Abstract
Long noncoding RNAs (lncRNAs) are emerging as important functional components in the establishment of long-range chromosomal interactions. In a recent paper published in Cell Research, Xiang et al. provide mechanistic insight into this phenomenon by characterizing the role of CCAT1-L, a colorectal cancer-specific lncRNA, in intra-chromosome looping between the MYC gene promoter and distal upstream enhancer elements that regulate MYC transcription.
Enhancer elements are the principal means through which cell type/context-specific gene expression is achieved. Enhancers contain binding sites for multiple transcription factors that work in concert to regulate the activity of specific gene promoters. Enhancers can be separated from the genes that they regulate by large genomic distances, but are brought into close physical proximity through a process known as chromosome looping. The mechanisms through which chromosome looping between enhancers and promoters is regulated are incompletely understood. However, several reports have described widespread transcription occurring within enhancer elements and have suggested that the resulting noncoding RNA transcripts may be involved in enhancer-promoter interactions1,2,3,4,5.
In a recent paper published in Cell Research, Xiang et al.6 characterize one such enhancer-derived long noncoding RNA (lncRNA), CCAT1-L, and conclude that it does indeed have a functional role in chromosome looping at the MYC locus. The authors first identified CCAT1-L, an alternative isoform of the previously described lncRNA CCAT1, after performing RNA-Sequencing (RNA-Seq) in colorectal cancer (CRC) patient tissue samples. As compared to normal tissue samples, CCAT1-L expression levels were significantly elevated in CRC tissue. Furthermore, CCAT1-L expression was detected in several CRC-derived cell lines but not in non-CRC cell lines, underscoring the specificity of CCAT1-L in CRC.
The CCAT1-L transcript is encoded within an enhancer located 515 kb upstream of the MYC gene. In general, enhancer-derived RNAs are not spliced or polyadenylated; however, CCAT1-L contains two exons and a poly-A tail1,3,7. The authors used a combination of cell fractionation and FISH experiments to show that CCAT1-L is enriched in the nucleus and localized at its site of transcription in CRC-derived cells. Subsequent knockdown experiments using antisense oligonucleotides (ASOs) revealed that depletion of CCAT1-L resulted in the reduction of MYC transcription. However, transient overexpression of CCAT1-L had no effect on MYC expression. Collectively, these observations suggest that CCAT1-L is involved in MYC regulation and that it functions in cis at the MYC enhancer.
Dissecting the mechanistic aspects of cis-functioning lncRNAs can be difficult because experiments require the perturbation of endogenous loci. Xiang et al. overcame this hurdle using genome editing to engineer two unique CRC-derived cell lines with modifications at the endogenous CCAT1-L locus. In one line a CMV promoter and an egfp gene were inserted upstream of CCAT1-L, resulting in the overexpression of an egfp-CCAT1-L fusion transcript. In the second line the egfp gene was followed by a double poly-A site, which allowed egfp expression as a control but prevented overexpression of the downstream CCAT1-L transcript. The authors found that overexpression of egfp-CCAT1-L resulted in increased MYC expression, supporting the role of CCAT1-L in MYC regulation and further suggesting that CCAT1-L functions in cis at that MYC enhancer.
To investigate the interactions between the CCAT1-L-encoding enhancer and the MYC promoter, the authors next utilized chromosome conformation capture (3C). The 3C assay is a proximity ligation-based approach that can detect interactions between genomic regions that are distant from one another in sequence space but in close physical proximity in the nucleus. Using this approach the authors validated interactions between the CCAT1-L locus and the MYC promoter. The authors also detected interactions between an additional MYC enhancer and both the MYC promoter and the CCAT1-L locus. Subsequent DNA FISH experiments confirmed that the MYC gene, the CCAT1-L locus, and the additional enhancer co-localize in the nucleus of CRC-derived cells. Moreover, RNA FISH revealed that the egfp-CCAT1-L RNA was also localized to these sites.
The authors turned to publically available ChIP-Seq datasets to gain insight into potential mechanisms through which CCAT1-L might be involved in chromosome looping at the MYC locus and noticed that CTCF-binding sites overlap the MYC promoter as well as both upstream enhancers. The authors depleted CRC-derived cells of either CCAT1-L or CTCF and observed a reduction in chromosome looping between the MYC promoter and its enhancers, which was accompanied by a decrease in MYC expression. Furthermore, a combination of biotin-labeled RNA pull-down and RNA immunoprecipitation experiments were used to characterize a direct interaction between CCAT1-L and CTCF. The authors concluded the study by demonstrating that depletion of CCAT1-L from CRC-derived cells resulted in a reduction in CTCF occupancy across the MYC locus.
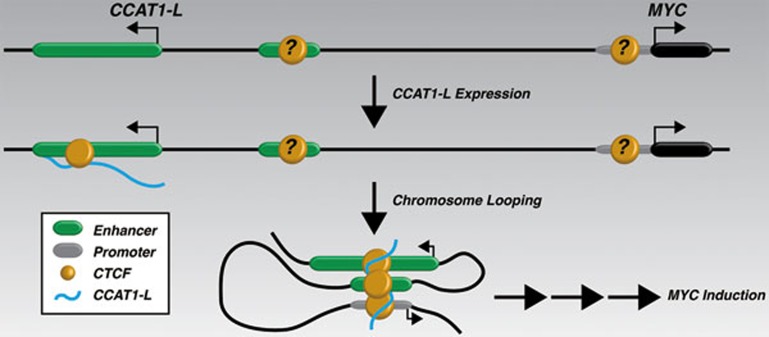
In summary, Xiang et al. provide compelling evidence to support a model whereby an enhancer-derived lncRNA, CCAT1-L, associates with CTCF and the resulting interaction facilitates chromosome looping across the MYC locus (Figure 1). The findings of Xiang et al. touch on a widely debated question in the study of enhancer-derived RNAs: are the RNAs transcribed from enhancers functional, are they simply a byproduct of open chromatin, or is it the act of their transcription that is required for enhancers to become accessible? In the case of CCAT1-L the answer remains unclear, although a strong argument could be made for the act of transcription being the key mechanistic feature. For example, increased transcription through the CCAT1-L locus led to an increase in enhancer activity. However, the interactions between the MYC promoter and its upstream enhancers, as well as the occupancy of CTCF across these regions, remained largely intact following the depletion of CCAT1-L RNA. Regardless of how CCAT1-L functions, Xiang et al. have illuminated previously unappreciated aspects of MYC regulation by distal enhancer elements, an advancement that has important implications in cancer biology.
Figure 1.
CCAT1-L is transcribed from a distal enhancer of MYC, associates with CTCF, and mediates chromosome looping between the MYC promoter and its upstream enhancers.
The observations of Xiang et al. also complement several recent reports on lncRNAs that facilitate chromosomal interactions and contribute to the proper establishment of nuclear architecture8,9,10. These reports include additional lncRNAs that are involved in intra-chromosomal looping as well as lncRNAs that mediate interactions between multiple chromosomes2,4,5,9,10,11. Collectively, these examples indicate that lncRNAs may be critical components of the molecular mechanisms important for shaping the three-dimensional structure of the genome.
References
- Kim TK, Hemberg M, Gray JM, et al. Nature. 2010. pp. 182–187. [DOI] [PMC free article] [PubMed]
- Ørom UA, Derrien T, Beringer M, et al. Cell. 2010. pp. 46–58. [DOI] [PMC free article] [PubMed]
- De Santa F, Barozzi I, Mietton F, et al. PLoS Biol. 2010. p. e 1000384. [DOI] [PMC free article] [PubMed]
- Ørom UA, Shiekhattar R. Cell. 2013. pp. 1190–1193. [DOI] [PMC free article] [PubMed]
- Wang KC, Yang YW, Liu B, et al. Nature. 2011. pp. 120–124. [DOI] [PMC free article] [PubMed]
- Xiang JF, Yin QF, Chen T, et al. Cell Res. 2014. pp. 513–531. [DOI] [PMC free article] [PubMed]
- Koch F, Fenouil R, Gut M, et al. Nat Struct Mol Biol. 2011. pp. 956–963. [DOI] [PubMed]
- Engreitz JM, Pandya-Jones A, McDonel P, et al. Science. 2013. pp. 1237973–1237973. [DOI] [PMC free article] [PubMed]
- Hacisuleyman E, Goff LA, Trapnell C, et al. Nat Struct Mol Biol. 2014. pp. 198–206. [DOI] [PMC free article] [PubMed]
- Simon MD, Pinter SF, Fang R, et al. Nature. 2013. pp. 465–469. [DOI] [PMC free article] [PubMed]
- Maass PG, Rump A, Schulz H, et al. J Clin Invest. 2012. pp. 3990–4002. [DOI] [PMC free article] [PubMed]