Abstract
Parkinson’s disease is a progressive, age-related, neurodegenerative disorder, and oxidative stress is an important mediator in its pathogenesis. DJ-1 is a causative gene of a familial form of Parkinson’s disease, namely PARK7, and plays a significant role in antioxidative defense to protect the cells from oxidative stress. DJ-1 undergoes preferential oxidation at the cysteine residue at position 106, Cys-106, under oxidative stress. The critical role of Cys-106 in the biological function of DJ-1 has been demonstrated, and recent studies indicate that DJ-1 acts as a sensor of oxidative stress by regulating the gene expression of antioxidative defense. Specific antibodies against Cys-106-oxidized DJ-1 have been developed, and the generation of oxidized DJ-1 in cellular and animal models of Parkinson’s disease has been investigated. This review focuses on the role of DJ-1 in antioxidative defense and the importance of oxidizable Cys-106 in its function. The significance of the identification of early-phase Parkinson’s disease biomarkers and the nature of oxidized DJ-1 as a biomarker for Parkinson’s disease are discussed here.
Keywords: DJ-1, Parkinson’s disease, oxidative stress, cysteine, sulfonic acid
Introduction
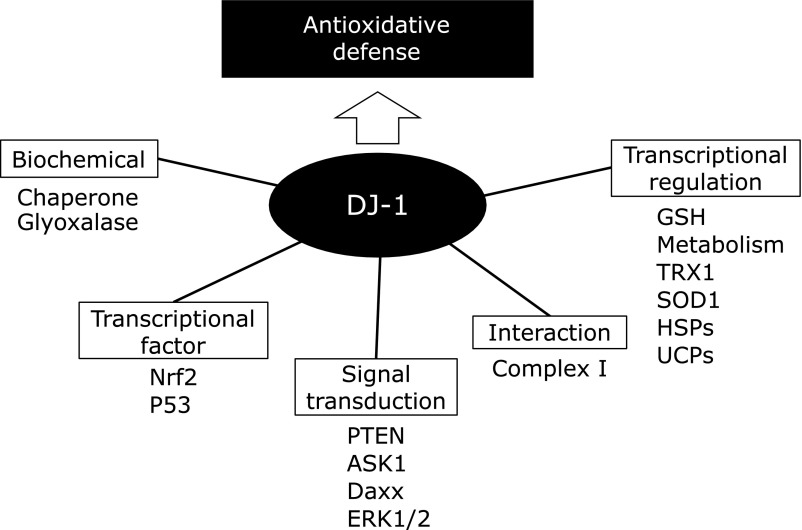
DJ-1 is a causative gene of a familial form of Parkinson’s disease (PD), namely PARK7, and plays a significant role in antioxidative defense to protect the cells from oxidative stress.(1–3) DJ-1 undergoes preferential oxidation at the cysteine residue at position 106 (Cys-106) under oxidative stress.(4) The critical role of Cys-106 in the biological function of DJ-1 has been demonstrated, and recent studies indicate that DJ-1 acts as a sensor of oxidative stress by changing the gene expression levels of antioxidative defense.(2,3,5) Specific antibodies against Cys-106-oxidized DJ-1 (oxDJ-1) have been developed, and the generation of oxDJ-1 in cellular and animal models of PD has been investigated.(6–8) This review focuses on the role of DJ-1 in antioxidative defense and the significance of oxidizable Cys-106 in its function (Fig. 1). The importance of the identification of PD biomarkers and the nature of oxDJ-1 as a biomarker for PD are discussed here.
Fig. 1.
Molecules releted to antioxidative function of DJ-1.
Parkinson’s Disease and Oxidative Stress
PD is a progressive, age-related, neurodegenerative disorder that is prevalent worldwide. PD is characterized by bradykinesia, rigidity, tremors, and gait dysfunction with postural instability.(9) The pathological hallmark of PD is the degeneration of dopamine neurons in the substantia nigra pars compacta and the subsequent depletion of striatal dopamine.(10) Autopsy studies have revealed that a pathological sign of PD is the presence of insoluble clumps of protein, called Lewy bodies, and a characteristic pattern of Lewy bodies in the PD-affected brain has been suggested.(11) Although the etiology of PD remains unknown, increasing evidence suggests that oxidative stress is an important mediator in its pathogenesis.(12,13) It is thought that nigral dopaminergic neurons are rich in reactive oxygen species (ROS) because the enzymatic and non-enzymatic metabolism of dopamine itself leads to the generation of ROS, including superoxide anions, hydrogen peroxide (H2O2), and hydroxyl radicals.(12–14) Monoamine oxidase, which is bound to the outer membrane of the mitochondria, catalyzes the oxidation of monoamines including dopamine, and enzymatically generates H2O2.(14,15) Furthermore, dopamine breakdown can occur spontaneously in the presence of iron, resulting in the generation of free radicals.(14) In addition, in nigral dopaminergic neurons, calcium entry through L-type channels occurs throughout the pacemaking cycle causing metabolic consumption of ATP, resulting in ROS generation.(16) There is abundant evidence, such as the increased levels of the oxidation products of lipids, proteins, and nuclear acids in nigral cells, to imply the role of oxidative stress in PD.(12–14) In addition, materials generating ROS and oxidative stress have been known to increase the risk of PD. Environmental factors including prolonged pesticide exposure, which can cause oxidative stress, have been observed to increase the risk of PD onset.(17) Altered levels of heavy metals such as copper and iron have also been found specifically in the substantia nigra of PD patients compared to healthy controls.(18) Moreover, a change in antioxidant defense, and decrease in the glutathione (GSH) content of the nigral lesion is known to occur in PD.(19) Phospholipid hydroperoxide GSH peroxidase (PH-GPx), which removes lipid hydroperoxide and H2O2 in the presence of GSH, has also been reported to colocalize with Lewy bodies in the substantia nigra of PD-affected brains.(20) Overall, PH-GPx was significantly reduced in the substantia nigra in PD, while higher expression levels of PH-GPx were observed in the surviving nigral cells.(20) Collectively, these reports indicate a pivotal role of oxidative stress in the onset and progression of PD. Thus, treatment with antioxidants is expected to slow or prevent the progression of PD, although it has not yet been developed.
Identification of the DJ-1 Gene as PARK7
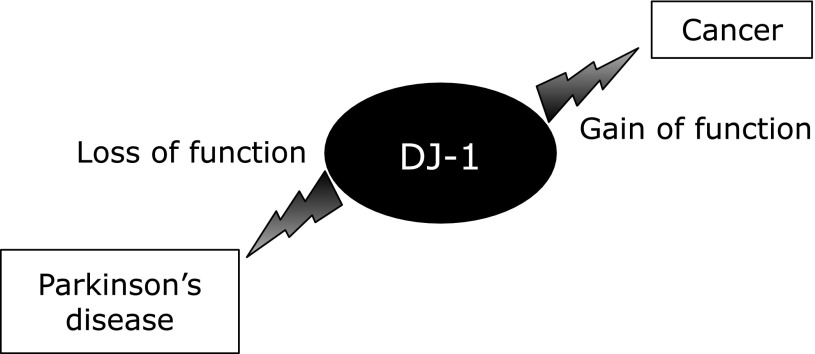
Recent genetic studies are yielding new insights into the molecular mechanisms underlying the pathogenesis of PD. In the last two decades, several genetic mutations have been identified that cause familial forms of PD with similar clinical and pathological features to idiopathic PD.(1–3,21) Mutation of α-synuclein has been identified as a causative gene in a familial form of PD, namely PARK1/4, and then α-synuclein was found to be the major fibrillar component of Lewy bodies, the pathological hallmark of familial and sporadic PD.(21,22) Parkin and PTEN-induced kinase 1 (PINK1) have also been identified as PARK2 and PARK6, respectively, and these genes play an important role in the maintenance of mitochondrial function. In 2003, the DJ-1 gene was identified as PARK7.(23) Mutations in PARK7 can cause autosomal recessive parkinsonism, and the early onset and slow progression of parkinsonism caused by PARK7 are similar to those seen in the other recessive PD syndromes, such as parkinsonism caused by PARK2 and PARK6. Several mutations of DJ-1 were found in familial forms of PD, and the point mutations such as L166P and M26I of DJ-1 have been reported to cause its severe destabilization of the DJ-1 protein and the loss of DJ-1 function (Fig. 2).(21,23) DJ-1 was first identified by Ariga et al.(24) as an oncogene that cooperates with ras in regulating cellular transformation. Therefore, the gain of DJ-1 function is related to cancer (Fig. 2). DJ-1 was also found to be a positive regulator of the androgen receptor, and is related to infertility.(25) DJ-1 possesses several functions, namely a multifunctional protein, that is involved in various physiological processes such as transcriptional regulation, antioxidative defense, mitochondrial function, and signal transduction.
Fig. 2.
DJ-1 function and related diseases.
Protein Characterization of DJ-1
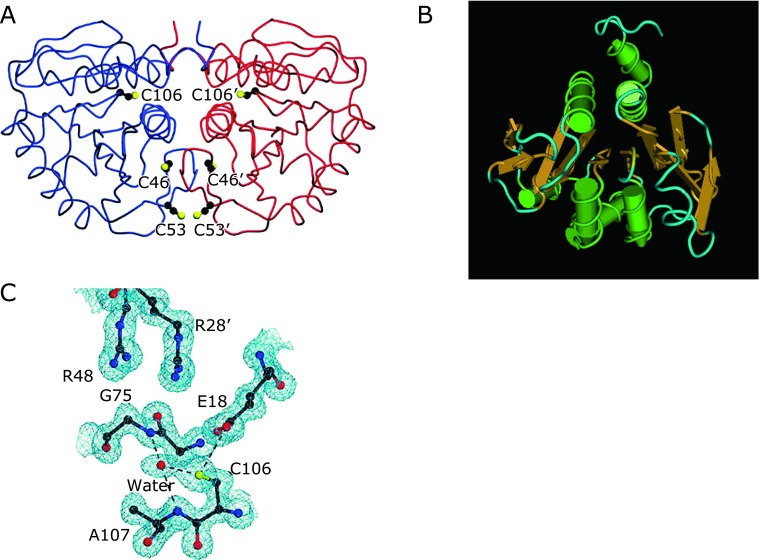
DJ-1 is homodimeric protein with each subunit comprising from 189 amino acids (Fig. 3A).(24) It is ubiquitously expressed throughout the body. DJ-1 is an abundant cellular protein and has been identified in several proteome studies using two dimensional-polyacrylamide gel electrophoresis (2D-PAGE) with protein staining.(4,26) The crystal structure of DJ-1 provided a great insight to this multi-functional protein.(27–29) The DJ-1 monomer takes a flavodoxin-like Rossmann fold, which contains a seven-strand parallel β-sheet as a core (Fig. 3B). This β-sheet is sandwiched by α-helices; therefore, the DJ-1 monomer has a three-layered structure. The tertiary structure of DJ-1 is similar to that of the bacterial cysteine protease PH1704, which is a member of the PfpI superfamily,(27,28) and DJ-1 belongs to this superfamily. However, DJ-1 has lost a catalytic triad that is essential for protease activity. Furthermore, the putative active site of DJ-1, Cys-106, is masked by an additional C-terminal helix. Several studies have suggested the protease activity of DJ-1; however, this protease activity is very low.(30) Both the cleavage of the C-terminal helix under oxidative stress and the increase in the protease activity of the C-terminal helix deletion mutant have been reported.(30) However, the physiological role of DJ-1 as a cysteine protease is still under debate. In addition, the tertiary structure of DJ-1 was similar to that of E. coli heat shock protein HSP31, a structural homolog of PH1704 protease.(28) It is interesting to note that DJ-1 has been reported to act as a redox-activated chaperone.(31,32) E. coli protein YajL, called as a member of the DJ-1/Hsp31/PfpI superfamily, functions as a covalent chaperones that is involved in the detection of sulfenylated proteins by forming mixed disulfide bonds with them. These disulfides are subsequently reduced by low molecular weight thiols.(32) In the case of DJ-1, similar covalent chaperone activity has been discovered. Many DJ-1-interacting proteins have been reported,(33) and the chaperone activity of DJ-1 might be responsible for the interactions with some of the identified proteins. Moreover, it has been discovered that HSP31 has glyoxalase activity, converting glyoxal or methylglyoxal to glycolic or lactic acid.(34) Similarly, the glyoxalase enzyme activity of DJ-1 has been demonstrated. As expected of a member of the DJ-1/Hsp31/PfpI superfamily, DJ-1 acts as a protease, molecular chaperone, and glyoxalase, and its character might be related to several cellular functions including the regulation of antioxidative defense.
Fig. 3.
Tertiary structure of DJ-1 protein. (A) Gross strucutre of DJ-1 protein. The DJ-1 dimer is shown with one monomer (blue) and the other (red). The three cysteine residues in DJ-1 are also shown. (B) Molecular model of monomer DJ-1. The green tube and brown arrow correspond to α-helix and β-sheet structures, respectively. The figure was made with Cn3D software. (C) The residues composing the environment of Cys-106. The thiol of Cys-106 (C106) makes two direct hydrogen bonds with surrounding atoms (dashed): one to Glu-18 (E18) and the other to an ordered water molecule. (A and C, reference (35); B, reference (28) with permission and modifications).
Cys-106 of DJ-1, the postulated active site of the cysteine protease, is structurally close to glutamate 18 (Glu-18), depressing pKa value; therefore, Cys-106 is susceptible to oxidation (Fig. 3D).(35) The point mutation of E18A depresses the pKa of Cys-106 and stabilizes the oxidized form of Cys-106.(35,36) The formation of the homodimer is important for the biological activity of DJ-1. The mutations related to the familial form of PD, such as L166P and M26I, destroy the dimer structure of DJ-1 and diminish its biological activity.(21,23,27,28) In addition to Cys-106, DJ-1 has two more cysteine residues, namely Cys-53 and Cys-46, which are located at the dimer interface (Fig. 3A). Although Cys-106 is the most susceptible to oxidation, these other cysteines are also thought to regulate the function of DJ-1 under oxidative stress.(37)
Oxidation of DJ-1 and its Antioxidative Function
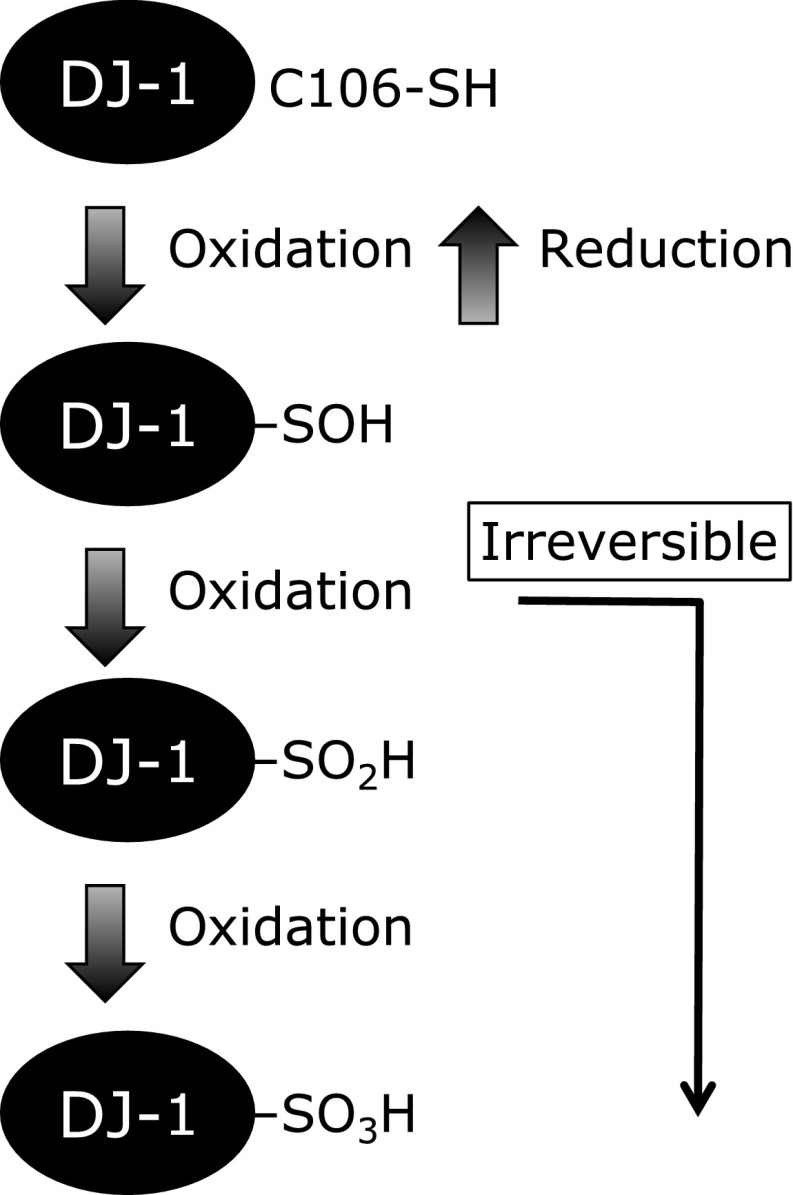
A proteomic study of cultured cells using 2D-PAGE revealed that the isoelectric point of DJ-1 showed an acidic mobility shift under oxidative stress.(4,26) The structural characterization of an acidic isoform of DJ-1 by using liquid chromatography-mass spectrometry revealed that the cysteines in DJ-1 were oxidized to cysteine sulfonic acid (Cys-SO3H) when cells were exposed to H2O2.(4) According to the information about the protein structure, Cys-106 of DJ-1 is preferentially oxidized in cells exposed to oxidative stress. Cys-106 is now accepted to be the key residue involved in the antioxidative action of DJ-1.(2,5) Cysteine forms three different oxidized species, namely cysteine-sulfenic acid (Cys-SOH), cysteine-sulfinic acid (Cys-SO2H), and Cys-SO3H through direct oxygen addition (Fig. 4). 2D-PAGE has shown an acidic spot shift of DJ-1 in cells under oxidative stress, and previous studies have shown that these acidic pI shifts are due to a post-translational process induced by the oxidation of the cysteine residue to Cys-SO2H or Cys-SO3H.(4) Cys-SO2H is chemically unstable and easily oxidized to Cys-SO3H. However, it has been reported that Cys-SO2H is stable in oxDJ-1 because of the surrounding amino acid residues.(36) The Cys-SO2H form of oxDJ-1 is likely to be the active form, and further oxidation to Cys-SO3H leads to loss of biological function.(5,31) DJ-1 can react with ROS such as H2O2. However, this reaction is not enzyme-catalyzed,(38) and might be less efficient at removing H2O2 compared with other antioxidative enzymes, such as GPxs, peroxiredoxins, and catalase. In this point of view, it is thought that DJ-1 is not effective antioxidant. Thus, it is reasonable to consider that DJ-1 plays a role in antioxidative defense via oxidation of Cys-106 to regulate transcription factors rather than removing ROS via direct oxidation of Cys-106.
Fig. 4.
Oxidation of cysteine residue in DJ-1. Cys-106 of DJ-1 is sequentially oxidized to SOH, SO2H, and SO3H.
Recent evidence indicates that DJ-1 acts as a sensor of oxidative stress to alter the expression levels of genes involved in antioxidative defense.(2,3,5) It has been reported that DJ-1 regulates glutathione metabolism and the expression of heat shock proteins and uncoupling proteins (UCP4 and UCP5).(16,39) It has also been reported that DJ-1 stabilizes NF-E2-related factor 2 (Nrf2), the master transcriptional factor of antioxidative defense: DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway.(40) Moreover, it has been demonstrated that DJ-1 regulates transcriptional factors p53 in a oxidation-dependent manner.(41) These lines of evidence suggest that oxidation physiologically activates DJ-1 to regulate these transcriptional factors related to antioxidative defense.
It has been known that DJ-1 regulates signal transduction related to oxidative stress response and cell death. The phosphoinositide 3-kinase (PI3K)/Akt pathway is the major signaling pathway for cell growth and survival.(42) Phosphatase and Tensin homolog deleted from chromosome 10 (PTEN) inhibits PI3K and acts as a negative regulator of the PI3K/Akt pathway.(42,43) DJ-1 directly binds to PTEN to inhibit its enzymatic activity: DJ-1 exhibit its cytoprotective action via the promotion of the PI3K/Akt pathway.(44,45) DJ-1 also regulates signal transduction of Apoptosis signal-regulating kinase 1 (ASK1) and death domain-associated protein (Daxx).(37,46) ASK1 is a member of mitogen activated protein (MAP) kinase kinase kinase family, and Daxx is associates with ASK1 in the cytoplasm to induce apoptosis under oxidative stress. DJ-1 binds to both ASK1 and Daxx to prevent the association between these signal molecules, thereby inhibiting oxidative stress-induced apoptosis.(37,46–48) In addition, DJ-1 regulates the expression of superoxide dismutase-1 (SOD1) through the extracellular signal-regulated kinases (ERK) pathway, one of MAP kinase signaling pathway.(49) Collectively, DJ-1 exhibits its antioxidative function to prevent oxidative stress-induced cel death by regulating various signaling pathways.
Cellular Function of DJ-1
DJ-1 is predominantly present in cytoplasm, but also exists in the nucleus and is associated with mitochondria. It has been reported that DJ-1 moves to the nucleus and the mitochondria under oxidative stress, while some mutants of DJ-1, including L166P and M26I, become monomers, and accumulate in the mitochondria.(50) The 12 N-terminal amino acids in DJ-1 are necessary for this localization, although DJ-1 has no mitochondrial target sequence. DJ-1 binds to subunits of mitochondrial complex I and regulates its activity.(51)
Recent evidences reveals the distinct role of DJ-1 in dopamine metabolism: DJ-1 upregulates the transcription of tyrosine hydroxylase, the rate-limiting enzyme of dopamine synthesis, by inhibiting the SUMOylation of pyrimidine tract binding protein-associated splicing factor (PSF).(52) DJ-1 also activates dopamine synthesis through interaction with dopamine biosynthetic enzymes such as tyrosine hydroxylase and 4-dihydroxy-l-phenylalanine decarboxylase, in an oxidation-dependent manner.(53) These observations suggest the predominant role of DJ-1 in the homeostasis of dopaminergic neurons. Slight oxidative stress inducing DJ-1 oxidation to Cys-106-SOH was found to upregulate dopamine synthesis, while extensive oxidative stress inducing DJ-1 oxidation to Cys-106-SO2H or Cys-106-SO3H did not upregulate dopamine production.(53) Dopaminergic cells are vulnerable and rich in ROS; therefore, DJ-1 in dopaminergic cells acts as a sensor of oxidative stress, which reflects the levels of dopamine synthesis.
Significance of Biomarker for PD
The identification of a biomarker for PD is vital for overcoming PD, particularly a biomarker for PD in its early phases.(54) Diagnosis of PD is dependent on the cardinal symptoms such as resting tremor, rigidity, and bradykinesia. However, more than half of the dopamine-producing neurons in the substantia nigra have been lost by the time the patient is diagnosed with PD.(9,10) Identification of a biomarker for PD in the early phases would have several benefits: researchers could identify preclinical PD patients by this biomarker, and longitudinal studies of preclinical PD patients could help researchers develop a novel drug to slow or prevent the progression of the disease. Besides, the effects of developed treatments will be evaluated by this biomarker for PD in its early phases. Biomarkers provide quantitative information about a living body, not only from body fluids but also from imaging. In the case of PD, several trials using an imaging biomarker have been conducted: changes in dopamine transporters and iron deposition in the substantia nigra have been identified by using imaging techniques such as magnetic resonance imaging (MRI).(55,56) Imaging baiomarkers have also been used to detect the change of peripheral tissues, which has been applied to the diagnosis of PD. 123I-meta-iodobenzylguanidine (MIBG) uptake in the myocardium has been used for the evaluation of the sympathetic nerve in the diagnosis of cardiac infarction. In the early phases of PD, cardiac sympathetic dysfunction and reduced cardiac 123I-MIBG uptake have been reported.(57) Therefore, 123I-MIBG scintillation has been used to evaluate the uptake of this probe in the myocardium, which in turn has been used for the diagnosis of PD. These imaging techniques provide reliable information for the diagnosis of PD. However, analysis using these biomarkers requires specific instruments as well as the administration of the probe. Therefore, there are lots of issues to be resolved before these techniques can be used clinically to predict PD.
Biochemical biomarkers for early-phase PD have been the subject of extensive studies, as with imaging biomarkers. For example, the abnormal oligomeric forms of α-synuclein, a major component of Lewy bodies, have been determined in blood and cerebrospinal fluid (CSF). Higher than normal levels of these oligomeric forms in PD patients have been reported.(58,59) However, a biochemical biomarker for early-phase PD has not yet been developed. The development of a biochemical biomarker for early phase-PD presents some difficulties.(60) PD is complex and can be difficult to diagnose, particularly in its early phases; it can sometimes be misdiagnosed as another diseases such as progressive supranuclear palsy (PSP). Therefore, it appears to be difficult to develop a diagnostic system using a single biomarker. It is important to examine several biomarker candidates, such as imaging and biochemical markers, simultaneously.(61) This strategy has also been tried in the research project known as Parkinson’s Progression Markers Initiative of the Michael J. Fox Foundation for PD.(62) The identification of an early-phase PD biomarker might require a large amount of funding, human resources, specialized instruments, and organized effort because researchers would have to evaluate several biomarker candidates in many patients over a long period.
oxDJ-1 as a Possible Biomarker for PD
DJ-1 is a promising candidate as a biomarker for PD. The biomarker stduies of DJ-1 in PD were summerized in Table 1. DJ-1 is induced by oxidative stress: an increase of DJ-1 protein is expected to reflect oxidative stress in PD patients.(38,63) The determination of DJ-1 content in blood and CSF has been studied. However, the results reported by several groups are controversial (Table 1).(64–66) DJ-1 content in erythrocytes is remarkably high, and hemolysis and contamination by erythrocytes greatly affects the DJ-1 level in plasma and CSF.(66,67) Thus, to determine the DJ-1 content in plasma and CSF, an evaluation of hemolysis and contamination by erythrocytes is necessary. Shi et al.(66) has been reported that using plasma without hemolysis, plasma DJ-1 is not useful as biomarkers for PD diagnosis or progression/severity. On the other hand, using CSF without contamination of blood cells, it has been demonstrated that DJ-1 and α-synuclein significantly decreased in PD patients compared with AD patients and control subjects.(67,68) It has also been repported that this change of DJ-1 in CSF does not differentiate among parkinsonian syndromes such as dementia with Lewy body (DLB), PSP, and multiple system atrophy (MSA).(69) Reduced concentrations of amyloid β peptide 1-42 in CSF of AD have been generally accepted.(70) At present, molecular mechanism of decrease in CSF DJ-1 has not been elucidated.
Table 1.
Biomarker study of DJ-1 in PD
| Study cited | Disease and number* | Material | Method | Outocome reported |
|---|---|---|---|---|
| Neurobiol Aging 2012; 33: 836. e5–e7 | LRRK2 (26) | CSF | Luminex assay | DJ-1 in LRRK2 CSF do not correlate with striatal dopaminergic function. |
| Parkin Rel Dis 2012; 18: 899–901(69) | PD (30), DLB (17), PSP (19), MSA (14), CBD (6), Unspecified (6) | CSF | ELISA | DJ-1 concentration in CSF does not differentiate among parkinsonian syndromes. |
| Sci Rep 2012; 2: 954(71) | PD (159), AD (14), Cont (60) | Whole blood | 2D-PAGE, WB | Blood levels of DJ-1 with 4-HNE modifications were altered in late-stage Parkinson disease |
| Ann Neurol 2011; 69: 570–580(68) | PD (126), AD (50), MSA (32), Cont (137) | CSF | Luminex assay | DJ-1 levels were decreased in PD versus Cont or AD. |
| Brain 2011; 134: 1–5 | PD (24), Cont (25) | Saliva | Luminex assay | DJ-1 increased in PD patients compared to control. |
| Neurosci Lett 2010; 480: 78–82(66) | PD (126), AD (33), Cont (122) | Plasma | Luminex assay | DJ-1 in plasma is not useful as biomarkers for PD diagnosis or progression/severity. |
| Brain 2010; 133: 713–726(67) | PD (117), AD (50), Cont (132) | CSF | Luminex assay | DJ-1 levels were decreased in PD patients versus Cont or AD patients. |
| Neurosci Lett 2009; 465: 1–5(6) | PD (unmedicated, 8; medicated, 7), Cont (18) | RBC | ELISA | Oxidized DJ-1 of unmedicated PD patients were higher than medicated PD patients and Cont. |
| Neurosci Lett 2008; 431: 86–89(64) | PD (95), Other Disease (30), Cont (24) | Serum | ELISA | There was no significant difference between the levels of serum DJ-1 in PD and Cont. |
| Neurosci Lett 2007; 425: 18–22(65) | PD (104), DLB (30), Cont (80) | Plasma | WB | Plasma DJ-1 levels in PD were significantly higher than Cont. |
| Biochem Biophys Res Comm 2006; 345: 967–972 | PD (40), Cont (38) | CSF | WB | The CSF DJ-1 levels in PD were significantly higher than Cont. |
*The number of determined samples was shown in parentheses. LRRK2; leucine-rich repeat kinase 2, CSF; cerebrospinal fluid, PD; Parkinson’s disease, DLB; dementia with Lewy body, PSP; progressive supranuclear palsy, MSA; multiple system atrophy, CBD; corticobasal degeneration, ELISA; enzyme-linked immunosorbent assay, AD; Alzheimer’s disease, 2D-PAGE; 2 dimensional-polyacrylamide gel electrophoresis, WB; western blot, RBC; red blood cell.
In addition to the quantity of DJ-1, its qualitative change, oxidation, is an interesting candidate for a PD biomarker. To determine oxDJ-1 in biological samples, our research group developed specific antibodies against oxDJ-1 and reported an increase in oxDJ-1 levels in erythrocytes and the brain.(6,7) Using a competitive enzyme-linked immunosorbent assay (ELISA) to detect oxDJ-1, it was discovered that the oxDJ-1 levels in the erythrocytes of unmedicated PD patients were markedly higher than those in the erythrocytes of medicated patients (treated with L-DOPA and/or dopamine agonist) or healthy subjects (Fig. 5A).(6) The term “unmedicated PD patients” refers to patients diagnosed with PD but not yet started on medications such as L-DOPA and/or dopamine agonist. Therefore, unmedicated PD patients are basically those with PD in its early phases. This result suggests that the ELISA system for oxDJ-1 detection is useful for the identification of PD in its early phases. Another group has recently reported the change of 4-hydroxy-2-nonenal-modified DJ-1 in the whole blood of PD patients.(71) In addition to the human study, we have reported that animal models of PD, developed by the administration of neurotoxins such as 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), are subject to the oxidative modification of DJ-1 in the brain and erythrocytes (Fig. 5B).(7) Based on immunohistochemical analyses of the substantia nigra of MPTP-treated mice, the number of oxDJ-1-positive cells exhibiting astrocyte-like morphology increases depending on the dose of neurotoxins. Collectively, these results suggest that DJ-1 oxidation in erythrocytes and the brain is a common phenomenon in PD patients and animal models of PD. It is not clear whether oxDJ-1 in erythrocytes of PD patients is derived from brain or erythrocytes. However, it is impossible to measure oxDJ-1 levels in human brain at present. The measurement of oxDJ-1 in erythrocyes have several benefits, such as handiness and versatility, as a biomarker of PD.
Fig. 5.
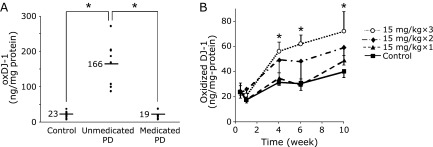
Oxidized DJ-1 levels in erythrocytes of PD patients and animal model of PD. (A) The levels of oxidized DJ-1 in erythrocytes of unmedicated PD patients were significantly higher than those in medicated PD patients and healthy subjects (*p<0.01 by ANOVA, Tukey). (B) After the administration of saline (control) and MPTP (15 mg/kg × 1, 2, and 3 i.p.), the levels of oxidized DJ-1 were quantified using a competitive ELISA system. The mean values of oxidized DJ-1 contents per total protein are shown with SD. (n = 6–8 in each group). *p<0.01 (Tukey, ANOVA) when compared with control. (A, reference (6); B, reference (7) with permission and modifications).
At present, the biological link between the onset of PD and the alteration of DJ-1 in erythrocytes has not been elucidated. The movement dysfunction associated with PD is considered to be just the tip of the iceberg, and several changes, including the formation of Lewy bodies in the peripheral tissues of PD patients, are known. The substantial relationship between central nerve system and peripheral tissues in PD pathology is still unclear. It is significant to elucidate this relationship not only for the understanding of PD pathology but also for the development of reliable biomarker of PD.
Conclusions
Several studies strongly indicates a relationship between DJ-1 oxidation, oxidative stress, and the onset and progress of PD. The importance of the prevention of oxidative stress in PD is understood. Elucidation of the timing and site of oxidative stress in PD might be required to develop the therapy using antioxidants. Recently, a correlation between oxDJ-1 levels in erythrocytes and MIBG scintillation in PD patients has been discovered. The examination of several PD biomarker candidates might lead to the diagnosis of PD in its early stages.
Abbreviations
- ASK1
apoptosis signal-regulating kinase 1
- CSF
cerebrospinal fluid
- Daxx
death domain-associated protein
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-regulated kinase
- GPx
glutathione peroxidase
- GSH
glutathione
- HSP
heat shock protein
- H2O2
hydrogen peroxide
- MAPK
mitogen activated protein kinase
- MIBG
meta-iodobenzylguanidine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MRI
magnetic resonance imaging
- Nrf2
NF-E2-related factor 2
- oxDJ-1
Cys-106-oxidized DJ-1
- PD
Parkinson’s disease
- PH-GPx
Phospholipid hydroperoxide glutathione peroxidase
- PINK1
PTEN-induced kinase 1
- PI3K
phosphoinositide 3-kinase
- PSF
pyrimidine tract binding protein-associated splicing factor
- PTEN
phosphatase and tensin homolog deleted from chromosome 10
- ROS
reactive oxygen species
- SOD1
superoxide dismutase-1
- SOH
sulfenic acid
- SO2H
sulfinic acid
- SO3H
sulfonic acid
- TRX
thioredoxin
- 2D-PAGE
two-dimensional polyacrylamide gel electrophoresis
- UCP
uncoupling protein
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Bonifati V. Autosomal recessive parkinsonism. Parkinsonism Relat Disord. 2012;18:S4–S6. doi: 10.1016/S1353-8020(11)70004-9. [DOI] [PubMed] [Google Scholar]
- 2.Ariga H, Takahashi-Niki K, Kato I, Maita H, Niki T, Iguchi-Ariga SM. Neuroprotective function of DJ-1 in Parkinson’s disease. Oxid Med Cell Longev. 2013;2013:683920. doi: 10.1155/2013/683920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahle PJ, Waak J, Gasser T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Rad Biol Med. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Kinumi T, Kimata J, Taira T, Ariga H, Niki E. Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;317:722–728. doi: 10.1016/j.bbrc.2004.03.110. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MA. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal. 2011;15:111–122. doi: 10.1089/ars.2010.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito Y, Hamakubo T, Yoshida Y, et al. Preparation and application of monoclonal antibodies against oxidized DJ-1. Significant elevation of oxidized DJ-1 in erythrocytes of early-stage Parkinson disease patients. Neurosci Lett. 2009;465:1–5. doi: 10.1016/j.neulet.2009.08.074. [DOI] [PubMed] [Google Scholar]
- 7.Akazawa YO, Saito Y, Hamakubo T, et al. Elevation of oxidized DJ-1 in the brain and erythrocytes of Parkinson disease model animals. Neurosci Lett. 2010;483:201–205. doi: 10.1016/j.neulet.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Miyama A, Saito Y, Yamanaka K, Hayashi K, Hamakubo T, Noguchi N. Oxidation of DJ-1 induced by 6-hydroxydopamine decreasing intracellular glutathione. PLoS One. 2011;6:e27883. doi: 10.1371/journal.pone.0027883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 12.Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- 13.Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinson Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 15.Maker HS, Weiss C, Silides DJ, Cohen G. Coupling of dopamine oxidation (monoamine oxidase activity) to glutathione oxidation via the generation of hydrogen peroxide in rat brain homogenates. J Neurochem. 1981;36:589–593. doi: 10.1111/j.1471-4159.1981.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 16.Guzman JN, Sanchez-Padilla J, Wokosin D, et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semchuk KM, Love EJ, Lee RG. Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42:1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- 18.Dexter DT, Jenner P, Schapira AH, Marsden CD. Alterations in levels of iron, ferritin, and other trace metals in neurodegenerative diseases affecting the basal ganglia. The Royal Kings and Queens Parkinson’s Disease Research Group. Ann Neurol. 1992;32 (Suppl):S94–S100. doi: 10.1002/ana.410320716. [DOI] [PubMed] [Google Scholar]
- 19.Perry TL, Godin DV, Hansen S. Parkinson’s disease: a disorder due to nigral glutathione deficiency? Neurosci Lett. 1982;33:305–310. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- 20.Bellinger FP, Bellinger MT, Seale LA, et al. Glutathione peroxidase 4 is associated with neuromelanin in substantia nigra and dystrophic axons in putamen of Parkinson’s brain. Mol Neurodegener. 2011;6:8. doi: 10.1186/1750-1326-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesage S, Brice A. Role of mendelian genes in “sporadic” Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:S66–S70. doi: 10.1016/S1353-8020(11)70022-0. [DOI] [PubMed] [Google Scholar]
- 22.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 23.Bonifati V, Rizzu P, van Baren MJ. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 24.Nagakubo D, Taira T, Kitaura H, et al. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 25.Okada M, Matsumoto K, Niki T, Taira T, Iguchi-Ariga SM, Ariga H. DJ-1, a target protein for an endocrine disrupter, participates in the fertilization in mice. Biol Pharm Bull. 2002;25:853–856. doi: 10.1248/bpb.25.853. [DOI] [PubMed] [Google Scholar]
- 26.Mitsumoto A, Nakagawa Y, Takeuchi A, Okawa K, Iwamatsu A, Takanezawa Y. Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic Res. 2001;35:301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- 27.Honbou K, Suzuki NN, Horiuchi M, et al. The crystal structure of DJ-1, a protein related to male fertility and Parkinson’s disease. J Biol Chem. 2003;278:31380–31384. doi: 10.1074/jbc.M305878200. [DOI] [PubMed] [Google Scholar]
- 28.Huai Q, Sun Y, Wang H, et al. Crystal structure of DJ-1/RS and implication on familial Parkinson’s disease. FEBS Lett. 2003;549:171–175. doi: 10.1016/s0014-5793(03)00764-6. [DOI] [PubMed] [Google Scholar]
- 29.Wilson MA, Ringe D, Petsko GA. The atomic resolution crystal structure of the YajL (ThiJ) protein from Escherichia coli: a close prokaryotic homologue of the Parkinsonism-associated protein DJ-1. J Mol Biol. 2005;353:678–691. doi: 10.1016/j.jmb.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 30.Mitsugi H, Niki T, Takahashi-Niki K, et al. Identification of the recognition sequence and target proteins for DJ-1 protease. FEBS Lett. 2013;587:2493–2499. doi: 10.1016/j.febslet.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 31.Zhou W, Zhu M, Wilson MA, Petsko GA, Fink AL. The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J Mol Biol. 2006;356:1036–1048. doi: 10.1016/j.jmb.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 32.Le HT, Gautier V, Kthiri F, et al. YajL, prokaryotic homolog of parkinsonism-associated protein DJ-1, functions as a covalent chaperone for thiol proteome. J Biol Chem. 2012;287:5861–5870. doi: 10.1074/jbc.M111.299198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knobbe CB, Revett TJ, Bai Y, et al. Choice of biological source material supersedes oxidative stress in its influence on DJ-1 in vivo interactions with Hsp90. J Proteome Res. 2011;10:4388–4404. doi: 10.1021/pr200225c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JY, Song J, Kwon K, et al. Human DJ-1 and its homologs are novel glyoxalases. Hum Mol Gen. 2012;21:3215–3225. doi: 10.1093/hmg/dds155. [DOI] [PubMed] [Google Scholar]
- 35.Witt AC, Lakshminarasimhan M, Remington BC, Hasim S, Pozharski E, Wilson MA. Cysteine pKa depression by a protonated glutamic acid in human DJ-1. Biochem. 2008;47:7430–7440. doi: 10.1021/bi800282d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackinton J, Lakshminarasimhan M, Thomas KJ, et al. Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1. J Biol Chem. 2009;284:6476–6485. doi: 10.1074/jbc.M806599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waak J, Weber SS, Görner K, et al. Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1. J Biol Chem. 2009;284:14245–14257. doi: 10.1074/jbc.M806902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 40.Im JY, Lee KW, Woo JM, Junn E, Mouradian MM. DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum Mol Gen. 2012;21:3013–3024. doi: 10.1093/hmg/dds131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato I, Maita H, Takahashi-Niki K, et al. Oxidized DJ-1 inhibits p53 by sequestering p53 from promoters in a DNA-binding affinity-dependent manner. Mol Cell Biol. 2013;33:340–359. doi: 10.1128/MCB.01350-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Kim RH, Peters M, Jang Y, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Kim YC, Kitaura H, Taira T, Iguchi-Ariga SM, Ariga H. Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int J Oncol. 2009;35:1331–1341. [PubMed] [Google Scholar]
- 46.Junn E, Taniguchi H, Jeong BS, Zhao X, Ichijo H, Mouradian MM. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc Nat Acad Sci U S A. 2005;102:9691–9696. doi: 10.1073/pnas.0409635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karunakaran S, Diwakar L, Saeed U, et al. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson’s disease: protection by alpha-lipoic acid. FASEB J. 2007;21:2226–2236. doi: 10.1096/fj.06-7580com. [DOI] [PubMed] [Google Scholar]
- 48.Klawitter J, Klawitter J, Agardi E, et al. Association of DJ-1/PTEN/AKT- and ASK1/p38-mediated cell signalling with ischaemic cardiomyopathy. Cardiovasc Res. 2013;97:66–76. doi: 10.1093/cvr/cvs302. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Liu J, Chen S, et al. DJ-1 modulates the expression of Cu/Zn-superoxide dismutase-1 through the Erk1/2-Elk1 pathway in neuroprotection. Ann Neurol. 2011;70:591–599. doi: 10.1002/ana.22514. [DOI] [PubMed] [Google Scholar]
- 50.Maita C, Maita H, Iguchi-Ariga SM, Ariga H. Monomer DJ-1 and its N-terminal sequence are necessary for mitochondrial localization of DJ-1 mutants. PLoS One. 2013;8:e54087. doi: 10.1371/journal.pone.0054087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayashi T, Ishimori C, Takahashi-Niki K, et al. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem Biophys Res Commun. 2009;390:667–672. doi: 10.1016/j.bbrc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 52.Zhong N, Kim CY, Rizzu P, et al. DJ-1 transcriptionally up-regulates the human tyrosine hydroxylase by inhibiting the sumoylation of pyrimidine tract-binding protein-associated splicing factor. J Biol Chem. 2006;281:20940–20948. doi: 10.1074/jbc.M601935200. [DOI] [PubMed] [Google Scholar]
- 53.Ishikawa S, Taira T, Niki T, et al. Oxidative status of DJ-1-dependent activation of dopamine synthesis through interaction of tyrosine hydroxylase and 4-dihydroxy-L-phenylalanine (L-DOPA) decarboxylase with DJ-1. J Biol Chem. 2009;284:28832–28844. doi: 10.1074/jbc.M109.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones R. Biomarkers: casting the net wide. Nature. 2010;466:S11–S12. doi: 10.1038/466S11a. [DOI] [PubMed] [Google Scholar]
- 55.Booij J, Tissingh G, Winogrodzka A, et al. Practical benefit of [123I]FP-CIT SPET in the demonstration of the dopaminergic deficit in Parkinson’s disease. Eur J Nucl Med. 1997;24:68–71. doi: 10.1007/BF01728311. [DOI] [PubMed] [Google Scholar]
- 56.Oakley AE, Collingwood JF, Dobson J, et al. Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology. 2007;68:1820–1825. doi: 10.1212/01.wnl.0000262033.01945.9a. [DOI] [PubMed] [Google Scholar]
- 57.Orimo S, Ozawa E, Nakade S, Sugimoto T, Mizusawa H. (123)I-metaiodobenzylguanidine myocardial scintigraphy in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67:189–194. doi: 10.1136/jnnp.67.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El-Agnaf OM, Salem SA, Paleologou KE, et al. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- 59.Mollenhauer B, El-Agnaf OM, Marcus K, Trenkwalder C, Schlossmacher MG. Quantification of α-synuclein in cerebrospinal fluid as a biomarker candidate: review of the literature and considerations for future studies. Biomark Med. 2010;4:683–699. doi: 10.2217/bmm.10.90. [DOI] [PubMed] [Google Scholar]
- 60.Parnetti L, Castrioto A, Chiasserini D, et al. Cerebrospinal fluid biomarkers in Parkinson disease. Nature Rev Neurol. 2013;9:131–140. doi: 10.1038/nrneurol.2013.10. [DOI] [PubMed] [Google Scholar]
- 61.Schapira AH. Recent developments in biomarkers in Parkinson disease. Curr Opin Neurol. 2013;26:395–400. doi: 10.1097/WCO.0b013e3283633741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yanagida T, Tsushima J, Kitamura Y, et al. Oxidative stress induction of DJ-1 protein in reactive astrocytes scavenges free radicals and reduces cell injury. Oxid Med Cell Longev. 2009;2:36–42. doi: 10.4161/oxim.2.1.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maita C, Tsuji S, Yabe I, et al. Secretion of DJ-1 into the serum of patients with Parkinson’s disease. Neurosci Lett. 2008;431:86–89. doi: 10.1016/j.neulet.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 65.Waragai M, Nakai M, Wei J, et al. Plasma levels of DJ-1 as a possible marker for progression of sporadic Parkinson’s disease. Neurosci Lett. 2007;425:18–22. doi: 10.1016/j.neulet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 66.Shi M, Zabetian CP, Hancock AM, et al. Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson’s disease. Neurosc Lett. 2010;480:78–82. doi: 10.1016/j.neulet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hong Z, Shi M, Chung KA, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain. 2010;133:713–726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi M, Bradner J, Hancock AM, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69:570–580. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salvesen L, Bech S, Lokkegaard A, et al. The DJ-1 concentration in cerebrospinal fluid does not differentiate among Parkinsonian syndromes. Parkinsonism Relat Disord. 2012;18:899–901. doi: 10.1016/j.parkreldis.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 70.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 71.Lin X, Cook TJ, Zabetian CP, et al. DJ-1 isoforms in whole blood as potential biomarkers of Parkinson disease. Sci Rep. 2012;2:954. doi: 10.1038/srep00954. [DOI] [PMC free article] [PubMed] [Google Scholar]