Abstract
The aim of this study was to investigate the hepatoprotective effect of BRP, a polysaccharide fraction isolated from Boschniakia rossica, against galactosamine and lipopolysaccharide induced fulminant hepatic failure. Mice were injected with a single dose of galactosamine/lipopolysaccharide with or without pretreatment of BRP. Results showed marked reduction of hepatic necrosis, serum marker enzymes and levels of tumor necrosis factor-α and interleukin-6 in BRP pretreated mice when compared with galactosamine/lipopolysaccharide-challenged mice. Mice pretreated with BRP decreased the activation of caspases-3 and caspase-8, and showed a reduced level of DNA fragmentation of liver cells. BRP also reduced hepatic lipid peroxidation, increased potential of hepatic antioxidative defense system, and reduced hepatic nitric oxide level which was elevated by galactosamine/lipopolysaccharide injection. Immunoblot analysis showed down-regulation of inducible nitric oxide synthase and cyclooxygenase-2 proteins of liver tissues in BRP pretreated group when compared with galactosamine/lipopolysaccharide-challenged group. Furthermore, treatment with galactosamine/lipopolysaccharide markedly increased toll-like receptor 4, nuclear level of nuclear factor-κB, and phosphorylation of both extracellular signal-regulated kinase and c-Jun N-terminal kinase in liver tissues. However, these increases were attenuated by pretreatment with BRP. The results suggest that BRP alleviates galactosamine/lipopolysaccharide-induced liver injury by enhancing antioxidative defense system, suppressing inflammatory responses and reducing apoptotic signaling.
Keywords: Boschniakia rossica, polysaccharide, hepatic failure, mice
Introduction
Fulminant hepatic failure (FHF) is a life-threatening clinical syndrome that results from severe impairment of liver function. It is associated with high mortality if not treated by liver transplantation.(1) A low dose of lipopolysaccharide (LPS), an outer membrane component of gram-negative bacteria, in combination with galactosamine (GalN), a hepatotoxin, has been shown to induce an experimental liver injury that is similar to clinical acute hepatic failure.(1–5) GalN mainly causes liver injury via the generation of free radicals and depletion of uridine triphosphate nucleotides, leading to inhibition of RNA and protein synthesis, and induction of apoptosis in the liver.(6,7) LPS directly activates macrophages, including Kupffer cells, the resident macrophages in the liver, to produce inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and nitric oxide (NO), causing hepatic injury.(4,8–10) It is also known that TNF-α is one of the major mediators which contributes to hepatocyte apoptosis and organ failure.(5,11–13) Overproduction of reactive oxidative species (ROS) also plays an etiological role in the process of FHF induced by GalN/LPS.(5,14–16) Consequently, interventions in the production of pro-inflammatory mediators and oxidative substances are potential strategies for the prevention or therapy of FHF.(5)
Boschniakia rossica Fedtsch. et Flerov (Orobanchaceae), a parasitic plant often parasitizing on the root of plants of the genus Alnus (Betulaceae), distributes widely in Changbai Mountain of China, Fuji Mountain of Japan, and the northern mountains of North Korea.(17) It is widely used in Chinese traditional medicine as a substitute for Cistanchis Herba, a famous staminal tonic agent. It is called an “anti senility herb” in China and is valued for its ability to invigorate the kidney, moistening intestines, nourishing body and strengthen Yang.(17) The pharmacognostical studies had identified three major groups of compounds in Boschniakia rossica, namely polysaccharides, iridoids and phenylpropanoids, displaying a variety of pharmacological activities.(18–23) In recent decades, polysaccharides isolated from botanical sources (mushrooms, algae, lichens and higher plants) have attracted a great deal of attention in the biomedical arena because of their relatively low toxicity and various biological properties.(22–26) Polysaccharides from Boschniakia rossica had been reported to have anti-oxidative and anti-inflammatory functions, and have characteristics of non-toxicity.(22,23,25,26) Furthermore, our previous experiment showed that oral administration of Boschniakia rossica polysaccharides play a protective role in CCl4-induced acute liver injury by inhibiting lipid peroxidation and suppressing inflammatory responses.(26)
Based on the above preliminary investigation, we hypothesize that polysaccharides from Boschniakia rossica may be effective in protecting against acute liver failure induced by galactosamine (GalN) and lipopolysaccharide (LPS). Here, we evaluated the protective effect and possible mechanism of BRP, a polysaccharide fraction isolated from Boschniakia rossica, in a GalN/LPS-induced FHF in vivo model. The effect of BRP on acute liver failure was compared to that of silymarin, which is used clinically in Europe and Asia for the treatment of liver diseases.(26)
Materials and Methods
Animals
Male ICR mice weighing 18–20 g were purchased from the animal house section of Yanbian University Health Science Center, China [Certification No: SCXK (Ji) 2008-0005]. Mice were housed in a controlled environment at 22 ± 2°C and a relative humidity of 50 ± 10%, under a 12 h light/dark cycle. Animals were fed with a standard laboratory diet and given water ad libitum. The composition of the mineral mixture was according to AIN-93G MX and that of the vitamin mixture to AIN-93G VX.(27) Animal experiments began after one week of adaptation. The experimental procedures were in accordance with internationally accepted guidelines for animal use and care (EEC Directive of 1986; 86/09/EEC; National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, revised 1988), which are in agreement with the Helsinki declaration, with the approval of the local ethics committee.
Reagents
GalN and LPS were obtained from Sigma Chemical Company (St. Louis, MO). Lipid hydroperoxide (LOOH) diagnostic kit was obtained from Kyowa Medex Company Ltd, Tokyo, Japan. Diagnostic kits to measure serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), malondialdehyde (MDA), reduced GSH, glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST) and NO were obtained from Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Nuclear and cytoplasmic protein extraction kit was purchased from Beyotime Institute of Biotechnology, Shanghai, China. ELISA kits for TNF-α and IL-6 were purchased from BD Bioscience, San Jose, CA. Antibodies against rabbit inducible nitric oxide synthase (iNOS), nuclear factor-κB (NF-κB) p65, phospho-NF-κB p65, toll-like receptor 4 (TLR4), extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase 1/2 (JNK1/2) and mouse heme oxygenase-1 (HO-1) were purchased from Abcam (HK) Ltd., Hongkong, China. Antibodies against mouse cyclooxygenase-2 (COX-2), cleaved caspase-3, cleaved caspase-8, and Lamin B1, rabbit glyceraldehyde 3-phosphate dehydrogenase (GAPDH), horseradish peroxidase-conjugated anti-rabbit IgG and anti-mouse IgG were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. A wizard genomic DNA purification kit, caspase-3 and caspase-8 activity assay kits were obtained from Kaiji Biotechnology Institute, Nanjing, China. All other chemicals were of analytical grade.
Polysaccharides preparation and preliminary chemical analysis
The whole plant of Boschniakia rossica was collected from Changbai Mountain of China, and identified and identified by Dr. Zongzhu Yin of Yanbian University, China. A voucher specimen was deposited in the Herbarium of Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang, China (No.13201001). The preparation of BRP was performed as previously described with a minor modification.(22,26,28) Briefly, the water-soluble crude polysaccharides were obtained from Boschniakia rossica by defatting with ethanol, hot water extraction, ethanol precipitation, deproteinization by Sevag method and repeated unfreezing process, dialysis against water and lyophilization. The yield of crude polysaccharide accounted for 3.3% of the whole plant. The polysaccharide fraction was further purified by DEAE-cellulose chromatography and gel filtration chromatography according to the method of Song.(28) The major polysaccharide fraction BRP with molecular weight of 4.9 × 104 Da was selected for the evaluation of the hepatoprotective effect. The total carbohydrate content in BRP was 97.4% (w/w) by the phenol-sulfuric acid method with glucose as the standard. In addition, BRP had a negative response in the Bradford test and exhibited no absorption at 280 nm in the UV spectrum, indicating the absence of protein.(22,29)
Animal treatment
Mice were randomly assigned to the following 5 groups: the normal control group, the model group, BRP (120 and 240 mg/kg) group, and silymarin (50 mg/kg) group. In BRP group, mice were administered BRP at 120 and 240 mg/kg intragastrically (i.g.) for 3 days prior to exposure to LPS and GalN, while other groups received an equivalent volume of saline as the control. The dose of BRP treatment was selected based on our previous experiments and other reports.(22,23,25,26) All animals were injected intraperitoneally (i.p.) with 700 mg/kg GalN and 10 µg/kg LPS dissolved in phosphate-buffered saline, except for the normal control. For sample, mice were anesthetized with ether and sacrificed by decapitation at 2 and 8 h after GalN/LPS injection, and blood and liver were collected for testing.
Biochemical analysis of serum
The serum ALT and AST levels at 8 h, and serum TNF-α and IL-6 levels at 2 h after GalN/LPS administration were measured. The serum ALT and AST were determined in accordance with the methods provided by the diagnostic kits. The serum TNF-α and IL-6 levels were measured using mouse TNF-α and IL-6 ELISA kits according to the corresponding protocols. The inhibitory ratios of BRP in ALT, AST, TNF-α and IL-6 were calculated by using the following equation.
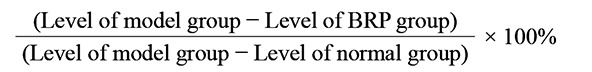 |
Histopathological examinations
Fresh liver tissues were collected 8 h after the induction of acute liver injury in mice, and then fixed in 10% paraformaldehyde (PFA)/PBS for 24 h. The fixed livers were then embedded in paraffin, sectioned, deparaffinized, and rehydrated using the standard techniques. Liver sections were processed for staining with hematoxylin and eosin (HE), using the standard techniques.
Detection of DNA fragmentation
Genomic DNA was extracted from liver tissues at 8 h after GalN/LPS administration using a genomic DNA purification kit according to the manufacturer’s instruction. DNA was separated on 1.5% agarose gel containing 0.5 mg/ml ethidium bromide. DNA fragmentation pattern was photographed under ultraviolet illumination.
Biochemical determination of liver
Liver tissues were collected 8 h after GalN/LPS administration, and homogenized in cold phosphate-buffered saline (pH 7.2), and then centrifuged at 12,000 × g for 10 min at 4°C and supernatants were collected. The total protein level was measured by the Bradford method according to manufacturer’s instructions with bovine serum albumin as the standard.(29) Liver lipid hydroperoxide (LOOH) was determined enzymatically, using a kit according to the manufacturer’s instruction. Thiobarbituric acid-reactive substance (TBARS) was determined according to the thiobarbituric acid method using an MDA test kit. Hepatic SOD, CAT, GSH-Px, GST and GSH levels were measured by the corresponding kits according to the manufacturer’s instructions. The values were normalized by the total protein concentrations of the same samples. Activations of caspase-3 and caspase-8 in liver tissues were quantified by colorimetric assay, using commercial test kits according to the manufacturer’s instructions. The relative ratios of caspase-3 and caspase-8 activities were normalized to normal control.
Immunoblot analysis
The protein expression of TLR4, JNK, ERK and NF-κB p65 at 2 h after GalN/LPS administration, and the expression of other proteins at 8 h after GalN/LPS administration in the liver were measured. The nuclear and cytoplasmic protein extraction kit was used for extract nuclear and cytosolic proteins in accordance with the manufacturer’s instructions. The electrophoretic separation of the proteins was performed using 10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and then transferred to nitrocellulose membranes, which was immunoblotted with anti-mouse HO-1, COX-2, caspase-3 and caspase-8 or anti-rabbit iNOS, 65, phospho-p65, TLR4, ERK1/2, phospho-ERK1/2, JNK1/2, phospho-JNK1/2 and GAPDH antibodies. Horseradish peroxidase conjugated goat anti-mouse IgG or anti-rabbit IgG were used as the secondary antibodies. The specific protein was detected using the enhanced chemiluminescence (ECL) reagent. The relative levels of protein expressions were normalized to GAPDH after quantitative estimation by using NIH Image software (Bethesda, MD) and expressed in arbitrary units.
Statistical analysis
Data from experiments are expressed as mean ± SD. Statistical analysis was conducted by a one-way analysis of variance followed by Tukey’s post hoc test using Statistics Package for Social Science 19.0 software (SPSS Inc., Chicago, IL). The significance level for all analyses was set at probability of less than or equal to 0.05.
Results
We first examined the effects of GalN/LPS alone on liver damage and cytokine production in the first several hours after GalN/LPS injection. We confirmed that serum ALT and AST levels peaked within 8 h after a single injection of LPS and GalN, and TNF-α and IL-6 levels were increased within 2 h and slowly decreased after GalN/LPS induction (data not shown). We therefore standardized the sampling time to 2 and 8 h after GalN/LPS injection for histological, molecular, and biochemical analyses.
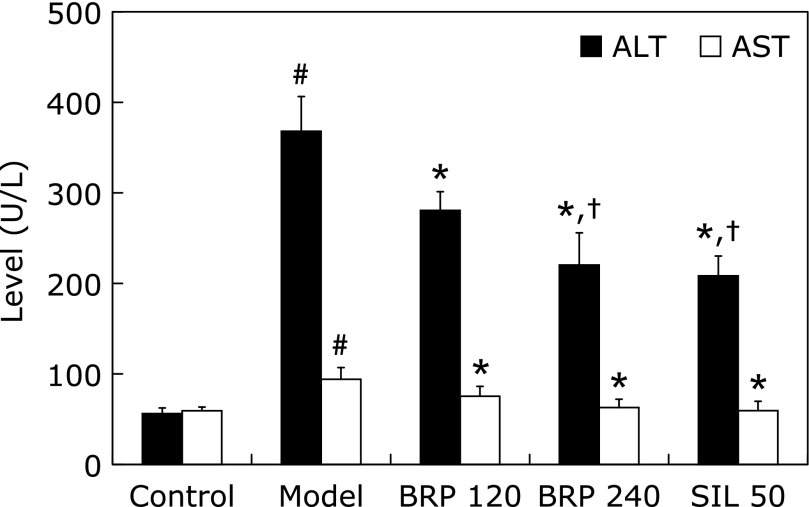
The protective effect of BRP on the GalN/LPS-induced elevation of serum ALT and AST is presented in Fig. 1. As expected, the administration of BRP at doses of 120 and 240 mg/kg prior to GalN/LPS challenge was observed to reverse the alterations of ALT by 28% and 47%, of AST by 53% and 90%, respectively (Fig. 1). Silymarin administration also reversed the alteration of ALT and AST by 52% and 99% when compared with the model group. The serum levels of ALT and AST in 240 mg/kg BRP group were comparable to those in 50 mg/kg silymarin group (Fig. 1).
Fig. 1.
BRP reduced serum ALT and AST of mice with GalN/LPS-induced liver injury. Mice were orally administered with BRP, silymarin or a vehicle for 3 days prior to administration with GalN/LPS. Blood samples were collected at 8 h after GalN/LPS exposure. Data are presented as mean ± SD, n = 12. #p<0.05 as compared with the control group; *p<0.05 as compared with the model group; †p<0.05, significantly different from 120 mg/kg BRP group. Control: normal control; Model: GalN/LPS alone; BRP: GalN/LPS plus BRP (120 and 240 mg/kg); SIL: GalN/LPS plus silymarin (50 mg/kg).
According to the light microscopic evaluation of the normal control group, a regular morphology of the liver parenchyma with well-designated hepatic cells and sinusoids was evident (Fig. 2A). However, massive necrosis, apoptosis, inflammatory cell infiltrate, and vacuolation appeared in the livers of GalN/LPS-treated mice. Many apoptotic bodies and nuclei with condensed chromatin appeared in the mice of the GalN/LPS group (Fig. 2B). These pathological changes were ameliorated both in BRP and silymarin-pretreated mice (Fig. 2C–E), suggesting that both BRP and silymarin have protective effects on GalN/LPS-induced hepatotoxicity.
Fig. 2.
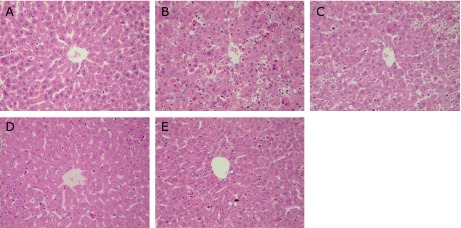
BRP improved liver histological alterations in GalN/LPS-challenged mice. Liver sections were stained with hematoxylin-eosin for morphological evaluation (200×). Mice were orally administered with BRP, silymarin or a vehicle for 3 days prior to administration with GalN/LPS. (A) Control; (B) Model; (C) BRP 120 mg/kg; (D) BRP 240 mg/kg; (E) Silymarin 50 mg/kg.
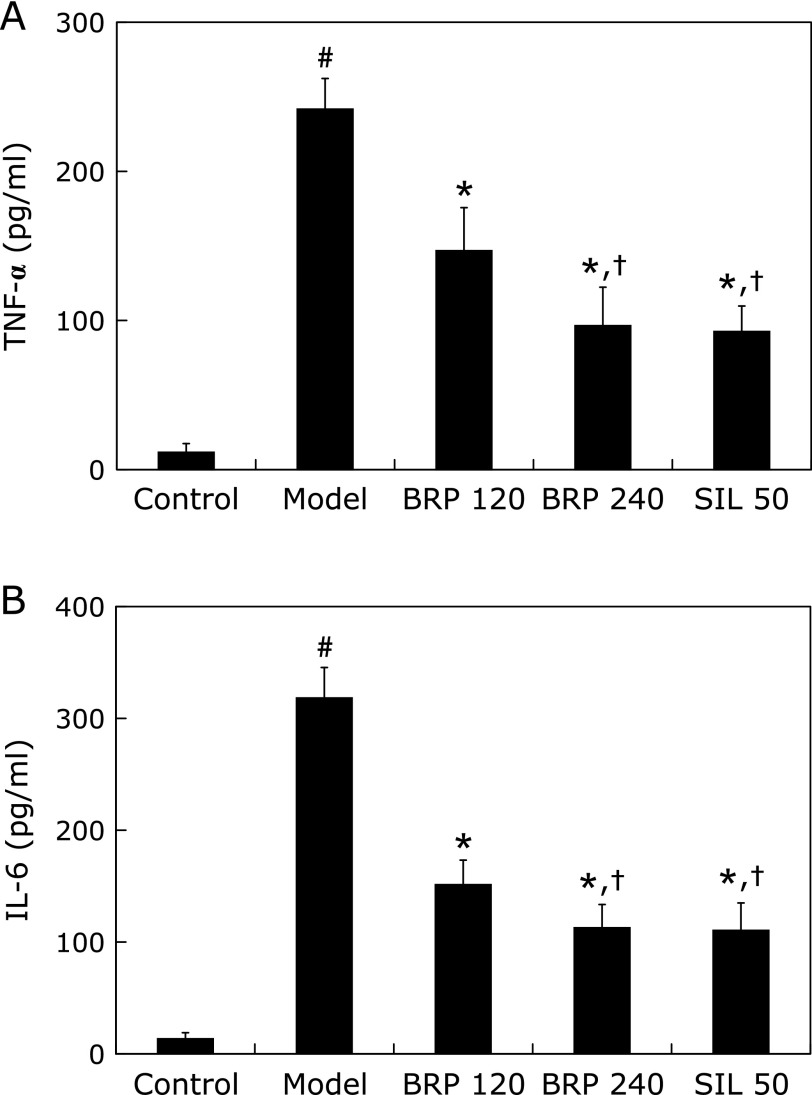
The effects of BRP pretreatment on GalN/LPS-induced elevation of serum TNF-α and IL-6 levels are presented in Fig. 3. Challenge with GalN/LPS caused a marked increase in serum TNF-α and IL-6 levels compared with that of the normal control group. However, pretreatment with BRP reduced serum TNF-α level to 41% and 62%, and IL-6 level to 54% and 66%, respectively, of those in GalN/LPS-treated mice. Silymarin also reduced the serum TNF-α and IL-6 levels when compared with the model group. The levels of two cytokines in both 240 mg/kg BRP and 50 mg/kg silymarin groups were comparable (Fig. 3). Thus, BRP at 240 mg/kg was selected as the optimal effective dose for evaluating the molecular mechanisms of BRP against GalN/LPS-induced hepatotoxicity.
Fig. 3.
BRP reduced serum TNF-α and IL-6 contents in GalN/LPS-challenged mice. Mice were orally administered with BRP, silymarin or a vehicle for 3 days prior to administration with GalN/LPS. Blood samples were collected at 2 h after GalN/LPS exposure for measurement of circulating serum TNF-α (A) and IL-6 (B). Data are presented as mean ± SD, n = 12. #p<0.05 as compared with the control group; *p<0.05 as compared with the model group; †p<0.05, significantly different from 120 mg/kg BRP group. Control: normal control; Model: GalN/LPS alone; BRP: GalN/LPS plus BRP (120 and 240 mg/kg); SIL: GalN/LPS plus silymarin (50 mg/kg).
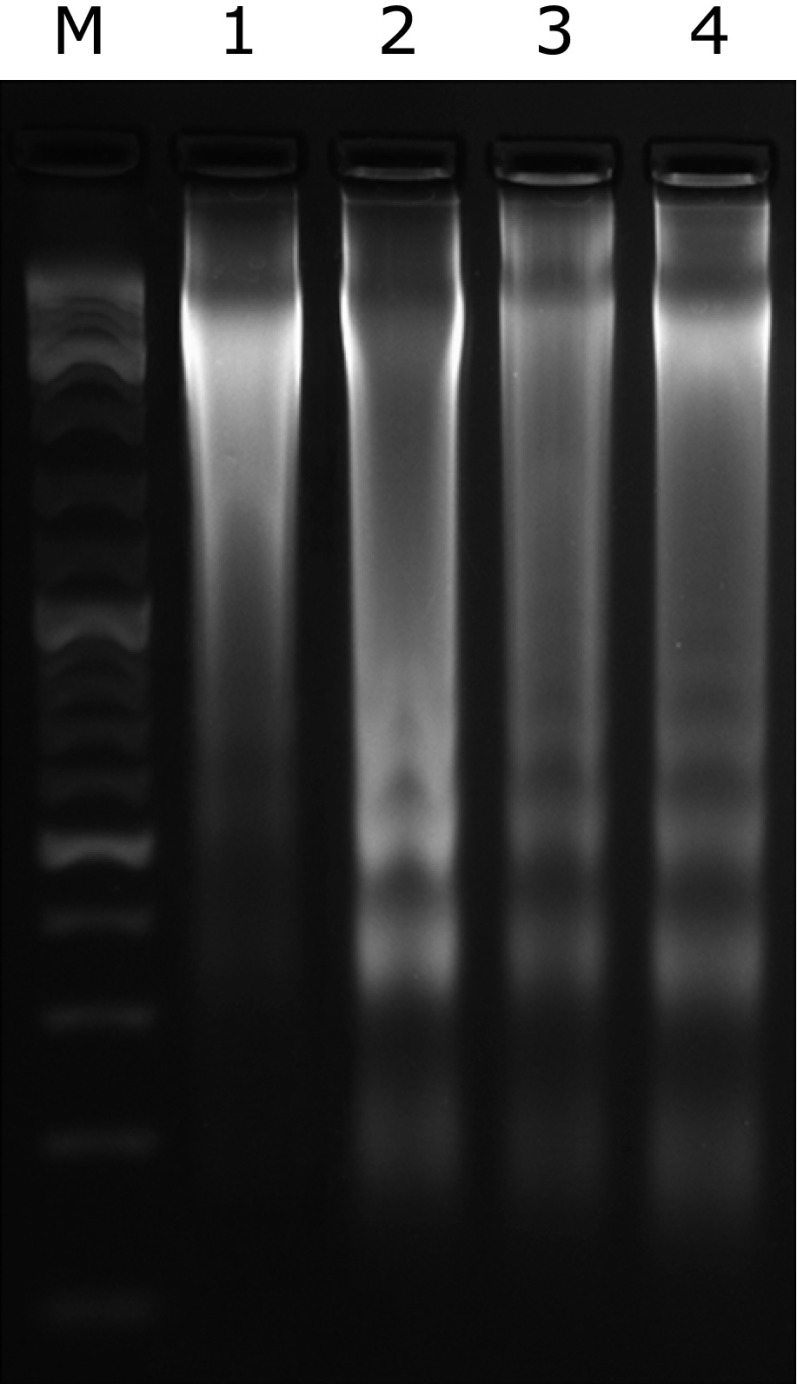
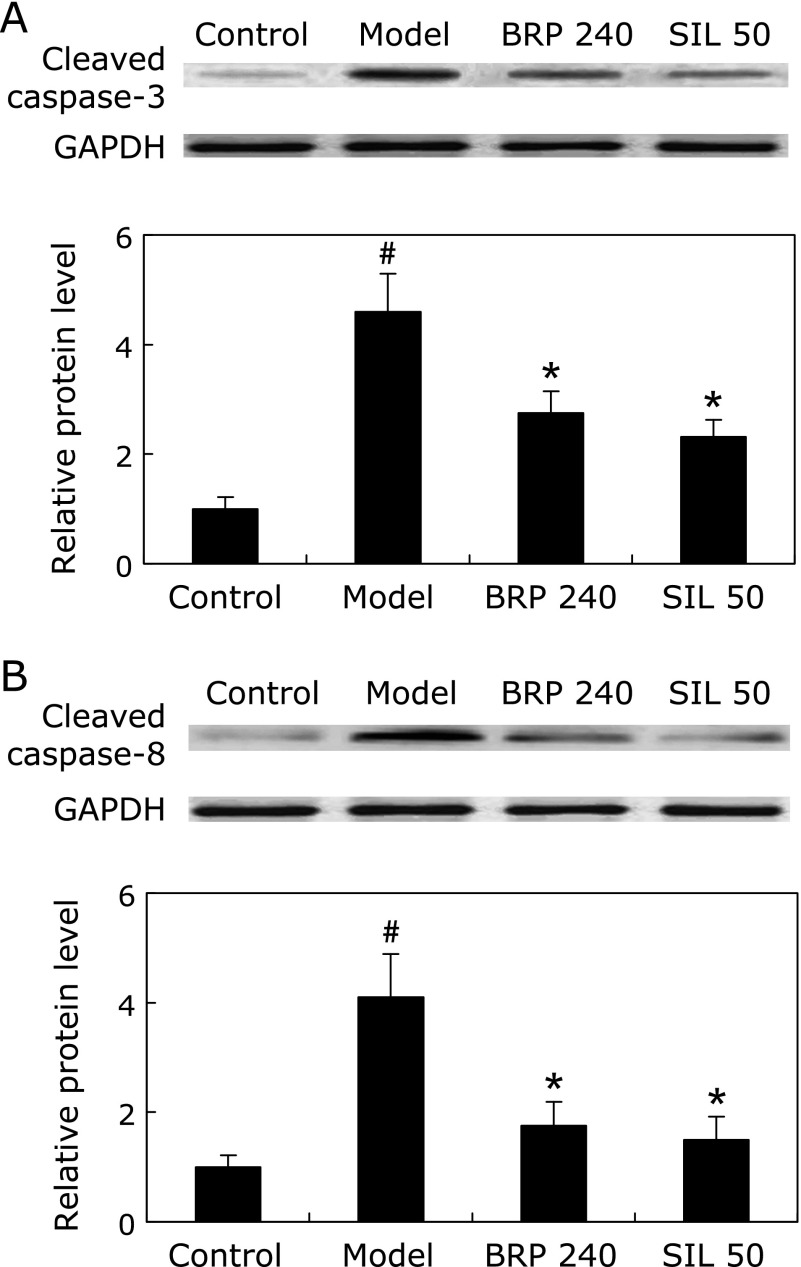
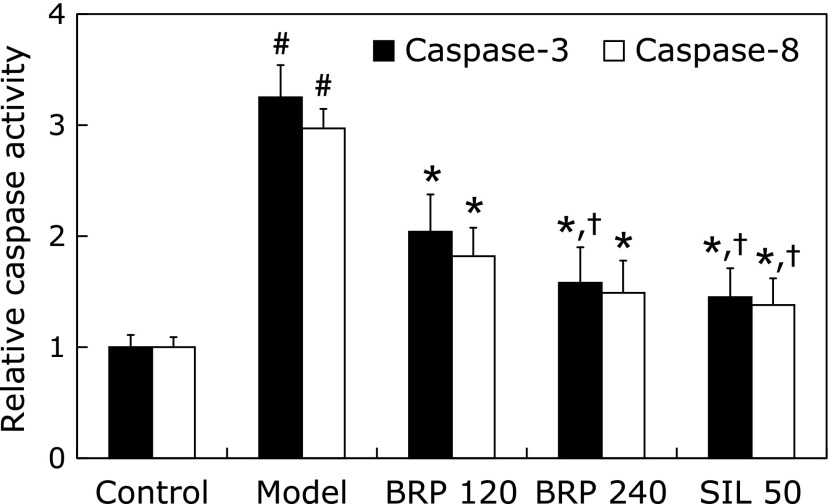
Livers from GalN/LPS-treated mice showed representative ladder pattern of liver oligonucleosomal DNA. As expected, pretreatment with BRP or silymarin resulted in suppression of internucleosomal DNA fragmentation, evidenced by a reduced level of detectable DNA ladders on agarose gels (Fig. 4). The levels of cleaved caspase-3 and caspase-8, were prominently increased in GalN/LPS-treated mice but were significantly reduced by BRP or silymarin treatment as determined by Immunoblot analysis (Fig. 5). Treatment with either BRP or silymarin also significantly decreased the activities of caspase-3 and caspase-8 in GalN/LPS-treated livers (Fig. 6).
Fig. 4.
BRP suppressed hepatic DNA fragmentation in GalN/LPS-challenged mice. Mice were orally administered with 240 mg/kg BRP, 50 mg/kg silymarin or a vehicle for 3 days prior to administration with GalN/LPS. The animals were sacrificed 8 h after GalN/LPS exposure. Lane M: marker; Lane 1: control; Lane 2: model; Lane 3: BRP 240 mg/kg; Lane 4: silymarin 50 mg/kg.
Fig. 5.
BRP suppressed hepatic caspase-3 and caspase-8 cleavages in GalN/LPS-challenged mice. Mice were orally administered with 240 mg/kg BRP, 50 mg/kg silymarin or a vehicle for 3 days prior to administration with GalN/LPS. Liver samples were collected at 8 h after GalN/LPS exposure for measurement of cleaved caspase-3 (A) and caspase-8 (B). GAPDH was used as an internal control and the relative level of protein expression was normalized to the control. Data are presented as mean ± SD, n = 6. #p<0.05 as compared with the control group; *p<0.05 as compared with the model group. Control: normal control; Model: GalN/LPS alone; BRP 240: GalN/LPS plus BRP (240 mg/kg); SIL: GalN/LPS plus silymarin (50 mg/kg).
Fig. 6.
BRP reduced hepatic caspase-3 and caspase-8 activities in GalN/LPS-challenged mice. Mice were orally administered with BRP, silymarin or a vehicle for 3 days prior to administration with GalN/LPS. Liver samples were collected at 8 h after GalN/LPS exposure. Data are presented as mean ± SD, n = 12. #p<0.05 as compared with the control group; *p<0.05 as compared with the model group; †p<0.05, significantly different from 120 mg/kg BRP group. Control: normal control; Model: GalN/LPS alone; BRP: GalN/LPS plus BRP (120 and 240 mg/kg); SIL: GalN/LPS plus silymarin (50 mg/kg).
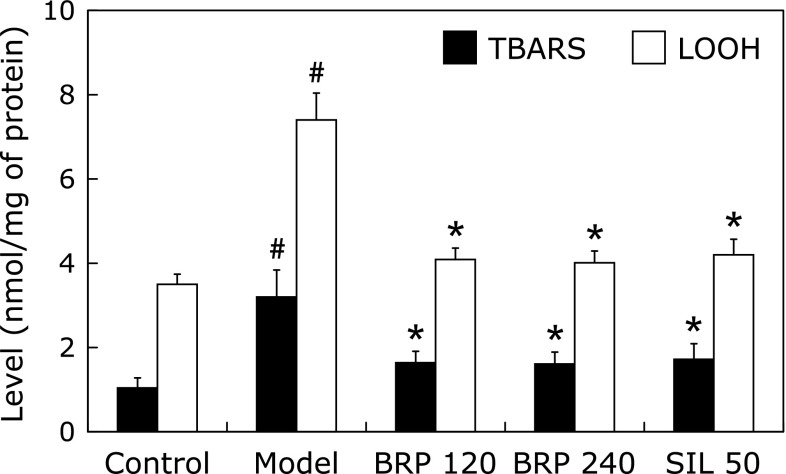
LOOH and TBARS are frequently used as markers for injuries induced by free radicals. Mice treated with GalN/LPS showed a marked increase in the levels of LOOH and TBARS as compared with the normal control. A significant reduction in the amount of LOOH and TBARS was observed in BRP or silymarin groups of mice when compared with the GalN/LPS group (Fig. 7). Remarkable changes were also observed in activities of the hepatic antioxidative and detoxicating enzymes. The data showed that decreased activities of SOD, GSH-Px and GST in liver tissues as the result of GalN/LPS injection were significantly increased both in BRP and silymarin groups (Table 1). In liver of GalN/LPS treated mice, a significant decrease in GSH levels relative to normal mice was observed. Treatment with BRP or silymarin resulted in GSH levels that were similar to the levels in normal mice. These results suggest that pretreatment with BRP or silymarin could enhance the hepatic antioxidant potential.
Fig. 7.
BRP reduced hepatic LOOH and TBARS contents in GalN/LPS-challenged mice. Mice were orally administered with BRP, silymarin or a vehicle for 3 days prior to administration with GalN/LPS. Liver samples were collected at 8 h after GalN/LPS exposure. Data are presented as mean ± SD, n = 12. #p<0.05 as compared with the control group; *p<0.05 as compared with the model group. Control: normal control; Model: GalN/LPS alone; BRP: GalN/LPS plus BRP (120 and 240 mg/kg); SIL: GalN/LPS plus silymarin (50 mg/kg).
Table 1.
Effect of BRP on SOD, CAT, GSH-Px, GST and GSH levels of liver tissues
| Group | Dosage (mg/kg) | SOD (U/mg) | CAT (U/mg) | GSH-Px (U/mg) | GST (U/mg) | GSH (nmol/mg) |
|---|---|---|---|---|---|---|
| Control | — | 170.9 ± 10.8 | 12.9 ± 1.8 | 240.9 ± 40.4 | 27.0 ± 7.8 | 23.6 ± 4.2 |
| Model | — | 107.6 ± 20.2# | 11.7 ± 1.9 | 142.4 ± 25.2# | 13.2 ± 6.1# | 11.4 ± 3.7# |
| BRP | 120 | 162.9 ± 7.8* | 13.4 ± 1.2 | 279.9 ± 38.2 | 19.8 ± 7.7* | 20.7 ± 3.9* |
| 240 | 162.3 ± 9.9* | 14.5 ± 2.0* | 285.3 ± 20.3* | 21.5 ± 7.2* | 21.8 ± 4.0* | |
| SIL | 50 | 168.5 ± 15.1* | 13.9 ± 1.1 | 258.0 ± 39.4* | 18.4 ± 6.3* | 21.3 ± 5.4* |
Mice were orally administered with BRP, silymarin or a vehicle for 3 days prior to administration with GalN/LPS. Blood samples were collected at 8 h after GalN/LPS exposure. Data are presented as mean ± SD, n = 12. #p<0.05 as compared with the control group; *p<0.05 as compared with the model group. Control: normal control; Model: GalN/LPS alone; BRP: GalN/LPS plus BRP (120 and 240 mg/kg); SIL: GalN/LPS plus silymarin (50 mg/kg).
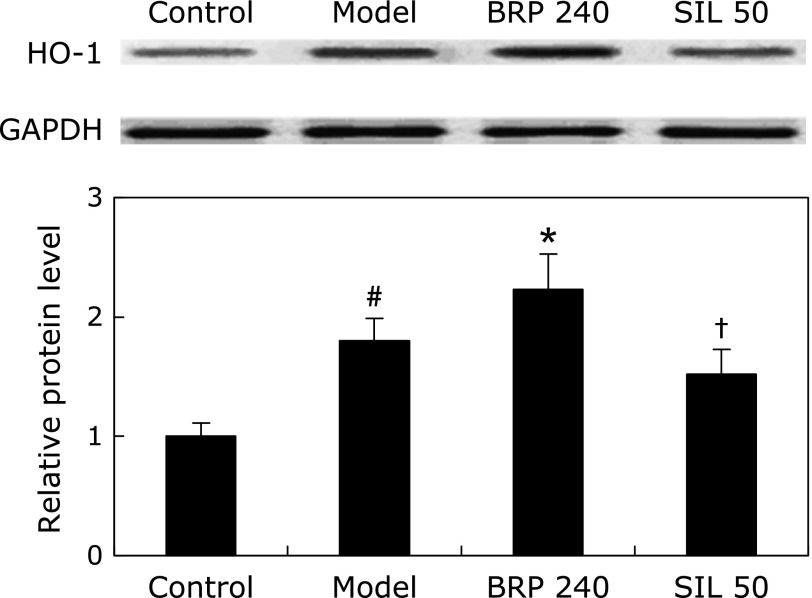
Treatment with BRP before GalN/LPS insult resulted in an increase in hepatic HO-1 protein level (Fig. 8). Interestingly, HO-1 protein expression was increased along with the treatment of BRP compared with that in the model group. In contrast, HO-1 protein level of silymarin group tended to decrease to a small extent, though the difference was not statistically significant as compared with the model group. Actually there was a significant difference in HO-1 protein level between the silymarin and BRP groups (Fig. 8).
Fig. 8.
BRP up-regulated hepatic HO-1 protein expression in GalN/LPS-challenged mice. Mice were orally administered with 240 mg/kg BRP, 50 mg/kg silymarin or a vehicle for 3 days prior to administration with GalN/LPS. Liver samples were collected at 8 h after GalN/LPS exposure. GAPDH was used as an internal control and the relative level of protein expression was normalized to the control. Data are presented as mean ± SD, n = 6. #p<0.05 as compared with the control group; *p<0.05 as compared with the model group; †p<0.05, significantly different from 240 mg/kg BRP group. Control: normal control; Model: GalN/LPS alone; BRP 240: GalN/LPS plus BRP (240 mg/kg); SIL: GalN/LPS plus silymarin (50 mg/kg).
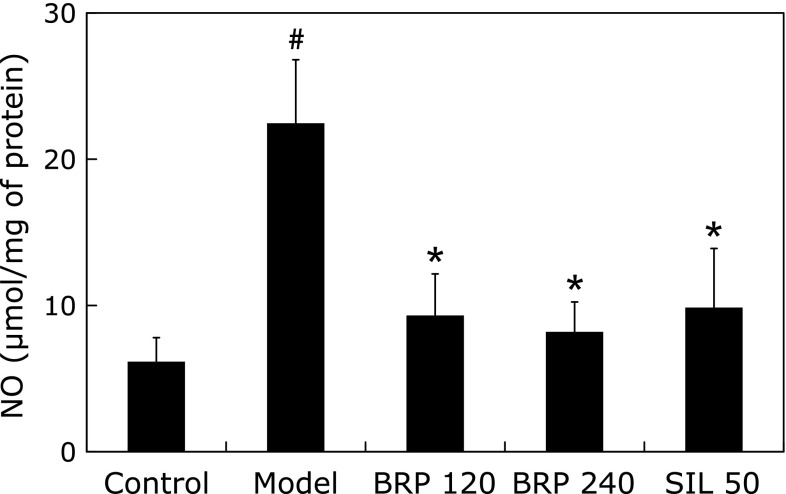
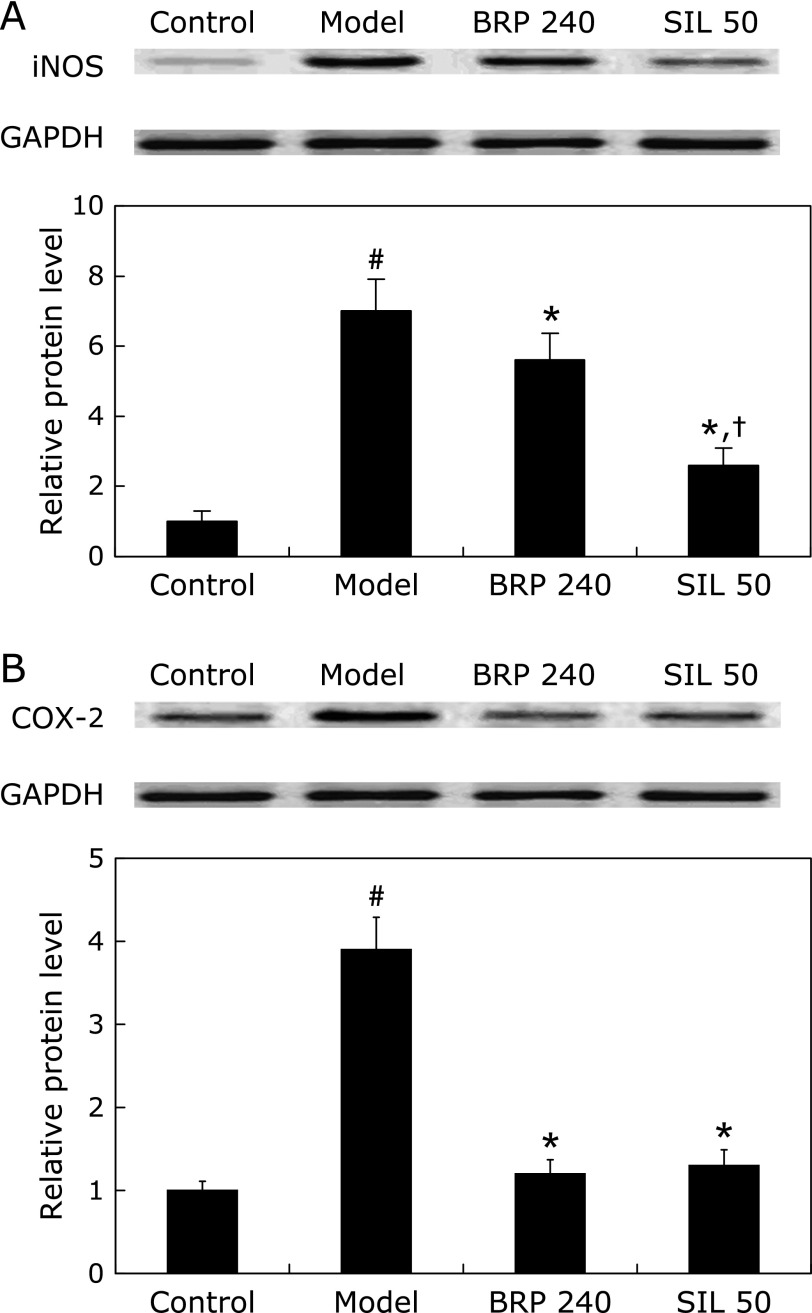
To further assess the hepatic nitrosative and inflammatory status of different groups, we measured the NO levels in the supernatants of liver homogenates. As shown in Fig. 9, hepatic NO was markedly increased after GalN/LPS treatment. However, pretreatment with BRP markedly suppressed the production of NO (Fig. 9). Immunoblot analysis with specific anti-iNOS and anti-COX-2 antibodies revealed up-regulation of both proteins in the liver cells after GalN/LPS challenge, while down-regulated iNOS or COX-2 proteins were found in the livers of BRP and silymarin groups (Fig. 10).
Fig. 9.
BRP reduced hepatic NO content in GalN/LPS-challenged mice. Mice were orally administered with BRP, silymarin or a vehicle for 3 days prior to administration with GalN/LPS. Liver samples were collected at 8 h after GalN/LPS exposure. Data are presented as mean ± SD, n = 12. #p<0.05 as compared with the control group; *p<0.05 as compared with the model group. Control: normal control; Model: GalN/LPS alone; BRP: GalN/LPS plus BRP (120 and 240 mg/kg); SIL: GalN/LPS plus silymarin (50 mg/kg).
Fig. 10.
BRP down-regulated hepatic iNOS and COX-2 protein expression in GalN/LPS-challenged mice. Mice were orally administered with 240 mg/kg BRP, 50 mg/kg silymarin or a vehicle for 3 days prior to administration with GalN/LPS. Liver samples were collected at 8 h after GalN/LPS exposure for measurement of iNOS (A) and COX-2 (B). GAPDH was used as an internal control and the relative level of protein expression was normalized to the control. Data are presented as mean ± SD, n = 6. #p<0.05 as compared with the control group; *p<0.05 as compared with the model group; †p<0.05, significantly different from 240 mg/kg BRP group. Control: normal control; Model: GalN/LPS alone; BRP 240: GalN/LPS plus BRP (240 mg/kg); SIL: GalN/LPS plus silymarin (50 mg/kg).
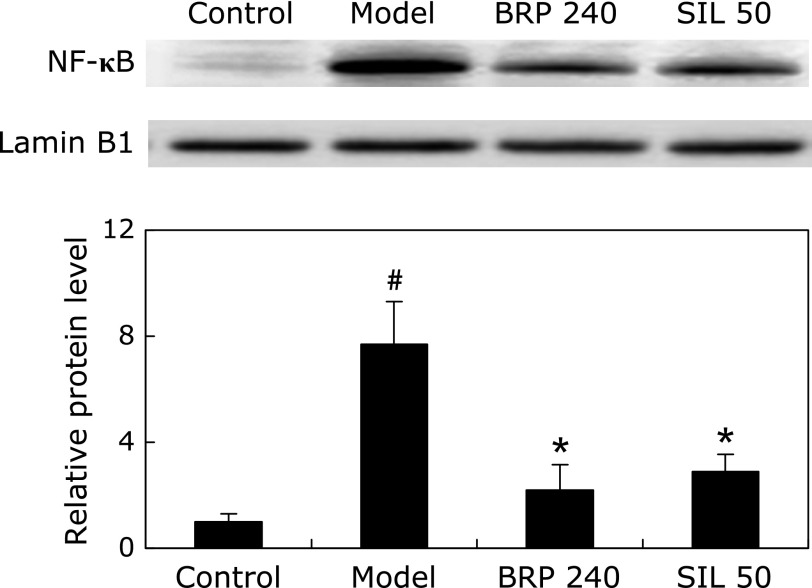
We examined the nuclear translocation of NF-κB by measuring the protein level of NF-κB p65 subunit in the nucleus. Immunoblot analysis revealed that the nuclear translocation of NF-κB p65 in liver tissues was significantly increased in GalN/LPS treated mice. However, BRP treatment significantly inhibited the translocation of p65 to nucleus in GalN/LPS-treated livers, similar to that observed in mice pretreated with silymarin (Fig. 11).
Fig. 11.
BRP down-regulated nuclear NF-κB p65 protein expression in livers of GalN/LPS-challenged mice. Mice were orally administered with 240 mg/kg BRP, 50 mg/kg silymarin or a vehicle for 3 days prior to administration with GalN/LPS. Liver samples were collected at 2 h after GalN/LPS exposure. Lamin B1 was used as an internal control and the relative level of protein expression was normalized to the control. Data are presented as mean ± SD, n = 6. #p<0.05 as compared with the control group; *p<0.05 as compared with the model group. Control: normal control; Model: GalN/LPS alone; BRP 240: GalN/LPS plus BRP (240 mg/kg); SIL: GalN/LPS plus silymarin (50 mg/kg).
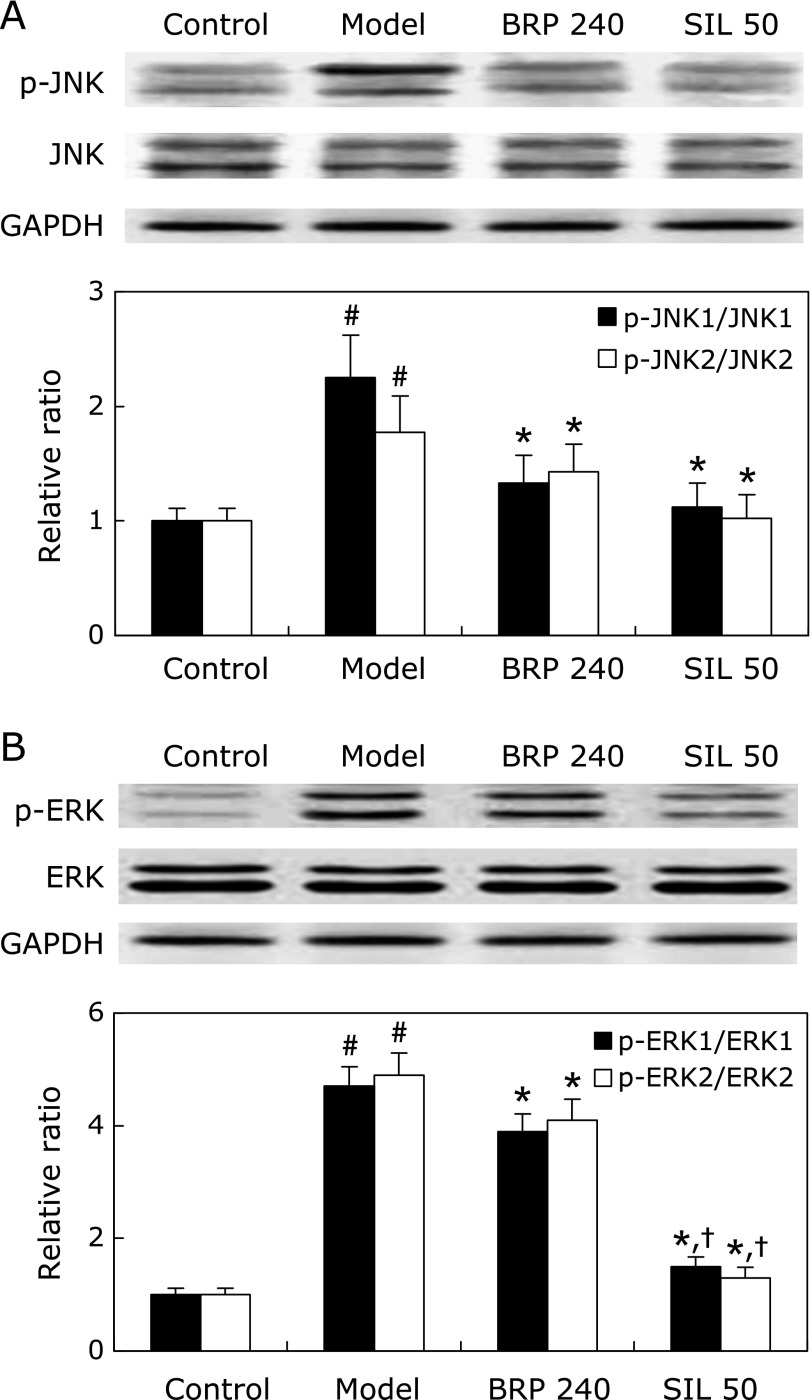
Results showed that activation of JNK and ERK by phosphorylation was increased in livers of mice challenged with GalN/LPS (Fig. 12). Treatment with BRP or silymarin, however, resulted in suppression of JNK and ERK phosphorylation, and the protein ratios of phosphorylated JNK and ERK to total JNK and ERK were significantly decreased, respectively (Fig. 12). However, the reduction of phospho-ERK in the silymarin group was much greater than the BRP group.
Fig. 12.
BRP suppressed hepatic JNK and ERK activation in GalN/LPS-challenged mice. Mice were orally administered with 240 mg/kg BRP, 50 mg/kg silymarin or a vehicle for 3 days prior to administration with GalN/LPS. Liver samples were collected at 2 h after GalN/LPS exposure for measurement of JNK (A) and ERK (B). GAPDH was used as an internal control and the relative level of protein expression was normalized to the control. Data are presented as mean ± SD, n = 6. #p<0.05 as compared with the control group; *p<0.05 as compared with the model group; †p<0.05, significantly different from 240 mg/kg BRP group. Control: normal control; Model: GalN/LPS alone; BRP 240: GalN/LPS plus BRP (240 mg/kg); SIL: GalN/LPS plus silymarin (50 mg/kg).
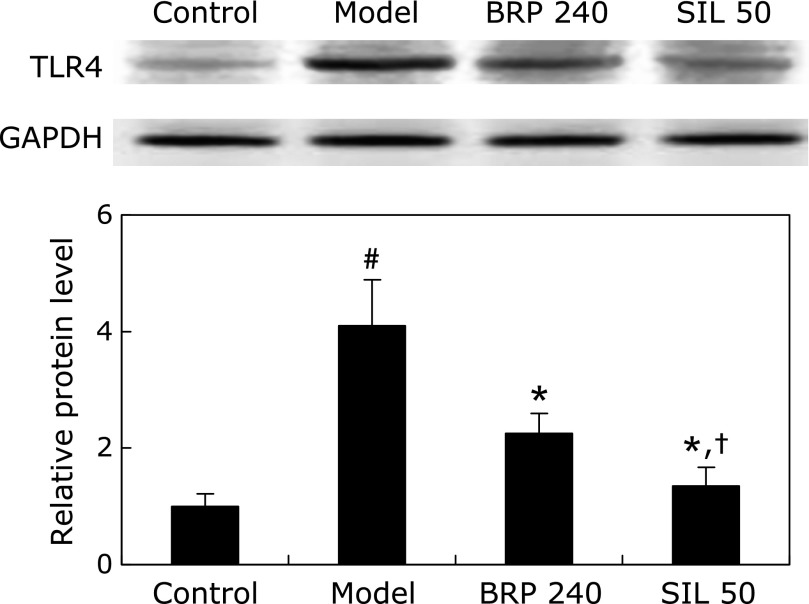
As shown in Fig. 13, the level of TLR4 protein expression increased to approximately 4-fold of that of the control group at 2 h after GalN/LPS injection. However, it was significantly abolished both in BRP and silymarin groups (Fig. 13).
Fig. 13.
BRP down-regulated TLR4 protein expression in livers of GalN/LPS-challenged mice. Mice were orally administered with 240 mg/kg BRP, 50 mg/kg silymarin or a vehicle for 3 days prior to administration with GalN/LPS. Liver samples were collected at 2 h after GalN/LPS exposure. GAPDH was used as an internal control and the relative level of protein expression was normalized to the control. Data are presented as mean ± SD, n = 6. #p<0.05 as compared with the control group; *p<0.05 as compared with the model group; †p<0.05, significantly different from 240 mg/kg BRP group. Control: normal control; Model: GalN/LPS alone; BRP 240: GalN/LPS plus BRP (240 mg/kg); SIL: GalN/LPS plus silymarin (50 mg/kg).
Discussion
Boschniakia rossica has been reported to exert several biological activities, including anti-oxidative, anti-tumor and anti-inflammatory actions, largely because its major ingredients, polysaccharides, phenylpropanoids and iridoids, have potent anti-oxidative and immunomodulatory activities.(19–23,26) Nowadays, the physiological functions of plant-derived polysaccharides have been extensively reported.(22–24,26) Most recently, we demonstrated that the aqueous fraction from Boschniakia rossica and its polysaccharide fraction could act as immunomodulators in mice with carbon tetrachloride-induced liver injury.(26,30) Here, we demonstrated that BRP has a protective effect in mice with GalN/LPS-induced FHF.
FHF is defined by the sudden onset of hepatic encephalopathy and often occurs in association with coagulopathy, jaundice and multisystem organ failure. GalN/LPS-induced liver failure is used widely as an experimental liver injury model for elucidating the mechanism of clinical liver dysfunction and for testing the efficacy of hepatoprotective agents. The advantage of the FHF model is that the combination of LPS and GalN selectively causes hepatic failure resulting in rapid death within a few days.(1–3) As observed in our preliminary study, mice challenged with GalN/LPS started to die at 12 h, and the mortality rate reached an extremely high level of 100% at 48 h (data not shown). At 8 h after the GalN/LPS injection, extensive apoptosis and necrosis injury occurred as a result of GalN/LPS-induced fulminant hepatic failure. This was also indicated by the significant increase in serum ALT and AST activities. The hepatoprotective effect of BRP was evidenced by its ability to decrease the ALT and AST levels after marked release of ALT and AST into the circulation during GalN/LPS intoxication.
The experimental model employed in this study depends upon cross-talk between macrophages and hepatocytes. Macrophages respond to LPS via TLR4 and produce TNF-α and IL-6, along with other mediators of inflammation in a nuclear import-dependent manner.(32) TLR4 is the recognition receptor of LPS, which is involved in the microbial immune response and cell activation. A number of studies have reported the impact of TLR4 on inflammatory diseases.(33,34) In addition, hepatic TLR4 expression has proved to be up-regulated in animals with GalN/LPS-induced liver injury.(4,5) In our study, the hepatic TLR4 protein level markedly increased after GalN/LPS injection as compared with the normal control. BRP attenuated the increased level of TLR4 protein, implicating that the protective effect of BRP might be associated with its inhibition on TLR4 over-expression. Still there is a possibility that down-regulation of TLR4 is the result of reduced macrophage numbers in liver and the mechanism needs to be further investigated.
Consistent with the above results, we also demonstrated that treatment with BRP decreased the serum levels of TNF-α and IL-6, and reduced hepatic inflammation and apoptotic tissue injury. The release of TNF-α, a pro-inflammatory mediator in liver apoptosis, is linked to cytotoxicity induced by GalN/LPS.(35) Apparently, TNF-α via its “death” receptor (TNFR1) evokes a different pro-apoptotic signaling in hepatocyte that is metabolically altered by GalN. A cascade of initiator and effector caspases is activated in hepatocytes and ultimately leads to the execution of a program of DNA fragmentation and chromatin condensation.(32) In this study, DNA fragmentation assay was examined to confirm the suppressive effect of BRP on apoptosis. Furthermore, the GalN/LPS exposure activated caspase-3 and caspase-8, and BRP significantly inhibited the activation of caspase-3 and caspase-8. These results showed that BRP suppressed the apoptotic signaling via modulation of TNF-α release.
It has been documented that challenge with LPS and GalN resulted in overproduction of ROS which plays a critical role in the development of FHF.(5) If there is an imbalance between the production of ROS and insufficient antioxidant defense systems, oxidative stress will occur and cause damage to cells through direct or indirect pathways.(5) Cells have a number of mechanisms to protect themselves from the toxic effects of free radicals, including free radical scavengers and chain reaction terminators such as SOD, GSH-Px, GST and GSH.(5,25) In this study, the GalN/LPS challenge caused a significant oxidative stress in the liver characterized by the formation of lipidperoxides as well as the oxidative end-products such as TBARS. BRP supplementation significantly inhibited the production of LOOH and TBARS while improving the hepatic levels of SOD, GSH-Px, GST and GSH in GalN/LPS challenged mice. It suggests that the beneficial effect of BRP is partially associated with its anti-oxidative capacities.
HO-1, a protein involved in the catabolism of heme into biliverdin, free iron and carbon monoxide, is an inducible stress-responsive protein with important cytoprotective effects (e.g., hepatoprotection) against oxidative stress and inflammation.(2) Previous studies have demonstrated that expression of HO-1 is rapidly up-regulated in the liver after administration of GalN/LPS.(1) This represents an adaptive response to the oxidative damage. This study confirmed a significant increase in the HO-1 protein expression after GalN/LPS injection. This alteration was augmented by the BRP treatment suggesting that BRP induces HO-1 protein expression and/or suppresses the degradation of this protein.
We were also interested in determining whether BRP can inhibit the production of NO, another important mediator in oxidative stress. Macrophages produce TNF-α in rapid response to tissue injury, and TNF-α stimulates the release of cytokines from macrophages and the production of NO, a short-lived free radical signaling molecule that mediates inflammation.(5) Cytotoxicity is usually correlated with NO produced by iNOS. NO could present a host defense against tumor cells, while over-expression of NO mediates inflammation. Therefore, selective inhibition of iNOS and reduced NO synthesis could be a useful therapy for various inflammatory diseases.(5,6) COX-2 is the enzyme responsible for the catalysis of prostaglandin E2 from arachidonic acid and the induction of COX-2 is also closely related to NO production.(26) In current study, we demonstrated that BRP is a potent anti-inflammatory agent that effectively reduces NO production and iNOS and COX-2 expression in the livers of GalN/LPS-treated mice.
The activation of NF-κB has been documented to play a vital role in the regulation of pro-inflammatory genes, including TNF-α.(2,5,6) Several studies have demonstrated that GalN/LPS challenge leads to increased NF-κB activation.(2,5,6) MAPK cascades also participate in many LPS-induced macrophage inflammatory processes, such as cell death, proliferation and inflammation. Although the involvement of the MAPK pathway in TNF-α regulation is firmly documented, the detailed mechanism is still unknown.(9) Although the three major MAPK proteins, p38, JNK and ERK, are thought to play different roles in inflammatory diseases in different capacities, the JNK signaling pathway is one of the most important apoptosis-signaling pathways, and is activated by various forms of liver injury.(9) Our results confirmed that GalN/LPS challenge resulted in increased phosphorylation of MAPKs including ERK and JNK, and nuclear expression of NF-κB. Notably, BRP administration inhibited the phosphorylation of ERK and JNK, and nuclear translocation of NF-κB. It is well known that NF-κB can be activated by TLR4 ligation with LPS.(4,5,32) As such, we consider that the down-regulation of TLR4/NF-κB signaling and JNK and ERK signaling pathways by BRP might be partly attributed to the amelioration of liver injury and changes in oxidative substances and cytokine production in mice.
In conclusion, our results indicated that BRP protect hepatocytes against GalN/LPS-induced injury through suppressing inflammatory responses and apoptotic signaling pathways, and enhancing oxidative defense system. Hence, we propose that BRP might be useful as a potential therapeutic medication for attenuating fulminant hepatic failure. This study provides evidence that BRP may be useful as a potential pharmacological therapy for the prevention of hepatic failure.
Acknowledgments
This study was supported by the grants from the National Natural Science Foundation of China (No.81160539, No.81360651).
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BRP
a polysaccharide fraction isolated from Boschniakia rossica
- COX-2
cyclooxygenase-2
- DEAE
2-diethylaminoethyl
- ERK
extracellular signal-regulated kinase
- FHF
fulminant hepatic failure
- GalN
D-galactosamine
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSH-Px
glutathione peroxidase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- GST
glutathione-S-transferase
- HE
hematoxylin and eosin
- HO-1
heme oxygenase-1
- IL-6
interleukin-6
- iNOS
inducible nitric oxide synthase
- JNK
c-Jun N-terminal kinase
- LOOH
lipid hydroperoxide
- LPS
lipopolysaccharide
- MDA
malondialdehyde
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- PBS
phosphate buffered saline
- PFA
paraformaldehyde
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid-reactive substances
- TLR4
toll-like receptor 4
- TNF-α
tumor necrosis factor-α
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kim SJ, Kim KM, Park J, Kwak JH, Kim YS, Lee SM. Geniposidic acid protects against D-galactosamine and lipopolysaccharide-induced hepatic failure in mice. J Ethnopharmacol. 2013;146:271–277. doi: 10.1016/j.jep.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 2.Huang CC, Lin KJ, Cheng YW, Hsu UA, Yang SS, Shyur LF. Hepatoprotective effect and mechanistic insights of deoxyelephantopin, a phyto-sesquiterpene lactone, against fulminant hepatitis. J Nutr Biochem. 2013;24:516–530. doi: 10.1016/j.jnutbio.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Ewaschuk J, Endersby R, Thiel D, et al. Probiotic bacteria prevent hepatic damage and maintain colonic barrier function in a mouse model of sepsis. Hepatology. 2007;46:841–850. doi: 10.1002/hep.21750. [DOI] [PubMed] [Google Scholar]
- 4.Kang JW, Kim DW, Choi JS, Kim YS, Lee SM. Scoparone attenuates D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure through inhibition of toll-like receptor 4 signaling in mice. Food Chem Toxicol. 2013;57:132–139. doi: 10.1016/j.fct.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Wang YZ, Li YX, Xie JM, et al. Protective effects of probiotic Lactobacillus casei Zhang against endotoxin and d-galactosamine-induced liver injury in rats via anti-oxidative and anti-inflammatory capacities. Int Immunopharmacol. 2013;15:30–37. doi: 10.1016/j.intimp.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Liong EC, Xiao J, Lau TY, Nanji AA, Tipoe GL. Cyclooxygenase inhibitors protect D-galactosamine/lipopolysaccharide induced acute hepatic injury in experimental mice model. Food Chem Toxicol. 2012;50:861–866. doi: 10.1016/j.fct.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Decker K, Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;71:77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- 8.Fiuza C, Suffredini AF. Human models of innate immunity: local and systemic inflammatory responses. J Endotoxin Res. 2001;7:385–388. [PubMed] [Google Scholar]
- 9.Lian LH, Wu YL, Wan Y, Li X, Xie WX, Nan JX. Anti-apoptotic activity of gentiopicroside in D-galactosamine/lipopolysaccharide-induced murine fulminant hepatic failure. Chem Biol Interact. 2010;188:127–133. doi: 10.1016/j.cbi.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Deutschman CS, Haber BA, Andrejko K, et al. Increased expression of cytokine-induced neutrophil chemoattactant in septic rat liver. Am J Physiol. 1996;271:R593–R600. doi: 10.1152/ajpregu.1996.271.3.R593. [DOI] [PubMed] [Google Scholar]
- 11.Hishinuma I, Nagakawa J, Hirota K, et al. Involvement of tumor necrosis factor-alpha in development of hepatic injury in galactosamine-sensitized mice. Hepatology. 1990;12:1187–1191. doi: 10.1002/hep.1840120518. [DOI] [PubMed] [Google Scholar]
- 12.Streetz K, Leifeld L, Grundmann D, et al. Tumor necrosis factor alpha in the pathogenesis of human and murine fulminant hepatic failure. Gastroenterology. 2000;119:446–460. doi: 10.1053/gast.2000.9364. [DOI] [PubMed] [Google Scholar]
- 13.Jambekar AA, Palma E, Nicolosi L, et al. A glutamine synthetase inhibitor increases survival and decreases cytokine response in a mouse model of acute liver failure. Liver Int. 2011;31:1209–1221. doi: 10.1111/j.1478-3231.2011.02553.x. [DOI] [PubMed] [Google Scholar]
- 14.Morikawa A, Koide N, Sugiyama T, et al. The enhancing action of D-galactosamine on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells. FEMS Immunol Med Microbiol. 2004;41:211–218. doi: 10.1016/j.femsim.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Wan Y, Wu YL, Feng XC, Lian LH, Jiang YZ, Nan JX. The protective effects of total saponins from Ornithogalum saundersiae (Liliaceae) on acute hepatic failure induced by lipopolysaccharide and D-galactosamine in mice. J Ethnopharmacol. 2010;132:450–455. doi: 10.1016/j.jep.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Lekić N, Cerný D, Hořínek A, Provazník Z, Martínek J, Farghali H. Differential oxidative stress responses to D-galactosamine-lipopolysaccharide hepatotoxicity based on real time PCR analysis of selected oxidant/antioxidant and apoptotic gene expressions in rat. Physiol Res. 2011;60:549–558. doi: 10.33549/physiolres.932041. [DOI] [PubMed] [Google Scholar]
- 17.Jiangsu New Medical College Zhong Yao Da Ci Dian [Dictionary of Chinese Materia Medica] Shanghai Scientific and Technological Publishers; Shanghai, 19791583 [Google Scholar]
- 18.Yin ZZ, Kim HS, Kim YH, Lee JJ. Iridoid compounds from Boschniakia rossica. Arch Pharm Res. 1999;22:78–80. doi: 10.1007/BF02976441. [DOI] [PubMed] [Google Scholar]
- 19.Quan J, Yin X, Xu H. Boschniakia rossica prevents the carbon tetrachloride-induced hepatotoxicity in rat. Exp Toxic Pathol. 2011;63:53–59. doi: 10.1016/j.etp.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Quan JS, Piao L, Xu H, Li T, Yin X. Protective effect of iridoid glucosides from Boschniakia rossica on acute liver injury induced by carbon tetrachloride in rats. Biosci Biotech Biochem. 2009;73:849–854. doi: 10.1271/bbb.80757. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Wu C, Guo L, et al. Triglyceride accumulation inhibitory effects of phenylpropanoid glycosides from Boschniakia rossica Fedtsch et Flerov. Fitoterapia. 2013;85:69–75. doi: 10.1016/j.fitote.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Sheng Y, Yuan GX, et al. Purification and physicochemical properties of different polysaccharide fractions from the water extract of Boschniakia rossica and their effect on macrophages activation. Int J Biol Macromol. 2011;49:1007–1011. doi: 10.1016/j.ijbiomac.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Wang ZH, Wu BJ, Zhang XH, et al. Purification of a polysaccharide from Boschniakia rossica and its synergistic antitumor effect combined with 5-Fluorouracil. Carbohyd Polym. 2012;89:31–35. doi: 10.1016/j.carbpol.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Guo C, Li R, Zheng N, Xu L, Liang T, He Q. Anti-diabetic effect of ramulus mori polysaccharides, isolated from Morus alba L., on STZ-diabetic mice through blocking inflammatory response and attenuating oxidative stress. Int Immunopharmacol. 2013;16:93–99. doi: 10.1016/j.intimp.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Fei R, Chi LC, Du JS, Fu JG, He Y, Xing SY. Toxicity of polysaccharides of Boschniakia rossica. Dong Bei Shi Da Xue Bao (Zi Ran Ke Xue Ban) 2008;40:98–100. [Google Scholar]
- 26.Quan J, Li T, Zhao WX, Xu H, Qiu D, Yin X. Hepatoprotective effect of polysaccharides from Boschniakia rossica on carbon tetrachloride-induced toxicity in mice. J Clin Biochem Nutr. 2013;52:244–252. doi: 10.3164/jcbn.12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 28.Song QS.Purification and characterization of the crude polysaccharide from the root and stem of Boschniakia rossica Fedtsch. et Flerov [Dissertation] Yanbian University; Yanji, 200518 [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 30.Zhao WX, Jin MH, Li T, Wang YJ, Quan JS. Intervention effect of aqueous fractions from Boschniakia rossica on hepatic oxidative stress in mice with liver injury induced by carbon tetrachloride. Zhongguo Zhong Yao Za Zhi. 2013;38:875–878. [PubMed] [Google Scholar]
- 31.Wu Z, Han M, Chen T, Yan W, Ning Q. Acute liver failure: mechanisms of immune-mediated liver injury. Liver Int. 2010;30:782–794. doi: 10.1111/j.1478-3231.2010.02262.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Li C, Chen Y, et al. Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide. J Biol Chem. 2004;279:48434–48442. doi: 10.1074/jbc.M407190200. [DOI] [PubMed] [Google Scholar]
- 33.Mandal P, Roychowdhury S, Park PH, Pratt BT, Roger T, Nagy LE. Adiponectin and heme oxygenase-1 suppress TLR4/MyD88-independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. J Immunol. 2010;185:4928–4937. doi: 10.4049/jimmunol.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Ari Z, Avlas O, Fallach R, et al. Ischemia and reperfusion liver injury is reduced in the absence of toll-like receptor 4. Cell Physiol Biochem. 2012;30:489–498. doi: 10.1159/000341432. [DOI] [PubMed] [Google Scholar]
- 35.Wu YL, Lian LH, Wan Y, Nan JX. Baicalein inhibits nuclear factor-κB and apoptosis via c-FLIP and MAPK in D-GalN/LPS induced acute liver failure in murine models. Chem Biol Interact. 2010;188:526–534. doi: 10.1016/j.cbi.2010.09.008. [DOI] [PubMed] [Google Scholar]