerotic actions of tea catechins. Biochem Biophys Res Commun. 2008;374:527–532. doi: 10.1016/j.bbrc.2008.07.086. [DOI] [PubMed] [Google Scholar]
- 27.Yan S, Chai H, Wang H, et al. Effects of lysophosphatidylcholine on monolayer cell permeability of human coronary artery endothelial cells. Surgery. 2005;138:464–473. doi: 10.1016/j.surg.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 28.Mochizuki M, Kajiya K, Terao J, et al. Effect of quercetin conjugates on vascular permeability and expression of adhesion molecules. Biofactors. 2004;22:201–204. doi: 10.1002/biof.5520220142. [DOI] [PubMed] [Google Scholar]
- 29.Ishisaka A, Kawabata K, Miki S, et al. Mitochondrial dysfunction leads to deconjugation of quercetin glucuronides in inflammatory macrophages. PLoS One. 2013;8:e80843. doi: 10.1371/journal.pone.0080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myöhänen TT, Männistö PT. Distribution and functions of catechol-O-methyltransferase proteins: do recent findings change the picture? Int Rev Neurobiol. 2010;95:29–47. doi: 10.1016/B978-0-12-381326-8.00003-X. [DOI] [PubMed] [Google Scholar]
- 31.Hartung HP, Kladetzky RG, Hennerici M. Chemically modified low density lipoproteins as inducers of enzyme release from macrophages. FEBS Lett. 1985;186:211–214. doi: 10.1016/0014-5793(85)80710-9. [DOI] [PubMed] [Google Scholar]
- 32.Tapper H, Sundler R. Protein kinase C and intracellular pH regulate zymosan-induced lysosomal enzyme secretion in macrophages. J Leukoc Biol. 1995;58:485–494. doi: 10.1002/jlb.58.4.485. [DOI] [PubMed] [Google Scholar]
- 33.Serhan CN, Korchak HM, Weissmann G. PGBX, a prostagandin derivative, mimics the action of the calcium ionophore A23187 on human neutrophils. J Immunol. 1980;125:2020–2024. [PubMed] [Google Scholar]
- 34.Smolen JE, Korchak HM, Weissmann G. Increased levels of cyclic adenosine-3',5'-monophosphate in human polymorphonuclear leukocytes after surface stimulation. J Clin Invest. 1980;65:1077–1085. doi: 10.1172/JCI109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimoi K, Saka N, Nozawa R, et al. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab Dispos. 2001;29:1521–1524. [PubMed] [Google Scholar]
- 36.O'Leary KA, Day AJ, Needs PW, Sly WS, O'Brien NM, Williamson G. Flavonoid glucuronides are substrates for human liver beta-glucuronidase. FEBS Lett. 2001;503:103–106. doi: 10.1016/s0014-5793(01)02684-9. [DOI] [PubMed] [Google Scholar]
- 37.Shimoi K, Nakayama T. Glucuronidase deconjugation in inflammation. Methods Enzymol. 2005;400:263–272. doi: 10.1016/S0076-6879(05)00015-7. [DOI] [PubMed] [Google Scholar]
- 38.Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 39.Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 40.Jung HS, Chung KW, Won Kim J. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Wu JJ, Quijano C, Chen E, et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY) 2009;1:425–437. doi: 10.18632/aging.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komatsu M, Kurokawa H, Waguri S, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 43.Takamura A, Komatsu M, Hara T, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakahira K, Haspel JA, Rathinam VA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 46.Oka T, Hikoso S, Yamaguchi O, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williamson G, Barron D, Shimoi K, Terao J. In vitro biological properties of flavonoid conjugates found in vivo. Free Radic Res. 2005;39:457–469. doi: 10.1080/10715760500053610. [DOI] [PubMed] [Google Scholar]
- 48.Mullen W, Boitier A, Stewart AJ, Crozier A. Flavonoid metabolites in human plasma and urine after the consumption of red onions: analysis by liquid chromatography with photodiode array and full scan tandem mass spectrometric detection. J Chromatogr A. 2004;1058:163–168. [PubMed] [Google Scholar]
- 49.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Galindo P, Rodriguez-Gómez I, González-Manzano S, et al. Glucuronidated quercetin lowers blood pressure in spontaneously hypertensive rats via deconjugation. PLoS One. 2012;7:e32673. doi: 10.1371/journal.pone.0032673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shirai M, Kawai Y, Yamanishi R, Kinoshita T, Chuman H, Terao J. Effect of a conjugated quercetin metabolite, quercetin 3-glucuronide, on lipid hydroperoxide-dependent formation of reactive oxygen species in differentiated PC-12 cells. Free Radic Res. 2006;40:1047–1053. doi: 10.1080/10715760600794287. [DOI] [PubMed] [Google Scholar]