Abstract
An explosion of new information about mitochondria reveals that their importance extends well beyond their time-honored function as the “powerhouse of the cell.” In this Perspectives article, we summarize new evidence showing that mitochondria are at the center of a reactive oxygen species (ROS)-dependent pathway governing the response to hypoxia and to mitochondrial quality control. The potential role of the mitochondrial genome as a sentinel molecule governing cytotoxic responses of lung cells to ROS stress also is highlighted. Additional attention is devoted to the fate of damaged mitochondrial DNA relative to its involvement as a damage-associated molecular pattern driving adverse lung and systemic cell responses in severe illness or trauma. Finally, emerging strategies for replenishing normal populations of mitochondria after damage, either through promotion of mitochondrial biogenesis or via mitochondrial transfer, are discussed.
Keywords: hypoxia, lung injury, mitochondrial biogenesis, mitochondrial transfer, mtDNA
“the powerhouse of the cell.” All of the contributors to the 2013 ATS symposium forming the basis of this Perspectives article, and perhaps many of the attendees, too, recalled this phrase as their first introduction to mitochondria and often their first memorable phrase in biology. Though still true, it now seems almost quaint given the explosion of information about mitochondrial functions unrelated to energy metabolism that has occurred over the past two decades or so. Our symposium was motivated partly by this “explosion” and partly by the equally rapid accumulation of evidence that mitochondria likely play crucial roles in lung disease with significant potential to serve as targets in pulmonary and critical care medicine.
This Perspectives article does not seek to comprehensively review all developments in the mitochondrial field that bear on lung disease. Rather, it highlights evolving concepts of how mitochondria contribute to normal lung health and how mitochondria could be targeted for specific intervention in lung disease. Having registered this, we present concise summaries pertaining to mitochondria, reactive oxygen species (ROS), and oxygen sensing in the lung, the role of the mitochondrial genome as a sentinel molecule governing lung cell survival in response to oxidant stress, the nature of mitochondrial DNA (mtDNA) damage-associated molecular patterns (DAMPs) originating from the lung, and their potential use and importance as biomarkers and proinflammatory alarm signals. Finally, we explore the intriguing notion that therapeutic strategies aimed at reconstituting normal mitochondrial population and function may comprise therapeutic approaches in acute lung injury (ALI).
Mitochondrial Oxygen Sensing
Mitochondria consume O2 at cytochrome c oxidase, the terminal complex in the electron transport chain (ETC). Electron flux along the ETC is linked to proton translocation across the inner mitochondrial membrane, resulting in the generation of an electrochemical gradient that is used by the ATP synthase to generate ATP during oxidative phosphorylation. As mitochondria convert O2 to water the intracellular O2 tension decreases, thereby generating a gradient for O2 diffusion into the cell from the extracellular space. Mitochondria are the principal site of oxygen consumption in the cell, so their oxygen tension is lower than that of any other organelle. This characteristic has led investigators to ask whether mitochondria function as oxygen sensors in the cell (64). A conceptually simple idea is that decreases in cellular O2 levels (tissue hypoxia) would limit mitochondrial oxygen consumption, leading to redox changes as the ETC complexes became saturated with electrons owing to the loss of cytochrome c oxidase activity (56). These redox changes would then be reflected in the redox status of systems that feed electrons into the ETC, such as the Krebs cycle. However, a conceptual problem with this idea arose from experimental studies showing that mitochondria do not become limited by oxygen availability until the cell is nearly anoxic (12, 95). Specifically, how could the mitochondria respond to physiological levels of hypoxia if their ability to signal via redox alterations was not activated until all of the oxygen was gone? For many years this paradox represented a conceptual barrier to the idea that mitochondria might be involved in oxygen sensing. Some investigators suggested that specialized oxygen sensors such as the glomus cells of the carotid body might circumvent this problem by expressing special isoforms of cytochrome c oxidase with low apparent affinity for O2 (59). In those cells, electron transport and oxygen consumption would become O2 supply limited at milder levels of hypoxia, allowing the mitochondria to detect subtle decreases in cellular oxygenation (58). However, no experimental evidence for the existence of such a low-affinity oxidase has been reported. Moreover, mammalian cells with high-affinity forms of cytochrome c oxidase are still capable of detecting and responding to hypoxia.
A second hypothesized model of oxygen sensing was promoted by Michelakis, Archer, and Weir, who sought to explain how pulmonary artery smooth muscle cells detect hypoxia and trigger acute hypoxic pulmonary vasoconstriction (4). Their model proposed the existence of an intracellular oxidase that would generate more ROS at high O2 levels and less under hypoxic conditions. According to that model, ROS would then activate voltage-dependent potassium (Kv) channels in the plasma membrane. Conversely, the lesser oxidation of these channels during hypoxia would promote channel closure, causing membrane depolarization that would lead to the entry of extracellular Ca2+ through L-type calcium channels. However, when they tested the effect of gp91-(phox) deletion on this response, using mice with genetic deletion of NOX2, they found that the cellular response to hypoxia was still present (3). They subsequently considered that mitochondria were the source of these ROS signals, based on studies using various mitochondrial inhibitors (57). However, a conceptual problem with that model has been the consistent failure of diverse chemical antioxidants or the expression of enzymatic antioxidant systems to activate hypoxic responses in a normoxic cell.
A third model of mitochondrial oxygen sensing (Fig. 1) arose from initial studies showing that intracellular ROS levels actually increase during hypoxia (13, 26). Parallel studies using chemical mitochondrial inhibitors implicated complex III as the likely source of ROS generation. This paradoxical response was initially criticized because it seemed counterintuitive and was based on the use of nonspecific fluorescent probes to detect intracellular ROS levels and pharmacological inhibitors to block the ETC (34, 90). Importantly, different laboratory groups using the same inhibitors at various concentrations reported conflicting responses. To address this, Waypa et al. (91) utilized a novel genetically encoded fluorescent protein sensor to detect intracellular ROS levels. The initial sensor, HSP-FRET, exhibited ratiometric behavior that obviated an important limitation of the fluorescent chemical probes. Specifically, ratiometric sensors provide a signal that is independent of the light intensity used to excite the probe. Importantly, studies using HSP-FRET expressed in pulmonary artery smooth muscle cells confirmed the previous observation that ROS levels increase in the cytosol during hypoxia (91). Moreover, the increase in ROS during hypoxia was shown to be required for the increase in cytosolic Ca2+ activation in pulmonary artery smooth muscle cells. Further advances in this hypoxia-induced ROS signaling model came with the introduction of roGFP, a redox-sensitive mutant of green fluorescent protein (24, 35). This ratiometric sensor provides a specific measure of protein thiol oxidation/reduction status in the cell. Two important features contributed to the importance of this advance. First, its oxidation is reversible in the cell, allowing it to be calibrated, analogous to the behavior of ratiometric Ca2+ sensors. Second, its expression can be targeted to specific intracellular compartments, allowing assessment of redox at the subcellular level. Using roGFP expressed in cytosol, mitochondrial intermembrane space (IMS), and the mitochondrial matrix, Waypa et al. (92) found that significant differences in basal oxidant status exist across these compartments in pulmonary artery smooth muscle cells (PASMC), with the cytosol existing in a more reduced state, the mitochondrial matrix in a highly oxidized state, and the IMS at an intermediate level. During acute hypoxia, oxidation status in the IMS and the cytosol increased, whereas oxidation in the mitochondrial matrix decreased. These findings were consistent with a model in which the release of superoxide from complex III to the IMS increases during hypoxia, allowing the ROS to potentially exit to the cytosol and initiate redox signaling involved in triggering the release of intracellular calcium stores (94). By contrast, hypoxia caused an attenuation of oxidant stress in mitochondrial matrix. The opposing redox responses to hypoxia in these two compartments could reflect a distinct operation of two independent mechanisms controlling ROS generation during hypoxia in the two domains. Alternatively, the possibility that ROS alters the direction of ROS secretion from the membrane, toward the matrix during normoxia and toward the IMS during hypoxia, has not been ruled out.
Fig. 1.
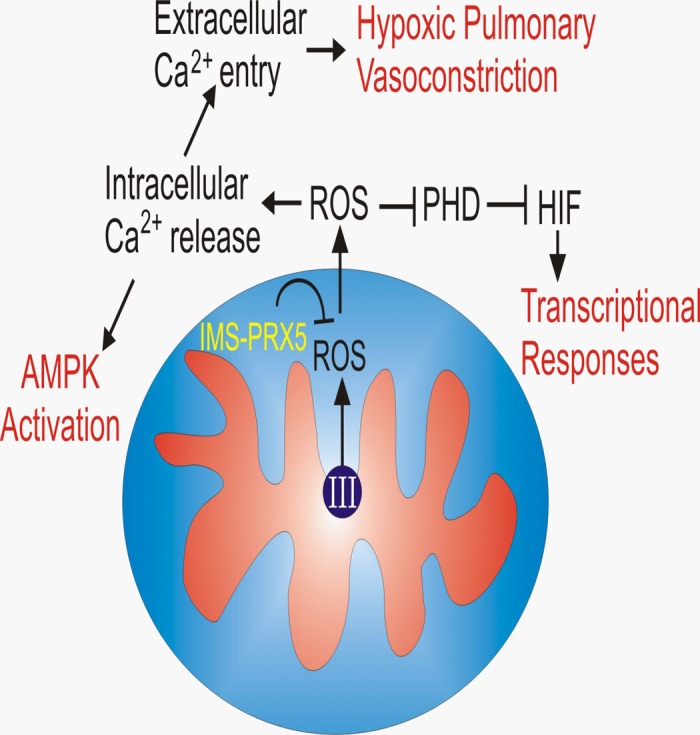
Conceptual model of oxygen sensing by mitochondria through the regulated generation of reactive oxygen species (ROS) that are released to the cytosol through the intermembrane space (IMS). IMS-PRDX5, peroxiredoxin-5 targeted to the IMS; PHD, hypoxia-inducible factor (HIF) prolyl hydroxylase; III, mitochondrial complex III.
In either case, studies were needed to more critically test the hypothesis that the mitochondrial electron transport chain is the source of ROS signaling during hypoxia. Accordingly, Waypa et al. (93) developed a genetic model by generating a mouse to permit conditional deletion of the Rieske iron-sulfur protein (RISP). This nuclear encoded protein is critical for the function of complex III, where it accepts an electron from ubiquinol, the carrier that moves electrons into complex III from complexes I and II (34). ROS generation from complex III can occur after RISP accepts an electron from ubiquinol, generating the intermediate free radical, ubisemiquinone. The remaining electron on ubisemiquinone is normally transferred to the b-cytochromes in the complex but can potentially be transferred instead to O2, generating superoxide. If RISP is deleted, ubisemiquinone, and thus superoxide, cannot be generated at complex III (33, 54). Accordingly, that group studied PASMC isolated from the conditional RISP mice and used Cre recombinase expression to delete the RISP gene. After loss of RISP was confirmed, subsequent roGFP studies demonstrated that the hypoxia-induced changes in redox status in the cytosol, IMS, and the mitochondrial matrix were abolished. These studies therefore demonstrated that a functional complex III was required for the changes in redox observed in different subcellular compartments during hypoxia (93). In parallel studies, it was found that the transient increase in cytosolic Ca2+ in PASMC during acute hypoxia was abrogated in cells where RISP had been deleted (93).
A technical limitation of studies using cultured PASMC is the possibility that the cells will undergo a phenotypic shift in culture. Moreover, studies in cultured cells cannot provide an assessment of the role of those responses in the intact lung. Accordingly, that group conducted in vivo studies in which RISP was deleted from smooth muscle cells by breeding the conditional RISP mice with a transgenic line expressing a smooth muscle-specific, tamoxifen-inducible Cre (96). The resulting mice were administered tamoxifen to activate nuclear localization of the Cre in smooth muscle cells, and subsequent deletion of RISP from smooth muscle was confirmed. The acute pulmonary vasoconstriction response was then compared in the knockout and control mice, by measuring the right ventricular systolic pressure (RVSP) response to acute alveolar hypoxia. Control mice exhibited a significant increase in RVSP during 5% O2 ventilation, which was significantly attenuated in the RISP-deficient mice (93). These findings indicate that a functional complex III is required for the acute vasopressor response to hypoxia in the lung.
Does the same mitochondrial ROS signal trigger other functional responses to hypoxia? Mungai et al. (60) tested the hypothesis that hypoxia activates the cellular energy sensor, AMP-dependent kinase (AMPK), through an ROS-dependent mechanism. In PASMC, they found that ROS generation during moderate hypoxia led to AMPK activation, even in the absence of any increase in AMP or ADP concentrations. Those findings suggest that mitochondrial ROS signals activate AMPK as an early response to hypoxia, allowing it to activate protective mechanisms before the cell progresses into a bioenergetic crisis where AMP and ADP concentrations are increased.
Do specific antioxidants block these responses? To test this, Sabharwal et al. (79) reasoned that the expression of an enzymatic scavenger of H2O2 exclusively in the mitochondrial IMS should abolish functional responses to hypoxia by preventing ROS released from complex III to reach the cytosol. Peroxiredoxin-5 (PRDX5) is an atypical 2-cysteine-containing peroxidase that is normally expressed in multiple subcellular compartments including the mitochondrial matrix (45). This enzyme functions as a monomer, whereas other H2O2 scavengers only function as multimers. Sabharwal et al. targeted its expression to the IMS using the NH2-terminal sequence that directs the SMAC/Diablo protein to that compartment (66) and confirmed correct localization using immunogold electron microscopy (79). When PRDX5 was expressed in the IMS of PASMC, it attenuated the increase in ROS in the IMS and the cytosol during hypoxia. Importantly, the PRDX5-expressing cells also showed a significant attenuation of the acute hypoxia-induced activation of cytosolic Ca2+. These findings provide an independent demonstration that ROS release from the mitochondrial inner membrane to the IMS and subsequently to the cytosol is critical for triggering acute responses to hypoxia in PASMC.
Previous studies by this group had shown that the stabilization of hypoxia-inducible factor-1α (HIF-1α) is triggered by hypoxia-induced release of ROS from mitochondria. In PASMC, hypoxia also triggers stabilization of HIF-1α by inhibiting prolyl hydroxylase, which targets the protein for degradation by hydroxylating conserved proline residues during normoxia (38, 41). In PASMC expressing graded levels of PRDX5 in the IMS, hypoxia-induced HIF-1α stabilization was inhibited in a dose-dependent manner (79).
Collectively, these findings indicate that mitochondria function as more than simple factories supplying ATP to the cell. Through the regulated release of ROS to the cytosol, these organelles have the capacity to orchestrate a diverse group of adaptive responses to hypoxia. In that regard, the moniker of “cellular oxygen sensors” clearly applies to these organelles.
Sentinel Functions of mtDNA in Pulmonary Vascular Cells
The nuclear and mitochondrial genomes differ in certain fundamental respects. For example, compared with the nuclear genome, the mitochondrial genome is tiny. Even if all the mtDNA molecules in cells with high mitochondrial densities are totaled, it is still a small fraction of the size of the nuclear genome. Following from this, mtDNA encodes for only a handful of proteins compared with the many thousands encoded by nuclear DNA. Second, the structure and regulation of the mitochondrial genome is comparatively simple. Unlike nuclear DNA, in which the state of compaction, its interactions with multiple transcription factors and coactivators, and epigenetic determinants are all exquisitely regulated processes culminating in precise control of gene expression, the mitochondrial genome is expressed as a single unit and its transcription is controlled by a handful of mtDNA binding proteins, particularly mitochondrial transcription factor A (TFAM) (10, 44). Instead of compaction within a nucleosome context, a single molecule of mtDNA is associated with ∼30 core proteins in a structure known as the nucleoid. Most if not all of the enzymes needed for mtDNA replication and mtRNA expression appear to be components of the nucleoid (9).
Another difference between mitochondrial and nuclear DNA particularly relevant to the present discussion is that mtDNA is ∼50-fold more sensitive to oxidative damage (7, 16, 32, 97). Reasons for this heightened sensitivity are not entirely known, but have been attributed to a comparatively simple process of mtDNA repair (mitochondria seem to contain only base excision repair whereas the nucleus has multiple, overlapping pathways) (8), and the prospect that the open structure of mtDNA is more accessible to DNA-damaging ROS. In light of the forgoing comments that mitochondrial ROS generation plays a central role in hypoxic signal transduction, the question can be raised as to whether mtDNA is damaged by oxidation in hypoxic environments. Although DNA integrity across the entire mitochondrial genome in hypoxic cells has not been examined, a large expanse of the mtDNA coding region, including the “common deletion sequence” known to be sensitive to oxidant stress (25), fails to display oxidative base modifications in hypoxia (31). Perhaps this is because in hypoxia, the matrix, where mtDNA resides, becomes more reduced in hypoxia as ROS are vectored into the IMS and cytosol (92).
In marked contrast to the lack of effect of hypoxia-induced mitochondrial ROS generation on mtDNA integrity, when cells or intact lung tissue are challenged with exogenous ROS the nuclear genome is largely spared of lesions whereas mtDNA is extensively damaged (7, 16, 32, 97). One question that emerges from this interesting observation pertains to the biological value in having a mitochondrial genome sensitive to damage by exogenous oxidants whereas the nuclear genome is relatively resistant. A potential answer, discussed subsequently, is that mtDNA functions as a “fuse” or a “sentinel molecule” such that before an externally applied oxidant stress rises to a level that threatens the nuclear genome with mutation, oxidative mtDNA damage triggers both death of the affected cell and promotes the dissemination of signals to “alarm” neighboring and itinerant cells.
Multiple lines of evidence support the idea that mtDNA serves as a molecular sentinel dictating cell survival in response to oxidant stress. As just noted, in virtually all cell types studied thus far mtDNA is highly sensitive to ROS-induced damage (7, 32, 97). There is also a conspicuous association between mtDNA damage and ROS-mediated cell death, with the propensity for cytotoxicity inversely related to the efficiency of mtDNA repair (32, 37). Genetic modulation of the first and rate-limiting step in mtDNA repair, mediated by Ogg1 (19), a DNA glycosylase that detects and excises oxidatively damaged purines, coordinately regulates ROS- and reactive nitrogen species (RNS)-induced mtDNA damage and cell death in all cultured cell populations so far examined (14, 21, 47, 67, 72, 73). Particularly relevant here are studies using mitochondrially targeted Ogg1 constructs, which eliminate the possibility that the biological consequences of Ogg1 overexpression are related to effects on nuclear DNA repair. Studies in pulmonary artery endothelial cells show that overexpression of mitochondrially targeted Ogg1 reduces sensitivities to both oxidative mtDNA damage and cytotoxicity evoked by exogenous ROS stressors (21, 77). Conversely, reduction in total cell Ogg1 by using siRNA, although not sensitizing nuclear DNA to oxidative damage, leads to persistence of ROS-induced damage to the mitochondrial genome, increased apoptosis, and cytotoxicity (78).
Although these observations suggest that mtDNA integrity is a determinant of ROS-induced cytotoxicity, many questions remain about the specific pathway(s) by which oxidative mtDNA damage dictates lung cell survival and function. The link between ROS-induced mtDNA damage and cell death traditionally has been ascribed to the idea that persistent damage leads to diminished mtDNA transcription, defective ETC function and to resulting formation of a regenerative cycle of excessive proapoptotic ROS production and increasing mtDNA damage that ultimately culminates in mitochondrially driven cell death (11, 39, 75). However, this traditional model has never been thoroughly explored, and, more importantly, emerging evidence suggests that other pathways are equally plausible. Protective actions of mtDNA repair enzymes against ROS-induced lung cell death and dysfunction might not be linked to mtDNA repair, but rather to their interaction with oxidatively damaged mtDNA or mtDNA damage-related proteins. In this regard, overexpression of a mitochondrially targeted DNA repair-defective Ogg1 mutant protected against asbestos-induced apoptosis in A549 cells (67). Because the repair-defective enzyme, like the wild-type enzyme, bound aconitase, the possibility was advanced that Ogg1 influences ROS-mediated signaling events downstream of mtDNA damage (80). In a related context, it has recently been shown that Ogg1 in complex with free 8-oxoguanine serves as a guanine exchange factor to activate Ras-mediated signaling (29), but whether this occurs in intact cells with ROS-induced mtDNA damage has not been established.
As a means of exploring involvement of oxidative mtDNA damage in intact lung and animal models of pulmonary disease, Wilson and colleagues (48) developed novel fusion-protein constructs targeting DNA repair glycosylases, either Ogg1 or the bacterial enzyme Endo III, to mitochondria. Early work showed that these fusion protein constructs prevented both hydrogen peroxide-induced mtDNA damage and increases in the vascular filtration coefficient in isolated perfused rat lungs (16). The Ogg1 construct also attenuated ventilator-induced lung injury and mortality in mice (36) and hyperoxia-induced dysmorphogenesis in fetal rat lung explants (28). Preliminary studies also suggest that mitochondrially targeted Ogg1 inhibits mortality and ALI evoked by intratracheal Pseudomonas aeruginosa in rats (15).
These observations on the effectiveness of increasing mtDNA repair in isolated lungs and animal models are a step toward determining whether mtDNA integrity is a legitimate pharmacological target in lung disease. However, there are many unresolved issues. As noted above, some pertain to the specific mechanism whereby increased mtDNA repair protects against cytotoxicity. As an extension of this, whereas protection of mtDNA integrity in cultured cells inhibits ROS-induced cytotoxicity, the involvement of cytotoxicity per se in intact animal models of ALI is complex. Taking this analysis one step further, it is easy to envision how oxidative mtDNA damage could, by different mechanisms, mediate different types of cellular responses in lung disease. For example, ROS-induced cytotoxicity, driven by persistent mtDNA damage, defective mtDNA transcription, and attendant bioenergetic insufficiency, could be lethal to some populations of lung cells, whereas oxidant-induced alterations in mtDNA-protein stability might acutely influence behavior of other lung cell populations secondary to alterations in cytoskeletal integrity (74). Additional studies will be required to determine the mechanism(s) of action and pharmacological utility of protecting mtDNA integrity in the many pulmonary disorders in which acute oxidant stress plays a pathogenic role.
It has been appreciated for some time that ROS contribute to the evolution of ALI/acute respiratory distress syndrome (ARDS) and other lung diseases, possibly through an adverse effect on mitochondrial function (11). Indeed, ∼40 clinical trials have examined the effect of antioxidant strategies in ALI and multiple organ system failure, but the therapeutic effects of antioxidant strategies have been unimpressive (88). These disappointing outcomes may be attributed in part to the heterogeneous nature and onset of ALI/ARDS that tend to confound design and interpretation of clinical trials. There also remains considerable scientific uncertainty about molecular targets of antioxidant drug action. For example, nonselective antioxidants may disrupt signaling required for physiological adaption, such as hypoxia-mediated ventilation-perfusion matching in the injured lung, lung cell survival, and recovery. Strategies currently available also may not target the key sentinel molecule(s) triggering adverse cellular effects of ROS. Perhaps, as suggested by the foregoing discussion, mtDNA could be such a sentinel molecule.
Mitochondrial DNA DAMP Release and Its Cellular Targets in Lung Disease
An intriguing question to emerge from the prospect that mtDNA damaged by oxidation in the setting of oxidant lung injury pertains to the fate of the molecule. As has been suggested from studies in other experimental systems (20), if mtDNA damage is severe and not promptly repaired, does the molecule fragment, after which it is exported from the organelle, possibly as part of mitophagy? And if oxidatively damaged mtDNA fragments are exported from mitochondria, what are the next destinations of the fragments and do they play a role in disease pathogenesis? These questions, depicted in Fig. 2, are discussed below.
Fig. 2.
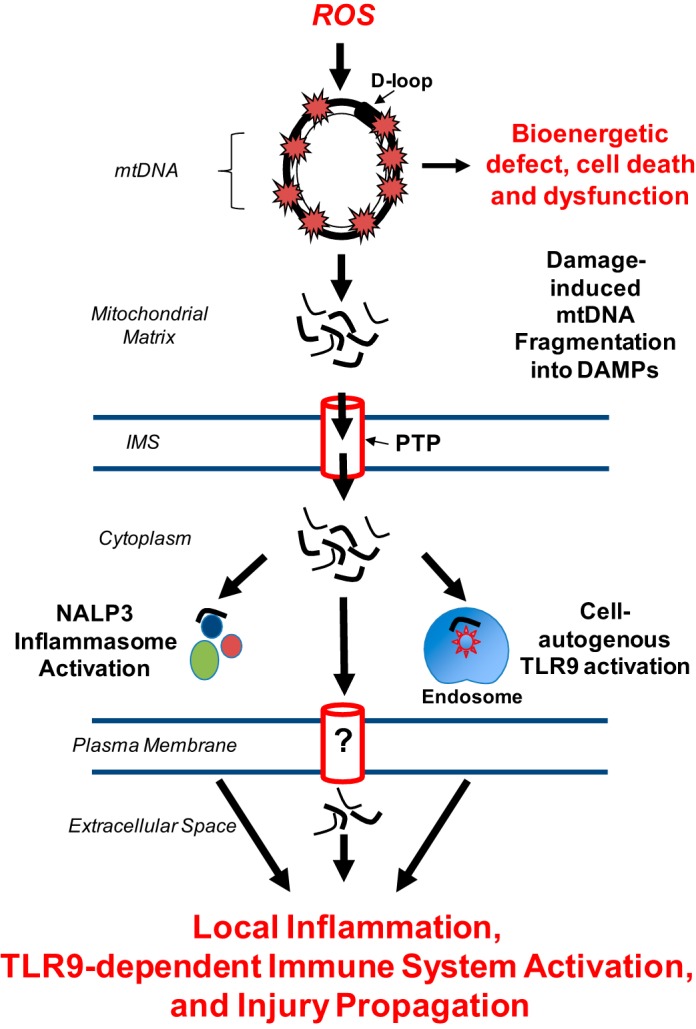
Fate and biological actions of the oxidatively damaged mitochondrial genome that warrant characterization of mtDNA as a “sentinel molecule.” See text for details. DAMPs, damage associated molecular patterns; PTP, permeability transition pore.
If unrepaired, damaged mtDNA is rapidly degraded (81). Interestingly, mtDNA so damaged displays a higher abundance of sugar-phosphate backbone lesions and abasic sites relative to oxidized base products, which would be expected to fragment the DNA rather then lead to persistent mutagenic lesions. There is scant evidence concerning the fate of mtDNA fragments formed as a consequence of oxidative damage. In a study using isolated mitochondria subjected to an exogenous oxidant stress, Garcia and Chavez (27) reported that mtDNA fragments less than 1,000 bp were lost from the organelle via the permeability transition pore. Studies in macrophages also indicate that the permeability transition pore is required for cytosolic accumulation of mtDNA after immunological stimulation (61), but whether there is some specificity for fragment size or other attributes is unknown. In cardiomyocytes subjected to hemodynamic stress-induced mitochondrial damage, mtDNA fragments accumulate in autolysosomes where, on the one hand, DNase II is capable of degrading the fragments to biological insignificance (65). On the other hand, mtDNA fragments escaping degradation appear capable of autonomously activating endosomal TLR9 (65). In immunologically stimulated macrophages, mtDNA fragments accumulating in the cytoplasm function as a coactivator in the NALP3-dependent activation of caspase 1 (61). Importantly, both TLR9 and NALP3 activation can initiate downstream proinflammatory signaling.
In severely injured (100) and critically ill human subjects (62) mtDNA accumulates in the circulation, where it functions as a DAMP and causes TLR9-dependent activation of the innate immune system. Here, too, the mechanism by which mtDNA fragments exit cells has received little study. In IL-5- or γ-IFN-primed eosinophils, lipopolysaccharide causes the ROS-dependent accumulation of mtDNA fragments in the extracellular space. The release process, described as “catapult-like,” occurred rapidly and was not dependent on death of the cells (98). Whether a similar process accounts for release of mtDNA from other types of oxidatively or mechanically stressed cells is entirely unknown. Indeed, whether mtDNA DAMP release is inextricably linked to autophagy, mitophagy, or cell death remains to be established.
As noted previously, mtDNA is normally localized in a multiprotein complex within the mitochondrial matrix, referred to as a nucleoid, which contains the essential transcription factor and structural protein TFAM (9, 30, 44). TFAM has a high binding affinity for mtDNA and remains associated when DNA is immunoprecipitated from lysed cells (43). TFAM is a functional and structural homologue of the nuclear DNA binding protein HMGB1, and both TFAM and HMGB1 are potent ligands for RAGE. In the context promoting inflammation, RAGE ligands are shown to amplify the immune responses to pattern-recognizing Toll-like receptors (43). In contrast to nuclear DNA, which is nonimmunogenic, mtDNA, like viral and bacterial DNA, is enriched with hypomethylated CpG motifs, which are natural ligands for TLR9, a highly specific intracellular (endosomal) pattern recognizing receptor (50). Given these properties of TFAM and mtDNA, it is not surprising that TFAM remains associated with mtDNA following cell necrosis, and this complex is particularly potent in terms of promoting TLR9-responsive immune cell activation (43).
Whereas many cell types are capable of responding to exogenous and endogenous danger signals, including neutrophils (PMNs), monocytes, and even epithelial cells (50), dendritic cells (DCs) are specialized for sensing and processing immunogenic antigens to initiate and coordinate complex and sustained immune responses. Despite being present in low abundance (<1%) in blood and other tissues, immature DCs, particularly plasmacytoid DCs (pDCs), and immature myeloid DCs (iDCs) are uniquely equipped to initiate and ultimately sustain sterile immune responses to immunogenic DNA, including viral and bacterial DNA. Immature DCs are unique in their capacity to simultaneously promote pro- and anti-inflammatory immune responses. They are distinguished from other immune cell lines by the lack of expression of common T cell, B cell, or myeloid cell surface markers and are further discriminated by their ability to produce large quantities of type I interferons in response to immunogenic (e.g., viral or bacterial) RNA and DNA, recognized by TLR7/8 and TLR9, respectively. Activated iDCs and pDCs, through the production of TNF-α, further promote “bystander” activation of regional macrophages, are induced to mature and are targeted to various tissues via expression of specific chemokine receptors [e.g., CCR2 targets DCs to lung (53), where they are capable of presenting antigen via MHC class II molecules to promote potent T cell-mediated adaptive and counterregulatory immune responses (Fig. 3)].
Fig. 3.
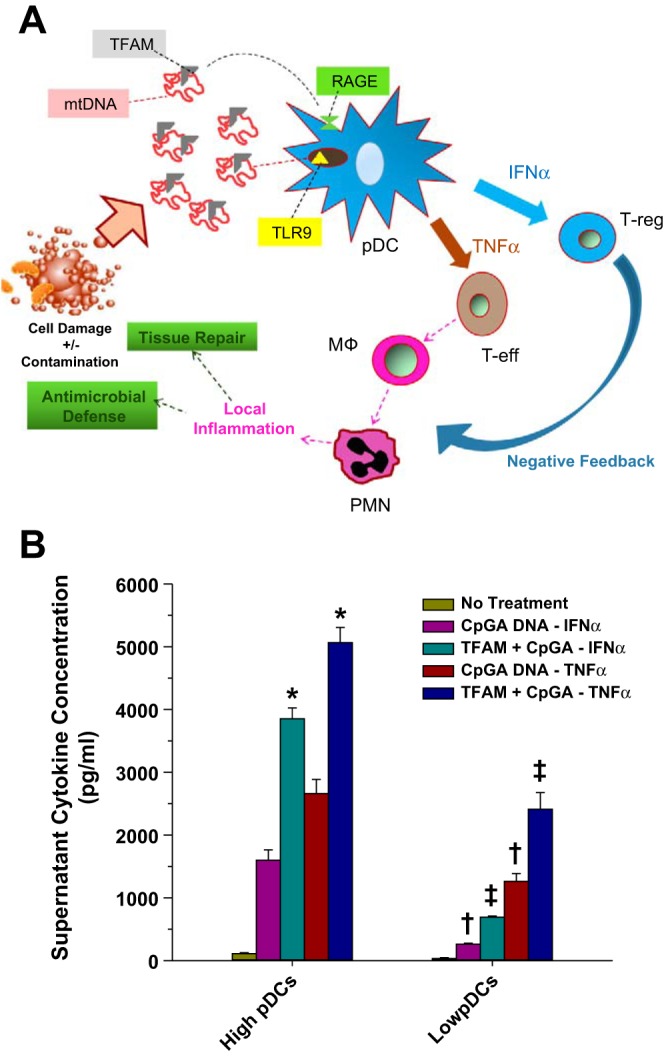
Plasmacytoid dendritic cells (pDCs) play a central role in orchestrating both pro- and anti-inflammatory responses. A: during acute tissue injury mitochondrial DNA (mtDNA) and mitochondrial transcription factor A (TFAM) released from damaged cells and contaminating bacteria is sensed by pDCs to promote simultaneous release of TNF-α and IFN-α, which promotes the simultaneous activation of proinflammatory and regulatory immune cells. Thus pDCs are poised to orchestrate a localized and self-limited inflammatory response. Presumably, excessive pDCs activation (e.g., massive cell injury) could promote systemic pro- and anti-inflammatory immune responses. B: in splenocyte cultures representing complex immune cell interactions typically occurring in vivo release of IFN-α and TNF-α in response to immunogenic (CpGA) DNA is enhanced by TFAM and depletion of pDCs [by magnetic MicroBead selection (Miltenyi Biotec, Auburn, CA)], representing <3% of the total cell population from the splenocyte cultures, is shown to greatly suppress the immune response. Thus pDCs are important “amplifiers” of the immune response to TFAM and DNA. *P < 0.01, compared with corresponding CpGA DNA treatment alone; †P < 0.01, relative to matching treatment in the High pDC group; and ‡P < 0.01, compared with corresponding CpGA DNA treatment alone and the matching treatment in the high-pDC group. PMN, neutrophil; MΦ, macrophage; T-eff, effector CD4+ T cell; T-reg, regulatory T cell.
In the context of acute cell damage, immature DCs respond specifically to mitochondrial danger signals; indeed, the TFAM/mtDNA complex has been shown to be sufficient to promote the simultaneous release of TNF-α and IFN-α from purified pDCs (42, 43) (Fig. 3A). Moreover, using a splenocyte culture model closely approximating the complex interaction of immune cells in vivo, TFAM was found to augment the immune response to CpGA DNA (a readily available surrogate for mtDNA). Furthermore, the depletion of pDCs, representing a small fraction of the total splenocyte culture population, dramatically suppressed the innate immune response to TFAM/CpG complex, demonstrating the capacity of pDCs to amplify both proinflammatory and counterregulatory immune pathways (Fig. 3B). Together, these data indicate that TFAM remains associated with mtDNA following acute cell damage, that TFAM serves to augment the immune response to mtDNA, and that immature DCs are important sensors for the TFAM/mtDNA complex and important regulators of the sterile immune response to these mitochondrial danger signals.
New evidence indicates that DCs, particularly pDC-like macrophages, are of critical importance during the pathogenesis of ALI in the context of sterile inflammation, and mitochondrial danger signals are incriminated as proximal mediators. Immunogenic DNA, such as is present in bacteria and mitochondria, is sufficient to promote ALI in animal models (46, 87), and mtDNA has been specifically linked to the pathogenesis of ALI in setting of sterile inflammation induced experimentally by acid aspiration (17), surgical trauma (100), and acute liver necrosis (55). PMNs, through activation of TLR9, appear to play a major role in initiating ALI under these conditions. By contrast, recruitment of pDC-like macrophages to the lung is shown to attenuate ALI induced by LPS, particularly in the acute phase of lung injury (49). These pDC-like macrophages express CCR2 and are recruited to the lung by CC chemokine ligand-2 (CCL2), whereupon they facilitate the clearance of damaged cells (52). In addition to enhanced clearance of damaged cells, pDC activation in the lungs results in the release of Type 1 interferons, which are capable of suppressing ALI by altering the balance between proinflammatory (e.g., IL-1β, IL-6) and anti-inflammatory (e.g., IL-10) cytokines (5). Finally, lung DCs present antigen to and thereby activate regulatory T cells (Tregs), which, in turn, contribute to the resolution of inflammation during the subacute phase of ALI (1) (Fig. 3A). Thus the current evidence suggests that the acute damage inflicted by activated PMNs is counterbalanced by pDCs in the context of ALI induced by mitochondrial danger signals.
Circulating Mitochondria DNA: A Biomarker of Sepsis and ARDS in the ICU
As noted above, accumulating evidence suggests that mitochondrial damage may engender the export mtDNA DAMPs that activate innate immune responses and possibly propagate injury to distant targets. In this regard, although biomarkers can be useful for identifying patients with critically illness or assessing the responses to the therapies for the patients in the intensive care unit (ICU), a small number of biomarkers reflecting the disease severity are commonly measured in clinical practice (e.g., lactate) (71). The question thus arises as to whether mtDNA DAMP may be a useful biomarker.
Inflammasomes are multiprotein complexes that activate caspase-1 and downstream immune responses, including the maturation and secretion of proinflammatory cytokines (i.e., IL-1β and IL-18) (18). Recent studies show that inflammasomes are involved in the pathogenesis of various diseases such as infection, cancer, and metabolic diseases (18). Inflammasomes are activated by a wide range of signals of pathogenic and nonpathogenic origins (host cell derived molecules including extracellular ATP, hyaluronan, amyloid-β fibrils, and uric acid crystals) (18).
To examine the involvement of inflammasome in critical illnesses, Choi and colleagues (22, 23) first performed gene expression profiling analysis of lungs from mice subjected to mechanical ventilation (MV), an experimental animal model of ALI. Specific changes in expression were identified for caspase-activator domain-10, IL-18 receptor-1 (Il18r1), and Il1r2 and IL-1β (Il1b) as genes following MV. Since genes representing inflammasome complex molecules and the downstream cytokines were regulated in animal models of ALI, it was then determined whether inflammasome family genes are also regulated in human critical illnesses such as ARDS, a lethal disease in ICU, and sepsis, a major cause of ARDS and a leading cause of death in ICU. Total blood RNA was extracted from patients admitted in medical ICU to determine the global gene expression profile of ICU controls, patients with systemic inflammatory response syndrome (SIRS), sepsis, and sepsis/ARDS. The gene expression profiling analysis revealed that inflammasome-associated genes (e.g., asc and Il1b) were upregulated in patients with sepsis/ARDS compared with SIRS (23). In addition, plasma levels of IL-18, a representative inflammasome-mediated proinflammatory cytokine, at the time of medical ICU admission were higher with increasing disease severity categories (control, SIRS, sepsis, and sepsis/ARDS) and the highest in sepsis/ARDS patients (23). Importantly, patients with elevated IL-18 levels at the time of medical ICU hospitalization had increased mortality (23). In mice, MV enhanced IL-18 levels in the serum and bronchoalveolar lavage fluid (23). Furthermore, IL-18 neutralizing antibody treatment, or genetic deletion of IL-18 or caspase-1, reduced lung injury in response to MV (23). These data suggest critical roles of inflammasome-mediated immune responses in ICU patients.
Recent studies suggest mtDNA is a potent inflammasome activator (61) and is released from mitochondria to the cytosol or the extracellular space as a mitochondrial DAMP. In addition, exogenous administration of disrupted mitochondria containing mtDNA induces acute inflammatory tissue injuries (100). Therefore, we examined whether the levels of circulating mtDNA are associated with disease severity or mortality in ICU (62). Circulating cell-free mtDNA level was higher in ICU patients who died within 28 days of medical ICU admission as well as in patients with sepsis or ARDS in the ICU. Medical ICU patients with an elevated mtDNA level had an increase in their odds of dying within 28 days of ICU admission, whereas no evidence for association was noted in nonmedical ICU patients (e.g., surgical ICU patients). There was no evidence that the association between mtDNA level and medical ICU mortality was attenuated in models adjusted for clinical covariates, including Acute Physiology and Chronic Health Evaluation (APACHE) II score, sepsis, or ARDS. The inclusion of an elevated mtDNA to a clinical model (including age, gender, race/ethnicity, APACHE II score, and sepsis) improved the net reclassification of 28-day mortality among medical ICU patients. The improvement in net reclassification remained significant even after additionally adjusting for measures of procalcitonin or lactate, commonly used biomarkers in ICU. Similar relationships between circulating mtDNA and outcome have been noted in patients with severe trauma (82) or patients with sepsis in the emergency room (51). Thus circulating mtDNA could serve as a viable plasma biomarker in medical ICU patients and may represent an important pathogenic determinant that contributes to a exaggerated systemic inflammatory response observed in patients with sepsis or ARDS.
Mitochondrial Biogenesis to the Rescue
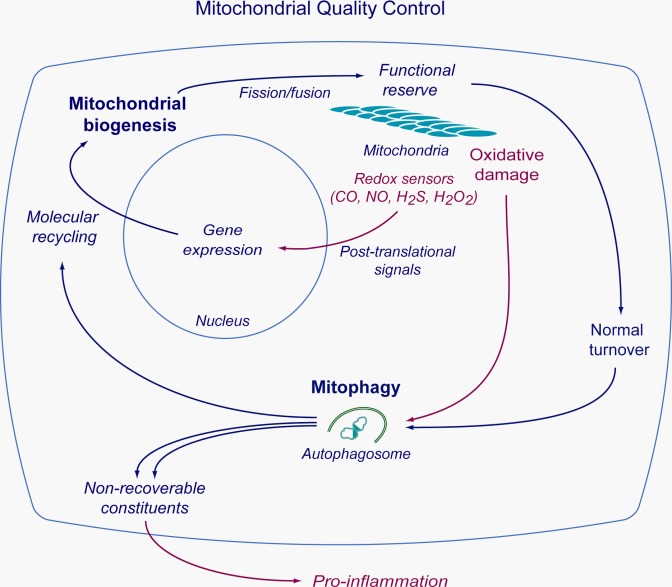
Decades of laboratory and clinical studies have revealed that acute systemic inflammatory syndromes, including sepsis, generate substantial macromolecular damage to mitochondria, particularly in the cells of highly metabolically active tissues like the liver, heart, and kidneys (83, 85). Such mitochondrial damage may be indiscernible in vivo because it does not necessarily activate cell death pathways, and some of the same factors that damage mitochondria, e.g., ROS, can also activate the process of mitochondrial biogenesis (86). Recently mitochondrial biogenesis has been detected even in distal lung cells, including alveolar type II epithelial cells (6). Indeed, all cells that rely on aerobic respiration and oxidative phosphorylation for energy conservation express a robust genetic program of mitochondrial quality control that is designed to maintain a fully functional complement of mitochondria through the conjoined activities of mitochondrial biogenesis and mitochondrial autophagy (mitophagy) (89). These closely regulated processes serve a prosurvival capacity by allowing cells to quickly replace metabolically dysfunctional mitochondria with fresh organelles from a pool of undamaged organelles before energy failure (Fig. 4).
Fig. 4.
Integrated process of mitochondrial quality control in the maintenance of mitochondrial homeostasis. The diagram illustrates the simplified relationship between mitochondrial biogenesis and selective mitochondrial autophagy or mitophagy. The cycle is accelerated by oxidative stress and is monitored and regulated by redox sensors including the physiological gases.
During inflammation mitochondrial damage is caused by the overproduction of reactive oxygen and nitrogen species (ROS/RNS) at multiple sites, leading to the inactivation of critical mitochondrial macromolecules through biochemical oxidation, nitration, and/or S-nitrosation. The critical mitochondrial targets are diverse, including many proteins, lipids, and mtDNA that embody integral mitochondrial processes including protein importation, transcription, protein synthesis, tricarboxylic acid cycle function, electron transport, heme synthesis, and control of the mitochondrial permeability transition pore (69). The upshot of damage from elevated ROS production is the compromised efficiency of oxidative phosphorylation, which may destabilize energy homeostasis and lead to further inflammation that may perpetuate the mitochondrial damage (101). However, the production of mitochondrial ROS also provides signals to activate redox-sensitive transcriptional pathways that regulate mitochondrial quality control and trigger the synthesis of counterinflammatory mediators, such as IL-10 (70). Moreover, the enhanced induction of enzymatic NO and CO production also promotes the expression of a large group of genes that is essential for mitochondrial biogenesis (63, 84).
A number of the transcriptional pathways activated by the NO synthase 2 (NOS2) and heme oxygenase-1 (HO-1) enzymes for the induction of mitochondrial biogenesis during inflammation have been worked out over the last several years. For both enzymes, the endogenous gaseous product (NO or CO) is directly involved in the induction mechanisms through the activation of redox-sensitive pathways. For NOS2, as for the other NOS isoforms, a key step involves the cyclic GMP-dependent phosphorylation of cyclic AMP-responsive element-binding protein 1 (CREB1), which undergoes translocation to the nucleus to activate the gene for PGC-1a, a critical coactivator of mitochondrial biogenesis and oxidative metabolism. Activated PGC-1a protein is a partner for nuclear respiratory factor-1 (NRF-1) and NRF-2 (GABPA); these are both essential transcription factors for mitochondrial biogenesis in most cells. For HO-1, heme catabolism releases CO, which binds to the reduced terminal oxidase of the respiratory chain and increases the mitochondrial H2O2 leak rate. The increased H2O2 production activates several prosurvival kinases, such as Akt, as well as the transcription factor Nfe2l2 (Nrf2) (68). Nrf2, in its gene transactivation function, binds to antioxidant response elements (ARE) in the NRF-1 gene promoter, whereas Akt phosphorylates GSK-3b, which relieves a restriction on the entry of Nrf2 into the nucleus. Akt phosphorylates the NRF-1 protein as well, allowing both transcription factors to gain entrance to the nucleus to induce an assemblage of genes required for mitochondrial biogenesis.
At counterpoise to mitochondrial biogenesis is the removal of senescent or damaged mitochondria that no longer perform oxidative phosphorylation or are triggering oxidative damage to nearby cellular structures. Such damaged mitochondria also propagate certain danger signals, for example, inflammasome activation (101). The deconstruction of obsolescent mitochondria by mitophagy, a form of selective macroautophagy, entails the envelopment of the mitochondrial cargo within double-membrane vesicles or autophagosomes, which fuse with lysosomes to degrade the cargo. The rate of mitophagy is governed by the extent of mitochondrial damage, but also by the cell's energy requirement and its capacity to meet this mitochondrial disposal function (99). Thus, from the cellular perspective, optimal stress-stabilizing strategies match energy requirements with the rates of mitophagy and mitochondrial biogenesis to ensure energy sufficiency and mitochondrial quality control.
In the mammalian lung, mitochondrial energy provision is an important function of many of the roughly 40 cell types present there. In the parenchyma, for instance, alveolar type II cells are enriched in mitochondria, which provide ATP to support surfactant synthesis, secretion, and recycling. These cells also initiate mitochondrial biogenesis during ALI, pneumonia, hyperoxia, and other stresses through induction of the HO-1/CO and NOS2 systems. These alveolar type II cell mitochondria also proliferate to support not only surfactant metabolism, but multiple other energy-dependent functions in the transition to type I epithelium, such as those involved in maintaining alveolar barrier integrity. In fact, mitochondrial proliferation precedes the proliferation of type II cells as well as their differentiation into type I epithelium. Under many of these same conditions, as discussed elsewhere in this Perspectives article, autophagy programs, including mitophagy, are activated to eliminate damaged proteins and other damaged organelles from the cell.
In the future, our understanding of the basic intracellular mechanisms of mitochondrial quality control in the lung may be brought into line with newly emerging roles for mitochondrial depletion, immune cell scavenging of exported mitochondrial components, and adoptive mitochondrial rescue-by-transfer during acute and chronic lung injury. The importance of these intricate extra- and intercellular processes will hinge on the relative effectiveness of mitochondrial biogenesis and mitophagy under pathogenic conditions of varying type, intensity, and severity that are currently being investigated in different experimental settings.
Mitochondrial Transfer to the Rescue
The foregoing discussion raises the prospect that mitochondrial oxygen sensing, maintenance of mtDNA integrity to prevent mtDNA fragmentation and release in the form of mtDNA DAMPs, and mitochondrial biogenesis may be important determinants of outcome in sepsis. A related and desirable goal of mitochondrial-directed therapy in severe illness is to prevent mitochondrial dysfunction (11). Clearly, the attendant decreased ATP production could causes failure of various aspects of cell function that in combination could lead to multiorgan failure in ALI/ARDS. The cause of mitochondrial failure is not well understood, but one possibility is that NF-κB activation, occurring secondary to TLR4 ligation by bacterial endotoxin, causes transcriptional upregulation of inducible nitric oxide synthase. The increased nitric oxide thus produced reacts with mitochondrial superoxide to cause peroxynitration, hence dysfunction of mitochondrial electron transport proteins (69). Although other mechanisms may also apply, the end result is failure of cellular bioenergetics as well documented by the reduction of tissue ATP levels. The therapeutic objective, therefore, would be to replenish dysfunctional mitochondria of host cells with fresh mitochondria.
The question is how does one replenish mitochondria? One possibility is to give exogenous mitochondria with the expectation that cells will take up the mitochondria by endocytosis and thereby repair the ATP deficit. However, the caveat here is that free mitochondria in the extracellular space might act as DAMPs that activate pattern recognition receptors that exacerbate tissue inflammation and injury (100).
The other option is to enable intercellular mitochondrial transfer, a process that has been reported in cultured cells (76). Islam et al. (40) now show that the process also occurs in vivo and that mitochondrial transfer from exogenously administered BMSCs (bone-marrow-derived stromal cells) to the alveolar epithelium protects against LPS-induced ALI. A more recent report by Ahmad et al. (2) also affirm that mitochondrial transfer from BMSCs to the airway epithelium occurs in vivo under inflammatory conditions. In this paper, the authors show that the mitochondrial transfer abrogated airway inflammation and protected against bronchial injury caused by mitochondrial dysfunction. These findings attest to the feasibility of mitochondrial transfer between specific cell types in vivo, indicating that it might be applicable as a therapeutic tool in that mitochondrial transfer might correct the deficit of mitochondrial function in affected cells through donation of fresh mitochondria from BMSCs.
Islam et al. (40) noted two important features regarding mitochondrial transfer. First, the transfer did not occur when BMSCs were instilled in control lungs not exposed to LPS, indicating that pathological stress to the alveolar epithelium was essential. Second, stabilization of BMSCs on the alveolar epithelium was also essential. The stabilization occurred through formation of gap junctional channels (GJCs) between the BMSCs and the alveolar epithelium. Knockdown of connexin 43, a GJC protein, blocked the BMSC stabilization and also diminished the survival benefit accruing from BMSC instillation. These findings indicate that for mitochondrial transfer to occur from a donor to a recipient cell, a preconditioning of the interacting cell surfaces is critical.
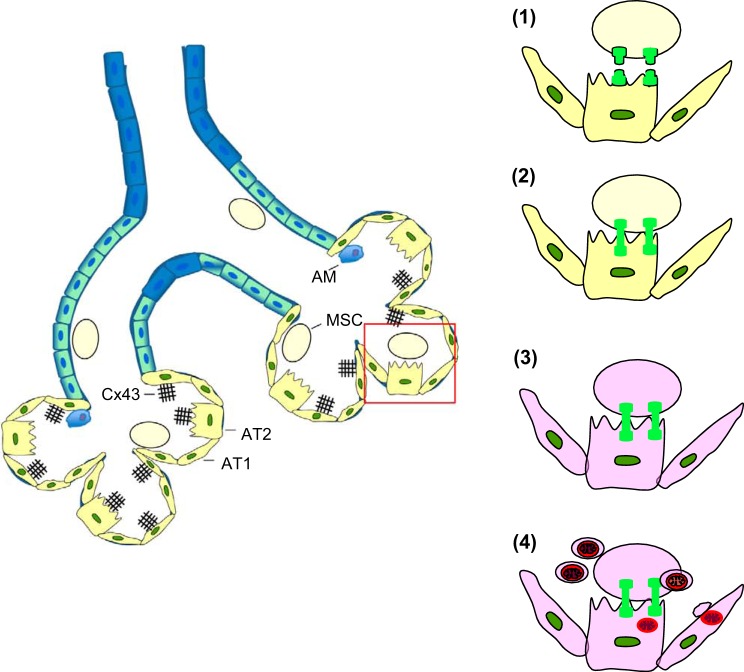
In the study by Islam et al. (40), the mechanism of mitochondrial transfer in alveoli occurred through a sequence in which BMSCs released mitochondria-containing nanotubes and microvesicles, and then the alveolar epithelium engulfed the microvesicles, internalizing the mitochondria (Fig. 5). Ahmad et al. (2) show that Miro1, a mitochondrial Rho-GTPase, is critical for this process since BMSCs lacking Miro1 fail to transfer mitochondria to the airway epithelium. The mechanisms that regulate microvesicle engulfment and release of the internalized mitochondria in the alveolar cytosol are not clear. However, Islam and coworkers found that the internalized mitochondria were functional, since progressive increases of ATP levels were detectable in the mitochondria-recipient epithelium. Islam et al. showed that endotoxin-induced mouse mortality decreased markedly following instillation of BMSCs carrying normal mitochondria, but not when the BMSC mitochondria were made dysfunctional by knockdown of the electron-transporting RISP that determines mitochondrial ATP production. Although mitochondrial transfer has been shown in vitro and in feral dogs (62), the reports by Islam et al. and Ahmad et al. indicate that the process occurs in vivo to promote tissue repair in organ systems undergoing pathological stress. The applicability of mitochondrial transfer as a viable therapeutic modality in inflammatory lung disease requires further consideration.
Fig. 5.
Sequence of events leading to mitochondrial transfer from alveolus-attached bone-marrow-derived stromal cells (BMSCs) to the alveolar epithelium. The sketch of the distal airway (left) shows the arrival of airway instilled BMSCs (MSC) in alveoli composed of alveolar type 1 and type 2 cells (AT2 and AT2, respectively) and alveolar macrophages (AM). Subsequent processes in the alveolus (right) are 1) BMSCs adhere to the alveolar epithelium through Cx43 interactions; 2) gap junctions form between BMSCs and the epithelium; 3) calcium levels increase in the BMSCs; and 4) BMSCs release mitochondria-containing microvesicles which enter the epithelium and release their mitochondria in the cytosol.
GRANTS
The authors' research described in this Perspectives article was supported by grants from the National Institutes of Health (R01 HL35440 and R01 HL122062 to P. T. Schumacker, R01 HL058234 and R0 1 HL113614 to M. N. Gillespie, R01 HL055330 and P01 HL114501 to A. M. K. Choi, R01 AI62740 and R01 AI083912 to E. D. Crouser, P01 HL108801 and R01 AI095424 C. A. Piantadosi, and RO1 HL36024 and RO1 HL57556 to J. Bhattacharya).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.T.S., M.N.G., E.D.C., and C.A.P. prepared figures; P.T.S., M.N.G., K.N., A.M.C., E.D.C., C.A.P., and J.B. drafted manuscript; P.T.S., M.N.G., K.N., A.M.C., E.D.C., C.A.P., and J.B. edited and revised manuscript; P.T.S., M.N.G., K.N., A.M.C., E.D.C., C.A.P., and J.B. approved final version of manuscript.
REFERENCES
- 1.Aggarwal NR, D'Alessio FR, Tsushima K, Sidhaye VK, Cheadle C, Grigoryev DN, Barnes KC, King LS. Regulatory T cell-mediated resolution of lung injury: identification of potential target genes via expression profiling. Physiol Genomics 41: 109–119, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, Roy SS, Mabalirajan U, Ghosh B, Agrawal A. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J 2014. February 6 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer SL, Reeve HL, Michelakis E, Puttagunta L, Waite R, Nelson DP, Dinauer MC, Weir EK. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc Natl Acad Sci USA 96: 7944–7949, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest 101: 2319–2330, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arimori Y, Nakamura R, Yamada H, Shibata K, Maeda N, Kase T, Yoshikai Y. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antiviral Res 99: 230–237, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Athale J, Ulrich A, Chou Macgarvey N, Bartz RR, Welty-Wolf KE, Suliman HB, Piantadosi CA. Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice. Free Radic Biol Med 53: 1584–1594, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, Runge MS. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res 86: 960–966, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Bogenhagen DF. Repair of mtDNA in vertebrates. Am J Hum Genet 64: 1276–1281, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogenhagen DF, Rousseau D, Burke S. The layered structure of human mitochondrial DNA nucleoids. J Biol Chem 283: 3665–3675, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Bonawitz ND, Clayton DA, Shadel GS. Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol Cell 24: 813–825, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Chandel N, Budinger GR, Kemp RA, Schumacker PT. Inhibition of cytochrome-c oxidase activity during prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 268: L918–L925, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 95: 11715–11720, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee A, Mambo E, Zhang Y, Deweese T, Sidransky D. Targeting of mutant hogg1 in mammalian mitochondria and nucleus: effect on cellular survival upon oxidative stress. BMC Cancer 6: 235, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chouteau J, Gorodnya O, Ruchko M, Obiako B, Wilson G, Gillespie M. Novel fusion protein constructs targeting DNA repair enzymes to mitochondria protect against pseudomonas aeruginosa-induced acute lung injury in intact rats (Abstract). Am J Respir Crit Care Med 279: A3763, 2011 [Google Scholar]
- 16.Chouteau JM, Obiako B, Gorodnya OM, Pastukh VM, Ruchko MV, Wright AJ, Wilson GL, Gillespie MN. Mitochondrial DNA integrity may be a determinant of endothelial barrier properties in oxidant-challenged rat lungs. Am J Physiol Lung Cell Mol Physiol 301: L892–L898, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson BA, Vethanayagam RR, Grimm MJ, Mullan BA, Raghavendran K, Blackwell TS, Freeman ML, Ayyasamy V, Singh KK, Sporn MB, Itagaki K, Hauser CJ, Knight PR, Segal BH. NADPH oxidase and Nrf2 regulate gastric aspiration-induced inflammation and acute lung injury. J Immunol 190: 1714–1724, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29: 707–735, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Souza-Pinto NC, Eide L, Hogue BA, Thybo T, Stevnsner T, Seeberg E, Klungland A, Bohr VA. Repair of 8-oxodeoxyguanosine lesions in mitochondrial DNA depends on the oxoguanine DNA glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial DNA of OGG1-defective mice. Cancer Res 61: 5378–5381, 2001 [PubMed] [Google Scholar]
- 20.Ding Z, Liu S, Wang X, Khaidakov M, Dai Y, Mehta JL. Oxidant stress in mitochondrial DNA damage, autophagy and inflammation in atherosclerosis. Sci Rep 3: 1077, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobson AW, Grishko V, LeDoux SP, Kelley MR, Wilson GL, Gillespie MN. Enhanced mtDNA repair capacity protects pulmonary artery endothelial cells from oxidant-mediated death. Am J Physiol Lung Cell Mol Physiol 283: L205–L210, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Dolinay T, Kaminski N, Felgendreher M, Kim HP, Reynolds P, Watkins SC, Karp D, Uhlig S, Choi AM. Gene expression profiling of target genes in ventilator-induced lung injury. Physiol Genomics 26: 68–75, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, Haspel JA, Landazury R, Eppanapally S, Christie JD, Meyer NJ, Ware LB, Christiani DC, Ryter SW, Baron RM, Choi AM. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 185: 1225–1234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem 279: 22284–22293, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Driggers WJ, Holmquist GP, LeDoux SP, Wilson GL. Mapping frequencies of endogenous oxidative damage and the kinetic response to oxidative stress in a region of rat mtDNA. Nucleic Acids Res 25: 4362–4369, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273: 11619–11624, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Garcia N, Chavez E. Mitochondrial DNA fragments released through the permeability transition pore correspond to specific gene size. Life Sci 81: 1160–1166, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Gebb SA, Decoux A, Waggoner A, Wilson GL, Gillespie MN. Mitochondrial DNA damage mediates hyperoxic dysmorphogenesis in rat fetal lung explants. Neonatology 103: 91–97, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.German P, Szaniszlo P, Hajas G, Radak Z, Bacsi A, Hazra TK, Hegde ML, Ba X, Boldogh I. Activation of cellular signaling by 8-oxoguanine DNA glycosylase-1-initiated DNA base excision repair. DNA Repair (Amst) 12: 856–863, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol 25: 1354–1366, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grishko V, Solomon M, Breit JF, Killilea DW, Ledoux SP, Wilson GL, Gillespie MN. Hypoxia promotes oxidative base modifications in the pulmonary artery endothelial cell VEGF gene. FASEB J 15: 1267–1269, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Grishko V, Solomon M, Wilson GL, LeDoux SP, Gillespie MN. Oxygen radical-induced mitochondrial DNA damage and repair in pulmonary vascular endothelial cell phenotypes. Am J Physiol Lung Cell Mol Physiol 280: L1300–L1308, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1: 401–408, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 91: 807–819, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem 279: 13044–13053, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Potter BJ, Wilson GL, Gillespie MN, Parker JC. Mitochondrial-targeted DNA repair enzyme 8-oxoguanine DNA glycosylase 1 protects against ventilator-induced lung injury in intact mice. Am J Physiol Lung Cell Mol Physiol 304: L287–L297, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollensworth SB, Shen C, Sim JE, Spitz DR, Wilson GL, LeDoux SP. Glial cell type-specific responses to menadione-induced oxidative stress. Free Radic Biol Med 28: 1161–1174, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Hon WC, Wilson MI, Harlos K, Claridge TD, Schofield CJ, Pugh CW, Maxwell PH, Ratcliffe PJ, Stuart DI, Jones EY. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature 417: 975–978, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 88: 529–535, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 18: 759–765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Julian MW, Shao G, Bao S, Knoell DL, Papenfuss TL, VanGundy ZC, Crouser ED. Mitochondrial transcription factor A serves as a danger signal by augmenting plasmacytoid dendritic cell responses to DNA. J Immunol 189: 433–443, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Julian MW, Shao G, Vangundy ZC, Papenfuss TL, Crouser ED. Mitochondrial transcription factor A, an endogenous danger signal, promotes TNFalpha release via RAGE- and TLR9-responsive plasmacytoid dendritic cells. PloS One 8: e72354, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang D, Hamasaki N. Mitochondrial transcription factor A in the maintenance of mitochondrial DNA: overview of its multiple roles. Ann NY Acad Sci 1042: 101–108, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Knoops B, Goemaere J, Van der Eecken V, Declercq JP. Peroxiredoxin 5: structure, mechanism, and function of the mammalian atypical 2-Cys peroxiredoxin. Antioxid Redox Signal 15: 817–829, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Knuefermann P, Baumgarten G, Koch A, Schwederski M, Velten M, Ehrentraut H, Mersmann J, Meyer R, Hoeft A, Zacharowski K, Grohe C. CpG oligonucleotide activates Toll-like receptor 9 and causes lung inflammation in vivo. Respir Res 8: 72, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koczor CA, Shokolenko IN, Boyd AK, Balk SP, Wilson GL, Ledoux SP. Mitochondrial DNA damage initiates a cell cycle arrest by a Chk2-associated mechanism in mammalian cells. J Biol Chem 284: 36191–36201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koczor CA, Snyder JW, Shokolenko IN, Dobson AW, Wilson GL, Ledoux SP. Targeting repair proteins to the mitochondria of mammalian cells through stable transfection, transient transfection, viral transduction, and TAT-mediated protein transduction. Methods Mol Biol 554: 233–249, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Kojima K, Arikawa T, Saita N, Goto E, Tsumura S, Tanaka R, Masunaga A, Niki T, Oomizu S, Hirashima M, Kohrogi H. Galectin-9 attenuates acute lung injury by expanding CD14- plasmacytoid dendritic cell-like macrophages. Am J Respir Crit Care Med 184: 328–339, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol 32: 157–164, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Kung CT, Hsiao SY, Tsai TC, Su CM, Chang WN, Huang CR, Wang HC, Lin WC, Chang HW, Lin YJ, Cheng BC, Su BY, Tsai NW, Lu CH. Plasma nuclear and mitochondrial DNA levels as predictors of outcome in severe sepsis patients in the emergency room. J Transl Med 10: 130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang J, Jung Y, Tighe RM, Xie T, Liu N, Leonard M, Gunn MD, Jiang D, Noble PW. A macrophage subpopulation recruited by CC chemokine ligand-2 clears apoptotic cells in noninfectious lung injury. Am J Physiol Lung Cell Mol Physiol 302: L933–L940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol 180: 2562–2572, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab 1: 393–399, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BH, Lopes GA, Russo RC, Avila TV, Melgaco JG, Oliveira AG, Pinto MA, Lima CX, De Paula AM, Cara DC, Leite MF, Teixeira MM, Menezes GB. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 56: 1971–1982, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Michelakis ED, Archer SL, Weir EK. Acute hypoxic pulmonary vasoconstriction: a model of oxygen sensing. Physiol Res 44: 361–367, 1995 [PubMed] [Google Scholar]
- 57.Michelakis ED, Rebeyka I, Wu X, Nsair A, Thebaud B, Hashimoto K, Dyck JR, Haromy A, Harry G, Barr A, Archer SL. O2 sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res 91: 478–486, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Mills E, Jobsis FF. Mitochondrial respiratory chain of carotid body and chemoreceptor response to changes in oxygen tension. J Neurophysiol 35: 405–428, 1972 [DOI] [PubMed] [Google Scholar]
- 59.Mills E, Jobsis FF. Simultaneous measurement of cytochrome a3 reduction and chemoreceptor afferent activity in the carotid body. Nature 225: 1147–1149, 1970 [DOI] [PubMed] [Google Scholar]
- 60.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol 31: 3531–3545, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12: 222–230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, Lawler LA, Christie JD, Meyer NJ, Mc Causland FR, Waikar SS, Waxman AB, Chung RT, Bueno R, Rosas IO, Fredenburgh LE, Baron RM, Christiani DC, Hunninghake GM, Choi AM. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med 10: e1001577; discussion e1001577, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299: 896–899, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Nuutinen EM, Nishiki K, Erecinska M, Wilson DF. Role of mitochondrial oxidative phosphorylation in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol 243: H159–H169, 1982 [DOI] [PubMed] [Google Scholar]
- 65.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485: 251–255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ozawa T, Natori Y, Sako Y, Kuroiwa H, Kuroiwa T, Umezawa Y. A minimal peptide sequence that targets fluorescent and functional proteins into the mitochondrial intermembrane space. ACS Chem Biol 2: 176–186, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Panduri V, Liu G, Surapureddi S, Kondapalli J, Soberanes S, de Souza-Pinto NC, Bohr VA, Budinger GR, Schumacker PT, Weitzman SA, Kamp DW. Role of mitochondrial hOGG1 and aconitase in oxidant-induced lung epithelial cell apoptosis. Free Radic Biol Med 47: 750–759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 103: 1232–1240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piantadosi CA, Suliman HB. Redox regulation of mitochondrial biogenesis. Free Radic Biol Med 53: 2043–2053, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Piantadosi CA, Withers CM, Bartz RR, MacGarvey NC, Fu P, Sweeney TE, Welty-Wolf KE, Suliman HB. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J Biol Chem 286: 16374–16385, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care 14: R15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rachek LI, Grishko VI, Ledoux SP, Wilson GL. Role of nitric oxide-induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Radic Biol Med 40: 754–762, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Rachek LI, Grishko VI, Musiyenko SI, Kelley MR, LeDoux SP, Wilson GL. Conditional targeting of the DNA repair enzyme hOGG1 into mitochondria. J Biol Chem 277: 44932–44937, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Reyes A, He J, Mao CC, Bailey LJ, Di Re M, Sembongi H, Kazak L, Dzionek K, Holmes JB, Cluett TJ, Harbour ME, Fearnley IM, Crouch RJ, Conti MA, Adelstein RS, Walker JE, Holt IJ. Actin and myosin contribute to mammalian mitochondrial DNA maintenance. Nucleic Acids Res 39: 5098–5108, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ricci C, Pastukh V, Leonard J, Turrens J, Wilson G, Schaffer D, Schaffer SW. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol Cell Physiol 294: C413–C422, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Rogers RS, Bhattacharya J. When cells become organelle donors. Physiology 28: 414–422, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Ruchko M, Gorodnya O, LeDoux SP, Alexeyev MF, Al-Mehdi AB, Gillespie MN. Mitochondrial DNA damage triggers mitochondrial dysfunction and apoptosis in oxidant-challenged lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 288: L530–L535, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Ruchko MV, Gorodnya OM, Zuleta A, Pastukh VM, Gillespie MN. The DNA glycosylase Ogg1 defends against oxidant-induced mtDNA damage and apoptosis in pulmonary artery endothelial cells. Free Radic Biol Med 50: 1107–1113, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sabharwal SS, Waypa GB, Marks JD, Schumacker PT. Peroxiredoxin-5 targeted to the mitochondrial intermembrane space attenuates hypoxia-induced reactive oxygen species signalling. Biochem J 456: 337–346, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shadel GS. Mitochondrial DNA, aconitase ‘wraps’ it up. Trends Biochem Sci 30: 294–296, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Shokolenko I, Venediktova N, Bochkareva A, Wilson GL, Alexeyev MF. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res 37: 2539–2548, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simmons JD, Lee YL, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg 258: 591–596; discussion 596–598, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suliman HB, Carraway MS, Piantadosi CA. Postlipopolysaccharide oxidative damage of mitochondrial DNA. Am J Respir Crit Care Med 167: 570–579, 2003 [DOI] [PubMed] [Google Scholar]
- 84.Suliman HB, Carraway MS, Tatro LG, Piantadosi CA. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci 120: 299–308, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Suliman HB, Welty-Wolf KE, Carraway M, Tatro L, Piantadosi CA. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res 64: 279–288, 2004 [DOI] [PubMed] [Google Scholar]
- 86.Suliman HB, Welty-Wolf KE, Carraway MS, Schwartz DA, Hollingsworth JW, Piantadosi CA. Toll-like receptor 4 mediates mitochondrial DNA damage and biogenic responses after heat-inactivated E. coli. FASEB J 19: 1531–1533, 2005 [DOI] [PubMed] [Google Scholar]
- 87.Tasaka S, Kamata H, Miyamoto K, Nakano Y, Shinoda H, Kimizuka Y, Fujiwara H, Hasegawa N, Fujishima S, Miyasho T, Ishizaka A. Intratracheal synthetic CpG oligodeoxynucleotide causes acute lung injury with systemic inflammatory response. Respir Res 10: 84, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsushima K, King LS, Aggarwal NR, De Gorordo A, D'Alessio FR, Kubo K. Acute lung injury review. Intern Med 48: 621–630, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Wang K, Klionsky DJ. Mitochondria removal by autophagy. Autophagy 7: 297–300, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res 88: 1259–1266, 2001 [DOI] [PubMed] [Google Scholar]
- 91.Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res 99: 970–978, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 106: 526–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, Schumacker PT. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med 187: 424–432, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Waypa GB, Schumacker PT. Hypoxia-induced changes in pulmonary and systemic vascular resistance: where is the O2 sensor? Respir Physiol Neurobiol 174: 201–211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilson DF, Erecinska M. Effect of oxygen concentration on cellular metabolism. Chest 88: 229S–232S, 1985 [DOI] [PubMed] [Google Scholar]
- 96.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12–G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 14: 64–68, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA 94: 514–519, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med 14: 949–953, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ 16: 939–946, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011 [DOI] [PubMed] [Google Scholar]