Abstract
For a coherent and meaningful life, conscious self-representation is mandatory. Such explicit “autonoetic consciousness” is thought to emerge by retrieval of memory of personally experienced events (“episodic memory”). During episodic retrieval, functional imaging studies consistently show differential activity in medial prefrontal and medial parietal cortices. With positron-emission tomography, we here show that these medial regions are functionally connected and interact with lateral regions that are activated according to the degree of self-reference. During retrieval of previous judgments of Oneself, Best Friend, and the Danish Queen, activation increased in the left lateral temporal cortex and decreased in the right inferior parietal region with decreasing self-reference. Functionally, the former region was preferentially connected to medial prefrontal cortex, the latter to medial parietal. The medial parietal region may, then, be conceived of as a nodal structure in self-representation, functionally connected to both the right parietal and the medial prefrontal cortices. To determine whether medial parietal cortex in this network is essential for episodic memory retrieval with self-representation, we used transcranial magnetic stimulation over the region to transiently disturb neuronal circuitry. There was a decrease in the efficiency of retrieval of previous judgment of mental Self compared with retrieval of judgment of Other with transcranial magnetic stimulation at a latency of 160 ms, confirming the hypothesis. This network is strikingly similar to the network of the resting conscious state, suggesting that self-monitoring is a core function in resting consciousness.
All subjective experience may be seen as self-conscious in the weak sense that there is something it feels like for the subject to have that experience. We may at times be self-conscious in a deep way, for example, when we are engaged in figuring out who we are and what we are going to do with our lives, a distinctly human experience giving organization, meaning, and structure to life. In its absence, our representation of ourselves and our world becomes kaleidoscopic and our life chaotic (1).
Such explicit “autonoetic consciousness” is thought to emerge by retrieval of memory of personally experienced events (episodic memory) (2, 3). The cerebral activation pattern of episodic memory retrieval differs from that of semantic retrieval (retrieval of common knowledge by “familiarity”): e.g., activation of medial parietal cortex is characteristic of the former and activation of left lateral temporal lobe is characteristic of the latter (4). First, we hypothesize that the cerebral activity pattern of retrieval of previous judgments of a person is determined not only by the episodic retrieval nature of the task, but also by the degree of self-reference of the judgment to be retrieved. Second, we hypothesize that the regions activated by retrieval of judgment of the mental Self are functionally interacting. This network would give a distinct neural correlate to the emergence of explicit representation of the mental Self in the mind. To these ends, we compare regional cerebral blood flow (rCBF) changes determined by positron-emission tomography (PET) during retrieval of judgment of three subjects with different degrees of self-relevance, Self, Best Friend, and the Danish Queen, by using a simple non-memory-loaded condition with identical input and output as a control.
Finally, not only may episodic memory retrieval and, hence, autonoetic consciousness be accompanied by activation in the medial parietal region, but this region could also be essential for the task. To test this third hypothesis, we use transcranial magnetic stimulation (TMS) (5) to transiently disrupt normal neural activity in the medial parietal region to see whether such disruption would affect the task.
Subjects and Methods
CBF. Thirteen right-handed participants of Danish nationality (7 females; age range, 20-38 yr, median age of 27 yr) first rated a series of 75 personality trait adjectives as to how well they applied to themselves, or, in separate series, their best friend or the Danish Queen, translated into Danish from ref. 6. The rating was done on a six-point scale to ensure adequate encoding (7). The adjectives were allocated to the series randomly, to achieve counterbalance for likeability, and were shown sequentially on a monitor. Scanning took place ≈5 min later while the series of adjectives was shown again, one at a time. The subjects should have recalled whether each adjective had initially been characterized as fitting the person or not. Accuracy was stressed, speed was not. The results were reported on a keyboard with a two-point scale by using the right index finger (2). Two scans were carried out for each of the three conditions for each participant. In addition, a control scan was done during presentation of a similar series of adjectives, the subjects being required to indicate whether the number of syllables was even. Conditions were counterbalanced. CBF was estimated with an Advance PET scanner (General Electric) operating in 3D mode, with collimated septa retracted. It produced 35 image slices with a distance of 4.25 mm after i.v. injection of 400-MBq H215O by using an automated injection system. The total axial field was 15 cm, with an approximate in-plane resolution of 5 mm. Each scan started just as the tracer reached cerebral circulation and lasted 90 s. SPM 2 software was used for analysis of the PET data (8). Parametric statistical models were assumed at each voxel, by using the general linear model to describe the variability in the data in terms of experimental and confounding effects, and residual variability. Hypotheses expressed in terms of the model parameters were assessed at each voxel with univariate statistics. The multiple comparisons problem of simultaneously assessing all of the voxel statistics was addressed by using the theory of continuous random fields. Results for the Euler characteristics led to corrected P values for each voxel hypothesis. Processing included spatial realignment of PET images, coregistration to a T1-weighted MRI scan, normalization to Talairach space, and smoothing with a 15-mm Gaussian kernel. First, a simple “conditions” analysis was performed, evaluating differences in the spatial pattern of rCBF between conditions. This analysis revealed areas in the brain that exhibit different rCBF patterns in the two conditions. Functional connectivities of the brain with (i) the medial parietal/posterior cingulate cortex and (ii) the medial prefrontal cortex were investigated in a subsequent covariate analysis. The two structures were defined from the activated areas found in the contrast between Self and the control condition (P < 0.05, corrected, SPM 2). The correlations between mean activity in these two regions and the rest of the brain, for all conditions and all subjects, were analyzed. This procedure revealed areas in the brain that exhibit a similar pattern of changes over time in activation for the two regions.
TMS. Twenty-five American subjects were studied (15 males; age range, 20-56 yr, median age of 24 yr; 3 left-handed). A series of 90 of the original personality trait adjectives (6) was presented in six blocks, response-encoded, and retrieved essentially as in PET, with the exception that the subjects were now required to respond as fast and as accurately as they could during retrieval, and only two conditions were used: retrieval of adjectives describing oneself and adjectives describing Best Friend. Retrieval was carried out without TMS and with TMS applied at one of three midline locations: Oz (occipital pole), Pz (medial parietal region, midway between the vertex and Oz), and Fz (region anterior to the vertex by 40% of the vertex-nasion distance) (International 10-20 system). TMS was coupled randomly to presentation of adjectives by latencies of 0, 80, 160, 240, and 480 ms. A Magstim 200 transcranial magnetic stimulator (Magstim, Whitland, Wales, U.K.) was used. We used a double cone coil, specifically designed for stimulation of medial cortical structures, with two angularly placed interconnected coils (diameter of 9 cm). The intersection of the coils was placed in the sagittal plane, with the current moving posteriorly-anteriorly, in close contact with the scalp on a nylon bathing cap with landmarks. The coil generated a peak magnetic field of 2 T, with an estimated rise time of 0.1 ms and duration of <1 ms. The TMS output was set at 150% of threshold for eliciting motor responses in the feet. The primary motor area is located at the medial cortical surface just anterior to the central sulcus, approximately at the same depth from the scalp as the medial parietal cortex. The intensity was 50-75% of maximal stimulator output (9-11).
Accuracy (percent of correct responses) and average reaction time (RT) in ms were calculated. Although Accuracy and RT may represent different aspects of the memory process (12), an “efficiency score” was calculated to counteract tradeoff between Accuracy and RT during the task (13). It was defined as the velocity [RT-(s-1)], corrected by a factor defined by Accuracy (acc) to be 0 at random responses and 1 at 100% correct responses: Efficiency = [(acc - 50)/50]·RT-1·s-1.
Results
During retrieval of judgment of mental characteristics, rCBF revealed differential activation predominantly in medial parietal/posterior cingulate and medial prefrontal regions, regardless of Self or Other (Queen) (Fig. 1 and Table 1). This medial core of hemodynamic response was accompanied by lateral regions according to the degree of self-reference (Fig. 2 and Table 2).
Fig. 1.
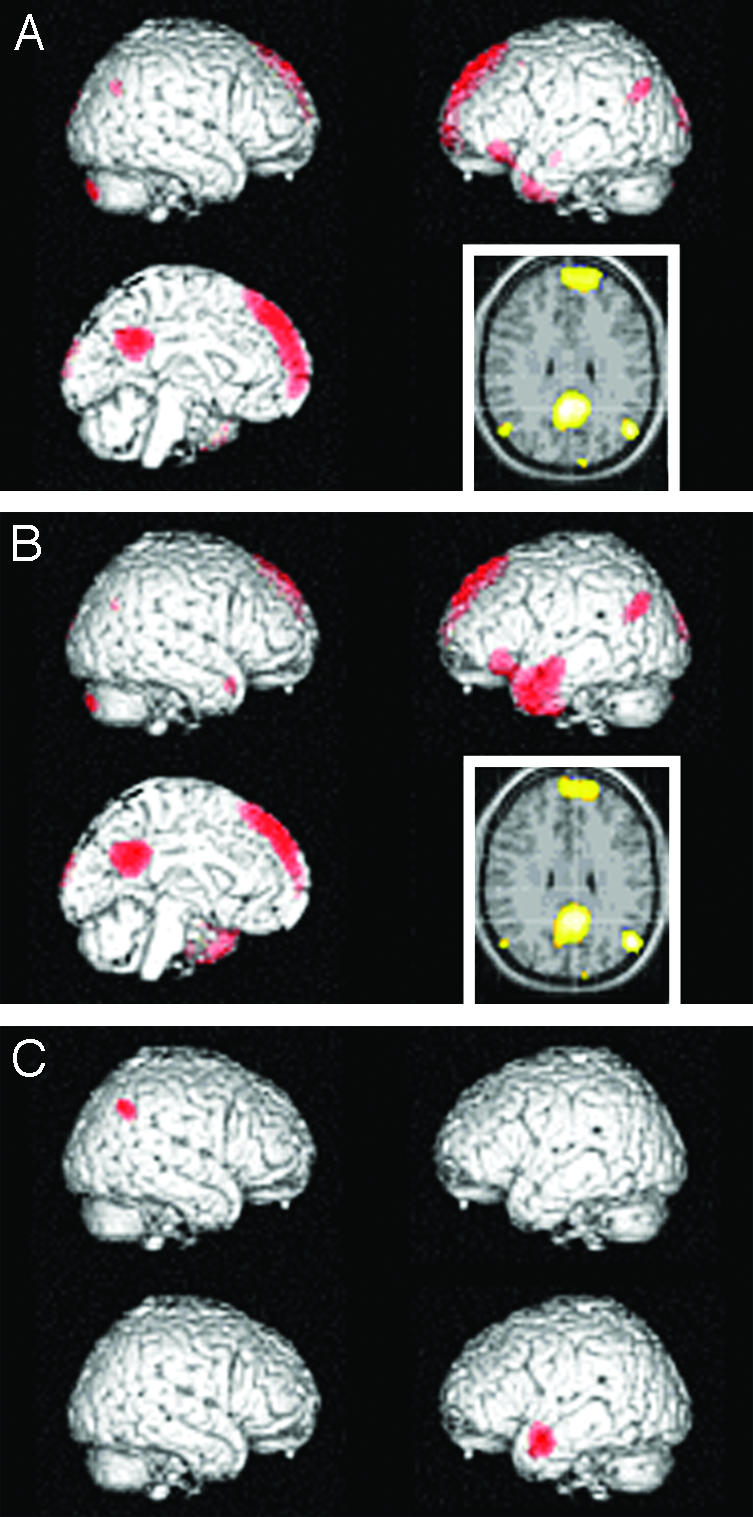
rCBF distribution in retrieval of previous judgment of mental characteristics, compared with control state. (A) Emergence of self-representation. Differential activity is noted in medial prefrontal and parietal/posterior cingulate regions, together with bilateral occipital and parietal regions, and a confluent left inferior prefrontal and temporal region (P < 0.05, corrected for multiple comparisons). (B) Emergence of representation of Other (Queen). Activation of nearly similar regions. (C) The relative contributions of two of the above regions are, however, different. For Self (Left), activity is comparatively high in right parietal region and low in left lateral temporal region (P < 0.001, uncorrected). (Insets) Left = right hemisphere.
Table 1. Regional [Brodmann area (BA)] activations by retrieval of judgment of mental Self and Other (Queen).
| BA | Max. z-score | Talairach coordinates (8) | |||
|---|---|---|---|---|---|
| Self minus Control | |||||
| Left med. sup. front. | 8/10 | 7.20 | −8 | 40 | 54 |
| Post. cing./precuneus | 31/7 | 6.70 | 4 | −50 | 30 |
| Left inf. par. | 39 | 6.18 | −48 | −66 | 30 |
| Left inf. front./m.t. pole | 47/21 | 5.69 | −44 | 30 | −10 |
| Right inf. par. | 39 | 5.26 | 52 | −70 | 34 |
| Left sup. occ. | 19/18 | 5.18 | −12 | −96 | 24 |
| Right sup. occ. | 19 | 5.16 | 22 | −88 | −38 |
| Control minus Self | |||||
| Right inf. temp. | 37 | Inf. | 58 | −56 | −14 |
| Left inf. temp. | 37 | 7.30 | −56 | −62 | −12 |
| Left inf. par. | 40 | 6.77 | −52 | −44 | 42 |
| Right inf./sup. par. | 40/7 | 6.04 | 58 | −38 | 48 |
| Left inf. par. | 40 | 5.15 | 42 | 36 | 32 |
| Right med. front. | 6 | 5.08 | 30 | −4 | 62 |
| Queen minus Control | |||||
| Left sup. front. | 9/10 | 7.20 | −8 | 38 | 54 |
| Left inf. par. | 39 | 7.04 | −48 | −66 | 28 |
| Left m.t./inf. f. | 21/47 | 6.84 | −54 | −2 | −22 |
| Post. cing./precuneus | 31/7 | 6.81 | −4 | −52 | 24 |
| Right sup. occ. | 19 | 5.51 | 22 | −88 | −38 |
| Right sup. temp. | 38 | 5.36 | 46 | 12 | −26 |
| Left sup. occ. | 18 | 5.29 | −12 | −98 | 22 |
| Control minus Queen | |||||
| Right inf. temp. | 37 | Inf. | 60 | −54 | −14 |
| Left inf. temp. | 37 | 7.45 | −56 | −62 | −12 |
| Right sup./inf. par. | 7/40 | 6.62 | 30 | −70 | 48 |
| Left inf. par. | 40 | 6.37 | −54 | −46 | 48 |
| Left sup. par. | 7 | 5.38 | −18 | −72 | 56 |
Max., maximum; sup., superior; post., posterior; front., frontal; cing., cingulate; inf., inferior; par., parietal; m.t., middle temporal; occ., occipital; temp., temporal; med., medial.
Fig. 2.
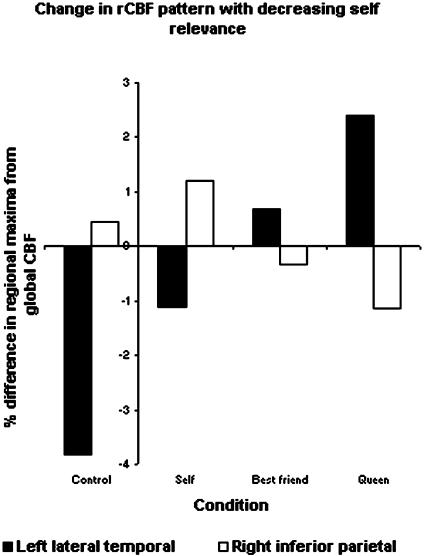
rCBF differences (%) from global CBF in sites of peak activity for Self, Best Friend, and Queen. For right inferior parietal region, the site is the voxel of maximal activity during the Self condition (x, y, z: 44, -58, 38). The differences between Self vs. Best Friend and Self vs. Queen are both significant (P = 0.021 and 0.0008, respectively). For left medial temporal region, the site is the voxel of maximal activity during the Queen condition (x, y, z: -50, 2, -20). The differences between Self and Best Friend, Best Friend and Queen, and Self and Queen are all significant (P = 0.014, 0.02, and < 0.0001, respectively).
Table 2. Activations during episodic retrieval.
| Comparisons, Self vs. Queen | BA | Max. z-score | Coordinates | ||
|---|---|---|---|---|---|
| Self-Queen | |||||
| Right inf. par. | 39 | 3.96 | 44 | −58 | 38 |
| Queen-Self | |||||
| Left middle temp. | 21 | 4.66 | −50 | 2 | −20 |
BA, Brodmann area; Max., maximum; inf., inferior; par., parietal; temp., temporal.
In retrieval of judgment of the mental Self, activity was high in the right inferior parietal cortex compared with that of Queen (SPM 2 analysis, Fig. 1C). After confirming normal distribution of the data, rCBF rates were compared for all conditions at the site of the peak voxel of the Self condition [Talairach coordinates (8), 44, -58, 38] with a random effect model (processing mixed in sas). Self condition was not significantly different from control condition: mean differences (%) from global CBF (SD) were 1.20 (1.08) and 0.44 (2.26), respectively. In contrast, rCBF in the Queen condition (-1.14; SD, 0.67) was significantly lower than in both the Self and control conditions (P = 0.0008 and 0.018, respectively), and rCBF in the Best Friend (-0.34; SD, 0.80) was lower than in the Self condition (P = 0.021) (Fig. 2).
In the left lateral temporal cortex, activity of Self was low compared with Queen condition (SPM 2 analysis, Fig. 1C). rCBF differences (%) from global CBF at the site of peak rCBF for the Queen condition in the left lateral temporal cortex (Talairach coordinates, -50, 2, -20) were compared for all conditions: control condition, -3.83 (2.30); Self, -1.10 (1.30); Best Friend, 0.70 (1.80); and Queen, 2.39 (1.55). Differences between each step were significant (P = 0.0004, 0.014, and 0.020, respectively).
Functional connectivity was studied by examining the degree of temporal synchronization of mean rCBF in medial prefrontal and medial parietal/posterior cingulate regions with the rest of the brain (SPM 2) in all conditions. We found the signal-to-noise relationship too weak to allow calculations for each condition separately. By combining all conditions, including the control state, a clear pattern appeared: Interaction between the anterior and posterior medial regions was highly significant. For medial prefrontal cortex a tight functional correlation with the left temporal cortex was found, with weaker correlation to the left inferior parietal cortex (Fig. 3 and Tables 3 and 4). In contrast, the medial parietal/posterior cingulate region was interacting with both lateral inferior parietal regions in addition to medial prefrontal cortex, without evidence of coupling to left temporal cortex. In conclusion, by interacting with both right inferior parietal and medial prefrontal cortices, the medial parietal/posterior cingulate region may be a nodal structure in a network of retrieval of self-reference.
Fig. 3.
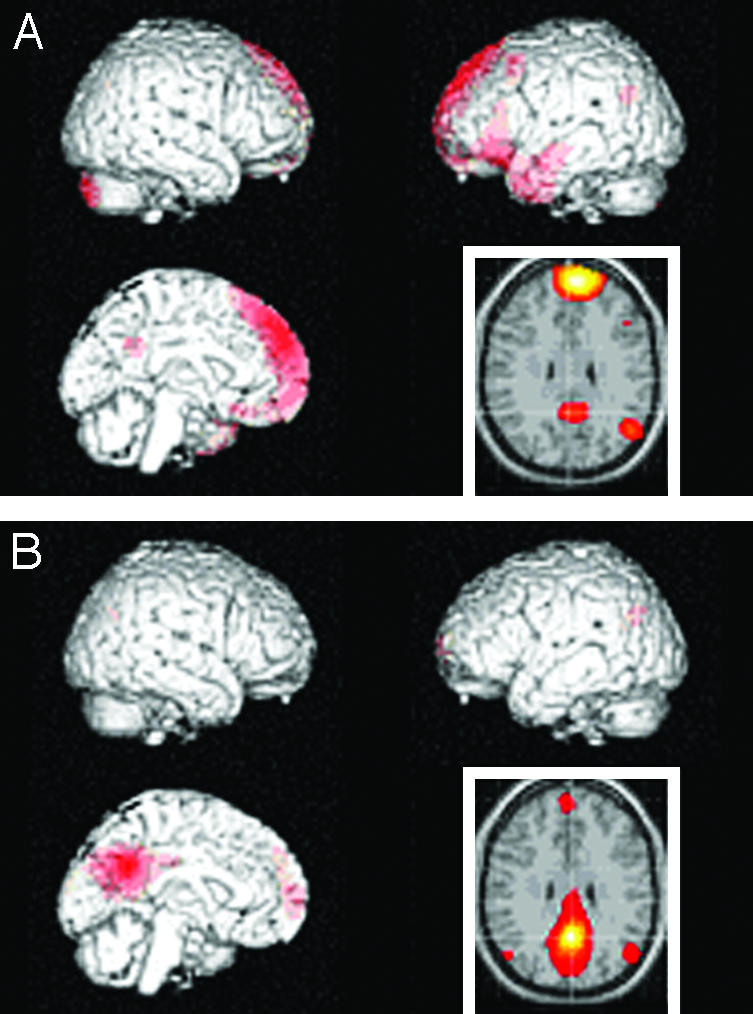
Connectivity patterns during combined tasks. The data are shown as z-score maps of synchronous activity in the rest of the brain across conditions with medial prefrontal region (A), and with medial parietal/posterior cingulate region (B). The former is mainly connected with medial parietal/posterior cingulate, left temporoprefrontal region, and left inferior parietal region; the latter is mainly connected with medial prefrontal cortex and bilateral inferior parietal cortices.
Table 3. Functional connectivity of medial prefrontal cortex during all conditions combined.
| Connected regions | BA | Max. z-score | Coordinates | ||
|---|---|---|---|---|---|
| Sup. medial front. | 9/8 | Inf. | −6 | 50 | 40 |
| Left inf. front./middle temp. | 47/20/21 | Inf. | −42 | 32 | −12 |
| Right cerebellar h. | 7.24 | 26 | −84 | −38 | |
| Left inf. par. | 39 | 6.57 | −48 | −64 | 30 |
| Post. cing./precuneus | 31/7 | 5.39 | 2 | −50 | 30 |
Sup., superior; front., frontal; inf., inferior; temp., temporal; par., parietal; post., posterior; cing., cingulate; h., hemisphere; BA, Brodmann area; Max., maximum.
Table 4. Functional connectivity of medial parietal cortex/posterior cingulate during all conditions combined.
| Connected regions | BA | Max. z-score | Coordinates | ||
|---|---|---|---|---|---|
| Post. cing./prec./cun. | 31/7/18/19 | inf. | 0 | −54 | −28 |
| Left inf. par. | 39 | 5.69 | −48 | −66 | 28 |
| Left medial front. | 10 | 5.66 | −14 | 66 | 8 |
| Right inf. par. | 39 | 4.87 | 50 | −70 | 32 |
BA, Brodmann area; Max., maximum; post., posterior; cing., cingulate; prec., precuneus; cun., cuneus; inf., inferior; par., parietal; front., frontal.
To determine whether this region is essential for emergence of self-representation, we used TMS to detect latency-specific effects on retrieval. In the first TMS experiment, the 13 participants had no TMS or TMS applied to one of two locations during recall, Oz or Pz (including precuneus). RT and Efficiency were significantly latency-dependent only at Pz and only for Self-judgment (P = 0.04 and 0.02, Friedman's test). Post hoc analysis revealed that TMS increased RT and decreased Efficiency at a latency of 160 ms after stimulus (P = 0.007 and 0.05, Wilcoxon's rank sum test), indicating an effect of TMS for Self at Pz specifically at a latency of 160 ms. A direct comparison between the two sites with repeated-measures multivariate ANOVA (MANOVA) revealed that only the Self vs. Other factor is significant (P < 0.02). The lack of significance for Site may be due to the proximity of the two sites, with overlapping magnetic fields and/or cellular populations.
To test these results in a second independent TMS experiment with a more distant control site, 12 additional participants were examined in a similar comparison of Pz and Fz (Table 5). The latter site is anterior to the vertex by 40% of the vertex-nasion distance in the midline. RT, Accuracy, and Efficiency were all latency-dependent, but only for Self and only at Pz (P = 0.04, 0.002, and 0.01, Friedman's test). RT increased at latencies of 80 and 160 ms (P = 0.02 and 0.04, Wilcoxon's test), and Accuracy and Efficiency decreased at a latency of 160 ms (P = 0.05 and 0.05, Wilcoxon's rank sum test). A direct comparison between the two sites revealed that only the Site factor was significant here (P < 0.025, MANOVA).
Table 5. Summary of Accuracy, RT, and Efficiency at latencies of 0-480 ms with SEM in the two TMS experiments (for definitions, see Subjects and Methods).
| 0 ms
|
80 ms
|
160 ms
|
240 ms
|
480 ms
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Self | Other | Self | Other | Self | Other | Self | Other | Self | Other | |
| Pz-Oz Comparison n = 13 | ||||||||||
| Pz | ||||||||||
| Accuracy, % | 92.3 ± 7.0 | 86.3 ± 12.3 | 92.7 ± 5.7 | 89.7 ± 9.6 | 88.0 ± 9.3 | 87.6 ± 7.6 | 93.2 ± 5.6 | 91.0 ± 7.4 | 93.6 ± 5.5 | 88.9 ± 9.6 |
| RT, ms | 935 ± 227 | 921 ± 280 | 943 ± 246 | 957 ± 246 | 1,017 ± 279 | 976 ± 257 | 959 ± 232 | 979 ± 285 | 960 ± 248 | 982 ± 274 |
| Efficiency | 0.96 ± 0.28 | 0.81 ± 0.34 | 0.95 ± 0.24 | 0.88 ± 0.29 | 0.79 ± 0.27 | 0.83 ± 0.27 | 0.94 ± 0.23 | 0.88 ± 0.20 | 0.95 ± 0.21 | 0.83 ± 0.22 |
| Oz | ||||||||||
| Accuracy, % | 88.0 ± 11.5 | 82.9 ± 12.1 | 87.2 ± 9.2 | 90.2 ± 7.6 | 85.5 ± 10.8 | 88.0 ± 11.1 | 84.2 ± 9.8 | 85.9 ± 10.5 | 88.0 ± 7.8 | 85.9 ± 8.7 |
| RT, ms | 936 ± 187 | 943 ± 229 | 979 ± 234 | 991 ± 234 | 986 ± 239 | 939 ± 223 | 980 ± 243 | 1,002 ± 297 | 975 ± 241 | 915 ± 232 |
| Efficiency | 0.83 ± 0.27 | 0.73 ± 0.24 | 0.80 ± 0.28 | 0.86 ± 0.24 | 0.74 ± 0.21 | 0.84 ± 0.24 | 0.73 ± 0.21 | 0.75 ± 0.24 | 0.83 ± 0.27 | 0.81 ± 0.24 |
| Pz-Fz Comparison n = 12 | ||||||||||
| Pz | ||||||||||
| Accuracy, % | 93.0 ± 7.2 | 90.7 ± 8.0 | 89.8 ± 8.1 | 91.2 ± 9.0 | 88.0 ± 8.5 | 88.4 ± 8.7 | 90.3 ± 10.9 | 88.9 ± 7.1 | 90.7 ± 7.2 | 90.7 ± 9.6 |
| RT, ms | 778 ± 140 | 899 ± 226 | 847 ± 181 | 892 ± 167 | 833 ± 169 | 899 ± 198 | 804 ± 136 | 900 ± 134 | 796 ± 146 | 893 ± 170 |
| Efficiency | 1.14 ± 0.28 | 0.93 ± 0.22 | 0.98 ± 0.29 | 0.95 ± 0.24 | 0.94 ± 0.25 | 0.90 ± 0.29 | 1.04 ± 0.34 | 0.88 ± 0.20 | 1.04 ± 0.22 | 0.94 ± 0.28 |
| Fz | ||||||||||
| Accuracy, % | 87.5 ± 8.6 | 90.7 ± 6.0 | 92.6 ± 6.4 | 88.0 ± 11.8 | 92.6 ± 12.6 | 90.3 ± 12.6 | 88.0 ± 11.6 | 88.4 ± 10.4 | 94.9 ± 4.4 | 86.6 ± 9.9 |
| RT, ms | 883 ± 131 | 920 ± 155 | 814 ± 147 | 908 ± 183 | 862 ± 151 | 921 ± 141 | 876 ± 152 | 964 ± 213 | 901 ± 161 | 942 ± 207 |
| Efficiency | 0.87 ± 0.26 | 0.91 ± 0.19 | 1.08 ± 0.26 | 0.88 ± 0.37 | 1.02 ± 0.36 | 0.90 ± 0.24 | 0.89 ± 0.32 | 0.84 ± 0.33 | 1.03 ± 0.21 | 0.83 ± 0.32 |
Efficiency, no TMS: Self, 2.09 ± 0.08; Other, 1.87 ± 0.08. P = 0.0005 (t test).
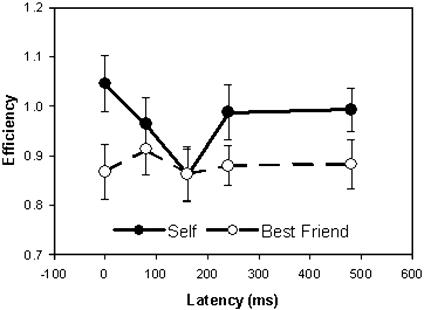
For Efficiency, the depression of Self-judgment across latencies of 160 ms at the Pz site is particularly evident in combined data including all 25 subjects, being significant in a direct comparison with Other, despite the variability of the responses (Fig. 4; for statistics, see legend). None of the studies had any systematic effect of gender or handedness (for TMS).
Fig. 4.
TMS at medial parietal site (Pz). Retrieval of self-judgment is less efficient with TMS at a latency of 160 ms than at a latency of 0 ms (P = 0.003), suggesting that neural activity at that time after stimulus presentation is particularly important for self-representation. This effect is not seen for retrieval of judgment of Best Friend. The difference between Self and Best Friend is significant (P < 0.05) (for details, see text and Table 5).
The efficiency of retrieval was much higher during TMS (Table 5) than PET, in which the usual mnemonic superiority for self-related material (13) was absent: Efficiency for retrieval of Self, Best Friend, and Queen was 0.49, 0.42, and 0.53 s-1, respectively [mean values, not significant (NS)]. Accuracies were similar to Accuracies measured in the TMS experiments: 88%, 86%, and 90% for Self, Best Friend, and Queen, respectively (mean values, NS). RTs were much longer, ensuring continuous stimulation during the scans: 1,550 ms (SD, 1023), 1,705 ms (SD, 1,133), and 1,507 ms (SD, 933), for Self, Best Friend, and Queen, respectively (mean values, NS).
Discussion
The major finding of the present study is the demonstration of a distinct neural correlate of the mental Self, or explicit autonoetic consciousness (3, 13, 14), causally related to activity in the parietal cortex. There is ample evidence for varying levels of memory performance according to the degree of self-relevance, for instance, Self > Other > Case or, in the present instance, Self > Best Friend > Queen. A comparatively recent metaanalysis concludes by stressing both the higher degree of organization and of elaboration of self-relevant memories as important determinants for greater memory performance (15). However, the question remains: is there something special about the neural organization of this effect? This question has behavioral scientists looking to imaging studies to provide an answer. Two important studies have addressed the issue by comparing activation patterns during the encoding process (13, 16), both concluding that self-referential processing is unique in terms of neural activity patterns. The present study of retrieval activation agrees with this interpretation. A few other studies have used related retrieval methodology but have not attempted to distinguish mental Self from Other (17-19).
Autonoetic consciousness is identified with episodic memory retrieval measured by remember responses, in contrast to semantic (or “familial” or “noetic” memory), measured by “know” responses (3). Both memory systems may contribute to performance in episodic memory tasks (3). Here, we use episodic retrieval tasks to investigate the neural mechanisms of retrieval of judgments of psychological traits in subjects graded with respect to self-reference, i.e., Self, Best Friend, and the Danish Queen, to identify a neurological correlate of emergence of awareness of the mental Self. The results were surprisingly clear: the massive activation of medial prefrontal and medial parietal/posterior cingulate region in all three tasks compared with the control condition agreed with their episodic nature. The medial prefrontal cortex is the classical region involved in self-reference (13). Early lesion studies have shown deficient self-awareness and self-control in lesions of this structure (20, 21), and increased activity has been seen in first-person reports of mental states like emotions, self-generated thoughts, and intentions to speak (4). In addition, “theory of the mind” and attributing mental states to others are functions that have been associated with activity in that region (22). Medial prefrontal cortex is also highly active during the resting state. It was earlier suggested by Ingvar (23) that this activity expressed a “rehearsal,” or “simulation of behavior,” whereas Frith and Frith (24) concluded that dorsal medial prefrontal regions are concerned with explicit representations of states of the self. In our study, we extend the responsibility for self-reference to a medial network of parietal/prefrontal regions interacting with the (right) inferior lateral parietal cortex.
The medial parietal/posterior cingulate region has repeatedly been associated with episodic memory retrieval (for review, see ref. 4). Functional MRI activation has been found during retrieval of previously encoded words according to a subjective quality (7), a paradigm closely related to the present one. It has been suggested that perfusion in the medial parietal region (precuneus) is correlated to linking new information with prior knowledge in a memory processing/retrieval system (25), with an important role for precuneus in retrieval of episodic memory compared with episodic encoding (4, 7, 16) and semantic retrieval (4, 26, 27). Here, we present evidence that the function of medial parietal cortex is particularly important to explicit self-representation despite our finding of similar hemodynamic activation during all three tasks. Thus, TMS impaired the retrieval of highly self-referential information selectively at this site with a latency of 160 ms. In other regions, neural activity with a latency of 160 ms includes non-phase-locked visually elicited γ oscillations, which are suggested to be important for the emergence of visual awareness of physical objects (28). The differential importance of medial parietal cortex for explicit self-representation agrees with our coherence analysis of synchrony, which showed functional correlation between precuneus and the right inferior parietal cortex, selectively active in explicit self-reference, and not with the left temporal region, selectively active in other-reference. Extended TMS studies will be needed to ascertain whether the effect of TMS at the medial parietal site is truly specific for explicit self-representation or a matter of degree compared with a smaller effect on episodic memory retrieval in general. There is a trend for an effect on RTs also on retrieval of judgment of Best Friend (Table 5), which leads us to favor the latter hypothesis. The question of an eventual effect of TMS on inferior right parietal and left lateral temporal cortices will also have to be referred to future studies.
The right inferior parietal cortex was particularly active during retrieval of self-referential information. This finding came to us as a surprise, but several studies provide circumstantial evidence for a role of the right inferior parietal region in self-representation: first, there is a right hemisphere preference for self-recognition (29), and, second, a number of recent studies on physical first-person perspective such as position in space, imagination of agency, and body representation, have shown activation in the right inferior parietal region (30-32). Last, illusory own-body perceptions have even been produced by direct electrical stimulation of the right inferior parietal cortex during surgical treatment for epilepsy (33). These studies point to a role for the right lateral parietal region in representation of the physical Self. With our present results showing that this is also the case for the mental Self, we conclude that the right inferior parietal cortex is selectively activated in self-representation in general. It should be noted, however, that the degree of activity was not significantly different from our simple control task and was solely apparent when compared with memory-loaded retrieval of representations of Other. Although the present study was not specifically designed to test the hypothesis of a medial prefrontal and lateral and medial parietal “default mode” system with high activity during rest and self-related activity (34), our results support that hypothesis.
An increasing contribution of the network of semantic retrieval with decreasing self-reference was apparent in increasing activity of the left lateral temporal region. Activity in this region is a hallmark of semantic retrieval. The region is activated not only for words but also for pictures (35-37) and faces (38, 39), in accordance with involvement in higher level semantic processes that are independent on input modality.
The idea that not only medial anterior, but also medial posterior, regions are essential for subjectivity is a recent development. The concept was proposed by Raichle and coworkers (34, 40, 41) with a metaanalysis of spontaneous rCBF and oxygen consumption in the resting brain. They stated that high activity in medial frontal and medial parietal regions is “consistent with the continuity of a stable, unified perspective of the organism relative to the environment (a `Self')” (41). The tonic activity decreases during engagement in non-self-referential goal-directed actions (i.e., default mode) (34). rCBF depends on afferent function (i.e., all aspects of presynaptic and postsynaptic processing) but is independent of the efferent function (i.e., the spike rate of the same region) (42). Even if that were not the case, conclusions on the increased cognitive expediency of a given region cannot be inferred from increased rCBF or oxidative metabolism. There are, in fact, several examples of the opposite (e.g., ref. 43). Therefore, these important suggestions from rCBF studies have lacked decisive experimental support.
This situation has changed with the present evidence for an essential role of medial parietal region in self-reference. It connects right (and left) lateral inferior parietal cortex with medial prefrontal cortex, already known to be essential for self-representation (21). There are abundant anatomical connections between the medial parietal/posterior cingulate region and medial prefrontal/anterior cingulate region (44), and these regions are functionally integrated in reflective self-awareness (45) and the resting conscious state (46). Together, these findings point to a principle of regulation of subjectivity and conscious self-monitoring.
The Self may act as a core in the unity of conscious experience (41). This finding agrees with the proposed role of these medial regions in such unity.¶ The medial structures not only integrate anterior and posterior brain, but also left and right hemispheres (47) and limbic and neocortical structures (20). They have also been shown here to provide gateways to spread information in the brain from lateral cortical regions. We speculate that such local brain activity may gain access in this way to a “global workspace” of consciousness, as proposed by Baars (48).
Acknowledgments
We thank Professors Vagn Andersen, Chris Frith, and Anders Gade for critical comments on earlier versions of the manuscript. This study was supported by the Lundbeck Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: rCBF, regional cerebral blood flow; PET, positron-emission tomography; TMS, transcranial magnetic stimulation; Oz, occipital pole; Pz, medial parietal region, midway between vertex and Oz; Fz, region anterior to the vertex by 40% of the vertex-nasion distance; RT, reaction time.
Footnotes
Kjaer, T. W. & Lou, H. C. (2000) Consc. Cognit. 9, S59 (abstr.).
References
- 1.Flanagan, O. (1995) Consciousness Reconsidered (MIT Press, Cambridge, MA).
- 2.Tulving, E. (1972) in Organization of Memory, eds. Tulving, E. & Donaldson, W. (Academic, New York), pp. 381-403.
- 3.Gardiner, J. M. (2001) Philos. Trans. R. Soc. London B 356, 1351-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabeza, R. & Nyberg, L. (2000) J. Cognit. Neurosci. 12, 1-47. [DOI] [PubMed] [Google Scholar]
- 5.Keenan, J. P. (2001) J. Consc. Stud. 3, 31-34. [Google Scholar]
- 6.Anderson, N. H. (1968) J. Pers. Soc. Psychol. 9, 272-279. [DOI] [PubMed] [Google Scholar]
- 7.McDermott, K. B., Ojemann, J. G., Petersen, S. E., Ollinger, J. M., Snyder, A. Z., Akbudaz, E., Conturo, T. E. & Raichle, M. E. (1999) Memory 7, 661-678. [DOI] [PubMed] [Google Scholar]
- 8.Frackowiak, R. S. J., Friston, K. J., Frith, C. R., Doland, R. J. & Maziotta, J. C. (1997) Human Brain Function (Academic, San Diego).
- 9.Pascual-Leone, A., Bartrez-Faz, D. & Keenan J. P. (1999) Philos. Trans. R. Soc. London B 354, 1229-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paus, T., Sipila P. K. & Strafella, A. P. (2001) J. Neurophysiol. 86, 1983-1990. [DOI] [PubMed] [Google Scholar]
- 11.Jalinous, R. (1991) J. Clin. Neurophysiol . 8, 10-25. [DOI] [PubMed] [Google Scholar]
- 12.Craik, F. I. M., Moroz, T. M., Moscovitch, M., Stuss, D. T., Wincour, G., Tulving, E. & Kapur, S. (1999) Psychol. Sci. 10, 26-34. [Google Scholar]
- 13.Matthews, N., Luber, B., Qian, N. & Lisanby, S. H. (2001) Exp. Brain Res. 140, 397-406. [DOI] [PubMed] [Google Scholar]
- 14.Tulving, E. (1987) Hum. Neurobiol. 6, 67-80. [PubMed] [Google Scholar]
- 15.Symons, C. S. & Johnson, B. T. (1997) Psychol. Bull. 121, 371-394. [DOI] [PubMed] [Google Scholar]
- 16.Kelley, W. M., Macrae, C. N., Wyland, C. L., Caglar, S., Inati, S. & Heatherton, T. F. (2002) J. Cognit. Neurosci. 14, 785-794. [DOI] [PubMed] [Google Scholar]
- 17.Andreasen, N. C., O'Leary, D. S., Cizadlo, T., Arndt, S., Rezai, K., Watkins, G. L. B., Ponto, L. L. & Hichwa, R. D. (1995) Am. J. Psychiatry 152, 1576-1585. [DOI] [PubMed] [Google Scholar]
- 18.Kircher, T. T., Senior, C., Phillips, M. L., Benson, P. J., Bullmore, E. T., Barummer, M., Simmonds, A., Williams, S. C. R., Bertels, M. & David, A. S. (2000) Cognit. Brain Res. 10, 133-144. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, S. C., Baxter, L. C., Wilder, L. S., Pine, J. G., Hiesemann, J. E. & Prigatano, G. P. (2002) Brain 125, 1808-1814. [DOI] [PubMed] [Google Scholar]
- 20.Posner, M. I. & Rothbart, M. K. (1998) Philos. Trans. R. Soc. London B 353, 1915-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuss, D. T. & Levine, B. (2002) Annu. Rev. Psychol. 53, 401-433. [DOI] [PubMed] [Google Scholar]
- 22.Vogeley, K., Bussfeld, P., Newen, A., Herrman, S., Happe, F., Falkei, P., Maier, W., Shah, N. J., Fink, G. R. & Zilles, K. (2001) NeuroImage 14, 170-181. [DOI] [PubMed] [Google Scholar]
- 23.Ingvar, D. H. (1979) Acta Neurol. Scand. 60, 12-25. [DOI] [PubMed] [Google Scholar]
- 24.Frith, C. D. & Frith, U. (1999) Science 286, 1692-1695. [DOI] [PubMed] [Google Scholar]
- 25.Maguire, E. A., Frith, C. D. & Morris, R. G. (1999) Brain 122, 1839-1850. [DOI] [PubMed] [Google Scholar]
- 26.Henson, R. N. A., Ruggs, M. D., Shallice, T., Josephs, O. & Dolan, R. J. (1999) J. Neurosci. 19, 3962-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiggs, C. L., Weisberg, J. & Martin, A. (1999) Neuropsychologia 37, 103-118. [DOI] [PubMed] [Google Scholar]
- 28.Tallon-Baudry, C., Bertrand, O., Delpuech, C. & Pernier, J. (1996) J. Neurosci. 16, 4240-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keenan, J. P., Nelson, A., O'Connor, M. & Pascual-Leone, A. (2001) Nature 409, 305. [DOI] [PubMed] [Google Scholar]
- 30.Maguire, E. A., Burgess, N., Donnett, J. G., Frackowiak, R. S., Frith, C. D. & O'Keefe, J. (1998) Science 280, 921-924. [DOI] [PubMed] [Google Scholar]
- 31.Maguire, E. A., Burgess, N. & O'Keefe, J. (1999) Curr. Opin. Neurobiol. 9, 171-177. [DOI] [PubMed] [Google Scholar]
- 32.Vogeley, K. & Fink, G. R. (2003) Trends Cognit. Sci. 7, 38-42. [DOI] [PubMed] [Google Scholar]
- 33.Blanke, O., Ortigue, S., Landis, T. & Seeck, M. (2002) Nature 149, 269-270. [DOI] [PubMed] [Google Scholar]
- 34.Gusnard, D. A., Akbudak, E., Shulman, G. & Raichle, M. E. (2001) Proc. Natl. Acad. Sci. USA 98, 4259-4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin, A., Haxby, J. V., Lalonde, F. M., Wiggs, C. & Ungerleider, L. G. (1995) Science 5233, 102-105. [DOI] [PubMed] [Google Scholar]
- 36.Martin, A., Wiggs, C. L., Ungerleider, L. G. & Haxby, J. V. (1996) Nature 379, 649-652. [DOI] [PubMed] [Google Scholar]
- 37.Vandenberghe, R., Price, C., Wise, R., Josephs, O. & Frackowiak, R. S. (1996) Nature 383, 254-256. [DOI] [PubMed] [Google Scholar]
- 38.Sergent, J., Ohta, S. & MacDonald, B. (1992) Brain 115, 15-36. [DOI] [PubMed] [Google Scholar]
- 39.Tempini, M. L., Price, C. J., Josephs, O., Vandenberghe, R., Cappa, S. F., Kapur, N. & Frackowiak, R. S. (1998) Brain 121, 2103-2118. [DOI] [PubMed] [Google Scholar]
- 40.Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A. & Shulman, G. L. (2001) Proc. Natl. Acad. Sci. USA 98, 676-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gusnard, D. A. & Raichle, M. E. (2001) Nat. Rev. Neurosci. 2, 685-694. [DOI] [PubMed] [Google Scholar]
- 42.Lauritzen, M. (2001) J. Cereb. Blood Flow Metab. 21, 1367-1383. [DOI] [PubMed] [Google Scholar]
- 43.Mehta, M. A., Owen, A. M., Sahakian, B. J., Mavaddat, N., Pickard, J. D. & Robbins, T. W. (2000) J. Neurosci. 20, 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavada, C. & Goldman-Rakic, P. S. (1989) J. Comp. Neurol. 287, 422-445. [DOI] [PubMed] [Google Scholar]
- 45.Kjaer, T. W., Nowak, M., Kjaer, K. W., Lou, A. R. & Lou, H. C. (2001) Conscious. Cogn. 10, 356-365. [DOI] [PubMed] [Google Scholar]
- 46.Greicius, M. D., Krasnow, B., Reiss, A. L. & Menon, V. (2003) Proc. Natl. Acad. Sci. USA 100, 253-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldman-Rakic, P. S. & Schwartz, P. S. (1982) Science 216, 755-757. [DOI] [PubMed] [Google Scholar]
- 48.Baars, B. J. (2002) Trends Cogn. Sci. 6, 47-51. [DOI] [PubMed] [Google Scholar]